Abstract
Rationale:
Neuroacanthocytosis (NA) is a heterogeneous group of inherited neurodegenerative disorders characterized by misshapen spiculated erythorcytes and symptoms that resemble Huntington's disease.
Patient concerns:
A 59-year-old female who developed hyperkinetic involuntary movements that became progressively more obvious during the course of a year.
Diagnoses:
Acanthocytes were observed in a peripheral blood smear. The patient had elevated levels of serum creatine kinase (CK). Gene sequencing did not reveal a genetic mutation.
Interventions:
The patient was administered oral tiapride, alprazolam, B1 and B12 Vitamins.
Outcomes:
After 2 months of treatment the patient's symptoms were obviously alleviated. At the 6 month follow-up, the patient could feed herself and walk without assistance.
Lessons:
The NA syndrome is extremely rare. It may be identified in the clinic based on abnormal orofacial movement, chorea, cognitive decline, elevated CK levels, and acanthocytosis. If available, protein- or genetic-based testing may provide a confirmatory diagnosis.
Keywords: chorea, erythrocyte acanthocytosis, neuroacanthocytosis
1. Introduction
Neuroacanthocytosis (NA) is a heterogeneous group of inherited neurodegenerative disorders characterized by misshapen spiculated erythorcytes (acanthocytes) and symptoms that resemble Huntington's disease. The NA syndromes present with movement disorders, such as choreoathetosis, orofacial dyskinesia, dystonia, and Parkinsonism,[1] as well as cognitive impairments, psychiatric symptoms, seizures, and axonal neuropathy. The NA pathophysiology involves progressive degeneration of the basal ganglia.
Core NA syndromes include chorea-acanthocytosis (ChAc) caused by mutations of the vacuolar protein sorting 13 homolog A (VPS13A) gene, and McLeod syndrome (MLS) caused by mutations’ of the Kx gene.[2] Other inherited neurodegenerative disorders that may present with acanthocytosis include Huntington's disease-like 2 (HDL2), pantothenate kinase associated neurodegeneration (PKAN),[3] and neurodegeneration with brain iron accumulation (NBIA).[4] Differential diagnosis among NA syndromes is based on their genotypic and phenotypic heterogeneity.
The NA syndromes are rare, with an estimated prevalence of 1 to 5 in 1 million individuals.[5] The NA syndromes may be underdiagnosed due to their low prevalence and high clinical variability. Here, we report the case of a Chinese patient with an NA syndrome. The patient presented with chorea, other movement disorders, and acanthocytosis.
2. Case report
A 59-year-old female patient presented to our institution with a 1-year history of involuntary tongue movements that affected eating and speech. She initially experienced symptoms in October 2016. The patient also had a 1-year history of anxiety, restlessness, and cognitive impairment. A psychiatrist diagnosed her as obsessive-compulsive disorder and provided psychotherapy. Subsequently, her symptoms fluctuated. During the next 3 months, the patient's hyperkinetic involuntary movements became progressively more obvious. She developed tongue protrusion, orofacial dyskinesia, dysarthria, dysphagia, generalized chorea, dystonic movements of the feet, and bilateral areflexia and hypotonia, without evident muscle weakness and muscle atrophy. The patient's temperature was normal and her past medical history was unremarkable. The Babinski, Chaddock, and meningeal irritation signs were negative.
Brain magnetic resonance imaging (MRI) revealed mild age-related atrophy of the bilateral globus pallidus, frontal lobe, temporal lobe, and cerebellum (Fig. 1). Electroencephalogram (EEG) and cerebrospinal fluid (CSF) analysis were normal. Serum creatine kinase (CK) level was 612U/L (normal range, 25–200U/L). Routine blood analysis, blood sedimentation rate, hepatic function, renal function, thyroid function, and serum levels of folacin, Vitamin B12, and tumor markers were normal. The patient was non reactive for anti-HIV antibodies and syphilis. Acanthocytes were observed in a peripheral blood smear (Fig. 2).
Figure 1.
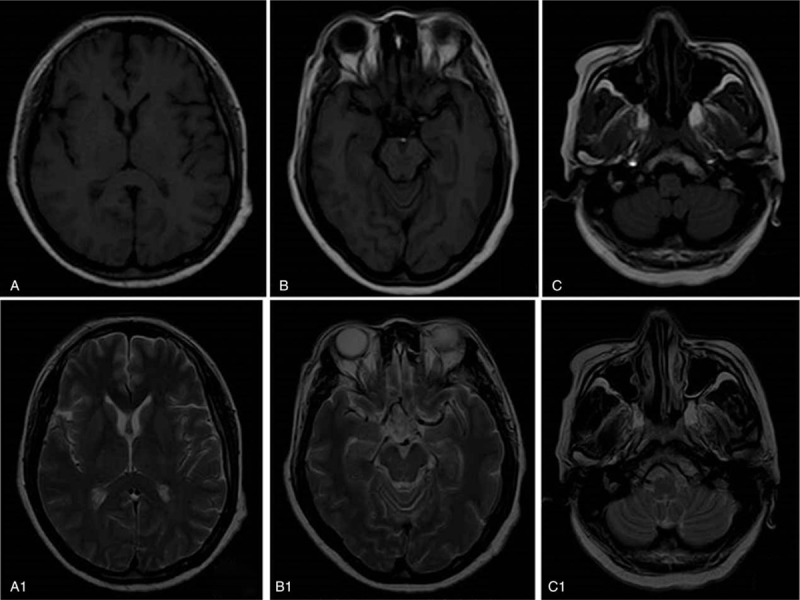
Neuroimaging: brain magnetic resonance imaging (MRI) T1WI (A, B and C) and T2WI (A1, B1 and C1) revealed mild atrophy of the bilateral globus pallidus (A, A1), frontal lobe (A, A1), temporal lobe (B, B1), and cerebellum (C, C1). MRI = magnetic resonance imaging.
Figure 2.
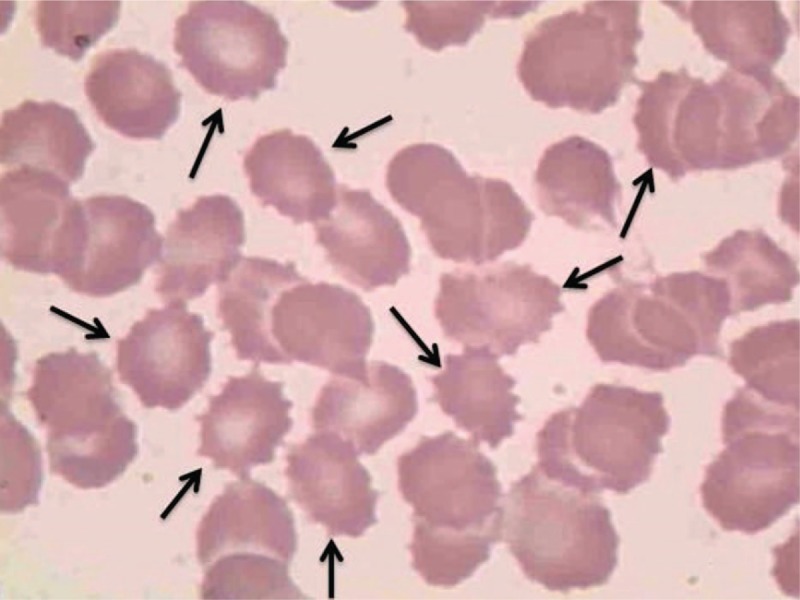
Peripheral blood smear showing acanthocytes as misshapen spiculated red blood cells (arrows) (×100).
Symptoms including tongue protrusion, orofacial dyskinesia, dysarthria, generalized chorea, dystonic movements of the feet, areflexia, absence of myopathy, cognitive impairment, neuropsychiatric involvement, elevated serum CK levels, and acanthocytes in a peripheral blood smear were considered consistent with a diagnosis of ChAc.
Genetic testing was performed, and no mutations were found in the XK, PANK2, APOB, SC5D, KCNN4, MTTP, VPS13A, or GATA1 genes.
The patient was administered oral tiapride (100 mg t.i.d) to treat chorea, alprazolam (0.8 mg q.d. at bedtime) to improve sleep, Vitamin B1 (20 mg, t.i.d), and Vitamin B12 (0.05 mg, t.id.). After 2 months of treatment the patient's symptoms were obviously alleviated. At the 6 month follow-up, the patient could feed herself and walk without assistance. The study was approved by the local ethics committee of Jilin University, Changchun, China. And the patient has provided informed consent for publication of the case.
3. Discussion
The NA refers to a heterogeneous group of inherited clinical syndromes that require protein-based or molecular genetic testing to make a final definitive diagnosis.[2] The diagnosis of NA in China is mainly based on clinical manifestations and an elevated proportion of acanthocytes in the blood, as well as the exclusion of other possible diseases. In a review of published Chinese case studies, hyperkinetic movements and orofacial dyskinesia were the most common symptoms of NA, reported in 88% and 80% of patients, respectively.[6] The NA syndromes are underdiagnosed, and generally there is a delay between the onset of symptoms and diagnosis, emphasizing the need to screen blood smears in uncertain cases.
Although diagnosis of a NA syndrome may be supported by reviewing a peripheral blood smear, the presence of acanthocytes in peripheral blood smears has limited utility as a diagnostic test. A negative result does not exclude a diagnosis of an NA syndrome, and the presence of acanthocytosis has not been associated with specific clinical symptoms in NA.[7] Furthermore, acanthocytosis can be seen in other conditions, including mitochondrial diseases, anorexia nervosa, and hypothyroidism,[2] several congenital hemolytic anemias characterized by mutations in erythrocyte cytoskeletal or membrane proteins, alterations in red blood cell shape, and deceased number of mature red cells in the circulation, and conditions involving movement disorders.[8] Acanthocytosis is characterized by abnormal shaped erythrocytes that have unevenly distributed irregular spicules in contrast to the biconcave shape of normal red blood cells.[5] An “acanthocytic state” is suggested by the presence of an acanthocytic cell shape and alterations in the function and interdependent membrane properties of erythrocytes. In NA syndromes, the occurrence of acanthocytes is not associated with anemia or other hematological symptoms. It is conceivable that a common factor/pathway influencing erythrocyte membrane shape homeostasis is present in all NA syndromes. The same factor/pathway may be active in neurons and its dysregulation may be associated with neurodegeneration.
The NA is very rare, but it is the most common form of hereditary chorea after Huntington's disease. The NA syndromes have phenotypes that resemble Huntington's disease.[5] Patients with Huntington's disease present with cognitive impairment, behavioral dysfunction, and movement disorders (e.g., chorea, dystonia). Patients with NA have additional symptoms, including orofacial self-mutilation, dyskinesia, seizures, and peripheral neuropathy. Neuropsychiatric symptoms are common in NA, including disinhibition, trichotillomania, depression, anxiety, and impairment of memory and executive skills ranging from minor abnormalities to dementia.[2]
Clinical investigations have identified CK and liver enzymes as possible biomarkers of NA. In a review of published Chinese case studies, 52% of Chinese NA cases had elevated CK levels.[6] However, these data should be interpreted with caution, as values within normal ranges are likely to have been omitted from many publications.[9]
In the current case, the patient presented with hyperkinetic movements, cognitive impairment, neuropsychiatric involvement, and acanthocytes in a peripheral blood smear, which is consistent with a diagnosis of ChAc. Published evidence indicates that ChAc has been reported in multiple countries, including Brazil,[10] China,[11] and Japan.[12] Mutations of the VPS13A gene are responsible for ChAc. The VPS13A gene encodes the protein chorein, which may control cycling of proteins through the trans-Golgi network to endosomes, lysosomes, and the plasma membrane.[13] In the current case, gene sequencing did not identify a mutation in the VPS13A gene, indicating that our patient's pathogenesis was not heredity.
The ChAc typically presents in early adulthood with hyperkinetic involuntary movements. The ChAc is characterized by chorea, but other movement disorders may also be present, including dystonia, Parkinsonism, and tics.[2] Tongue-protrusion dystonia during eating accompanied by involuntary biting of the tongue and lips are very suggestive of ChAc, with tongue-and lip-biting occurring in 40% of cases.[14,15,16] Self-mutilation caused by tongue and lip biting may be avoided by keeping an object such as a stick between the teeth. This may function as a mechanical block or a sensory trick. In the current case, the patient's son provided similar proprioceptive maneuvers to protect his mother.
Cognitive decline and neuropsychological abnormalities (especially frontal lobe disturbances) are found in 2/3 of patients with ChAc.[16] In the current case, the patient initially presented with hyperkinetic movements. She experienced progressive cognitive impairment during the next year. Swallowing and speech dysfunction were evident and debilitating, due to increasing loss of the muscle control required for these functions. Executive functioning became challenging, and the patient was unable to perform activities of daily living or communicate with others. The patient's cognitive impairment was dramatically reversed after symptomatic treatment. Due to the fluctuating nature of the patient's neuropsychiatric symptoms, she required a relatively long follow up to determine the evolution of symptoms.
The neuropathology of ChAc is characterized by an obvious decrease in neurons, predominantly in the striatum, accompanied by reactive astrogliosis and activation of microglia. Macroscopically, there is atrophy of cortical and subcortical structures. Autopsy studies have shown mild atrophy of the bilateral caudate nuclei and involvement of the putamen, globus pallidus, substantia nigra, and pyramida.[17] One autopsy study investigating pathological changes in 2 brothers with ChAc and Parkinsonism without chorea demonstrated preferential loss of striatal substance P-containing projection neurons and parvalbumen positive interneurons (PVIs) in the striatum. There was a reduced number of PVIs in the cerebral cortex, and the most severe loss occurred in the temporal lobe.[18] In these cases, the substantia nigra was intact.[18]
Neuroimaging in ChAc with brain MRI shows a symmetric reduction in gray matter volume in the caudate nucleus,[16] which is in accordance with the clinical presentation of extrapyramidal motor disturbances and cognitive symptoms. An increased T2 signal is common in the bilateral putamen and caudate nucleus. Tracer imaging studies with single-photon emission computed tomography (SPECT) and positron emission tomography (PET) showed the reduction of striatal tracer uptake was greater in the caudate nucleus than in the putamen, indicating that hypometabolism proceeds MRI signal changes and gray-matter atrophy in the caudate nucleus and putamen. There are occasional reports of iron deposition in the striatum and globus pallidus or cerebellar atrophy.[19] In the current case, brain MRI showed mild atrophy of the bilateral globus pallidus, frontal lobe, temporal lobe, and cerebellum. There was no obvious atrophy in the caudate nucleus and putamen.
This study suggests that NA syndromes may be identified in the clinic based on abnormal orofacial movement, chorea, cognitive decline, elevated CK levels, and acanthocytosis, with or without characteristic findings on brain MRI. If available, protein- or genetic-based testing may provide a confirmatory diagnosis.
Author contributions
Conceptualization: Jingyao Liu.
Data curation: Teng Zhao, Hui Zhu.
Formal analysis: Jingyao Liu, Hui Zhu.
Investigation: Teng Zhao, Hui Zhu.
Methodology: Xuemin Feng.
Project administration: Jingyao Liu.
Resources: Hui Zhu.
Supervision: Xuemin Feng.
Writing – original draft: Xue-min Feng, Hui Zhu
Writing – review & editing: Jingyao Liu.
Footnotes
Abbreviations: ChAc = chorea-acanthocytosis, CK = creatine kinase, CSF = cerebrospinal fluid, CT = computed tomography, EEG = electroencephalogram, HDL2 = Huntington's disease-like 2, MLS = McLeod syndrome, MRI = magnetic resonance imaging, NA = neuroacanthocytosis, NBIA = neurodegeneration with brain iron accumulation, PET = positron emission tomography, PKAN = pantothenate kinase associated neurodegeneration, PVIs = parvalbumen positive interneurons, SPECT = single-photon emission computed tomography.
HZ and X-mF These authors contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Jung HH, Danek A, Walker RH. Neuroacanthocytosis syndromes. Orphanet J Rare Dis 2011;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walker RH. Untangling the thorns: advances in the neuroacanthocytosis syndromes. J Mov Disord 2015;8:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walker RH, Jung HH, Dobson-Stone C, et al. Neurologic phenotypes associated with acanthocytosis. Neurology 2007;68:92–8. [DOI] [PubMed] [Google Scholar]
- [4].Chen PY, Lai SC, Yang CC, et al. A novel XK gene mutation in a Taiwanese family with McLeod syndrome. J Neurol Sci 2014;340:221–4. [DOI] [PubMed] [Google Scholar]
- [5].Dulski J, Sołtan W, Schinwelski M, et al. Clinical variability of neuroacanthocytosis syndromes—a series of sixpatients with long follow-up. Clin Neurol Neurosurg 2016;147:78–83. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Bader B, Danek A. Neuroacanthocytosis in China: a review of published reports. Tremor Other Hyperkinet Mov (NY) 2014;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schiessl-Weyer J, Roa P, Laccone F, et al. Acanthocytosis and the c.680 A>G mutation in the PANK2 gene: a study enrolling a Cohort of PKAN patients from the Dominican Republic. PloS One 2015;10:e0125861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bader B, Walker RH, Vogel M, et al. Tongueprotrusion and feeding dystonia: a hallmark of chorea-acanthocytosis. Mov Disord 2010. 127–9. [DOI] [PubMed] [Google Scholar]
- [9].Bader B, Danek A, Walker RH. Walker RH. Chorea-acanthocytosis. The differential diagnosis of chorea. New York: Oxford University Press; 2011. 122–48. [Google Scholar]
- [10].Ueno S, Maruki Y, Nakamura M, et al. The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nat Genet 2001;28:121–2. [DOI] [PubMed] [Google Scholar]
- [11].Rodrigues GR, Walker RH, Bader B, et al. Chorea-acanthocytosis: report of two Brazilian cases. Mov Disord 2008;23:2090–3. [DOI] [PubMed] [Google Scholar]
- [12].Ong B, Devathasan G, Chong PN. Choreoacanthocytosis in a Chinese patient—a case report. Singapore Med J 1989;30:506–8. [PubMed] [Google Scholar]
- [13].Hirose G. Walker RH, Saiki S, Danek A. Neuroacanthocytosis in Japan-review of the literature and cases. Neuroacanthocytosis Syndromes II. 1st ed.Germany: Springer Berlin Heidelberg; 2008. 75–84. [Google Scholar]
- [14].Schneider SA, Aggarwal A, Bhatt M, et al. Severe tongue protrusion dystonia: clinical syndromes and possible treatment. Neurology 2006;67:940–3. [DOI] [PubMed] [Google Scholar]
- [15].Gooneratne IK, Weeratunga PN, Gamage R. Teaching video neuroimages: orofacial dyskinesia and oral ulceration due to involuntary biting in neuroacanthocytosis. Neurology 2014;82:e70. [DOI] [PubMed] [Google Scholar]
- [16].Danek A, Jung HH, Melone MAB, et al. Neuroacanthocytosis: new developments in a neglected group of dementing disorders. J Neurol Sci 2005;229-230:171–86. [DOI] [PubMed] [Google Scholar]
- [17].Miki Y, Nishie M, Ichiba M, et al. Chorea-acanthocytosis with upper motor neuron degeneration and 3419_3420 delCA and 3970_3973 delAGTC VPS13A mutations. Acta Neuropathol 2010;119:271–3. [DOI] [PubMed] [Google Scholar]
- [18].Connolly BS, Hazrati LN, Lang AE. Neuropathological findings in chorea-acanthocytosis: new insights into mechanisms underlying parkinsonism and seizures. Acta Neuropathol 2014;127:613–5. [DOI] [PubMed] [Google Scholar]
- [19].Henkel K, Danek A, Grafman J, et al. Head of the caudate nucleus is most vulnerable in chorea–acanthocytosis: a Voxel-based morphometry study. Mov Disord 2006;21:1728–31. [DOI] [PubMed] [Google Scholar]


