Abstract
Rationale:
Pneumatosis intestinalis (PI) and hepatic portal venous gas (HPVG) are rare but potentially lethal conditions in which gas pathologically accumulates in the portal vein and intestinal wall, respectively. Proposed mechanisms include flatus escaping through an injured intestinal mucosa into the submucosa and thence into the portal venous system, or bacterial translocation (BT) of gas-forming enteric microorganisms from the gut into and through the intestinal wall to other organs. However, there has been no clear histopathological evidence to support these hypotheses.
Patient concerns:
A 61-year-old man underwent sigmoidectomy for colonic adenocarcinoma. Postoperatively, he developed paralytic ileus and then had a sudden cardiopulmonary arrest.
Diagnoses:
PI and HPVG were found at autopsy, presumably caused by the postoperative paralytic ileus and associated with BT of gas-forming organisms.
Interventions:
Cardiopulmonary resuscitation was unsuccessful.
Outcomes:
Postmortem imaging indicated the presence of massive PI and HPVG. At autopsy, there was marked intestinal emphysema with diffuse ischemic mucosal necrosis and severe pneumatosis in the stomach and intestine and marked gaseous dilation of the intrahepatic portal veins. Postmortem bacterial cultures revealed enteric bacteria in the peripheral blood and liver tissue.
Lessons:
Postoperative ileus leading to intestinal mucosal damage may be associated with BT of gas-forming enteric bacteria and the rapid onset of PI and HPVG with a lethal outcome.
Keywords: bacterial translocation, gas-forming bacteria, hepatic portal venous gas, paralytic ileus, pneumatosis intestinalis
1. Introduction
Pneumatosis intestinalis (PI) and hepatic portal venous gas (HPVG) are abnormal accumulations of gas in the intestinal wall and portal vein, respectively. Bacterial translocation (BT) is defined as the passage of bacteria and bacterial endotoxins from the gut to other organs.[1] Experimental studies have suggested that PI, HPVG, and BT share a similar pathogenetic mechanism in which mucosal disruption allows for the invasion of flatus and infectious agents into tissues where they are not normally present.[2,3] However, these mechanisms have not been fully understood because of insufficient histopathologic and postmortem evidence in humans. Moreover, little has been reported on the clinical and pathologic relationship between BT and pneumatoses such as PI and HPVG.
We present the case of a 61-year-old Japanese man who died after a sigmoidectomy complicated by postoperative paralytic ileus, with the subsequent rapid onset of BT, PI, and HPVG. We review the ante- and postmortem clinical, radiological, and histopathological findings.
2. Case report
A 61-year-old Japanese man, with no relevant previous history of disease, was admitted with suspected sigmoid colon cancer. He had been evaluated at another hospital with computed tomography after a positive fecal occult blood test. Physical examination of the patient on admission to our hospital revealed obesity (body mass index: 34.2 kg/m2) with normal vital signs, a blood pressure of 125/73 mmHg, and a pulse rate of 67 with a regular rhythm. Laboratory data on admission were within reference ranges, except for slight hyperglycemia (hemoglobin A1c 6.3%, reference range: 4.6–6.2%) and mild renal dysfunction (creatinine 1.14 mg/dL, reference range: 0.6–1.1 mg/dL). Computed tomography revealed a well-demarcated solid mass in the sigmoid colon. Endoscopic biopsy of the mass demonstrated a well to moderately differentiated adenocarcinoma.
Sigmoidectomy and regional lymph node dissection were performed. After the resection of the sigmoid, an initial attempt to perform colorectal anastomosis using a 29-mm diameter intraluminal stapler failed, as the intestinal wall was partially torn because of excessive tensile strength. Eventually, anastomosis was successfully completed using a 25-mm diameter stapler with no air leak. Pathological examination of the resected specimen revealed a moderately differentiated adenocarcinoma of the sigmoid colon, pT1, pN0, M0, stage I (according to the 8th edition of the TNM classification), which had been completely resected (R0).
The postoperative course was uneventful until 5 days after the operation. The patient had begun drinking water on the 6th postoperative day, but he vomited twice and complained of abdominal discomfort at that day. An upright abdominal radiograph showed marked gaseous dilatation of the stomach, small intestine, and colon with air-fluid levels, indicating paralytic ileus (Fig. 1). Therefore, the patient again resumed fasting that evening. The nursing staff noted that he was in bed and conscious at 10:30 pm. However, at 1:50 am, he was found in the toilet in cardiopulmonary arrest. Resuscitation was started immediately but was unsuccessful, and he was pronounced dead.
Figure 1.
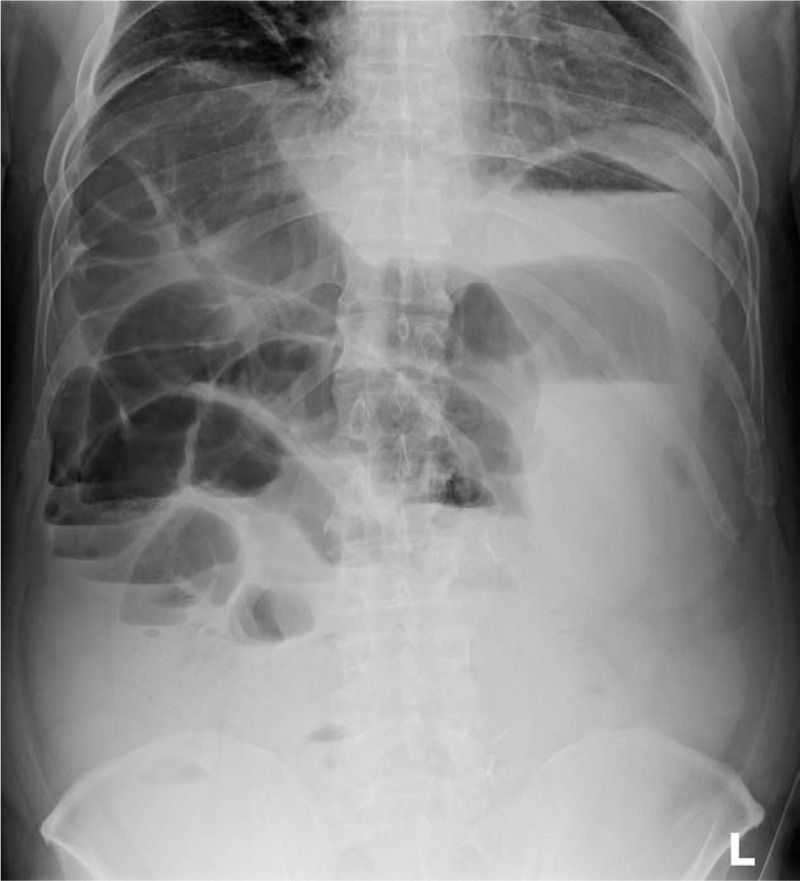
Abdominal upright radiograph 5 days after the operation. The stomach and intestine are markedly dilated with gas, and air-fluid levels are seen, indicating paralytic ileus.
An autopsy was performed. Computed tomography revealed marked PI, gastric pneumatosis, and HPVG (Fig. 2), but it did not indicate any clear cause of these pneumatosis. There was abdominal distention, but the surgical wound appeared normal for that stage postoperatively. There was sanguineous ascites in the abdominal cavity. However, there was no leakage from the colorectal anastomosis, nor was any residual or metastatic colon cancer found, indicating that the sigmoidectomy itself did not appear to have contributed to the outcome. However, there was marked gastric and intestinal pneumatosis, especially from the jejunum to the ileum, with the intestines spilling out as soon as the abdomen was opened (Fig. 3A and B). Histologically, there were ischemic changes indicated by mucosal thinning and necrosis of the gastrointestinal wall, as well as marked pneumatosis in the mucosal lamina propria and submucosa (Fig. 3C–F). The intrahepatic portal veins were markedly dilated due to gas accumulation, but the portal vein walls themselves were structurally normal (Fig. 4). There was centrilobular liver cell congestion and necrosis. Enterococcus faecalis and Klebsiella oxytoca were detected in blood drawn from the heart and also in tissue harvested from the liver, indicating BT and subsequent bacteremia. Informed written consent was obtained from the patient for publication of this case report and accompanying images.
Figure 2.
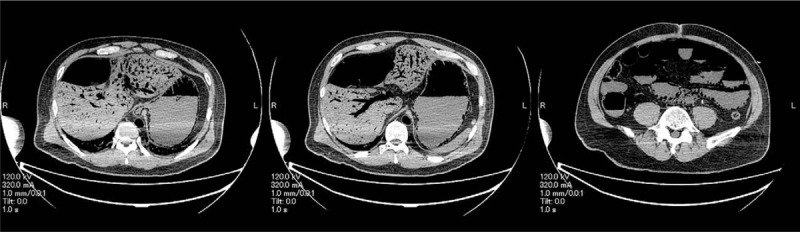
Postmortem computed tomography reveals extensive dilatation of intrahepatic portal veins in left and right branches and central and peripheral vessels and marked gastric and intestinal distention with fine, bubble-like aerocysts in the walls. These radiological findings indicate the presence of hepatic portal venous gas, pneumatosis intestinalis, and gastric pneumatosis.
Figure 3.
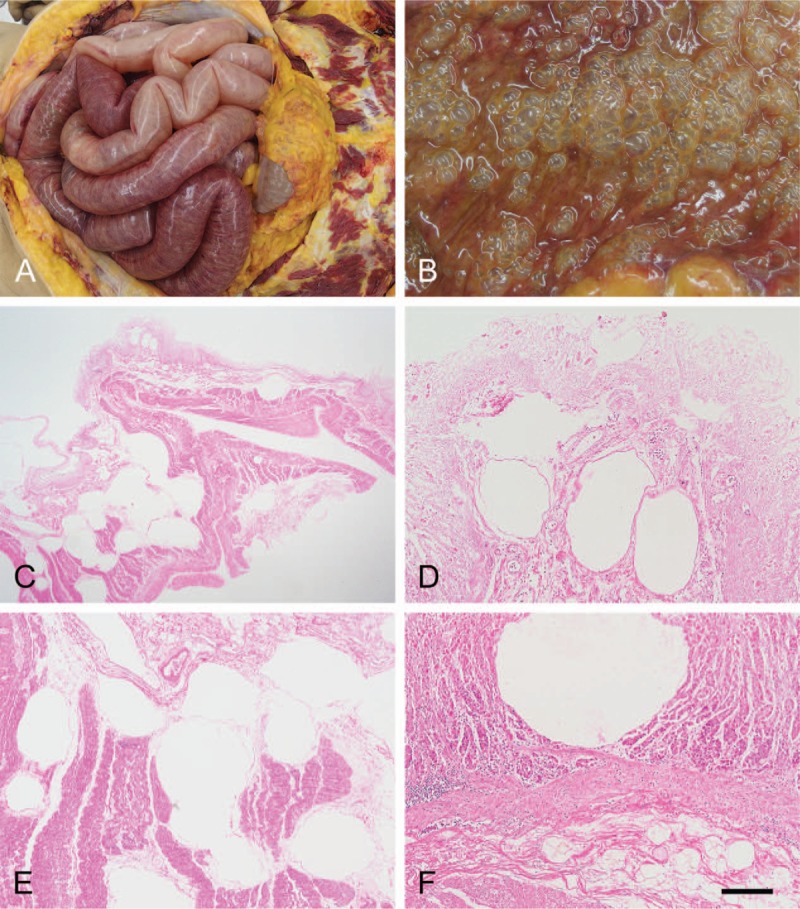
Autopsy findings in the intestine and stomach. (A) Markedly dilated and necrotic small intestine that spilled out as the abdomen was opened, suggesting an increased intestinal and intra-abdominal pressure. (B) Numerous bubble-like aerocysts are diffusely present in the intestinal mucosa. (C–F) Histologically, aerocysts are identified particularly in the lamina propria mucosae and submucosa of the small intestine (C–E) and stomach walls (F). Hematoxylin and eosin staining. Bar = 1.5 mm for C, and 200 μm for D, E, and F.
Figure 4.
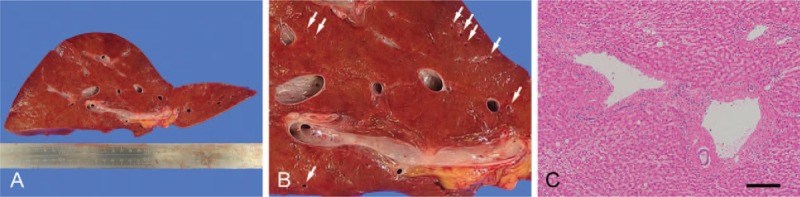
Autopsy findings in the liver. (A, B) Central and peripheral (arrow) portal veins are markedly dilated. (C) Histologically, the portal veins in Glisson's sheaths are dilated, but the venous walls are structurally normal. Hematoxylin and eosin staining. Bar = 200 μm.
3. Discussion
This is a rare autopsy case of PI and HPVG along with BT, which may itself have contributed to the pneumatosis. These were the findings in a patient who underwent an apparently uneventful sigmoidectomy but then developed paralytic ileus, with subsequent intestinal necrosis and bacteremia. The patient's only known risk for postoperative complications was obesity. His sudden deterioration was completely unexpected. Although there have been a few reports of PI and HPVG associated with BT, they were based only on clinical and radiological observations rather than histopathological findings.[4–7]
BT is defined as passage of bacteria and bacterial endotoxins from the gut through the mucosa to other organs.[1] Intraluminal microorganisms are able to breach the intestinal mucosal barrier in an event that can be seen in cases of intestinal obstruction, inflammatory bowel disease, jaundice, acute pancreatitis, organ transplantation, Stevens–Johnson syndrome, and malignant neoplasms.[8–16] In the present case, the postmortem morphologic analysis revealed extensive necrosis of the mucosal epithelia in the small and large intestine. The intestinal crypts overlying the gas-distended submucosa were partially desquamated. There was no increase in the numbers of lymphocytes and plasma cells over what are usually seen in normal, uninflamed mucosa. These histological changes were compatible with a diagnosis of ischemic enterocolitis. In the presence of ileus, as seen in our presenting patient, a rapid increase in intestinal luminal and intra-abdominal pressure can lead to multisystem organ failure, including intestinal ischemia and hepatic fragility.[17] The autopsy findings of the patient suggest that postoperative paralytic ileus may have led to widespread ischemic intestinal necrosis and subsequent BT.
Generally, BT is clinically confirmed by blood cultures positive for intestinal bacterial flora.[18] The autopsy in this patient revealed that E faecalis was isolated not only from the blood but also from the liver tissue, suggesting that BT may have led to lethal bacteremia. Enterococcus species, including E faecalis, are part of the normal flora of the human gut.[19] However, life-threatening E faecalis infections, including urinary tract infections, intra-abdominal and pelvic infections, meningitis, and endocarditis, are sometimes seen in hospitalized patients with weakened immune systems as a result of surgery, cancer treatment, dialysis, organ transplantation, and immunodeficiency.[20–23] As discussed below, PI and HPVG may be also induced in a patient postoperatively by BT by gas-forming E faecalis. The aggressiveness of E faecalis depends on a patient's clinical status independent of the virulence of the bacterial strains.[24] Thus, postoperative patients are unusually vulnerable to E faecalis infection, which worsens disease severity and clinical outcome.[25,26]
PI and HPVG are rare but potentially lethal conditions mostly associated with several acute abdominal conditions such as inflammatory bowel disease, infections, graft-versus-host disease, diverticulitis, bowel obstruction, and ischemic enterocolitis.[27–29] Although the mechanism is not fully understood, many researchers hold to a mechanical theory, in which PI and HPVG stem from flatus escaping through a damaged intestinal mucosa into the submucosal stroma and vessels and, from there, into the portal venous system. An association between BT and PI and HPVG has rarely been described in the literature. However, in an experimental study, PI was induced artificially by injecting gas-forming enteric bacilli into the bowel wall in rats.[3] The present autopsy case may support the theory that this same mechanism may occur in humans, namely, that PI and HPVG may arise from BT of gas-forming microorganisms such as Enterobacter and Klebsiella species. Normally, the intestinal mucosal barrier physiologically resists such invasion, but mucosal necrosis disrupts the barrier's integrity, which can predispose to BT. Thus, both the mechanical pressure from the distended gut and BT by gas-forming bacteria may act synergistically to produce PI and HPVG, a potentially lethal condition even in patients with no known risks. In patients with postoperative ileus, close attention to possible bacterial infections are warranted to avoid clinical deterioration.
Author contributions
Sayumi Tahara wrote the manuscript; Yasuhiro Sakai wrote and organized the manuscript; and Hidetoshi Katsuno, Makoto Urano, Makoto Kuroda, and Tetsuya Tsukamoto critically reviewed the manuscript and had useful professional suggestions. All authors reviewed the final version of the manuscript.
Conceptualization: Yasuhiro Sakai, Makoto Kuroda, Tetsuya Tsukamoto.
Supervision: Makoto Kuroda, Tetsuya Tsukamoto.
Validation: Hidetoshi Katsuno, Makoto Urano, Makoto Kuroda, Tetsuya Tsukamoto.
Writing – original draft: Sayumi Tahara.
Writing – review & editing: Yasuhiro Sakai.
Yasuhiro Sakai orcid: 0000-0001-7210-9249.
Footnotes
Abbreviations: BT = bacterial translocation, HPVG = hepatic portal venous gas, PI = pneumatosis intestinalis.
The authors have no funding or conflicts of interest to disclose.
References
- [1].Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979;23:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kay-Butler JJ. Interstitial emphysema of the caecum. Gut 1962;3:267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yale CE, Balish E, Wu JP. The bacterial etiology of pneumatosis cystoides intestinalis. Arch Surg 1974;109:89–94. [DOI] [PubMed] [Google Scholar]
- [4].Ginesu GC, Barmina M, Cossu ML, et al. Conservative approach to hepatic portal venous gas: a case report. Int J Surg Case Rep 2017;30:183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Slawinski C, Parkin E, Casey P, et al. Hepatic portal venous gas complicating Bickerstaff's encephalitis with Guillain–Barré overlap. BMJ Case Rep 2015;2015:bcr2015211514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sadatomo A, Koinuma K, Kanamaru R, et al. Hepatic portal venous gas after endoscopy in a patient with anastomotic obstruction. World J Gastrointest Surg 2015;7:21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Venkatesh B, Tesar P. Hepatic portal venous gas in a critically ill patient. Anaesth Intensive Care 1998;26:575–8. [DOI] [PubMed] [Google Scholar]
- [8].Sagar PM, MacFie J, Sedman P, et al. Intestinal obstruction promotes gut translocation of bacteria. Dis Colon Rectum 1995;38:640–4. [DOI] [PubMed] [Google Scholar]
- [9].Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg 1989;124:699–701. [DOI] [PubMed] [Google Scholar]
- [10].Kabaroudis A, Papaziogas B, Koutelidakis I, et al. Disruption of the small-intestine mucosal barrier after intestinal occlusion: a study with light and electron microscopy. J Invest Surg 2003;16:23–8. [PubMed] [Google Scholar]
- [11].Takesue Y, Ohge H, Uemura K, et al. Bacterial translocation in patients with Crohn's disease undergoing surgery. Dis Colon Rectum 2002;45:1665–71. [DOI] [PubMed] [Google Scholar]
- [12].Sakrak O, Akpinar M, Bedirli A, et al. Short and long-term effects of bacterial translocation due to obstructive jaundice on liver damage. Hepatogastroenterology 2003;50:1542–6. [PubMed] [Google Scholar]
- [13].Kuzu MA, Kale IT, Col C, et al. Obstructive jaundice promotes bacterial translocation in humans. Hepatogastroenterology 1999;46:2159–64. [PubMed] [Google Scholar]
- [14].Deitch EA, Sittig K, Li M, et al. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg 1990;159:79–84. [DOI] [PubMed] [Google Scholar]
- [15].Schoeffel U, Pelz K, Haring RU, et al. Inflammatory consequences of the translocation of bacteria and endotoxin to mesenteric lymph nodes. Am J Surg 2000;180:65–72. [DOI] [PubMed] [Google Scholar]
- [16].Takesue Y, Kakehashi M, Ohge H, et al. Bacterial translocation: not a clinically relevant phenomenon in colorectal cancer. World J Surg 2005;29:198–202. [DOI] [PubMed] [Google Scholar]
- [17].Sugerman HJ, Bloomfield GL, Saggi BW. Multisystem organ failure secondary to increased intraabdominal pressure. Infection 1999;27:61–6. [DOI] [PubMed] [Google Scholar]
- [18].MacFie J. Bacterial translocation in surgical patients. Ann R Coll Surg Engl 1997;79:183–9. [PMC free article] [PubMed] [Google Scholar]
- [19].Layton BA, Walters SP, Lam LH, et al. Enterococcus species distribution among human and animal hosts using multiplex PCR. J Appl Microbiol 2010;109:539–47. [DOI] [PubMed] [Google Scholar]
- [20].Arias CA, Murray BE. Bennett JE, Dolin R, Blaser MJ. Enterococcus species, Streptococcus gallolyticus group, and Leuconostoc species. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia, PA: Saunders; 2015. 2328–39. [Google Scholar]
- [21].Pintado V, Cabellos C, Moreno S, et al. Enterococcal meningitis: a clinical study of 39 cases and review of the literature. Medicine (Baltimore) 2003;82:346–64. [DOI] [PubMed] [Google Scholar]
- [22].Hill EE, Herijgers P, Claus P, et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007;28:196–203. [DOI] [PubMed] [Google Scholar]
- [23].Garrison RN, Fry DE, Berberich S, et al. Enterococcal bacteremia: clinical implications and determinants of death. Ann Surg 1982;196:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ceci M, Delpech G, Sparo M, et al. Clinical and microbiological features of bacteremia caused by Enterococcus faecalis. J Infect Dev Countr 2015;30:1195–203. [DOI] [PubMed] [Google Scholar]
- [25].Barie PS, Christou NV, Dellinger EP, et al. Pathogenicity of the enterococcus in surgical infections. Ann Surg 1990;212:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sitges-Serra A, López MJ, Girvent M, et al. Postoperative enterococcal infection after treatment of complicated intra-abdominal sepsis. Br J Surg 2002;89:361–7. [DOI] [PubMed] [Google Scholar]
- [27].Koss LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol 1952;53:523–49. [PubMed] [Google Scholar]
- [28].Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg 1990;212:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abboud B, El Hachem J, Yazbeck T, et al. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J Gastroenterol 2009;15:3585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


