Abstract
To analyze whether neoadjuvant chemotherapy (NAC) changes the expression rates of invasive ductal carcinoma (IDC) markers: estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), Ki67, and P53.
This was a retrospective study of 112 IDC patients who underwent NAC (docetaxel+epirubicin/pirarubicin+cyclophosphamide) but without pathological complete response (pCR) in 2012 to 2013 at the First Affiliated Hospital of Chongqing Medical University. The IDC subtypes and tumor protein markers were analyzed by immunohistochemistry (IHC). Specific changes in tumor protein markers before/after NAC were compared.
The decrease in the positive rate of Ki-67 was the most significant, from 75.9% before NAC to 41.1% after NAC (P < .001). The positive rate of HER2 decreased from 42.0% before NAC to 32.1% after NAC (P = .04). The positive rate of ER decreased from 66.1% before NAC to 56.2% after NAC (P = .04). Increased number of metastatic lymph nodes (P = .006) and body mass index (BMI) (P = .028) seemed to be related to conversion of PR (positive to negative). There was statistical association between the Ki-67 (positive to negative) with the age greater or equal to 50 (P = .015). The BMI greater or equal to 24 (P = .021), age greater or equal to 50 (P = .047), and blood type A (P = .038) were independently associated with conversion of P53 (positive to negative). The BMI greater or equal to 24 (P = .004), number of metastatic lymph nodes greater or equal to 1 (P = .029) and TNM stages I–II (P = .008) were statistically associated with change of HER2 (positive to negative).
In patients without pCR, NAC leads to changes in Ki-67, HER2, and hormone receptor (HR) expression. Age, BMI, number of metastatic lymph nodes, and TNM stage are associated with some changes of markers.
Keywords: breast cancer subtype, IDC, markers, neoadjuvant chemotherapy
1. Introduction
Conversion of the hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) status after neoadjuvant chemotherapy (NAC) can be used to predict the prognosis of breast cancer patients.[1] However, there is a lack of data about markers’ exact conversions in patients with invasive ductal carcinoma (IDC) without pathological complete response (pCR).
Breast cancer (BC) is a heterogeneous disease encompassing all malignant lesions arising in the breast, including IDC, invasive lobular carcinoma (ILC), and ductal carcinoma in situ (DCIS). In the United States, BC is the most common cancer among women and the 2nd most common cause of death among women.[2] The lifetime risk of BC among American women is 12.4%.[2] In China, BC remains the most common cancer in women, but its incidence in China is lower than in the United States (21.6 vs 76.0 per 100,000 women),[3] suggesting differences in risk factors and tumor biology. Nevertheless, BC remains an important health issue in China.
The NAC has been established as a standard treatment strategy for patients with locally advanced BC and operable BC that have to be down-sized to improve respectability.[4] The NAC has some advantages, such as a reduction in the extent of surgery, providing information on the sensitivity to chemotherapy,[5] reducing the size of the tumor, down-staging the tumor, improving the probability of breast conserving surgery, and destroying distant micrometastatic lesions.[6]
Besides the histological subtypes, BC can also be classified according to protein expression and molecular profile. Using immunohistochemistry (IHC), BC can be classified as these subtypes: HR-positive, HER2-positive, and triple-negative; these subtypes have distinct natural history and therapeutic approaches.[7–10] Using a genome-wide approach, it is now known that BC can be classified into 7 biologic subtypes: luminal A, luminal B, luminal C, HER2-enriched, basal-like, claudin-low, and normal breast-like.[7] Luminal A BC represents 40% of all cases; they are sensible to hormonal manipulations, but less to chemotherapy; their prognosis is favorable.[11] Luminal B BC represents 20% of all cases; they are characterized by genomic instability, poor response to NAC, and a poorer prognosis than luminal A BC.[11] The HER2-enriched BC is characterized by HER2 overexpression and represents 20 to 30% of all BC; their prognosis is poorer than luminal A BC.[11] Basal-like BC represents about 15% of all BC; they are generally ER-negative, PR-negative, and HER2-negative; their prognosis is poor.[11] Genomic profiling is expensive and not available everywhere, but IHC can be used to estimate the genomic profile (Table 1).[12]
Table 1.
The 13th St Gallen international expert consensus on breast cancer subtype[12].
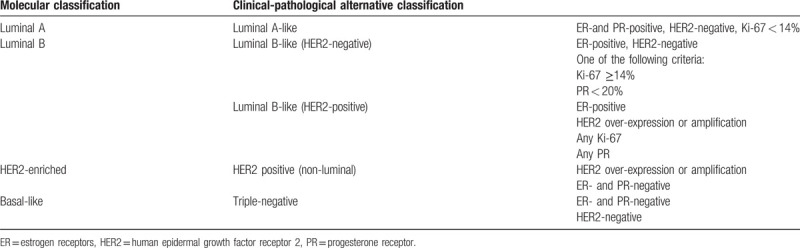
The 2013 St Gallen Consensus Conference focused on the choice of treatment options, based on BC subtypes, for the treatment of HER2-positive BC and triple-negative BC, and the original recommendation for systemic treatment was basically maintained.[13] The expert group considered that the main purpose to distinguish between luminal A (sensitive to endocrine therapy, indolent, with good prognosis) and luminal B (insensitive to endocrine therapy, with strong invasion, and poor prognosis) is to determine whether adjuvant cytotoxic chemotherapy is effective in these patients.[13] Therefore, tumor protein markers play a very important role in determining BC subtypes, in order to directly determine the treatment strategy and prognosis of patients with BC.
Some studies revealed that NAC affects the tumor protein markers expression and status.[14–21] The classification of BC subtypes based on tumor protein markers plays a very important role in systemic therapy and prognosis.[22] Therefore, because of these changes, chemotherapy, endocrine, and/or targeted therapy cannot be made based only on the IHC results obtained before NAC. Instead, systemic therapy should be guided by the results of multiple IHC from before and after NAC, and from eventual recurrences.[23]
Most studies examined the prognosis impact of these changes in tumors with pCR to NAC.[13–20] Therefore, the purpose of the present study was to analyze the pathological data of patients without pCR after NAC and to analyze the actual changes of tumor protein markers of IDC.
2. Material and methods
2.1. Study design and patients
This was a retrospective study of patients with IDC treated between July 2012 and December 2013 at the Endocrine and Breast Surgery of the First Affiliated Hospital of Chongqing Medical University (China). The inclusion criteria were: First, underwent core needle biopsy (CNB) before NAC. Second, received 4 courses of standard NAC (docetaxel, 75 mg/m2 iv d1, epirubicin/pirarubicin, 50 mg/m2 iv dl, cyclophosphamide, 500 mg/m2 iv dl, q21d). Third, female. Fourth, available clinical and radiologic assessments, pathology reports, and operative reports. The exclusion criteria were: First, inflammatory BC. Second, de novo metastatic. Third, bilateral BC. Fourth, accepted other types of therapies, such as endocrine therapy, radiation therapy, targeted therapy, etc. Fifth, achieved pCR after NAC. The PCR was determined by microscopic examination of the resected tumor and lymph nodes after NAC. The PCR was defined as the disappearance of all invasive lesions from the breast and lymph nodes. The presence of DCIS only after NAC was considered as pCR.[24,25]
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University. The need for individual consent was waived by the Board because of the retrospective nature of the study.
2.2. Data collection
Age, body mass index (BMI), body surface area (BSA), menstruation, American Joint Committee on Cancer (AJCC) TNM stage,[26] Nottingham grade, and tumor location were collected from the medical charts. According to the classification criteria of China, BMI > 24.0 was defined as overweight.[27] For each sample, the histological type and the Nottingham grade of the tumor were determined according to the criteria of Elston and Ellis:[28] The 3 to 5 points was regarded as grade I (high differentiation), 6 to 7 points was regarded as grade II (moderate differentiation), and 8 to 9 points was regarded as grade III (poor differentiation).
2.3. Immunohistochemistry
The histological diagnosis was performed on formalin-fixed (within 30 minutes of sampling) and paraffin-embedded breast tissue blocks from pretreatment biopsies and mastectomies. All IHC analyses were carried out at the Pathology Department of Chongqing Medical University and evaluated by light microscopy blindly and independently by 2 pathologists (assistant professor and professor); in case of disagreement, the case was reviewed by a committee of 5 pathologists (professors). The antibodies were: monoclonal mouse antibody against ER-α (clone 1D5; 1:200; Dako, Glostrup, Denmark); monoclonal mouse antibody against PR (clone pgR636; 1:200; Dako, Glostrup, Denmark); monoclonal antibody against Ki67 (clone MIB-1; 1:200; Dako, Glostrup, Denmark); polyclonal antibody against HER2/neu (1:200; Dako, Glostrup, Denmark); and monoclonal antibody against human p53 (DO-7; 1: 200; Dako, Glostrup, Denmark). The cutoff value for estrogen receptor (ER) and progesterone receptor (PR) positivity was 10% positive tumor cells with nuclear staining.[29] The HR negativity was defined as negative for both ER and PR.
The HER2 status was determined as 0, 1+, 2+, or 3+ in accordance with the guidelines published by Sauter et al[30]. Tumors with a score of 0 or 1+ were regarded as HER2-negative and those with a score of 3+ were regarded as HER2-positive. Tumors with a 2+ staining were tested for gene copy numbers of Her2 by in-situ hybridization. Using a kit with 2 probes of different colors (ZytoDot, 2C SPEC HER2/CEN17, Zyto Vision Ltd, Bremerhaven, Germany), the gene copy numbers of HER2 and centromeres of the corresponding chromosome 17 were retrieved. A HER2/CEP17 ratio of ≥ 2.2 was considered as amplification of HER2.
According to the 2014 St Gallen Consensus,[31] PR > 20% helps improve the accuracy of distinguishing between luminal A and luminal B BC. A P53 expression > 10% is considered as a positive expression.[32] Ki67 was scored as the percentage of nuclei-stained cells out of all cancer cells in the invasive front of the tumor regardless of the intensity in ×400 high-power fields; 500 to 1000 tumor cells were counted in each case. For Ki-67, ≥14% was considered as positive.[13] Classification criteria for BC subtypes were based on the 2013 St Gallen Consensus (Table 1).[12]
2.4. Statistical analysis
Analyses were conducted using SPSS 16.0 (IBM, Armonk, NY). The clinicopathological parameters (age, BMI, BSA, and tumor protein markers expression rates) were continuous data and expressed as mean ± standard deviation. Menopausal status, number of offspring, blood group, TNM stage, and the number of changes in tumor protein markers were categorical data and expressed as number and percentage. The average expression rates of ER, PR, P53, and Ki-67 before and after NAC were evaluated using the paired t test. Changes in tumor protein markers and BC subtypes before and after NAC were paired categorical data and analyzed using the McNemar and McNemar-Bowker tests. Multivariate regression analysis was used to determine the predictor of markers changing after NAC. Two-sided P-values < .05 were considered statistically significant.
3. Results
3.1. Characsteristics of the patients
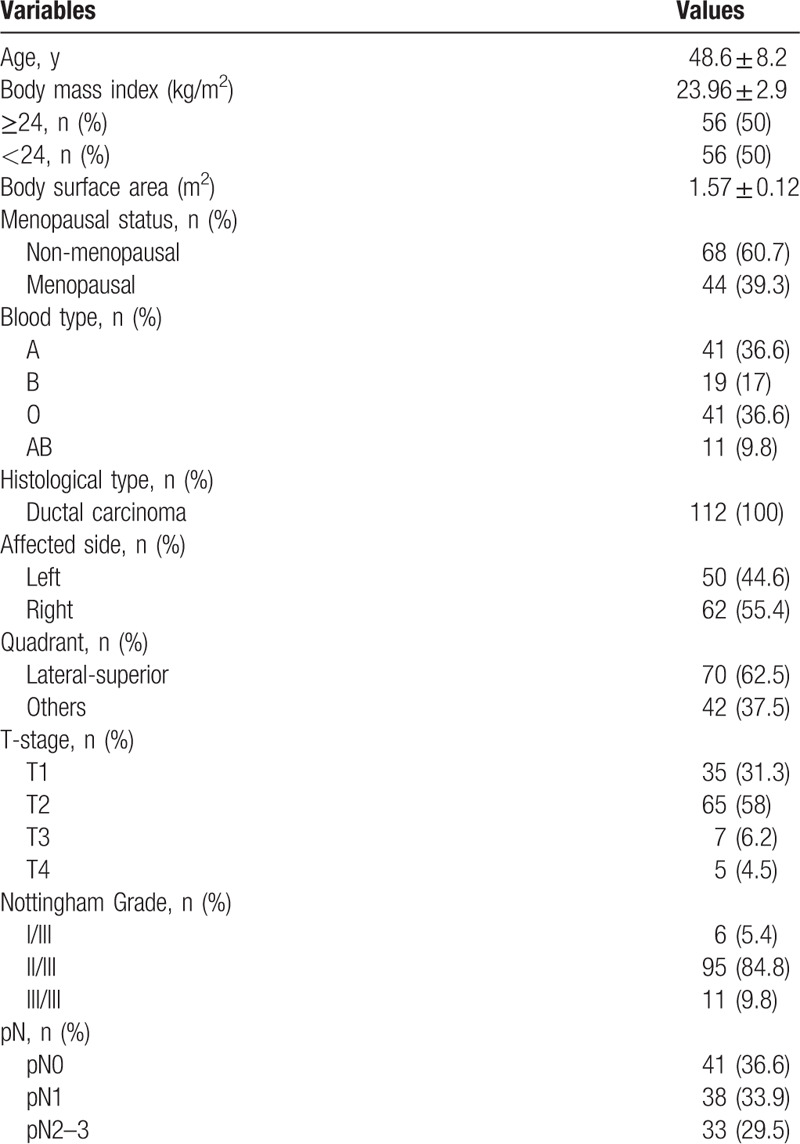
During the study period, 157 patients completed NAC, and all underwent breast surgery within a week. Among them, 45 patients achieved a pCR and 112 did not. Table 2 presents the characteristics of the study patients. Mean age was 48.6 ± 8.2 years, mean BMI was 24.0 ± 2.9 kg/m2, and BSA was 1.57 ± 0.12 m2. Among the 112 patients, 44 (39.3%) were menopausal; 112 (100%) had IDC; 35 (31.3%) were stage I, 65 (58.0%) were stage II, 7 (6.2%) were stage III, and 5 (4.5%) were stage IV; 6 (5.4%) were grade I/III, 95 (84.8%) were grade II/III, and 11 (9.8%) were grade III/III; 41 (36.6%) were pN0, 38 (33.9%) were pN1, and 33 (29.5%) were pN2-3.
Table 2.
Characteristics of the patients.
3.2. Expression of tumor protein markers before and after NAC
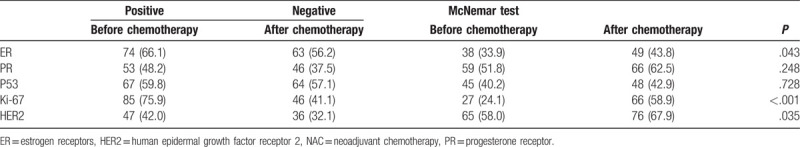
The IHC staining (Fig. 1) before NAC revealed that the highest and lowest positive rates were for Ki-67 and HER2, respectively (75.9% and 42%); while the highest and lowest positive rates were for ER and HER2 (56.2% and 32.1%) after NAC. The decrease in the positive rate of Ki-67 after NAC was the more important, from 75.9% before NAC to 41.1% after NAC (P < .001). The positive rate for HER2 decreased from 42.0% before NAC to 32.1% after NAC (P = .04). The positive rate of ER decreased from 66.1% before NAC to 56.2% after NAC (P = .04). There was no significant change in PR and P53 (Table 3).
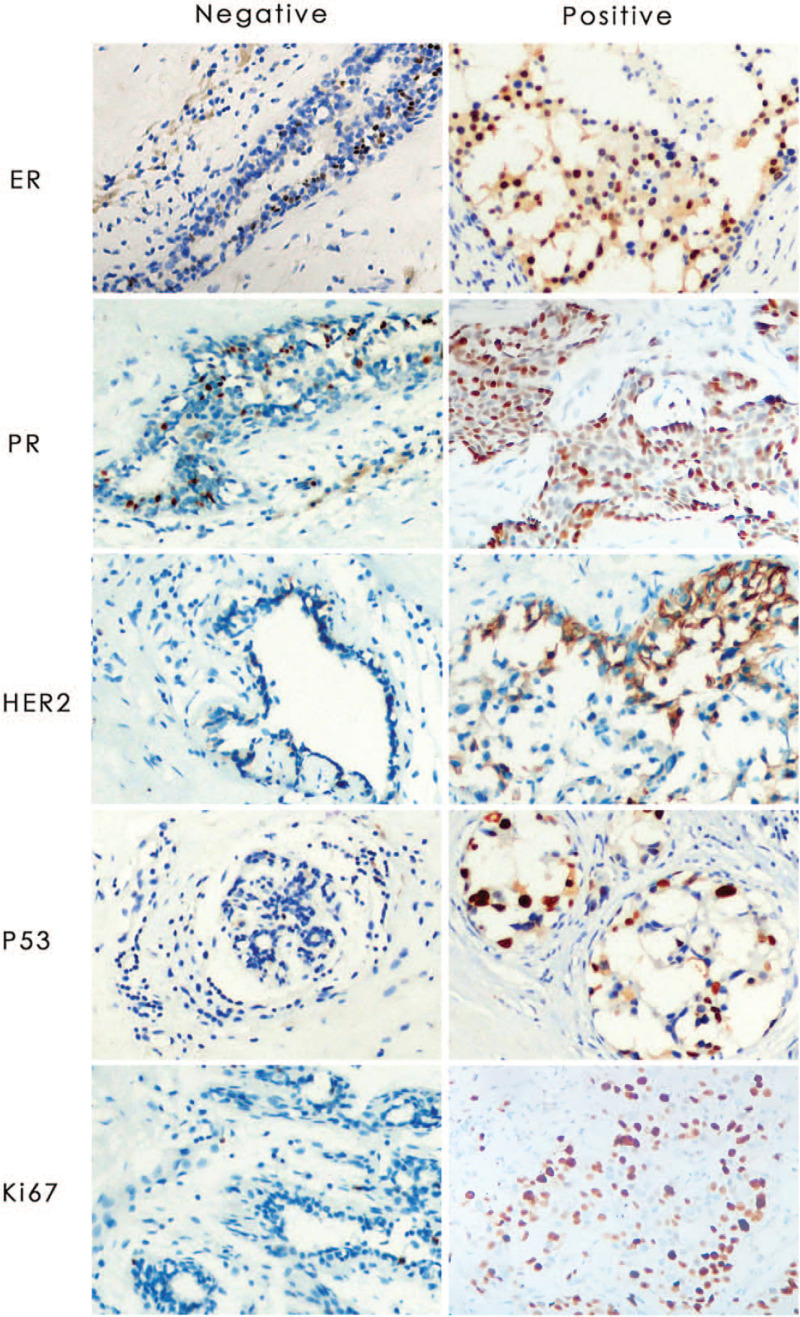
Figure 1.
Representative images of IHC for the markers of IDC tested in breast cancer. Left row is negative staining and right row is positive staining. All the slides were analyzed in x 200 microscope objective and the scale is 50 μm. IDC = invasive ductal carcinoma, IHC = immunohistochemistry.
Table 3.
Tumor immunohistochemistry before and after NAC.
3.3. Analysis of changes in tumor protein markers before and after NAC
The analysis of changes in individual tumor protein marker is shown in Table 4 and revealed that before and after NAC, tumor protein markers changed at different degrees. The smallest and largest changes occurred in HER2 (20.5%) and Ki-67 (40.2%), respectively. The highest positive-to-negative change rate occurred for Ki-67 (37.5%), while the smallest change was for HER2 (15.2%). In addition, the negative-to-positive changes were just the opposite that is the largest change occurred in P53 (13.3%), while the smallest occurred in Ki-67 (2.7%).
Table 4.
Changes of immunohistochemistry markers before and after NAC.
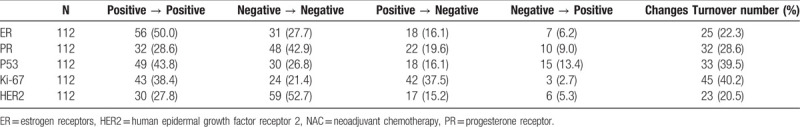
Before and after NAC, only the average expression rate of Ki-67 were decreased (from 28.6 ± 19.2% to 19.7 ± 18.5%, P < .001). There were no significant differences in ER (from 39.0 ± 33.7 to 42.2 ± 37.3%, P = .30), PR (from 27.0 ± 31.3% to 24.1 ± 31.7%, P = .27), or P53 (from 29.6 ± 28.0% to 31.4 ± 29.7%, P = .51).
3.4. Analysis of BC subtypes before and after NAC
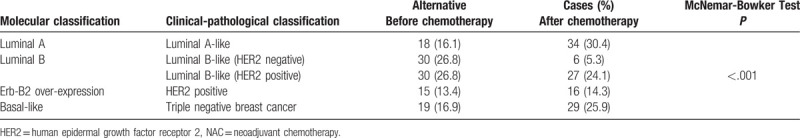
The proportions of luminal A-like, HER2-positive, and triple-negative BC subtypes increased after NAC, while that of luminal B-like decreased. The proportion of luminal A-like BC increased from 16.1% to 30.4% after NAC. The largest change occurred in luminal B-like (HER2-negative), which reduced from 26.8% to 5.3% (P < .001) (Table 5).
Table 5.
Analysis of breast cancer subtype before and after NAC.
3.5. Multivariate regression analysis to determine the predictor of markers change after NAC.
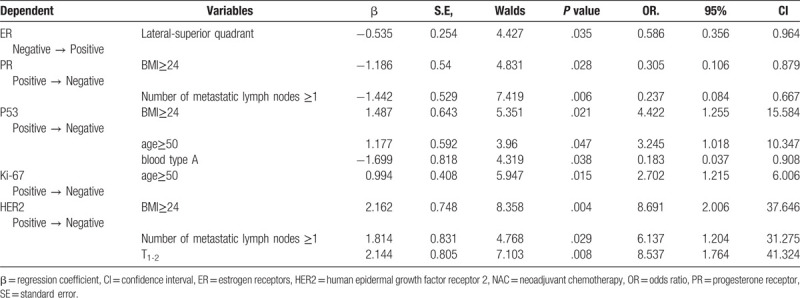
In the subsequent multivariate regression analysis, changes of markers were defined as the dependent variables. Lateral-superior Quadrant (OR = 0.586, P = .035) was observed to be independently associated with change in ER (Negative→Positive) (Table 6). Increased number of lymph nodes (OR = 0.237, P = .006) and BMI (OR = 0.305, P = .028) seemed to be related to conversion of PR (Positive→Negative). And, there was statistical association between the Ki-67 (Positive→Negative) and the age≥50 (OR = 2.702, P = .015). The BMI ≥ 24 (OR = 4.422, P = .021), age ≥ 50 (OR = 3.245, P = .047) and blood type A (OR = 0.183, P = .038) were independently associated with conversion of P53 (Positive→Negative). The BMI ≥ 24 (OR = 8.691, P = .004), number of lymph nodes ≥1 (OR = 6.137, P = .029) and TNM 1-2 (OR = 8.537, P = .008) were statistically associated with changes in HER2 (Positive→Negative). All other tested variables were not associated with the conversion of markers (P > .05)
Table 6.
Multivariate regression analysis to determine the predictor of markers’ change after NAC.
4. Discussion
Conversion of the HR status and HER2 status after NAC can be used to predict the prognosis of BC patients,[1] but there is a lack of data about these changes in patients without pCR since most studies examined patients with pCR.[14–21] Changes in markers may benefit patients with some subtypes of BC. According to the 2013 St Gallen Consensus Conference, if IHC results of patients who undergo core biopsy are negative for both ER and PR, while the postoperative IHC results are positive for ER or (and) PR, these patients will be able to receive treatment with tamoxifen (premenopausal) or aromatase inhibitors (postmenopausal). If the IHC result after core biopsy is negative for Her2, but after surgery, the IHC result is positive for Her2, or FISH result is amplification of CerBb2, these patients will be able to receive treatment with Herceptin, which will greatly improve the overall survival of patients. Therefore, a close observation of the changes in markers will bring very great benefits to the treatment and prognosis of patients. These results imply that the optimal course of treatments for BC should be based on tumor characteristics before and after NAC.[33,34]
Van De Ven et al[23] pointed out in a meta-analysis that HR may change in 8 to 33% of patients after NAC. Hirata et al[35] reported that changes in ER and PR occurred in 23% of patients after NAC. Furthermore, the positive-to-negative rate of change in HR and HER2 were 8.2% and 6%, respectively; and the negative-to-positive rate of change was 7.9% and 3.5%, respectively. Nevertheless, direct comparisons among studies cannot be made because pCR has to be considered. Indeed, in the present study, ER changed in 22.3% of patients, PR changed in 28.6% of patients, and the positive-to-negative and negative-to-positive rates of change for HER2 were 15.2% and 5.3%, respectively. Van De Ven et al[23] revealed that after NAC that included trastuzumab, negative-to-positive change for HER2 was observed in 5.3% of patients. For patients who require targeted therapy, since the rate of change seems to be higher, IHC should be carried out again on the specimens after surgical resection in order to avoid missing HER2-positive patients. The amplification of the HER2 gene is an important factor of prognosis. The HER2 positive patients can achieve a clinical response (CR) or pCR after NAC combined with trastuzumab treatment.[36,37]
An important source of bias is the correlation of IHC results between coarse needle biopsy (CNB) and surgical specimens. Nevertheless, among patients who did not undergo NAC, Arnedos et al[38] reported that the accordance rates of ER, PR, and HER2 were 98.2%, 85.0%, and 98.8%, respectively. The changes observed after NAC in the present study are all higher than the non-accordance rate observed by Arnedos et al,[38] suggesting that the changes observed in the present study were probably caused by NAC, as observed in previous studies.[14–21] Nevertheless, source of biases include tumor heterogeneity,[39] the time interval between biopsy and surgery, technical issues such as the fixation delay, and differences in the subjective evaluation from different pathologists.
The present study revealed that ER, Ki-67, and HER2 were significantly changed after NAC. According to the literatures,[17,35] HRs either change with HER2, or both do not change. In samples in which PR and ER expression rates increased, HER2 expression would be downregulated accordingly; while in samples in which HER2 expression increased, ER and PR expression rates would be reduced accordingly. Similar results were also obtained with the use of NAC combined with trastuzumab.[40] For BC that has a positive HER2 result in CNB only or surgery only, the heterogeneity of HER2 expression does not need to be considered and anti-HER2 treatment should be given[41] or less.[42]
Many different polygene analysis techniques have provided prognostic information for BC, and this information is mainly derived from proliferation-related genes.[43] A study proposed that moderate or strong PR expression should act as an additional condition for the definition of the luminal alternative classification.[44] As a marker of cell proliferation, Ki-67 expression levels are also important in the definition of luminal A.[32] There is evidence that strongly positive PR (>20%) is helpful for improving the accuracy of distinguishing between luminal A and B BC.[44] Due to the addition of this condition, the number of patients classified as luminal A BC should be reduced and the number of patients who are suggested to undergo chemotherapy would increase.[13] In the present study, PR > 20% was used as the threshold for BC subtype classification. Luminal A-like BC increased from 16.1% to 30.4% and luminal B-like (HER2 negative) BC decreased from 26.8% to 5.3%, supporting that luminal B-like (HER2 negative) BC was sensitive to chemotherapy, and luminal A-like is less sensitive to chemotherapy.
The high expression of Ki-67 indicates poor prognosis, but the highly proliferative tumor cells are more sensitive to anthracycline chemotherapy.[45] Studies have confirmed that the expression of Ki-67 was reduced after NAC,[45] endocrine therapy,[46,47] or chemotherapy combined with endocrine therapy.[48] Burcombe et al[49] reported that the median value of the expression rate of Ki-67 decreased from 24.9% before chemotherapy to 18.1% after chemotherapy. These results support the results of the present study.
The P53 is a cancer suppressor gene. The P53 mutations can result in a variety of tumors and are closely correlated with anthracycline resistance.[50] However, the average value of the P53 expression rate was not statistically significant before and after NAC in the present study. In addition, positive-to-negative conversion of P53 all occurred in BMI ≥ 24 (OR = 4.422, P = .021), age ≥ 50 (OR = 3.245, P = .047), and blood type A (OR = 0.183, P = .038). These suggest that when the patients are overweight or older, mutant P53 cells actively proliferate. Highly proliferating cells are sensitive to cytotoxic chemotherapy drugs, which could lead to a decrease in P53- and Ki-67-positive cells.
From the perspective of recurrence and poor prognosis of BC, obesity is widely considered as a risk factor.[51] There is evidence that suggests that pluripotent stem cells in adipose tissues may affect tumor angiogenesis.[52] In preclinical studies, this kind of cells has been proven to promote the occurrence and development of BC.[53] In the present study, obese patients more easily presented a positive-to-negative conversion of PR (P = .028), HER2 (P = .004) and P53 (P = .021). Lymph node metastasis is also a very important prognostic factor. In this study, patients with axillary cavity lymph node metastasis after NAC are more prone to a positive-to-negative conversion of HER2 (P = .029) and PR (P = .006).
The determination of tumor markers is a useful tool for clinical management in cancer patients, assisting in diagnosis, staging, evaluation of therapeutic response, detection of recurrence and metastasis, and development of new treatment modalities.[21] For example, after NAC, the number of patients whose ER and PR becoming positive is 7 and 10 respectively (Table 4). The percentage of patients luminal A subtype increased from 18 (16.1%) to 34 (30.4%), 16 new luminal A patients would be thought to be treated with hormonal therapy (Table 5). Luminal A patients are sensitive to hormonal manipulations, but less sensitive to chemotherapy, their prognosis is favorable.[11] So new treatment modalities should be developed for these patients who can benefit from the new treatment of breast cancer.
The present study is not without limitations. The sample size was small and from a single center. The small sample size also prevented multivariable analyses. The IHC analysis is somewhat subjective and differences among pathologists could lead to some bias. The retrospective nature of the study prevented us from analyzing factors that were not reported in the medical charts. Finally, no molecular mechanisms could be explored.
In conclusion, our observational study demonstrated the existence of discordance in the HR status and markers’ status after NAC and the predictors of the conversion. These findings might help optimize the choice of sequential adjuvant therapy and improve treatment and prognosis. The administration of NAC might be the main reason for the change in receptor status, but the mechanism needs to be elucidated. In the future, further studies are required to identify the mechanism for this switch in receptor status after NAC and to validate the prognostic impact associated with this switch.
Acknowledgments
The authors acknowledge the help of Dr. Yanlin Chen, et al and the committee of pathologists, from the Pathology Department of Chongqing Medical University, for kindly providing pathological service and suggestions.
Author contributions
Conceptualization: Jian-Heng Peng, Hong-Yuan Li, Yong-Hong Wang.
Data curation: Jian-Heng Peng, Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo.
Formal analysis: Jian-Heng Peng, Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo.
Investigation: Jian-Heng Peng, Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo, Yong-Hong Wang.
Methodology: Jian-Heng Peng, Hong-Yuan Li, Yong-Hong Wang.
Project administration: Hong-Yuan Li, Yong-Hong Wang.
Resources: Jian-Heng Peng, Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo.
Software: Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo.
Supervision: Yong-Hong Wang.
Writing – original draft: Jian-Heng Peng.
Writing – review & editing: Xiang Zhang, Jun-Long Song, Liang Ran, Rong Luo, Hong-Yuan Li, Yong-Hong Wang.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, BC = breast cancer, BMI = body mass index, BSA = body surface area, CNB = coarse needle biopsy, DCIS = ductal carcinoma in situ, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = hormone receptor, IDC = invasive ductal carcinoma, IHC = immunohistochemistry, ILC = invasive lobular carcinoma, NAC = neoadjuvant chemotherapy, pCR = pathological complete response, PR = progesterone receptor.
Funding: This study was funded by Cultivating Fund of the First Affiliated Hospital of Chongqing Medical University.
The authors have no conflicts of interest to disclose.
References
- [1].Xi Jin, Yi-Zhou Jiang, Sheng Chen, et al. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget 2015;6:9600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011;103:714–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang YC, Wei LJ, Liu JT, et al. Comparison of cancer incidence between China and the USA. Cancer Biol Med 2012;9:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].NCCN Clinical Practuce Guidelines in Oncology (NCCN Guidelines). Breast cancer. Version 1.2017. Fort Washington: National Comprehensive Cancer Network. 2017. in press. [Google Scholar]
- [5].von Minckwitz G, Fontanella C. Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast 2013;22(Suppl 2):S149–151. [DOI] [PubMed] [Google Scholar]
- [6].Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg 2015;220:1063–9. [DOI] [PubMed] [Google Scholar]
- [7].Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 2007;8:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang E, Cheng SH, Dressman H, et al. Gene expression predictors of breast cancer outcomes. Lancet 2003;361:1590–6. [DOI] [PubMed] [Google Scholar]
- [11].Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer 2013;5:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harbeck N, Thomssen C, Gnant M, St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel) 2013;8:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Avci N, Deligonul A, Tolunay S, et al. Neoadjuvant chemotherapy-induced changes in immunohistochemical expression of estrogen receptor, progesterone receptor, HER2, and Ki-67 in patients with breast cancer. J BUON 2015;20:45–9. [PubMed] [Google Scholar]
- [15].Lee HC, Ko H, Seol H, et al. Expression of immunohistochemical markers before and after neoadjuvant chemotherapy in breast carcinoma, and their use as predictors of response. J Breast Cancer 2013;16:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Faneyte IF, Schrama JG, Peterse JL, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer 2003;88:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res 2008;28:1797–804. [PubMed] [Google Scholar]
- [18].Chuah BY, Putti T, Salto-Tellez M, et al. Serial changes in the expression of breast cancer-related proteins in response to neoadjuvant chemotherapy. Ann Oncol 2011;22:1748–54. [DOI] [PubMed] [Google Scholar]
- [19].Tiezzi DG, Andrade JM, Ribeiro-Silva A, et al. HER-2, p53, p21 and hormonal receptors proteins expression as predictive factors of response and prognosis in locally advanced breast cancer treated with neoadjuvant docetaxel plus epirubicin combination. BMC Cancer 2007;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Magbanua MJ, Wolf DM, Yau C, et al. Serial expression analysis of breast tumors during neoadjuvant chemotherapy reveals changes in cell cycle and immune pathways associated with recurrence and response. Breast Cancer Res 2015;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Banin Hirata BK, Oda JM, Losi Guembarovski R, et al. Molecular markers for breast cancer: prediction on tumor behavior. Dis Markers 2014;2014:513158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res 2008;14:8019–26. [DOI] [PubMed] [Google Scholar]
- [23].van de Ven S, Smit VT, Dekker TJ, et al. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 2011;37:422–30. [DOI] [PubMed] [Google Scholar]
- [24].Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–81. [DOI] [PubMed] [Google Scholar]
- [25].Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- [26].Sinn HP, Helmchen B, Wittekind CH. TNM classification of breast cancer: changes and comments on the 7th edition. Pathologe 2010;31:361–6. [DOI] [PubMed] [Google Scholar]
- [27].Hao S, Liu Y, Yu KD, et al. Overweight as a prognostic factor for triple-negative breast cancers in Chinese women. PLoS One 2015;10:e0129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- [29].Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174–83. [DOI] [PubMed] [Google Scholar]
- [30].Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol 2009;27:1323–33. [DOI] [PubMed] [Google Scholar]
- [31].Gnant M, Thomssen C, Harbeck N, St. Gallen/Vienna 2015: a brief summary of the consensus discussion. Breast Care (Basel) 2015;10:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang YF, Liao YY, Li LQ, et al. Changes in ER, PR and HER2 receptors status after neoadjuvant chemotherapy in breast cancer. Pathol Res Pract 2013;209:797–802. [DOI] [PubMed] [Google Scholar]
- [34].Gahlaut R, Bennett A, Fatayer H, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression—implications for the practising oncologist. Eur J Cancer 2016;60:40–8. [DOI] [PubMed] [Google Scholar]
- [35].Hirata T, Shimizu C, Yonemori K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer 2009;101:1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol 2010;28:2024–31. [DOI] [PubMed] [Google Scholar]
- [37].Untch M, von Minckwitz G. Recent advances in systemic therapy: advances in neoadjuvant (primary) systemic therapy with cytotoxic agents. Breast Cancer Res 2009;11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arnedos M, Nerurkar A, Osin P, et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol 2009;20:1948–52. [DOI] [PubMed] [Google Scholar]
- [39].Burki TK. High genetic heterogeneity in some breast cancer tumours. Lancet Oncol 2015;16:e529. [DOI] [PubMed] [Google Scholar]
- [40].Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 2009;15:7381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021–8. [DOI] [PubMed] [Google Scholar]
- [42].Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14:741–8. [DOI] [PubMed] [Google Scholar]
- [43].Wirapati P, Sotiriou C, Kunkel S, et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 2008;10:R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Prat A, Cheang MC, Martín M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013;31:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 2005;23:2477–92. [DOI] [PubMed] [Google Scholar]
- [46].Tanei T, Shimomura A, Shimazu K, et al. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol 2011;37:155–61. [DOI] [PubMed] [Google Scholar]
- [47].Delpech Y, Wu Y, Hess KR, et al. Ki67 expression in the primary tumor predicts for clinical benefit and time to progression on first-line endocrine therapy in estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat 2012;135:619–27. [DOI] [PubMed] [Google Scholar]
- [48].Kurebayashi J, Kanomata N, Shimo T, et al. Marked lymphovascular invasion, progesterone receptor negativity, and high Ki67 labeling index predict poor outcome in breast cancer patients treated with endocrine therapy alone. Breast Cancer 2014;21:214–22. [DOI] [PubMed] [Google Scholar]
- [49].Burcombe RJ, Makris A, Richman PI, et al. Evaluation of ER, PgR, HER-2 and Ki-67 as predictors of response to neoadjuvant anthracycline chemotherapy for operable breast cancer. Br J Cancer 2005;92:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Geisler S, Lønning PE, Aas T, et al. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res 2001;61:2505–12. [PubMed] [Google Scholar]
- [51].Niraula S, Ocana A, Ennis M, et al. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769–81. [DOI] [PubMed] [Google Scholar]
- [52].Bertolini F. Adipose tissue and breast cancer progression: a link between metabolism and cancer. Breast 2013;22(Suppl 2):S48–49. [DOI] [PubMed] [Google Scholar]
- [53].Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol 2012;23:582–8. [DOI] [PubMed] [Google Scholar]