Abstract
Background:
In patients with hepatocellular carcinoma (HCC), the clinicopathologic and prognostic roles of tumor-infiltrating CD8+ T cells for survival are still controversial. A meta-analysis was performed to resolve this issue.
Methods:
We identified studies from PubMed, Embase, and the Cochrane Library to evaluate the clinicopathologic and prognostic value of tumor-infiltrating CD8+ T cells in patients with HCC. A meta-analysis was conducted to estimate clinicopathologic characteristics, overall survival (OS), and disease-free survival. The hazard ratio (HR) and 95% confidence interval (CI) were calculated employing fixed-effect or random-effect models depending on the heterogeneity of the included trials.
Results:
A total of 3509 patients from 21 observational studies were enrolled. The meta-analysis revealed that high levels of intratumoral CD8+ tumor-infiltrating lymphocytes (TILs) were associated with better OS (OS; HR = 0.676, P = .001) and disease-free survival (disease-free survival [DFS]; HR = 0.712, P = .002). The pooled analysis also demonstrated high density of infiltration of CD8+ TILs in margin of tumor (MT) was statistically significant associated with better OS (HR = 0.577; P <.001). Moreover, the patients with low CD8+ TILs infiltration had negative HBSAg (OR = 1.67, P = .02), large tumor size (OR = 1.74, P <.01), and later TNM stage (OR = 1.70, P <.01).
Conclusions:
Our findings suggested that low levels of CD8+ TILs predict large tumor size, later TNM stage and might be a promising prognostic factor of HCC especially for Asian patients. High-quality randomized controlled trials are needed to determine if CD8+ TILs could serve as targets for immunotherapy in hepatocellular carcinoma.
Keywords: CD8, clinicopathologic, hepatocellular carcinoma, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common primary malignancies of the liver, representing the third leading cause of global cancer deaths and the incidence is rising.[1] Current treatments include surgical resection, liver transplantation, transarterial chemoembolization, radiofrequency ablation, and Chinese medicinal therapy.[2] However, the outcome of HCC remains poor due to low radical resection rates and high recurrence rates. Besides early diagnosis, prompt treatment plans identified from the prediction of patients’ outcomes also contribute to successful treatment of liver cancer. Therefore, further investigation is needed to discovery precise tumor biomarkers with higher sensitivity and specificity in HCC to determine the optimal treatment programs and predict the prognosis of HCC.
Tumor-infiltrating lymphocytes (TILs), including T cells, B cells, and natural killer (NK) cells, are one of the representative components of host antitumor immune responses.[3] Increased numbers of TILs, particularly activated cytotoxic T lymphocytes (CTLs), are reported to correlate with better survival in some malignant tumors including HCC.[4,5] CD8+ TILs play an important role in host immune defense against tumor progression. Ramzan et al revealed that patients with high density of CD8+ T cell had better outcome,[6] but Chew et al reported the opposite results.[7] Therefore, whether CD8+ TILs have prognostic value in patients with HCC remains controversial.
Although a meta-analysis on prognosis has been conducted, they merely focused on intratumoral CD8+ TILs and the prognostic value of CD8+ TILs in peritumoral regions and tumor margin was ignored.[8] Moreover, whether CD8+ TILs are associated with clinicopathologic features in patients with HCC has not been analyzed systematically. For these reasons, we performed this meta-analysis to make a more precise estimation of the clinicopathologic and prognostic value of CD8+ TILs in patients with HCC.
2. Materials and methods
2.1. Literature search
Relevant articles were identified by 2 reviewers via an electronic search of PubMed, EMBASE, and Cochrane using the following keywords: (tumour or tumor or cancer or carcinoma), (hepatic or liver), (prognostic or prognosis or outcome or survival or clinicopathologic), and CD8. And the search time period of the electronic database was from inception to August 20, 2018. Additionally, pertinent studies were searched in reference lists of selected reports and reviews. Unpublished literature was not performed to search. Disagreement on article inclusion between the 2 reviewers was resolved via a third reviewer. A third researcher would determine the final results about the disagreement of the 2 reviewers.
2.2. Inclusion and exclusion criteria
Inclusion criteria for this meta-analysis were as follows:
-
(1)
studies researching the prognostic effect of TILs or associated TIL subsets in HCC;
-
(2)
report of TILs or associated TIL subsets in tumor surgical specimens;
-
(3)
hazard ratio (HR) and 95% confidence interval (CI) could be extracted;
-
(4)
when the same author or group reported results obtained from the same crowd in more than one paper, the most recent report or the most informative report was selected.
Exclusion criteria for this meta-analysis were as follows:
-
(1)
letters, reviews, case reports, animal trials, abstracts, and expert opinion were excluded;
-
(2)
articles in which have no information on overall survival (OS) or disease-free survival (DFS);
-
(3)
patients who only treated with immunotherapy chemotherapy, radiotherapy, interventional therapy or liver transplantation;
-
(4)
non-primary tumor, such as metastatic tumor or recurrent tumor. Names of authors or journals of the articles were not considered in excluding or including the articles.
2.3. Statistical analysis
HR and its 95% CI were used to assess the association between TILs and prognosis of HCC. If a direct report of OS and DFS were not available, then extract the survival data from Kaplan-Meier curves by Engauge Digitizer version 4.1 as described previously.[9] The extraction was performed by 2 independent researchers to decrease error. STATA version 14.0 was performed to merge the results of studies. Statistical heterogeneity between trials was assessed by Chi-square test and was suggested significant when I2 > 50% and/or P <.05. The fixed-effect model was used when no heterogeneity was detected among studies, while the random-effect model was preferred when variance existed. The cumulative analysis was performed according to publication time. Sensitivity analysis was conducted to assess the stability and reliability of this review. Egger test was performed to evaluate the publication bias.[10]
3. Results
3.1. Study selection and characteristics
A total of 1692 articles were initially gathered after duplicate removal. Among them, 27 studies reported the association between CD8+ TILs and clinicopathologic characteristics or prognosis of HCC. After further screening, 6 types of research were excluded (one studying the same crowd, 4 lacking enough useful data and one treating with immunotherapy). Finally, a total of 21 retrospective studies including 3509 patients from America, China, France, Germany, Japan, Netherlands and Singaporean were included in this meta-analysis (Fig. 1).[6,7,11–29]
Figure 1.
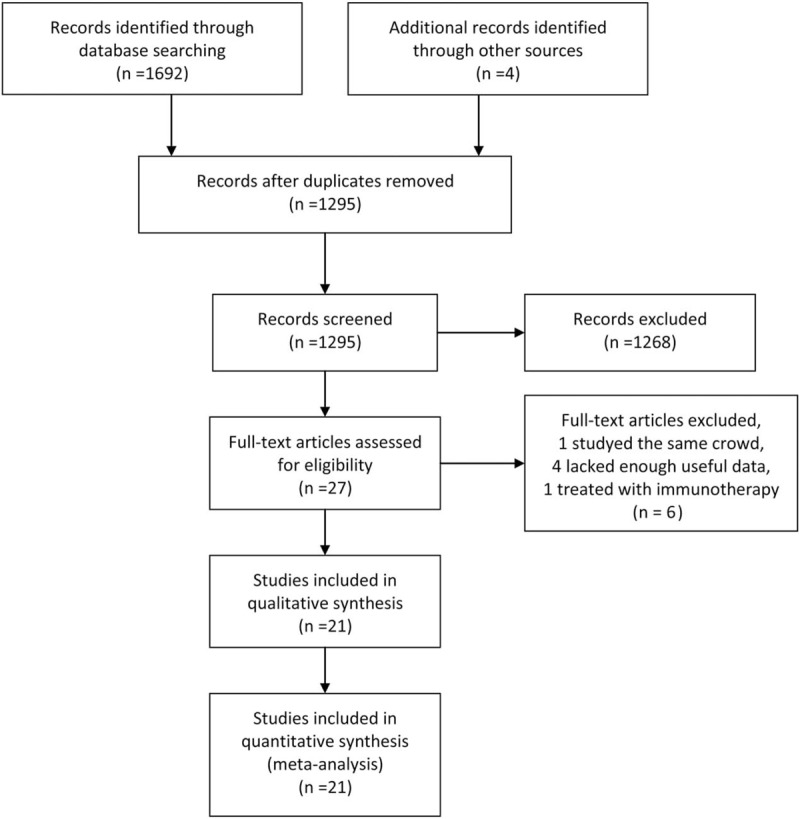
Flow diagram of study selection.
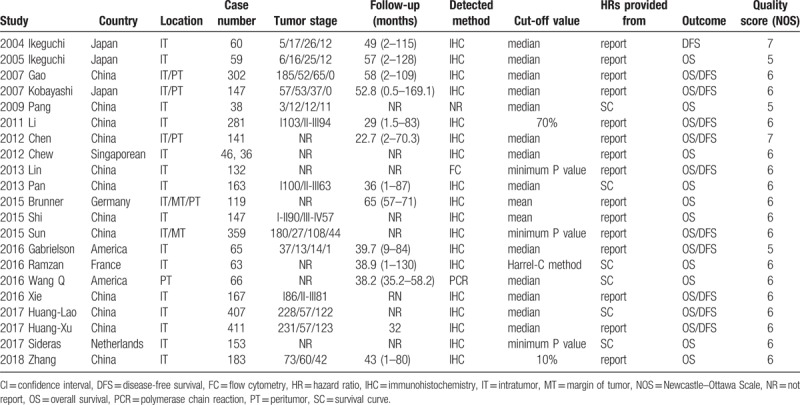
The main characteristics of included studies were displayed in Table 1. The prognostic role of TILs in intratumor (IT), margin of tumor (MT), peritumor (PT) were evaluated in 21, 2 and 5 articles, respectively. Sample sizes of included studies ranged from 38 to 411 patients. The details of tumor stage were offered in 14 articles, and the classifications were diverse. Follow-up time was provided in 13 articles. CD8+ TILs were detected by methods of flow cytometry (FC), immunohistochemistry (IHC) and polymerase chain reaction (PCR), respectively. All 21 types of research were considered to be of adequate quality for the meta-analysis according to Newcastle–Ottawa Scale (NOS) assessment (score ≥5 points) (Table 1).
Table 1.
Main characteristics of all studies included in the meta-analysis.
3.2. Prognostic effect of CD8+ TILs on survival
A total of 20 articles, including 3102 patients with HCC, researched the association between clinical outcome and the density of CD8+ TILs.[6,7,11–24,26–29]
Eighteen articles focused on the relationship between OS and the density of CD8+ TILs in IT.[6,7,12–22,24,26–29] 2 articles focused on the association between OS and the density of CD8+ TILs in MT.[19,21] 5 articles focused on the association between OS and the density of CD8+ TILs in PT.[13,14,16,19,23] A random model was used because of a significant heterogeneity (P <.001, I2 = 69.5%), and the result demonstrated patients with high infiltration of CD8+ TILs in IT had better OS (pooled HR = 0.676; 95% CI = 0.540–0.845; P = .001). The pooled analysis revealed that high density of infiltration of CD8+ TILs in MT was statistically significant associated with better OS (pooled HR = 0.577; 95% CI = 0.437–0.760; P <.001). But, there was no statistical significance between the density of CD8+ TILs in PT and OS (P = .531) (Fig. 2).
Figure 2.
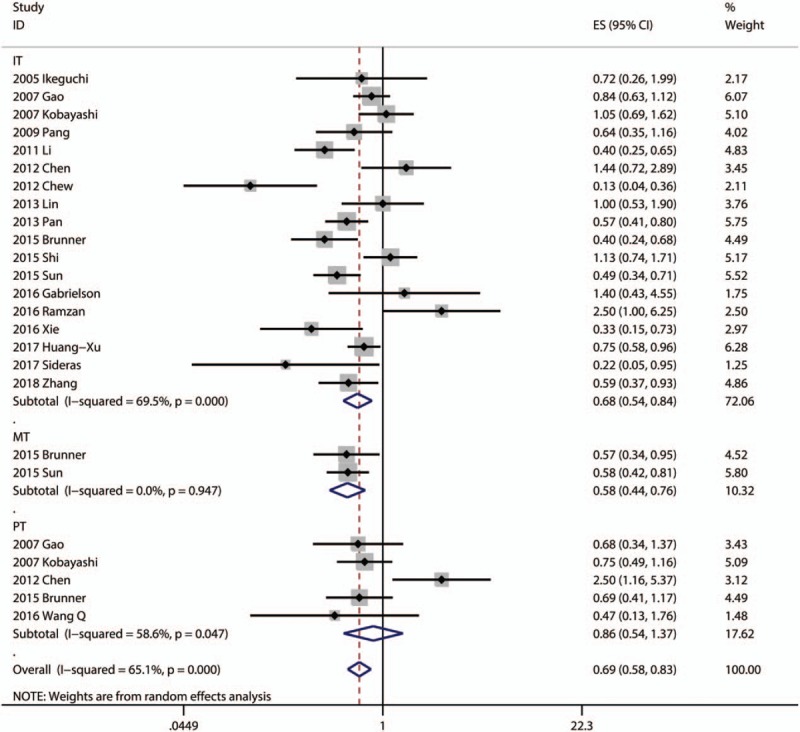
Forest plots of studies evaluating the association between CD8+ lymphocytes and OS of HCC patients. OS = overall survival, HCC = hepatocellular carcinoma.
Ten articles focused on the relationship between DFS and the density of CD8+ TILs in IT.[11,13–17,21,22,24,26] Only one article focused on the association between DFS and the density of CD8+ TILs in MT.[21] Three articles focused on the association between DFS and the density of CD8+ TILs in PT.[13,14,16] The pooled analysis revealed that high density of infiltration of CD8+ TILs in IT was statistically significant associated with better OS (pooled HR = 0.712; 95% CI = 0.574–0.883; P = .002). There was no statistical significance between DFS and CD8+ TILs in PT (P = .160) (Fig. 3).
Figure 3.
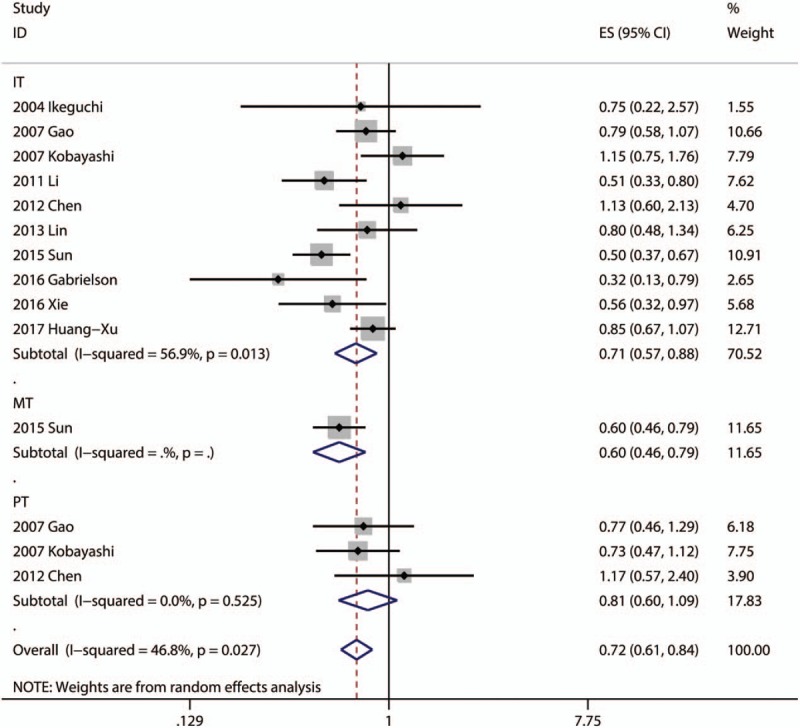
Forest plots of studies evaluating the association between CD8+ lymphocytes and DFS of HCC patients. DFS = disease free survival, HCC = hepatocellular carcinoma.
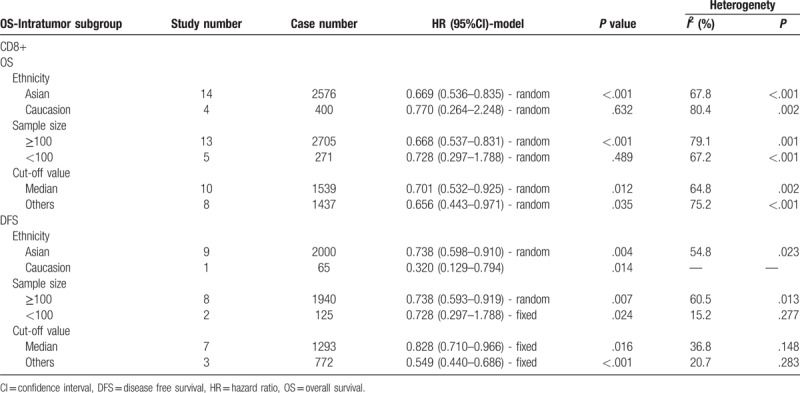
3.3. Subgroup analyses of the prognostic effect of CD8+ TILs
Subgroup analysis showed that patients with high infiltration of CD8+ T lymphocytes had better OS in the groups with Asian patients (pooled HR = 0.669, 95% CI = 0.536–0.835; P <.001), large sample size (≥100; pooled HR = 0.668, 95% CI = 0.537–0.831; P <.001), median cutoff values (pooled HR = 0.701, 95% CI = 0.532–0.925; P = .012), and other cutoff values (pooled HR = 0.656, 95% CI = 0.443–0.971; P = .035). Moreover, patients with high infiltration of CD8+ T lymphocytes had better DFS in all subgroups, including ethnicity, sample size and cut-off value (Table 2).
Table 2.
Pooled hazard ratios for OS according to subgroup analyses.
The cumulative meta-analysis indicated that the results of the association between intratumoral CD8+ lymphocytes and OS (Fig. 4A) and DFS (Fig. 4B) got more and more stable and the CI got narrowed since the Sun's research in 2015.[21] It is convinced that CD8+ lymphocytes in IT were associated with better prognostic for HCC.
Figure 4.
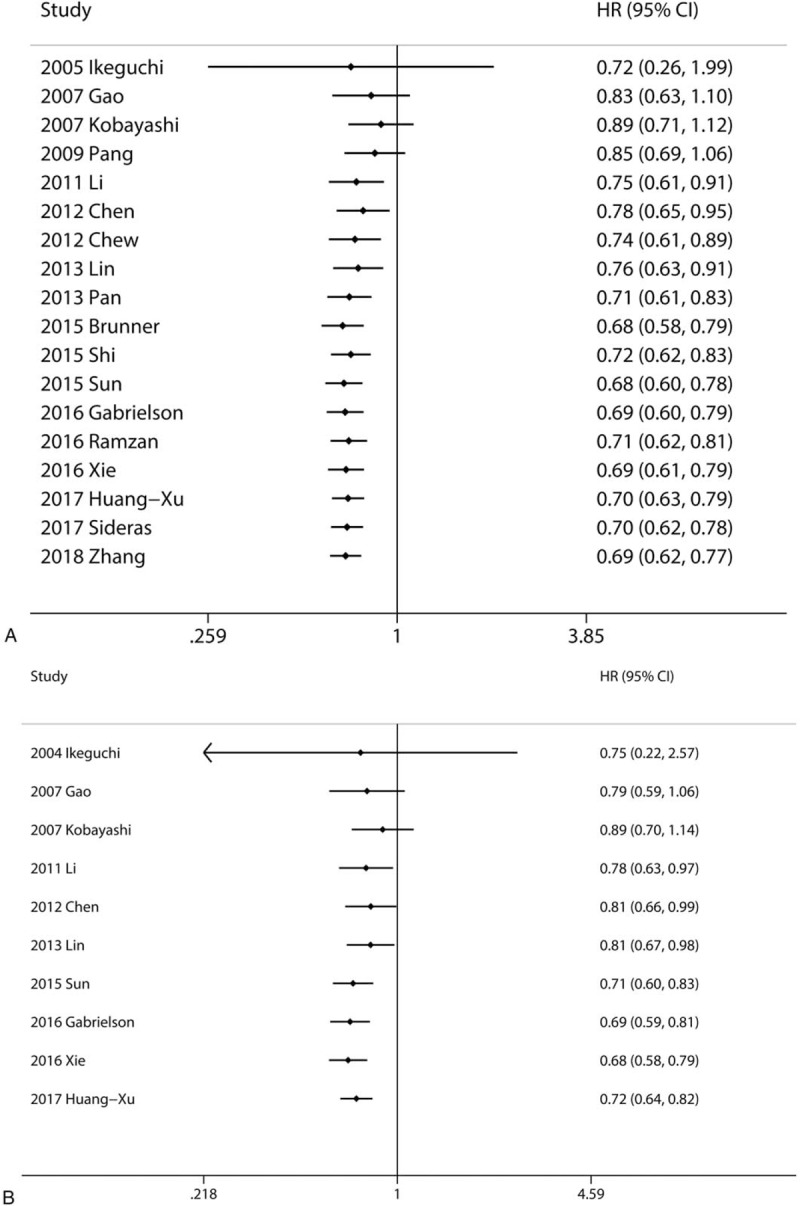
Cumulative meta-analysis of the association between CD8+ TILs and prognosis. (A) Intratumoral CD8+ lymphocytes and OS; (B) Intratumoral CD8+ lymphocytes and DFS. DFS = disease free survival, OS = overall survival, TILs = tumor-infiltrating lymphocytes.
3.4. Relationship between CD8+ TILs and clinicopathologic characteristics
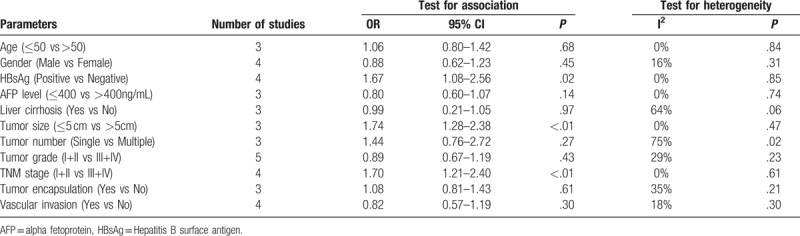
Five studies reported the association between CD8+ TILs and clinicopathologic parameters. A total of 11 features were analyzed, including HBsAg, Tumor size, TNM stage. The information for various clinicopathologic parameters and their correlation with CD8+ TILs is concluded in Table 3. The results of meta-analysis demonstrated that patients with negative HBsAg (pooled OR = 1.67, 95% CI = 1.08–2.56; P = .02), large tumor size (pooled OR = 1.74, 95% CI = 1.28–2.38; P <.01), later TNM stage (pooled OR = 1.70, 95% CI = 1.21–2.40; P <.01) had low CD8+ TILs levels. Besides, the results of meta-analysis demonstrated no correlation between infiltration of CD8+ TILs and age, gender, AFP level, liver cirrhosis, tumor number, tumor grade, tumor encapsulation, and vascular invasion (Table 3).
Table 3.
Meta-analysis of reported clinicopathologic characteristics in the included studies.
3.5. Sensitivity analyses and publication bias
Sensitivity analyses showed that the association between CD8+ TILs and prognosis was robust (Fig. 5). Egger test was performed to assess the publication bias of this meta-analysis. As shown in Figure 5, there was no statistical significance of publication bias in the studies about CD8+ TILs in IT with OS (P = .653) (Fig. 6A) or with DFS (P = .688) (Fig. 6B).
Figure 5.
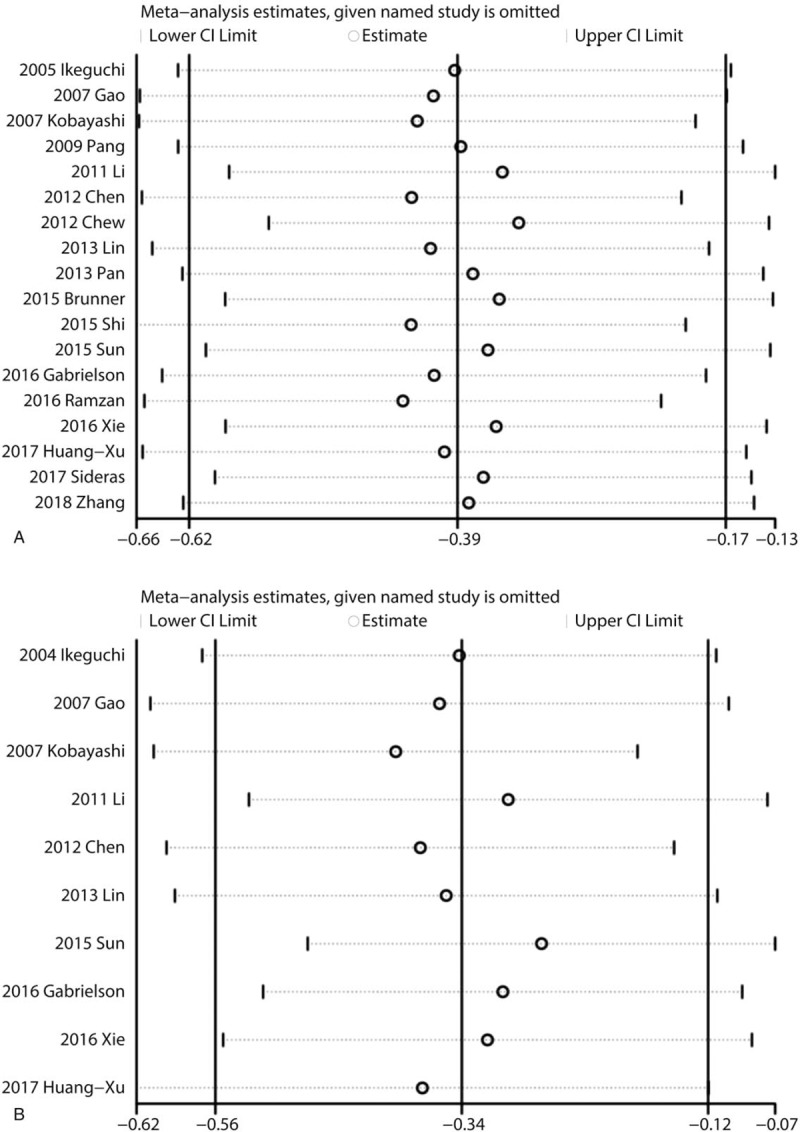
Sensitivity analyses of the association between CD8+ TILs and prognosis. (A) Sensitivity analysis of the association between CD8+ lymphocytes in IT and OS; (B) Sensitivity analysis of the association between CD8+ lymphocytes in IT and DFS. DFS = disease free survival, IT = intratumor, OS = overall survival, TILs = tumor-infiltrating lymphocytes.
Figure 6.
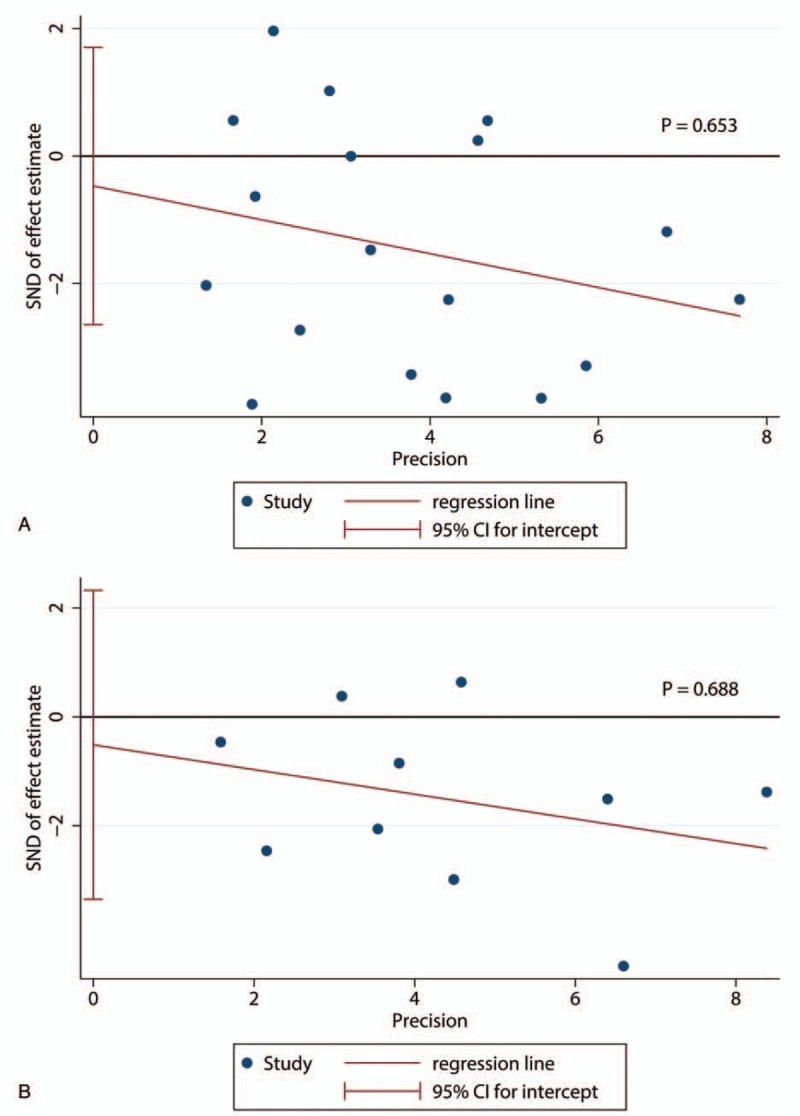
Egger's tests of the association between CD8+ TILs and prognosis. (A) Egger's tests of the association between CD8+ lymphocytes in IT and OS; (B) Egger's tests of the association between CD8+ lymphocytes in IT and DFS. DFS = disease free survival, IT = intratumor, OS = overall survival, TILs = tumor-infiltrating lymphocytes.
4. Discussion
A high CD8+ TILs level was associated with better prognosis in both gastric cancer, breast cancer, and colorectal cancer.[30–32] In HCC, most researchers[7,19,21,24,27] approved that CD8+ TILs were protective factors, but a few researchers[6,16] insisted that CD8+ TILs were dangerous factors. This study addressed the prognostic value of CD8+ TILs in HCC. We included a larger number of studies in this meta-analysis and drew the same conclusion that a higher CD8+ TILs level was associated with significantly higher OS and DFS in patients with HCC in IT or in MT. By subgroup analysis, we found that Asian patients with high infiltration of CD8+ T lymphocytes were associated with better OS than non-Asian patients, which was considered to be related to the epidemiological features that Hepatitis B remains the primary cause of HCC in Asia. However, there was controversy over whether high CD8+ lymphocytes level in other sites, such as peripheral blood and peritumoral regions has the same effect on prognosis. We found that CD8+ lymphocytes in peritumoral regions have no significant association with prognosis. Overall, a higher level of CD8+ lymphocytes in IT and MT might improve survival and reduce recurrence of HCC, and may be a promising therapeutic strategy for HCC.
Tumor-infiltrating CD8+ lymphocytes were believed to be front fighters against cancers and play a positive prognostic effect in some cancers.[33] Generally, most malignant cells express antigens that can be recognized by host CD8+ T-lymphocytes, which trigger antitumor immune responses.[34] Present researches considered that these tumor-associated antigens (TAA)-specific CD8+ T-cells responses positively influence survival in HCC.[35] The TAA-specific cytotoxic CD8 T-lymphocytes are the key factors in antitumor immune response. In early-stage colorectal cancer, the presence of activated CD8+ T cells both in IT and in PT has been shown to have significant positive prognostic import.[36] The mechanisms discussed above may lead to the association between CD8+ TILs and prognosis in HCC.
We also explored the relationship between clinicopathologic characteristics of HCC patients and CD8+ lymphocytes. Our study including some eligible studies showed that lower CD8+ TILs level was associated with some clinicopathologic parameters, such as negative HBsAg, large tumor size, and later TNM stage. This conclusion further supported the meta-analysis results on OS and DFS because patients with large tumor size and later TNM stage have poor prognosis. But Sohn et al[37] reported that the HBV DNA levels and HBsAg levels were associated with recurrence after curative resection in HBV-related HCC. It is contradictory with our result. Further researches were needed to prove the relationship between CD8+ lymphocytes in HCC and HBSAg. According to Chen's study,[16] lower CD8+ TILs level was associated with multiple tumors. However, our present meta-analysis demonstrated no association between CD8+ TILs and tumor number. As for AFP level, tumor grade, vascular invasion, and tumor encapsulation, more studies need to be included in the review.
There are several limitations which should be taken into consideration in interpreting the conclusions of this study. First, studies included used different cut-off values that could reduce the accuracy of CD8+ TILs in the process of estimating prognosis. Second, when several HRs could not be directly acquired in the original article, we obtained them by calculating the data extracted from the survival curves (SCs). Third, some data comes from univariate analysis, which may overestimate the effect sizes of some TIL subsets compared to multivariate analysis. Fourth, there are many reasons for potential heterogeneity. CD8 markers, follow-up time, and cut-off value were defined differently among studies. However, we tried to reduce the impact of heterogeneity through subgroup analyses. Fifth, the number of included studies reporting CD8+ TILs and clinicopathologic characteristics was relatively small. Finally, no prospective randomized trials are reported; therefore, future research should be directed at performing prospective randomized trials.
Despite the limitations of our study, our meta-analysis is meaningful for demonstrating the correlation between CD8+ TILs and prognosis, especially CD8+ TILs in intratumoral regions. Besides, this is the first comprehensive analysis of the association between CD8+ TILs and clinicopathologic characteristics in HCC patients. And sensitivity analyses revealed that the results were robust.
5. Conclusions
In conclusion, our meta-analysis provides strong evidence that high density of CD8+ TILs in IT was associated with better OS and DFS especially for Asian patients, and CD8+ TILs would serve as an effective marker for evaluating the prognosis of patients with HCC. Patients with lower CD8+ TILs level tended to have large tumor size and later TNM stage. Nevertheless, further well designed clinical studies are needed to elucidate the exact relationship and the underlying mechanism and more non-Asian studies were needed to be enrolled to decrease heterogeneity.
Acknowledgments
We thank all our colleagues at the Department of General Surgery, Wujin Hospital, affiliated with Jiangsu University.
Author contributions
Tan YL and Xu XZ contributed equally to the work as co-first authors; Ding W and Tan YL designed the study and wrote the manuscript; Ding W and Xu XZ designed the research; Qan Y, Xue WB and Wang YB performed the research and analyzed the data; Du JG and Jin L contributed analytical tools; and Ding W designed the study and edited the manuscript as the corresponding author.
Conceptualization: Xuezhong Xu, Yulin Tan, Wei Ding.
Data curation: Yan Qian, Wenbo Xue, Yibo Wang, Jianguo Du, Lei Jin.
Writing – original draft: Yulin Tan, Wei Ding.
Writing – review & editing: Wei Ding.
Wei Ding orcid: 0000-0001-6990-8621.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease free survival, HCC = hepatocellular carcinoma, HR = hazard ratio, IT = intratumor, MT = margin of tumor, OS = overall survival, PT = peritumor, TILs = tumor-infiltrating lymphocytes.
XX and YT contributed equally to this work.
This study was supported by the Science and Technology Project of Wujin (WS201515).
The study received grants from the Science and Technology Bureau of Changzhou Municipal Wujin District.
The authors declare no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Rasool M, Rashid S, Arooj M, et al. New possibilities in hepatocellular carcinoma treatment. Anticancer Res 2014;34:1563–71. [PubMed] [Google Scholar]
- [3].Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol 2010;15:544–51. [DOI] [PubMed] [Google Scholar]
- [4].Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013;58:139–49. [DOI] [PubMed] [Google Scholar]
- [5].Wada Y, Nakashima O, Kutami R, et al. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998;27:407–14. [DOI] [PubMed] [Google Scholar]
- [6].Ramzan M, Sturm N, Decaens T, et al. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int Off J Int Assoc Study Liver 2016;36:434–44. [DOI] [PubMed] [Google Scholar]
- [7].Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yao W, He JC, Yang Y, et al. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep 2017;7: 7525. doi: 10.1038/s41598-017-08128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Galon J, Pages F, Marincola FM, et al. Cancer classification using the immunoscore: a worldwide task force. J Transl Med 2012;10: 205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ikeguchi M, Oi K, Hirooka Y, et al. CD8+ lymphocyte infiltration and apoptosis in hepatocellular carcinoma. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2004;30:53–7. [DOI] [PubMed] [Google Scholar]
- [12].Ikeguchi M, Hirooka Y. Interleukin-2 gene expression is a new biological prognostic marker in hepatocellular carcinomas. Onkologie 2005;28:255–9. [DOI] [PubMed] [Google Scholar]
- [13].Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol Off J Am Soc Clin Oncol 2007;25:2586–93. [DOI] [PubMed] [Google Scholar]
- [14].Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res Off J Am Assoc Cancer Res 2007;13:902–11. [DOI] [PubMed] [Google Scholar]
- [15].Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497–505. [DOI] [PubMed] [Google Scholar]
- [16].Chen KJ, Zhou L, Xie HY, et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol (Northwood, Lond, Engl) 2012;29:1817–26. [DOI] [PubMed] [Google Scholar]
- [17].Lin SZ, Chen KJ, Xu ZY, et al. Prediction of recurrence and survival in hepatocellular carcinoma based on two cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prevent Res 2013;6:594–602. [DOI] [PubMed] [Google Scholar]
- [18].Pan QZ, Pan K, Zhao JJ, et al. Decreased expression of interleukin-36α correlates with poor prognosis in hepatocellular carcinoma. Cancer Immunol Immunother 2013;62:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brunner SM, Rubner C, Kesselring R, et al. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 2015;61:1957–67. [DOI] [PubMed] [Google Scholar]
- [20].Shi J, Dong Q, Qin L, et al. Phenotype and distribution of infiltrating lymphocytes in liver cancer tissues. Chin J Clin Oncol 2015;42:559–63. [Google Scholar]
- [21].Sun C, Xu J, Song J, et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 2015;6:35602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res 2016;4:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Q, Luan W, Warren L, et al. Prognostic role of immune cells in hepatitis b-associated hepatocellular carcinoma following surgical resection depends on their localization and tumor size. J Immunother (Hagerstown, Md: 1997) 2016;39:36–44. [DOI] [PubMed] [Google Scholar]
- [24].Xie QK, Zhao YJ, Pan T, et al. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. OncoImmunology 2016;5: e1181252. doi: 10.1080/2162402X.2016.1181252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang CY, Wang H, Liao W, et al. Transforming growth factor (is a poor prognostic factor and inhibits the favorable prognostic value of CD8+ CTL in human hepatocellular carcinoma. J Immunother 2017;40:175–86. [DOI] [PubMed] [Google Scholar]
- [26].Huang CY, Wang Y, Luo GY, et al. Relationship between PD-L1 expression and CD8+ T-cell immune responses in hepatocellular carcinoma. J Immunother (Hagerstown, Md: 1997) 2017;40:323–33. [DOI] [PubMed] [Google Scholar]
- [27].Sideras K, Biermann K, Verheij J, et al. PD-L1, Galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. OncoImmunology 2017;6: e1273309. doi: 10.1080/2162402X.2016.1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang B, Shen L, Wang K, et al. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int 2018;38:1449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang M, Pang HJ, Zhao W, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer 2018;18: 511. doi: 10.1186/s12885-018-4435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zheng X, Song X, Shao Y, et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget 2017;8:57386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015;6:17462–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuan CH, Sun XM, Zhu CL, et al. Amphiregulin activates regulatory T lymphocytes and suppresses CD8+ T cell-mediated anti-tumor response in hepatocellular carcinoma cells. Oncotarget 2015;6:32138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gajewski TF, Fuertes MB, Woo SR. Innate immune sensing of cancer: clues from an identified role for type I IFNs. Cancer Immunol Immunother CII 2012;61:1343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, N Y ) 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- [37].Sohn W, Paik YH, Kim JM, et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol 2014;21:2429–35. [DOI] [PubMed] [Google Scholar]


