Abstract
High adoption rates of single-gene Bacillus thuringiensis (Bt) Cry1Ac soybean impose selection pressure for resistance in the soybean looper, Chrysodeixis includens, a major defoliator in soybean and cotton crops. To anticipate and characterize resistance profiles that can evolve, soybean looper larvae collected from field crops in Brazil in 2013 were selected for resistance to Cry1Ac. Using two methods of selection viz., chronic exposure to Cry1Ac cotton leaves and the seven-day larval exposure to purified Cry1Ac on the artificial diet, 31 and 127-fold resistance was obtained in 11 and 6 generations of selection, respectively. The resistance trait had realized heritability of 0.66 and 0.72, respectively, indicating that most of the phenotypic variation in Cry1Ac susceptibility of the soybean looper larvae was due to additive genetic variation. The Cry1Ac-selected populations showed positive cross-resistance to Cry1Ab (6.7–8.7 fold), likely because these Bt toxins have a very similar molecular structure. Importantly, the Cry1Ac-selected populations became more susceptible to Cry2Aa and Cry1Fa, showing negative cross-resistance (up to 6-fold, P < 0.05). These results indicate that Cry1Ac, Cry1Fa, and Cry2A are compatible in a multi-toxin approach to minimize the risk of rapid adaptation of the soybean looper to Bt toxins.
Introduction
Plant biotechnology has helped in the development of pest-resistant cultivars, which are increasingly important in the face of growing pressure to reduce broadcast pesticides typically used in crop protection1. Transgenic crops producing Bacillus thuringiensis (Bt) insecticidal toxins have been extensively used as a major tool for controlling insect pests worldwide2–4. In two decades of use, these crops have been reported to provide substantial agronomic, environmental, economic, and societal benefits5,6. Nevertheless, the sustainable use of these crops is threatened by the rapid evolution of resistance7,8.
Historically, Bt cultivars of corn (Zea mays) and cotton (Gossypium sp.) were commercially introduced prior to those of soybean (Glycine max)2,9. While Bt cotton and corn expressing more than one toxin were launched in the 2000s4, single-trait Bt soybean was introduced a decade later10–12, although new multi-trait Bt soybean are in the process of commercial release13,14. The single-toxin Bt soybean produces Cry1Ac, which is also produced in some Bt cotton technologies, such as Bollgard (Cry1Ac) and Bollgard II (Cry1Ac + Cry2Ab), and all are simultaneously deployed in some countries9,11,15. The Bt Cry1Ac soybeans were first commercialized in 2013 in South America12, which accounts for more than half of the global production16. Soybean cultivars carrying this Bt technology have been extensively adopted in Brazil, Argentina, Paraguay, and Uruguay2, targeting the main Lepidoptera pests like the velvetbean caterpillar, Anticarsia gemmatalis (Erebidae) and the soybean looper, Chrysodeixis includens (Noctuidae), in spite of not reaching the high-dose condition for the latter10.
The soybean looper is a notorious pest not only in soybean but also in cotton, where a relatively high number of larvae can survive and reproduce on Bt cotton foliage producing Cry1Ac17,18. In such a less-than-high-dose scenario, even though some Bt susceptible larvae may recover from sub-lethal exposure to the toxin and transmit susceptibility alleles to subsequent generations19, some carriers of resistance alleles may pass the resistance allele to the next generation and increase the rate of Bt resistance evolution faster than expected from current resistance management modeling20,21. Bt soybean grown predominantly over 60% of 39.1 million ha and Bt cotton occupying 70% of 1.2 million ha2, both producing the same Cry1Ac toxin, exert tremendous selection pressure on common pests to evolve resistance. This is particularly the case for the soybean looper, which is relatively less susceptible to Cry1 toxins and biopesticides10,22,23. The risk of Bt resistance in the soybean looper is further increased because the larvae typically have sheltered feeding habits within the plant canopy, which is challenging for effective insecticidal sprays that may be needed for integrated pest/resistance management.
In order to manage pest adaptation (often referred to as insect resistance management) to Bt crops, the primary strategy has been to ensure that effective refuges of non-Bt host plants occur near Bt crops. Ideally, these crops must have toxicity that is high enough to render resistance recessive (i.e., to kill nearly all insects heterozygous for Bt resistance), although for some pests the low innate susceptibility may make it difficult to reach a high-dose condition21,24. Other approaches that can be used with refuges are “pyramids”, which are plants that produce two or more Bt toxins effective against the same pest21,25–28, and alternating with new toxins for which no resistance is reported24. Structurally distinct toxins can be effective for resistance management when pest populations are susceptible to all toxins deployed in the Bt crop24,28 and there is negligible cross-resistance among them in order to meet the redundant-killing principle21,26. Also appropriate would be if individuals that have alleles conferring resistance to a single-toxin Bt crop are hypersusceptible to another toxin, in which case there would be negative cross-resistance between the two toxins29.
Resistant populations of insects targeted by Bt crops provide an opportunity to assess the conditions that favor the success of resistance management strategies, including the genetic basis of the resistance30,31, cross-resistance7,32,33, fitness costs34, and frequency of resistance alleles in the field35,36. Selection experiments that generate resistance similar to field resistance31,37,38 are useful in assessing the risk of resistance evolution39–43. Such efforts help to elucidate the mode of action of Bt toxins, assess the risk of field-level resistance and develop resistance management strategies30,44. Here, we report on the ability of the soybean looper to respond to selection for resistance to Cry1Ac in the laboratory and its respective realized heritability. Importantly, we also report negative cross resistance in the Cry1Ac selected soybean looper to Cry2Aa and Cry1F, which may have important implications for resistance management in Bt crops, especially soybean.
Results
Response to selection for Cry1Ac resistance
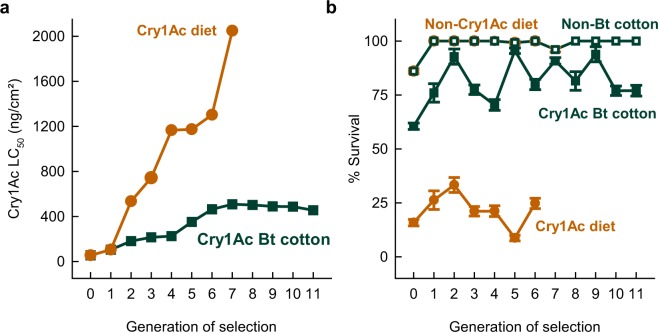
Estimates of median lethal concentration (LC50) values and survival rates over the generations of selection (Fig. 1) show that soybean looper larvae increased the resistance to Cry1Ac when exposed to the Bt cotton leaf tissues throughout larval development, or to the toxin on the surface of the artificial diet for seven days of exposure. Selection with Cry1Ac overlaid on the surface of the diet resulted in higher resistance in each generation of selection (Fig. 1a), as it imposed a greater intensity of selection than did the Bt cotton leaf tissues producing Cry1Ac (Fig. 1b: mean selected proportion of 27% and 81%, respectively). In fact, with only two generations of selection using the purified toxin, the increase in the LC50 values was similar to that achieved in six generations of selection using the Bt cotton leaves (Fig. 1a).
Figure 1.
Response to selection for resistance to the Cry1Ac Bacillus thuringiensis (Bt) toxin in larvae of the soybean looper as affected by two methods of selection, namely chronic exposure to Cry1Ac cotton leaves and the seven-day larval exposure to purified Cry1Ac on the artificial diet. (a) Increase in the median lethal concentration (LC50) for larvae of the individuals selected in the previous generation. (b) Larval survival rates (±SE) for the selected individuals (i.e., survivors on Bt Cry1Ac cotton or diet overlaid with Cry1Ac) as compared to those reared on plain food (i.e., non-Bt isoline cotton or artificial diet).
Realized heritability
Realized heritability (h2) estimates were 0.66 and 0.72 for the respective Cry1Ac and Bt-cotton selected populations, and the corresponding number of generations needed for a 10-fold increase in LC50 values were ca 3 and 8 generations (Table 1). After eleven generations of selection with leaf tissues of Bt cotton, Cry1Ac LC50 values increased from 19 to 503 ng/cm2 and the slope of the probit line increased from 1.04 to 2.34, while after six generations of selection with Cry1Ac, toxin LC50 values increased from 19 to 2048 ng/cm2 and the slope increased from 1.04 to 2.11 (Table 1).
Table 1.
Estimation of realized heritability (h2) and number of generations to a 10-fold increase in resistance to the Cry1Ac Bacillus thuringiensis toxin in two populations of soybean looper selected using chronic exposure to Cry1Ac Bt cotton leaves (BG1-Sel) or seven-day larval exposure to purified Cry1Ac on the artificial diet (1Ac-Sel).
| Population of soybean looper | Number of gene- rations selected | Estimate of mean response per generation | Estimate of mean selection differential per generation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial LC50 (log) | Final LC50 (log) | Response to selection (R) | Mean Percent survival after selection | Intensity of selectiona | Initial slope | Final slope | Phenotypic standard deviation | Selection differential (S) | Realized heritability (h²) | Number of generations to a 10-fold increase in resistance | ||
| BG1-Sel | 11 | 1.283 | 2.701 | 0.129 | 81 | 0.33 | 1.04 | 2.49 | 0.59 | 0.22 | 0.66 | 8 |
| 1Ac-Sel | 6 | 1.283 | 3.311 | 0.338 | 27 | 1.23 | 1.04 | 2.38 | 0.59 | 0.82 | 0.72 | 3 |
Level of resistance and cross-resistance
As indicated by the ratios between the LC50 values for the selected and unselected larvae (Table 2), selection with Cry1Ac cotton leaves produced a 31-fold resistance in 11 generations of selection, while exposure to purified Cry1Ac produced a 127-fold resistance to Cry1Ac in only 6 generations of selection (Table 2). Cry1Ab LC50 values increased 7–9 fold in both selected insect populations, indicating positive cross-resistance between Cry1Ab and Cry1Ac in the soybean looper (Table 2). In contrast, for larvae selected with Cry1Ac cotton leaves, there was a significant reduction in Cry2Aa and Cry1Fa LC50 values of 4 and 6-fold, respectively (P < 0.05, based on the likelihood ratio test and non-overlapping fiducial limits); likewise, there was a 6-fold decrease in Cry2Aa LC50 value for the population selected with purified Cry1Ac toxin. These results indicate negative cross-resistance between Cry1Ac and Cry2Aa or Cry1Fa in the soybean looper, even if a conservartive criterion is used (i.e., lack of overlap between fiducial limits of the LC50 values for unselected and selected strains) (Table 2).
Table 2.
Resistance and cross-resistance to Bacillus thuringiensis (Bt) toxins in two soybean looper populations, one selected using chronic exposure to Cry1Ac Bt cotton leaves (BG1-Sel) and the other using seven-day larval exposure to purified Cry1Ac on the artificial diet (1Ac-Sel). The bioassays were conducted during the last generation of selection.
| Toxin | Population | Slope ± SE | LC50 (95% fiducial limits)a ng/cm² | Resistance ratiob (95% confidence limits) | χ 2 c | P | Nd |
|---|---|---|---|---|---|---|---|
| Cry1Ac | Bt-Unsel | 1.85 ± 0.14 | 16.1 (13.22–19.71) | 1 | 2.66 | 0.752 | 545 |
| BG1-Sel | 2.34 ± 0.27 | 502.82 (398.51–636.50) | 31.10 (22.82–42.35) | 1.27 | 0.937 | 236 | |
| 1Ac-Sel | 2.11 ± 0.23 | 2048.4 (1567.8–2893.4) | 126.67 (94.10–170.51) | 5.43 | 0.365 | 437 | |
| Cry1Ab | Bt-Unsel | 3.39 ± 0.64 | 86.40 (64.25–107.27) | 1 | 2.21 | 0.697 | 230 |
| BG1-Sel | 1.25 ± 0.16 | 576.90 (358.90–1763.00) | 6.70 (4.11–10.83) | 9.02 | 0.108 | 359 | |
| 1Ac-Sel | 0.76 ± 0.10 | 748.00 (304.96–3863.40) | 8.66 (3.95–18.99) | 6.67 | 0.246 | 301 | |
| Cry1Fa | Bt-Unsel | 5.43 ± 0.81 | 117. (101.25–135.88) | 1 | 2.24 | 0.691 | 256 |
| BG1-Sel | 2.77 ± 0.30 | 22.12 (18.25–25.84) | 0.20 (0.16–0.23) | 0.97 | 0.965 | 539 | |
| Cry2Aa | Bt-Unsel | 4.03 ± 0.68 | 24.30 (19.51–30.60) | 1 | 0.23 | 0.998 | 248 |
| BG1-Sel | 3.92 ± 0.54 | 6.13 (5.15–7.32) | 0.25 (0.19–0.33) | 3.28 | 0.657 | 252 | |
| 1Ac-Sel | 2.52 ± 0.31 | 3.99 (2.50–5.80) | 0.17 (0.12–0.28) | 10.36 | 0.065 | 443 |
aLC50, (Lethal Concentration causing 50% mortality, in ng/cm2) was estimated by probit analysis using Polo-Plus79.
bResistance ratio = LC50 selected population/LC50 for control population, indicates the level of resistance or cross-resistance, that is, how many times the selected population is less susceptible than the control, unselected population to a particular toxin; values in parentheses represent the 95% confidence limits for the resistance ratio79.
cChi-square statistic with its P value for df = 5.
dNumber of insects tested in the bioassays.
Survival assays on soybean leaf tissue
Despite the 127-fold resistance to Cry1Ac in the 1Ac-Sel soybean looper population, their larvae did not survive on Cry1Ac soybean foliage (Fig. 2). The was no significant difference in larval survival of the three insect populations on either non-Bt or Bt soybean (F2, 110 = 1.37, P = 0.32; F2, 109 = 0.01, P = 0.99). However, overall insect survival differed significantly between Bt and non-Bt soybean (F1, 110 = 13545.8, P < 0.01) (Fig. 2). Most larvae of any selected or unselected populations died in 3 days on Bt soybean. On non-Bt soybean, the survival rates were similar for both selected and unselected control populations (Fig. 2), indicating no fitness cost of resistance to early-instar larval survival.
Figure 2.
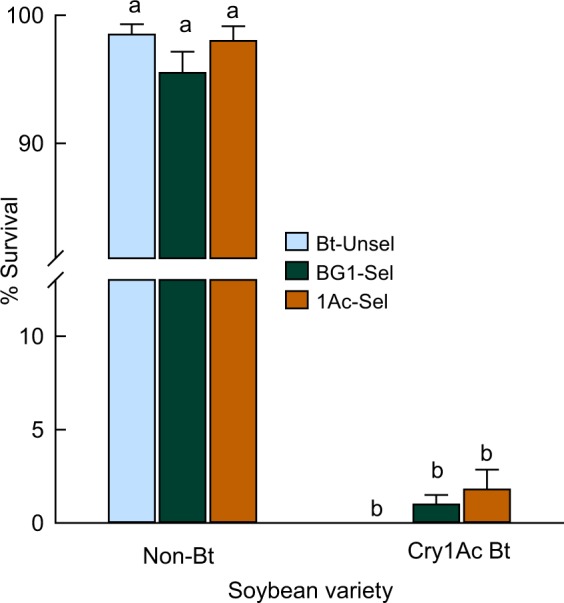
Testing whether soybean looper larvae from the Cry1Ac-selected (BG1-Sel and 1Ac-Sel) and unselected populations (Bt-Unsel) survive on foliage of Cry1Ac Bt soybean. Shown are mean (±SE) 3-day survival rates for neonates (n = 200) released on foliage excised from Cry1Ac-producing and near-isoline soybean plants. Columns with same letter are not significantly different (α = 0.05, Fisher’s LSD procedure after ANOVA).
Discussion
Field-derived larvae of the soybean looper responded to selection for resistance to Cry1Ac in the laboratory, generating up to 127-fold resistance to the toxin, which is consistent with other selection experiments using purified Bt toxin30,45–48 and plant leaf tissue producing Bt toxin7,31,38,39,49. These results indicate that either method of selection (i.e., leaf tissues of Bt plants or larval diet containing Bt toxin) can be used to produce resistant insect populations, which are important tools for anticipating the risk assessment of resistance development in field settings7,30,47.
Whereas using purified Bt toxin on the larval diet may allow better control of the intensity of selection and avoid confounding effects of plant allelochemicals, using the Bt plant foliage may better represent the larval exposure under field settings50, selecting for a more realistic resistance profile. Gossypol interaction with Cry1Ac in Bt cotton may have affected the ability of soybean looper to evolve resistance as fast as with pure Cry1Ac51. In addition, Cry1Ac protein levels may have varied in the cotton foliage52,53, even though we meticulously grew cotton plants in optimized soil conditions and standardized the growth stage and the leaf age used in the experiment. To our knowledge, this is the first report of selection of Bt resistant populations of soybean looper. Importantly, despite the 127-fold resistance to Cry1Ac in the 1Ac-Sel soybean looper population, their larvae did not survive on Cry1Ac soybean foliage. The low survival of 1Ac-Sel larvae on Cry1Ac soybean foliage may be due to the relatively high titer of Bt toxin10,54,55 or its synergism with soybean allelochemicals56.
The higher level of resistance reached by the 1Ac-Sel population indicates that the rate of resistance evolution to Cry1Ac in soybean looper is linked to the intensity of the selection57. The realized heritability (h2) values for Cry1Ac resistance were quite high (0.66 and 0.72), which means that most of the phenotypic variation for Cry1Ac resistance in the selected soybean looper populations is due to genetic variation50, and indicates that the soybean looper has high potential to develop resistance to Cry1Ac. The higher heritability value for the selection using Cry1Ac on artificial diet is indicative of a greater contribution of genes as compared to that for selection using Bt cotton leaves. The latter reflects that low concentration of Cry1Ac and presence of allelochemicals. The slow resistance evolution in the soybean looper using Bt cotton may reflect a likely scenario for field-evolved resistance in the near future in a soybean agroecosystem, particularly if Bt soybean cultivars carry natural resistance to the looper and/or expresses high Bt toxin content.
Despite trying to obtain resistant strains representative of those found in the field by mimicking field conditions in our laboratory selection experiment50,57 we cannot guarantee that the resistance is exactly like that which may evolve in the field58, because the conditions will differ under field settings. Nevertheless, laboratory selection experiments have often produced resistance similar to field-evolved resistance8,31,34,36,37,59. Our results indicate that the pre-existing Cry1Ac resistance alleles12 may not be so rare in the field (i.e., >0.001), such that the risk of populations of soybean looper to evolve field resistance to Cry1Ac soybean should be further investigated.
Selection for Cry1Ac resistance resulted in different levels of cross-resistance to other Cry toxins regardless of the method of toxin exposure. Importantly, our estimated LC50 values are comparable to those reported for soybean loopers in Brazil60 and the United States61. This latter study also reported that Cry1Ac and Cry1Fa toxins have specific binding sites on the midgut brush border membrane vesicles of soybean looper larvae, although the toxins share some, but not all, binding sites in the insect midgut61. Here, Cry1Ac and Cry1Ab showed low but significant (i.e., 6.7–8.7 fold) positive cross-resistance, which is not surprising given the likely resistance mechanism62 and the similarity in the amino acid sequences of domains II and III (i.e., 99% and 51%, respectively)32. Despite a paucity of published reports on competition binding assays between Cry1Ac and Cry1Ab in the soybean looper, these toxins do share binding sites63,64 or show positive cross-resistance33,65 in other Lepidoptera.
In addition, there was no positive cross-resistance between Cry1Ac and Cry1Fa or Cry2Aa in the two selected soybean looper populations, supporting an absence of common binding sites for these pairs of toxins on brush border membrane of the midgut based on competitive and specific binding studies61. Interestingly, our data indicate that the resistance to Cry1Ac negatively correlates with resistance to Cry1Fa or Cry2Aa (i.e., the Cry1Ac-selected soybean loopers became more susceptible to Cry1Fa and Cry2Aa). This is one of the few empirical evidences for negative cross-resistance or negatively correlated resistance involving Bt toxins (reviewed in29), deserving investigation of its genetic basis (i.e., if caused by a single or different genes29), which in this case may encode altered receptor proteins interacting with these Bt toxins in their intoxication route66. In practical terms, negative cross-resistance may be exploited for resistance management29; for example, Cry1F and Cry2A toxins may be used to preferentially kill soybean loopers that are resistant to Cry1Ac.
Although some variation exists in the patterns of cross-resistance between Cry1A and Cry2A47,67–69, these toxins seem to be compatible for resistance management in most pest species. Here, the clear absence of positive cross-resistance to Cry1Fa and Cry2Aa provides empirical evidence that these toxins do not share binding sites in receptor proteins61,70. These findings are relevant because Cry2A or Cry1F toxins are pyramided with Cry1Ac in second-generation Bt cotton71,72 and soybean13,14 in the Americas. Most importantly, our results indicate that Cry2A and/or Cry1F are compatible with Cry1Ac in a multi-toxin approach for resistance management of soybean looper. This is critical in the stewardship and optimal management strategy24 of new Bt soybean varieties13,14 that are to be introduced in the market for controlling soybean looper and other lepidopteran pests.
Material and Methods
Insect collection and rearing
Field populations of soybean looper (ca. 500 third to fifth instar larvae) were collected from non-Bt soybean (G. max) and dry beans (Phaseolus vulgaris) on farms of the Federal University of Viçosa, in Viçosa and Coimbra counties, Minas Gerais state, Brazil, in April 2014. The larvae were brought to the laboratory and reared individually on leaves of the respective host crops (i.e., non-Bt soybean or dry beans). Two to three batches of pupae (80♂ + 80♀) were placed in two polyvinylchloride cages, 20 cm diameter × 30 cm height, lined internally with sulfite paper as substrate for oviposition. Adults were fed a 10% honey solution in distilled water, and eggs were collected daily and stored in an incubator until hatching. Neonates were reared at 27 ± 1 °C, 70 ± 10% r.h. with 16:8 (light:dark) cycle on artificial diet73. In the F1 generation, the larvae were divided in three subpopulations; one was selected with purified Cry1Ac toxin (1Ac-Sel), another was selected with Cry1Ac cotton leaf tissue (BG1-Sel), and a third subpopulation was left unselected (Bt-Unsel) and maintained on artificial diet using methods described above, keeping the population size at approximately 200 adults per generation.
Source of cotton for selection and soybean for leaf-bioassays
Transgenic plants producing Cry1Ac used were Bollgard cotton (event MON 531, Monsanto do Brasil, São Paulo, SP) and Intacta soybean (event MON 87701 x MON 89788, Monsanto do Brasil, São Paulo, SP). Isoline or near isoline non-Bt cotton (Delta OPAL, Monsanto do Brasil, São Paulo, SP) and non-Bt soybean (MSOY 8866, Monsanto do Brasil, São Paulo, SP) were used as controls. To obtain appropriate leaves for the bioassays, cotton and soybean plants were cultivated in the greenhouse following standard cultivation practices74,75. Cotton was sown every three months in 15-L pots with substrate composed of 3 parts of soil, 2 parts cattle manure, and 2 parts of sand to obtain plants with normal levels of Bt protein expression52,53,76. The plants were irrigated twice or three times a day depending on soil moisture conditions, and leaves were collected from cotton plants 45–50 d after emergence. Soybean plants were field-grown using cultivation practices as recommended for the crop, and leaves were excised when plants were in the R2-R4 growth stages74. Soil fertilization was as recommended for cotton75 or soybean crops74. The plants were inspected weekly for mechanical pest control or disposal of infested plants when needed. Immunodetection assays using ImmunoStrip STX 74500 kit (Agdia Inc., Elkhart, IN) were used according to the manufacturer’s instructions to confirm the presence or absence of the Cry1Ac trait in the Bt or non-Bt isoline plants from which foliage was excised.
Bt toxins and bioassays
The Cry1A toxins (Cry1Ab, Cry1Ac and Cry1Fa) and Cry2Aa protoxin used in the experiments were obtained from Dr. Marianne P. Carey (Case Western Reserve University, OH). The proteins were purified on HPLC, shipped as lyophilized powder, and stored at −80 °C until use, when fresh dilutions were prepared as follows. Cry1 toxins were solubilized in 100 mM Na2CO3 buffer (pH 10.3, containing 10 mM DTT) and Cry2A protoxin in 50 mM Na2CO3 buffer (pH 12.1, containing 5 mM EDTA and 10 mM EGTA) to produce the stock concentration (1 mg/ml) for each Bt toxin. These were further diluted with 0.1% Triton-X 100 to obtain the appropriate concentrations used in the bioassays.
All bioassays used in the selection experiment and cross-resistance study were repeated twice and included at least seven different concentrations of purified Cry toxin plus a control (i.e., 0.1% Triton-X 100 only) applied to the diet surface77. A single neonate (<24 h after hatching) was placed in each well of a 128 well-tray (CD International, Pitman, NJ), and held for seven days at 27 ± 1 °C, 24 h scotophase, and 80% r.h. until assessment of larval mortality45,77. Larvae of the Cry1Ac-selected population were not bioassayed against purified Cry1Fa as they were low in availability when the assays were conducted.
Selection experiment using purified Cry1Ac toxin
This experiment was conducted in parallel with that using Bt cotton leaf sections. Methods were adapted from Gould et al.47 and Pereira et al.45. Initial bioassays to determine the population susceptibility to Cry1Ac were conducted as previously described, using the same toxin source that was later used in the selection experiment. Bioassays were done using graded Cry1Ac concentrations applied on the surface of artificial diet77. Larval mortality was recorded after seven days of exposure and analyzed by probit regression to determine the concentration that kills 90% of the larvae (LC90), which was used as the concentration applied on the diet surface for the next generation of selection. The following Cry1Ac concentrations were used from the first to sixth generation of selection, respectively: 215, 535, 1568, 1868, 3004, and 4058 ng/cm2 (Table S1). At least 2,500 neonates were exposed to Cry1Ac every generation of selection, and after seven days of feeding on the toxin-treated diet, the larvae with size (i.e., weight) similar to those of the control were selected. These larvae were transferred to the untreated artificial diet and reared until pupation. The adults were held in mating cages as previously described. A portion of the offspring from the parents selected in the previous generation was bioassayed as above to estimate the gain by selection. This process was repeated during six generations of selection.
Selection experiment using Cry1Ac cotton foliage
A chronic selection experiment using Cry1Ac cotton leaves throughout larval development was started in the first generation of the field-collected laboratory colony in 2014. In each generation of selection, we transferred 160 batches of 10 neonates to 16-well-plastic trays (Advento do Brasil, Diadema, São Paulo) (10 larvae/well), each well containing a cotton leaf section. After three days, we assessed the number of survivors, and they were transferred to new 16-well trays (3 larvae/well). From then on, cotton leaves were replaced every three days until pupation, when the insects were transferred to mating cages and held as previously described. The selection experiment took place during 11 generations. Soybean looper larvae were also reared in parallel on non-Bt cotton leaves to estimate natural mortality in the absence of selection. In each generation of selection, the gain by selection (i.e., increase in resistance) was estimated using bioassays with purified Cry1Ac protein as described above.
Leaf tissue assay using Cry1Ac soybean
We tested the hypothesis that the Cry1Ac resistance developed in the laboratory-selected soybean looper populations would be high enough to allow for survival on leaf tissues of the Cry1Ac Bt soybean. In a completely randomized experiment with 20 replications, we exposed larvae of the two selected and the control populations to foliage of Bt soybean (Intacta) and its non-Bt near isoline (MSOY8866, Monsanto do Brasil, São Paulo, SP). Soybean foliage was excised in the R2-R4 growth stages, quickly placed in buckets with water, brought to the laboratory, thoroughly rinsed with distilled water, and placed on paper towels to dry for 15 min at room temperature. The Cry1Ac or control soybean foliage was cut into 3-cm2 pieces and placed in 50-ml plastic cups. Batches of 10 neonates (<1 day old) were transferred to each cup and maintained as described previously. After three days, we recorded the number of survivors and calculated the survival rate for each experimental unit (i.e., each cup, total n = 120). The total number of larvae tested in the bioassay was 1200 (400 per insect population).
Realized heritability
Following Falconer and Mackay78 and Tabashnik50, we calculated the realized heritability (h2) as h2 = Response to selection ÷ Selection differential. In this equation, the response to selection (R) was calculated as: R = [Log (final LC50)−Log (initial LC50)]/n, where the final LC50 is the LC50 of the population after six generation of selection with purified toxin or Bt cotton foliage, respectively; the initial LC50 is the LC50 of the base parental population before selection, and n is the number of generations selected with Bt cotton or the purified toxin. The selection differential was calculated as follows: Selection differential = i × σp, where i is intensity of selection calculated according to Falconer and Mackay78, and σp is the phenotypic standard deviation, which was calculated as follows: σp = [0.5 × (initial slope + final slope)]−1. Finally, the number of generations required for a 10-fold increase in LC50 (G) was calculated as: G = 1/R.
Statistical analyses
The probit model was fit to bioassay data using Polo-Plus software79 to estimate the concentration causing 50% mortality (LC50) and their 95% fiducial limits as well as the slope of the concentration-response lines and their standard errors. The data were adjusted for natural mortality relative to controls when needed. Lethal concentration ratios (i.e., resistance or cross-resistance ratios) and their respective 95% confidence limits79 were determined using the unselected control population as reference for comparison, and considered significantly different (P < 0.05) when they did not include the value one. The significance of cross-resistance between toxins was also assessed by the failure of 95% fiducial limits at LC50 estimates to overlap, which is quite conservative79,80. The data from leaf tissue assays were analyzed using a two-way analysis of variance; the two main effects were insect population and plant phenotype (Bt or non-Bt soybean), both considered fixed factors. Means were separated using a comparison wise error rate (α) of 0.0581.
Electronic supplementary material
Acknowledgements
We appreciate the financial support provided by the National Council of Scientific and Technological Development (CNPq), the CAPES Foundation (Brazilian Ministry of Education), and the Minas Gerais State Foundation for Research Aid (FAPEMIG). We thank Dr. Thomas E. Hunt for reviewing our manuscript for clarity and readability as well as the undergraduate research assistants that helped with insect rearing, plant cultivation, and general lab maintenance. The thoughtful comments and suggestions provided by three anonymous reviewers were greatly appreciated and helped improve the manuscript.
Author Contributions
E.J.G.P. and N.R.-S. designed the study. A.F.C., D.F.O., A.F.T. and O.F.S.-A. performed the experiments and assisted with insect rearing and data input in spreadsheets. N.R.-S. and E.J.G.P. analyzed data and wrote the manuscript. M.C.P. provided valuable insights and resources for the experiments and assisted with manuscript preparation. All authors have read and approved the manuscript for publication.
Availability of Materials and Data
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
E.J.G.P. is coauthor of a patent application on combinations of Bt toxins for resistance management, “Combinations of Cry1Ab and Cry1Fa as an insect resistance management tool” (patent application publication number US20070006340). Monsanto, Pioneer, Dow AgroSciences, Syngenta and Bayer CropScience did not provide funding to support this work, but may be affected financially by publication of this paper and some of them have funded other work by E.J.G.P. The other authors declare no potential conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35965-5.
References
- 1.Johnson, S. N., Karley, A. J., Gregory, P. J. & Brennan, R. M. Crop traits for defense against pests and disease: durability, breakdown and future prospects. Front. Plant Sci. 8 (2017). [DOI] [PMC free article] [PubMed]
- 2.ISAAA Global Status of Commercialized Biotech/GM Crops in 2017: Biotech Crop Adoption Surges as Economic Benefits Accumulate in 22 Years. ISAAA Brief No. 53. (ISAAA, Ithaca, NY; 2017).
- 3.Schnepf E, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanahuja G, Banakar R, Twyman RM, Capell T, Christou P. Bacillus thuringiensis: a century of research, development and commercial applications. Plant Biotech. J. 2011;9:283–300. doi: 10.1111/j.1467-7652.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 5.Klumper, W. & Qaim, M. A meta-analysis of the impacts of genetically modified crops. PLoS ONE9 (2014). [DOI] [PMC free article] [PubMed]
- 6.National Academies of Sciences Engineering and Medicine (ed.) Genetically Engineered Crops: Experiences and Prospects. (National Academies Press, Washington; 2016). [PubMed]
- 7.Santos-Amaya, O. F. et al. Resistance to dual-gene Bt maize in Spodoptera frugiperda: selection, inheritance, and cross-resistance to other transgenic events. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed]
- 8.Tabashnik BE, Carrière Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotech. 2017;35:926–935. doi: 10.1038/nbt.3974. [DOI] [PubMed] [Google Scholar]
- 9.Brookes, G. & Barfoot, P., Edn. 2018 204 (PG Economics Ltd., Dorchester, United Kingdom; 2018).
- 10.Bernardi O, et al. Assessment of the high‐dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2012;68:1083–1091. doi: 10.1002/ps.3271. [DOI] [PubMed] [Google Scholar]
- 11.Crop Biotech Update (ISAAA, 2010).
- 12.Yano SAC, et al. High susceptibility and low resistance allele frequency of Chrysodeixis includens (Lepidoptera: Noctuidae) field populations to Cry1Ac in Brazil. Pest Manag. Sci. 2016;72:1578–1584. doi: 10.1002/ps.4191. [DOI] [PubMed] [Google Scholar]
- 13.Marques LH, et al. Field evaluation of soybean transgenic event DAS-81419-2 expressing Cry1F and Cry1Ac proteins for the control of secondary lepidopteran pests in Brazil. Crop Prot. 2017;96:109–115. doi: 10.1016/j.cropro.2017.02.014. [DOI] [Google Scholar]
- 14.Marques LH, et al. Efficacy of soybean’s event DAS-81419-2 expressing Cry1F and Cry1Ac to manage key tropical lepidopteran pests under field conditions in Brazil. J. Econ. Entomol. 2016;109:1922–1928. doi: 10.1093/jee/tow153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CTNBio, Vol. 2017, Edn. 01/23/2017 Summary of GM crops deregulated in Brazil (Ministério da Ciência, Tecnologia, Inovações e Comunicação, Brasilia; 2017).
- 16.Chang, W. -S., Lee, H. -I. & Hungria, M. In Principles of Plant-Microbe Interactions. (ed. B. Lugtenberg) 393–400 (Springer, New York; 2015).
- 17.Funichello M, Grigolli JF, de Souza BHS, Junior ALB, Busoli AC. Effect of transgenic and non-transgenic cotton cultivars on the development and survival of Pseudoplusia includens (Walker) (Lepidoptera: Noctuidae) Afr. J. Agric. Res. 2013;8:5424–5428. [Google Scholar]
- 18.Ashfaq M, Young S, McNew R. Larval mortality and development of Pseudoplusia includens (Lepidoptera: Noctuidae) reared on a transgenic Bacillus thuringiensis-cotton cultivar expressing CryIAc insecticidal protein. J. Econ. Entomol. 2001;94:1053–1058. doi: 10.1603/0022-0493-94.5.1053. [DOI] [PubMed] [Google Scholar]
- 19.Sousa, F. F. et al. Life-history traits of Spodoptera frugiperda populations exposed to low-dose Bt maize. PLoS ONE11 (2016). [DOI] [PMC free article] [PubMed]
- 20.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Ann. Rev. Entomol. 1994;39:47–79. doi: 10.1146/annurev.en.39.010194.000403. [DOI] [Google Scholar]
- 21.Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Ann. Rev. Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 22.Morales, L. et al. Suscetibilidade de Anticarsia gemmatalis Hübner e Chrysodeixis includens (Walker)(Lepidoptera: Noctuidae), a Bacillus thuringiensis (Berliner). Ann. Soc Entomol. Brasil (1995).
- 23.Mascarenhas R, Boethel D, Leonard B, Boyd M, Clemens C. Resistance monitoring to Bacillus thuringiensis insecticides for soybean loopers (Lepidoptera: Noctuidae) collected from soybean and transgenic Bt-cotton. J. Econ. Entomol. 1998;91:1044–1050. doi: 10.1093/jee/91.5.1044. [DOI] [Google Scholar]
- 24.Sudo M, Takahashi D, Andow DA, Suzuki Y, Yamanaka T. Optimal management strategy of insecticide resistance under various insect life histories: heterogeneous timing of selection and interpatch dispersal. Evol. App. 2018;11:271–283. doi: 10.1111/eva.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc. B. 1998;353:1777–1786. doi: 10.1098/rstb.1998.0330. [DOI] [Google Scholar]
- 26.Zhao JZ, et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotech. 2003;21:1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
- 27.Bates SL, Zhao J-Z, Roush RT, Shelton AM. Insect resistance management in GM crops: past, present and future. Nat. Biotech. 2005;23:57–62. doi: 10.1038/nbt1056. [DOI] [PubMed] [Google Scholar]
- 28.Carrière, Y., Fabrick, J. A. & Tabashnik, B. E. In Advances in Insect Control and Resistance Management 263–286 (Springer, 2016).
- 29.Pittendrigh, B. R. et al. In Insect Resistance Management, Edn. 2 373–401 (Academic Press, San Diego; 2014).
- 30.Pickett, B. R., Gulzar, A., Ferre, J. & Wright, D. J. Bacillus thuringiensis Vip3Aa toxin resistance in Heliothis virescens (Lepidoptera: Noctuidae). App. Environ. Microbiol. 83 (2017). [DOI] [PMC free article] [PubMed]
- 31.Santos-Amaya OF, et al. Genetic basis of Cry1F resistance in two Brazilian populations of fall armyworm. Spodoptera frugiperda. Crop Prot. 2016;81:154–162. doi: 10.1016/j.cropro.2015.12.014. [DOI] [Google Scholar]
- 32.Carrière Y, Crickmore N, Tabashnik BE. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotech. 2015;33:161–168. doi: 10.1038/nbt.3099. [DOI] [PubMed] [Google Scholar]
- 33.Siqueira HAA, Moellenbeck D, Spencer T, Siegfried BD. Cross-resistance of Cry1Ab-selected Ostrinia nubilalis (Lepidoptera: Crambidae) to Bacillus thuringiensis delta-endotoxins. J. Econ. Entomol. 2004;97:1049–1057. doi: 10.1093/jee/97.3.1049. [DOI] [PubMed] [Google Scholar]
- 34.Santos-Amaya OF, et al. Fitness costs and stability of Cry1Fa resistance in Brazilian populations of Spodoptera frugiperda. Pest Manag. Sci. 2017;73:35–43. doi: 10.1002/ps.4312. [DOI] [PubMed] [Google Scholar]
- 35.Gould F, et al. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proc. Nat. Acad. Sci. USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos-Amaya OF, et al. Magnitude and allele frequency of Cry1F resistance in field populations of the fall armyworm (Lepidoptera: Noctuidae) in Brazil. J. Econ. Entomol. 2017;110:1770–1778. doi: 10.1093/jee/tox146. [DOI] [PubMed] [Google Scholar]
- 37.Farias JR, et al. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014;64:150–158. doi: 10.1016/j.cropro.2014.06.019. [DOI] [Google Scholar]
- 38.Leite NA, et al. Rapid selection and characterization of Cry1F resistance in a Brazilian strain of fall armyworm. Entomol. Exp. App. 2016;158:236–247. doi: 10.1111/eea.12399. [DOI] [Google Scholar]
- 39.Meihls LN, et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc. Nat. Acad. Sci. USA. 2008;105:19177–19182. doi: 10.1073/pnas.0805565105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meihls LN, Higdon ML, Ellersieck M, Hibbard BE. Selection for resistance to mCry3A-expressing transgenic corn in western corn rootworm. J. Econ. Entomol. 2011;104:1045–1054. doi: 10.1603/EC10320. [DOI] [PubMed] [Google Scholar]
- 41.Oswald KJ, French BW, Nielson C, Bagley M. Selection for Cry3Bb1 resistance in a genetically diverse population of nondiapausing western corn rootworm (Coleoptera: Chrysomelidae) J. Econ. Entomol. 2011;104:1038–1044. doi: 10.1603/EC10312. [DOI] [PubMed] [Google Scholar]
- 42.Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE. 2011;6:e22629. doi: 10.1371/journal.pone.0022629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gassmann AJ. Field-evolved resistance to Bt maize by western corn rootworm: predictions from the laboratory and effects in the field. J. Invert. Pathol. 2012;110:287–293. doi: 10.1016/j.jip.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Devos Y, Meihls LN, Kiss J, Hibbard BE. Resistance evolution to the first generation of genetically modified Diabrotica-active Bt-maize events by western corn rootworm: management and monitoring considerations. Transg. Res. 2013;22:269–299. doi: 10.1007/s11248-012-9657-4. [DOI] [PubMed] [Google Scholar]
- 45.Pereira EJ, Lang BA, Storer NP, Siegfried BD. Selection for Cry1F resistance in the European corn borer and cross‐resistance to other Cry toxins. Entomol. Exp. App. 2008;126:115–121. doi: 10.1111/j.1570-7458.2007.00642.x. [DOI] [Google Scholar]
- 46.Gong Y, Wang C, Yang Y, Wu S, Wu Y. Characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in Plutella xylostella from China. J. Invert. Pathol. 2010;104:90–96. doi: 10.1016/j.jip.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 1995;88:1545–1559. doi: 10.1093/jee/88.6.1545. [DOI] [Google Scholar]
- 48.Liu LP, et al. Resistance to Bacillus thuringiensis toxin Cry2Ab and survival on single-toxin and pyramided cotton in cotton bollworm from China. Evol. App. 2017;10:170–179. doi: 10.1111/eva.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girón-Pérez K, Oliveira A, Teixeira A, Guedes R, Pereira E. Susceptibility of Brazilian populations of Diatraea saccharalis to Cry1Ab and response to selection for resistance. Crop Prot. 2014;62:124–128. doi: 10.1016/j.cropro.2014.04.004. [DOI] [Google Scholar]
- 50.Tabashnik BE. Resistance risk assessment: realized heritability of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae), tobacco budworm (Lepidoptera: Noctuidae), and Colorado potato beetle (Coleoptera: Chrysomelidae) J. Econ. Entomol. 1992;85:1551–1559. doi: 10.1093/jee/85.5.1551. [DOI] [Google Scholar]
- 51.Carrière Y, et al. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. J. Econ. Entomol. 2004;97:1710–1718. doi: 10.1603/0022-0493-97.5.1710. [DOI] [PubMed] [Google Scholar]
- 52.Greenplate JT. Quantification of Bacillus thuringiensis insect control protein Cry1Ac over time in Bollgard cotton fruit and terminals. J. Econ. Entomol. 1999;92:1377–1383. doi: 10.1093/jee/92.6.1377. [DOI] [Google Scholar]
- 53.Dong H, Li W. Variability of endotoxin expression in Bt transgenic cotton. J. Agron. Crop Sci. 2007;193:21–29. doi: 10.1111/j.1439-037X.2006.00240.x. [DOI] [Google Scholar]
- 54.Yu H, Li Y, Li X, Romeis J, Wu K. Expression of Cry1Ac in transgenic Bt soybean lines and their efficiency in controlling lepidopteran pests. Pest Manag. Sci. 2013;69:1326–1333. doi: 10.1002/ps.3508. [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Romeis J, Li Y, Li X, Wu K. Acquisition of Cry1Ac protein by non-target arthropods in Bt soybean fields. PLoS ONE. 2014;9:e103973. doi: 10.1371/journal.pone.0103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith CM, Fischer N. Chemical factors of an insect resistant soybean genotype affecting growth and survival of the soybean looper. Entomol. Exp. App. 1983;33:343–345. doi: 10.1111/j.1570-7458.1983.tb03278.x. [DOI] [Google Scholar]
- 57.Groeters FR, Tabashnik BE. Roles of selection intensity, major genes, and minor genes in evolution of insecticide resistance. J. Econ. Entomol. 2000;93:1580–1587. doi: 10.1603/0022-0493-93.6.1580. [DOI] [PubMed] [Google Scholar]
- 58.ffrench-Constant RH. The molecular genetics of insecticide resistance. Genetics. 2013;194:807–815. doi: 10.1534/genetics.112.141895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roush RT, McKenzie JA. Ecological genetics of insecticide and acaricide resistance. Ann. Rev. Entomol. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- 60.Crialesi-Legori PCB, et al. Interação de proteínas Cry1 e Vip3A de Bacillus thuringiensis para controle de lepidópteros-praga. Pesq. Agropec. Bras. 2014;49:79–87. doi: 10.1590/S0100-204X2014000200001. [DOI] [Google Scholar]
- 61.Bel Y, Sheets JJ, Tan SY, Narva KE, Escriche B. Toxicity and binding studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C, and Cry2A proteins in the soybean pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens. App. Environ. Microbiol. 2017;83:e00326–00317. doi: 10.1128/AEM.00326-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, Y. In Advances in Insect Physiology, Vol. 47. (eds. T.S. Dhadialla & S. S. Gill) 297–242 (Academic Press, London; 2014).
- 63.Jurat-Fuentes JL, Adang MJ. Importance of Cry1 δ-endotoxin domain II loops for binding specificity in Heliothis virescens (L.) App. Environ. Microbiol. 2001;67:323–329. doi: 10.1128/AEM.67.1.323-329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernández-Rodríguez CS, Hernández-Martínez P, Van Rie J, Escriche B, Ferré J. Shared midgut binding sites for Cry1A. 105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS ONE. 2013;8:e68164. doi: 10.1371/journal.pone.0068164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang T, et al. Inheritance patterns, dominance and cross-resistance of Cry1Ab-and Cry1Ac-selected Ostrinia furnacalis (Guenée) Toxins. 2014;6:2694–2707. doi: 10.3390/toxins6092694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jurat-Fuentes JL, Crickmore N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invert. Pathol. 2017;142:5–10. doi: 10.1016/j.jip.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 67.Luo S, et al. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J. Econ. Entomol. 2007;100:909–915. doi: 10.1093/jee/100.3.909. [DOI] [PubMed] [Google Scholar]
- 68.Tabashnik BE, et al. Asymmetrical cross-resistance between Bacillus thuringiensis toxins Cry1Ac and Cry2Ab in pink bollworm. Proc. Nat. Acad. Sci. USA. 2009;106:11889–11894. doi: 10.1073/pnas.0901351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jurat-Fuentes JL, Gould FL, Adang MJ. Altered glycosylation of 63-and 68-kilodalton microvillar proteins in Heliothis virescens correlates with reduced Cry1 toxin binding, decreased pore formation, and increased resistance to Bacillus thuringiensis Cry1 toxins. App. Environ. Microbiol. 2002;68:5711–5717. doi: 10.1128/AEM.68.11.5711-5717.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MK, Rajamohan F, Gould F, Dean D. Resistance to Bacillus thuringiensis CryIA delta-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. App. Environ. Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tindall KV, Siebert MW, Leonard BR, All J, Haile FJ. J. Econ. Entomol. 2009. Efficacy of Cry1ac:Cry1F proteins in cotton leaf tissue against fall armyworm, beet armyworm, and soybean looper (Lepidoptera: Noctuidae) pp. 1497–1505. [DOI] [PubMed] [Google Scholar]
- 72.Viana DD, Netto JC, Aguirre-Gil OJ, Busoli AC. Biological parameters of soybean looper in cotton cultivars with the Cry1Ac and Cry1F proteins. Pesq. Agropec. Bras. 2014;49:569–572. doi: 10.1590/S0100-204X2014000700010. [DOI] [Google Scholar]
- 73.Greene G, Leppla N, Dickerson W. Velvetbean caterpillar: a rearing procedure and artificial medium. J. Econ. Entomol. 1976;69:487–488. doi: 10.1093/jee/69.4.487. [DOI] [Google Scholar]
- 74.Sediyama, T., Silva, F. & Borém, A. Soja: do Plantio à Colheita. (Universidade Federal de Viçosa, Viçosa, Brazil; 2015).
- 75.Borém, A. & Freire, E. C. Algodão do Plantio à Colheita. (Universidade Federal de Viçosa, Viçosa, Brazil; 2014).
- 76.Wan P, Zhang Y, Wu K, Huang M. Seasonal expression profiles of insecticidal protein and control efficacy against Helicoverpa armigera for Bt cotton in the Yangtze River valley of China. J. Econ. Entomol. 2005;98:195–201. doi: 10.1093/jee/98.1.195. [DOI] [PubMed] [Google Scholar]
- 77.Marçon PC, Young LJ, Steffey KL, Siegfried BD. Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J. Econ. Entomol. 1999;92:279–285. doi: 10.1093/jee/92.2.279. [DOI] [Google Scholar]
- 78.Falconer, D. S. & Mackay, T. F. C. Introduction to Quantitative Genetics, Edn. 4. (Longman, London; 1996).
- 79.Robertson, J. L., Savin, N., Preisler, H. K. & Russell, R. M. Bioassays with Arthropods. (CRC press, Boca Raton; 2007).
- 80.Wheeler MW, Park RM, Bailer AJ. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006;25:1441–1444. doi: 10.1897/05-320R.1. [DOI] [PubMed] [Google Scholar]
- 81.SAS Institute SAS/STAT User’s Guide. (SAS Institute Inc., Cary, NC, USA; 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.