Abstract
Poorly soluble small molecules typically pose translational hurdles owing to their low solubility, low bioavailability, and formulation challenges. Nanocrystallization is a versatile method for salvaging poorly soluble drugs with the added benefit of a carrier‐free delivery system. In this review, we provide a comprehensive analysis of nanocrystals with emphasis on their clinical translation. Additionally, the review sheds light on clinically approved nanocrystal drug products as well as those in development.
Keywords: clinical trials, nanocrystals, nanomedicine, nanoparticles, nanotherapeutics, translational medicine
1. INTRODUCTION
Over the years, nanoparticles (NPs) made of both organic and inorganic materials have been engineered to circumvent the biological barriers and deliver drugs for a variety of indications.1, 2 Water‐insoluble or hydrophobic drugs, pose a challenge in terms of achieving optimal bioavailability and thereby, adequate efficacy.3 As reported in 2015, 40% of drugs on the market and 90% of drugs within the discovery pipeline face solubility issues.4 Other statistics, cite 40% of all potential drug candidates were shelved as a result of intrinsic aqueous solubility issues.5 Thus, a number of hydrophobic drugs, which could potentially be useful for treatments are in need of clinically acceptable carriers.6
For the purpose of this review article, drug nanocrystals may be defined as pure solid particles with a mean diameter <1 μm and a crystalline character. The platform offers an exceptional opportunity to deliver hydrophobic drugs (Figure 1). Its uniqueness originates from the fact that nanocrystals are composed entirely of 100% drug or the payload thereby eliminating the ancillary role of a carrier.7 In addition, surfactants or stabilizers are commonly used to stabilize the crystalline dispersions in liquid media.
Figure 1.
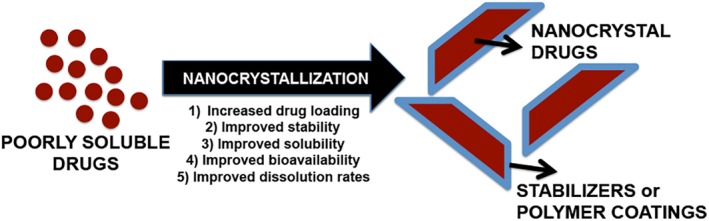
Nanocrystallization of poorly soluble drugs improves physicochemical stability and drug bioavailability
Nanocrystalline drug technology improves the solubility of hydrophobic drugs due to an increased surface area to volume ratio and improved dissolution rates (i.e., dissolution velocity) associated with nanosizing.8 The drug crystals are singularly well‐suited for the rehabilitation of previously unsuccessful Biopharmaceutics Classification System (BCS) Class II and IV drugs (low solubility drugs).9 The BCS classification system is an experimental model that measures permeability and solubility under prescribed conditions. The system divides the drugs into four classes. While Class I drugs have high solubility and high permeability, Class II molecules have low solubility and high permeability, Class III identifies with high solubility and low permeability, and drugs in Class IV have low solubility and low permeability.4
Nanocrystal drug formulations have also been shown to be stable in suspensions and are often referred to as nanocrystal colloidal dispersions (NCD's). The dispersions provide a platform for easy scale‐up and manufacturing of highly stable and marketable products. Their synthesis and scale‐up considerations have been described at length elsewhere.10, 11 Commonly used synthesis techniques include the use of microfluidic based platforms or the milling method, which, among others, is both flexible and tunable.7, 12, 13, 14, 15, 16, 17 Taken together, the nanocrystal drug technology has been studied extensively and is well positioned for further exploration in the field of drug delivery.
Several hydrophobic drugs have been salvaged via the nanocrystal formulation method. The drugs were successfully developed, and approved by the FDA to treat a variety of indications ranging from dental disorders to cancer in the clinic.14, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Depending on the disease, the approved formulations can be administered via different routes including oral, dermal, and parenteral. This highlights the versatility of a nanocrystal drug platform. Pharmacokinetic, biodistribution, and bioavailability data for organs involved in delivery routes tested using nanocrystal technology have been addressed at length previously.10, 13, 18, 24, 25, 29, 30, 31, 32, 33, 34 Specifically, the reviews of Lu et al. 2016 and 2017 delve into the biodistribution pattern of nanocrystal drugs in the blood, heart, liver, spleen, lung, kidney, tumor, and thymus (i.e., the organs involved in clearance/circulation and host immune responses).24, 35
Several articles have been published, discussing the techniques used to synthesize nanocrystal drugs; the type of stabilizers or surfactants involved; and the methods adopted for physicochemical and biological characterization.10, 19, 36, 37 However, a wide translational gap exists between this highly promising platform and its clinical approval. In this review, we discuss the nanocrystal drug technology and its development from a translational perspective. We speak to the paucity of FDA approved products despite the platform's obvious strengths. We discuss the challenges involved in their successful translation to the clinic.
2. PREPARATION AND CHARACTERIZATION OF NANOCRYSTALS
Properties such as crystallinity, size, shape, surface charge, and the type of stabilizers or polymer coatings used during formulation influence the therapeutic outcome of nanocrystal drug products. Other physicochemical properties currently under investigation for their influence on preclinical (i.e., in vivo/in vitro) performance assays include stiffness and surface texture.
2.1. Nanocrystal drug dissolution: Concept and theory
Although often overlooked, crystallinity is a foundational parameter for drug nanocrystals. It can provide insights into the structure of the final formulation. Assessing crystallinity is critical in verifying the successful integration of stabilizers, surface polymers (chemically conjugated or physically adsorbed), and targeting ligands. Further, nanocrystals with an amorphous crystalline substructure have an increased dissolution rate and are better suited for delivering multiple hydrophobic drugs.21, 38, 39 This is better explained using the “spring and parachute” concept adapted to describe dissolution rates of amorphous, crystalline, or co‐crystalline drugs.
Co‐crystalline drugs are often composed of multiple components, including a hydrophobic drug and a stabilizer. Stabilizers are supplementary molecules which when added during formulation, can control the nanocrystal size, agglomeration, and its overall biodistribution in vivo.8, 14 Stabilizers are generally 50–500 fold more soluble in water than the drug in its free powder form. When exposed to an aqueous environment, the stabilizer first begins to leach into solution and leaves behind the drug particles. Subsequently, the loosely self‐aggregated drug particles form supramolecular aggregates. The co‐crystal at this point is in an amorphous crystalline state resulting in a high peak concentration (Figure 2). The highly soluble amorphous co‐crystal slowly transforms to a stable species following Ostwald's law that promotes dissolution into a free form of the drug (Curve 1). The time required for the drug to move through the high‐energy aggregate stage to a low‐energy solubilized state exhibit a “parachute effect.” Here, a high dissolution rate is achieved over an extended period of time. In absence of the stabilizer, the drug precipitates rapidly to a stable polymorph via the so‐called “spring effect.” This results in a modest improvement in solubility which is short‐lived and not sustained for long‐term dose regimens. The stable crystalline drug is almost insoluble as shown by Curve 1.
Figure 2.
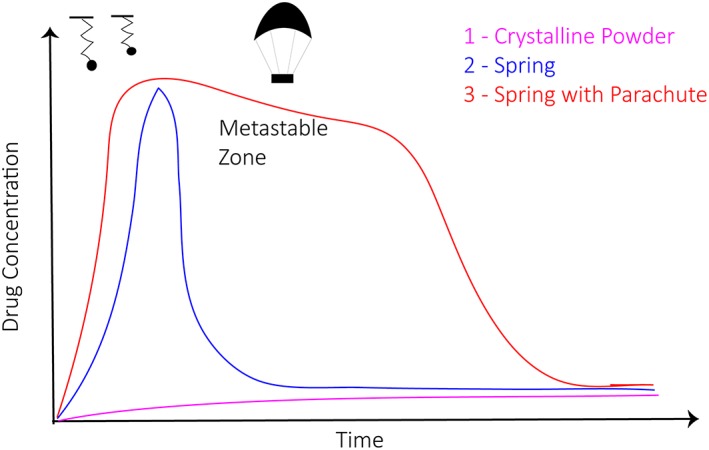
Schematic depicting spring and parachute model for nanocrystal dissolution as a function of time and drug concentration (Adapted from N. Babu and A. Nangia, 2011, Cryst Growth Des, 11, 2662–2679)
2.2. Physical analytical methods to characterize nanocrystal drug products
Stabilizers that are widely used in nanocrystal drug formulations are presented in Table 1. These are amphiphilic molecules that increase the nanocrystal's surface wettability, and when used at optimal concentrations, do not interfere with the crystal growth. Its successful integration into the final formulation can be confirmed simultaneously with the crystal's substructure using X‐Ray spectroscopy methods such as Small‐angle X‐ray scattering, X‐ray diffraction, and X‐ray photoelectron spectroscopy.
Table 1.
Examples of drug/stabilizer combinations in nanocrystal formulations (compiled from Refs. 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50)
| Nanocrystal Core molecule | Stabilizer | Process |
|---|---|---|
| Glibizide | Sodium lauryl sulfate, Polyvinyl pyrrolidone K30, Pluronics F68 and F127, Tween 80, hydroxypropyl methylcellulose | Milling, Antisolvent precipitation |
| MTKi‐327 | Pluronic F108, Lipid S75, | Milling |
| Beclomethasone diproprionate | Hydrophobin | Antisolvent precipitation |
| Naproxen | Vitamin E tocopherol polyethylene glycol succinate, Pluronic F127, sodium lauryl sulfate, di(2‐ethylhexyl) sulfosuccinate, | Milling |
| Paclitaxel | Hydroxylpropyl methylcellulose, polyvinylpyrrolidone, polyethylene glycol 400, Pluronics F127 and F68, sodium lauryl sulfate, Tween 20 and 80, transferrin, immunoglobulin G, human serum albumin | Antisolvent precipitation, sonication |
| Indomethacin | α‐, β‐, and γ‐ cyclodextrans, Pluronics F68, 17R4, and L64, Tetronics 908 and 1107 | Emulsion solvent diffusion, milling |
| Budesonide | Lecithin, Pluronic F68 | Milling |
| Curcumin | Polyvinyl alcohol, polyvinyl pyrrolidone, vitamin E tocopherol polyethylene glycol succinate, sodium lauryl sulfate, Carboxymethylcellulose sodium | High pressure homogenization |
| Nitrendipine | Polyvinyl alcohol | Antisolvent precipitation, ultrasonication |
| Brinzolamide | Tween 80, Pluronics F68 and F127, Hydroxypropyl methylcellulose | Milling |
| Fenofibrate | Hydroxypropyl methylcellulose, Soluplus | Milling |
| Nimodipine | Pluronic F127, Hydroxypropylmethylcellulose | Microprecipitation, high pressure homogenization |
| Loviridine, cinnarizine, griseofulvin, mebendazole, phenylbutazone, phenytoin | Polyvinyl pyrrolidone, polyvinyl alcohol‐polyethylene glycol, Pluronic F68, tocopherol polyethylene glycol succinate, hydroxypropyl methylcellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, methylcellulose, carboxymethylcellulose sodium, polyvinyl alcohol, sodium alginate, tween 80 | Milling |
| Cellulose | Tetradecyltrimethylammonium bromide, dodecyldimethylammonium bromide, cetyltrimethylammonium bromide, Hexadecyltrimethylammonium | Miniemulsion polymerization, Pickering emulsions |
| PH‐797804 MAPK inhibitor nanocrystal‐polymer particle | Polylactic acid | Miniemulsion polymerization |
| Gold | Cetyltrimethylammonium bromide, benzyldimethylhexadecylammonium chloride, hexamethylenetetramine, polyvinyl pyrrolidone, polyethylene glycol, β‐cyclodextrins | Self‐assembly, antisolvent precipitation |
| Cobalt | Trioctylphosphine oxide and oleic acid | Self‐assembly, antisolvent precipitation |
| Cyclosporin A | Imwitor®900, Tagat®S, sodium cholate | High pressure homogenization |
| Dexamethasone, ibuprofen, tacrolimus | Poloxamer 407, Poloxamer 188, vitamin E‐TPGS, lecithin, Plantacare 2000 UP | Wet bead milling |
| IONP | Poly(ethylene glycol)–poly(vinylphosphonic acid) block copolymers (PEG‐b‐PVPA) | Antisolvent precipitation |
Nanocrystal drug sample preparation for X‐Ray spectroscopy analysis often involves freeze‐drying. The effects of freeze‐drying on the agglomeration of nanocrystals and its subsequent re‐dispersibility have been studied and reported previously.51 Liquid nanocrystal dispersions designed for dosing in their solid form must be freeze‐dried above the critical freezing rates. Using lower rates would increase particle aggregation and affect formulation redispersability.52 Critical freezing rates may be determined by treating re‐dispersibility as a result of competition between the freezing speed and the particle collision frequency. With increase in drug concentrations, the average inter‐particle distance decreases, thereby increasing the frequency of collisions. This results in an overall increase in the critical freezing rate.52 Thus, understanding the role of freeze‐drying in determining nanocrystal drug stability is critical during translation.
Methods commonly used to confirm the incorporation of stabilizer, polymer coating, or targeting ligands during co‐crystallization include Fourier Transform Infrared spectroscopy, Raman spectroscopy, and Nuclear Magnetic Resonance spectroscopy. The aforementioned techniques are among the most preferred methods for characterizing nanocrystal compositions. In a review by Luykx et al.,53 analytical techniques that may be used to characterize size, shape, charge, and the composition of NP drugs have been described. Some of these methods are deemed as facile and are not as widely used in nanocrystal drug development. These include field flow fractionation for size, desorption electrospray ionization‐mass spectrometry, and ion‐mobility spectrometry‐mass spectrometry for mass and composition, photon correlation spectroscopy for size and distribution, and analytical ultracentrifugation for size, shape, and structural analysis.
Previous studies have probed the effects of NP size, shape, surface morphology, and charge on therapeutic outcome. It has been shown that these parameters influence phagocytosis, immune responses, endothelial targeting, adhesion under flow, transport mechanisms, and intracellular delivery.54, 55, 56, 57, 58, 59, 60, 61, 62, 63 As an example, Mitragotri and co‐workers described the differences in internalization rate and the pathways of NPs differing in shape, size, and aspect ratio in mouse peritoneal macrophages.58 Large particles (>100 nm) are usually internalized via non‐specific pathways such as phagocytosis and macropinocytosis. However, nanocrystal drug surfaces can be modified to minimize non‐specific uptake and facilitate entry via specific pathways such as receptor‐mediated endocytosis. This can be achieved by coating the surface with polymers or surfactants such as PEG, PEG derivatives, polydopamine, and Pluronic F127 or with antibody coatings. Chung et al. showed that coating iron oxide NPs with positively charged multi‐arm PEG derivatives could reduce mass aggregates and used to uniquely label mesenchymal stem cells.64 Jiang et al. showed that a positive charge on iron oxide NPs coated with lipids can deliver nucleic acid payloads to cells.65 Sonvico et al. showed that dextran coatings on maghemite NPs can achieve comparable disaggregation properties to PEG coatings and can be used to conjugate targeting moieties.4 It has also been shown that surface modifications can significantly affect the dissolution kinetics of pure drug NPs.66, 67, 68, 69 Stiffness and surface texture are also known impact the in vivo performance of NPs.62 Eliaz et al. and Lorenzetti et al. showed that nanocrystals developed for bone grafting and other bone related therapeutic applications (i.e., joint reconstruction) are particularly sensitive to stiffness (i.e., rigidity), surface texture (i.e., roughness), and hardness. The properties are influenced by bone‐forming cells and are components crucial to the successful integration or rejection of these materials.70, 71 These findings highlight the importance of translational aspects of physical forces and properties that impact the biological performance and its clinical relevance. Table 2 summarizes the different sizes and geometries which have had success in preclinical research models. The table highlights the diversity of size domains, morphologies, and type of nanocrystal (i.e., organic/inorganic).
Table 2.
Nanocrystal variants with a varied range of properties including composition, size, and geometry
| Type of nanocrystal | Size | Geometry | References |
|---|---|---|---|
| Iron oxide | 11–16 nm | Spheres | 72 |
| Copper nanocrystals | 2–10 nm | Spheres Cylinders Tetrahedra Cubes |
73 |
| Gold nanocrystals | 1–4 nm | Spheres | 74 |
| Cellulose nanocrystals | 141–1,073 nm (length) 12–28 nm (width) 1.12–1.45 nm (pitch) |
Rods Ribbons |
75 |
| Hydroxyapatite | 10–12 nm (width) | Plate‐like crystals | 76 |
| Hydroxyapatite | 200 × 40 nm | Plate‐like crystals | 77 |
| Camptothecin | 200–700 nm | Rods Needles |
78 |
| Lutein nanocrystal | 429–560 nm 1–3 μm |
Spheres in suspension Spherical capsules |
79 |
| Chitosan/LaF3:Eu3+ | 13–18 nm | Spheres | 80 |
| DNA‐Au nanocrystal hybrids | <20 nm | Spheres | 81 |
The influence of surface charge on the in vivo fate of NPs has been extensively researched and reported. For instance, positively charged iron oxide nanocrystals have been found to induce cytotoxic effects in vitro in a charge‐dependent manner. This is believed to result from increased endocytosis due to the strong binding between the positively charged surface on the crystal to the negatively charged glycolipid membrane. The internalized positively charged nanocrystals further interact with the negatively charged organelles and DNA.82 A similar observation was also noted for paclitaxel nanocrystals where a positive charge led to higher cell uptake and cytotoxicity compared to negatively charged particles.66 On the contrary, negatively charged nanocrystals exhibited significant uptake via clathrin‐ and caveolae‐mediated uptake mechanisms.83 Additionally, excessive positive and negative surface charges on NPs have been shown to induce higher rates of opsonization and capture by the immune cells in vivo.83 A vastly diverse array of nanocrystalline material fabricated with various surface charges for drug delivery purposes has been explored and reported in the literature (Table 3). Finally, a majority of nanocrystal drug products approved for use in the clinic and or in clinical trials are delivered via oral or intravenous administration. Figure 3 depicts the challenges faced by these products to overcome the various biological barriers in vivo and properties that influence its biodistribution and site‐specific delivery.
Table 3.
Nanocrystals with varied surface charges
| Type of nanocrystal | Preclinical model (in vitro) | Surface charges | References |
|---|---|---|---|
| Magnetite | HCT116, NIH3T3 |
Reported as neutral | 84 |
| SPIONSa | HUVEC MCF7 |
+9.4 mV, −8.3 mV | 85 |
| Cellulose‐FITC Cellulose‐RBITC |
HEK 293, Sf9 |
+3.9 mV, −46.4 mV, −48.7 mV +9.0 mV, 8.7 mV, 8.6 mV |
86 |
| Cellulose | KU‐7 | 0 mV to −55 mV | 87 |
| Cellulose | MDCK, HeLa, Caco‐2, J774 |
−40 mV to 100 mV | 88 |
| Camptothecin | Eahy926 4T1 |
−4.67 mV, −9.66 mV, −30.4 mV | 89 |
| Paclitaxel | A549 | +19.3 mV, −2.4 mV, −22.7 mV | 90 |
| Paclitaxel | MCF‐7, HaCaT |
−2.73 mV, −15.9 mV, −16.6 mV | 91 |
| Hydroxyapatite | MC3T3‐E1 | −12.5 mV, −23.3 mV | 92 |
| Hydroxyapatite | MC3T3‐E1 | +48.6 mV, −11.4 mV, −28.3 mV | 93 |
| NC‐quantum dots | Vero | +40.52 mV, +36.2 mV, −48.12 mV −55.15 mV, −58.75 mV |
94 |
| Lanthanide Doped |
HT29, OVCAR3, Wistar rats |
Reported as positive | 95 |
| CdSe quantum Dot NC–MUA ligand |
NHBE cells | −21 mV, −53.5 mV, −71.8 mV | 96 |
| CdSe quantum Dot NC–MPA ligand |
NHBE cells | −29.4 mV, −39 mV, −56 mV | 96 |
| CdSe quantum Dot NC–AUT ligand |
NHBE cells | +86.8 mV, 73.7 mV, 60.6 mV | 96 |
| CdSe quantum Dot NC–CYST ligand |
NHBE cells | +57.4 mV, 46.7 mV, 43.4 mV | 96 |
| Cerium oxide (nanoceria) |
H9c2, HEK293, A549, MCF‐7 |
+30 mV, 0 mV, −45 mV | 97 |
SPIONS, superparamagnetic iron oxide nanoparticles. Surface charges listed left to right as positive, neutral, negative; respectively.
Figure 3.
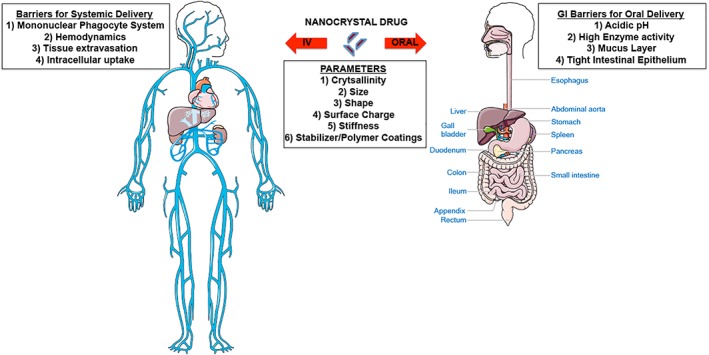
A schematic depicting the in vivo barriers and properties that influence in vivo biodistribution and site‐specific delivery of nanocrystal drug products administered orally or intravenously (IV)
3. NANOCRYSTAL‐DRUG PRODUCTS
Nanocrystals, on account of their high‐drug loading efficiency, steady dissolution rates, enhanced structural stability, and extended circulation times, have been a topic of high research and development activity. Several products are already in the market, and a number of other formulations are undergoing clinical trials.
3.1. Fabrication techniques for nanocrystal‐drug products
Nanocrystals are typically produced using two approaches: (a) top‐down and (b) bottom‐up approaches. Top‐down approaches include methods such as media milling (pearl milling) and high‐pressure homogenization (HPH). Here the large‐sized particles are broken down to a smaller size. In media milling, the milling chamber is filled with milling pearls, the stabilizer, drug, and the dispersion media. The milling pearls are usually made of stainless steel, glass, zirconium oxide, or highly cross‐linked polystyrene resin. The drug‐crystals are nanosized by a combination of collision with the milling pearls; the milling chamber; and high shear forces. During HPH, drug particles—raw or dispersed in aqueous media with surfactant—are transformed into micronized suspensions by a sonicator, homogenizer, mortar, and pestle or jet mill.98, 99, 100, 101, 102 The suspensions are then exposed to a series of collisions, strong cavitation, and high shear forces generated by passing through a narrow gap. This causes it to boil due to change in pressure differences. For a bottom‐up approach, the most common method is precipitation. Here, nanosuspensions are engineered from completely dissolved small molecules in their corresponding antisolvent. It involves two steps—nucleation and crystal growth. Other methods include the use of an acid–base neutralization step. Here nanosuspensions are prepared from a drug dissolved in an acid‐organic solvent mix and then added gradually to a base until the solution is neutralized.103 Nanosuspensions have also been prepared using techniques such as microfluidic nanoprecipitation process, spray drying, electrospraying, and aerosol flow reactor.104, 105, 106, 107, 108 The pros and cons of both top‐down and bottom‐up approaches are summarized in Table 4.
Table 4.
Top‐down versus bottom‐up approaches for nanocrystal‐drug products
| Technique | Merits | Limitations | |
|---|---|---|---|
| Top‐down approaches | Media milling (MM) | 1. Works for drugs that are insoluble in both aqueous and non‐aqueous solvent. 2. no organic solvents are used 3. ease of scale‐up 4. minimal batch to batch variation 5. narrow size distribution of particles 6. high drug loading efficiency |
1. Costly manufacturing process 2. high energy requirements with long durations for milling 3. could destabilize the drugs due to high shear forces and temperature 4. risks for contamination from the dispersion media 5. unwanted drug loss |
| High‐pressure homogenization (HPH) | ‐ Same as MM ‐ | 1. Particles need to be micronized and form suspensions 2. risk of contamination including machine debris 3. high energy requirements |
|
| Bottom‐up approach | Precipitation | 1. Simple and less expensive 2. minimal energy requirements 3. ease of scale‐up 4. possible non‐stop production |
1. Extensive optimization required selecting solvent/antisolvent 2. possible growth of particles with time 3. inadequate purification process or removal of toxic solvents |
3.2. Nanocrystal‐drug products in the market
Since 1995, the FDA has approved ~50 nanodrugs, mostly based on liposomes, polymers, and nanocrystals for various indications.109, 110 Nanocrystallization is an effective way to formulate and develop poorly soluble drugs. The commercial value of this technology is further enhanced by the relatively short span of time to clinical approval. While liposomes took almost 25 years to commercialize, the relative development time for Emend® was just 10 years. Emend's first patent application was filed in 1990 and the product was launched in 2000.29 Thus, compared to other nanosized platforms, a large number of nanocrystal drug products have been developed and launched successfully within a limited span.29
Rapamune®, a poorly soluble immunosuppressant Sirolimus (SRL), was the first marketed nanocrystal drug product, introduced by Wyeth Pharmaceuticals (Madison, NJ) in the year 2000. Rapamune was formulated using the pearl mill technology method, and its oral bioavailability was found to be 21% higher than SRL in its conventional oral solution form. This was followed by the launch of Emend (Aprepitant), in 2003 by Merck (Winehouse Station, NJ). Emend was formulated from Aprepitant—a poorly water soluble anti‐emetic medication, which can only be absorbed in the upper gastrointestinal tract and has a narrow absorption window. Nanoionization of Aprepitant via the pearl mill technology increased its oral bioavailability by making it more soluble in water. Tricor®, launched by Abbott Laboratories in 2003, was formulated from fenofibrate—a lipophilic medication for Hypercholestremia—using the pearl mill technology method. Formulating fenofibrate into nanocrystals increased its adhesiveness to the gut wall and improved its oral bioavailability by 9% independent of fed or fasted state. This made way for a simplified, flexible dosing regimen for patients. Another nanocrystal drug product, derived from fenofibrate is Triglide® which was launched by Skyepharma in 2005. The Triglide nanocrystals were produced using the HPH method and provided benefits similar to Tricor. Triglide achieved an improved bioavailability that was independent of the fed or fasted state with increased adhesiveness to the gut wall. Triglide is currently marketed by Sciele Pharma Inc. (Atlanta, GA). Another nanocrystal product is Megace ES® and was launched by Par Pharmaceutical Companies, Inc. (Spring Valley, NY) in 2005. Megace ES was formulated into nanocrystals from megestrol acetate—an appetite stimulant using the pearl mill method. This improved its dissolution rate and reduced the single dose volume by four times, thereby improving its oral bioavailability and patient compliance when compared to the highly viscous megestrol acetate oral suspension. Other approved nanocrystal drug products are listed in Table 5 and media milling is the most widely accepted method used to produce a majority of the marketed products.
Table 5.
Nanocrystal drug products in the market29
| Trade name | Company | Drug | Indication | Technology | Delivery route | Approval year |
|---|---|---|---|---|---|---|
| Rapamune | Wyeth | Rapamycin/sirolimus | Immunosuppressive | Coprecipitation | Oral | 2000 |
| Emend | Merck | Aprepitant | Anti‐emetic | Media milling | Oral | 2003 |
| Tricor | Abbott | Fenofibrate | Hypercholesterolemia | Media milling | Oral | 2004 |
| Triglide | Skye Pharma | Fenofibrate | Hypercholesterolemia | High pressure homogenization | Oral | 2005 |
| Megace®ES | Par Pharma | Megestrol acetate | Appetite stimulant | Media milling | Oral | 2005 |
| Invega Sustenna® | Johnson & Johnson | Paliperidone palmitate | Antidepressant | High pressure homogenization | Parenteral | 2009 |
| Cesamet® | Lilly | Nabilone | Anti‐emetic | Coprecipitation | Oral | 2009 |
| Avinza® | King Pharma | Morphine sulfate | Anti‐chronic pain | Media milling | Oral | 2002 |
| Naprelan® | Wyeth | Naproxen sodium | Anti‐inflammation | Media milling | Oral | 2006 |
| Ritalin LA® | Novartis | Methylphenidate hydrochloride | Anti‐psychotic | Media milling | Oral | 2002 |
3.3. Nanocrystal drug‐products in clinical trials
As seen in Table 5, a majority of nanocrystal drug products are currently approved for oral ingestion and treating diseases other than cancer. The market for oral administration is enormous and, the path to commercialization is easier compared to injectables. Since the product is primarily composed of the drug and can be incorporated with GRAS approved stabilizers and excipients, the regulatory approval process for nanocrystal drug products is easier. Thus considering the feasibility for rapid development and commercialization, there are several nanocrystal drug products currently in clinical trials, as referred to in Table 6. Semapimod® nanocrystals from Cytokine Pharamsciences (CPSI) is a synthetic guanylhydrazone and was found to act as an immunomodulator, preventing the production of TNF‐α, a proinflammatory cytokine, involved in inflammation‐associated carcinogenesis during a Phase I study in cancer patients.111 CPSI also showed the drug to be effective in treating psoriasis and moderate‐to‐severe Crohn's disease during a preliminary clinical trial. Another nanocrystal drug currently in clinical trials is PAXCEED™ from Angiotech Pharmaceuticals, Inc..112 PAXCEED is formulated from paclitaxel and is a cremophor EL‐free systemic formulation. This could potentially reduce hypersensitivity in patients treated for cancer or chronic inflammation. Theralux™ from Celmed BioSciences Inc., (Saint‐Laurent, QC) is a photodynamic therapy‐based treatment system consisting of thymectacin, which is poorly soluble and has minimal bioavailability.113 It is currently being evaluated in autoimmune diseases, non‐Hodgkin's lymphoma, colon cancer and prevention of graft‐versus‐host disease. Nucryst Pharmaceuticals (Wakefield, MA) has developed a cream formulation based on a proprietary substance NPI 32101, which is primarily composed of silver nanocrystals.114, 115 The drug displayed promising anti‐inflammatory and antimicrobial properties.116 NPI 32101 is currently undergoing Phase II clinical trials for atopic dermatitis.117 Panzem® NCDs were formulated from 2‐Methoxyestradiol (2‐ME2)—a natural metabolite of estradiol (EntreMed, Inc.). 2‐ME2 showed promising antiproliferative and antiangiogenic properties during preclinical trials. Subsequently, Entremed moved ahead with Panzem to test its activity against ovarian cancer, and other solid carcinomas. However, it did not proceed beyond Phase II, and all clinical development of Panzem was suspended.118
Table 6.
Nanocrystal drug products in clinical trials
| Trade name | Company | Drug | Indication | Technology | Delivery route | Clinical status |
|---|---|---|---|---|---|---|
| Semapimod | Cytokine Phamasciences | Guanylhydrazone | TNF‐α Inhibitor |
Self‐developed | Intravenous | II |
| Paxceed® | Angiotech | Paclitaxel | Anti‐inflammatory | Unknown | Intravenous | III |
| Theralux | Celmed | Themectacin | Autoimmune diseases and cancer | Media milling | Intravenous | II |
| Nucryst® | Nucryst Pharmaceuticals | Silver | Atopic dermatitis | Self‐developed | Topical | II |
| PanzemNCD | EntreMed | 2‐methoxy estradiol | Ovarian cancer | Media milling | Oral | II |
3.4. Challenges and criteria in the clinical development of nanocrystal‐drug products
The past two decades witnessed major strides being made in the development of nanocrystal drug technology. A majority of the active pharmaceutical ingredient (API) used for these formulations have low water‐solubility. This, in turn, affects the drugs' systemic bioavailability post‐administration causing inconvenient dosing regimens for patients. Nanoionization of such APIs can result in low particle size, with increase in surface/volume ratio and rate of dissolution. Consequently, this could improve dose proportionality, linear pharmacokinetics, and bioavailability when compared to its original composition. Physicians could then predict the therapeutic response and customize dosing regimens for individual patients. However, issues pertaining to the quality, Chemistry, Manufacturing, and Controls, or bioequivalence (BE) of nanocrystal drug products still exist during scale‐up and development. This could delay the clinical advancement of promising nanocrystal drug products for a variety of indications.
One such issue is determining the most appropriate conditions for testing in vitro dissolution rates of newly formulated products. Nanocrystal drug dissolution usually occurs in two steps—(a) the drug molecules are released from the crystal surface into the surrounding dissolution media to create a saturated layer close to its surface; (b) the released molecules then diffuse through the solvent from a region of high concentration (i.e., the saturated layer) to a region of low concentration. Assessing the dissolution rate of a newly formulated nanocrystal drug product is vital and useful during its development and manufacturing process. It would meet the requisites for worldwide regulatory standards while establishing safety, efficacy, and quality. Depending on the delivery route, dissolution studies must be carried out in vivo or in conditions that simulate in vivo. For instance, if delivered orally, the drugs need to be released from the crystal, absorbed by the gastrointestinal (GI) tract and circulate in the blood to reach its site of action. The study design should take into account the harsh gastric conditions including the acidic pH (1–3), constant churning, the intestinal compartment's pH (5–7), and so forth.
The Noyes–Whitney's equation is often used to describe the drug dissolution rate at a specific time.119
where Dx/dt is dissolution rate, A is surface area of the dissolving particle, D is the diffusion rate constant, δ is thickness of the stagnant layer surrounding the particle, Cs is saturation solubility of the drug, X d is amount of drug dissolved at time t, and V is volume of the dissolution media.
Thus, if the dissolution media increases the drug solubility, it should also increase the crystal's dissolution rate. Thus, it is crucial to use conditions that closely mimic the physiological environment under in vitro conditions. Current dissolution techniques such as the paddle‐method combined with UV spectroscopy or HPLC do not mimic physiological conditions. A high degree of variability therefore exists between in vitro dissolution and in vivo bioavailability data. Use of microfluidic co‐culture devices that take physiological considerations into account could facilitate a smoother translation into the clinic.69, 120, 121 It would be useful to assess the quality of the product and predict its in vivo performance. Consequently, this will reduce the number of BE studies performed in humans during the clinical development, scale‐up, and post‐approval changes.
Other challenges that exist during product development relate to: (a) control of drug substance, (b) control of drug product, (c) manufacturing process, and (d) stability of drug substance/drug product. Depending on the purity, drug substance, is referred to as the API without excipients and achieves the end therapeutic effect. The effect depends on physical attributes such as size and crystallinity. This could affect the manufacturing process and quality of the final product. Since particle size and distribution depend on the dispersion media and stabilizers used during formulation, values are reported in terms of particle size distributions (D values). The D values represent the midpoint values and range during submissions. When suitable, specifications are expressed as intensity‐weighted harmonic mean (Z‐average) and polydispersity index with histograms. Another concern would be identifying the ideal technique to validate particle size. To ensure reliability, at least two analytical methods that support each other should be used to determine particle size and distribution. Dependence on pH may also be taken into account since nanocrystal size and stability are often affected by pH of the dispersion media. In terms of crystallinity, change in structure to amorphous or polymorphic variants could affect its dissolution, stability, and bioavailability. It is therefore important to ensure that the change in crystalline structure is monitored and controlled during manufacture and shelf life of the final product.122, 123, 124, 125, 126, 127, 128, 129
Drug product is referred to as the prototype or the marketed dosage form of the drug substance formulated with excipients. Its control refers to factors that affect the quality and in vivo performance of the final product. This is often affected by changes in viscosity, dissolution rate, specific gravity, content uniformity, and redispersability. The factors are also influenced by the presence of impurities formed during manufacturing. Hence, due consideration must be given for assays that test the purity and continuously monitor degradation products formed during manufacture or shelf life of the final product.
Factors critical during the product's manufacturing process include determining the most appropriate tests or controls to monitor particle size distribution, agglomeration, and presence of contaminants at various steps during the process. It is therefore important to continuously track impurities generated during the process.29, 130 In case of a top‐down approach as in wet milling, impurities depend on the milling media used, the milling material that comes in contact with the drug, the milling mechanism and the number of milling cycles used for the process. Further, other factors such as product and chiller temperature as a function of time, the drive motor speed, shape, aspect ratio, viscosity of product dispersion, and so forth may affect the process and particle size distribution.27, 29, 130 In case of a bottom‐up approach, care should be taken to ensure that (a) a uniform dispersion of the drug is maintained and agglomeration is prevented while adding the drug slowly in to the melt, (b) an optimal viscosity is maintained for the molten material, (c) consistency is sustained during sampling and the solidification/cooling procedure and finally, (d) solvent residues and other impurities in the drug substance/product are tracked and isolated on a constant basis.131 Deviations in any of the above steps could significantly impact product quality, and affect its in vivo performance.
Since crystallinity of the final product could significantly impact its quality and in vivo performance, it is important to ensure that structural stability is retained post‐manufacturing and throughout its shelf life. As stated previously, techniques such as X‐ray powder diffraction, differential scanning calorimetry, or spectroscopic methods, and so forth can be used to study and compare the structure of the initial molecule with the final product and at the end of its shelf life. Additional studies may be designed concerning short or long‐term stability, or its dosage form since particles tend to aggregate due to sedimentation, redispersion, or caking, and so forth.
4. NANOCRYSTAL DRUGS: A CARRIER‐FREE THERAPEUTIC PLATFORM FOR CANCER AND OTHER DISEASES?
By the year 2021, nanocrystal drug products are estimated to account for 60% of the total NP‐based drug delivery market.132 This is valued to be at ~$82 billion. While nanocrystal technology is attractive due to its ease of formulation, uniform composition, and attractive pharmacoeconomic values, it also has the potential to overcome some of the biggest challenges for drug development. Poor solubility could result in abysmal bioavailability, thereby affecting optimal delivery of the drug. By formulating poorly soluble drugs into nanocrystals, the resulting increase in surface/volume ratio, saturation solubility, and the rate of dissolution can ensure an enhanced bioavailability of most insoluble drugs irrespective of its route of administration. Clinical efficacy of nanocrystal drugs depend on several factors including the size, morphology, surface charge, amount of drug loaded, the type of excipient used, the degree of redispersability, and site‐specific targeting. Also, multimodal theranostic nanocrystal drug products would be vital to assess and monitor in vivo bioavailability. A majority of nanocrystal drug products are currently approved for oral ingestion and for treating diseases other than cancer. The manufacturing process for such oral products is consistent and form crystals at sizes well above the 100–200 nm range. Since nanocrystals are not expected to undergo rapid dissolution in the blood due to a minimum volume of distribution; they can be injected intravenously. However, crystals with dimensions >100–200 nm could promote macrophage‐mediated phagocytosis, rapid blood clearance, and minimal efficacy compared to the drugs' conventional form. Efforts must, therefore, be directed to develop crystal products at sizes well below the 100 nm range with surface modifications to avoid renal clearance and sequestration by the mononuclear phagocytic system. Besides, it is vital to understand their intracellular and intratumoral fate. Overall, nanocrystals have the potential to open up new frontiers in the field of therapeutics. The ability to reformulate off‐patent drugs into novel nanocrystal drug products for clinical use offers a clear competitive edge for companies in the market.
ACKNOWLEDGMENT
Image templates made freely available by Servier Medical Art (http://smart.servier.com) were used for the preparation of Figure 3.
Jarvis M, Krishnan V, Mitragotri S. Nanocrystals: A perspective on translational research and clinical studies. Bioengineering & Translational Medicine. 2019;4:5–16. 10.1002/btm2.10122
REFERENCES
- 1. De Jong WH, Borm PJA. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3:133‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs – a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113:151‐170. [DOI] [PubMed] [Google Scholar]
- 4. Sonvico F, Mornet S, Vasseur S, et al. Folate‐conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments. Bioconj Chem. 2005;16:1181‐1188. 10.1021/BC050050Z. [DOI] [PubMed] [Google Scholar]
- 5. Savjani KT, Gajjar AK, Savjani JK. Drug dsolubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Junghanns J, Müller R. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuomela A, Saarinen J, Strachan CJ, Hirvonen J. Production, applications and in vivo fate of drug nanocrystals. J Drug Deliv Sci Technol. 2016;34:21‐31. [Google Scholar]
- 9. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research . Waiver of in vivo bioavailability and bioequivalence studies for immediate‐release solid Oral dosage forms based on a biopharmaceutics classification system guidance for industry. Biopharmaceutics. 2017;1‐19. [Google Scholar]
- 10. Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in‐vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51‐66. [DOI] [PubMed] [Google Scholar]
- 11. George J, Sabapathi SN. Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl. 2015;8:45‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Zhuang J, Peng Q, Li Y. A general strategy for nanocrystal synthesis. Nature. 2005;437:121‐124. [DOI] [PubMed] [Google Scholar]
- 13. Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23:534‐547. 10.1016/J.DRUDIS.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 14. Lu Y, Chen Y, Gemeinhart RA, Wu W, Li T. Developing nanocrystals for cancer treatment. Nanomedicine. 2015;10:2537‐2552. [DOI] [PubMed] [Google Scholar]
- 15. Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top‐down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64‐75. [DOI] [PubMed] [Google Scholar]
- 16. Boleininger J, Kurz A, Reuss V, Sönnichsen C. Microfluidic continuous flow synthesis of rod‐shaped gold and silver nanocrystals. Phys Chem Chem Phys. 2006;8:3824‐3827. [DOI] [PubMed] [Google Scholar]
- 17. Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62:1569‐1579. [DOI] [PubMed] [Google Scholar]
- 18. Gao L, Liu G, Ma J, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30:307‐324. [DOI] [PubMed] [Google Scholar]
- 19. Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals: in vivo performances. J Control Release. 2012;160:418‐430. [DOI] [PubMed] [Google Scholar]
- 20. Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water‐soluble drugs. Drug Discov Today. 2011;16:354‐360. [DOI] [PubMed] [Google Scholar]
- 21. Brough C, Williams RO. Amorphous solid dispersions and nano‐crystal technologies for poorly water‐soluble drug delivery. Int J Pharm. 2013;453:157‐166. [DOI] [PubMed] [Google Scholar]
- 22. Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: boundaries and opportunities. Chem Eng Sci. 2015;125:158‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6:106‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merisko‐Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly‐water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63:427‐440. [DOI] [PubMed] [Google Scholar]
- 26. Miao X, Yang W, Feng T, Lin J, Huang P. Drug nanocrystals for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;10:e1499 10.1002/wnan.1499. [DOI] [PubMed] [Google Scholar]
- 27. Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453:142‐156. [DOI] [PubMed] [Google Scholar]
- 28. Peltonen L, Hirvonen J. Drug nanocrystals – versatile option for formulation of poorly soluble materials. Int J Pharm. 2018;537:73‐83. [DOI] [PubMed] [Google Scholar]
- 29. Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399:129‐139. [DOI] [PubMed] [Google Scholar]
- 30. Su H, Wang Y, Gu Y, Bowman L, Zhao J, Ding M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J Appl Toxicol. 2018;38:3‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu PS, Inbaraj BS, Chen BH. Determination of oral bioavailability of curcuminoid dispersions and nanoemulsions prepared from Curcuma longa Linnaeus. J Sci Food Agric. 2018;98:51‐63. [DOI] [PubMed] [Google Scholar]
- 32. Maudens P, Seemayer CA, Thauvin C, Gabay C, Jordan O, Allémann E. Nanocrystal‐polymer particles: extended delivery carriers for osteoarthritis treatment. Small. 2018;14:1703108 10.1002/smll.201703108. [DOI] [PubMed] [Google Scholar]
- 33. He Y, Xia DN, Li QX, Tao JS, Gan Y, Wang C. Enhancement of cellular uptake, transport and oral absorption of protease inhibitor saquinavir by nanocrystal formulation. Acta Pharmacol Sin. 2015;36:1151‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koshani R, Madadlou A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci Technol. 2018;71:268‐273. [Google Scholar]
- 35. Lu Y, Qi J, Dong X, Zhao W, Wu W. The in vivo fate of nanocrystals. Drug Discov Today. 2017;22:744‐750. [DOI] [PubMed] [Google Scholar]
- 36. Möschwitzer JP, Müller RH. Factors influencing the release kinetics of drug nanocrystal‐loaded pellet formulations. Drug Dev Ind Pharm. 2013;39:762‐769. [DOI] [PubMed] [Google Scholar]
- 37. Mura S, Nicolas J, Couvreur P. Stimuli‐responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991‐1003. [DOI] [PubMed] [Google Scholar]
- 38. Bates S, Zografi G, Engers D, Morris K, Crowley K, Newman A. Analysis of amorphous and nanocrystalline solids from their x‐ray diffraction patterns. Pharm Res. 2006;23:2333‐2349. [DOI] [PubMed] [Google Scholar]
- 39. Babu NJ, Nangia A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des. 2011;11:2662‐2679. [Google Scholar]
- 40. Markiewicz KH, Seiler L, Misztalewska I, et al. Advantages of poly(vinyl phosphonic acid)‐based double hydrophilic block copolymers for the stabilization of iron oxide nanoparticles. Polym Chem. 2016;7:6391‐6399. [Google Scholar]
- 41. Puntes V, Krishnan K, Alivisatos AP. Colloidal nanocrystal shape and size control: the case of cobalt. Science. 2001;5511:2115‐2117. [DOI] [PubMed] [Google Scholar]
- 42. Xiao J, Qi L. Surfactant‐assisted, shape‐controlled synthesis of gold nanocrystals. Nanoscale. 2011;3:1383‐1396. [DOI] [PubMed] [Google Scholar]
- 43. Tuomela A, Hirvonen J, Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y, Liu X, Zhang Z, et al. Adaptive structured pickering emulsions and porous materials based on cellulose nanocrystal surfactants. Angew Chem. 2018;130:13748‐13752. [DOI] [PubMed] [Google Scholar]
- 45. Kaboorani A, Riedl B. Surface modification of cellulose nanocrystals (CNC) by a cationic surfactant. Ind Crops Prod. 2015;65:45‐55. [Google Scholar]
- 46. Hu P, Zhuang J, Chou LY, et al. Surfactant‐directed atomic to mesoscale alignment: metal nanocrystals encased individually in single‐crystalline porous nanostructures. J Am Chem Soc. 2014;136:10561‐10564. [DOI] [PubMed] [Google Scholar]
- 47. Maudens P, Seemayer CA, Pfefferlé F, Jordan O, Allémann E. Nanocrystals of a potent p38 MAPK inhibitor embedded in microparticles: therapeutic effects in inflammatory and mechanistic murine models of osteoarthritis. J Control Release. 2018;276:102‐112. [DOI] [PubMed] [Google Scholar]
- 48. Kedzior SA, Marway HS, Cranston ED. Tailoring cellulose nanocrystal and surfactant behavior in Miniemulsion polymerization. Macromolecules. 2017;50(58):2645‐2655. [Google Scholar]
- 49. Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN®) versus drug nanocrystals. Int J Pharm. 2006;317:82‐89. [DOI] [PubMed] [Google Scholar]
- 50. Colombo M, Staufenbiel S, Rühl E, Bodmeier R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int J Pharm. 2017;521:156‐166. [DOI] [PubMed] [Google Scholar]
- 51. Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze‐drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688‐1713. [DOI] [PubMed] [Google Scholar]
- 52. Lee J, Cheng Y. Critical freezing rate in freeze drying nanocrystal dispersions. J Control Release. 2006;111:185‐192. [DOI] [PubMed] [Google Scholar]
- 53. Luykx DMAM, Peters RJB, van Ruth SM, Bouwmeester H. A review of analytical methods for the identification and characterization of Nano delivery systems in food. J Agric Food Chem. 2008;56:8231‐8247. [DOI] [PubMed] [Google Scholar]
- 54. Champion J, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PLoS One. 2010;5:e10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM‐1‐targeted carriers. Mol Ther. 2008;16(8):1450‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Doshi N, Prabhakarpandian B, Rea‐Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Contol Release. 2010;146(2):196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Champion J, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930‐4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Champion J, Katare Y, Mitragotri S. Particle shape: a new design parameter for micro‐and nanoscale drug delivery carriers. J Control Release. 2007;121:3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma G, Valenta DT, Altman Y, et al. Polymer particle shape independently influences binding and internalization by macrophages. J Control Release. 2010;147(3):408‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size‐dependent immune response to antigen‐carrying nanoparticles. J Control Release. 2015;220:141‐148. [DOI] [PubMed] [Google Scholar]
- 62. Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jarvis M, Arnold M, Ott J, et al. Detachment of ligands from nanoparticle surface under flow and endothelial cell contact: assessment using microfluidic devices. Bioeng Transl Med. 2018;3:148‐155. 10.1002/btm2.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chung HJ, Lee H, Bae KH, et al. Facile synthetic route for surface‐functionalized magnetic nanoparticles: cell labeling and magnetic resonance imaging studies. ACS Nano. 2011;5:4329‐4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG. Lipidoid‐coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013;13:1059‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krishnan V, Sarode A, Bhatt R, et al. Surface‐functionalized carrier‐free drug nanorods for leukemia. Adv. Ther. 2018;1:1800010 10.1002/adtp.201800010. [DOI] [Google Scholar]
- 67. Chen Y, Li T. Cellular uptake mechanism of paclitaxel nanocrystals determined by confocal imaging and kinetic measurement. AAPS J. 2015;17:1126‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Krishnan V, Xu X, Kelly D, et al. CD19‐targeted Nanodelivery of doxorubicin enhances therapeutic efficacy in B‐cell acute lymphoblastic leukemia. Mol Pharm. 2015;12:2101‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jarvis M, Arnold M, Ott J, Pant K, Prabhakarpandian B, Mitragotri S. Microfluidic co‐culture devices to assess penetration of nanoparticles into cancer cell mass. Bioeng Transl Med. 2017;2:268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lorenzetti M, Biglino D, Novak S, Kobe S. Photoinduced properties of nanocrystalline TiO2‐anatase coating on Ti‐based bone implants. Mater Sci Eng C. 2014;37:390‐398. [DOI] [PubMed] [Google Scholar]
- 71. Eliaz N, Shmueli S, Shur I, Benayahu D, Aronov D, Rosenman G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone‐forming cells. Acta Biomater. 2009;5:3178‐3191. [DOI] [PubMed] [Google Scholar]
- 72. Hyeon T, Lee SS, Park J, Chung Y, Na HB. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size‐selection process. 2001;123(51):12798‐12801. [DOI] [PubMed] [Google Scholar]
- 73. Pileni M‐P, Pileni M‐P. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat Mater. 2003;2:145‐150. [DOI] [PubMed] [Google Scholar]
- 74. Jana NR, Peng X. Single‐phase and gram‐scale routes toward nearly monodisperse Au and other noble metal nanocrystals. J Am Chem Soc. 2003;125:14280‐14281. [DOI] [PubMed] [Google Scholar]
- 75. Elazzouzi‐Hafraoui S, Nishiyama Y, Putaux JL, Heux L, Dubreuil F, Rochas C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules. 2008;9:57‐65. [DOI] [PubMed] [Google Scholar]
- 76. Roveri N, Falini G, Sidoti MC, et al. Biologically inspired growth of hydroxyapatite nanocrystals inside self‐assembled collagen fibers. Mater Sci Eng C. 2003;23:441‐446. [Google Scholar]
- 77. Bigi A, Boanini E, Capuccini C, Gazzano M. Strontium‐substituted hydroxyapatite nanocrystals. Inorg Chim Acta. 2006;360:1009‐1016. 10.1016/j.ica.2006.07.074. [DOI] [Google Scholar]
- 78. Zhang H, Hollis CP, Zhang Q, Li T. Preparation and antitumor study of camptothecin nanocrystals. Int J Pharm. 2011;415:293‐300. [DOI] [PubMed] [Google Scholar]
- 79. Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420:141‐146. [DOI] [PubMed] [Google Scholar]
- 80. Wang F, Zhang Y, Fan X, Wang M. One‐pot synthesis of chitosan/LaF3:Eu 3+ nanocrystals for bio‐applications. Nanotechnology. 2006;17:1527‐1532. [Google Scholar]
- 81. Alivisatos AP, Johnsson KP, Peng X, et al. Organization of ‘nanocrystal molecules’ using DNA. Nature. 1996;382:609‐611. [DOI] [PubMed] [Google Scholar]
- 82. Huang Y‐W, Cambre M, Lee H‐J. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int J Mol Sci. 2017;18:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657‐3666. [DOI] [PubMed] [Google Scholar]
- 84. Lee Y, Lee H, Kim YB, et al. Bioinspired surface immobilization of hyaluronic acid on monodisperse magnetite nanocrystals for targeted cancer imaging. Adv Mater. 2008;20:4154‐4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Osaka T, Nakanishi T, Shanmugam S, Takahama S, Zhang H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf B. 2009;71:325‐330. [DOI] [PubMed] [Google Scholar]
- 86. Mahmoud KA, Mena JA, Male KB, Hrapovic S, Kamen A, Luong JHT. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl Mater Interfaces. 2010;2:2924‐2932. [DOI] [PubMed] [Google Scholar]
- 87. Jackson JK, Letchford K, Wasserman BZ, Ye L, Hamad WY, Burt HM. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int J Nanomed. 2011;6:321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hosseinidoust Z, Alam MN, Sim G, Tufenkji N, van de Ven TGM. Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale. 2015;7:16647‐16657. [DOI] [PubMed] [Google Scholar]
- 89. Jarvis M, Arnold M, Ott J, Pant K, Prabhakarpandian B, Mitragotri S. Microfluidic co‐culture devices comprising breast cancer and endothelial cells to assess nanoparticle interactions and cancerous cell mass penetration. Bioeng. Transl. Med. 2017;2:268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Choi J‐S, Park J‐S. Effects of paclitaxel nanocrystals surface charge on cell internalization. Eur J Pharm Sci. 2016;93:90‐96. [DOI] [PubMed] [Google Scholar]
- 91. Sohn JS, Yoon D‐S, Sohn JY, Park J‐S, Choi J‐S. Development and evaluation of targeting ligands surface modified paclitaxel nanocrystals. Mater Sci Eng C. 2017;72:228‐237. [DOI] [PubMed] [Google Scholar]
- 92. Ribeiro N, Sousa SR, Monteiro FJ. Influence of crystallite size of nanophased hydroxyapatite on fibronectin and osteonectin adsorption and on MC3T3‐E1 osteoblast adhesion and morphology. J Colloid Interface Sci. 2010;351:398‐406. [DOI] [PubMed] [Google Scholar]
- 93. Chen L, Mccrate JM, Lee JC‐M, Li H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology. 2011;22:105708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hoshino A, Fujioka K, Oku T, et al. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163‐2169. [Google Scholar]
- 95. Chatterjee DK, Rufaihah AJ, Zhang Y. Upconversion fluorescence imaging of cells and small animals using lanthanide doped nanocrystals. Biomaterials. 2008;29:937‐943. [DOI] [PubMed] [Google Scholar]
- 96. Nagy A, Steinbrück A, Gao J, Doggett N, Hollingsworth JA, Iyer R. Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano. 2012;6:4748‐4762. [DOI] [PubMed] [Google Scholar]
- 97. Asati A, Santra S, Kaittanis C, Perez JM. Surface‐charge‐dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano. 2010;4:5321‐5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47:3‐19. [DOI] [PubMed] [Google Scholar]
- 99. Pardeike J, Strohmeier DM, Schrödl N, et al. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int J Pharm. 2011, 2011;420:93‐100. [DOI] [PubMed] [Google Scholar]
- 100. Lai F, Pini E, Angioni G, et al. Nanocrystals as tool to improve piroxicam dissolution rate in novel orally disintegrating tablets. J Pharm Biopharm. 2011;79:552‐558. [DOI] [PubMed] [Google Scholar]
- 101. Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005, 2005;299:167‐177. [DOI] [PubMed] [Google Scholar]
- 102. Sharma P, Denny W, Garg S. Effect of wet milling process on the solid state of indomethacin and simvastatin. Int J Pharm. 2009;380:40‐48. [DOI] [PubMed] [Google Scholar]
- 103. Mou D, Chen H, Wan J, Xu H, Yang X. Potent dried drug nanosuspensions for oral bioavailability enhancement of poorly soluble drugs with pH‐dependent solubility. Int J Pharm. 2011;413:237‐244. [DOI] [PubMed] [Google Scholar]
- 104. Eerikäinen H, Watanabe W, Kauppinen EI, Ahonen PP. Aerosol flow reactor method for synthesis of drug nanoparticles. Eur J Pharm Biopharm. 2003;55:357‐360. [DOI] [PubMed] [Google Scholar]
- 105. Wang M, Rutledge GC, Myerson AS, Trout BL. Production and characterization of carbamazepine nanocrystals by electrospraying for continuous pharmaceutical manufacturing. J Pharm Sci. 2012;101:1178‐1188. [DOI] [PubMed] [Google Scholar]
- 106. Lee S, Heng D, Ng WK, Chan HK, Tan RB. Nano spray drying: a novel method for preparing protein nanoparticles for protein therapy. Int J Pharm. 2011;403:192‐200. [DOI] [PubMed] [Google Scholar]
- 107. Ali H, York P, Ali A, Blagden N. Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release. 2011;149:175‐181. [DOI] [PubMed] [Google Scholar]
- 108. Ali H, York P, Blagden N. Preparation of hydrocortisone nanosuspension through a bottom‐up nanoprecipitation technique using microfluidic reactors. Int J Pharm. 2009;375:107‐113. [DOI] [PubMed] [Google Scholar]
- 109. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle‐based medicines: a review of FDA‐approved materials and clinical trials to date. Pharm Res. 2016;33:2373‐2387. [DOI] [PubMed] [Google Scholar]
- 110. Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1416. [DOI] [PubMed] [Google Scholar]
- 111.Home ‐ Ferring Corporate. https://www.ferring.com/. Accessed December 19, 2018.
- 112. Raghava Srivalli KM, Mishra B. Drug nanocrystals: A way toward scale‐up. Saudi Pharm J. 2016;24(4):386‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.New Biotic. https://www.newbiotic.com/. Accessed December 19, 2018.
- 114. Bhol KC, Alroy J, Schechter PJ. Anti‐inflammatory effect of topical nanocrystalline silver cream on allergic contact dermatitis in a Guinea pig model. Clin Exp Dermatol. 2004;29:282‐287. [DOI] [PubMed] [Google Scholar]
- 115. Bhol KC, Schechter PJ. Topical nanocrystalline silver cream inhibits expression of matrix metalloproteinase‐9 in animal models of allergic contact dermatitis. J Invest Dermatol. 2005;1244:117. [Google Scholar]
- 116. Lyczak J, Schechter P. Nanocrystalline silver inhibits antibiotic‐, antiseptic‐resistant bacteria. Clin Pharmacol Ther. 2005;77:P60‐P60. [Google Scholar]
- 117. Bhol KC, Schechter PJ. Topical nanocrystalline silver cream suppresses inflammatory cytokines and induces apoptosis of inflammatory cells in a murine model of allergic contact dermatitis. Br J Dermatol. 2005;152:1235‐1242. [DOI] [PubMed] [Google Scholar]
- 118. Harrison MR, Hahn NM, Pili R, et al. A phase II study of 2‐methoxyestradiol (2ME2) NanoCrystal® dispersion (NCD) in patients with taxane‐refractory, metastatic castrate‐resistant prostate cancer (CRPC). Invest New Drugs. 2011;29:1465‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Walther N. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem. 1904;47:52‐55. [Google Scholar]
- 120. Deschamps B, Musaji N, Gillespie JA. Food effect on the bioavailability of two distinct formulations of megestrol acetate oral suspension. Int J Nanomed. 2009;4:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schmidt LE, Dalhoff K. Food‐drug interactions. Drugs. 2002;62:1481‐1502. [DOI] [PubMed] [Google Scholar]
- 122.Guidances (Drugs).
- 123.ISO/TC 24/SC 4 ‐ Particle characterization
- 124.Committee E56 on Nanotechnology. https://www.astm.org/COMMITTEE/E56.htm. Accessed December 18, 2018.
- 125. Assay Cascade Protocols | Nanotechnology Characterization Lab (NCL) . https://nanolab.cancer.gov/resources/assay-cascade-protocols. Accessed December 18, 2018.
- 126. Taurozzi JS, Hackley VA, Wiesner MR. Reporting Guidelines for the Preparation of Aqueous Nanoparticle Dispersions from Dry Materials ‐ Version 2.1; 2012. doi: 10.6028/NIST.SP.1200-1. [DOI]
- 127. ISO/TS 16195 :2013. Nanotechnologies ‐‐ Guidance for developing representative test materials consisting of nano‐objects in dry powder form.
- 128. Roebben G, Ramirez‐Garcia S, Hackley VA, et al. Interlaboratory comparison of size and surface charge measurements on nanoparticles prior to biological impact assessment. J Nanopart Res. 2011;13:2675‐2687. [Google Scholar]
- 129. Patel VR, Agrawal YK. Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hu J, Johnston KP, Williams RO. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm. 2004;30:233‐245. [DOI] [PubMed] [Google Scholar]
- 131. de Waard H, Frijlink HW, Hinrichs WLJ. Bottom‐up preparation techniques for nanocrystals of lipophilic drugs. Pharm Res. 2011;28:1220‐1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nanotechnology for drug delivery: global market for nanocrystals. Available at: https://www.researchandmarkets.com/research/ths3db/nanotechnology_for. Accessed June 26, 2018.


