Abstract
Background
Human immunodeficiency virus–positive (HIV+) individuals have higher rates of cognitive impairment and cerebrovascular disease compared with uninfected populations. We hypothesize that cerebrovascular disease, specifically brain large artery disease, may play a role in HIV-associated neurocognitive disorders (HAND).
Methods
Participants (N = 94) in the Manhattan HIV Brain Bank study were followed on average 32 ± 33 months with repeated neuropsychological examinations until death. We used five cognitive domains (motor, processing speed, working memory, verbal fluency, and executive functioning) to assess ante mortem performance. We quantified the diameter of the lumen and arterial wall thickness obtained during autopsy. The diagnoses of HAND were attributed using the American Academy of Neurology nosology. We used generalized linear mixed model to account for repeated measures, follow-up time, and codependence between arteries. Models were adjusted for demographics, viral loads, CD4 counts, history of opportunistic infections, and vascular risks.
Results
We included 94 HIV+ individuals (mean age 56 ± 8.3, 68% men, 54% African American). In adjusted models, there was an association between arterial wall thickness and global cognitive score (B = −0.176, P value = .03), processing speed (B = −0.175, P = .05), and verbal fluency (B = −0.253, P = .02). Participants with incident or worsening HAND had thicker brain arterial walls (B = 0.523 ± 0.234, P = .03) and smaller arterial lumen (B = −0.633 ± 0.252, P = .01).
Conclusions
We report here a novel association between brain arterial wall thickening and poorer ante mortem cognitive performance and diagnosis of incident or worsening HAND at death. Strategies to preserve the arterial lumen or to prevent wall thickening may impact HAND.
Keywords: HIV-associated neurocognitive disorders, brain arterial remodeling, dementia, HIV, cerebrovascular disease
A thicker brain arterial wall at death relates to poorer ante mortem overall cognition, processing speed, and verbal fluency. A thicker brain arterial wall relates to human immunodeficiency virus–associated cognitive disorders (HAND). Strategies to prevent brain arterial wall thickening could impact HAND.
Cognitive impairment has been a neurological hallmark of human immunodeficiency virus (HIV) disease [1, 2]. Early in the course of the epidemic, cognitive impairment was reported in a fourth of individuals with Centers for Disease Control and Prevention stage A and half of those with stage C HIV infection [3]. With the advent of combination antiretroviral therapy (cART), opportunistic infections and neoplasms have decreased while non-AIDS related diseases have increased [4]. Cognitive impairment remains a frequent condition in some cART era cohorts [3, 5]. One argument for the persistence of cognitive deficits is that as individuals with HIV get older, other processes related to aging per se occur [6, 7]. These processes may be responsible for, or augment an underlying susceptibility to, cognitive impairment among HIV+ populations. For example, HIV+ individuals have a higher rate of dementia compared with uninfected populations [8]. Aging with HIV among carriers of apolipoprotein E-4 is also associated with poorer cognitive performance, especially among individuals with lower CD4 counts [9].
Another explanation for the increased rate of cognitive impairment and dementia among HIV+ individuals may relate to their higher burden of cerebrovascular disease. There is substantial evidence that cerebrovascular disease, defined as clinically overt stroke, silent brain infarct, or large or small artery disease, increases the risk of dementia, either of the Alzheimer type or of vascular dementia, in uninfected populations [10, 11]. There is also evidence that HIV+ individuals have a higher rate of stroke compared with HIV− individuals [12] and that brain arterial pathologies such as intracranial atherosclerosis and dolichoectasia are common among aging HIV+ individuals [13, 14]. Based on this, it is plausible that cerebrovascular disease may also contribute to the increased rate of cognitive impairment among HIV+ individuals.
The purpose of the study is to evaluate the relationship between ante mortem cognitive performance and brain large artery disease at the time of death in a group of HIV+ individuals. We hypothesize that brain large artery disease is associated with HIV-associated neurocognitive impairment (HAND) at the time of death and that ante mortem cognitive performance will be poorer among people with evidence of brain large artery disease at death.
METHODS
Participants for this study were enrolled in the Manhattan HIV Brain Bank, which operates under institutional review board approval at the Icahn School of Medicine at Mount Sinai. Upon consent and enrollment, participants are prospectively followed with semiannual or annual neuropsychological, neuromedical, and psychiatric examinations; upon death, participants agree to be brain donors for the purposes of neuro-HIV research. Immunovirologic data (CD4 counts, viral loads) were recovered from the medical charts in the first epoch of the study and run as standard laboratories during study visits at later years. Other information assessed via self-report and when available, review of medical records, includes history of vascular risk factors such as hypertension, diabetes, dyslipidemia, and smoking or evidence of medication use for these vascular risk factors.
Neurocognitive Testing
During full study visits, participants undergo an extensive neurocognitive battery as previously described [5, 15]. For this study, we used the entry visit Wide Range Achievement Test (WRAT), 3rd edition, reading subtest as an indicator of premorbid literacy [16]. We analyzed the following 5 putative cognitive domains derived from aggregated neuropsychological tests: motor domain (derived from grooved pegboard dominant and nondominant hands), processing speed (derived from Trail Making Test-Part A, Wechsler Adult Intelligence Scale-III [WAIS] Digit Symbol, WAIS Symbol Search), working memory (derived from WAIS-III Letter Number Sequencing and Paced Auditory Serial Addition Task), verbal fluency (derived from Controlled Oral Word Association Test), and executive functioning (derived from Trail Making Test-Part B and Wisconsin Card Sorting Test-Perseverative Responses). Norms were applied to the raw scores to obtain T scores, taking into account age, education, sex, and ethnicity as appropriate [17]. A composite global score was obtained by adding and averaging domain T scores.
Neurocognitive Diagnoses
The neurocognitive diagnoses were ascertained during a multidisciplinary meeting by consensus of at least 1 physician and 1 neuropsychologist. The diagnoses of HIV-associated dementia (HAD) and minor cognitive-motor disorder (MCMD) were attributed using the American Academy of Neurology nosology [1]. Subsyndromic neuropsychological impairment (NPI, equivalent to asymptomatic neuropsychological impairment in the Frascati classification of HAND) [18] and NPI deemed due to other causes (NPI-O) were defined as published by the National NeuroAIDS Tissue Consortium [19].
Brain Large Artery Definitions
The methods and definitions used for brain large arteries have been described elsewhere [13, 20]. Briefly, we identified arterial segments forming part of the circle of Willis (ie, first portion of the middle, anterior, and posterior cerebral arteries, etc.) and cut 5-mm segments from their most proximal and distal portions. Arterial segments were paraffin-embedded and stained with hematoxylin-eosin and elastin Van Gieson. Each section was digitized using an Olympus VS110 high-speed, high-resolution scanner with Olympus Soft Imaging Solutions software and a microscope with constant illumination, with 10× magnification and scale = 0.643 μm/pixel. We quantified the diameter of the lumen and arterial wall using color-based thresholding, with inter- and intrarater >0.90 for both measures [21]. Given the prior observation that the morphometry of each brain artery is strongly influenced by artery type and sex (as closest surrogates of head size) [22], we normalized the lumen diameter and arterial wall thickness into normal scores in subsamples of artery type and sex. Integrity of the internal elastic lamina and presence of atheroma (used here as sine qua non of atherosclerosis) was defined by visual observation with a kappa value of 0.80 and 0.72, respectively [23]. Dolichoectasia was defined as a normalized lumen-to-wall ratio ≥95th percentile [13].
Statistical Analyses
The data structure for the analyses consists of repeated measures of neurocognitive T scores (by domain) and CD4 counts over time (up to the time of death). We used age at the time of entry. For the other demographic, clinical, and pathological variables, we used their status at the time of death. Each participant had a variable number of brain large arteries. To fit this data structure, we used a generalized linear mixed model, which takes into account repeated measures over time, a random statement to account for the codependence between arteries from within the same individual, and a random statement for the follow-up time to assess trends.
First, we assessed whether the 2 main continuous variables (wall thickness and lumen diameter) were collinear using variance inflammation and condition index. In this case, there was no more than 19% variance inflammation and a maximum condition index of 3.0. Therefore, we built statistical models using the 2 continuous variables together. We started with a simple model using baseline WRAT scores, age, sex, and ethnicity. We assessed the change in beta estimates with subsequent adjustment for HIV-related variables (CD4 counts over time, use of cART, and viral load at time of death) in model 2, vascular risk factors (hypertension, smoking, dyslipidemia, diabetes) in model 3, and brain infarcts in model 4 to be able to assess the confounding effects of each of these cluster of variables in the beta estimate. We also substituted wall thickness by atherosclerosis and dolichoectasia as phenotypes of arterial disease in model 3. Due to collinearity between NPI-O and WRAT scores (low literacy may be a condition that contributes to NPI-O), we ran a sensitivity analysis excluding NPI-O. Finally, we used a generalized linear model to evaluate the cross-sectional relationship between neurocognitive diagnosis at death and brain arterial measurements. A P value ≤ 0.05 was considered statistically significant. The statistical software used for the analysis was SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Sample Description
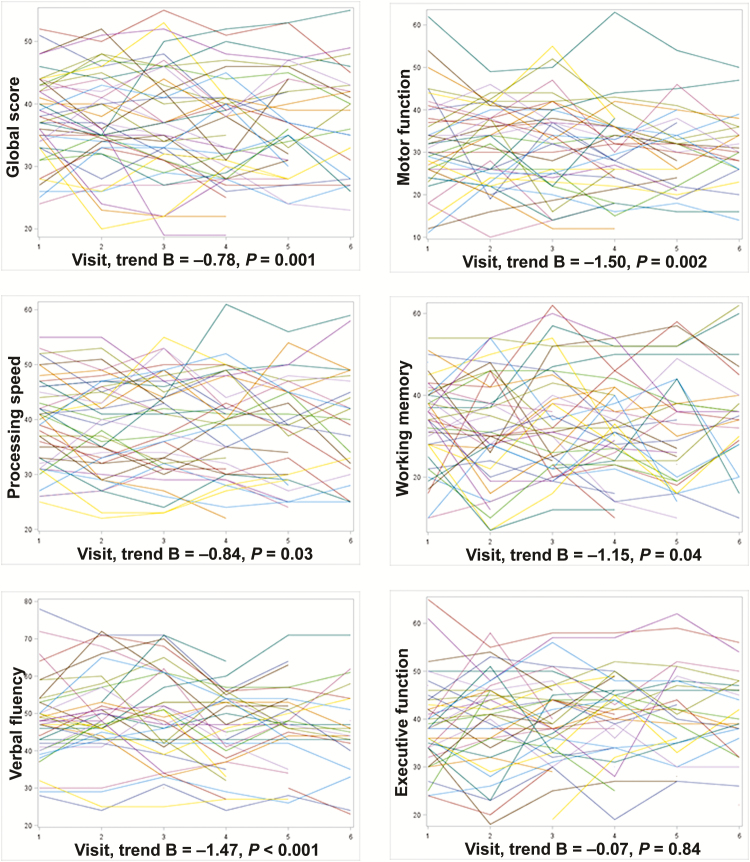
We included 94 HIV+ individuals (mean age, 49.2 ± 8.8 years; 68% men; 54% non-Hispanic black). The sample characteristics at the time of death are described in Table 1. Participants were followed on average 32 ± 33 months (median, 21 months; interquartile range [IQR], 7–75). During this period, participants had, on average, 4 visits at which neuropsychological testing was conducted (median, 3; IQR, 1–6), with a total of 417 neurocognitive data points. The median time between the latest cognitive test and the time of death was 4 months. The cognitive performance in the sample worsened over time, with the exception of executive function (Figure 1). From all participants, we obtained 703 brain large arteries (mean per participants, 7; median, 6; IQR, 5–9) at the time of autopsy.
Table 1.
Characteristics of the Sample at Death (N = 94)
| Age (in Years, SD, Median, Interquartile Range) | 56 (8.3, 57, 50–63) |
|---|---|
| Male sex (%) | 68 |
| Ethnicity (%) | |
| Non-Hispanic white | 20 |
| Non-Hispanic black | 54 |
| Hispanic | 26 |
| Hypertension (%) | 61 |
| Diabetes (%) | 19 |
| Dyslipidemia (%) | 30 |
| Smoking (%) | 56 |
| Coinfection (%) | |
| Hepatitis B | 49 |
| Hepatitis C | 44 |
| Combination antiretroviral therapy use at death (%) | 68 |
| Brain infarcts (%) | 20 |
| Prior opportunistic infections (%) | 70 |
| Nadir CD4 (per mm3, SD, median, interquartile range) | 127 (146, 78, 6–191) |
| CD4 count at enrollment (per mm3, SD, median, interquartile range) |
230 (287, 119, 20–331) |
| CD4 count at death (per mm3, SD, median, interquartile range) |
268 (217, 219, 73–493) |
| Viral load at death >50 copies/mL (%) | 72 |
Abbreviation: SD, standard deviation.
Figure 1.
Longitudinal T scores in various cognitive domains suggestive of an overall negative trend in cognitive performance over time. Each line represents an individual performance over time (x axis) visits. The negative trend was confirmed for all domains except for executive function using multivariate analyses adjusting for age, sex, age at the first visit, and follow-up time.
Brain Large Artery Measurements and Ante Mortem Cognitive Performance
We used arterial wall thickness and lumen diameter as continuous measures of brain arterial disease and atherosclerosis and dolichoectasia as categorical variables of arterial wall disease. In the first simple model (Table 2), there was an association between thicker arterial wall and poorer ante mortem global score (B = −0.177, P value = .03), processing speed (B = −0.175, P = .05), and verbal fluency (B = −0.291, P = .01) but not with motor, working memory, or executive function scores. Further adjustment for viral loads, CD4 counts, history of opportunistic infections, and vascular risk factors did not change the significance of the association. Greater lumen diameter was overall associated with better ante mortem cognitive scores but it did not reach significance in any of the comparisons. Substituting wall thickness with atherosclerosis and dolichoectasia showed a negative association with both brain large artery phenotypes but it did not reach significance in any of the comparisons.
Table 2.
Brain Arterial Pathological Correlates of Pre Mortem Cognitive Performance Among Human Immunodeficiency Virus–positive Individuals
| Global | Motor | Processing Speed | Memory (Retrieval) |
Verbal Fluency | Executive Function | ||
|---|---|---|---|---|---|---|---|
| Beta Estimate ± Standard Error | |||||||
| Lumen diameter | Model 1 | 0.143 ± 0.081 | 0.046 ± 0.107 | 0.141 ± 0.087 | 0.206 ± 0.164 | 0.210 ± 0.115 | 0.109 ± 0.115 |
| Model 2 | 0.128 ± 0.080 | 0.027 ± 0.106 | 0.127 ± 0.086 | 0.199 ± 0.164 | 0.198 ± 0.113 | 0.098 ± 0.114 | |
| Model 3 | 0.128 ± 0.080 | 0.028 ± 0.107 | 0.128 ± 0.086 | 0.198 ± 0.166 | 0.198 ± 0.114 | 0.102 ± 0.116 | |
| Model 4 | 0.126 ± 0.080 | 0.027 ± 0.107 | 0.125 ± 0.086 | 0.194 ± 0.166 | 0.197 ± 0.113 | 0.113 ± 0.114 | |
| Wall thickness | Model 1 | −0.185 ± 0.082a | −0.063 ± 0.110 | −0.187 ± 0.088a | −0.257 ± 0.166 | −0.296 ± 0.118a | −0.154 ± 0.117 |
| Model 2 | −0.169 ± 0.081a | −0.038 ± 0.108 | −0.171 ± 0.087 | −0.253 ± 0.167 | −0.281 ± 0.116a | −0.141 ± 0.117 | |
| Model 3 | −0.176 ± 0.082a | −0.039 ± 0.110 | −0.174 ± 0.089a | −0.266 ± 0.170 | −0.253 ± 0.114a | −0.149 ± 0.119 | |
| Model 4 | −0.177 ± 0.082a | −0.037 ± 0.110 | −0.174 ± 0.089 | −0.269 ± 0.170 | −0.280 ± 0.116 | −0.134 ± 0.116 | |
| Model 3 | Lumen diameters | 0.064 ± 0.074 | 0.013 ± 0.099 | 0.063 ± 0.079 | 0.103 ± 0.152 | 0.093 ± 0.105 | 0.047 ± 0.105 |
| Atherosclerosis | −0.027 ± 0.226 | −0.023 ± 0.328 | −0.023 ± 0.237 | −0.026 ± 0.461 | −0.017 ± 0.322 | −0.013 ± 0.314 | |
| Dolichoectasia | −0.022 ± 0.267 | −0.005 ± 0.346 | −0.029 ± 0.293 | −0.058 ± 0.543 | −0.039 ± 0.382 | −0.027 ± 0.383 | |
Model 1: adjusting for age, sex, race/ethnicity, baseline Wide Range Achievement Test scores, and confounders for cognitive assessments. Model 2: model 1 plus nadir CD4, CD4 trend during follow-up, and use of combination antiretroviral therapy. Model 3: model 2 plus hypertension, diabetes, dyslipidemia, and smoking. Model 4: model 2 plus pathology-based brain infarcts.
a P value .05–.01.
Using model 3, we confirmed that during follow-up, there was poorer cognitive performance in all domains except executive function (Table 3). Higher CD4 counts over time, however, were associated with better cognitive performance in all domains. Other relevant associations included literacy (as assessed by the WRAT score) with better cognitive performance over time and nadir CD4 associated with poorer cognitive performance. Men had poorer processing speed and verbal fluency compared with women, and hypertension was associated with poorer motor performance. There was a persistent, but not significant, negative association between diabetes and cognitive performance and dyslipidemia with better cognitive performance. In a sensitivity analyses, removing NPI-O from model 3 did not affect the results in any of the models.
Table 3.
Clinical and Pathological Correlates of Premortem Cognitive Performance Among Human Immunodeficiency Virus–positive Individuals
| Global | Motor | Processing Speed | Memory (Retrieval) |
Verbal Fluency | Executive Function | |
|---|---|---|---|---|---|---|
| Age (years) | 0.085 ± 0.133 | 0.154 ± 0.208 | 0.127 ± 0.157 | -0.050 ± 0.204 | 0.234 ± 0.201 | 0.111 ± 0.171 |
| Male sex | -2.118 ± 1.734 | 3.512 ± 2.731 | -5.502 ± 2.049b | -2.650 ± 2.672 | -9.064 ± 2.682 | -1.227 ± 2.230 |
| Nonwhite ethnicity | 0.229 ± 2.437 | -3.509 ± 4.051 | -3.231 ± 2.891 | 6.340 ± 3.762 | -0.397 ± 3.924 | -5.079 ± 3.141 |
| Hypertension | -0.237 ± 1.820 | -5.525 ± 2.887 | -0.742 ± 2.156 | 1.676 ± 2.799 | -3.702 ± 2.838 | 0.201 ± 2.347 |
| Diabetes | -3.216 ± 2.141 | -3.354 ± 3.331 | -2.057 ± 2.533 | -5.705 ± 3.266 | -2.477 ± 3.218 | -1.512 ± 2.751 |
| Dyslipidemia | 1.684 ± 1.805 | 2.422 ± 2.818 | 2.779 ± 2.137 | 0.350 ± 2.772 | 0.163 ± 2.763 | 2.787 ± 2.321 |
| Smoking | -0.522 ± 1.804 | 2.254 ± 2.823 | -1.175 ± 2.138 | -0.733 ± 2.786 | 3.107 ± 2.812 | -0.575 ± 2.323 |
| Combination antiretroviral therapy use at death | 1.628 ± 1.884 | 0.338 ± 2.953 | 1.885 ± 2.231 | 2.576 ± 2.900 | 2.769 ± 2.889 | 0.663 ± 2.425 |
| Unsuppressed viral load | -0.945 ± 2.258 | 0.713 ± 3.526 | -1.595 ± 2.667 | -3.567 ± 3.444 | 2.694 ± 3.402 | 3.312 ± 2.910 |
| CD4 nadir | -0.022 ± 0.011a | -0.033 ± 0.016a | -0.030 ± 0.013a | -0.008 ± 0.016 | -0.033 ± 0.016a | -0.031 ± 0.014a |
| CD4 count at entry | 0.008 ± 0.005 | 0.010 ± 0.007 | 0.004 ± 0.005 | 0.011 ± 0.007 | 0.010 ± 0.169 | 0.010 ± 0.006 |
| Follow-up time (visits) | -1.008 ± 0.247c | -1.884 ± 0.386c | -1.011 ± 0.382b | -1.207 ± 0.596a | -2.010 ± 0.409c | -0.160 ± 0.395 |
| CD4 trend (CD4avisit) | 0.001 ± 0.0001c | 0.001 ± 0.0001c | 0.001 ± 0.0001c | 0.001 ± 0.0001b | 0.001 ± 0.0001c | 0.001 ± 0.0001b |
| Wide Range Achievement Test score at entry | 0.319 ± 0.076c | 0.053 ± 0.123 | 0.242 ± 0.091b | 0.549 ± 0.117c | 0.468 ± 0.120c | 0.097 ± 0.098 |
Beta estimates obtained from model 3 represented in Table 2.
a P value .05–.01.
b P value < .01–.001.
c P value < .001.
Brain Large Artery Disease and HAND Status at the Time of Death
At the time of enrollment, 20% (N = 19) were cognitively normal, 15% (N = 15) had subsyndromic NPI, 12% (N = 11) had MCMD, and 3% (N = 3) had HAD. Half of the sample had NPI-O confounded by drug use, acute medical illness, central nervous system (CNS) opportunistic infection, poor vision, or incomplete battery tests or low literacy. Upon death, 10% (N = 9) remained cognitively normal, 78% (N = 74) persisted cognitively impaired (either with NPI or HAD), and 12% (N = 11) had incident MCMD/HAD or progressed to HAD from less severe forms of HAND. Using an adjusted multiordinal hierarchical regression (with variables from model 3 in Table 2), the probability of having any cognitive impairment at death was higher with thicker arterial walls (B = 0.262 ± 0.211, P = .20) and lower with larger arterial lumen (B = −0.409 ± 0.249, P = .10). Categorizing cognitive impairment at the last antemortem visit as incident (ie, participants cognitively normal at baseline developed MCMD/HAD during follow-up) or worsening cognitive impairment (ie, participants with MCMD progressed to HAD) vs cognitively stable (ie, participants remained cognitively normal or remained with stable MCMD) demonstrated an independent significant association between thicker arterial walls (B = 0.523 ± 0.234, P = .03) and inverse association of larger arterial lumen (B = −0.633 ± 0.252, P = .01) with incident or worsening HAND.
In a post hoc analysis using model 3 from Table 2, we found that at the time of death, hypertension (B = 0.288 ± 0.119, P = .01) was associated with wall thickness, and older age had a marginal association (B = 0.013 ± 0.007, P = .06). Use of cART at the time of death (B = 0.452 ± 0.135, P = .001) and unsuppressed viral load (B = −0.290 ± 0.148, P = .05) were associated with lumen diameter. None of the other covariates were statistically related to arterial wall thickness or luminal diameters.
DISCUSSION
In this study, we present novel evidence of an association between brain large artery morphometry with ante mortem cognitive performance trajectories and with neurocognitive diagnoses at the time of death. A thicker arterial wall at the time of death was associated with poorer ante mortem cognitive performance independent of vascular risk factors and immunovirological variables such as CD4 counts, viral load, and use of cART. In addition, a thicker arterial wall correlated with HAND at the time of death, in particular worsening HAND. The relationship between brain large artery disease and HAND has not been studied, but data from this study suggest that there are cerebrovascular contributions to HAND that may merit further study. Lumen diameter, which is directly proportional to blood flow, appears to counteract the effects of a thicker arterial wall. Dolichoectasia, however, was also associated with poorer cognitive performance, but the study lacked the power to validate this observation.
The observed association between brain arterial thickness and lumen with cognitive performance and diagnoses deserves further discussion. It is possible that a thicker arterial wall represents end organ damage after lifetime exposure to vascular risk factors. This may be even more important when we lack continuous measures of vascular risk factor control over time. However, the minimal attenuation after controlling for vascular risk factor (model 2 to model 3) suggests that alternative explanations may exist. Brain arterial wall thickening is inherently associated with aging, and it may relate to Alzheimer dementia in uninfected populations [20]. Thickening of the arterial wall is related to atherosclerosis, but not exclusively. Compensatory intimal thickening with no atheroma may result from chronic blood flow, resulting in wall thickening and increased arterial stiffness [20, 23]. A preserved lumen but a thicker wall would result in greater stiffness [24], particularly if accompanied by elastin loss [25], which in turn may cause parenchymal damage due to arteriolar remodeling and blood–brain barrier dysfunction [26–28]. In fact, blood–brain barrier dysfunction is consistently associated with axonal damage and HAND [29]. It is possible that HIV-related immune activation may be an effect modifier in the association between wall thickness and cognition, but we lack measures of systemic inflammation to test this hypothesis. Maintenance of viral suppression and cART use at the time of death were associated with larger luminal diameters in this sample. It is not clear from our data whether the effects of viral suppression on cognition are mediated by lumen preservation or whether lumen preservation is an epiphenomenon related to less CNS inflammation and neuronal degeneration [30]. Elucidation of this relationship may further the understanding of the cerebrovascular contributions to HAND. Strategies to maintain the lumen and prevent wall thickening may plausibly modify the natural history of HAND. Based on the data presented here, aiming at normotension and maintaining viral suppression are probably the easiest and least controversial measures to protect the brain against stroke, small artery disease, and possible HAND. Whether other strategies may provide additional brain protection by modifying the effects of HIV on brain large arteries remains unknown, and their effect may be marginal.
Our study also needs to be contextualized with prior reports in order to be in agreement with the current literature. For example, diabetes has been associated with HAND in other cohorts [31, 32]. We did not confirm an association between diabetes and cognitive scores. However, the most likely explanation is that categorical variables require larger samples for achieving statistical significance. Alternatively, our cohort may not have the severity of end organ complications from diabetes that is seen in other populations. It is possible that diabetes also predisposes to thicker arterial walls by increasing the risk of atherosclerosis [33, 34], which is a frequent underlying phenotype of arterial wall thickening (Figure 2). Hypertension was an overall negative influence for global cognition, processing speed, and verbal fluency, but it was significant only for motor performance. This may be due to the greatest impact of hypertension in subcortical white matter disease and lacunar infarcts, which in turn may be related to poorer performance in grooved pegboard test performance [35, 36]. The effects of vascular risk factors over cognitive performance seem to be even more relevant for cohorts with higher CD4 medians compared to ours [37]. Consequently, the higher prevalence of persistent immunosuppression in our sample may have attenuated the strength of association of vascular risk factors with cognitive performance. Avoiding low nadir CD4 and maintaining high CD4 counts with continuous use of cART may preserve cognition, in addition to decreasing cardiovascular morbidity and mortality in HIV [12, 38, 39].
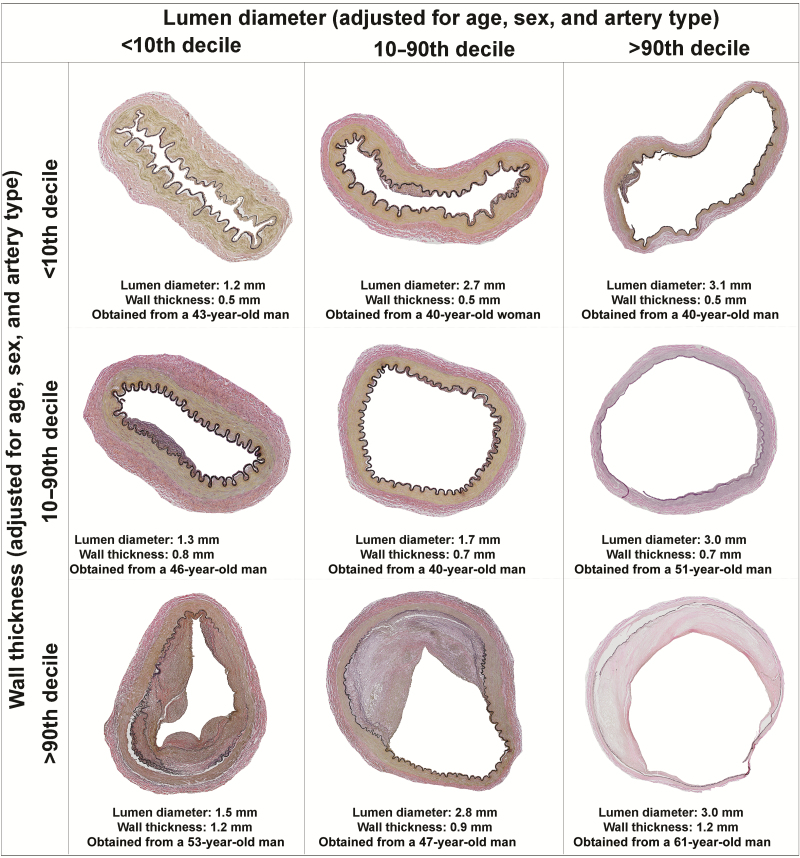
Figure 2.
Examples of the heterogeneity of brain arterial remodeling phenotypes by combining the distribution of the lumen and the arterial wall. A thick arterial wall is often accompanied by fibrotic intimal proliferation and disruption of the internal elastic lamina with no or minimal cholesterol deposition (far left and bottom) or by atherosclerosis (defined by an atheroma; mid and far right bottom). Dolichoectasia is consistent with a thin arterial wall and dilated lumen (far right and top). Atherosclerosis and dolichoectasia are extremes of brain arterial remodeling with possible negative effects on cognition.
The results from this study should be contextualized to our cohort, which represents an ethnically diverse urban population with advanced HIV. The rates of cART use in our cohort are similar to the rates reported throughout the United States [40]. Similarly, the relatively lower nadir and subsequent CD4 counts reflect the immunological status of underserved populations, which now account for the majority of the existing and new HIV cases in the United States [40, 41].
Categorical variables, either as an outcome or as covariates, require large samples to detect statistical significance. Additionally, hierarchical models used to account for the data structure limit the power of this study. Consequently, it is possible that we missed relevant associations with HAND. Accounting for the complex data structure, however, increases the validity of the associations detected. Other limitations include the relatively small sample size and selection bias related to autopsy consent.
In summary, we report a novel association between brain arterial wall thickening and poorer ante mortem cognitive performance and diagnosis of incident or worsening HAND at death. Preservation of the arterial lumen and, with it, the brain flow may be related to better ante mortem cognitive performance and is negatively associated with the diagnosis of incident or worsening HAND. In addition to diligent control of blood pressure, avoidance of low nadir CD4, and maintenance of CD4 and viral suppression, strategies to prevent or slow down brain arterial wall thickening and to preserve the arterial lumen may plausibly alter the natural history of HAND.
Notes
Financial support. This study was supported in part by the National Institutes of Health (NIH; U24MH100931, 5T32AI007387-27), the Manhattan HIV Brain Bank (member of the National NeuroAIDS Tissue Consortium), and the Campbell Foundation.
Potential conflicts of interest. M. T. Y. reports personal fees from Gilead Sciences and Viiv outside the submitted work. J. G. reports grants from the Campbell Foundation and the NIH during the conduct of the study and personal fees from Pfizer and ProPhase, outside the submitted work. He has also received personal fees while working as a medico-legal consultant outside the submitted work. All other authors report no conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Marder K, Albert S, Dooneief G, et al. Clinical confirmation of the American Academy of Neurology algorithm for HIV-associated cognitive/motor disorder. Neurology 1996; 47:1247–53. [DOI] [PubMed] [Google Scholar]
- 2. McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993; 43:2245–52. [DOI] [PubMed] [Google Scholar]
- 3. Heaton RK, Franklin DR, Ellis RJ, et al. ; CHARTER Group; HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber R, Ruppik M, Rickenbach M, et al. ; Swiss HIV Cohort Study.. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 5. Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S; Manhattan HIV Brain Bank Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Arch Neurol 2008; 65:1096–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004; 63:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology 2009; 73:1982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mateen FJ, Shinohara RT, Carone M, et al. Neurologic disorders incidence in HIV+ vs HIV− men: Multicenter AIDS Cohort Study, 1996–2011. Neurology 2012; 79:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panos SE, Hinkin CH, Singer EJ, et al. Apolipoprotein-E genotype and human immunodeficiency virus-associated neurocognitive disorder: the modulating effects of older age and disease severity. Neurobehav HIV Med 2013; 5:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging Cohort. Ann Neurol 2010; 68:231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348:1215–22. [DOI] [PubMed] [Google Scholar]
- 12. Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One 2017; 12:e0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gutierrez J, Goldman J, Dwork AJ, Elkind MS, Marshall RS, Morgello S. Brain arterial remodeling contribution to nonembolic brain infarcts in patients with HIV. Neurology 2015; 85:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the southeastern United States. AIDS Res Hum Retroviruses 2013; 29:1068–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs MM, Murray J, Byrd DA, Hurd YL, Morgello S. HIV-related cognitive impairment shows bi-directional association with dopamine receptor DRD1 and DRD2 polymorphisms in substance-dependent and substance-independent populations. J Neurovirol 2013; 19:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson GS Wilkinson, GS. Wide range achievement test: WRAT3: Wide Range. 1993. [Google Scholar]
- 17. Heaton RK, Grant I, Matthews CG.. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications; with a supplement for the Wechsler Adult Intelligence Scale-Revised (WAIS-R): Psychological Assessment Resources. 1991. [Google Scholar]
- 18. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004; 26:759–78. [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez J, Honig L, Elkind MS, et al. Brain arterial aging and its relationship to Alzheimer dementia. Neurology 2016; 86:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gutierrez J, Elkind MS, Petito C, Chung DY, Dwork AJ, Marshall RS. The contribution of HIV infection to intracranial arterial remodeling: a pilot study. Neuropathology 2013; 33:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gutierrez J, Elkind MS, Gomez-Schneider M, et al. Compensatory intracranial arterial dilatation in extracranial carotid atherosclerosis: the Northern Manhattan Study. Int J Stroke 2015; 10:843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutierrez J, Elkind MS, Virmani R, et al. A pathological perspective on the natural history of cerebral atherosclerosis. Int J Stroke 2015; 10:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nichols WW, O’Rourke MF, McDonald DA. Special circulations. In: Nichols WW, O’Rourke MF, McDonald DA. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 6th ed London: Hodder Arnold, 2011:xiv, 755 p. [Google Scholar]
- 25. Dobrin PB. Mechanical properties of arteries. Physiol Rev 1978; 58:397–460. [DOI] [PubMed] [Google Scholar]
- 26. Ott C, Raff U, Harazny JM, Michelson G, Schmieder RE. Central pulse pressure is an independent determinant of vascular remodeling in the retinal circulation. Hypertension 2013; 61:1340–5. [DOI] [PubMed] [Google Scholar]
- 27. Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension 1989; 13:968–72. [DOI] [PubMed] [Google Scholar]
- 28. Gutierrez J, Murray J, Chon C, Morgello S. Relationship between brain large artery characteristics and their downstream arterioles. J Neurovirol 2018; 24:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petito CK, Cash KS. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol 1992; 32:658–66. [DOI] [PubMed] [Google Scholar]
- 30. Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 2012; 60:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valcour VG, Shikuma CM, Shiramizu BT, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr 2005; 38:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCutchan JA, Marquie-Beck JA, Fitzsimons CA, et al. ; CHARTER Group Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 2012; 78:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rincon F, Sacco RL, Kranwinkel G, et al. Incidence and risk factors of intracranial atherosclerotic stroke: the Northern Manhattan Stroke Study. Cerebrovasc Dis 2009; 28:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities Study. Stroke 2006; 37:2493–8. [DOI] [PubMed] [Google Scholar]
- 35. Waldstein SR, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med 2005; 29:174–80. [DOI] [PubMed] [Google Scholar]
- 36. Nyquist PA, Yanek LR, Bilgel M, et al. Effect of white matter lesions on manual dexterity in healthy middle-aged persons. Neurology 2015; 84:1920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright EJ, Grund B, Robertson K, et al. ; INSIGHT SMART Study Group Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 2010; 75:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Department of Health and Human Services. Panel on antiretroviral guidelines for adults and adolescents: guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: DHHS, 2016. [Google Scholar]
- 39. El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 40. Rosenberg ES, Grey JA, Sanchez TH, Sullivan PS. Rates of prevalent HIV infection, prevalent diagnoses, and new diagnoses among men who have sex with men in US states, metropolitan statistical areas, and counties, 2012–2013. JMIR Public Health Surveill 2016; 2:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vital signs: HIV infection, testing, and risk behaviors among youths—United States. MMWR Morb Mortal Wkly Rep 2012; 61:971–6. [PubMed] [Google Scholar]