Abstract
Background
Lyme disease is the most common reportable zoonotic infection in the United States. Recent data suggest spread of the Ixodes tick vector and increasing incidence of Lyme disease in several states, including Pennsylvania. We sought to determine the clinical presentation and healthcare use patterns for pediatric Lyme disease in western Pennsylvania.
Methods
The electronic medical records of all patients with an International Classification of Disease, Ninth Revision, diagnosis of Lyme disease between 2003 and 2013 at Children’s Hospital of Pittsburgh were individually reviewed to identify confirmed cases of Lyme disease. The records of 773 patients meeting these criteria were retrospectively analyzed for patient demographics, disease manifestations, and healthcare use.
Results
An Lyme disease increased exponentially in the pediatric population of western Pennsylvania. There was a southwestward migration of Lyme disease cases, with a shift from rural to nonrural zip codes. Healthcare provider involvement evolved from subspecialists to primary care pediatricians and emergency departments (EDs). Patients from nonrural zip codes more commonly presented to the ED, while patients from rural zip codes used primary care pediatricians and EDs equally.
Conclusions
The current study details the conversion of western Pennsylvania from a Lyme-naive to a Lyme-epidemic area, highlighting changes in clinical presentation and healthcare use over time. Presenting symptoms and provider type differed between those from rural and nonrural zip codes. By elucidating the temporospatial epidemiology and healthcare use for pediatric Lyme disease, the current study may inform public health measures regionally while serving as an archetype for other areas at-risk for Lyme disease epidemics.
Keywords: Lyme disease, epidemic, epidemiology, pediatric, geographic spread
We identified 773 children with Lyme disease in western Pennsylvania, a previously Lyme-naive region. Over 10 years, the number of cases increased exponentially, with the highest burden of infection shifting from rural to nonrural areas and affecting healthcare use.
Lyme disease is the most common reportable vector-borne infection in the United States [1]. Since its initial description in 1977 during an epidemic of inflammatory arthritis in children in Connecticut, epidemiologic studies have identified the complex zoonotic life cycle of the infectious agent, Borrelia burgdorferi [2–4]. Over the past 4 decades, Lyme disease has become increasingly common throughout the Northeastern and Mid-Atlantic states, as well as regions of Minnesota and Wisconsin [5–10]. In the Mid-Atlantic region, Lyme disease has been largely restricted to areas east of the Appalachian Mountains [11, 12]. Within western Pennsylvania, populations of the tick vector, Ixodes scapularis, have expanded in number and become increasingly infected with B. burgdorferi [13, 14]. Ticks infected with B. burgdorferi are now detectable in every county in Pennsylvania, with infection rates similar to that of endemic Northeastern states [13, 14].
In clinical practice, we observed an increase in the number of patients diagnosed with Lyme disease at Children’s Hospital of Pittsburgh (CHP) of the University of Pittsburgh Medical Center, which prompted our investigation to assess the burden of Lyme disease in children. Specifically, we used our electronic medical record (EMR) to perform a retrospective analysis of Lyme disease cases in patients evaluated at CHP and associated community practices. We used these data to determine the clinical presentation, healthcare use, and geographic location of cases. We sought to better understand the changing characteristics of pediatric Lyme disease and the implications for healthcare use.
METHODS
Case Ascertainment
The CHP EMR system was queried for all patients from 2003 to 2013 to identify potential cases of Lyme disease based on an International Classification of Disease, Ninth Revision (ICD-9) code of 088.81. After 908 patients with an ICD-9 code for Lyme disease were identified, their medical records ere individually evaluated using Centers for Disease Control and Prevention (CDC) 2011 criteria for Lyme disease [1, 15]. These criteria for a confirmed case of Lyme disease included a case of erythema migrans (EM) with a known exposure, a case of EM with laboratory evidence of infection without a known exposure, and any case with ≥1 late manifestation (eg, joint, nervous system, or cardiovascular involvement) with laboratory evidence of infection [15]. Exposure is defined as having been in wooded, brushy, or grassy areas in a county in which Lyme disease is endemic (>2 cases) less than 30 days before disease presentation [15]. Laboratory evidence of infection was defined as positive results of 2-tier testing by enzyme-linked immunosorbent assay and Western blot analysis, per CDC guidelines [16]. A total of 773 patients meeting these criteria were included. Each excluded patient (n = 135) was reviewed by ≥2 physician authors before exclusion.
Patient Information
Patient demographics, symptoms, laboratory data, healthcare provider type, and disease outcome were recorded from the EMR. The location of diagnosis was classified as the physician ordering serologic studies or making the clinical diagnosis of Lyme disease. All patient information was handled securely, as approved by the institutional review board (protocol number PRO17110480). Data were deidentified, with the exception of zip code.
Geographic Characteristics
Patient zip codes were classified as rural using Federal Office of Rural Health Policy guidelines (ie, if >50% of the population resided in either a nonmetropolitan county or a rural census tract). The zip codes corresponding to Pittsburgh, Pennsylvania, in the United States Postal Service system were used to identify Pittsburgh residents. Patient zip code data were then analyzed using Tableau software, version 10.4 (Tableau Desktop Professional edition 10.4).
Statistical Analysis
Normally distributed continuous variables were presented as means with interquartile range (IQR); nonparametric continuous and count variables, as medians with IQR; and categorical variables, as proportions. Univariable logistic regressions were used to evaluate the association between sex and year and tick bite and year, with time serving as a categorical variable. To determine the association between categorical age, seasonality, and categorical time we used ordered logistic regression. Multinomial logistic regression was used to evaluate the association between race (white, African American, or Asian, unknown) and categorical time. All P values for the relationship between categorical time were based on trend.
Linear regression was performed on rural/nonrural symptoms by year as well as multiple subspecialty involvement and admissions by year. Slopes were analyzed for significant deviation from zero. In addition, slopes of the linear regression lines comparing rural and nonrural symptoms were analyzed for significant differences. We used nonlinear, exponential regression to analyze the number of cases over time and performed χ2 analysis was performed of the incidence of symptoms by geographic location. All statistical tests were evaluated using an α value of .05 and performed using StataSE (version 14; StataCorp) or GraphPad Prism (version 7.0; GraphPad) software.
RESULTS
Demographics and Laboratory Findings of Pediatric Lyme Disease
A total of 908 cases had an ICD-9 diagnosis of Lyme disease at CHP from 2003 to 2013. Of patients with this diagnosis, 773 met the 2011 CDC criteria for Lyme disease (Table 1). Of these patients, 262 had Lyme disease diagnosed clinically based on EM rash alone. Serologic diagnosis using CDC 2-tiered testing was performed in 511 patients, with 193 immunoglobulin M (IgM)+/immunoglobulin G (IgG)−, 121 IgG+/IgM−, and 197 IgM+/IgG+ positive Western blots.
Table 1.
Case Ascertainment, Patient Demographics, Laboratory Data, and Treatment Profiles of Diagnosed Lyme Disease Cases
| Case ascertainment, No. (%) | |
| ICD-9 code (088.81) | 908 |
| Met CDC definitiona | 773 |
| Clinical diagnosisb | 262 (34) |
| IgM+/IgG− | 193 (25) |
| IgM−/IgG+ | 121 (16) |
| IgM+/IgG+ | 197 (25) |
| Demographics, No. (%) | |
| Age, y | |
| 0–4 | 171 (22) |
| 5–9 | 347 (45) |
| ≥10 | 255 (33) |
| Sex | |
| Male | 457 (59) |
| Female | 316 (41) |
| Race | |
| White | 718 (93) |
| African American | 22 (3) |
| Asian | 1 (<1) |
| Unknown | 32 (4) |
| Tick bite | |
| Yes | 222 (29) |
| No | 551 (71) |
| Month of diagnosis | |
| January–April | 77 (10) |
| May–August | 478 (62) |
| September–December | 218 (28) |
| Laboratory data, median (IQR) No. (%) | |
| CSF (n = 61) | |
| WBCs/µL | 21 (2–78) |
| Neutrophils, % | 6 (3–22) |
| Lymphocytes, % | 71 (52–81) |
| Monocytes, % | 12 (6–20) |
| Joint (n = 86) | |
| WBCs/µL | 41000 (25000–63000) |
| Neutrophils, % | 90 (82–95) |
| Lymphocytes, % | 4 (2–6) |
| Monocytes, % | 6 (4–11) |
| Treatment, No. (%) | |
| Doxycycline | 367 (47) |
| Amoxicillin | 346 (45) |
| Cephalosporin | 31 (4) |
| Home intravenous antibiotics | 16 (2) |
Abbreviations: CDC, Centers for Disease Control and Prevention; CSF, cerebrospinal fluid; ICD-9, International Classification of Disease, Ninth Revision; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; WBCs, white blood cells.
aCDC case definition according to 2011 guidelines.
bAll clinically diagnosed cases had a single erythema migrans rash without serologic diagnosis.
Forty-five percent of patients were between 5 and 9 years of age, consistent with previously published CDC surveillance data (Table 1 and Supplementary Table 1) [1, 17]. The patient population was predominately male (59%) and white (93%). Twenty-nine percent of patients reported a history of tick bite. Lyme disease was more commonly diagnosed in May through August, accounting for 62% of cases.
The study population had several laboratory findings consistent with previously published clinical observations of pediatric Lyme disease [18–20]. A total of 61 patients underwent lumbar puncture with subsequent cerebrospinal fluid analysis that demonstrated a lymphocytic pleocytosis. Likewise, elevated synovial fluid white blood cell counts with neutrophilic predominance were observed in the 89 arthrocenteses performed (Table 1).
All 773 patients received treatment for Lyme disease, with doxycycline (47%) and amoxicillin (44%) the most commonly prescribed oral antibiotics. Outpatient intravenous antibiotics were used to treat 15 cases of Lyme meningitis and 1 case of Lyme arthritis (Table 1).
Symptoms of Pediatric Lyme Disease by Age and Month of Presentation
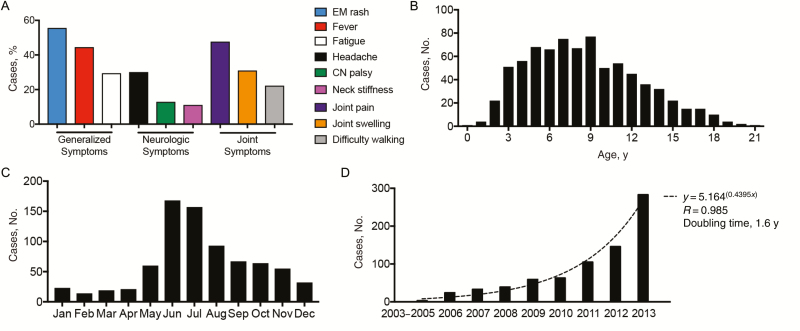
The most common symptom reported or observed in the total study population was EM rash (56%), followed by joint pain (47%) and fever (45%) (Figure 1A). Fatigue and headache were present in 30% of cases, and joint swelling and difficulty walking were present in 31% and 22%, respectively. Neurologic symptoms, such as cranial nerve (CN) palsy (12%) and neck stiffness (11%), were less common.
Figure 1.
Symptoms of Lyme disease by age, month, and year of diagnosis in children. A, Incidence of individual symptoms in all cases of Lyme disease. B, Number of cases by age during the study period. C, Number of cases by month during the study period. D, Number of cases by year during the study period, with a nonlinear exponential regression trend line. Abbreviations: Apr, April; Aug, August; CN, cranial nerve; Dec, December; EM, erythema migrans; Feb, February; Jan, January; Jul, July; Jun, June; Mar, March; Nov, November; Oct, October; Sep, September.
The median age at diagnosis was 8.1 years (IQR, 5.3–11.3 years) (Figure 1B). Lyme disease was diagnosed in all months of the year, but diagnoses peaked in June and July, with a nadir in February (Figure 1C). There was an exponential increase in the number of cases of pediatric Lyme disease over the study period, with a calculated doubling time of 1.6 years (Figure 1D).
The median (IQR) age was 7.7 (4.9–11.1) years in children presenting with EM, and 8.2 (5.5–11.4) years in those with fever. Fatigue was reported in patients with a median (IQR) age of 8.6 (6.5–11.8) years (Supplementary Figure 1A). Children with neurologic manifestations, such as headache, CN palsy, and neck stiffness, had median ages at presentation of 9.2, 9.3, and 9.0 years, respectively (Supplementary Figure 1B). Joint pain, joint swelling, and difficulty walking were found in similar age ranges (median age [IQR], 8.5 [5.8–11.4], 8.3 [5.8–11.3], and 7.3 [4.9–10.5] years, respectively) (Supplementary Figure 1C).
EM, fever, and fatigue were more commonly seen in May, June, and July (Supplementary Figure 1D). Neurologic findings, such as CN palsy and neck stiffness were more common in April and May and continued into the summer months (Supplementary Figure 1E). Joint pain was seen in both summer and winter months, and joint swelling and difficulty walking were more common in winter (Supplementary Figure 1F).
Healthcare Use in Patients With Lyme Disease in Western Pennsylvania
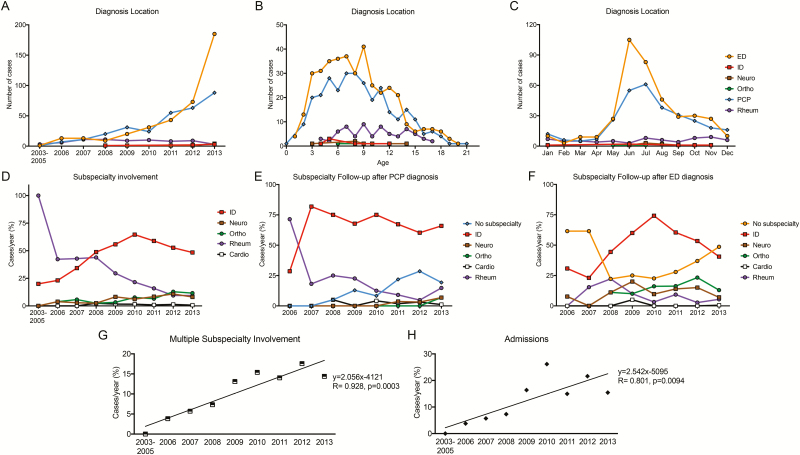
As the incidence of Lyme disease increased over time, the type of provider changed. The number of diagnoses made at a pediatric subspecialty clinic was comparable to emergency department (ED) and primary care pediatrician (PCP) diagnoses until 2008 (Figure 2A). From 2008–2012, diagnoses made at PCP clinics and EDs increased equally over time, whereas the frequency of diagnoses by subspecialty providers was unchanged (Figure 2A). In 2013, there was a surge in ED visits compared with PCP visits (Figure 2A). Patients at ED and PCP sites had similar age ranges, whereas those with Lyme disease diagnosed at a subspecialist had a more constricted age range (Figure 2B). ED diagnoses peaked in June and July, whereas PCP visits had a more blunted increase in these months (Figure 2C). Subspecialist diagnosis was more consistent throughout the year, albeit less common (Figure 2C).
Figure 2.
Healthcare use in children with Lyme disease over time. A–C, Location of diagnosis by year, age, and month of disease presentation. D, Percentage of cases per year with infectious diseases (ID), neurology (Neuro), orthopedic surgery (Ortho), rheumatology (Rheum), or cardiology (Cardio) involvement. E, Percentage of cases per year with subspecialty follow-up after diagnosis was made at the office of the primary care pediatrician (PCP). F, Percentage of cases per year with subspecialty follow-up after diagnosis in the emergency department (ED). G, Percentage of cases per year with multiple subspecialists involved in management. H, Percentage of cases per year admitted for Lyme disease. Abbreviations: Apr, April; Aug, August; Dec, December; Feb, February; Jan, January; Jul, July; Jun, June; Mar, March; Nov, November; Oct, October; Sep, September.
A number of subspecialists were involved in the care of children with Lyme disease. Early in the epidemic of Lyme disease in western Pennsylvania, joint symptoms were the predominant complaint. This was reflected by rheumatology being the most common subspecialty involved in care (Figure 2D). Over time, infectious diseases (ID) specialists became increasingly used, reaching a peak in 2010 (Figure 2D). Other subspecialties, such as orthopedic surgery, neurology, and cardiology, had smaller increases in use over time (Figure 2D).
Interestingly, the percentage of patients with subspecialty follow-up was associated with the location of the initial diagnosis. Nearly 75% of patients with Lyme disease diagnosed at the PCP office had follow-up with ID physicians, and this practice was adopted quickly (Figure 2E). Over time, the number of cases managed at the PCP office without subspecialty follow-up also increased (Figure 2E). In contrast, patients with Lyme disease diagnosed in the ED were less likely to be seen by a subspecialist early in the epidemic, though there was a peak in ID follow-up in 2010 (Figure 2F). The number of ED diagnoses without follow-up also rose in the latter half of the study period, becoming the most common outcome in ED-diagnosed cases in 2013 (Figure 2F).
Over the course of the epidemic, the proportion of patients requiring care by multiple subspecialists increased (Figure 2G). Likewise, the number of cases per year requiring admission increased over the study period (Figure 2H). Admissions were due to arthritis (47%), meningitis (38%), CN palsy (9%), rash with fever (4%), and carditis (2%) and had a median duration of 3 days.
Geographic Expansion of Lyme Disease From Rural to Nonrural Zip Codes in 2003–2013
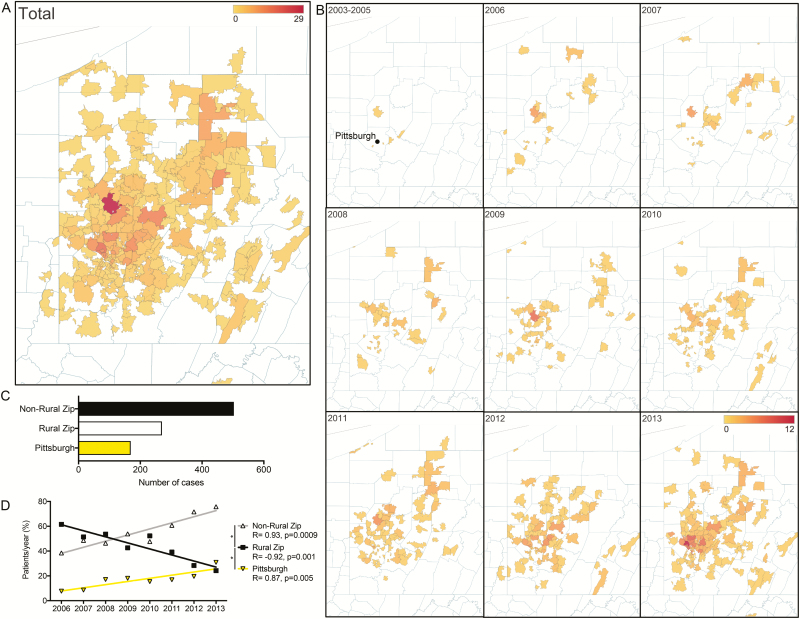
The catchment area for Lyme disease diagnosis within the CHP system encompassed nearly all of western Pennsylvania (Figure 3A). Cases of Lyme disease were also recorded from adjacent areas of Ohio, West Virginia, Maryland, and New York (data not shown). Over time, there was an observable southwestward expansion of Lyme disease cases (Figure 3B and Supplementary Figure 2). Butler County, north of Pittsburgh, was the most common site of Lyme disease in 2008–2010, which shifted to Allegheny County and Pittsburgh by 2011–2013 (Figure 3B). Interestingly, between 2011 and 2013, Allegheny County had a total of 12 reported cases of Lyme disease based on CDC surveillance data, a striking difference from the 221 cases of pediatric Lyme disease identified by the retrospective chart review [1, 21].
Figure 3.
Geographic expansion of Lyme disease in Western Pennsylvania from 2003 to 2013. A, All cases of Lyme disease by zip code, demonstrating a broad catchment area. B, Increase in Lyme disease cases in a westward and southward direction over time. C, Number of cases originating from rural zip codes, nonrural zip codes, and zip codes corresponding to the city of Pittsburgh. D, Linear regression of the number of cases per year for rural, nonrural, and Pittsburgh zip codes over time demonstrating significantly different slopes ****P < .001.
During the study period, 270 cases of Lyme disease came from rural zip codes, as classified by the Federal Office of Rural Health Policy guidelines, and 503 came from nonrural zip codes (Figure 3C). The number of Lyme disease cases seen within the CHP system from nonrural zip codes increased significantly over time, with a concomitant significant decrease in cases from rural zip codes (Figure 3D). In addition, 169 cases of Lyme disease originated in the city of Pittsburgh, with a higher frequency of cases over time (Figure 3C and 3D).
Differences in Healthcare Use for Lyme Disease in Rural vs Nonrural Communities
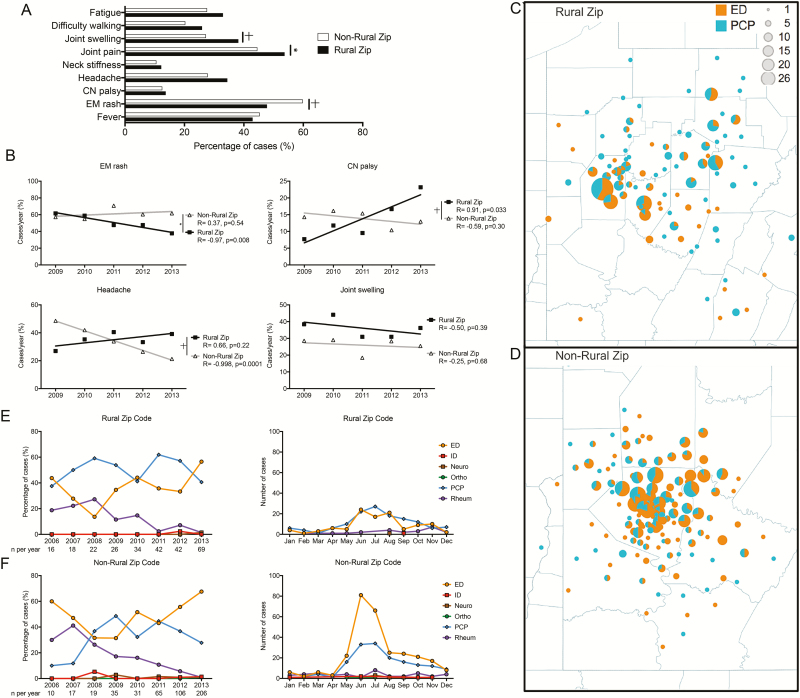
Residents of rural zip codes had a significantly higher percentage of cases with joint pain and joint swelling than those from nonrural zip codes (Figure 4A). Conversely, patients from nonrural zip code had a higher incidence of EM (Figure 4A). Over the second half of the study period, patients from rural zip codes had a significant decrease in cases with EM, while nonrural patients maintained a steady incidence (Figure 4B). Patients from rural zip codes also had a significant increase in cases with CN palsy over time, and those from nonrural zip codes had a significant decrease in cases with headache (Figure 4B). Joint swelling was more commonly reported each year in rural patients (Figure 4B).
Figure 4.
Presentation of children in rural and nonrural zip codes differs in both presentation and healthcare use patterns. A, Symptom frequency in nonrural and rural zip codes. *P < .05; †P < .01 (χ2 test). B, Individual symptoms over time in rural and nonrural zip codes demonstrating significant differences in the slopes of erythema migrans (EM) rash, cranial nerve (CN) palsy, and headache. *P < .01; †P < .001. C–D, Geographic representation of healthcare use in rural and nonrural zip codes. E–F, Diagnostic location by year and month for rural and nonrural zip codes. Abbreviations: Apr, April; Aug, August; Cardio, cardiology; Dec, December; ED, emergency department; Feb, February; ID, infectious diseases; Jan, January; Jul, July; Jun, June; Mar, March; Nov, November; Oct, October; Ortho, orthopedic surgery; PCP, primary care pediatrician; Rheum, rheumatology; Sep, September.
In rural zip codes, Lyme disease was more commonly diagnosed at the PCP office (Figure 4C). In contrast, patients from nonrural zip codes more frequently presented to the ED (Figure 4D). Cases from rural zip codes were diagnosed equally within PCP offices and EDs over the study period, with increases for both sites in the summer months (Figure 4E). However, 68% of patients from nonrural zip codes were seen in the ED in 2013, compared with 28% in the PCP office (Figure 4F). In addition, this higher volume of ED cases was largely seen the summer months (Figure 4F).
Similar presentations and healthcare use patterns were seen in patients from the city of Pittsburgh and those in rural zip codes. Patients within the city of Pittsburgh were more likely than rural patients to have EM and less likely to have joint pain and swelling (Supplementary Figure 3A). The primary location of diagnosis for patients from Pittsburgh was the ED (Supplementary Figure 3B). In 2011–2013, the ED during the summer months was the most common location for diagnosis of Lyme disease for patients from Pittsburgh (Supplementary Figure 3C and 3D).
DISCUSSION
The incidence of Lyme disease is increasing across the United States. Several studies have explored the geographic expansion of Lyme disease through Health Department reporting data and tick surveillance (Table 2). Health department studies in New York, Minnesota, Virginia, and Michigan have all indicated an increase in reported Lyme disease cases over the past few decades [5, 7–9, 22]. Moreover, recent entomologic studies have demonstrated the increased presence of B. burgdorferi–infected Ixodes ticks in previously Lyme-naive areas, such as Illinois, Ohio, North Dakota, and Iowa [11, 23–27]. One such study in Pennsylvania found B. burgdorferi–infected ticks in every county from 2012 to 2014, a change from prior tick surveillance studies [13, 14]. Little is known, however, about the consequences of such geographic expansion on how patients with Lyme disease interface with the healthcare system.
Table 2.
Summary of Recent Literature Describing the Epidemiology of Lyme Disease
| Study | Years of Study | Region | Conclusions |
|---|---|---|---|
| CDC surveillance studies | |||
| Bacon et al (2008) [17] | 1992–2006 | United States | Describes Lyme disease symptoms and location over time |
| Schwartz et al (2017) [1] | 2008–2015 | United States | Builds on Bacon et al [17]; geographic distribution of Lyme disease is expanding |
| Health Department studies | |||
| Chen et al (2005) [22] | 1990–2000 | New York | Mapping/modeling of spread in New York |
| Brinkerhoff et al (2014) [5] | 2000–2011 | Virginia | Changes in spatial distribution |
| Robinson et al (2015) [9] | 1996–2011 | Minnesota | Increase in tick-borne diseases |
| Lantos et al (2015) [8] | 2000–2014 | Virginia, North Carolina | Expansion of Lyme disease cases in Virginia |
| Lantos et al (2017) [7] | 2000–2014 | Michigan | Expansion of Lyme disease cases |
| Tick-based studies | |||
| Jobe et al (2007) [26] | 2006–2007 | Chicago, Illinois | Detected Borrelia-infected ticks |
| Wang et al (2014) [24] | 1989–2012 | Ohio | Borrelia life cycle present in Ohio |
| Stone et al (2015) [23] | 2012 | North Dakota | Detected Borrelia-infected ticks |
| Hutchinson et al (2015) [13] | 2012–2014 | Pennsylvania | Borrelia-infected ticks in every county |
| Eisen et al (2016) [11] | 1996–2015 | United States | Map of Borrelia-infected ticks in county |
| Oliver et al (2017) [27] | 1990–2013 | Iowa | Detected Borrelia-infected ticks |
| Clow et al (2017) [25] | 2014–2016 | Ontario, Canada | Ongoing expansion of Borrelia |
| Retrospective clinical studies | |||
| Current study | 2003–2013 | Western Pennsylvania | Pediatric healthcare use for patients with Lyme disease and rural/nonrural Lyme disease presentations |
Abbreviation: CDC, Centers for Disease Control and Prevention.
The current retrospective study, in addition to mapping the geographic spread of Lyme disease, sought to characterize how pediatric patients with Lyme disease present and use healthcare during the setting of an epidemic. The observed incidences of disease manifestations such as EM (56%), arthritis (31%), and carditis (<1%) were similar to those in nationwide CDC surveillance studies [1, 17]. CN palsies (13%) and meningitis (7%) were seen at high incidences, probably reflecting the higher frequency of neuroborreliosis in children [28–30]. In addition, boys aged 5–9 years had the highest incidence of disease, and we observed a similar seasonality of cases, with a peak in June and July [1, 17]. In total, our study population had similar demographics and disease manifestations compared to the greater US Lyme disease epidemic.
However, our study examined clinical data, including specific provider involvement and zip code of residence, extracted from the comprehensive EMR of a pediatric, tertiary-care hospital. This enabled us to comprehensively describe disease presentation and healthcare use for Lyme disease throughout western Pennsylvania during the conversion from a Lyme-naive area to a Lyme disease epidemic over a 10-year period. The study of the pediatric population at a single tertiary-care hospital also uniquely allowed for the consolidation and concentration of cases across a large geographic region.
From 2003 to 2005, five children with arthritis were seen by rheumatologists for Lyme disease, acting as sentinel events reminiscent of the original description by Steere et al [2] in Old Lyme, Connecticut. As the epidemic progressed, ID and other subspecialties (eg, orthopedic surgery, neurology) became increasing involved. An increased rate of Lyme disease–related admissions and subspecialty involvement reflected care of patients with meningitis, CN palsy, arthritis, and carditis, as the total burden of Lyme disease rose exponentially. In the latter years of the epidemic, as the burden of Lyme disease reached its highest during the study period, most cases were diagnosed at ED and PCP sites. These observations illustrate the impact of the geographic expansion of Lyme disease on presentation and provider involvement.
As Lyme disease spread in southwestward, a greater expansion of cases was observed in nonrural zip codes, including zip codes corresponding to the city of Pittsburgh, clarifying that this epidemic was a dynamic geographic phenomenon. Interestingly, Allegheny County, which includes Pittsburgh, had 12 total cases of Lyme disease reported at the height of the epidemic, compared with the 221 cases identified in the current retrospective chart review [1, 21]. This finding corroborates previous studies suggesting underreporting of Lyme disease occurs frequently, while demonstrating the utility of multiple methods of evaluating Lyme disease burden, including retrospective chart reviews [1, 31–34].
By integrating temporospatial epidemiology with clinical data, we observed that patients from rural and nonrural zip codes presented with differing symptoms and patterns of provider use. Patients from nonrural zip codes presented to the ED more frequently than patients from rural zip codes, often with manifestations of early Lyme disease (eg, rash). Conversely, patients from rural zip codes were more likely to seek care from a PCP with symptoms of late Lyme disease (eg, arthritis). This may reflect limited access to care in rural communities or may represent differing referral patters of rural and nonrural pediatricians. These observations suggest that targeted provider education and public health awareness based on community urbanization may be an effective strategy to enhance care as the geographic expansion of Lyme disease progresses.
The current study does have limitations. In a retrospective study, cases of Lyme disease may be excluded owing to inherent biases such as misclassification, because cases lacking an ICD-9 diagnosis would not have been included in the original search. In addition, most but not all children in western Pennsylvania are included in our integrated EMR, so our results probably underrepresent the true case numbers for pediatric Lyme disease. In attempting to understand the geographic trends, we used US postal zip codes as a geographic unit, which may not accurately capture the heterogeneity in demographics and urbanization within specific communities.
The current study does complement the existing body of literature surrounding the spread of Lyme disease by addressing how an epidemic of Lyme disease is experienced by a local healthcare system. Specifically, several areas that were once Lyme naive, including Ohio, Illinois, North Dakota, and Iowa, now have an increasing B. burgdorferi-infected tick population and are at-risk for expansion of Lyme disease cases, similar to how Pennsylvania was at the time of our study [13, 23–27]. In addition, our data from western Pennsylvania suggest a key role for pediatric referral facilities in detecting this expansion. An understanding of epidemic changes over time with regard to disease manifestation and healthcare provider use in western Pennsylvania could serve as a model for both rural and nonrural communities that may see an increase in Lyme disease cases.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Heart Lung and Blood Institute (grant K08HL128809 to B. T. C.) and the National Institute for Allergy and Infectious Diseases (grant F30AI114146 to T. E.), National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ 2017; 66:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 1977; 20:7–17. [DOI] [PubMed] [Google Scholar]
- 3. Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest 2004; 113:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med 2003; 348:2472–4. [DOI] [PubMed] [Google Scholar]
- 5. Brinkerhoff RJ, Gilliam WF, Gaines D. Lyme disease, Virginia, USA, 2000–2011. Emerging Infect Dis 2014; 20:1661–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steere AC. Lyme disease: a growing threat to urban populations. Proc Natl Acad Sci U S A 1994; 91:2378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lantos PM, Tsao J, Nigrovic LE, et al. Geographic expansion of Lyme disease in Michigan, 2000–2014. Open Forum Infect. Dis 2017; 4:ofw269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lantos PM, Nigrovic LE, Auwaerter PG, et al. Geographic expansion of Lyme disease in the Southeastern United States, 2000–2014. Open Forum Infect Dis 2015; 2:ofv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson SJ, Neitzel DF, Moen RA, et al. Disease risk in a dynamic environment: the spread of tick-borne pathogens in Minnesota, USA. Ecohealth 2015; 12:152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubin R. Lyme disease spreading beyond states with historically high incidence. JAMA 2017; 318:2420. [DOI] [PubMed] [Google Scholar]
- 11. Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016; 53:349–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis 2015; 21:1455–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutchinson ML, Strohecker MD, Simmons TW, Kyle AD, Helwig MW. Prevalence rates of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in host-seeking Ixodes scapularis (Acari: Ixodidae) from Pennsylvania. J Med Entomol 2015; 52:693–98. [DOI] [PubMed] [Google Scholar]
- 14. Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol 1998; 35:629–38. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 2011 case definition Available at: https://wwwn.cdc.gov/nndss/conditions/lyme-disease/case-definition/2011/. Accessed 11 May 2018.
- 16. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep 1995; 44:590–91. [PubMed] [Google Scholar]
- 17. Bacon RM, Kugeler KJ, Mead PS; Centers for Disease Control and Prevention. Surveillance for Lyme disease—United States, 1992–2006. MMWR Surveill Summ 2008; 57:1–9. [PubMed] [Google Scholar]
- 18. Deanehan JK, Kimia AA, Tan Tanny SP, et al. Distinguishing Lyme from septic knee monoarthritis in Lyme disease-endemic areas. Pediatrics 2013; 131:e695–701. [DOI] [PubMed] [Google Scholar]
- 19. Ogrinc K, Lusa L, Lotrič-Furlan S, et al. Course and outcome of early European Lyme neuroborreliosis (Bannwarth syndrome): clinical and laboratory findings. Clin Infect Dis 2016; 63:346–53. [DOI] [PubMed] [Google Scholar]
- 20. Esposito S, Bosis S, Sabatini C, Tagliaferri L, Principi N. Borrelia burgdorferi infection and Lyme disease in children. Int J Infect Dis 2013; 17:e153–8. [DOI] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Lyme disease: data and statistics Available at: https://www.cdc.gov/lyme/stats/. Accessed 17 November 2017.
- 22. Chen H, White DJ, Caraco TB, Stratton HH. Epidemic and spatial dynamics of Lyme disease in New York State, 1990–2000. J Med Entomol 2005; 42:899–08. [DOI] [PubMed] [Google Scholar]
- 23. Stone BL, Russart NM, Gaultney RA, Floden AM, Vaughan JA, Brissette CA. The Western progression of Lyme disease: infectious and nonclonal Borrelia burgdorferi sensu lato populations in Grand Forks County, North Dakota. Appl Environ Microbiol 2015; 81:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang P, Glowacki MN, Hoet AE, et al. Emergence of Ixodes scapularis and Borrelia burgdorferi, the Lyme disease vector and agent, in Ohio. Front Cell Infect Microbiol 2014; 4:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clow KM, Leighton PA, Ogden NH, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One 2017; 12:e0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jobe DA, Nelson JA, Adam MD, Martin SA Jr. Lyme disease in urban areas, Chicago. Emerg Infect Dis 2007; 13:1799–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliver JD, Bennett SW, Beati L, Bartholomay LC. Range expansion and increasing Borrelia burgdorferi infection of the tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J Med Entomol 2017; 54:1727–34. [DOI] [PubMed] [Google Scholar]
- 28. Eppes SC, Nelson DK, Lewis LL, Klein JD. Characterization of Lyme meningitis and comparison with viral meningitis in children. Pediatrics 1999; 103:957–60. [DOI] [PubMed] [Google Scholar]
- 29. Bingham PM, Galetta SL, Athreya B, Sladky J. Neurologic manifestations in children with Lyme disease. Pediatrics 1995; 96:1053–6. [PubMed] [Google Scholar]
- 30. Eppes SC. Diagnosis, treatment, and prevention of Lyme disease in children. Paediatr Drugs 2003; 5:363–72. [DOI] [PubMed] [Google Scholar]
- 31. Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am 2015; 29:187–210. [DOI] [PubMed] [Google Scholar]
- 32. Nelson CA, Saha S, Kugeler KJ, et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerging Infect Dis 2015; 21:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention (CDC). Physician reporting of Lyme disease—Connecticut, 1991–1992. MMWR Morb Mortal Wkly Rep 1993; 42:348–50. [PubMed] [Google Scholar]
- 34. Young JD. Underreporting of Lyme disease. N Engl J Med 1998; 338:1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.