Abstract
Background
The effect of depressive symptoms on progression through the human immunodeficiency virus (HIV) treatment cascade is poorly characterized.
Methods
We included participants from the Centers for AIDS Research Network of Integrated Clinic Systems cohort who were antiretroviral therapy (ART) naive, had at least 1 viral load and HIV appointment measure after ART initiation, and a depressive symptom measure within 6 months of ART initiation. Recent depressive symptoms were measured using the Patient Health Questionnaire-9 (PHQ-9) and categorized using a validated cut point (PHQ-9 ≥10). We followed participants from ART initiation through the first of the following events: loss to follow-up (>12 months with no HIV appointment), death, administrative censoring (2011–2014), or 5 years of follow-up. We used log binomial models with generalized estimating equations to estimate associations between recent depressive symptoms and having a detectable viral load (≥75 copies/mL) or missing an HIV visit over time.
Results
We included 1057 HIV-infected adults who contributed 2424 person-years. At ART initiation, 30% of participants reported depressive symptoms. In multivariable analysis, recent depressive symptoms increased the risk of having a detectable viral load (risk ratio [RR], 1.28; 95% confidence interval [CI], 1.07, 1.53) over time. The association between depressive symptoms and missing an HIV visit (RR, 1.20; 95% CI, 1.05, 1.36) moved to the null after adjustment for preexisting mental health conditions (RR, 1.00; 95% CI, 0.85, 1.18).
Conclusions
Recent depressive symptoms are a risk factor for unsuppressed viral load, while preexisting mental health conditions may influence HIV appointment adherence.
Keywords: HIV, depression, HIV treatment cascade, mental health, viral load
In a cohort of new antiretroviral therapy users, mental health adversely affected engagement in human immunodeficiency virus (HIV) care. Recent depressive symptoms increased the risk of detectable viral load, while preexisting mental health diagnoses influenced HIV appointment adherence over time.
Depression affects 20%–30% of adults living with human immunodeficiency virus (HIV), negatively influencing outcomes across the HIV treatment cascade [1, 2]. Depression has been associated with risk behaviors for sexual HIV transmission [3], reduced antiretroviral therapy (ART) adherence [4, 5], unsuppressed viral load [6–8], and increased mortality [6, 9–13]. Depression is also frequently undiagnosed, untreated, or undertreated in HIV-infected populations [14–16], raising the likelihood that depression will adversely affect outcomes across the HIV treatment cascade.
Engagement in care is essential to improve outcomes across the HIV treatment cascade. Viral suppression has long been recognized as a biological indicator of ART adherence and the ultimate goal of HIV treatment [17, 18]. However, achieving sustained viral suppression also requires attendance at regularly scheduled HIV clinic visits. HIV appointment adherence has increasingly been recognized as an important, and distinct, indicator of engagement in care along the HIV treatment cascade [19–21]. Missed HIV clinic visits have been associated with delays in starting ART and achieving viral suppression, as well as development of AIDS-defining illnesses and mortality [22, 23]. Understanding how depressive symptoms affect both viral suppression and HIV appointment adherence over time is therefore essential to optimize outcomes across the HIV treatment cascade.
In this analysis, we used data from a large cohort of HIV-infected adults in the United States to investigate how presenting with recent, clinically meaningful depressive symptoms affects the risk of the following 2 important markers of engagement in HIV care over time: having a detectable viral load and missing an HIV appointment. We hypothesize that recent depressive symptoms may affect these distinct aspects of engagement in care differently over time.
METHODS
Data for the present analysis come from the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort. The CNICS cohort includes more than 32000 HIV-infected adults in routine HIV clinical care at 8 academic medical centers across the United States [24]. Since 1997, CNICS has collected detailed information on demographic characteristics, medication (including ART) prescriptions, HIV/AIDS clinical events, comorbid conditions, CD4 count, HIV viral load, and vital status of patients who consent to participate. Between 2005 and 2011, CNICS introduced self-administered questionnaires, called patient‐reported outcomes (PROs), across 7 of the 8 sites for patients to complete on touch-screen tablets or personal computers every 4–6 months as part of routine clinical visits. PRO assessments typically begin around entry into CNICS care, with some variation by site. Participants provided written informed consent to participate in CNICS. The institutional review board at each CNICS site provided ethical approval for the use of routinely collected clinical data.
Study Population
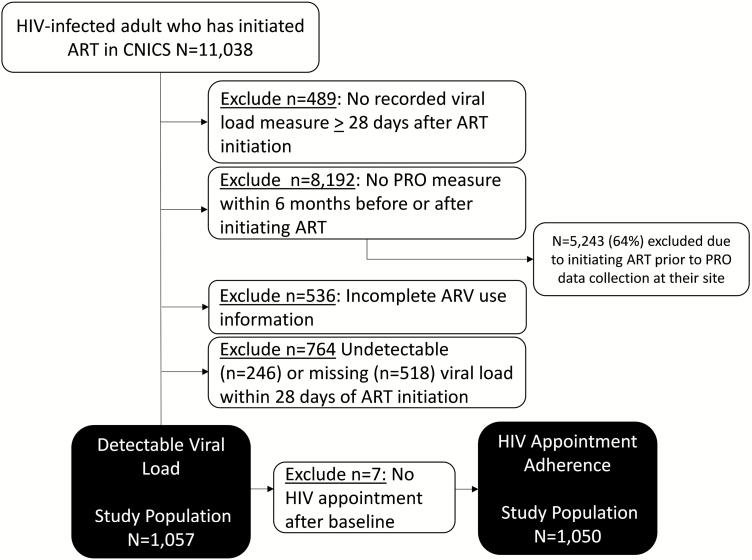
For the present analysis, we included HIV-infected adults in CNICS initiating ART (n = 11038) with a viral load measured at least 28 days after ART initiation (n = 10549) and at least 1 PRO measure in the 6 months before or after initiating ART (n = 2357). The majority of participants excluded for not having a PRO measure (64%) were participants who initiated ART prior to PRO data collection beginning at their CNICS site. To identify ART-naive participants, we excluded participants with incomplete information on ART use (n = 1821); those with an undetectable viral load (<75 copies/mL) at ART “initiation” (n = 246 excluded), possibly indicating prior ART use; and those who did not have a viral load measure within 28 days of initiating ART (n = 518 excluded). Our final study population when examining detectable viral load (henceforth, “detectable viral load analysis”) included 1057 participants. Of the 1057 participants, 7 did not have an HIV appointment scheduled after ART initiation and were excluded from the analysis of HIV appointment adherence (henceforth, “appointment adherence analysis”; n = 1050 participants; Figure 1). Participants from 6 of the 8 CNICS sites were included in our study population (1 site did not collect PRO information and 1 site did not collect PROs around the time of ART initiation.
Figure 1.
Study population for new antiretroviral therapy users in the Centers for AIDS Research Network of Integrated Clinical Systems included in the detectable viral load (n = 1057) and human immunodeficiency virus appointment adherence (n = 1050) analyses. Abbreviations: ART; antiretroviral therapy; ARV, antiretroviral; CNICS, Centers for AIDS Research Network of Integrated Clinical Systems; HIV, human immunodeficiency virus; PRO, patient‐reported outcome.
Participants were followed from ART initiation through the first of the following events: lost to follow-up from CNICS (defined as the date 12 months after their last HIV care appointment), death, administrative censoring (2014–2015 depending on site), or 5 years of follow-up. We assessed multiple outcomes for each participant using a repeated measures framework.
Depressive Symptoms Exposure
For both the detectable viral load and appointment adherence analyses, the exposure of interest was a binary measure of recent depressive symptoms, measured using the Patient Health Questionnaire-9 (PHQ-9) [25]. The PHQ-9 is used to assess the presence of the 9 Diagnostic and Statistical Manual of Mental Disorders-V criteria symptoms for depression in the past 2 weeks and has been widely validated, including in HIV-infected populations [26]. The PHQ-9 ranges from 0 to 27, with higher scores indicating more severe depressive symptoms. Participants with a score of ≥10 were considered to have clinically meaningful depressive symptoms; a cutoff of ≥10 has 88% sensitivity and 88% specificity to indicate probable major depressive disorder and is considered a clinical threshold for beginning antidepressant treatment [25, 27]. We considered a continuous measure of recent depressive symptoms as a secondary exposure, modeled using both a linear term and locally weighted scatterplot smoothing regression after investigating the functional form.
Depressive symptoms are measured in CNICS approximately every 6 months, during the PRO administration. Therefore, we allowed depressive symptom measures to be valid (eg, carried forward) for up to 6 months (eg, 183 days) or until the patient’s next PRO measure; we defined this as recent depressive symptoms. One pathway that depressive symptoms affect having a detectable viral load is through reduced ART adherence [4, 5], a process that may take several weeks to become evident. Therefore, for the detectable viral load analysis, we assessed each participant’s depressive symptoms 30 days prior to when each viral load measurement was taken. For the HIV appointment adherence analysis, we assessed depressive symptoms on the day of a given attended HIV appointment to estimate the likelihood that the next scheduled appointment would be kept or missed. For all observations without a valid depressive symptoms measure, the exposure was considered missing and accounted for in sensitivity analysis.
HIV Treatment Cascade Outcomes
The outcome of interest for the detectable viral load analysis was having a detectable viral load, defined as ≥75 copies of HIV-1 RNA per milliliter of plasma. In the HIV appointment adherence analysis, the outcome of interest was missing, as opposed to keeping, the next scheduled HIV appointment (walk-in or rescheduled appointments were excluded). Multiple outcomes per participant were assessed over time.
Covariates
Both the detectable viral load and appointment adherence analyses included time-fixed and time-varying covariates. Time-fixed covariates were measured at enrollment into CNICS or ART initiation (baseline) and included site, gender, race/ethnicity (white, non-Hispanic; black, non-Hispanic; Hispanic; or other), HIV acquisition risk group (intravenous drug user, male-to-male sexual contact, heterosexual contact, or other), and having a preexisting mental health, diabetes, or hypertension diagnosis documented by a provider, likely around the time of HIV care initiation, in the medical record at the time of ART initiation. Preexisting mental health diagnosis was defined as any previously documented anxiety, depression, bipolar disorder, post-traumatic stress disorder, psychosis, or other uncategorized mood or mental health disorders. Chronic comorbid medical diagnoses may also affects patients’ depressive symptoms status and engagement in clinical care [28–30]. Therefore, we also controlled for chart-documented comorbid medical diagnoses available in our data, namely, hypertension and diabetes.
Time-varying covariates collected via CNICS PROs included symptoms of panic disorder (measured using the PHQ-5, defined as no panic symptoms, some panic symptoms, or panic disorder) [31]; high-risk alcohol use (measured using the alcohol use disorders identification test [AUDIT-C], defined as an AUDIT-C score ≥4 for males and ≥3 for females) [32]; and current, past, or no illicit drug use, excluding marijuana (measured using the alcohol, smoking, and substance involvement screening test) [33, 34]. CNICS also collects time-updated information on whether a participant has a current antidepressant prescription, CD4 count, and viral load laboratory values (viral load was only used as a covariate in the appointment adherence analysis). Poor ART adherence is more likely to result from depressive symptoms rather than precede them; therefore, ART adherence (measured via PROs) was not considered as a potential confounder in our analysis [35, 36]. Information on counseling or other behavioral health treatment is not available in CNICS.
Values for all time-varying covariates were considered valid (eg, carried forward) for 6 months. To ensure appropriate temporal ordering, all time-varying covariate values were lagged and assessed 6 months prior to each depressive symptom measure. If time-varying covariate information was not available 6-months prior, the time-varying covariate values were considered missing and accounted for in sensitivity analyses.
Statistical Analysis
The goal of our analysis was to estimate how time-varying recent depressive symptoms affect engagement in care over time. We estimated the relationship between recent depressive symptoms and having a detectable viral load over time and separately estimated the relationship between recent depressive symptoms and missing a subsequent HIV appointment over time. For both analyses, we estimated unadjusted and adjusted risk ratios (RRs) and 95% confidence intervals (CIs) using generalized estimating equations for log binomial models, with a robust variance estimator and an exchangeable correlation matrix. All covariates considered for inclusion in multivariable models were identified a priori using directed acyclic graphs [37].
Due to model convergence issues, we empirically investigated which time-fixed and time-varying covariates were associated with both recent depressive symptoms and each outcome (ie, were confounders). For the detectable viral load analysis, multivariable models included site; preexisting chart-documented mental health, diabetes, and hypertension diagnoses; HIV acquisition group; and lagged PRO measures of depressive symptoms, anxiety, drug use, alcohol risk, as well as lagged antidepressant prescription information. For the appointment adherence analysis, we considered 2 adjustment sets. The first included preexisting chart-documented diabetes or hypertension diagnoses, gender, race/ethnicity, age, HIV acquisition group, and lagged PRO measures of drug use and alcohol risk, as well as lagged antidepressant prescription, CD4 count, and detectable viral load. The second adjustment set additionally included preexisting chart-documented mental health diagnoses and lagged PRO measures of anxiety and depressive symptoms in order to understand how including preexisting and lagged mental health–related factors influenced effect estimates. Time-fixed and time-varying continuous confounders were modeled using restricted cubic splines with 4 knots.
In sensitivity analysis, we used multiple imputation to account for all missing data and inverse probability of visit weights (IPVW) to account for the fact that some participants had more frequent viral load measures or HIV appointments than others [38]. Additional details about sensitivity analysis are available in the Supplementary Materials. All statistical analyses were conducted in Stata, version 13 (StataCorp, College Station, TX) or SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
We included 1057 HIV-infected adults who initiated ART in CNICS between 2005 and 2015 with a valid viral load measure and 1050 who had both a valid viral load and HIV appointment measure (Figure 1). Participants contributed 2424 person-years (median follow-up time, 735 days) to the detectable viral load analysis and 2416 person-years (median follow-up time, 744 days) to the appointment adherence analysis.
At ART initiation, 30% of participants reported clinically meaningful depressive symptoms (Table 1). Over the follow-up period, 1057 participants had 7733 viral load measures, 28% of which indicated a detectable viral load (≥75 copies/mL), and 1050 participants had 14131 HIV appointments, 18% of which were missed. Participants had a median of 5 viral load measures (interquartile range [IQR], 3–8), with a median of 104 days (IQR, 64–161) between measures, and a median of 9 HIV appointments (IQR, 4–18), with a median of 41 days between appointments (IQR, 15–92). More than half (53%) of participants were administratively censored, 29% were lost to follow-up, 1% died, and 17% were censored at 5 years of follow-up.
Table 1.
Characteristics at Antiretroviral Therapy (ART) Initiation and Over Follow-up of 1057 Human Immunodeficiency Virus–Infected Adults Who Initiated ART Between 2005 and 2015 in the Centers for AIDS Research Network of Integrated Clinical Systems Cohort
| Characteristic | At Antiretroviral Therapy Initiation, | Over Follow-up, |
|---|---|---|
| (n = 1057) Participants | (n = 2424) Person-Years | |
| n (%) | n (%) | |
| Time-fixed | ||
| Site | ||
| Fenway | 103 (9.7) | … |
| University of Alabama, Birmingham | 286 (27.1) | … |
| University of North Carolina, Chapel Hill | 20 (1.9) | … |
| University of California, San Diego | 427 (40.4) | … |
| University of California, San Francisco | 54 (5.1) | … |
| University of Washington | 167 (15.8) | … |
| Age in years, median (IQR) | 36 (28, 45) | … |
| Gender | ||
| Male | 929 (87.9) | … |
| Female | 128 (12.1) | … |
| Race/ethnicity | ||
| White, non-Hispanic | 509 (48.6) | … |
| Black, non-Hispanic | 288 (27.5) | … |
| Hispanic | 191 (18.2) | … |
| Other | 59 (5.6) | … |
| HIV risk group | ||
| Intravenous drug user | 109 (10.4) | … |
| Men who have sex with men | 721 (68.7) | … |
| Heterosexual | 187 (17.8) | … |
| Other | 33 (3.1) | … |
| Previous mental health diagnosis | ||
| No | 687 (65.0) | … |
| Yes | 370 (35.0) | … |
| Previous medical diagnosis | ||
| No | 941 (89.0) | … |
| Yes | 116 (11.0) | … |
| Time-updated | ||
| Depressive symptoms | ||
| No (PHQ-9, <10) | 737 (69.7) | 3755 (74.3) |
| Yes (PHQ-9, ≥ 10) | 320 (30.3) | 1299 (25.7) |
| Viral load | ||
| Undetectable (<75 copies/mL) | 0 (0.0) | 5964 (77.1) |
| Detectable (≥75 copies/mL) | 1057 (100.0) | 1769 (22.9) |
| HIV appointment status | ||
| Missed appointment | … | 2492 (17.6) |
| Kept appointment | … | 11639 (82.4) |
| Depressive symptom score (PHQ-9, range 0–27), median (IQR) | 5 (1, 11) | 4 (1, 10) |
| Panic disorder | ||
| No symptoms | 716 (68.9) | 4032 (71.5) |
| Some symptoms | 179 (17.2) | 864 (15.3) |
| Panic disorder | 145(13.9) | 746 (13.2) |
| Antidepressant prescription | ||
| Not on antidepressants | 900 (85.2) | 6101 (78.9) |
| On antidepressants | 157 (14.9) | 1632 (21.1) |
| Drug use | ||
| No current use | 437 (46.2) | 2676 (50.2) |
| Current use | 218 (23.0) | 998 (18.7) |
| Past use | 291 (30.8) | 1656(31.1) |
| Alcohol usea | ||
| Not at risk drinking | 816 (80.5) | 4710 (84.9) |
| At risk drinking | 198 (19.5) | 840 (15.1) |
| CD4 count, cells/mm3, median (IQR) | 334 (167, 488) | 426 (258, 616) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; PHQ-9, Patient Health Questionnaire-9.
aDefined as alcohol use disorders identification test score >4 for males and >3 for females.
Study participants were primarily male (88%), white non-Hispanic (49%), and reported contracting HIV through male-to-male sexual contact (69%; Table 1). At ART initiation (baseline), 35% of participants had a preexisting mental health diagnosis, 14% had PRO-measured panic disorder, and 15% had an antidepressant prescription. Of the 320 participants (30%) with depressive symptoms at baseline, 47% had a preexisting mental health diagnosis documented in their chart at the time of ART initiation and 27% had a preexisting chart-documented depression diagnosis. Nearly a quarter (23%) of participants reported current drug use, and 20% reported at-risk alcohol use on PRO measures at baseline.
Undetectable Viral Load Analysis
In the unadjusted (crude) model, recent depressive symptoms were associated with a 37% average increase in the risk of having a detectable viral load over time (RR, 1.37; 95% CI, 1.20, 1.56). After adjustment for time-fixed and time-varying confounders, recent depressive symptoms remained associated with an increased risk of having a detectable viral load over time (RR, 1.28; 95% CI, 1.07, 1.53; Table 2 and Supplementary Table 1). Effect estimates did not change meaningfully when missing data were imputed (RR, 1.22; 95% CI, 1.04 1.42) or when IPVW were used (RR, 1.23; 95% CI, 1.01, 1.49; Supplementary Table 2). When depressive symptoms were considered as a continuous measure, higher (worse) depressive symptom scores were associated with an increasing risk of having a detectable viral load. However, data were sparse at the highest depressive symptom scores (Supplementary Figure 1).
Table 2.
Risk Ratios and 95% Confidence Intervals for the Association Between Having Recent Depressive Symptoms and the Risk of an Adverse Outcome Over Time Along the Human Immunodeficiency Virus Treatment Cascade
| Depressive Symptom Status | Detectable Viral Loada | Missed Human Immunodeficiency Virus Visit | |||
|---|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjusted Set 1c | Adjusted Set 2d | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| No depressive symptoms (PHQ-9 <10) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Depressive symptoms (PHQ-9 ≥ 10) | 1.37 (1.20, 1.56) | 1.28 (1.07, 1.53) | 1.24 (1.07, 1.39) | 1.20 (1.05, 1.36) | 1.00 (0.85, 1.18) |
RRs and 95% CIs estimated from a generalized estimating equation log binomial model with a robust variance estimator and an exchangeable correlation matrix to account for repeated measures.
Abbreviations: CI, confidence interval; PHQ-9, Patient Health Questionnaire-9; RR, risk ratio.
aDetectable viral load defined as ≥75 copies/mL.
bAdjusted for site; preexisting mental health, hypertension, or diabetes diagnoses; human immunodeficiency virus (HIV) acquisition group; lagged anxiety level; lagged drug use; lagged alcohol risk; lagged antidepressant use; and lagged depressive symptoms.
cAdjustment set 1: preexisting hypertension or diabetes diagnoses, gender, race/ethnicity, age, HIV acquisition group, lagged CD4 count, lagged drug use, lagged alcohol risk, lagged antidepressant use, and lagged detectable viral load.
dAdjustment set 2: all variables in adjustment set 1, as well as preexisting mental health diagnoses, lagged anxiety level, and lagged depressive symptoms.
Appointment Adherence Analysis
In the unadjusted model, recent depressive symptoms were associated with a 24% average increase in the risk of missing a subsequent HIV appointment over time (RR, 1.24; 95% CI, 1.07, 1.39). The effect estimate was similar (RR, 1.20; 95% CI, 1.05, 1.36; Table 2 and Supplementary Table 1) in multivariable analysis that did not include lagged PRO measures of depressive or anxiety symptoms and preexisting mental health diagnoses (adjustment set 1). When these variables were additionally included (adjustment set 2), the RR for the association between recent depressive symptoms and HIV appointment adherence moved to the null (RR, 1.00; 95% CI, 0.85, 1.18). Effect estimates with the full multivariable adjustment set (adjustment set 2) did not change meaningfully when missing data were imputed (RR, 1.07; 95% CI, 0.95, 1.21) or when IPVW were used (RR, 1.10; 95% CI, 0.97, 1.23; Supplementary Table 2).
DISCUSSION
In a large cohort of HIV-infected adults initiating ART, we observed that impaired mental health adversely affected engagement in care along the HIV treatment cascade. Over a median of approximately 2 years on ART, recent, clinically meaningful depressive symptoms were associated with an increased risk of having a detectable viral load over time, controlling for sociodemographic and clinical factors and for preexisting mental health diagnoses. For HIV appointment adherence, recent depressive symptoms were also associated with an increased risk of missing the next HIV visit after adjustment for sociodemographic and clinical confounders. However, this association moved to the null after further adjustment for preexisting mental health diagnoses and lagged measures of depressive and anxiety symptoms. These results suggest that for new ART users, recent depressive symptoms are a risk factor for unsuppressed viral load, while preexisting mental health conditions may play a larger role in HIV appointment adherence.
For clinicians who treat people living with HIV, understanding how depression affects engagement in HIV care and progression through the HIV treatment cascade is complex. Depressive symptoms change over time and frequently reoccur, particularly among HIV-infected adults [39–41]. The episodic nature of depression raises questions for clinicians about whether a patient’s depression status at a given HIV visit or mental health history is a more clinically relevant risk factor for disengagement from HIV care. Further, patients may disengage from HIV care in multiple ways, all of which may have distinct risk factors. Our analysis helps to clarify these important relationships by demonstrating that recent depressive symptoms and preexisting mental health conditions are likely both risk factors for adverse outcomes but affect aspects of engagement in HIV care differently.
In our analysis, recent depressive symptoms consistent with major depressive disorders were associated with an increased risk of detectable viral load but not with HIV appointment adherence. Depressive symptoms have been linked to reduced ART adherence [4, 5] and subsequent unsuppressed viral load in the past [6–8]. However, the extent to which suboptimal adherence may affect clinic attendance or whether missing HIV visits also increases the risk of detectable viral load is not clear. Our results suggest that the increased risk of detectable viral load among persons with current depressive symptoms is most likely driven by reduced ART adherence or possibly the deleterious effect of depression on viral replication [8, 42], and not on an inability to regularly attend HIV visits. Conversely, preexisting mental health conditions, rather than recent depressive symptoms, may be more likely to affect HIV appointment adherence.
Our analysis has several strengths and limitations. Strengths include the use of a large, diverse cohort of HIV-infected adults across the United States; the ability to account for recent depressive symptoms, as well as preexisting mental health diagnoses and comorbid panic disorder symptoms; and the use of a validated measure for clinically meaningful depressive symptoms, as well as a range of other time-fixed and time-varying covariates. Limitations of our analysis include not having information on counseling or other behavioral health assistance, income, or other measures of instability, such as homelessness or insurance status. Participants in our analysis were enrolled in routine HIV care at 6 large, academic medical centers across the United States. Our results may be less generalizable to care settings that serve different populations of HIV-infected adults (ie, a larger population of HIV-infected women).
CONCLUSIONS
When it comes to engaging HIV-infected adults in care across the HIV treatment cascade, both recent and preexisting mental health conditions matter. For new ART users, recent clinically meaningful depressive symptoms may be a risk factor for detectable viral load over time, while having preexisting mental health conditions may be more likely to affect HIV appointment adherence over time. In order to keep HIV-infected adults engaged in care and virally suppressed, routine, ongoing depressive symptom screening and treatment by HIV providers are essential. For patients with a history of mental health conditions, even if they are not currently depressed, additional supportive services such as peer navigators and transportation assistance may be important to help ensure regular HIV visit attendance. A number of interventions exist to improve depression in HIV-infected adults, including counseling and pharmacotherapy [43–45]. Future studies should explore how such interventions could be combined with additional supportive services to optimize engagement and HIV care outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. We thank the National Institutes of Health (NIH; grants R01MH100970, R24AI067039, L30 MH110572, K99MH112413) for their support of this work. We also thank the Center for AIDS Research sites involved in CNICS including the University of Alabama at Birmingham (P30 AI027767), University of Washington (P30 AI027757), University of California–San Diego (P30 AI036214), University of California–San Francisco (P30 AI027763), Case Western Reserve University (P30 AI036219), Johns Hopkins University (P30 AI094189, U01 DA036935), Fenway Health/Harvard (P30 AI060354), and University of North Carolina Chapel Hill (P30 AI50410).
Potential conflicts of interest. K. C. reports grants from the NIH during the conduct of the study and grants and personal fees from Gilead and personal fees from Roche outside the submitted work. H. C. reports grants from the NIH during the conduct of the study and grants from the NIH and Patient-Centered Outcomes Research Institute, and from VIIV, outside the submitted work. All other authors report no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001; 58:721–8. [DOI] [PubMed] [Google Scholar]
- 2. Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 2001; 158:725–30. [DOI] [PubMed] [Google Scholar]
- 3. O’Cleirigh C, Newcomb ME, Mayer KH, Skeer M, Traeger L, Safren SA. Moderate levels of depression predict sexual transmission risk in HIV-infected MSM: a longitudinal analysis of data from six sites involved in a “prevention for positives” study. AIDS Behav 2013; 17:1764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr 2011; 58:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr 2008; 47:384–90. [DOI] [PubMed] [Google Scholar]
- 6. Ickovics JR, Hamburger ME, Vlahov D, et al. ; HIV Epidemiology Research Study Group Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA 2001; 285:1466–74. [DOI] [PubMed] [Google Scholar]
- 7. Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med 2005; 67:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leserman J, Jackson ED, Petitto JM, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med 1999; 61:397–406. [DOI] [PubMed] [Google Scholar]
- 9. Murphy K, Hoover DR, Shi Q, et al. Association of self-reported race with AIDS death in continuous HAART users in a cohort of HIV-infected women in the United States. AIDS 2013; 27:2413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr 2009; 51:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Villes V, Spire B, Lewden C, et al. ; ANRS CO-8 APROCO-COPILOTE Study Group The effect of depressive symptoms at ART initiation on HIV clinical progression and mortality: implications in clinical practice. Antivir Ther 2007; 12:1067–74. [PubMed] [Google Scholar]
- 12. Antelman G, Kaaya S, Wei R, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr 2007; 44:470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Todd JV, Cole SR, Pence BW, et al. Effects of antiretroviral therapy and depressive symptoms on all-cause mortality among HIV-infected women. Am J Epidemiol 2017; 185:869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asch SM, Kilbourne AM, Gifford AL, et al. ; HCSUS Consortium Underdiagnosis of depression in HIV: who are we missing?J Gen Intern Med 2003; 18:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pence BW, O’Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep 2012; 14:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pence BW, O’Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 2012; 26:656–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011; 52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. UNAIDS. 90-90-90: treatment for all. Available at: http://www.unaids.org/en/resources/909090. Accessed 5 January 2018. [Google Scholar]
- 19. Gardner LI, Giordano TP, Marks G, et al. ; Retention in Care Study Group Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis 2014; 59:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis 2013; 57:1164–71. [DOI] [PubMed] [Google Scholar]
- 21. Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012; 156:817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis 2009; 48:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giordano TP, Gifford AL, White AC Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 2007; 44:1493–9. [DOI] [PubMed] [Google Scholar]
- 24. Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crane PK, Gibbons LE, Willig JH, et al. Measuring depression levels in HIV-infected patients as part of routine clinical care using the nine-item Patient Health Questionnaire (PHQ-9). AIDS Care 2010; 22:874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiat Ann 2002; 32:509–15. [Google Scholar]
- 28. Cassell A, Edwards D, Harshfield A, et al. The epidemiology of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2018; 68:e245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park C, Fang J, Hawkins NA, Wang G. Comorbidity status and annual total medical expenditures in U.S. hypertensive adults. Am J Prev Med 2017; 53:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care 2013; 25:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282:1737–44. [DOI] [PubMed] [Google Scholar]
- 32. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG.. AUDIT, the alcohol use disorders identification test. Guidelines for use in primary care. Geneva, Switzerland: World Health Organization, 2001. [Google Scholar]
- 33. WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction 2002; 97:1183–94. [DOI] [PubMed] [Google Scholar]
- 34. Humeniuk R, Ali R, Babor TF, et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008; 103:1039–47. [DOI] [PubMed] [Google Scholar]
- 35. Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014; 11:291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. VanderWeele TJ, Hernán MA, Robins JM. Causal directed acyclic graphs and the direction of unmeasured confounding bias. Epidemiology 2008; 19:720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med 2011; 8:e1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carta MG, Angst J, Moro MF, et al. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord 2012; 141:361–6. [DOI] [PubMed] [Google Scholar]
- 40. Choi SK, Boyle E, Cairney J, et al. Prevalence, recurrence, and incidence of current depressive symptoms among people living with HIV in Ontario, Canada: results from the Ontario HIV Treatment Network Cohort Study. PLoS One 2016; 11:e0165816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson JG, Rabkin JG, Lipsitz JD, Williams JB, Remien RH. Recurrent major depressive disorder among human immunodeficiency virus (HIV)-positive and HIV-negative intravenous drug users: findings of a 3-year longitudinal study. Compr Psychiatry 1999; 40:31–4. [DOI] [PubMed] [Google Scholar]
- 42. Leserman J, Petitto JM, Gu H, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med 2002; 32:1059–73. [DOI] [PubMed] [Google Scholar]
- 43. Spies G, Asmal L, Seedat S. Cognitive-behavioural interventions for mood and anxiety disorders in HIV: a systematic review. J Affect Disord 2013; 150:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep 2015; 17:530. [DOI] [PubMed] [Google Scholar]
- 45. Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev 2018; 1:Cd008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.