Abstract
Alzheimer's disease (AD) is an age-related neurodegenerative disorder characterized by cognitive deficits and neuronal loss. Deposition of beta-amyloid peptide (Aβ) causes neurotoxicity through the formation of plaques in brains of Alzheimer's disease. Numerous studies have indicated that the neuropeptides including ghrelin, neurotensin, pituitary adenylate cyclase-activating polypeptide (PACAP), neuropeptide Y, substance P and orexin are closely related to the pathophysiology of Alzheimer's disease. The levels of neuropeptides and their receptors change in Alzheimer's disease. These neuropeptides exert neuroprotective roles mainly through preventing Aβ accumulation, increasing neuronal glucose transport, increasing the production of neurotrophins, inhibiting endoplasmic reticulum stress and autophagy, modulating potassium channel activity and hippocampal long-term potentiation. Therefore, the neuropeptides may function as potential drug targets in the prevention and cure of Alzheimer's disease.
Keywords: neuropeptide, Alzheimer's disease, ghrelin, neurotensin, pituitary adenylate cyclase-activating polypeptide, neuropeptide Y, substance P, orexin
Introduction
Alzheimer's disease (AD) is an age-related neurodegenerative disorder which is clinically characterized by cognitive deficits, memory impairment, disorientation, and behavioral issues (Burns and Iliffe, 2009). Commonly the Alzheimer's disease begins in people over the age of 65 years. The neuropathological features in Alzheimer's disease are amyloid plaques, neurofibrillary tangles, and neuronal loss (Tiraboschi et al., 2004). Beta-amyloid peptide (Aβ) oligomers have been reported to be the primary pathogenic forms of Aβ, which change the structure of synapses and eventually disrupt neuronal communication (Lacor et al., 2007). However, the pathogenesis remains unknown.
Neuropeptides are molecules which function as endogenous active substances within central nervous system and peripheral nervous system. Neuropeptides play important roles in a wide range of brain functions, including food intake, metabolism, reproduction, social behaviors, reward, learning and memory, sleep and wakefulness. Recent studies revealed that many neuropeptides including ghrelin, neurotensin, pituitary adenylate cyclase-activating polypeptide (PACAP), neuropeptide Y, substance P, and orexin may be associated with the pathophysiology and potential therapy of Alzheimer's disease. In this article, we review the recent advances about the involvement of neuropeptides in Alzheimer's disease.
Ghrelin and Alzheimer's Disease
Ghrelin, a 28-amino acid brain-gut peptide, activates the growth hormone secretagogue receptors which are expressed widely in the brain (Guan et al., 1997). Recent studies revealed that the receptor expression is developmental, with a stronger staining in the early stages and a weaker expression in the later stages of development (Lattuada et al., 2013). Ghrelin exerts critical roles in the regulation of energy homeostasis, neuroendocrine and neurodegenerative processes, especially in higher brain functions, such as learning and memory consolidation (Spitznagel et al., 2010; Rak-Mardyla, 2013; Murray et al., 2014; Panagopoulos and Ralevski, 2014; Jiao et al., 2017). Ghrelin is also involved in mitochondrial respiration and neuroprotection, which can be developed as biomarkers or drug targets for prevention and treatment of neurological disorders, including Parkinson's disease, stroke, epilepsy and Alzheimer's disease (dos Santos et al., 2013a; Shi et al., 2014, 2017; Stoyanova, 2014).
Ghrelin has been demonstrated to have a close relationship with Alzheimer's disease. One of the single nucleotide polymorphisms of the ghrelin gene, rs4684677 (Leu90Gln), has been proved to be associated with the onset age of Alzheimer's disease (Shibata et al., 2011). Early study revealed that the level of serum ghrelin is negatively related to several cognitive domains in older adults (Spitznagel et al., 2010). The level of the functional form of ghrelin, acylated ghrelin, is associated with Alzheimer's disease risk factors and mild cognitive impairment (Gahete et al., 2010; Cao et al., 2018). However, a significant reduction of ghrelin mRNA was observed in the temporal gyrus of Alzheimer's disease patients, suggesting the possible involvement of ghrelin in the cognitive deficit of Alzheimer's disease (Gahete et al., 2010).
Administration of ghrelin or ghrelin agonist decreases the level of Aβ and attenuates Alzheimer's disease-related cognitive impairment (Gahete et al., 2010, 2011; Dhurandhar et al., 2013; Kunath et al., 2015). Recent in vitro study demonstrated that pre-administration of ghrelin or ghrelin analog protects SH-SY5Y cellular models of Alzheimer's disease against methylglyoxal-induced toxicity and apoptosis, suggesting the potential treatment for neurodegenerative disorders (Cecarini et al., 2016; Popelová et al., 2018). Ghrelin exerts neuroprotective effects through different mechanisms. Central application of acylated ghrelin prevents Aβ-induced impairments of memory and energy and glucose metabolisms, probably through increase of AMPK and GSK phosphorylation and decrease of tau phosphorylation (Kang et al., 2015). Further study revealed that acylated ghrelin also blunts Aβ-induced depression of long-term potentiation (LTP) in hippocampus and therefore prevents impairments of recognition and spatial orientation (Santos et al., 2017). Ghrelin ameliorates neurogenesis impairment in hippocampus and improves memory deficits by prevention of synaptic degeneration including cholinergic fiber loss (Moon et al., 2011, 2014). Recent studies revealed that ghrelin exerts protective effects against Aβ-induced toxicity via preventing superoxide production, calcium elevation and mitochondrial membrane depolarization (Martins et al., 2013; Gomes et al., 2014). In addition, ghrelin restores the proteasome functionality in Alzheimer's disease and thus contributes to the elimination of toxic aggregates (Cecarini et al., 2016). It is known that brain insulin resistance is closely related to the cognitive impairment and neurodegeneration, particularly within Alzheimer's disease. Recently, it is demonstrated that ghrelin increases neuronal glucose uptake and improves tau hyperphosphrylation by activating Akt and GSK-3 beta phosphorylation in cultured hippocampal neurons (Chen et al., 2010).
In summary, the levels of ghrelin change in Alzheimer's disease. The functional form of ghrelin, acylated ghrelin, increases in mild cognitive impairment. A single nucleotide polymorphism of ghrelin gene is associated with the onset age of Alzheimer's disease. Ghrelin protects against Aβ-induced neurotoxicity through multiple pathways. Ghrelin prevents calcium elevation, superoxide production and mitochondrial membrane depolarization. Moreover, ghrelin increases neuronal glucose uptake and improves Aβ-induced deterioration of memory through activating AMPK and GSK phosphorylation and decreasing tau phosphorylation. Furthermore, ghrelin prevents cholinergic synaptic degeneration. Therefore, ghrelin is considered as a potential drug in the treatment of Alzheimer's disease (Figure 1).
Figure 1.
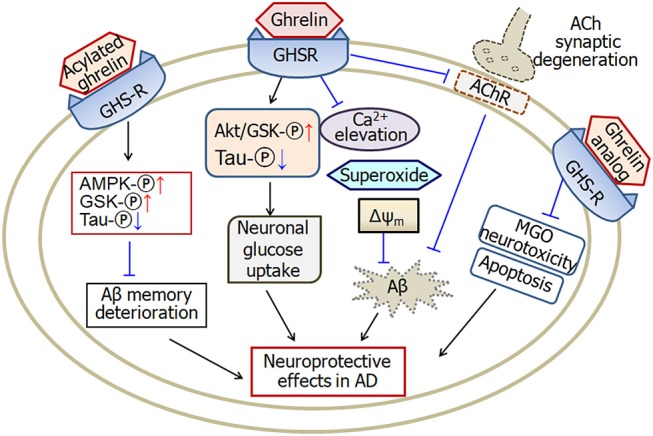
A schematic diagram describing the possible pathways of ghrelin-induced neuroprotective effects in Alzheimer's disease. Ghrelin protects against Aβ-induced neurotoxicity through prevention of calcium elevation, superoxide production and mitochondrial membrane depolarization. Ghrelin also increases neuronal glucose uptake by activating Akt/GSK phosphorylation and improving tau hyperphosphrylation. Moreover, ghrelin prevents cholinergic synaptic degeneration and therefore protects against Aβ-induced memory deficits. Acylated ghrelin improves Aβ-induced deterioration of memory through increase of AMPK and GSK phosphorylation and decrease of tau phosphorylation. Analog of ghrelin protects against MGO-induced neurotoxicity and apoptosis in cellular models of Alzheimer's disease. GHSR, growth hormone secretagogue receptors, also known as ghrelin receptors; Δψm, mitochondrial membrane potential; MGO, methylglyoxal; ACh, acetylcholine; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; GSK, glycogen synthase kinase; AChR, cholinergic receptors; -℗, phosphrylation. The internal and external circles represent the inner and outer leaflets of the cellular membrane. The dotted line in ACh synapse represents the degenerated synapse.
Neurotensin and Alzheimer's Disease
Neurotensin is a tridecapeptide which binds with two neurotensin receptors, type-1 and type-2, in the brain. Neurotensin plays multiple effects in central nervous system and is involved in the pathophysiology of several central nervous system disorders, including schizophrenia (Garver et al., 1991; Kinkead and Nemeroff, 2004), Parkinson's disease (Bissette et al., 1985; Fernandez et al., 1994), as well as Alzheimer's disease (Constantinidis et al., 1983; Struble et al., 1987; Gahete et al., 2010; Xiao et al., 2014). The levels of neurotensin and neurotensin receptors change in several brain regions of Alzheimer's disease patients. Neurotensin influences the formation of senile plaques and therefore is associated with the pathogenesis of Alzheimer's disease through different pathways (Figure 2).
Figure 2.
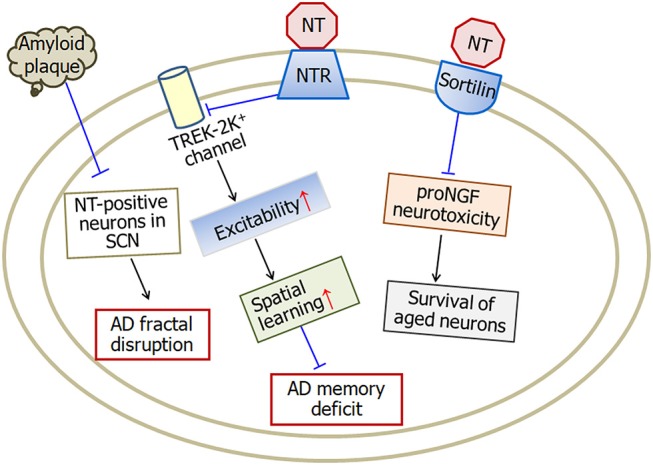
A model illustrating the neuroprotective effects of neurotensin in Alzheimer's disease. Neurotensin increases the excitability of neurons by inhibiting TREK-2K+ channel, which therefore improves memory status in Alzheimer's disease mice. Being a sortilin ligand, neurotensin rescues the survival of aged neurons through blocking sortilin-induced proNGF neurotoxicity. In addition, amyloid plaque density in the occipital cortex is negatively associated with neurotensin-positive neurons in the suprachiasmatic nucleus suggesting the involvement of neurotensin in fractal activity disruption in Alzheimer's disease. NT, neurotensin; NTR, neurotensin receptors; SCN, suprachiasmatic nucleus. The internal and external circles represent the inner and outer leaflets of the cellular membrane.
Previous studies revealed a decreased level of neurotensin in the septum (Ferrier et al., 1983), suprachiasmatic nucleus (Hu et al., 2013) and amygdala (Benzing et al., 1990), and a low level of neurotensin receptors in the entorhinal area (Jansen et al., 1990), dentate gyrus (Rowe et al., 2006) and temporal gyrus (Gahete et al., 2010) of Alzheimer's disease. In Alzheimer's disease patients, the amyloid plaque density in the occipital cortex is negatively associated with the neurotensin-positive neurons in the suprachiasmatic nucleus, i.e., the more plaques, the fewer neurotensin-positive neurons, which suggests the involvement of neurotensin in fractal activity disruption in Alzheimer's disease (Hu et al., 2013). The levels of amygdala neurotensin in males are significantly higher than that in females (Biggins et al., 1983), which suggests that the intrinsic function of the amygdala may be associated with hormonal regulation of other sex-dependent mechanism. For example, Skup et al. (2011) reported that the males and the females show different patterns in amygdala volume decline over time. Previous studies revealed that men exhibit greater volumes and neuronal densities in amygdala (Giedd et al., 1996; Goldstein et al., 2001; Witte et al., 2010). Furthermore, the density of neurotensin and acetylcholine containing fibers is reduced dramatically in the regions of amygdala with greatest senile plaques (Benzing et al., 1992, 1993). However, in the case of high plaque non-demented, no significant reduction of neurotensinergic fibers and other neurotransmitter fibers was observed in the amygdala (Benzing et al., 1993).
It is well-known that the entorhinal cortex is a crucial brain region involved in the earliest pathological change of Alzheimer's disease (Mann, 1989; Beach et al., 1997). Recently, Xiao et al. (2014) found that neurotensin persistently increases the spontaneous firing rate of neurons in the entorhinal cortex. This facilitation is mediated by neurotensin type 1 receptor and TREK-2K+ channels. Further behavioral studies revealed that activation of neurotensin type 1 receptors enhances spatial learning and therefore improves memory status in APP/PS1 Alzheimer's disease mice model. Recent electrophysiological studies further demonstrated that neurotensin increases glutamate release and spontaneous firing rate of dentate gyrus through both presynaptic and post-synaptic neurotensin receptors, respectively (Zhang et al., 2015, 2016).
It has recently been shown that the precursor form of nerve growth factor (proNGF) forming complex with p75 and sortilin contributes to neuronal death in basal forebrain neurons (Al-Shawi et al., 2007, 2008). Sortilin plays an important role in proNGF-mediated neurotoxicity. As a sortilin ligand, neurotensin blocks the sortilin-induced effects of proNGF and further rescues the survival of old neurons (Al-Shawi et al., 2008).
In summary, the levels of neurotensin and neurotensin receptors decrease in many brain areas of Alzheimer's disease. The amyloid plaque density in the occipital cortex is negatively associated with the neurotensin-positive neurons in the suprachiasmatic nucleus. Neurotensin increases the excitability of entorhinal cortex neurons and therefore improves spatial learning and memory. Furthermore, neurotensin rescues the survival of aged neurons through blocking sortilin-induced proNGF neurotoxicity.
PACAP and Alzheimer's Disease
PACAP belongs to the superfamily of secretin/glucagon/vasoactive intestinal polypeptide (Miyata et al., 1989). High level of PACAP is expressed in the hypothalamus, hippocampus, cerebellum and several brainstem nuclei (Hannibal, 2002). By activating three types of receptors (PAC1, VPAC1, and VPAC2), PACAP functions as a neurohormone, neurotransmitter, or neurotrophic factor in central nervous system (Lee and Seo, 2014). There is growing evidence (Reglodi et al., 2011) suggests that PACAP is closely associated with the pathology of Alzheimer's disease (Figure 3).
Figure 3.
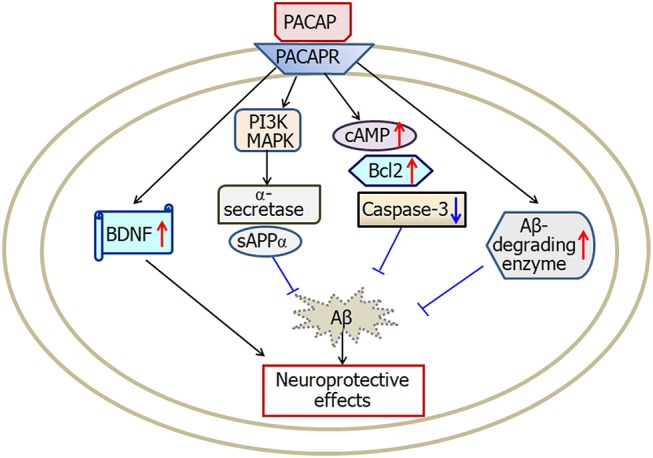
A scheme describing the possible mechanisms of PACAP-induced neuroprotective effects in Alzheimer's disease. PACAP protects against Aβ-induced neurotoxicity by activation of cAMP, BDNF, Bcl-2, Aβ-degrading enzyme and deactivation of caspase-3. Furthermore, PACAP increases α-secretase activation and then enhances secretion of sAPPα through both the MAPK and PI3K pathways. PACAP, pituitary adenylate cyclase-activating polypeptide; PACAPR, pituitary adenylate cyclase-activating polypeptide receptors; BDNF, brain-derived neurotrophic factor. The internal and external circles represent the inner and outer leaflets of the cellular membrane.
By using three different mouse models of Alzheimer's disease, early study showed downregulation of PACAP genes (Wu et al., 2006). Moreover, the PACAP levels are reduced in brain areas including entorhinal cortex, middle temporal gyrus, superior frontal gyrus, and primary visual cortex in Alzheimer's disease patients (Han et al., 2014a). Further study revealed that the lower PACAP levels are correlated with higher amyloid burden, tau protein, and the declined recognition memory with aging (Han et al., 2014b; An et al., 2017).
It is demonstrated that PACAP exerts neuroprotective effects through multiple mechanisms in Alzheimer's disease patients and mouse models (Vaudry et al., 2004; Rat et al., 2011). Onoue et al. (2002) first demonstrated that PACAP protects against Aβ-induced neuronal toxicity. Treatment with PACAP rescues 80% of Aβ-induced reduction of cell viability through increase of cAMP and deactivation of caspase-3 in PC12 cells. Intranasal application of PACAP increases the levels of brain-derived neurotrophic factor (BDNF) and antiapoptotic Bcl-2 protein. In addition, PACAP also increases the level of Aβ-degrading enzyme (Rat et al., 2011). Therefore, intranasal application of PACAP could be a useful therapeutic approach in treating Alzheimer's disease. It is known that, in the non-amyloidogenic pathway, the α-secretase cleaves the amyloid precursor protein and prevents amyloid plaque formation (Postina, 2012). Activation of PAC1 receptors increases APPs alpha secretion and enhances α-secretase cleavage of APP. Furthermore, both the MAPK and PI3K pathways are involved in PACAP-mediated α-secretase activation (Kojro et al., 2006).
Ginsenoside is a major component of the traditional herb ginseng which has been proved to exert neurotrophic and neuroprotective effects via preventing neuronal degeneration. Ginsenosides Rh2 increases the gene expression of PACAP in astrocytes of the brain and therefore ameliorates Aβ-induced growth inhibition of astrocytes (Shieh et al., 2008).
In summary, the level of PACAP decreases in brain areas of Alzheimer's disease patients and mouse models, which is correlated with higher amyloid burden, tau protein, and the declined recognition memory. PACAP protects against Aβ-induced neuronal toxicity through activation of cAMP, Bcl2, BDNF, Aβ-degrading enzyme, and deactivation of caspase-3. In addition, PACAP enhances α-secretase cleavage of APP via both MAPK and PI3K pathways.
Neuropeptide Y and Alzheimer's Disease
Neuropeptide Y is a 36-amino acid neuropeptide. Neuropeptide Y receptors are classified into five subtypes: Y1, Y2, Y4, Y5, and Y6 (Hsieh et al., 2013; Mittapalli and Roberts, 2014; Pérez-Fernández et al., 2014). Neuropeptide Y plays functions associated with modulation of food ingestion, mood, learning and memory (dos Santos et al., 2013b) and also plays an important role in neuroprotection against neurodegenerative diseases (Figure 4).
Figure 4.
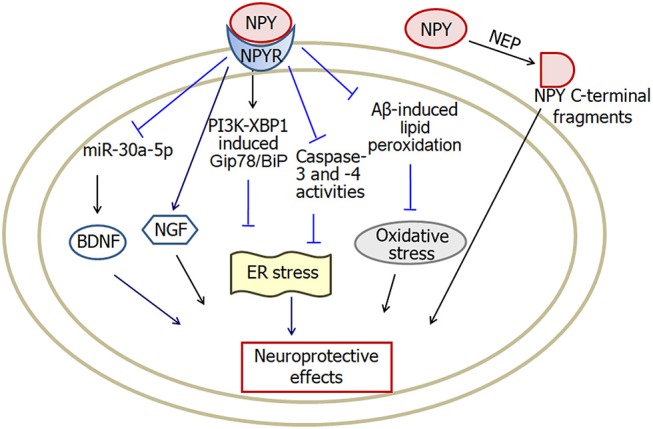
A model showing the possible pathways of neuropeptide Y-induced neuroprotective effects in Alzheimer's disease. Neuropeptide Y inhibits Aβ-induced lipid peroxidation and prevents intracellular oxidative stress. Activation of PI3K-XBP1 pathway may also be involved in neuropeptide Y-induced neuroprotection against endoplasmic reticulum stress. Moreover, both NGF and BDNF are involved in neuropeptide Y-induced neuroprotective effects. In addition, NEP cleaves neuropeptide Y into C-terminal fragments, which protect against the neurodegenerative pathology in Alzheimer's disease. NPY, neuropeptide Y; NPYR, neuropeptide Y receptors; NEP, neutral endopeptidase; ER, endoplasmic reticulum; BDNF, brain-derived neurotrophic factor; NGF, nerve growth factor. The internal and external circles represent the inner and outer leaflets of the cellular membrane.
The expression of neuropeptide Y changes under neurodegenerative diseases including Alzheimer's disease (Duarte-Neves et al., 2016). An anomalous high expression level of neuropeptide Y with aging was detected in the hippocampal circuits of mouse model of Alzheimer's disease (Diez et al., 2003; Krezymon et al., 2013). Recently, Mahar et al. (2017) reported that the number of neuropeptide Y immunoreactive hippocampal interneurons reduces in presymptomatic TgCRND8 Alzheimer's disease mouse model.
Neuropeptide Y has neuroprotective effects in Alzheimer's disease (Croce et al., 2011, 2012, 2013; Angelucci et al., 2014; Duarte-Neves et al., 2016; Spencer et al., 2016). Intracerebroventricular application of neuropeptide Y prevents Aβ1−40-induced depressive-like symptoms and spatial memory impairments through Y2 receptors. Neuropeptide Y produces the neuroprotection through inhibition of Aβ-induced lipid peroxidation, indicating the involvement of prevention of intracellular oxidative stress (dos Santos et al., 2013b). Moreover, neuropeptide Y induces protective effects against endoplasmic reticulum stress-mediated cell loss through activation of PI3K-XBP1-induced Gip78/BiP pathway as well as inhibition of caspase-3 and caspase-4 activities (Lee et al., 2018). Neuropeptide Y-induced neuroprotective effects are also associated with the production of neurotrophin family (Croce et al., 2011, 2012, 2013; Angelucci et al., 2014). Pretreatment with neuropeptide Y protects neurons against Aβ neurotoxicity which is accompanied by an increased intracellular level of NGF (Croce et al., 2012) and BDNF (Croce et al., 2013). Moreover, decrease of miR-30-5p (a membrane of miR-30a family regulating BDNF tuning expression) levels is involved in neuropeptide Y-induced modulation of BDNF.
The neutral endopeptidase (NEP) neprilysin is an Aβ degrading enzyme which is therefore involved in the pathogenesis of Alzheimer's disease. However, NEP has also been proved to cleave neuropeptide Y into C-terminal fragments. The NEP-produced C-terminal fragments of neuropeptide Y attenuate the neurodegenerative process in both transgenic Alzheimer's disease mice and Aβ treated human neurons (Rose et al., 2009). It is well-known that Aβ accumulation, senile plaque formation and neuronal dysfunction contribute to the cognitive declination in Alzheimer's disease. However, weight loss is an early sign of Alzheimer's disease. It is reported that high level of Aβ potentially devastates hypothalamic arcuate neuropeptide Y neurons and downregulates the leptin state in the early disease process, which may lead to the weight loss (dos Santos et al., 2013b; Ishii et al., 2014).
In summary, the level of neuropeptide Y increases significantly in hippocampus of mouse Alzheimer's disease models. Neuropeptide Y exerts neuroprotective effects through multiple pathways. Neuropeptide Y mitigates endoplasmic reticulum stress-induced neuronal cell death through activation of PI3K-XBP1-induced Gip78/BiP pathway and inhibition of caspase-3 and caspase-4 activities. Furthermore, neuropeptide Y suppresses oxidative stress via inhibition of Aβ-induced lipid peroxidation. In addition, neuropeptide Y plays neuroprotection via increasing the levels of BDNF and NGF.
Substance P and Alzheimer's Disease
The tachykinin family includes substance P, neurokinin A and neurokinin B. By activating neurokinin receptors, tachykinin plays important roles including pain, depression, nausea and emesis (Mantyh, 2002). In addition, tachykinin protects against the neurotoxic processes of Alzheimer's disease by multiple pathways (Severini et al., 2016).
Early studies revealed that the expression of substance P changes in different brain regions of Alzheimer's disease. The level of substance P decreases in the cortex, hippocampus and striatum in Alzheimer's disease patients and animal models (Bouras et al., 1990; Quigley and Kowall, 1991; Nag et al., 1999; Ahmed et al., 2004), but increases in the pallidum and substantia nigra (Bouras et al., 1990). However, Willis et al. (2007) showed that in old age of Alzheimer's disease transgenic mice, the substance P-immunoreactivity exists in astrocytes of hippocampus and thalamus. In late onset Alzheimer's disease patients, the level of substance P in cerebrospinal fluid increases significantly (Rösler et al., 2001). Furthermore, the increased level of substance P is positively associated with the level of Aβ1−42 in Alzheimer's disease patients (Johansson et al., 2015). In addition to the change of substance P, the activity of neuropeptidases to metabolize substance P also alters in Alzheimer's disease. Waters and David (1995) demonstrated that the activity of neuropeptidases is decreased in the temporal cortex of senile dementia of the Alzheimer's disease, which therefore increases the metabolic half-life of substance P.
Substance P exerts neuroprotective effects in a variety of in vitro and in vivo studies (Figure 5). Potassium channel dysfunction is a possible mechanism underlying the pathophysiology of Alzheimer's disease. Some kinds of voltage-gated potassium channels are involved in substance P-induced neuroprotective effects. Application of substance P prevents Aβ-induced impairment of cognitive processes through inhibition of Aβ-induced overexpression of potassium channel subunits (Campolongo et al., 2013), as well as Aβ-induced enhancement of A-type K+ currents (Pieri et al., 2010). Substance P also protects cerebellar granule cells against Aβ-induced apoptosis through inhibition of caspase-3-induced PARP-1 cleavage (Pieri et al., 2010). In addition, substance P plays non-amyloidogenic effect through decreasing Aβ1−42, increasing sAPPα and α-secretase activity (Marolda et al., 2012). Aβ25−35 reduces the expression of substance P in hippocampus before the neuronal loss of Alzheimer's disease. Memantine, a non-competitive NMDA receptor antagonist, attenuates Aβ25−35-induced decrease of substance P (Arif et al., 2009). Furthermore, in ibotenic acid-treated Alzheimer's disease model, memantine treatment recovers the decreased substance P expression (Ahmed et al., 2004). Recently, Fernandes et al. (2018) reported that a substance P receptor antagonist attenuates aluminum-induced spatial memory deficit probably through blockade of substance P-mediated neuroinflammation. It is known that the dysfunction of metal ions, such as copper is a feature of Alzheimer's disease. Neurokinin B protects against copper-induced calcium channel opening (Russino et al., 2013), as well as the synaptic homeostasis (Grosas et al., 2014).
Figure 5.
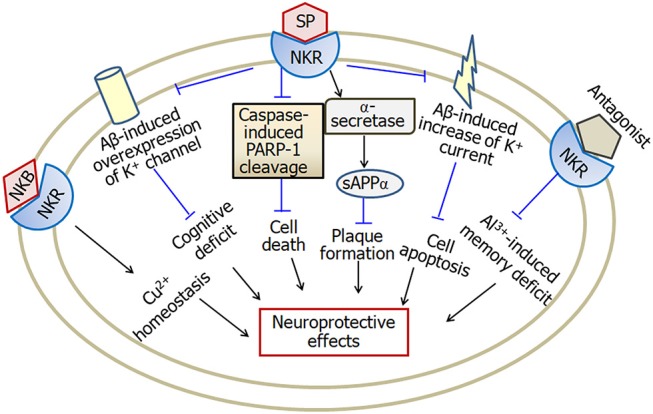
A model describing the multiple effects of substance P in Alzheimer's disease. Substance P inhibits Aβ-induced overexpression of K+ channel and Aβ-induced increase of K+ current, and therefore attenuates cognitive deficit and apoptosis in Alzheimer's disease. Furthermore, substance P exerts neuroprotective effects through inhibition of caspase-3-induced PARP-1 cleavage and enhancement of α-secretase activity. Neurokinin B plays a role in copper homeostasis. However, substance P receptor antagonist attenuates aluminum-induced spatial memory deficit probably through blockade of substance P-mediated neuroinflammation. SP, substance P; NKR, neurokinin receptors; PARP-1, poly ADP-ribose polymerase-1; sAPPα, soluble amyloid precursor protein α; NKB, neurokinin B; Cu, copper; Al, aluminum. The internal and external circles represent the inner and outer leaflets of the cellular membrane.
In summary, the levels of substance P decrease in brain regions including cortex and hippocampus, but increase in cerebrospinal fluid of late onset Alzheimer's disease patients. Substance P exerts neuroprotective effects through inhibition of Aβ-induced over- expression and activity of K+ channel. Furthermore, substance P inhibits caspase-3-induced PARP-1 cleavage and increases α-secretase activity, and is therefore involved in neuroprotection in Alzheimer's disease.
Orexin and Alzheimer's Disease
Orexin is a hypothalamic neuropeptide which plays an important role in maintaining wakefulness. It is well-known that sleep disturbances are common clinical symptom in neurodegenerative disorders. Recently people focus on the involvement of orexinergic system in the pathophysiology, especially sleep disturbances, of Alzheimer's disease (Ferini-Strambi, 2014; Malkki, 2014; Liguori, 2017; Liguori et al., 2017). Early morphological study revealed that the orexinergic neurons are reduced significantly in postmortem hypothalamus of Alzheimer's disease patients (Fronczek et al., 2012). However, it is demonstrated recently that the level of orexin-A in cerebrospinal fluid is higher in Alzheimer's disease patients which is positively associated with Alzheimer's disease biomarkers including Aβ and Tau (Liguori et al., 2016; Osorio et al., 2016; Gabelle et al., 2017). In both Alzheimer's disease patients and Aβ42 treated-SH-SY5Y cells, amyloid deposition and tau phosphorylation decrease the expression of orexin-1 and orexin-2 receptors (Davies et al., 2015). In vivo microdialysis revealed that infusion of orexin-A increases the amount of Aβ in brain interstitial fluid, while orexin receptor antagonist suppresses Aβ levels and reduces Aβ plaque deposition in the cortex (Kang et al., 2009). In Aβ-treated microglial cells, both orexin-A and orexin-B reduce the uptake of Aβ through downregulation of phagocytosis regulating molecules including PI3K, Akt and p38-MAPK. In addition, orexins suppress autophagosome-lysosome fusion and lead to impaired Aβ degradation (An et al., 2017). Furthermore, the orexin receptors 2 gene, rs2653349 polymorphism, is likely to be a risk factor of Alzheimer's disease (Gallone et al., 2014). In APP/PS1 transgenic Alzheimer's disease mice, orexin gene knock out markedly decreases the amount of Aβ pathology, while rescue of orexinergic neurons increases the amount of Aβ pathology in the brain (Roh et al., 2014). Recent study revealed that orexinergic system modulates both the hippocampal clock and clock-controlled-genes, Bace1 and Bace2. Both the two genes are correlated with the production of Aβ (Ma et al., 2016).
In addition to the inhibition of Aβ uptake and degradation in microglial cells (An et al., 2017), orexin may also exert neuroprotective effects. Orexinergic neuron degeneration impairs long-term social memory in mice (Yang et al., 2013). Intracerebroventricular microinjection of orexin-A improves memory in SAMP8 Alzheimer disease mice (Jaeger et al., 2002). Orexin receptors have been demonstrated to exert neuroprotective effects in Alzheimer's disease via heterodimerization with GPR103. ERK is a key molecule in the prevention of neurodegeneration. Treatment with orexins induces ERK1/2 phosphorylation suggesting the neuroprotection of the heterodimerization. Microarray showed that orexin-A augments NF-κB signaling and orexin-B up-regulates PI3K-Akt and Jak-STAT signaling, respectively (Davies et al., 2015).
The hippocampus is a crucial brain region that plays important roles in learning and memory. Intra-hippocampal administration of orexin-A attenuates pain-induced impairment of learning and memory (Raoof et al., 2015), while blocking orexin receptors in hippocampal CA1 regions impairs spatial memory retrieval (Akbari et al., 2006, 2008). It has been demonstrated that activation of cholinergic transmission increases the neuronal excitability of CA1 pyramidal neurons and therefore enhances hippocampus-dependent learning in Alzheimer disease animal model (Disterhoft and Oh, 2006). Moreover, decreasing the firing rate of CA1 pyramidal neurons by potassium channel activator impairs associative learning in rats (McKay et al., 2012). Therefore, our recent study that orexin-A increases the firing rate of hippocampal CA1 neurons may provide direct in vivo electrophysiological evidence for the possible involvement of orexin-A in Alzheimer disease (Chen et al., 2017).
In summary, Aβ deposition and tau phosphorylation decrease the expression of orexin and the receptors in hypothalamus, while the levels of orexins in cerebrospinal fluid increase in Alzheimer's disease patients. In microglial cells, orexins increase Aβ level through suppression of Aβ uptake and degradation. However, in cells forming OXRs and GPR103 heterodimers, orexins exert neuroprotective effects through upregulation of NF-κB, PI3K-Akt, Jak-STAT signaling, as well as ERK1/2 phosphorylation. Figure 6 illustrated the complicated effects of orexin in Alzheimer's disease.
Figure 6.
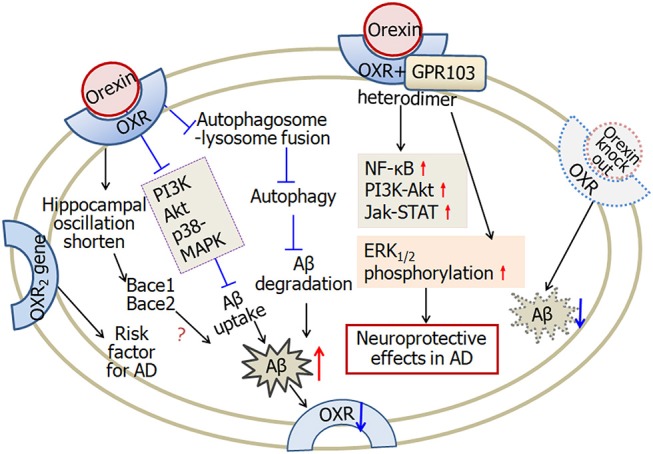
A scheme describing the complicated effects of orexin in Alzheimer's disease. Firstly, in microglial cells, orexin suppresses autophagosome-lysosome fusion process, leading to impaired Aβ degradation. Furthermore, orexin suppresses Aβ uptake through downregulating phagocytosis regulating molecules, such as PI3K, Akt, and p38-MAPK. Aβ-plaque formation and tau hyper-phosphorylation decrease the expression of orexin receptors in Alzheimer's disease. Secondly, orexin receptors and GPR103 form functional heterodimers. Orexin augments NF-κB, PI3K-Akt, Jak-STAT signaling and induces ERK1/2 phosphorylation, and therefore is involved in neuroprotective functions. Finally, one of the orexin receptor 2 gene is likely a risk factor for Alzheimer's disease. In APP/PS1 Alzheimer's disease mice, orexin gene knock out decreases the amount of Aβ. Orexin modulates the hippocampal oscillation and the expression of clock-controlled-genes, Bace1 and Bace2, which are associated with the production of Aβ. OXR, orexin receptors; AD, Alzheimer's disease. The internal and external circles represent the inner and outer leaflets of the cellular membrane.
Conclusion
In this review article, we provide a description of recent advances of neuropeptides including ghrelin, neurotensin, PACAP, neuropeptide Y, substance P and orexin in Alzheimer's disease. Based on current studies, the levels of these neuropeptides and their receptors change in Alzheimer's disease (Table 1). Neuropeptides exert significant neuroprotective effects against Aβ-induced neuronal toxicity (Table 2). Multiple intracellular mechanisms, including activation of α-secretases and Aβ-degrading enzyme, production of neurotrophins, increase of neuronal glucose uptake, upregulation of NF-κB, PI3K-Akt, MAPK, Jak-STAT, ERK1/2 phosphorylation and downregulation of caspase-3, inhibition of endoplasmic reticulum stress and autophagy, modulation of LTP and potassium channel activity, appear to be involved in neuropeptide-induced neuroprotection in Alzheimer's disease. As the levels of neuropeptides and the receptors may change before Aβ deposition and neuronal loss, as well as positively/negatively correlate with cognitive impairment, future studies would focus on detection of neuropeptides as biomarkers in early diagnosis and treatment evaluation of Alzheimer's disease. Furthermore, for the notable neuroprotective effects, further researches are needed to explore the potential use of neuropeptides, especially via convenient administration method like intranasal application, in the prevention and cure of Alzheimer's disease.
Table 1.
Changes in the level of neuropeptides and receptors in Alzheimer's disease.
| Neuropeptides | Receptors | Levels | Biological sample | State of disease | References | |
|---|---|---|---|---|---|---|
| Human | Animal models | |||||
| Ghrelin mRNA | ↓ | Temporal gyrus | AD patient | Gahete et al., 2010 | ||
| Acylated ghrelin | ↑ | Serum | MCI patient | Cao et al., 2018 | ||
| GHS-R1a | ↓ | Temporal gyrus | AD patient | Gahete et al., 2010 | ||
| GHS-R1b | ↑ | Temporal gyrus | AD patient | Gahete et al., 2010 | ||
| Neurotensin | ↓ | Amygdala | AD patient | Benzing et al., 1990, 1992, 1993 | ||
| Neurotensin | ↓ | Septum | AD patient | Ferrier et al., 1983 | ||
| Neurotensin mRNA | ↓ | Temporal gyrus | AD patient | Gahete et al., 2010 | ||
| NTSR1, NTSR2 | ↓ | Temporal gyrus | AD patient | Gahete et al., 2010 | ||
| NTSR | ↓ | Entorhinal area | AD patient | Jansen et al., 1990 | ||
| NTSR | ↓ | Dentate gyrus, SNc, VTA, PVNh |
Aged (24–25 months) rats with or without memory impairment | Rowe et al., 2006 | ||
| PACAP gene | ↓ | Cortex | APP/PS-1 (18 months) and Tg2576/PS-1 (12 months) mice with Aβ deposition | Wu et al., 2006 | ||
| Temporal cortex | AD patients | |||||
| PACAP | ↓ | Entorhinal cortex, middle temporal gyrus, superior frontal gyrus, primary visual cortex | AD patient | Han et al., 2014a | ||
| PACAP | ↓ | Hippocampus, cortex around hippocampus | hAPP mice in each age group | Han et al., 2017 | ||
| Neuropeptide Y | ↑ | Dentate gyrus | Tg2576 mice (18 months) | Krezymon et al., 2013 | ||
| Neuropeptide Y | ↑ | Hippocampus, cortex | APP23 mice (27 months) with Aβ plaques | Diez et al., 2003 | ||
| Neuropeptide Y | ↓ | Hippocampal interneurons | TgCRND8 mice (1 month before amyloid deposition) | Mahar et al., 2017 | ||
| Substance P | ↓ | Dentate gyrus | AD patient | Quigley and Kowall, 1991 | ||
| Substance P | ↓ | Cortex, hippocampus | Rats with Aβ infusion | Nag et al., 1999 | ||
| Substance P | ↓ | Cortex, hippocampus | AD patient | Bouras et al., 1990 | ||
| Substance P | ↑ | Pallidum, substantia nigra | AD patient | Bouras et al., 1990 | ||
| Substance P | ↑ | Astrocytes in hippocampal formation and thalamus | TgAPP751 mice (12 months with Aβ plaques) | Willis et al., 2007 | ||
| Substance P | ↑ | CSF | Late onset AD patient | Rösler et al., 2001; Johansson et al., 2015 | ||
| Orexin | ↓ | Hypothalamus | AD patient | Fronczek et al., 2012 | ||
| Orexin | ↑ | CSF | AD patient | Liguori et al., 2016; Osorio et al., 2016; Gabelle et al., 2017 | ||
| Orexin precursor gene | ↑ | Hypothalamus | APP/PS1dE9 mice (12–15 months) | Ma et al., 2016 | ||
| OX1R, OX2R | ↓ | Hippocampus, Aβ42 treated SH-SY5Y cells |
AD patient | Aβ plaques and tau phosphorylation | Davies et al., 2015 | |
AD, Alzheimer's disease; CSF, cerebrospinal fluid; GHS-R, ghrelin G-protein coupled receptor; MCI, mild cognitive impairment; NTSR, neurotensin receptor; OXR, orexin receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; PVNh, paraventricular nucleus of the hypothalamus; SNc, substantia nigra zona compacta; VTA, ventral tegmental area.
Table 2.
Neuroprotective effects and possible mechanisms of neuropeptides in Alzheimer's disease.
| Neuropeptides | Reagents or treatments | Concentrations and times used | Neuroprotective effects | Mechanisms | Cellular and animal models | References |
|---|---|---|---|---|---|---|
| Ghrelin | Ghrelin agonist: LY444711 | 30 mg/kg/day (in a chocolate pill) for 4 months | Improves cognition | Reduces Aβ and microglial inflammation in dentate gyrus; Impairs glucose tolerance immediately | Tg APPSwDI mice | Dhurandhar et al., 2013; Kunath et al., 2015 |
| Ghrelin | 0.1 and 1 μM for 24 h | Growth-promoting effect on neuronal cells | Induces GHS-R1 expression; Activate the proteasome; Deregulates autophagy | APP-transfected SH-SY5Y cells | Cecarini et al., 2016 | |
| Acyl-ghrelin or DES-acyl ghrelin | 0.2 nmol/h for 3 weeks | Reverses impairments of cognition and energy and glucose metabolism | Suppresses Aβ deposition; Increases the phosphorylation of AMPK and GSK, decreases the phosphorylation of tau | Rats with i.c.v infusion of Aβ | Kang et al., 2015 | |
| Acyl-ghrelin | 0.3 mg/kg i.p. daily for 7 days | Prevents impairment of recognition and spatial orientation | Blunts Aβ-induced depression of LTP in hippocampus | Mice with i.c.v infusion of Aβ | Santos et al., 2017 | |
| Ghrelin | 80 μg/kg i.p. daily for 7 days | Rescues memory deficits | Decreases microgliosis in hippocampus; Attenuates hippocampal neuronal loss; Prevents synaptic degeneration including cholinergic fiber loss |
Intrahippocampal injection of AβO | Moon et al., 2011 | |
| Ghrelin | 80 μg/kg i.p. every 2 days for 30 days | Ameliorates neurogenesis impairment in hippocampus | N/A | 5XFAD mice | Moon et al., 2014 | |
| Ghrelin | 0.1–0.5 μM for 24 h | Improves cell survival | Reduces superoxide production and mitochondrial membrane depolarization; Prevents GSK 3β |
AβO treated primary hippocampal neurons and hypothalamic N42 cell line | Martins et al., 2013; Gomes et al., 2014 | |
| Ghrelin | 10 nM for 1 h | Augments neuronal glucose uptake | Decreases tau phosphoralation; Increases Akt/GSK phosphorylation |
Primary hippocampal neurons treated with glucose | Chen et al., 2010 | |
| Neurotensin | Neurotensin or NTS1 agonist: PD149163 | 0.25 μM microinjection or bath application | Improves spatial learning and memory; Increases firing rate of AP in stellate neurons of EC |
Inhibits TREK-2K channels via PLC/PKC pathway | APP/PS1 mice | Xiao et al., 2014 |
| Neurotensin | 40 μM for 2 h | Rescues the survival of aged neurons | Blocks sortilin-mediated neurotoxic role of proNGF in old age; Increases proNGF expression in frontal cortex and hippocampus |
Aged mice BFN neurons; AD patients |
Al-Shawi et al., 2007, 2008 | |
| PACAP | PACAP38 | 10 μg daily for 3 months intranasal | Rescues impaired recognition; Stimulates the non-amyloidogenic processing of APP; Reduces Aβ40 and Aβ42 |
Enhances gene expression of α-secretases; Enhances Aβ-degrading enzyme neprilysin; Increases PACAP and PAC1 expression; Increases expression of BDNF; Increases expression of antiapoptotic Bcl-2 |
APP[V717I]- transgenic mice; SK-N-MC PAC1 cells (BDNF study) |
Rat et al., 2011 |
| PACAP27 | 1 nM for 72 h | Rescues Aβ-induced cell death | Increases cAMP formation; Decreases caspase-3 activity |
Aβ-treated PC12 cells | Onoue et al., 2002 | |
| PACAP27 | 1 μM for 4 h 300 nM for 4 h |
Enhances secretion of sAPPα | Stimulates MAPK pathway and PI3K | SK-N-MC cells; HEK 293 cells |
Kojro et al., 2006 | |
| Neuropeptide Y | Neuropeptide Y | 0.0234 μM/μL, i.c.v. | Prevents depressive-like behavior and spatial memory deficits | Blunts Aβ-induced increase in lipid peroxidation in hippocampus and prefrontal cortex | Mice treated with i.c.v. Aβ1−40 | dos Santos et al., 2013b |
| Neuropeptide Y | 100 nM for 12h | Attenuates ER stress-induced cell death | Decreases caspase-3 and−4 activities; Suppresses the activation of three major ER stress sensors; Activates PI3K-XBP1 pathway | SK-N-SH cells; Mouse cortical neurons | Lee et al., 2018 | |
| Neuropeptide Y | 0.5, 1, and 2 μM for 24 h | Rescues Aβ-induced cell death | Increases NGF synthesis and restores NGF release; Increases the levels of BDNF via inhibiting miR-30-5p |
Aβ25−35-treated SH-SY5Y cells; Aβ25−35-treated primary cortical neurons | Croce et al., 2012, 2013 | |
| Amidated NPY CTFs (NPY21–36 and 31–36) | 120 μM i.c.v. for 28 days in vivo; 10 nM for 24 h in vitro | Ameliorates neurodegenerative pathology; Protects human neuronal cultures | NPY CTFs generated during NEP-mediated proteolysis exerts neuroprotective effects in vivo | APP tg mice; Aβ1−42-treated primary human cortical neurons |
Rose et al., 2009 | |
| Substance P | Substance P | 50 mg/kg, i.p. daily for 7 days | Prevents cognitive impairments | Reduces Aβ-induced overexpression of Kv1.4 in hippocampus and cerebral cortex |
Rats with i.c.v infusion of Aβ25−35 | Campolongo et al., 2013 |
| Substance P | 200 nM | Reverses cell death; Reverses Aβ-induced increase of IKA current |
Inhibits caspase-3-induced PARP-1 cleavage through Akt-dependent mechanism; Prevents Aβ-induced upregulation of Kv4.2 and Kv4.3 | Aβ-treated rat cerebellar granule cells | Pieri et al., 2010 | |
| Substance P | 200 nM | Reverses K+-induced apoptotic cell death and amyloidogenic processing of APP | Enhances α-secretase activity; Increases sAPPα; Reduces Aβ1−42 | Rat cerebellar granule cells | Marolda et al., 2012 | |
| Orexin | Orexin-A Dual orexin receptor antagonist: almorexant |
1.5 pM i.c.v for 6 h 13.9 nM i.c.v for 24 h i.p. for 8 weeks |
Increases ISF Aβ levels; Suppresses ISF Aβ levels; Reduces Aβ plaque deposition in the cortex | N/A | Human APP transgenic (Tg2576) mice; APPswe/PS1dE9 mice | Kang et al., 2009 |
| Orexin-A; Orexin-B | 1 μM for 24 h | Suppresses the uptake of Aβ; Reduces the degradation of Aβ | Downregulates phagocytosis regulating molecules: PI3K, Akt and p38-MAPK; Interferes autophagosome- lysosome fusion, and then suppresses autophogic flux |
Aβ-treated BV2 microglial cells | An et al., 2017 | |
| Orexin-A; Orexin-B | 100 nM for 24 h 100 nM for 1 h |
Augments NF-κB signaling (OXA); Up-regulates PI3K-Akt and Jak-STAT signaling (OXB); Induces ERK1/2 phosphorylation |
NF-κB regulates cell survival, neurogenesis, learning and memory; PI3K-Akt is involved in neuroprotective functions; ERK is a key molecule in prevention of neurodegeneration |
SH-SY5Y cells treated with retinoic acid (microarray); HEK293 cells with OXRs and GPR103 form functional heterodimers |
Davies et al., 2015 |
AβO, amyloid-β oligomers; AD, Alzheimer's disease; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; AP, action potential; APP, amyloid precursor protein; BDNF, brain-derived neurotrophic factor; BFN, basal forebrain nuclei; CTFs, C-terminal fragments; EC, entorhinal cortex; ER, endoplasmic reticulum; GHS-R1, growth hormone secretagogue receptor type 1; GSK, glycogen synthase kinase; i.c.v., intracerebroventricular; IKA current, A-type K+ current; ISF, interstitial fluid; LTP, long-term potentiation; MAPK, mitogen-activated protein kinase; NEP, neutral endopeptidase; NGF, nerve growth factor; NPY, neuropeptide Y; NTS1, neurotensin receptor 1; OXA, orexin-A; OXB, orexin-B; PACAP, pituitary adenylate cyclase-activating polypeptide; PARP-1, poly ADP-ribose polymerase-1; PI3K, phosphatidylinositol 3-kinase; sAPPα, soluble amyloid precursor protein α; SK-N-MC PAC1 cells, human neuroblastoma SK-N-MC cells overexpressing PAC1 receptor.
Author Contributions
X-YC wrote the manuscript. Y-FD and LC revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from the National Natural Science Foundation of China (31671076, 81772448, 81200872), and Taishan Scholars Construction Project.
References
- Ahmed M. M., Hoshino H., Chikuma T., Yamada M., Kato T. (2004). Effect of memantine on the levels of glial cells, neuropeptides, and peptide-degrading enzymes in rat brain regions of ibotenic acid-treated Alzheimer's disease model. Neuroscience 126, 639–649. 10.1016/j.neuroscience.2004.04.024 [DOI] [PubMed] [Google Scholar]
- Akbari E., Motamedi F., Naghdi N., Noorbakhshnia M. (2008). The effect of antagonization of orexin 1 receptors in CA1 and dentate gyrus regions on memory processing in passive avoidance task. Behav. Brain Res. 187, 172–177. 10.1016/j.bbr.2007.09.019 [DOI] [PubMed] [Google Scholar]
- Akbari E., Naghdi N., Motamedi F. (2006). Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res. 173, 47–52. 10.1016/j.bbr.2006.05.028 [DOI] [PubMed] [Google Scholar]
- Al-Shawi R., Hafner A., Chun S., Raza S., Crutcher K., Thrasivoulou C., et al. (2007). ProNGF, sortilin, and age-related neurodegeneration. Ann. N. Y. Acad. Sci. 1119, 208–215. 10.1196/annals.1404.024 [DOI] [PubMed] [Google Scholar]
- Al-Shawi R., Hafner A., Olsen J., Chun S., Raza S., Thrasivoulou C., et al. (2008). Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur. J. Neurosci. 27, 2103–2114. 10.1111/j.1460-9568.2008.06152.x [DOI] [PubMed] [Google Scholar]
- An H., Cho M. H., Kim D. H., Chung S., Yoon S. Y. (2017). Orexin impairs the phagocytosis and degradation of amyloid-β fibrils by microglial cells. J. Alzheimers Dis. 58, 253–261. 10.3233/JAD-170108 [DOI] [PubMed] [Google Scholar]
- Angelucci F., Gelfo F., Fiore M., Croce N., Mathé A. A., Bernardini S., et al. (2014). The effect of neuropeptide Y on cell survival and neurotrophin expression in in-vitro models of Alzheimer's disease. Can. J. Physiol. Pharmacol. 92, 621–630. 10.1139/cjpp-2014-0099 [DOI] [PubMed] [Google Scholar]
- Arif M., Chikuma T., Ahmed M. M., Nakazato M., Smith M. A., Kato T. (2009). Effects of memantine on soluble Alphabeta(25-35)-induced changes in peptidergic and glial cells in Alzheimer's disease model rat brain regions. Neuroscience 164, 1199–1209. 10.1016/j.neuroscience.2009.08.063 [DOI] [PubMed] [Google Scholar]
- Beach T. G., Honer W. G., Hughes L. H. (1997). Cholinergic fibre loss associated with diffuse plaques in the non-demented elderly: the preclinical stage of Alzheimer's disease? Acta Neuropathol. 93, 146–153. 10.1007/s004010050595 [DOI] [PubMed] [Google Scholar]
- Benzing W. C., Mufson E. J., Armstrong D. M. (1993). Immunocytochemical distribution of peptidergic and cholinergic fibers in the human amygdala: their depletion in Alzheimer's disease and morphologic alteration in non-demented elderly with numerous senile plaques. Brain Res. 625, 125–138. 10.1016/0006-8993(93)90145-d [DOI] [PubMed] [Google Scholar]
- Benzing W. C., Mufson E. J., Jennes L., Armstrong D. M. (1990). Reduction of neurotensin immunoreactivity in the amygdala in Alzheimer's disease. Brain Res. 537, 298–302. 10.1016/0006-8993(90)90372-I [DOI] [PubMed] [Google Scholar]
- Benzing W. C., Mufson E. J., Jennes L., Stopa E. G., Armstrong D. M. (1992). Distribution of neurotensin immunoreactivity within the human amygdaloid complex: a comparison with acetylcholinesterase- and Nissl-stained tissue sections. J. Comp. Neurol. 317, 283–297. 10.1002/cne.903170306 [DOI] [PubMed] [Google Scholar]
- Biggins J. A., Perry E. K., McDermott J. R., Smith A. I., Perry R. H., Edwardson J. A. (1983). Post mortem levels of thyrotropin-releasing hormone and neurotensin in the amygdala in Alzheimer's disease, schizophrenia and depression. J. Neurol. Sci. 58, 117–122. 10.1016/0022-510X(83)90114-4 [DOI] [PubMed] [Google Scholar]
- Bissette G., Nemeroff C. B., Decker M. W., Kizer J. S., Agid Y., Javoy-Agid F. (1985). Alterations in regional brain concentrations of neurotensin and bombesin in Parkinson's disease. Ann. Neurol. 17, 324–328. 10.1002/ana.410170403 [DOI] [PubMed] [Google Scholar]
- Bouras C., Vallet P. G., Hof P. R., Charnay Y., Golaz J., Constantinidis J. (1990). Substance P immunoreactivity in Alzheimer disease: a study in cases presenting symmetric or asymmetric cortical atrophy. Alzheimer Dis. Assoc. Disord. 4, 24–34. 10.1097/00002093-19904010000003 [DOI] [PubMed] [Google Scholar]
- Burns A., Iliffe S. (2009). Alzheimer's disease. BMJ 338:b158. 10.1136/bmj.b158 [DOI] [PubMed] [Google Scholar]
- Campolongo P., Ratano P., Ciotti M. T., Florenzano F., Nori S. L., Marolda R., et al. (2013). Systemic administration of substance P recovers beta amyloid-induced cognitive deficits in rat: involvement of Kv potassium channels. PLoS ONE 8:e78036. 10.1371/journal.pone.0078036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Zhu M., He Y., Chu W., Du Y., Du H. (2018). Increased serum acylated ghrelin levels in patients with mild cognitive impairment. J. Alzheimers Dis. 61, 545–552. 10.3233/JAD-170721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V., Bonfili L., Cuccioloni M., Keller J. N., Bruce-Keller A. J., Eleuteri A. M. (2016). Effects of ghrelin on the proteolytic pathways of Alzheimer's disease neuronal cells. Mol. Neurobiol. 53, 3168–3178. 10.1007/s12035-015-9227-x [DOI] [PubMed] [Google Scholar]
- Chen X. Y., Chen L., Du Y. F. (2017). Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. J. Neurosci. Res. 95, 1415–1426. 10.1002/jnr.23975 [DOI] [PubMed] [Google Scholar]
- Chen Y., Cao C. P., Li C. R., Wang W., Zhang D., Han L. L., et al. (2010). Ghrelin modulates insulin sensitivity and tau phosphorylation in high glucose-induced hippocampal neurons. Biol. Pharm. Bull. 33, 1165–1169. 10.1248/bpb.33.1165 [DOI] [PubMed] [Google Scholar]
- Constantinidis J., Bouras C., Richard J. (1983). Putative peptide neurotransmitters in human neuropathology: a review of topography and clinical implications. Clin. Neuropathol. 2, 47–54. [PubMed] [Google Scholar]
- Croce N., Ciotti M. T., Gelfo F., Cortelli S., Federici G., Caltagirone C., et al. (2012). Neuropeptide Y protects rat cortical neurons against β-amyloid toxicity and re-establishes synthesis and release of nerve growth factor. ACS Chem. Neurosci. 3, 312–318. 10.1021/cn200127e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce N., Dinallo V., Ricci V., Federici G., Caltagirone C., Bernardini S., et al. (2011). Neuroprotective effect of neuropeptide Y against β-amyloid 25-35 toxicity in SH-SY5Y neuroblastoma cells is associated with increased neurotrophin production. Neurodegener. Dis. 8, 300–309. 10.1159/000323468 [DOI] [PubMed] [Google Scholar]
- Croce N., Gelfo F., Ciotti M. T., Federici G., Caltagirone C., Bernardini S., et al. (2013). NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol. Cell Biochem. 376, 189–195. 10.1007/s11010-013-1567-0 [DOI] [PubMed] [Google Scholar]
- Davies J., Chen J., Pink R., Carter D., Saunders N., Sotiriadis G., et al. (2015). Orexin receptors exert a neuroprotective effect in Alzheimer's disease (AD) via heterodimerization with GPR103. Sci. Rep. 5:12584. 10.1038/srep12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar E. J., Allison D. B., van Groen T., Kadish I. (2013). Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer's disease pathology in a mouse model. PLoS ONE 8:e60437. 10.1371/journal.pone.0060437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez M., Danner S., Frey P., Sommer B., Staufenbiel M., Wiederhold K. H., et al. (2003). Neuropeptide alterations in the hippocampal formation and cortex of transgenic mice overexpressing beta-amyloid precursor protein (APP) with the Swedish double mutation (APP23). Neurobiol. Dis. 14, 579–594. 10.1016/j.nbd.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Disterhoft J. F., Oh M. M. (2006). Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease. J. Physiol. Paris 99, 180–192. 10.1016/j.jphysparis.2005.12.079 [DOI] [PubMed] [Google Scholar]
- dos Santos V. V., Rodrigues A. L., De Lima T. C., de Barioglio S. R., Raisman-Vozari R., Prediger R. D. (2013a). Ghrelin as a neuroprotective and palliative agent in Alzheimer's and Parkinson's disease. Curr. Pharm. Des. 19, 6773–6790. 10.2174/13816128113199990411 [DOI] [PubMed] [Google Scholar]
- dos Santos V. V., Santos D. B., Lach G., Rodrigues A. L., Farina M., De Lima T. C., et al. (2013b). Neuropeptide Y (NPY) prevents depressive-like behavior, spatial memory deficits and oxidative stress following amyloid-β [Aβ(1-40)] administration in mice. Behav. Brain Res. 244, 107–115. 10.1016/j.bbr.2013.01.039 [DOI] [PubMed] [Google Scholar]
- Duarte-Neves J., Pereira de Almeida L., Cavadas C. (2016). Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol. Dis. 95, 210–224. 10.1016/j.nbd.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L. (2014). Possible role of orexin in the pathogenesis of Alzheimer disease. JAMA Neurol. 71, 1478–1480. 10.1001/jamaneurol.2014.2819 [DOI] [PubMed] [Google Scholar]
- Fernandes J., Mudgal J., Rao C. M., Arora D., Basu Mallik S., Pai K. S. R., et al. (2018). N-acetyl-L-tryptophan, a substance-P receptor antagonist attenuates aluminum-induced spatial memory deficit in rats. Toxicol. Mech. Methods 28, 328–334. 10.1080/15376516.2017.1411412 [DOI] [PubMed] [Google Scholar]
- Fernandez A., de Ceballos M. L., Jenner P., Marsden C. D. (1994). Neurotensin, substance P, delta and mu opioid receptors are decreased in basal ganglia of Parkinson's disease patients. Neuroscience 61, 73–79. 10.1016/0306-4522(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Ferrier I. N., Cross A. J., Johnson J. A., Roberts G. W., Crow T. J., Corsellis J. A., et al. (1983). Neuropeptides in Alzheimer type dementia. J. Neurol. Sci. 62, 159–170. 10.1016/0022-510X(83)90196-X [DOI] [PubMed] [Google Scholar]
- Fronczek R., van Geest S., Frölich M., Overeem S., Roelandse F. W., Lammers G. J., et al. (2012). Hypocretin (orexin) loss in Alzheimer's disease. Neurobiol. Aging 33, 1642–1650. 10.1016/j.neurobiolaging.2011.03.014 [DOI] [PubMed] [Google Scholar]
- Gabelle A., Jaussent I., Hirtz C., Vialaret J., Navucet S., Grasselli C., et al. (2017). Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol. Aging 53, 59–66. 10.1016/j.neurobiolaging.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Gahete M. D., Córdoba-Chacón J., Kineman R. D., Luque R. M., Castaño J. P. (2011). Role of ghrelin system in neuroprotection and cognitive functions: implications in Alzheimer's disease. Peptides 32, 2225–2228. 10.1016/j.peptides.2011.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahete M. D., Rubio A., Córdoba-Chacón J., Gracia-Navarro F., Kineman R. D., Avila J., et al. (2010). Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer's disease patients. J. Alzheimers Dis. 22, 819–828. 10.3233/JAD-2010-100873 [DOI] [PubMed] [Google Scholar]
- Gallone S., Boschi S., Rubino E., De Martino P., Scarpini E., Galimberti D., et al. (2014). Is HCRTR2 a genetic risk factor for Alzheimer's disease? Dement. Geriatr. Cogn. Disord. 38, 245–253. 10.1159/000359964 [DOI] [PubMed] [Google Scholar]
- Garver D. L., Bissette G., Yao J. K., Nemeroff C. B. (1991). Relation of CSF neurotensin concentrations to symptoms and drug response of psychotic patients. Am. J. Psychiatry 148, 484–488. 10.1176/ajp.148.4.484 [DOI] [PubMed] [Google Scholar]
- Giedd J. N., Snell J. W., Lange N., Rajapakse J. C., Casey B. J., Kozuch P. L., et al. (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb. Cortex 6, 551–560. 10.1093/cercor/6.4.551 [DOI] [PubMed] [Google Scholar]
- Goldstein J., Seidman L., Horton N., Makris N., Kennedy D., Caviness V., et al. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex 11, 490–497. 10.1093/cercor/11.6.490 [DOI] [PubMed] [Google Scholar]
- Gomes S., Martins I., Fonseca A. C., Oliveira C. R., Resende R., Pereira C. M. (2014). Protective effect of leptin and ghrelin against toxicity induced by amyloid-β oligomers in a hypothalamic cell line. J. Neuroendocrinol. 26, 176–185. 10.1111/jne.12138 [DOI] [PubMed] [Google Scholar]
- Grosas A. B., Kalimuthu P., Smith A. C., Williams P. A., Millar T. J., Bernhardt P. V., et al. (2014). The tachykinin peptide neurokinin B binds copper(I) and silver(I) and undergoes quasi-reversible electrochemistry: towards a new function for the peptide in the brain. Neurochem. Int. 70, 1–9. 10.1016/j.neuint.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Guan X. M., Yu H., Palyha O. C., McKee K. K., Feighner S. D., Sirinathsinghji D. J., et al. (1997). Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 48, 23–29. 10.1016/S0169-328X(97)00071-5 [DOI] [PubMed] [Google Scholar]
- Han P., Liang W., Baxter L. C., Yin J., Tang Z., Beach T. G., et al. (2014a). Pituitary adenylate cyclase-activating polypeptide is reduced in Alzheimer disease. Neurology 82, 1724–1728. 10.1212/wnl.0000000000000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Nielsen M., Song M., Yin J., Permenter M. R., Vogt J. A., et al. (2017). The impact of aging on brain pituitary adenylate cyclase activating polypeptide, pathology and cognition in mice and rhesus macaques. Front. Aging Neurosci. 9:180. 10.3389/fnagi.2017.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Tang Z., Yin J., Maalouf M., Beach T. G., Reiman E. M., et al. (2014b). Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 35, 2064–2071. 10.1016/j.neurobiolaging.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Hannibal J. (2002). Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J. Comp. Neurol. 453, 389–417. 10.1002/cne.10418 [DOI] [PubMed] [Google Scholar]
- Hsieh Y. S., Chen P. N., Yu C. H., Liao J. M., Kuo D. Y. (2013). The neuropeptide Y Y1 receptor knockdown modulates activator protein 1-involved feeding behavior in amphetamine-treated rats. Mol. Brain 6:46. 10.1186/1756-6606-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Harper D. G., Shea S. A., Stopa E. G., Scheer F. A. (2013). Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci. Rep. 3:2229. 10.1038/srep02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M., Wang G., Racchumi G., Dyke J. P., Iadecola C. (2014). Transgenic mice overexpressing amyloid precursor protein exhibit early metabolic deficits and a pathologically low leptin state associated with hypothalamic dysfunction in arcuate neuropeptide Y neurons. J. Neurosci. 34, 9096–9106. 10.1523/jneurosci.0872-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger L. B., Farr S. A., Banks W. A., Morley J. E. (2002). Effects of orexin-A on memory processing. Peptides 23, 1683–1688. 10.1016/s0196-9781(02)00110-9 [DOI] [PubMed] [Google Scholar]
- Jansen K. L., Faull R. L., Dragunow M., Synek B. L. (1990). Alzheimer's disease: changes in hippocampal N-methyl-D-aspartate, quisqualate, neurotensin, adenosine, benzodiazepine, serotonin and opioid receptors–an autoradiographic study. Neuroscience 39, 613–627. 10.1016/0306-4522(90)90246-Z [DOI] [PubMed] [Google Scholar]
- Jiao Q., Du X., Li Y., Gong B., Shi L., Tang T., et al. (2017). The neurological effects of ghrelin in brain diseases: Beyond metabolic functions. Neurosci. Biobehav. Rev. 73, 98–111. 10.1016/j.neubiorev.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Johansson P., Almqvist E. G., Wallin A., Johansson J. O., Andreasson U., Blennow K., et al. (2015). Cerebrospinal fluid substance P concentrations are elevated in patients with Alzheimer's disease. Neurosci. Lett. 609, 58–62. 10.1016/j.neulet.2015.10.006 [DOI] [PubMed] [Google Scholar]
- Kang J. E., Lim M. M., Bateman R. J., Lee J. J., Smyth L. P., Cirrito J. R., et al. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. 10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Moon N. R., Kim D. S., Kim S. H., Park S. (2015). Central acylated ghrelin improves memory function and hippocampal AMPK activation and partly reverses the impairment of energy and glucose metabolism in rats infused with β-amyloid. Peptides 71, 84–93. 10.1016/j.peptides.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Kinkead B., Nemeroff C. B. (2004). Neurotensin, schizophrenia, and antipsychotic drug action. Int. Rev. Neurobiol. 59, 327–349. 10.1016/S0074-7742(04)59013-X [DOI] [PubMed] [Google Scholar]
- Kojro E., Postina R., Buro C., Meiringer C., Gehrig-Burger K., Fahrenholz F. (2006). The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 20, 512–514. 10.1096/fj.05-4812fje [DOI] [PubMed] [Google Scholar]
- Krezymon A., Richetin K., Halley H., Roybon L., Lassalle J. M., Francès B., et al. (2013). Modifications of hippocampal circuits and early disruption of adult neurogenesis in the tg2576 mouse model of Alzheimer's disease. PLoS ONE 8:e76497. 10.1371/journal.pone.0076497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath N., van Groen T., Allison D. B., Kumar A., Dozier-Sharpe M., Kadish I. (2015). Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer's disease mouse model. Sci. Rep. 5:11452 10.1038/srep11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor P. N., Buniel M. C., Furlow P. W., Clemente A. S., Velasco P. T., Wood M., et al. (2007). Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J. Neurosci. 27, 796–807. 10.1523/jneurosci.3501-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattuada D., Crotta K., Tonna N., Casnici C., Benfante R., Fornasari D., et al. (2013). The expression of GHS-R in primary neurons is dependent upon maturation stage and regional localization. PLoS ONE 8:e64183. 10.1371/journal.pone.0064183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Y., Hong S. H., Kim B., Lee D. S., Yu K., Lee K. S. (2018). Neuropeptide Y mitigates ER stress-induced neuronal cell death by activating the PI3K-XBP1 pathway. Eur. J. Cell Biol. 97, 339–348. 10.1016/j.ejcb.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Lee E. H., Seo S. R. (2014). Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 47, 369–375. 10.5483/BMBRep.2014.47.7.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C. (2017). Orexin and Alzheimer's disease. Curr. Top. Behav. Neurosci. 33, 305–322. 10.1007/7854_2016_50 [DOI] [PubMed] [Google Scholar]
- Liguori C., Chiaravalloti A., Nuccetelli M., Izzi F., Sancesario G., Cimini A., et al. (2017). Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer's disease. J. Neurol. 264, 2215–2223. 10.1007/s00415-017-8613-x [DOI] [PubMed] [Google Scholar]
- Liguori C., Nuccetelli M., Izzi F., Sancesario G., Romigi A., Martorana A., et al. (2016). Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer's disease. Neurobiol. Aging 40, 120–126. 10.1016/j.neurobiolaging.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Ma Z., Jiang W., Zhang E. E. (2016). Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer's disease-risk genes. Sci. Rep. 6:36035. 10.1038/srep36035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I., Albuquerque M. S., Mondragon-Rodriguez S., Cavanagh C., Davoli M. A., Chabot J. G., et al. (2017). Phenotypic alterations in hippocampal NPY- and PV-expressing interneurons in a presymptomatic transgenic mouse model of Alzheimer's disease. Front. Aging Neurosci. 8:327. 10.3389/fnagi.2016.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkki H. (2014). Alzheimer disease: increased orexin level correlates with sleep disruption and cognitive decline in Alzheimer disease. Nat. Rev. Neurol. 10:672. 10.1038/nrneurol.2014.209 [DOI] [PubMed] [Google Scholar]
- Mann D. M. (1989). The pathogenesis and progression of the pathological changes of Alzheimer's disease. Ann. Med. 21, 133–136. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W. (2002). Neurobiology of substance P and the NK1 receptor. J. Clin. Psychiatry 63 (Suppl. 11), 6–10. [PubMed] [Google Scholar]
- Marolda R., Ciotti M. T., Matrone C., Possenti R., Calissano P., Cavallaro S., et al. (2012). Substance P activates ADAM9 mRNA expression and induces α-secretase-mediated amyloid precursor protein cleavage. Neuropharmacology 62, 1954–1963. 10.1016/j.neuropharm.2011.12.025 [DOI] [PubMed] [Google Scholar]
- Martins I., Gomes S., Costa R. O., Otvos L., Oliveira C. R., Resende R., et al. (2013). Leptin and ghrelin prevent hippocampal dysfunction induced by Aβ oligomers. Neuroscience 241, 41–51. 10.1016/j.neuroscience.2013.02.062 [DOI] [PubMed] [Google Scholar]
- McKay B. M., Oh M. M., Galvez R., Burgdorf J., Kroes R. A., Weiss C. (2012). Increasing SK2 channel activity impairs associative learning. J. Neurophysiol. 108, 863–870. 10.1152/jn.00025.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittapalli G. K., Roberts E. (2014). Ligands of the neuropeptide Y Y2 receptor. Bioorg. Med. Chem. Lett. 24, 430–441. 10.1016/j.bmcl.2013.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., et al. (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 164, 567–574. 10.1016/0006-291X(89)91757-9 [DOI] [PubMed] [Google Scholar]
- Moon M., Cha M. Y., Mook-Jung I. (2014). Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J. Alzheimers Dis. 41, 233–241. 10.3233/jad-132417 [DOI] [PubMed] [Google Scholar]
- Moon M., Choi J. G., Nam D. W., Hong H. S., Choi Y. J., Oh M. S., et al. (2011). Ghrelin ameliorates cognitive dysfunction and neurodegeneration in intrahippocampal amyloid-β1-42 oligomer-injected mice. J. Alzheimers Dis. 23, 147–159. 10.3233/jad-2010-101263 [DOI] [PubMed] [Google Scholar]
- Murray S., Tulloch A., Gold M. S., Avena N. M. (2014). Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat. Rev. Endocrinol. 10, 540–552. 10.1038/nrendo.2014.91 [DOI] [PubMed] [Google Scholar]
- Nag S., Yee B. K., Tang F. (1999). Reduction in somatostatin and substance P levels and choline acetyltransferase activity in the cortex and hippocampus of the rat after chronic intracerebroventricular infusion of beta-amyloid (1-40). Brain Res. Bull. 50, 251–262. 10.1016/S0361-9230(99)00196-3 [DOI] [PubMed] [Google Scholar]
- Onoue S., Endo K., Ohshima K., Yajima T., Kashimoto K. (2002). The neuropeptide PACAP attenuates beta-amyloid (1-42)-induced toxicity in PC12 cells. Peptides 23, 1471–1478. 10.1016/S0196-9781(02)00085-2 [DOI] [PubMed] [Google Scholar]
- Osorio R. S., Ducca E. L., Wohlleber M. E., Tanzi E. B., Gumb T., Twumasi A., et al. (2016). Orexin-A is associated with increases in cerebrospinal fluid phosphorylated-tau in cognitively normal elderly subjects. Sleep 39, 1253–1260. 10.5665/sleep.5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos V. N., Ralevski E. (2014). The role of ghrelin in addiction: a review. Psychopharmacology (Berl). 231, 2725–2740. 10.1007/s00213-014-3640-0 [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández J., Megías M., Pombal M. A. (2014). Cloning, phylogeny, and regional expression of a Y5 receptor mRNA in the brain of the sea lamprey (Petromyzon marinus). J. Comp. Neurol. 522, 1132–1154. 10.1002/cne.23481 [DOI] [PubMed] [Google Scholar]
- Pieri M., Amadoro G., Carunchio I., Ciotti M. T., Quaresima S., Florenzano F., et al. (2010). SP protects cerebellar granule cells against beta-amyloid-induced apoptosis by down-regulation and reduced activity of Kv4 potassium channels. Neuropharmacology 58, 268–276. 10.1016/j.neuropharm.2009.06.029 [DOI] [PubMed] [Google Scholar]
- Popelová A., Kákonová A., Hrubá L., Kuneš J., Maletínská L., Železná B. (2018). Potential neuroprotective and anti-apoptotic properties of a long-lasting stable analog of ghrelin: an in vitro study using SH-SY5Y cells. Physiol. Res. 67, 339–346. [DOI] [PubMed] [Google Scholar]
- Postina R. (2012). Activation of α-secretase cleavage. J. Neurochem. 120 Suppl. 1, 46–54. 10.1111/j.1471-4159.2011.07459.x [DOI] [PubMed] [Google Scholar]
- Quigley B. J., Jr., Kowall N. W. (1991). Substance P-like immunoreactive neurons are depleted in Alzheimer's disease cerebral cortex. Neuroscience 41, 41–60. 10.1016/0306-4522(91)90199-X [DOI] [PubMed] [Google Scholar]
- Rak-Mardyla A. (2013). Ghrelin role in hypothalamus-pituitary-ovarian axis. J. Physiol. Pharmacol. 64, 695–704. [PubMed] [Google Scholar]
- Raoof R., Esmaeili-Mahani S., Abbasnejad M., Raoof M., Sheibani V., Kooshki R., et al. (2015). Changes in hippocampal orexin 1 receptor expression involved in tooth pain-induced learning and memory impairment in rats. Neuropeptides 50, 9–16. 10.1016/j.npep.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Rat D., Schmitt U., Tippmann F., Dewachter I., Theunis C., Wieczerzak E., et al. (2011). Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 25, 3208–3218. 10.1096/fj.10-180133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D., Kiss P., Lubics A., Tamas A. (2011). Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 17, 962–972. 10.2174/138161211795589355 [DOI] [PubMed] [Google Scholar]
- Roh J. H., Jiang H., Finn M. B., Stewart F. R., Mahan T. E., Cirrito J. R., et al. (2014). Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J. Exp. Med. 211, 2487–2496. 10.1084/jem.20141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. B., Crews L., Rockenstein E., Adame A., Mante M., Hersh L. B., et al. (2009). Neuropeptide Y fragments derived from neprilysin processing are neuroprotective in a transgenic model of Alzheimer's disease. J. Neurosci. 29, 1115–1125. 10.1523/jneurosci.4220-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler N., Wichart I., Jellinger K. A. (2001). Ex vivo lumbar and post mortem ventricular cerebrospinal fluid substance P-immunoreactivity in Alzheimer's disease patients. Neurosci. Lett. 299, 117–120. 10.1016/S0304-3940(01)01514-2 [DOI] [PubMed] [Google Scholar]
- Rowe W. B., Kar S., Meaney M. J., Quirion R. (2006). Neurotensin receptor levels as a function of brain aging and cognitive performance in the Morris water maze task in the rat. Peptides 27, 2415–2423. 10.1016/j.peptides.2006.03.036 [DOI] [PubMed] [Google Scholar]
- Russino D., McDonald E., Hejazi L., Hanson G. R., Jones C. E. (2013). The tachykinin peptide neurokinin B binds copper forming an unusual [CuII(NKB)2] complex and inhibits copper uptake into 1321N1 astrocytoma cells. ACS Chem. Neurosci. 4, 1371–1381. 10.1021/cn4000988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos V. V., Stark R., Rial D., Silva H. B., Bayliss J. A., Lemus M. B., et al. (2017). Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1-40) administration in mice. J. Neuroendocrinol. 29,1–11. 10.1111/jne.12476 [DOI] [PubMed] [Google Scholar]
- Severini C., Petrella C., Calissano P. (2016). Substance P and Alzheimer's disease: emerging novel roles. Curr. Alzheimer Res. 13, 964–972. 10.2174/1567205013666160401114039 [DOI] [PubMed] [Google Scholar]
- Shi L., Du X., Jiang H., Xie J. (2017). Ghrelin and neurodegenerative disorders-a review. Mol. Neurobiol. 54, 1144–1155. 10.1007/s12035-016-9729-1 [DOI] [PubMed] [Google Scholar]
- Shi L. M., Jiang H., Xie J. X. (2014). Neuroprotective effects of ghrelin in Parkinsons disease and Alzheimer's disease. Sheng Li Ke Xue Jin Zhan. 45, 140–144. [PubMed] [Google Scholar]
- Shibata N., Ohnuma T., Kuerban B., Komatsu M., Arai H. (2011). Genetic association between ghrelin polymorphisms and Alzheimer's disease in a Japanese population. Dement. Geriatr. Cogn. Disord. 32, 178–181. 10.1159/000333075 [DOI] [PubMed] [Google Scholar]
- Shieh P. C., Tsao C. W., Li J. S., Wu H. T., Wen Y. J., Kou D. H., et al. (2008). Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci. Lett. 434, 1–5. 10.1016/j.neulet.2007.12.032 [DOI] [PubMed] [Google Scholar]
- Skup M., Zhu H., Wang Y., Giovanello K. S., Lin J. A., Shen D., et al. (2011). Sex differences in grey matter atrophy patterns among AD and aMCI patients: results from ADNI. Neuroimage 56, 890–906. 10.1016/j.neuroimage.2011.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B., Potkar R., Metcalf J., Thrin I., Adame A., Rockenstein E., et al. (2016). Systemic central nervous system (CNS)-targeted delivery of neuropeptide Y (NPY) reduces neurodegeneration and increases neural precursor cell proliferation in a mouse model of Alzheimer disease. J. Biol. Chem. 291, 1905–1920. 10.1074/jbc.M115.678185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel M. B., Benitez A., Updegraff J., Potter V., Alexander T., Glickman E., et al. (2010). Serum ghrelin is inversely associated with cognitive function in a sample of non-demented elderly. Psychiatry Clin. Neurosci. 64, 608–611. 10.1111/j.1440-1819.2010.02145.x [DOI] [PubMed] [Google Scholar]
- Stoyanova I. I. (2014). Ghrelin: a link between ageing, metabolism and neurodegenerative disorders. Neurobiol. Dis. 72(Pt A), 72–83. 10.1016/j.nbd.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Struble R. G., Powers R. E., Casanova M. F., Kitt C. A., Brown E. C., Price D. L. (1987). Neuropeptidergic systems in plaques of Alzheimer's disease. J. Neuropathol. Exp. Neurol. 46, 567–584. 10.1097/00005072-198709000-00006 [DOI] [PubMed] [Google Scholar]
- Tiraboschi P., Hansen L. A., Thal L. J., Corey-Bloom J. (2004). The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology 62, 1984–1989. 10.1212/01.wnl.0000129697.01779 [DOI] [PubMed] [Google Scholar]
- Vaudry D., Cottet-Rousselle C., Basille M., Falluel-Morel A., Fournier A., Vaudry H., et al. (2004). Pituitary adenylate cyclase-activating polypeptide inhibits caspase-3 activity but does not protect cerebellar granule neurons against beta-amyloid (25-35)-induced apoptosis. Regul. Pept. 123, 43–49. 10.1016/j.regpep.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Waters S. M., David T. P. (1995). Alterations of substance P metabolism and neuropeptidases in Alzheimer's disease. J. Gerontol. A. Biol. Sci. Med. Sci. 50, B315–B319. 10.1093/gerona/50a.5.b315 [DOI] [PubMed] [Google Scholar]
- Willis M., Hutter-Paier B., Wietzorrek G., Windisch M., Humpel C., Knaus H. G., et al. (2007). Localization and expression of substance P in transgenic mice overexpressing human APP751 with the London (V717I) and Swedish (K670M/N671L) mutations. Brain Res. 1143, 199–207. 10.1016/j.brainres.2007.01.080 [DOI] [PubMed] [Google Scholar]
- Witte A. V., Savli M., Holik A., Kasper S., Lanzenberger R. (2010). Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage 49, 1205–1212. 10.1016/j.neuroimage.2009.09.046 [DOI] [PubMed] [Google Scholar]
- Wu Z. L., Ciallella J. R., Flood D. G., O'Kane T. M., Bozyczko-Coyne D., Savage M. J. (2006). Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol. Aging 27, 377–386. 10.1016/j.neurobiolaging.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Cilz N. I., Kurada L., Hu B., Yang C., Wada E., et al. (2014). Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: beneficial actions in an Alzheimer's disease model. J. Neurosci. 34, 7027–7042. 10.1523/jneurosci.0408-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zou B., Xiong X., Pascual C., Xie J., Malik A., et al. (2013). Hypocretin/orexin neurons contribute to hippocampusdependent social memory and synaptic plasticity in mice. J. Neurosci. 33, 5275–5284. 10.1523/jneurosci.3200-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Dong H., Cilz N. I., Kurada L., Hu B., Wada E., et al. (2016). Neurotensinergic excitation of dentate gyrus granule cells via Gαq-coupled inhibition of TASK-3 channels. Cereb. Cortex 26, 977–990. 10.1093/cercor/bhu267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Dong H., Lei S. (2015). Neurotensinergic augmentation of glutamate release at the perforant path-granule cell synapse in rat dentate gyrus: Roles of L-Type Ca2+ channels, calmodulin and myosin light-chain kinase. Neuropharmacology 95, 252–260. 10.1016/j.neuropharm.2015.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]


