Abstract
Objectives:
Open-angle glaucoma (OAG) imposes high disease burden in South Korea. Although various effective interventions are available to manage the progression of OAG, there is limited data on the cost-effectiveness of these treatment strategies in South Korea.
Methods:
Using a Markov cohort model, we evaluated the cost-effectiveness of 3 major treatment strategies (medication, laser trabeculoplasty, and trabeculectomy) for South Korean patients with OAG. We projected a 25-year time horizon to study a hypothetical cohort of 10,000 patients of age 40 with mild OAG. The outcome measures were quality-adjusted life-years (QALYs) gained, cost from the societal perspective, and the incremental cost-effectiveness ratio (ICER) of medication, laser trabeculoplasty, and trabeculectomy. Interventions were evaluated at a willingness-to-pay (WTP) threshold of 30,000,000 KRW ($29,152) per QALY gained. Deterministic and probabilistic sensitivity analyses were conducted to address the model uncertainty.
Results:
The mean costs for medication, laser trabeculoplasty, and trabeculectomy were 29,661,740 KRW, 17,34,1342 KRW, and 22,275,438 KRW, respectively. The mean QALYs gained were 15.7, 15.3, and 14.8 for medication, laser trabeculoplasty, and trabeculectomy, respectively. Surgery was strongly dominated because it generated fewer expected QALYs but incurred greater expected cost than laser. The ICER was 30,885,179 KRW per QALY for medication versus laser trabeculoplasty. Laser was cost-effective, however, at a lower WTP threshold of 21,000,000 KRW per QALY gained or below. The results were most sensitive to the progression rates from mild to moderate glaucoma under laser treatment.
Conclusion:
Under the WTP threshold of 30,000,000 KRW per QALY, medication was cost-effective compared with laser trabeculoplasty and trabeculectomy for treating mild OAG in South Korean population. Laser, however, can be a cost-effective alternative in more resource-limited settings.
Keywords: cost-effectiveness, glaucoma medication, laser, life-years, open-angle glaucoma, open-angle glaucoma management, quality-adjusted, trabeculectomy, trabeculoplasty
1. Introduction
Glaucoma is the second leading cause of blindness worldwide[1]. It is estimated that 64.3 million people aged 40 to 80 years have glaucoma, and the number is projected to grow to 79.6 million by 2020.[1–3] The most common type of glaucoma is an open-angle glaucoma (OAG), which damages the optic nerve fiber from increased eye pressure and causes subsequent visual field loss, eventually leading to blindness. Randomized controlled trials have provided evidence that lowering the intraocular pressure (IOP) reduces the progression of visual field loss from OAG.[4–7] Major treatment modalities to control the IOP include topical medication, laser trabeculoplasty, and glaucoma drainage surgery such as trabeculectomy. In general, medical treatment is the first-line treatment option for patients with mild OAG, and laser or surgical intervention is considered after medication has failed to control the IOP. However, due to high cost of medication and the challenges with patient adherence,[8–11] providing medication as a first-line treatment may not be feasible, effective, or cost-effective.
South Korea bears a disproportionate burden of glaucoma, with higher OAG prevalence than worldwide.[12,13] The prevalence of glaucoma among South Korean population has increased by 54% between 2008 and 2013, and the cost of care for glaucoma has nearly doubled from $16.5 million in 2008 to $29.2 million 2013.[13,14] With the aging Korean population and projected growth in disease burden from glaucoma,[12] identifying the effective and cost-effective glaucoma treatment strategy is imperative to both policy makers and patients. However, there is no published data on the long-term cost-effectiveness of glaucoma treatment options for the South Korean population.
We conducted a cost-effective analysis for the management of mild OAG using medication, laser trabeculoplasty, and trabeculectomy among the South Korean population using a Markov model. Existing data on the cost-effectiveness of treatment options for glaucoma is limited to comparisons among 2 interventions and some results are conflicting.[15–17] A study comparing surgery and medication in the Brazilian population concluded that surgery is more cost-effective than medication,[15] whereas another recent study comparing medication and laser in the US population showed that medical treatment is cost-effective compared to laser treatment when optimal medical adherence is assumed.[16] To our knowledge, there is no long-term data that consider the cost-effectiveness of all 3 major treatment modalities for OAG. We compared the 3 major treatment strategies for a clinically homogeneous population and the health care system to adjust for the potential variations in the assumption on the model parameters. Our results can be relevant the policy makers in South Korea as well as those in countries that face high glaucoma disease burden.
2. Methods
2.1. Overview
The study adhered to the tenets of the Declaration of Helsinki and the protocol was approved by the Institutional Review/Ethics Board of the Catholic University of Korea. We constructed a Markov model to project the health benefits and costs of medical treatment, laser trabeculoplasty, and trabeculectomy for treating glaucoma for South Korean patients with newly diagnosed mild OAG. Our model was a modification of a previously validated glaucoma disease progression model by Stein et al.[16] A Markov model can simulate health outcomes and costs of chronic medical conditions with multiple disease stages over the lifetime. We projected the quality-adjusted life-years (QALYs) and health care costs from a societal perspective and compared the incremental cost-effectiveness of 3 interventions with one another to determine the cost-effectiveness. All clinical and economic parameters are based on published studies or expert opinion. The parameter values and the ranges for sensitivity analyses are listed in Table 1.
Table 1.
Summary of key model parameters. All costs are reported in 2017 South Korean Won (KRW).
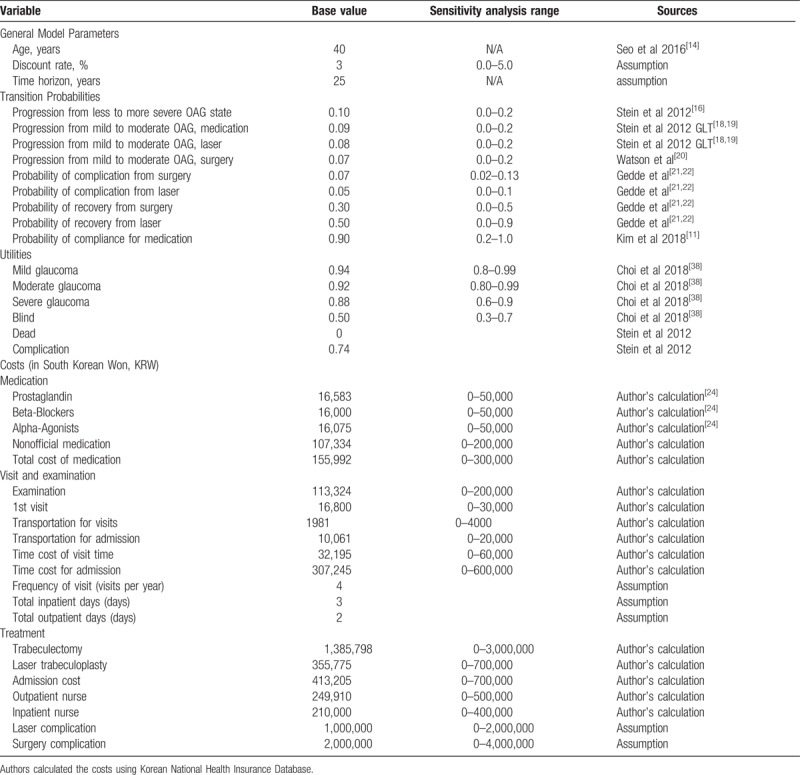
Our study population is a 100,000 hypothetical cohort of South Korean male and female patients aged 40 years with mild OAG over a 25-year horizon. We chose 40 as the starting age since the prevalence of OAG for South Korean population increases at age 40.[14,25] We chose 25 years as the time horizon to capture the slow progression rate of OAG and to project the cohort. TreeAge Pro 2016 Health Care (TreeAge Software) was used for analysis.
2.2. Overview of treatment strategies
Medical treatment (“medication”) strategy was chosen as a status quo because it is currently the most common practice for mild OAG patients in South Korea.[10,11] Medication was compared to the 2 alternative strategies, laser trabeculoplasty (“laser”) and trabeculectomy (“surgery”). In medication, patients were assumed to use all 4 main classes of topical drops (β-blocker, carbonic anhydrase inhibitor, α-adrenergic agonist, and prostaglandin) for glaucoma treatment. In laser, patients receive trabeculoplasty of either argon laser, or diode laser, which are shown to have similar effectiveness and cost.[26,27] In surgery, patients receive a trabeculectomy, the standard form of surgical treatment for OAG that creates an alternative route for aqueous humor outflow to reduce the IOP. We assumed that the patients with successful laser or surgical interventions do not require medication.
2.3. Markov model
Our model consists of Markov states of glaucoma disease stages, complications, and death. Glaucoma disease stages include mild, moderate, severe glaucoma, and blindness (Fig. 1). Following the literature, mild, moderate, and severe glaucoma were defined as glaucomatous damage with a mean deviation of −6 dB or less, between −6 dB and −12 dB, and −12 dB or worse of visual field loss on standard automated perimetry, respectively.[16] Blindness was defined as having best-corrected visual acuity of less than 20/200 due to glaucoma in at least 1 eye caused by glaucoma.
Figure 1.
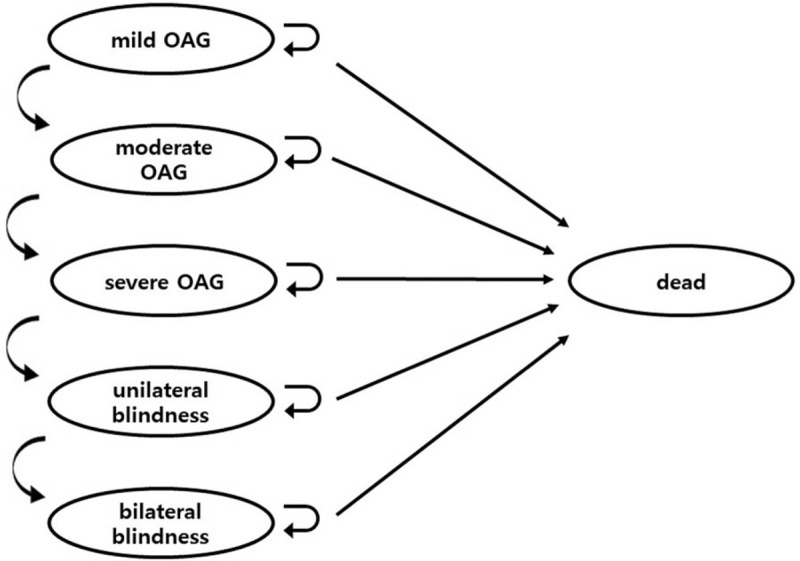
Markov model of glaucoma. OAG indicates open-angle glaucoma. OAG = open angle glaucoma.
We assumed that all population starts in mild OAG. At each cycle, a patient accumulates health utility and cost based on the current disease stage, and s/he either stays in the current state or transitions to a different state according to the transition probabilities associated with the current health state. Patients can only progress from less severe to more severe disease stage, and the transition probabilities differ by the treatment modality. The cycle length of the model was 1 year.
The central feature of our model that captures the differences between the treatment strategies are the disease progression rate from mild to moderate OAG. The progression rates from mild to moderate OAG for medication and laser treatment were estimated from the Glaucoma Laser Trial (GLT) study.[18,19] The GLT is a randomized clinical trial to assess the efficacy of treatment for OAG with argon laser trabeculoplasty versus topical medication.[18,19] The progression rates from mild to moderate OAG for surgery was estimated from a prospective randomized block study which compared the efficacy of reducing the IOP for managing OAG with laser trabeculoplasty versus surgery.[20] Because we estimated the model parameters on treatment efficacy from 2 different sources, we explored the impact of varying assumptions on these model parameters on our result in the sensitivity analysis.
We estimated the baseline probability of progression from less severe to more severe disease states from the Early Manifest Glaucoma Trial data.[6] Given that 53% of mild OAG patients from the trial progressed to more severe stages after 6 years under no treatment, we were able to estimate the instantaneous progression rate r from the equation r = –In (1–P)/t, where P is the probability of progression, and t years. Then we were able to estimate the annual probability of progression as 0.1 using the equation P=1–exp (–rt). We assumed that the interventions on mild OAG patients will mostly affect the transition probabilities from mild to moderate stages, and thus the progression probabilities from moderate to severe and severe to blind states remain constant. We addressed our assumption by varying the progression probabilities in the sensitivity analysis. The mortality rate was from 2015 Korean Life Table. Because glaucoma does not increase mortality rate, we assumed that there was no additional disease-specific mortality for glaucoma patients.
2.4. Complications
Patients who receive laser or surgery have a risk of complication, including endophthalmitis, suprachoroidal hemorrhage, bleb leak that required revision, hypotony maculopathy, or corneal edema that required penetrating keratoplasty.[22,28] The probability of developing any surgical complications was estimated from the 1-year result of the Tube vs. Trabeculectomy Trial as 5% and 7%, respectively.[22,28–31] Once a patient develops complication, he or she either stays in the complication state or recovers, and those treated with laser have a higher chance of recovery than those treated with surgery. For medication, however, we assumed that there is no severe complication for patients treated with medication because the reported side-effects are relatively mild.[32] Instead, we estimated the probability of compliance for medication, because side effects from medication may cause adherence problem.[11] In sensitivity analyses, we examined the probability of developing complication after laser treatment and surgery for a reasonable range. We did not assume any additional risk of late complication or death for patients who develop complications.[28] However, developing complications will lower the patient's quality of life, which is reflected in the QALY weights.
2.5. Costs
Taking societal perspective, we included both direct and indirect costs of associated with glaucoma management. Direct costs were estimated from calculating the average reimbursement rates for related procedures from the 2016 Korean National Health Insurance Database.[24] Direct medical costs of glaucoma care included the costs of inpatient and outpatient visits to ophthalmologists, tests to monitor patients, and the costs of interventions. We also estimated the indirect costs of patients’ time and transportation associated with visits. We did not include the indirect societal cost from the loss of productivity due to impaired vision gave the majority of the study population was over age 60.
We assumed that the costs for regular visits and diagnostic tests applied to all patients, regardless of the types of interventions received. This included the cost of full examination, first visit for consultation, and the transportation and time for consultation. The cost of visit included the slit-lamp examination, gonioscopy, automated visual field testing, optical coherence tomography, and fundus photography. During the first year, individuals in all cohorts were assumed to pay 3 follow-up examinations. The procedures and costs for treating complications were estimated from the literature assuming that patients undergo standard management.[33,34] The cost of complication included the total cost of hospitalization (treatment, boarding, and the personnel cost) as well as the patient's time and transportation costs. There were additional costs of acquiring low vision aids for individuals who have progressed to unilateral or bilateral blindness, which was estimated from the findings by Lee et al.[35] In sensitivity analyses, we examined the influence of a range of treatment and medication costs as well as the indirect costs on our results.
2.6. Utilities
The utility weights represent health-related quality of life associated with each disease state, ranging from death (=0) to perfect health (=1). The weights were based on published studies on common ophthalmic diseases and blindness,[23,36–38] and all utility weights were varied in sensitivity analyses.
2.7. Model validation
We evaluated the internal validity of the model by comparing the glaucoma outcomes with the outcomes from the GLT study.[18,19] We evaluated the external validity of the model by comparing the model outcomes to the observed outcomes from an IOP reduction randomized trial that was not used to inform our model parameters.[39]
2.8. Economic analysis
We calculated an incremental cost-effectiveness ratio (ICER) for laser and surgery to medication using the projected QALYs and costs to determine the cost-effectiveness. The ICER was compared to a cost-effectiveness threshold of 30,000,000 KRW per QALY gained, which is an approximate GPD per capital of South Korea in 2017.[40] All costs were in 2017 South Korean Won (KRW) and we assumed a 3% discounting rate per year.
2.9. Sensitivity analyses
We conducted a 1-way sensitivity analysis on all model parameters to identify the parameters to which the model results were most sensitive. Incorporating the results from 1-way sensitivity analysis and the clinical expertise, we performed a 2-way sensitivity analyses on select parameters. We evaluated the parameter uncertainty by conducting a probabilistic sensitivity analysis, where we had 10,000 random draws from beta distributions for each influential variable. We used a willingness-to-pay (WTP) threshold of 30,000,000 KRW per QALY. WTP threshold is the amount of additional cost the payer is willing to pay for 1 additional gain of QALY.
3. Results
3.1. Model validation
Within 10 years of initial treatment, about 70% of the patients treated with medication had mild or moderate glaucoma, whereas about 45% of patients treated with laser had mild or moderate glaucoma, consistent with the comparative effectiveness of laser versus medication from the GLT study result (Fig. 2).[18,19] About 43% of the untreated patients with mild OAG progressed into more severe states after 3 years, which is comparable to the outcomes of the IOP reduction randomized study where 39% of the untreated cohort experienced visual field progression.[39]
Figure 2.
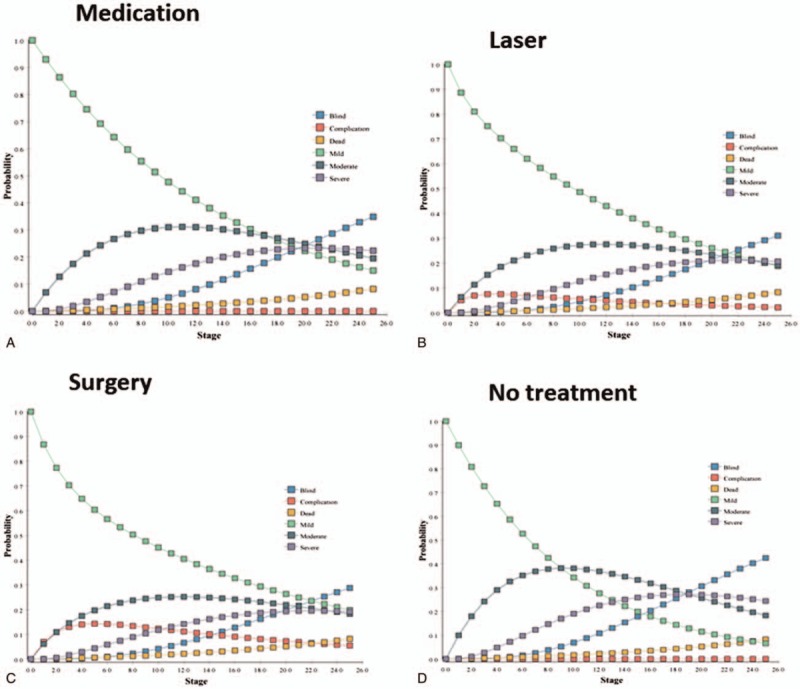
Markov tracings of disease states over time for the (a) medication, (b) laser, (c) surgery, and (d) no treatment cohort.
3.2. Base model
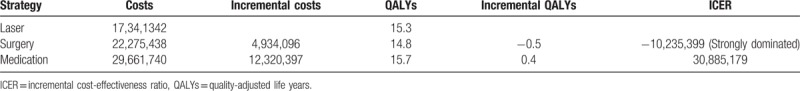
Figure 3 and Table 2 show the cost-effectiveness of treating newly diagnosed mild OAG using medication, laser, or surgery. Treating a single mild OAG patient via medication incurred expected cost of 29,661,740 KRW and expected QALY of 15.7. Compared to medication, both laser and surgery generated less expected cost (17,34,1342 KRW for laser and 22,275,438 KRW for surgery) and fewer QALYs gained (15.3 for laser and 14.8 for surgery). Because surgery had fewer expected QALYs gained but greater expected cost than laser, surgery was strongly dominated. Compared to laser, medication had higher cost and effectiveness, with ICER of 30,885,179 KRW per QALY gained.
Figure 3.
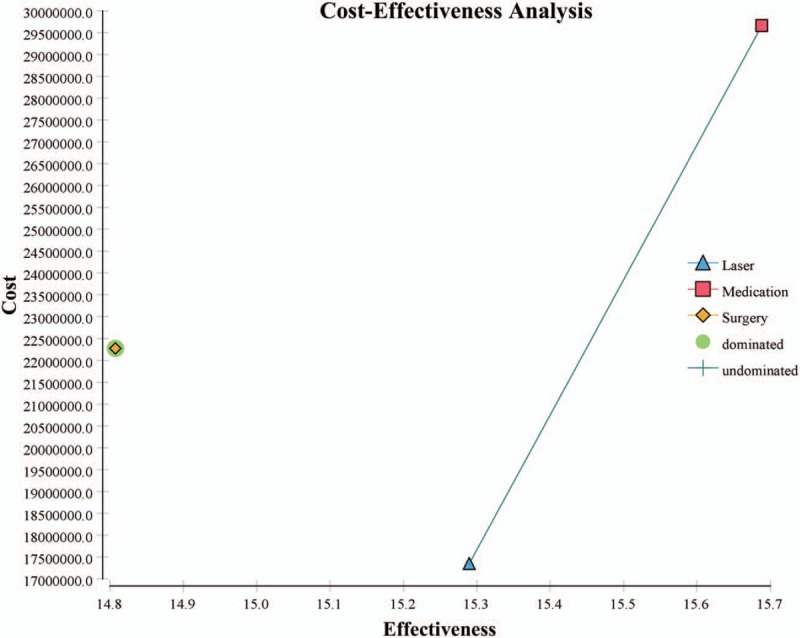
Cost-effectiveness plane comparing the effectiveness of medication, laser, and surgery. Note: cost was measured in South Korean Won (KRW) and effectiveness was measured in QALYs. QALYs = quality-adjusted life years.
Table 2.
Costs (KRW), QALYs, and ICERs (KRW/QALY) for treating newly diagnosed mild open-angle glaucoma using medication, laser, or surgery.
3.3. Sensitivity analysis
Figure 4 shows the result of 1-way sensitivity analysis on the 13 most influential model parameters on the model result. The outcomes are represented as the net monetary benefits when medication was compared to laser treatment at WTP threshold 30,000,000 KRW per QALY. The diagram shows that disease progression rates, the utilities of health states and cost of examinations and medications are influential on the model outcomes. We conducted additional 1-way sensitivity analyses by calculating the ICER of medication in comparison to laser for each influential parameter. The results of 1-way sensitivity analyses on the key parameters show that the parameters examined within the reasonable sensitivity ranges produced the result qualitatively equivalent to our main conclusion that the surgery is dominated and the medication incurs greater costs and benefits in comparison to the laser treatment.
Figure 4.
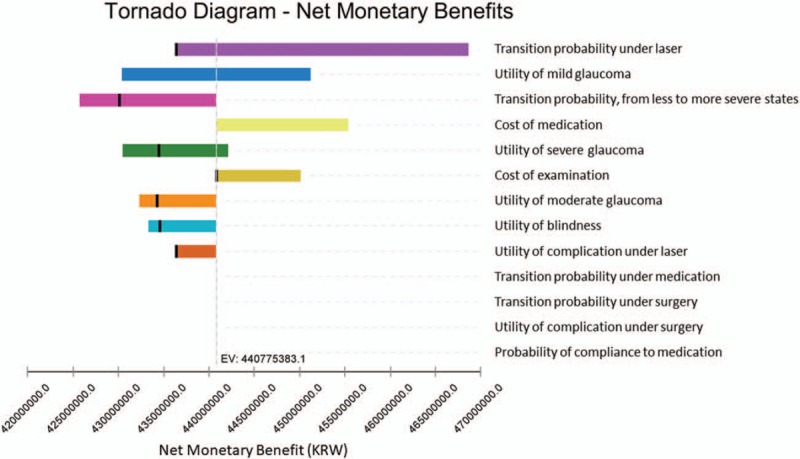
Tornado diagram of the range of net monetary benefits at willingness-to-pay threshold 30,000,000 KRW per QALY for all major model parameters. QALYs = quality-adjusted life years.
Because the progression rates from mild to moderate glaucoma under laser treatment and cost of medication are central to our model, we conducted 2-way sensitivity analyses on these parameters. When we compared the progression rates for laser to cost of medication (Fig. 5), medication was the dominant strategy in the red region, that is, when the progression rate under laser was high or cost of medication was low. Since cost of medication for glaucoma differs according to countries, the results of 2-way sensitivity analyses warrant further investigation in other countries with different health care systems. In the probabilistic sensitivity analysis that incorporates the parameter uncertainty (Fig. 6), the probability of each strategy being cost-effective was plotted against the WTP threshold ranges. Medication was a dominant strategy in 64% of the 10,000 iterations when the WTP threshold was 30,000,000 KRW per QALY. Laser was the dominant strategy, however, for greater proportions of iterations when the WTP threshold was lower, that is, under 21,000,000 KRW per QALY.
Figure 5.
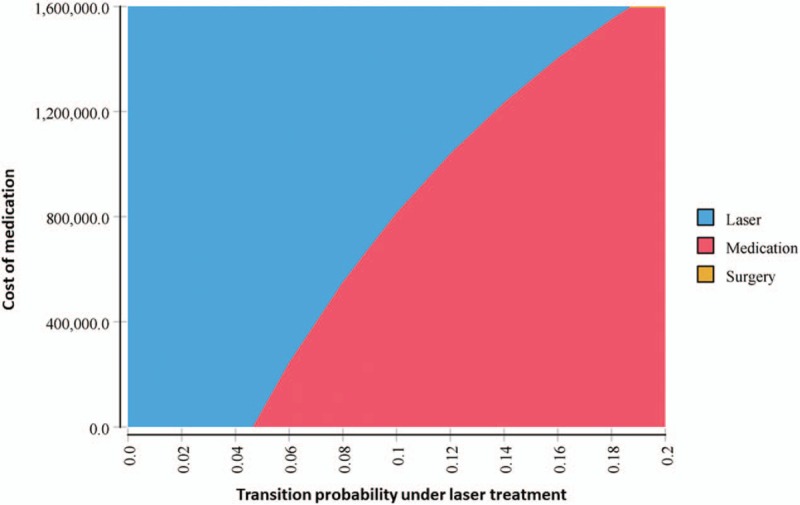
Two-way sensitivity analysis results for progression rates from mild to moderate glaucoma under medication and laser. QALYs = quality-adjusted life years.
Figure 6.
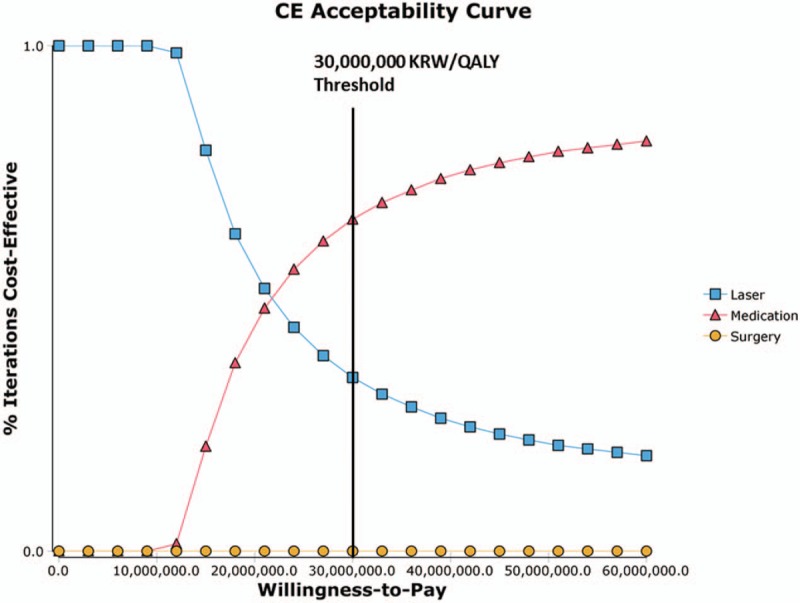
Cost-effectiveness acceptability curve from the probabilistic sensitivity analysis. Note: Willingness-to-pay threshold is in South Korean Won (KRW)/QALY. QALYs = quality-adjusted life years.
4. Discussion
We conducted a cost-effective analysis for the management of mild OAG by comparing medication, laser therapy, and surgery among South Korean patients. Although there are multiple effective interventions to control the IOP and slow down the progression of OAG, limited information exists on the cost-effectiveness of the comprehensive treatment strategies.[17] We found that surgery is not a viable initial treatment strategy for the patients with mild OAG, as the risk of complications and the high cost of surgery makes it both costlier and less effective than medication and laser. Laser was cheaper than medication but gained fewer expected QALYs. The ICER of medication in comparison to laser was 30,885,179 KRW (27,092 USD) per QALY gained, which was lower than the gross domestic product (GDP) per capita of South Korea (29,152 USD in 2017). Based on the World Health Organization (WHO)'s cost-effectiveness threshold of 1 to 3 times the GDP per capita of the country, medication is cost-effective and acceptable in health care system of Korea. The probabilistic sensitivity analysis showed that laser was more likely to be cost-effective than medication under low WTP threshold. It has been noted that medication is not available or feasible for long-term use in developing countries, in which case laser or surgery was suggested as alternative treatment option. Our model suggests that surgery is not cost-effective at any WTP threshold, but laser could be a cost-effective treatment option under low WTP in resource-limited settings.
We used 25-year time horizon for the cost projection in this study. Ideally, the time horizon of an analysis should extend far enough into the future to capture major economic and health outcomes.[41] However, extreme high timeline is hard for modeling parameters to be constant. Thus, most cost-effectiveness studies use time-horizon of 25 to 30 years. The results of this study should be interpreted with caution. As the study population was a cohort of patients with newly diagnosed mild OAG, results of this study can be applied only to patients with mild OAG, not to severe glaucoma. For patients with severe glaucoma, medications alone may not be as effective.
There are multiple limitations to our study. First, we made some important assumptions on the model structure. We assumed that patients do not receive repetitive treatments once treated with either surgery or laser treatment, and there are no underlying clinical differences between the patients who develop complications then recover and those who never develop complications. Second, there are limitations on our model parameters. We estimated the disease progression rates and the intervention effectiveness by combining information from multiple sources. Although we evaluated the internal and external validity of our model results, our assumptions can still be subject to multiple biases. Third, medication was assumed to have no complications. However, side effects form topical medication may cause adherence problem or dry eye disease in the case of benzalkonium chloride. We did not capture this complication because the reported side-effects are relatively mild. Instead, we have incorporated adherence problem into the model to compensate for the complication of topical medication. Fourth, the result of this study would be confined to patients with early stages of glaucoma. For those with severe stage, medications alone may not be as effective. Last, the cost values used in our model are from South Korea, which limits the generalizability of our findings. South Korea has a single-payer national health insurance system with universal coverage. The government manages the schedule of fees paid to providers, where most health care services are delivered by private providers. Our results can be informative in settings similar to South Korea in terms of the health care system and the level of resources, but the results may be less applicable under different settings, and our results should be interpreted with caution.
Glaucoma incidence and disease burden have been increasing worldwide, but there is limited scientific evidence on the cost-effective of treatment strategies. Previous studies were limited in providing a comprehensive comparison of 3 major interventions for managing OAG. We developed a Markov model for patients with OAG in South Korea and found that under the cost-effectiveness threshold of 30,000,000 KRW per QALY, medication is a cost-effective strategy compared to laser or surgery under reasonable assumptions on disease progression and treatment effectiveness. Our results also suggest that laser can be a cost-effective alternative under resource-limited settings.
Author contributions
Conceptualization: Sang Min Park, Jin Woo Kwon, Donghyun Jee.
Data curation: Jin A Choi, Lina D. Song, Seulggie Choi, Donghyun Jee.
Formal analysis: Jin A Choi, Lina D. Song, Seulggie Choi, Sang Min Park, Jin Woo Kwon, Donghyun Jee.
Funding acquisition: Donghyun Jee.
Investigation: Jin A Choi, Lina D. Song, Sang Min Park, Jin Woo Kwon, Donghyun Jee.
Methodology: Seulggie Choi.
Project administration: Donghyun Jee.
Supervision: Sang Min Park, Donghyun Jee.
Validation: Donghyun Jee.
Writing – original draft: Jin A Choi, Donghyun Jee.
Writing – review & editing: Lina D. Song, Seulggie Choi, Sang Min Park, Jin Woo Kwon, Donghyun Jee.
Footnotes
Abbreviations: GLT = Glaucoma Laser Trial, ICER = incremental cost-effectiveness ratio, IOP = intraocular pressure, OAG = open-angle glaucoma, QALY = quality-adjusted life-year, WTP = willingness-to-pay.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC16C2299) and by financial support of the National Research Foundation of Korea Grant funded by the Korean government (MSIP) (No. NRF2016R1D1A1B03932606). The content is solely the responsibility of the authors and does not necessarily represent the official views of the KHIDI.
The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
References
- [1].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014;121:2081–90. [DOI] [PubMed] [Google Scholar]
- [3].Varma R, Lee PP, Goldberg I, et al. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol 2011;152:515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130:429–40. [DOI] [PubMed] [Google Scholar]
- [5].Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–13. [DOI] [PubMed] [Google Scholar]
- [6].Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48–56. [DOI] [PubMed] [Google Scholar]
- [7].Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943–53. [DOI] [PubMed] [Google Scholar]
- [8].Azuara-Blanco A, Burr J. The rising cost of glaucoma drugs. Br J Ophthalmol 2006;90:130–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol 2006;17:190–5. [DOI] [PubMed] [Google Scholar]
- [10].Park MH, Kang KD, Moon J, et al. Noncompliance with glaucoma medication in Korean patients: a multicenter qualitative study. Jpn J Ophthalmol 2013;57:47–56. [DOI] [PubMed] [Google Scholar]
- [11].Kim CY, Park KH, Ahn J, et al. Treatment patterns and medication adherence of patients with glaucoma in South Korea. Br J Ophthalmol 2017;101:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan EW, Li X, Tham YC, et al. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol 2016;100:78–85. [DOI] [PubMed] [Google Scholar]
- [13].Park J, Lee JS, Jang YA, et al. A comparison of food and nutrient intake between instant noodle consumers and non-instant noodle consumers in Korean adults. Nutr Res Pract 2011;5:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seo SJ, Lee YH, Lee SY, et al. Estimated prevalence of glaucoma in South Korea using the national claims database. J Ophthalmol 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Guedes RA, Guedes VM, Chaoubah A. Cost-effectiveness comparison between non-penetrating deep sclerectomy and maximum-tolerated medical therapy for glaucoma within the Brazilian National Health System (SUS). Arq Bras Oftalmol 2012;75:11–5. [DOI] [PubMed] [Google Scholar]
- [16].Stein JD, Kim DD, Peck WW, et al. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol 2012;130:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ting NS, Li Yim JF, Ng JY. Different strategies and cost-effectiveness in the treatment of primary open angle glaucoma. Clinicoecon Outcomes Res 2014;6:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].The Glaucoma Laser Trial (GLT) and glaucoma laser trial follow-up study: 7. Results. Glaucoma Laser Trial Research Group. Am J Ophthalmol 1995;120:718–31. [DOI] [PubMed] [Google Scholar]
- [19].The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. The Glaucoma Laser Trial Research Group. Ophthalmology 1990;97:1403–13. [PubMed] [Google Scholar]
- [20].Watson PG, Allen ED, Graham CM, et al. Argon laser trabeculoplasty or trabeculectomy a prospective randomised block study. Trans Ophthalmol Soc U K 1985;104:55–61. [PubMed] [Google Scholar]
- [21].Gedde SJ, Feuer WJ, Shi W, et al. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology 2018;125:650–63. [DOI] [PubMed] [Google Scholar]
- [22].Gedde SJ, Herndon LW, Brandt JD, et al. Surgical complications in the tube versus trabeculectomy Study during the first year of follow-up. Am J Ophthalmol 2007;143:23–31. [DOI] [PubMed] [Google Scholar]
- [23].Brown MM, Brown GC, Sharma S, et al. Utility values associated with blindness in an adult population. Br J Ophthalmol 2001;85:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shin DW, Cho B, Guallar E. Korean national health insurance database. JAMA Intern Med 2016;176:138. [DOI] [PubMed] [Google Scholar]
- [25].Kim M, Kim TW, Park KH, et al. Risk factors for primary open-angle glaucoma in South Korea: the Namil study. Jpn J Ophthalmol 2012;56:324–9. [DOI] [PubMed] [Google Scholar]
- [26].Bergea B, Bodin L, Svedbergh B. Primary argon laser trabeculoplasty vs pilocarpine. II: Long-term effects on intraocular pressure and facility of outflow. Study design and additional therapy. Acta Ophthalmol (Copenh) 1994;72:145–54. [DOI] [PubMed] [Google Scholar]
- [27].Brancato R, Carassa R, Trabucchi G. Diode laser compared with argon laser for trabeculoplasty. Am J Ophthalmol 1991;112:50–5. [DOI] [PubMed] [Google Scholar]
- [28].Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol 2012;153:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kirwan JF, Cousens S, Venter L, et al. Effect of beta radiation on success of glaucoma drainage surgery in South Africa: randomised controlled trial. BMJ 2006;333:942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 2009;148:670–84. [DOI] [PubMed] [Google Scholar]
- [31].Wormald R, Wilkins MR, Bunce C. Post-operative 5-Fluorouracil for glaucoma surgery. Cochrane Database Syst Rev 2001;1132–67. [DOI] [PubMed] [Google Scholar]
- [32].Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg 1995;26:233–6. [PubMed] [Google Scholar]
- [33].Alvarenga LS, Mannis MJ, Brandt JD, et al. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol 2004;138:200–5. [DOI] [PubMed] [Google Scholar]
- [34].Suner IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology 1997;104:207–14. [DOI] [PubMed] [Google Scholar]
- [35].Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol 2006;124:12–9. [DOI] [PubMed] [Google Scholar]
- [36].Lee BS, Kymes SM, Nease RF, Jr, et al. The impact of anchor point on utilities for 5 common ophthalmic diseases. Ophthalmology 2008;115:898–903. [DOI] [PubMed] [Google Scholar]
- [37].Rein DB, Wirth KE, Johnson CA, et al. Estimating quality-adjusted life year losses associated with visual field deficits using methodological approaches. Ophthalmic Epidemiol 2007;14:258–64. [DOI] [PubMed] [Google Scholar]
- [38].Choi S, Choi JA, Kwon JW, et al. Utility values for glaucoma patients in Korea. PLoS One 2018;13:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998; 126:498–505. [DOI] [PubMed] [Google Scholar]
- [40].Data O. Gross domestic product (GDP). South Korea 2017. [Google Scholar]
- [41].Kim DD, Wilkinson CL, Pope EF, et al. The influence of time horizon on results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2017;17:615–23. [DOI] [PubMed] [Google Scholar]


