Supplemental Digital Content is available in the text
Keywords: chronic heart failure, Qishenyiqi, traditional Chinese medicine
Abstract
Background:
Qishenyiqi dripping pill for chronic heart failure (CHF) remains controversial due to lack of high-quality trials. Therefore, we conduct this pooled-analysis to evaluate the efficacy and safety of Qishenyiqi in CHF patients.
Methods:
We searched for randomized clinical trials for Qishenyiqi dripping pill in treating CHF up to August 2018 through China National Knowledge Infrastructure (CNKI), the PubMed Database, the Wanfang Database, the China Scientific Journal Database (VIP), and the Chinese Biomedicine Literature Service System. RevMan 5.3 was used for pooled analyses. Based on the New York Heart Association (NYHA) classification, the clinical therapeutic effect was collected as the primary endpoint.
Results:
The efficacy and safety of Qishenyiqi combined with routine treatment significantly increased NYHA functional classification, left ventricular ejection fraction, cardiac index, and 6-minute walking test and decreased brain natriuretic peptide, left ventricular end-diastolic, and end-systolic dimensions with no obvious side effects in comparison with routine therapy alone.
Conclusions:
Together these results provide important insights into Qishenyiqi is effective and safe in improving ventricular remodeling and function of CHF patients.
PROSPERO registration number:
PROSPERO106695.
1. Introduction
Chronic heart failure (CHF), as the end stage of various kinds of cardiovascular disease, is a progressive clinical syndrome in which adequate cardiac output can not be supplied by the heart for the body's metabolic need and accommodating venous return.[1] Epidemiological studies showed that CHF has become more and more common in huge population worldwide, resulting in high rate of mortality and hospitalization, low life quality, and poor prognosis.[2–4] Therefore, inhibiting the occurrence and development of CHF plays a key role in the treatment of patients with cardiovascular disease.
Qishenyiqi dripping pill, one traditional Chinese medicine (TCM) formulation, was composed of astragalus membranaceus, salvia miltiorrhiza, panax notoginseng, and rosewood heart wood, and has been widely applied for coronary artery disease and CHF. Existing pharmacological researches showed that Qishenyiqi could inhibit cardiac ischemia-reperfusion injury and ventricular remodeling through regulating energy metabolism and rennin-angiotensin-aldosterone system.[5] Currently, there have been some randomized controlled trials (RCTs) about the cure effect of combination of Qishenyiqi and conventional Western medicine therapy on CHF, indicating that this TCM has relatively high clinical value in improving symptoms and prognosis of CHF patients. However, most of the RCTs had low methodological qualities. On the other hand, few studies focused on the association with Qishenyiqi treatment with ventricular remodeling. Thus, we need more convincing evidence to confirm the efficacy of Qishenyiqi in cardiac function improvement, especially in delaying ventricular remodeling. Here, we aimed to assess Qishenyiqi's efficacy and safety in CHF patients by searching for the most up-to-date RCT literature and using predefined and strict selection criteria.
2. Methods
2.1. Inclusion criteria for study selection
2.1.1. Types of studies
All RCTs regarding the efficacy and/or safety of Qishenyiqi in the treatment for CHF, with language in English or Chinese, were included. No publication-status restrictions were applied in our study.
2.1.2. Types of patients
Studies involving CHF patients with definite diagnosis were included. Age, sex and race were not be limited.
2.1.3. Types of interventions
Based on the treatment guidelines of CHF, the patients in the Qishenyiqi group were treated with the conventional Western medications and Qishenyiqi dripping pills, and those in the control group received the same conventional Western medications.
2.1.4. Types of outcome measures
2.1.4.1. Primary outcomes
Clinical therapeutic effect according to the New York Heart Association (NYHA) functional classification
2.1.4.2. Secondary outcomes
Left ventricular ejection fraction (LVEF)
Left ventricular end-diastolic dimension (LVEDD)
Left ventricular end-systolic dimension (LVESD)
Cardiac index (CI)
Brain natriuretic peptide (BNP)/N-terminal prohormone of BNP (NT-proBNP)
minute walking test (6MWT)
adverse reactions
2.1.5. Ethics and dissemination
The ethical approval is not necessary because our meta-analysis is secondary study and the data were extracted from other people's work.
2.2. Search methods for the identification of studies
2.2.1. Electronic searches
Two investigators (MZC and ZWZ) electronically and independently retrieved 6 databases (PubMed, Web of Science, EMBASE, Chinese National Knowledge Infrastructure [CNKI], Wanfang Database, and VIP Database) from their inception to August 2018. The searched items will be used as follows: “Qishenyiqi” [All Fields], “Chronic heart failure” [All Fields] and “Randomized controlled trial” [All Fields] without any additional filters. The detailed search strategy used in PubMed was shown in appendix 1 and similar but reasonable search terms were applied to the other databases.
2.2.2. Searching other resources
Related systematic reviews and meta-analyses of RCTs were electronically searched and the reference lists of included studies were manually reviewed. Also, we particularly retrieved the relevant conference proceedings for more recently published papers.
2.3. Data collection and analysis
2.3.1. Selection of studies
Two investigators (MZC and ZWZ) searched all the databases independently and screened the titles and abstracts of related studies to remove the duplicates, animal experiments, studies with self-contrast or focusing on acute heart failure and acute myocardial infarction, and trials about other TCM application. Then full texts were read and eligible studies were selected according to the inclusion standards. All disagreements were discussed together with the third investigator (PH). Details of the selection process of meta-analysis will be shown in a PRISMA flow chart (Fig. 1).
Figure 1.
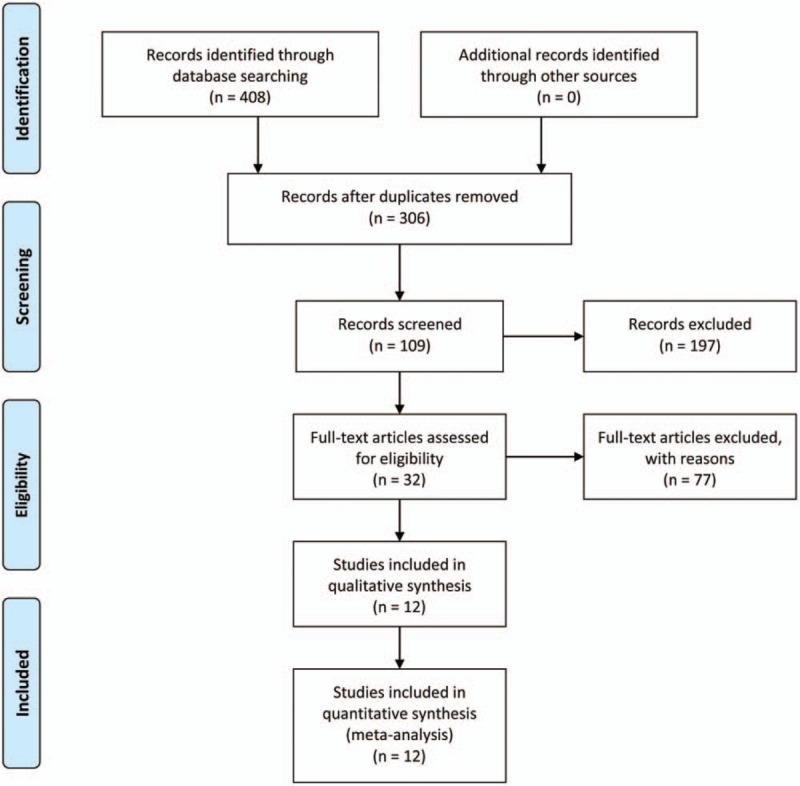
Flow diagram of the study selection process.
2.3.2. Data collection and management
Two investigators (MZC and ZWZ) read all the included articles and independently collected data via a standardized eligibility form. Available data in the retrieved articles, including the first author, year of publication, study design, sample size, age, sex, the severity of disease, intervention, and treatment applied in the control and case group, were extracted. Outcome measures and further information such as results of the study, adverse events, and conflicts of interest were extracted as well. Any divergence of the data extraction was discussed and resolved between the 2 investigators. The final results of the data extraction were collated by the third investigator (PPH). When the data were insufficient ambiguous, one of the reviewers contacted the original author to acquire additional and detailed information by telephone or E-mail.
2.4. Statistical analysis
RevMan 5.3 was used for pooled analyses. Chi-square and I2 tests were used to assess the heterogeneity among selected trials. We adopted fixed effects models for the pooled analyses with no heterogeneity (I2 < 50%) and random effects models for those with heterogeneity (I2 ≥50%). We calculated and evaluated odd ratio (OR) with 95% confidence interval (CI) for enumeration data and weighted mean difference (WMD) with 95% CI for continuous data. Two-tailed P < .05 was considered as statistically significant. Moreover, we evaluated publication bias using the fail-safe number (Nfs). The significance of Nfs was set at 0.05 for each pooled analysis (Nfs0.05 = [ΣZ/1.64]2 – k, k is the number of trials included in this analysis).
3. Results
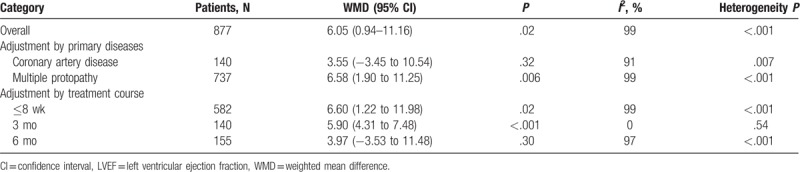
Eventually, we included and reviewed 12 RCTs for our pooled analysis.[6–17] Four among them were of high quality (Jadad score ≥3).[7,9,11,17] The baseline characteristics of the patients in the 12 trials were similar and the NYHA cardiac functions of the patients enrolled in these RCTs were graded >2 before treatment (Supplementary Table 1). Recommended dosage of Qishenyiqi in these patients was 0.5 g once orally, 3 times daily. Treatment with Qishenyiqi plus routine Western drugs could provide a better curative effect on cardiac function than routine Western drugs alone. Qishenyiqi therapy significantly increased NYHA functional class compared with control group (odds ratio [OR] 2.97, 95% confidence interval [CI] 1.86–4.75, P < .001) without significant heterogeneity (χ2 = 3.59, I2 = 0%, P = .83). There were significant heterogeneity in terms of left ventricular ejection fraction (LVEF) (χ2 = 1756.27, I2 = 99%, P < .001), cardiac index (χ2 = 26.48, I2 = 92%, P < .001), BNP (χ2 = 2423.45, I2 = 100%, P < .001), and 6MWT (χ2 = 37.17, I2 = 87%, P < .001), thus the random effects model was applied to compare Qishenyiqi combined with routine Western drugs and routine therapy alone for these endpoints. Qishenyiqi significantly increased LVEF (%) (WMD 6.05, 95% CI 0.94–11.16, P = .02), cardiac index (L min−1 m−2) (WMD 0.71, 95% CI 0.17–1.25, P = .01), 6MWT (m) (WMD 65.83, 95% CI 44.35–87.30, P < .001), and lowered BNP (pg/mL) (WMD −155.38, 95% CI −290.07 to –20.68, P = .02) as compared with routine therapy alone (Table 1). Moreover, Nfs0.05 in terms of each of the outcomes above was higher than the number of studies in the respective analysis, indicating a low risk of publication bias. In addition, we conducted subgroup analysis according to LVEF. The heterogeneity among trials was explained in part by the variability in primary diseases and treatment courses (Table 2).
Table 1.
Therapeutic effect of Qishenyiqi on ventricular function and remodeling in patients with chronic heart failure.
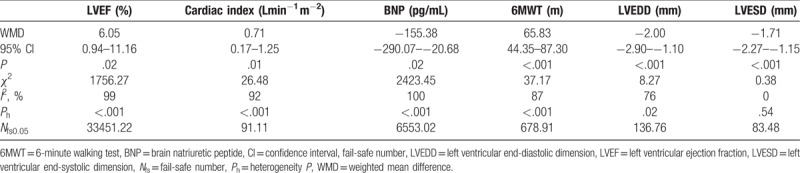
Table 2.
Subgroup analyses of the effect of Qishenyiqi on left ventricular ejection fraction.
Left ventricular end-diastolic dimension (LVEDD) and end-systolic dimension (LVESD) were selected as indices of left ventricular remodeling. Treatment with Qishenyiqi dripping pill significantly reduced LVEDD (mm) compared with control group (WMD −2.00, 95% CI −2.90 to −1.10, P < .001). Random effects model was used in this analysis as heterogeneity existed among studies (χ2 = 8.27, I2 = 76%, P = .02). Moreover, Qishenyiqi therapy significantly reduced LVESD (mm) compared with control group (WMD −1.71, 95% CI −2.27 to −1.15, P < .001) without significant heterogeneity (χ2 = 0.38, I2 = 0%, P = .54). Nfs0.05 for LVEDD and LVESD was calculated to be 136.76 and 83.48, respectively, demonstrating a negligible publication bias (Table 1).
All the included RCTs reported that neither obvious drug-related adverse reactions (such as headache, gastrointestinal discomfort, and arrhythmia) nor abnormal inspection results (hepatic and renal function, blood, urine and stool routine tests, and electrocardiogram examination) were observed in patients treated with TCM, which suggested that Qishenyiqi had a fairly good safety.
4. Discussion
To further explore the efficacy of Qishenyiqi dripping pills in CHF patients, our study investigated both the overall and sub-grouped analysis divided by primary diseases and treatment course. Significant association was found between taking Qishenyiqi dripping pills and heart-function improvements in CHF patients. Unknown high heterogeneity of LVEF might reflect the opinion of pathogenesis of CHF: its treatment is affected by a variety of underlying causes.[18]
Most RCTs included in those literature about Qishenyiqi's effects on cardiac function and ventricular remodeling were carried out before 2014 with relatively low quality. We have searched most recent publications of RCTs, only Jadad scale scored ≥2 were selected and analyzed, and totally 12 RCTs with more rigorous designs were included in this study.
Cardiac function parameters involving of LVEF, BNP, 6MWT, and cardiac index were used to analyze how Qishenyiqi dripping pills would help to normalize the cardiac function and increase the pumping force of patients’ hearts and exercise capacities. The LVEDD and LVESD were set as analyzed targets for ventricular remodeling.
Based on our analyses, we found that Qishenyiqi combined with routine Western drugs was more effective in improving LVEF, 6MWT, and cardiac index and reduced the levels of BNP, LVEDD, and LVESD with no adverse events with very low risk of publication bias, in comparison with routine Western drugs only. Our results provided strong evidence about Qishenyiqi's auxiliary effects on CHF sufferers’ outcomes, especially on restraining ventricular remodeling with high safety. As per literature, ventricular remodeling has been believed as the most important mechanism for the occurrence of CHF[19] and the insufficient sample capacity in the analysis for LVEDD and LVESD has suggested that further investigations on Qishenyiqi's effect on postponing ventricular remodeling are expected.
No adverse events associated with Qishenyiqi therapy were reported in the included RCTs. Meanwhile, despite the alleviation and advance of symptoms in the treatment for CHF, there are still adverse events that limit the use of the traditional drugs. For example, when patients take some antihypertensive drugs, some common adverse effects such as headache and bradycardia frequently occur.
We didn’t dig into the analysis results for LVEF, 6MWT, BNP, and cardiac index intensively due to their high heterogeneity. Though the included studies’ baselines were similar and comparable, we analyzed carefully and drew a conclusion that the noteworthy heterogeneity were potentially generated by patients’ ages, primary diseases, courses of diseases, original cardiac function, courses of treatment, and therapies of control groups. Only primary diseases and treatment course met the condition of subgroup analysis. Unknown high heterogeneity of LVEF might be caused by a variety of baseline factors of patients including age, sex, the primary disease, and medication differences. In addition, different therapy courses could be a better concern since no one had focused on it before. Furthermore, the association of Qishenyiqi and these basic factors were still not clear and more studies should be done to see Qishenyiqi's specific effects on patients with diverse basic illness backgrounds and to provide more rational administration of Qishenyiqi for CHF patients accordingly.
Several limitations of this study should be considered. Firstly, to pursue the high qualities of included RCTs, we had rejected most of the studies obtained from the first search and so the sample size of our study was not large enough. Secondly, 4 of the 12 finally included RCTs had high qualities (Jadad score ≥3) and the others didn’t do well in randomization concealment and double blindness with unclear description for exit and loss to follow-up. To address these problems, randomization concealment, double blindness, and description of exit and loss of follow-up have been taken into account when the quality assessment was conducted. In addition, we did not perform subgroup analyses according to subtypes and causes of heart failure due to insufficient data.
In conclusion, our results have showed that Qishenyiqi dripping pill might be effective and safe in improving ventricular remodeling and heart function in CHF patients. However, the efficacy and safety of Qishenyiqi for CHF patients still need further investigation with more large-sample, high-quality, and multicenter clinical trials.
Author contributions
Conceptualization: Mingzheng Chang.
Data curation: Mingzheng Chang, Lei Cheng.
Formal analysis: Yaqian Shen, Ying Zhang.
Funding acquisition: Panpan Hao, Zhongwen Zhang.
Methodology: Mingzheng Chang, Lei Cheng.
Project administration: Panpan Hao.
Panpan Hao orcid: 0000-0001-6903-1233.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 6MWT = 6-minute walking test, BNP= brain natriuretic peptide, CHF = chronic heart failure, CI = cardiac index, LVEDD = left ventricular end-diastolic dimension, LVEF = left ventricular ejection fraction, LVESD = left ventricular end-systolic dimension, NT-proBNP = N-terminal prohormone of BNP, NYHA = New York Heart Association, RCTs = randomized controlled trials, TCM = traditional Chinese medicine.
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2016HQ26 to ZWZ), the Key Research & Development Plan of Shandong Province (No. 2018GSF118176 to ZWZ and No. 2018GSF118234 to PPH), the National Natural Science Foundation of China (No. 81400284 to PPH), the Bethune-Merck's Diabetes Research Foundation (No. G2016014 to ZWZ and No. G2018030 to PPH), the China Cardiovascular Association-Cardiac Rehabilitation and Metabolic Therapy Research Fund (to PPH), and the Dyslipidemia and Atherosclerosis Research Fund (to PPH).
The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Shantsila E, Wrigley BJ, Shantsila A, et al. Monocyte-derived and CD34+/KDR+ endothelial progenitor cells in heart failure. J Thromb Haemost 2012;10:1252–61. [DOI] [PubMed] [Google Scholar]
- [2].Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chun L, Yong W, Qi Q, et al. Qishenyiqi protects ligation-induced left ventricular remodeling by attenuating inflammation and fibrosis via STAT3 and NF-kB signaling pathway. PLoS ONE 2014;9:e104255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368–76. [DOI] [PubMed] [Google Scholar]
- [5].Lin SQ, Wei XH, Huang P, et al. QiShenYiQi Pills® prevent cardiac ischemia-reperfusion injury via energy modulation. Int J Cardiol 2013;168:967–74. [DOI] [PubMed] [Google Scholar]
- [6].Sui S. Effect of Qishenyiqi dripping pill on plasma brain natriuretic peptide and cardiac function in patients with chronic heart failure [in Chinese] [master's thesis]. Shenyang, China: China Medical University; 2009. [Google Scholar]
- [7].Wu T. A clinical study on the treatment of heart failure caused by coronary heart disease using Qishenyiqi dripping pill [in Chinese] [master's thesis]. Guiyang, China: GuiYang College of Traditional Chinese Medicine; 2013. [Google Scholar]
- [8].Zhang B. An observation study on the treatment of chronic heart failure with Qishenyiqi dripping pill [in Chinese] [master's thesis]. Shenyang, China: Liaoning University of Traditional Chinese Medicine; 2013. [Google Scholar]
- [9].Lin G. Clinical observation of Qishenyiqi pills in the treatment of heart failure with qi deficiency and blood stasis [in Chinese] [master's thesis]. Guangzhou, China: Guangzhou University of Chinese Medicine; 2014. [Google Scholar]
- [10].Zhang Z, Yuan Y. Effect of Qishenyiqi dripping pill on cardiac function in elderly patients with chronic heart failure [in Chinese]. Med Innov China 2013;10:26–8. [Google Scholar]
- [11].Shao Z, Dai X, Mao J, et al. Effects of Qishenyiqi pills on cardiac function and high-sensitivity C-reactive protein in patients with chronic heart failure [in Chinese]. Chin J Exp Trad Med Formulae 2015;21:152–5. [Google Scholar]
- [12].Guan X, Deng B, Zhou X, et al. Effects of Qishenyiqi pills on ventricular remodeling and inflammatory factors in patients with chronic heart failure [in Chinese]. Lishizhen Med Mater Med Res 2013;24:681–2. [Google Scholar]
- [13].Jia H, Zhang K. Effects of Qishenyiqi dripping pills on cardiac function and NT-proBNP in patients with ischemic cardiomyopathy [in Chinese]. Chin J Exp Trad Med Formulae 2012;18:228–30. [Google Scholar]
- [14].Lv G. Effects of Qishenyiqi dripping pills on chronic congestive heart failure [in Chinese]. Clin Med 2012;32:113–5. [Google Scholar]
- [15].Sun Y. Clinical observation on the treatment of 60 patients with chronic heart failure with Qishenyiqi dripping pills [in Chinese]. Hunan J Trad Chin Med 2014;30:45–7. [Google Scholar]
- [16].Wang C. Efficacy of astragali radix bolus for replenishing qi in patients with chronic heart failure [in Chinese]. China Med Pharm 2016;6:62,207–4,207. [Google Scholar]
- [17].Wang H. Treatment of chronic congestive heart failure with integrated traditional Chinese and Western medicine [in Chinese]. Hunan J Trad Chin Med 2012;28:21–3. [Google Scholar]
- [18].Ingrid H, Kellie E. Chronic heart failure. Austral Prescriber 2017;40:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fertin M, Dubois E, Belliard A, et al. Usefulness of circulating biomarkers for the prediction of left ventricular remodeling after myocardial infarction. Am J Cardiol 2012;110:277–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


