Abstract
Erythropoiesis-stimulating agents (ESAs) are frequently used among patients with renal anemia, while a significant proportion of patients exhibit ESA hyporesponsiveness despite adequate dosing. Previous studies have suggested an inverse association between ESA hyporesponsiveness and statin use among patients receiving dialysis therapy. However, studies based on predialysis patients are extremely limited.
Based on electronic medical records of a tertiary hospital in Beijing, China between April 2010 and April 2015, we investigated the association between statin use and ESA hyporesponsiveness among patients with predialysis-chronic kidney disease (CKD).
Altogether 232 patients with CKD initiating ESA therapy and with hemoglobin levels monitored for at least 6 months were included in our analyses. Among them, 77 (38.5%) were long-term statin users (regular statin treatment for more than 3 months) before ESA initiation. Overall, 6.5% of the statin users and 17.1% of nonusers were considered to have ESA hyporesponsiveness. Long-term statin therapy was significantly associated with a lower proportion of ESA hyporesponsiveness in fully adjusted model (odds ratio 0.15, 95% confidence interval: 0.03–0.64).
We found that long-term statin therapy was inversely associated with ESA hyporesponsiveness among predialysis patients with CKD. Further studies are needed to validate our observations, and to explore the potential mechanisms between ESA resistance and statin therapy.
Keywords: chronic kidney disease, erythropoiesis-stimulating agent hyporesponsiveness, renal anemia, statin treatment
1. Introduction
Chronic kidney disease (CKD) has emerged as a leading public health issue with a high prevalence, poor outcome, and a heavy economic burden to society. Anemia is a highly prevalent complication among patients with CKD and is associated with an increased risk for death.[1] Previous epidemiologic studies have suggested that the prevalence of anemia is 12.3% in CKD stage 3, 22.7% in CKD stage 4, and more than 50% in CKD stage 5 patients.[2] The deficiency of erythropoietin (EPO) secretion is regarded to be the primary mechanism of renal anemia. As a result, the use of synthetic erythropoiesis-stimulating agents (ESAs) has been a mainstay of CKD-related anemia management, with some patients experiencing improved patient quality of life[3] and a reduced need for blood transfusion,[4] although randomized trial data have not shown a systematic benefit of ESAs for prolonging life or preventing cardiovascular events. In addition, an estimated 5% to 10% of patients with renal anemia exhibit an insufficient response to ESAs, as manifested by the persistence of anemia despite adequate dosing, or the requirement of higher than normal doses to achieve currently recommended hemoglobin targets.[5,6] Identification of mechanisms that affect EPO responsiveness could assist with development to strategies to optimize the management of anemia and improve the safety of ESA therapy among patients with CKD.
Several studies have reported an inverse association between ESA resistance and statin treatment. Sirken et al[7] reported that statin treatment successfully reduced ESA requirements by 25% in maintenance hemodialysis (MHD) patients. Chiang et al[8] also concluded from an interventional study that low-dose atorvastatin could improve ESA hyporesponsiveness in patients with end-stage renal disease (ESRD) receiving chronic dialysis. They postulated that the possible mechanism could be through the effect of statins on inflammation that in turn leads to ESA hyporesponsiveness among dialysis-dependent patients. Most existing studies have been conducted among dialysis-dependent patients with ESRD, but ESA therapy is also recommended in nondialysis-treated patients with CKD with hemoglobin concentration <100 g/L after the correction of other deficiency states (e.g., iron).[9] Moreover, poor ESA responders with CKD experience higher rates of cardiovascular events, death, and worse renal survival than patients with a better response.[6,10] Therefore, the reversal of ESA hyporesponsiveness among patients with CKD may be particularly important as it may provide an opportunity to alter the progression of renal failure and favorably impact mortality and cardiovascular outcomes. Despite the increasing use of statins in patients with CKD, the potential relation between statin treatment and ESA hyporesponsiveness is not well understood among these patients. The objective of this retrospective real world study was to examine the association of statin therapy with ESA hyporesponsiveness among Chinese patients with CKD. We hypothesized that long-term statin therapy may improve ESA hyporesponsiveness in these patients.
2. Methods
2.1. Source population and cohort assembly
The source population was derived from baseline and follow-up EMR data obtained between April 2010 and April 2015 from adult patients diagnosed with CKD managed in the nephrology department at Peking University People's Hospital, one of the largest tertiary healthcare facilities in Beijing. A retrospective cohort of prevalent adult patients with CKD who had newly initiated ESA therapy, continued ESA treatment for at least 6 months, and had hemoglobin levels monitored for at least 6 months and at intervals not >3 months were included in the study. The index date was the date of ESA initiation. We excluded patients who experienced any of the following conditions before the index date or during study period: diagnosis of malignant tumors or hematologic disorders (e.g., aplastic anemia and other chronic bleeding disorders), receipt of blood transfusion(s) during the study period, or missing information on statin use. Of note, all patients were receiving usual clinical care throughout the whole study period.
2.2. Data collection and definitions
The EMR data included key variables such as patient demographics, medical history, laboratory tests, and drug prescriptions. Information on age, gender, presumed cause of CKD, serum creatinine, albumin, high-sensitive CRP, hemoglobin level, serum iron, transferrin saturation and ferritin levels, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride levels, ESA dosing, statin use and doses, and other medication use were extracted from the EMR before and during the study period, with linking of data elements at the individual patient level.
Estimated glomerular filtration rate (eGFR) was based on the CKD-EPI equation[11] using baseline outpatient serum creatinine concentration and classified as stage 1 (eGFR ≥ 90 mL/min), stage 2 (eGFR 60–89 mL/min), stage 3 (eGFR 30–59 mL/min), stage 4 (eGFR 15–29 mL/min), and stage 5 (eGFR < 15 mL/min and not receiving renal replacement therapy).
Patients were considered statin nonusers if they had not previously received statin treatment and did not receive it during the study period. Patients were considered statin users if they were receiving statin treatment for at least 3 months prior to the index date and remained on statin treatment during follow-up.
The ESA hyporesponsiveness was defined as a failure, in the presence of adequate iron stores, to achieve and maintain the target hemoglobin level (110 g/L) at an ESA dose of or >300 IU/kg/wk when administered subcutaneously.[12]
2.3. Statistical analysis
Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC). Continuous variables were reported as mean values with standard deviations unless otherwise specified. Comparisons of continuous variables between 2 groups were performed using the t test or Wilcoxon signed rank tests where appropriate. The Chi-squared test was used to compare the categorical variables. Multivariable logistic regression models were constructed to assess the independent association between statin use and ESA hyporesponsiveness using a nested modeling approach that adjusted for different sets of clinical parameters including demographic, laboratory, and diagnostic measures. Specifically, the models progressively included patient demographics, baseline hemoglobin level, eGFR, serum albumin, baseline lipid profile, iron storage status, and receipt of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) treatment (Table 1). Odds ratio (OR) estimates were reported with 95% confidence intervals (CIs). Results were regarded as significant at the P < .05 level.
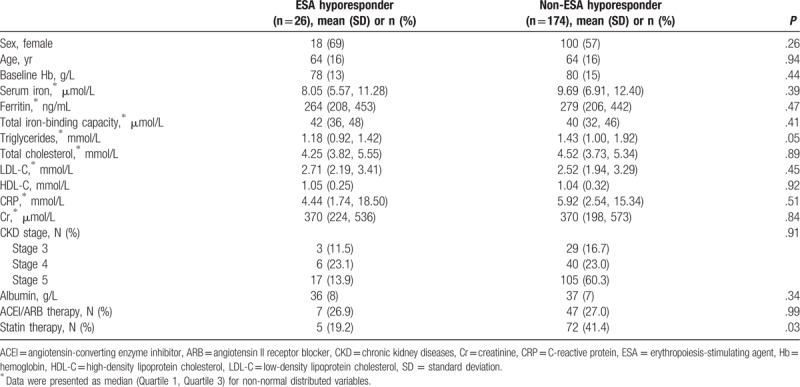
Table 1.
Univariate analysis of clinical factors associated with ESA hyporesponsiveness in adults with CKD.
3. Results
3.1. Study subjects
Among 2923 nondialysis-dependent patients with CKD who were older than 18 years and were followed up regularly at nephrology clinics between April 2010 and April 2015, 232 anemic patients who started ESA treatment for the 1st time and had hemoglobin levels monitored for at least 6 months and at intervals not >3 months were identified. Among these patients, 18 were excluded due to history of malignancy or hematologic disorders, 11 were excluded for receipt of blood transfusions during the study period, and 3 patients were excluded due to missing information related to statin usage prior to the index date or during the follow-up. Overall, 200 patients were included in the final analysis (Fig. 1).
Figure 1.
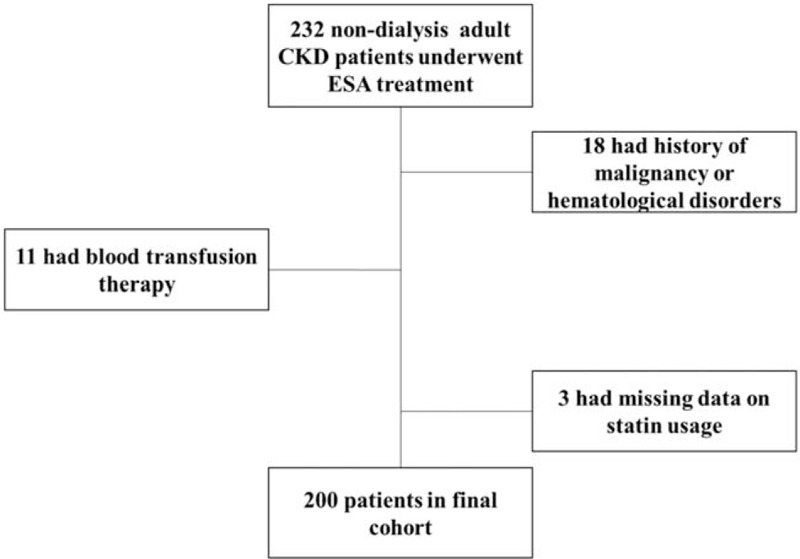
Assembly of cohort of Chinese adults with chronic kidney disease (CKD) initiating erythropoiesis-stimulating agent (ESA) therapy.
The mean age of patients with CKD included in this study was 64 (±16) years, ranging from 20 to 98, and 59% were women. Of these patients, 69 had diabetic nephropathy (34.5%), 66 had glomerulonephritis (33%), 41 had hypertensive kidney damage (21.5%), and 24 had other diseases (12%). At baseline, 16.0%, 23.0%, and 61.0% of subjects selected were in CKD stages 3, 4, and 5, respectively (Table 2). The mean follow-up duration was 23.6 (±13.4) months, ranging from 6 to 56 months. Median number of hemoglobin tests was 6 (interquartile range [IQR] = 3–10), and the median interval between 2 consecutive hemoglobin tests was 41 days (IQR = 28–88 days).
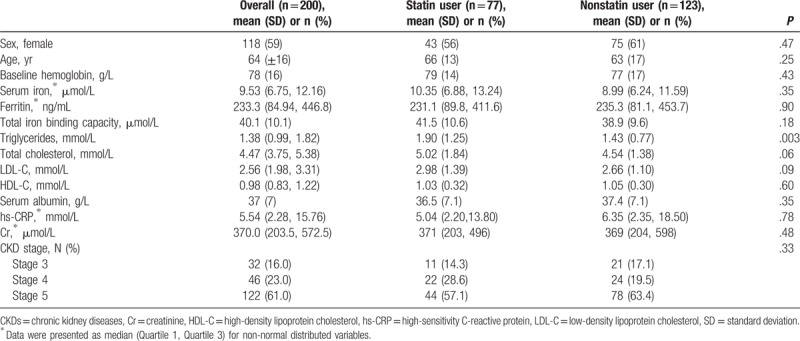
Table 2.
Baseline characteristics for patients with chronic kidney disease initiating erythropoiesis-stimulating agent therapy, overall and by statin treatment.
3.2. Statin treatment
A total of 123 (61.5%) patients with CKD were defined as statin nonusers and 77 (38.5%) were considered statin users. Among statin users, 55 (71.4%), 4 (5.2%), and 18 (23.4%) patients were treated for coronary heart disease, stroke, and dyslipidemia, respectively. The most common statin prescriptions were for atorvastatin 20 mg/d (45.5%), atorvastatin 10 mg/d (14.3%), rosuvastatin 10 mg/d (9.1%), simvastatin 20 mg/d (7.8%), and simvastatin 40 mg/d (14.3%). There were no significant differences across baseline clinical characteristics for statin users and nonusers, except for higher total cholesterol level among statin users at baseline (1.90 mmol/L vs 1.43 mmol/L, P = .003) (Table 2). Of note, highly sensitive C-reactive protein (hs-CRP) level did not differ by statin usage (P = .78).
3.3. ESA hyporesponsiveness
Among all studied subjects, 165 (82.5%) were on regular iron supplements before initiation of ESA therapy and during follow-up. Among patients receiving iron supplementation, 12 (7.3% of iron users) patients received intravenous iron and 153 (92.7% of iron users) were taking oral supplementation. At baseline, no patients had absolute iron deficiency, which was defined as ferritin level <200 ng/mL.[12] Baseline hemoglobin level was 78 (±16) g/L. The median dose of ESA for all patients was 133 IU/kg/wk (IQR = 100–282 IU/kg/wk).
Overall, 26 (13.0%) patients failed to reach target hemoglobin levels at a mean ESA dose of 348 ± 41 IU/kg/wk and were therefore classified as hyporesponders to ESA therapy. The rest of the patients with CKD (n = 174, 87.0%) achieved hemoglobin target within 6 months after ESA initiation at a mean dose of 220 ± 79 IU/kg/wk.
3.4. Effect of statin on ESA hyporesponsiveness
Overall, 6.5% of the statin users (5/77) and 17.1% of nonusers (21/123) were considered to have hyporesponsiveness to ESA therapy. In the univariate analysis, statin therapy was the only statistically significant factor associated with ESA hyporesponsiveness (P = .03, Table 1), while other previously reported factors associated with ESA hyporesponsiveness (e.g., iron insufficiency, inflammation, male gender, and ACEIs and/or ARB therapy[13–15]) were not found to be significantly associated with ESA hyporesponsiveness in our sample.
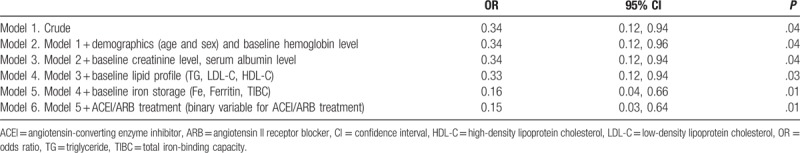
In logistic regression modeling, long-term statin treatment showed a protective association with ESA hyporesponsiveness in the unadjusted model (model 1, OR = 0.34, 95% CI: 0.12–0.96), and the association persisted after fully adjusting for all measured potential confounders (model 6, OR = 0.15, 95% CI: 0.03–0.64) (Table 3).
Table 3.
Nested logistic regression models assessing statin therapy with erythropoiesis-stimulating agent (ESA) hyporesponsiveness in patients with CKD.
4. Discussion
Among a cohort of Chinese adults with nondialysis-dependent CKD and anemia initiating ESA therapy, we found lower adjusted odds of ESA hyporesponsiveness with concomitant statin treatment. This association persisted after adjustment for patient demographics, baseline hemoglobin level, renal function, serum albumin, iron storage, lipid profile, and ACEI/ARB treatment.
Our study supports and extends previous studies that have reported an association between statin treatment and a lower risk of ESA hyporesponsiveness among patients with ESRD receiving chronic dialysis.[7,8,16] Sirken et al[7] reported that statin treatment could reduce ESA requirements by 25% in hemodialysis patients. Chiang et al[8] also observed that low-dose statin therapy (atorvastatin 10 mg/d) improved ESA responsiveness in dialysis-treated patients with ESRD in a prospective study. Our study builds upon these findings by exploring the association in less severe, nondialysis-dependent patients with CKD. It has been reported that poor ESA responders among patients with CKD are at a higher risk of cardiovascular events, death, and renal disease progression compared with patients that have a better response profile.[6,10] Therefore, the enhancement of ESA responsiveness among patients with CKD could potentially have important implications for clinical practice.
Other studies have consistently demonstrated a mechanistic link between chronic inflammation and ESA hyporesponsiveness, highlighting the association between increased inflammatory markers and a reduced response to ESA therapy.[16–19] In contrast, we did not observe an association between increased hs-CRP levels and ESA hyporesponsiveness as reported in other studies.[18,19] Reasons for this are not clear but may be explained, in part, by the timing of these measures, as baseline hs-CRP levels for statin users were taken and recorded after initiating statin treatment. Moreover, interleukin 6 (IL-6) may predict ESA responsiveness better than other biomarkers in patients with CKD.[17,20] It is also hypothesized that chronic inflammation can induce an enhanced state of T-cell activation, and the overproduction of erythropoiesis-inhibiting cytokines, such as IL-6 and tumor necrosis factor alpha (TNF-α), which may account for hyporesponsiveness to ESA therapy in patients with CKD. Furthermore, proinflammatory cytokines, including IL-6 and TNF-α, have been noted to be significantly suppressed after 12 weeks of statin therapy, resulting in an improved ESA responsiveness.[8] Unfortunately, information on these cytokines were unavailable in our study. Therefore, future studies should more thoroughly explore these and other potential contributing pathways that lead to ESA hyporesponsiveness in the setting of CKD.
One of the strengths of our study is inclusion of adults with CKD that were closely monitored after initiation of ESA therapy and who had complete clinical evaluations. We also adjusted for a wide range of possible confounders but still found a strong, favorable association between statin treatment and ESA hyporesponsiveness in Chinese patients with CKD.
Several limitations should also be considered. As an observational, retrospective study, we cannot prove a causal relation between statin treatment and improved ESA hyporesponsiveness. We classified patients’ potential responses to ESA as hyporesponsive vs nonhyporesponsive. However, given the complexity of longitudinal response possibilities to ESA, this simplification may have reduced the power to identify risk factors. In other studies, ESA-resistance index (ERI) was based on monthly average hemoglobin levels and in addition, ESA doses were frequently used to try to classify ESA responsiveness.[12] This index was not employed in our study because patients had hemoglobin measurements taken at varying time intervals, and ERI is considered to be more strongly related to EPO dose instead of patient-specific conditions.[21] Finally, our relatively small sample size restricted our ability to stratify patients further by statin type and dose. Therefore, we consider our study as a hypothesis-generating study that adds further support to the potential link between statin treatment and ESA responsiveness in patients with CKD.
5. Conclusion
Statin therapy reduces lipid concentrations and cardiovascular endpoints across the severity of CKD[22] and also reduces all-cause mortality in nondialysis-dependent patients with CKD.[23] We found that long-term statin treatment is independently and inversely associated with ESA hyporesponsiveness among Chinese adults with CKD, which suggests potential additional benefits of statin therapy in this population. Large-scale, prospective studies are warranted to directly address the effect of statin therapy on ESA hyporesponsiveness and to delineate modifiable mechanisms underlying ESA hyporesponsiveness in the setting of CKD.
Acknowledgments
The authors express their appreciation to the steering committee members of AstraZeneca, Jiemin Wang, MD, MPH, Claudia Cabrera, PhD, Jia Wei, PhD, and Alan S. Go. The authors are also grateful to the study nurses, physicians, and medical directors for the time and energy they have contributed to this study.
Author contributions
ZS and MW analyzed, interpreted the patient data and were major contributors in writing the manuscript. LZ reviewed all patients’ clinical information and verified diagnoses. All authors contributed to the design of the study, interpretation of results and writing of the manuscript. All authors read and approved the final manuscript.
Conceptualization: Zhun Sui, Mi Wang, Li Zuo.
Data curation: Mi Wang.
Formal analysis: Zhun Sui, Mi Wang.
Investigation: Zhun Sui, Mi Wang.
Methodology: Zhun Sui, Mi Wang.
Project administration: Zhun Sui, Mi Wang.
Resources: Zhun Sui, Mi Wang.
Software: Zhun Sui, Mi Wang.
Writing – original draft: Zhun Sui, Mi Wang.
Writing – review & editing: Li Zuo.
Footnotes
Abbreviations: ACEI = angiotensin-converting-enzyme inhibitor, ARB = angiotensin II receptor blocker, CKD = chronic kidney disease, EMR = electronical medical record, EPO = erythropoietin, ERI = erythropoiesis-stimulating agent resistance index, ESA = erythropoiesis-stimulating agent, ESRD = end-stage renal disease, eGFR = estimated glomerular filtration rate, HDL = high-density lipoprotein, hs-CRP = high sensitivity C-reactive protein, LDL = low-density lipoprotein.
ZS and MW contributed equally to this study.
The study protocol was approved by the ethics committee of the Peking University People's Hospital and performed in accordance with International Conference on Harmonization Good Clinical Practice (GCP, E6) and Declaration of Helsinki. As the study was retrospective, requirement of informed consent was waivered. Confidentiality of the patient data was maintained.
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Pisoni RL, Bragg-Gresham JL, Fuller DS, et al. Facility-level interpatient hemoglobin variability in hemodialysis centers participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS): associations with mortality, patient characteristics, and facility practices. Am J Kidney Dis 2011;57:266–75. [DOI] [PubMed] [Google Scholar]
- [2].Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed] [Google Scholar]
- [3].Gandra SR, Finkelstein FO, Bennett AV, et al. Impact of erythropoiesis-stimulating agents on energy and physical function in nondialysis CKD patients with anemia: a systematic review. Am J Kidney Dis 2010;55:519–34. [DOI] [PubMed] [Google Scholar]
- [4].Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 1989;111:992–1000. [DOI] [PubMed] [Google Scholar]
- [5].Kanbay M, Perazella MA, Kasapoglu B, et al. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif 2010;29:1–2. [DOI] [PubMed] [Google Scholar]
- [6].Minutolo R, Conte G, Cianciaruso B, et al. Hyporesponsiveness to erythropoiesis-stimulating agents and renal survival in non-dialysis CKD patients. Nephrol Dial Transplant 2012;27:2880–6. [DOI] [PubMed] [Google Scholar]
- [7].Sirken G, Kung SC, Raja R. Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. ASAIO J 2003;49:422–5. [PubMed] [Google Scholar]
- [8].Chiang CK, Yang SY, Peng YS, et al. Atorvastatin increases erythropoietin-stimulating agent hyporesponsiveness in maintenance hemodialysis patients: role of anti-inflammation effects. Am J Nephrol 2009;29:392–7. [DOI] [PubMed] [Google Scholar]
- [9].AHEMIÏ К. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Inter Suppl 2012;2:279. [Google Scholar]
- [10].Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010;363:1146–55. [DOI] [PubMed] [Google Scholar]
- [11].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].KDOQI; National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006;47:S11–45. [DOI] [PubMed] [Google Scholar]
- [13].Rossert J, Gassmann-Mayer C, Frei D, et al. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant 2007;22:794–800. [DOI] [PubMed] [Google Scholar]
- [14].Mallick S, Rafiroiu A, Kanthety R, et al. Factors predicting erythropoietin resistance among maintenance hemodialysis patients. Blood Purif 2012;33:238–44. [DOI] [PubMed] [Google Scholar]
- [15].Kalantar-Zadeh K, Lee GH, Miller JE, et al. Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis 2009;53:823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koc M, Dogan C, Arinsoy T, et al. Statin use is associated with lower inflammation and erythropoietin responsiveness index in hemodialysis patients. Hemodial Int 2011;15:366–73. [DOI] [PubMed] [Google Scholar]
- [17].Won HS, Kim HG, Yun YS, et al. IL-6 is an independent risk factor for resistance to erythropoiesis-stimulating agents in hemodialysis patients without iron deficiency. Hemodial Int 2012;16:31–7. [DOI] [PubMed] [Google Scholar]
- [18].Barany P, Divino Filho JC, Bergstrom J. High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 1997;29:565–8. [DOI] [PubMed] [Google Scholar]
- [19].Gunnell J, Yeun JY, Depner TA, et al. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 1999;33:63–72. [DOI] [PubMed] [Google Scholar]
- [20].Panichi V, Rosati A, Bigazzi R, et al. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant 2011;26:2641–8. [DOI] [PubMed] [Google Scholar]
- [21].Chait Y, Kalim S, Horowitz J, et al. The greatly misunderstood erythropoietin resistance index and the case for a new responsiveness measure. Hemodial Int 2016;20:392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Strippoli GF, Navaneethan SD, Johnson DW, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ 2008;336:645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barylski M, Nikfar S, Mikhailidis DP, et al. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy--a meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacol Res 2013;72:35–44. [DOI] [PubMed] [Google Scholar]


