Abstract
Pancreatitis is an inflammatory disease characterized by the induction of several proinflammatory cytokines like interleukin (IL)-6, IL-8, IL-1β, and IL-1. Recently, the multifunctional innate cytokine IL-15 has been implicated in the protection of several diseases, including cancer. Tissue fibrosis is one of the major problems in successfully treating chronic pancreatitis pathogenesis. Therefore, we tested the hypothesis that recombinant IL-15 (rIL-15) treatment may induce innate tissue responses and its overexpression will improve the pathogenesis of cerulein-induced chronic pancreatitis, associated remodeling, and fibrosis. We observed atrophy of acinar cells, increased inflammation, and increased deposition of perivascular collagen, the upregulated protein level of transforming growth factor (TGF)-β1, α-smooth muscle actin (α-SMA), and collagen-1 in cerulein-induced chronic pancreatitis in mice. Furthermore, we reported that rIL-15 treatment protects mice from the cerulein-induced chronic pancreatitis pathogenesis, including acinar cell atrophy, and perivascular accumulation of tissue collagen followed by downregulation of profibrotic genes such as TGF-β1, α-SMA, collagen-1, collagen-3, and fibronectin in cerulein-induced chronic pancreatitis in mice. Mechanistically, we show that IL-15-mediated increase of interferon-γ-responsive invariant natural killer T (iNKT) cells in the blood and tissue protects cerulein-induced pancreatic pathogenesis in mice. Of note, a reduction in iNKT cells was also observed in human chronic pancreatitis compared with normal individuals. Taken together, these data suggest that IL-15 treatment may be a novel therapeutic strategy for treating chronic pancreatitis pathogenesis.
NEW & NOTEWORTHY Pancreatic fibrosis is a major concern for the successful treatment of chronic pancreatitis and pancreatic cancer. Therefore, restriction in the progression of fibrosis is the promising approach to manage the pancreatitis pathogenesis. Herein, we present in vivo evidences that pharmacological treatment of recombinant interleukin-15 improves remodeling and fibrosis in cerulein-induced chronic pancreatitis in mice. Our observations indicate that interleukin-15 immunotherapy may be a possible and potential strategy for restricting the progression of fibrosis in chronic pancreatitis.
Keywords: fibrosis, inflammation, interleukin-15, NKT cells, pancreatitis
INTRODUCTION
Pancreatitis is the inflammation of the pancreas and defined as acute pancreatitis or chronic pancreatitis (12, 25, 31). The pathogenesis of chronic pancreatitis is yet not fully understood and believed to be a repeated episode of acute pancreatitis. Chronic pancreatitis is a slowly progressive disease characterized by fibrosis and calcification of pancreatic tissue that lead extensive loss of quality of life from chronic abdominal pain, malnutrition, nausea, diarrhea; the prevalence of chronic pancreatitis ranges from 4.4 to 11.9 per 100,000 patients each year (1, 31, 53). Patients with chronic pancreatitis have a reduced survival rate compared with normal individuals, and progression of chronic pancreatitis involves a local inflammatory autocrine, endocrine, and paracrine signaling cascade orchestrated by the release of several pro- and anti-inflammatory cytokines and chemokines (29, 31). These inflammatory signals recruit granulocytes (neutrophils, eosinophils), monocytes, macrophages, and lymphocytes (30, 49, 50, 55) that instigate the activation of pancreatic stellate cells (PSCs). PSCs are the major cells involved in the progression of pancreatic fibrosis, which is the primary pathological feature of chronic pancreatitis (46). Pancreatic fibrosis is a major concern for the successful treatment of chronic pancreatitis (4). Restraint in the progression of the fibrosis would be a promising approach for treatment of pancreatitis pathogenesis. A previous report revealed that the serum interleukin (IL)-15 level acts as a predictor of complication and mortality in severe acute pancreatitis (20). Moreover, the expression of IL-15 was reported in a rat model of severe acute pancreatitis, and IL-15 serve as a protective factor against organ injury (42, 43). Most recently, an in vitro study revealed that IL-15-activated natural killer (NK) cells have potential to kill human PSCs and pancreatic cancer cell lines compared with resting NK cells (44). IL-15 is a growth and survival factor for NK cells (7) and invariant natural killer T (iNKT) cells, which is evident from the abnormal generation of NK and NKT cells in IL-15-deficient (21) and IL-15Ra receptor-deficient mice (27). NK cells are functionally characterized by their ability to kill certain tumor cells without prior sensitization and to produce proinflammatory cytokines, especially interferon-γ (IFN-γ). Similarly, NKT cells developed in the thymus and expressed a rearranged T cell receptor (TCR). In contrast to typical T cells, NKT cells respond to antigen presented by the atypical major histocompatibility complex class I molecule, CD1d, and express intermediate levels of TCR. In addition, NKT cells are either CD4+ or CD4−CD8− in contrast to typical CD8+ class I restricted T cells (16, 38). Furthermore, several reports implicated NK and NKT cell-derived IFN-γ (2, 36, 45) in the improvement of acute pancreatitis (14). In this report, we show that IL-15-deficient mice have abnormal pancreatic acinar cell morphology and induced collagen in the pancreas compared with wild-type mice. Accordingly, we tested the hypothesis that IL-15 overexpression may be a novel strategy to restrict or reverse the pancreatitis fibrosis. Our approach indicated that recombinant (r) IL-15 pretreatment indeed restricts the progression of acinar cell atrophy and the accumulation of perivascular collagen and downregulates levels of profibrotic cytokines such as transforming growth factor (TGF)-β1, α-smooth muscle actin (α-SMA), collagen-1, collagen 3, and fibronectin in cerulein-induced chronic pancreatitis.
MATERIALS AND METHODS
Patient tissue samples.
Normal (n = 3) human pancreas with no malignancy and chronic pancreatitis (n = 3) tissue samples were obtained from the Biospecimen Core facility, Louisiana Cancer Research Consortium. Patients’ pancreatic tissues were collected during surgical procedures performed in chronic pancreatitis. Approximately 100-mg segments of pancreatic tissue were taken and immediately frozen in liquid nitrogen or 4% formaldehyde. All fixed tissues were used for paraffin embedding, sectioning, and processing for immunostaining and routine light microscopy. The specimen characteristics from normal portions of human nonmalignant pancreatic tissue showed no histopathological abnormalities, and the samples of chronic pancreatitis patient’s analysis showed no visible tumor. Patient details are provided in Table 1.
Table 1.
Details of human pancreatic biopsies
| Subject No. | Age, yr | Sex | Lymphovascular Invasion | Perineural Invasion | Tumor, % | Pathological Status |
|---|---|---|---|---|---|---|
| 1 | 45 | M | — | — | — | Normal tissue |
| 2 | 63 | F | — | — | — | Normal tissue |
| 3 | 57 | F | — | — | — | Normal tissue |
| 4 | 50 | F | — | — | — | Chronic pancreatitis |
| 5 | 67 | F | — | — | — | Chronic pancreatitis |
| 6 | 63 | M | — | ND | — | Chronic pancreatitis |
M, male; F, female; −, absent; ND, not defined.
Mice.
Specific pathogen-free Balb/C mice were obtained from Jackson Laboratory (Bar Harbor, ME). IL-15 gene-deficient Balb/c background mice were obtained from the laboratory of Dr. Fed Finkelman (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH). We used all male mice for our study because, as per the literature, chronic pancreatitis is more common in males compared with females (51, 52). We choose Balb/c mice for our studies because of earlier reports that Balbc mice show modest severity and less necrosis compared with C57BL6, which show mild severity and the least necrosis (47). These mice were maintained in a pathogen-free barrier facility. All experimental mice were age (6–8 wk) and sex matched. The Institutional Animal Care and Use Committee approved the animal protocol in accordance with the National Institute of Health guidelines. We performed all experiments according to the animal ethical rules and regulations.
Experimental pancreatitis.
Chronic pancreatitis was induced by repetitive cerulein injections as described (30, 50). Cerulein (Sigma-Aldrich, St. Louis, MO) was given by repetitive intraperitoneal injections as reported earlier (50 μg/kg, 6 hourly injections/day; 3 days/wk) along with 5 μg in 100 μl saline·wk−1·mice−1 intravenous injection of murine rIL-15 (Peprotech) for up to 4 wk; control mice received 100 μl saline. In brief, the treatment protocol is rIL-15 on day 1 followed by six intraperitoneal cerulein injections on days 2, 4, and 6 with rest on day 7; the schedule was repeated for up to 4 wk. Mice were sacrificed 3 days after the last cerulein injection (Fig. 2A), and tissue was immediately frozen in liquid nitrogen and stored at −80°C until used.
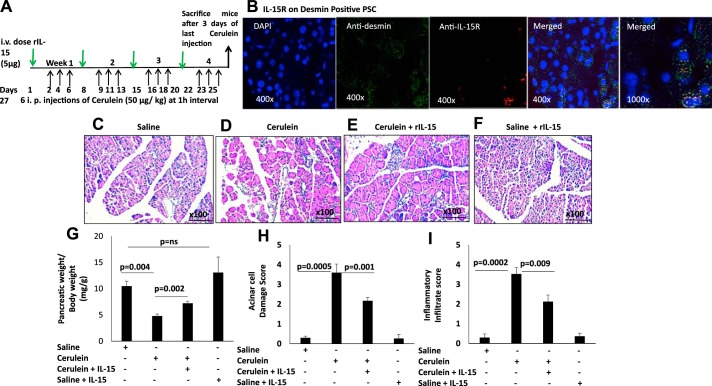
Fig. 2.
Interleukin (IL)-15 pretreatment ameliorates the pathogenesis of cerulein-induced chronic pancreatitis. A: experimental protocol of cerulein-induced chronic pancreatitis in a mouse model. Green arrows, dose of recombinant (r) IL-15; black arrows, ip cerulein injection during the experimental protocol. B: a representative immunofluorescence analysis detected IL-15 receptor (IL-15R) on anti-desmin-positive pancreatic stellate cells (PSCs); original magnification ×400 and ×1,000. C–F: photomicrograph of hematoxylin and eosin (H&E)-stained representative pancreatic tissue section of saline-treated (C), cerulein-treated (D), cerulein with rIL-15-treated (E), and saline + rIL-15 treated (F) mice. G–I: ratio of pancreas/mouse body weight (G), acinar cell damage (H), and inflammatory cell (I) analysis on a 0–4 scale in saline-, cerulein-, cerulein with IL-15-, and saline + IL-15-treated mice. Data are presented as means ± SD; n = 6–8 mice/group. All photomicrographs shown have the original magnification of ×100.
Histopathological analysis.
Mice and human pancreatic tissue specimens were fixed with 4% paraformaldehyde and embedded in paraffin using standard techniques. The paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin (H&E) to analyze the histopathological characteristics in tissue sections of experimental pancreatitis. Histologically, acinar cell damage was analyzed in H&E-stained mouse pancreatic tissue sections using light microscopy (×100 magnification) for saline-injected, cerulein-injected, rIL-15 + cerulein-injected, and rIL-15 + saline-injected groups. All H&E-stained tissue section slides from each group were coded properly, and four to five randomly chosen microscopic fields from each tissue section slide were graded blindly on the scale of 0 (absent) to 3 (severe). The parameters included were acinar cell damage and accumulation of inflammatory cells in tissue as described earlier (40). Acinar cell damage was also quantitated based on the severity of damage score on the scale of 0 to 3, i.e., from 0 = no damage, 1 = mild damage, 2 = moderate damage, and 3 = severe damage.
Tissue collagen analysis.
Pancreatic tissue sections were fixed with 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and fixed to positively charged slides. Collagen staining was then performed on tissue sections by the Masson's trichrome staining (Poly Scientific R&D) method for the detection of collagen fibers according to the manufacturer's recommendations, and collagen tissue thickness was measured using the video assistant integrated computer software program Image Pro software analyzer (Media Cybernetics, Warrendale, PA). The software measure accumulated collagen around the vessels or duct total area, and the positive area is expressed as micrometers squared (30, 33).
Immunofluorescence analysis.
Paraffin-coated mouse and human pancreatic tissue sections were deparaffinized, blocked with normal goat serum to reduce nonspecific binding, and incubated with anti-α-SMA antibody (1:250; Sigma Aldrich) overnight followed by anti-mouse IgG- and PE-labeled (Biolegend, San Diego, CA) secondary antibody. Additionally, CD49b PE-labeled, CD3 PE-labeled, CD3 FITC-labeled, IFN-γ FITC-labeled, fibroblast-specific protein 1 (FSP1)-FITC, F4/80-PE, and Vα24Jα18 (iNKT cell)-PE-labeled (1:200; Biolegend) primary antibodies were used to analyze the expression of CD49b, CD3, IFN-γ, and iNKT cells. The α-SMA+-, CD49b+-, CD3+-, IFN-γ+-, FSP1+-, and Vα24Jα18 (iNKT cell)+-immunostained sections were mounted with nuclear staining DAPI mounting material. The pancreatic sections were pretreated with 0.1% trypsin (Sigma-Aldrich) for 60 min at 37°C for immunostaining for IFN-γ as described earlier (24). The images were captured using an Olympus BX51 microscope with appropriate filters, and photomicrographs are presented as original magnification ×400. Each mouse slide was examined for four to five random sections at ×400 magnification. There were six to eight mice in each group.
Real-time polymerase chain reaction analysis.
RNA was isolated from the pancreas by the TRIZOL method (30). In brief, RNA samples (500 ng) were subjected to reverse transcription analysis using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Collagen 1, collagen 3, α- SMA, and fibronectin were quantified by real-time polymerase chain reaction (PCR) using the CFX connect Real-Time System (Bio-Rad) and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Results were normalized with GAPDH amplified from the same cDNA mix and expressed as relative expression compared with controls. cDNA was amplified using the primers listed in Table 2.
Table 2.
List of primers used in qPCR analysis
| Subject No. | Name of Primer | Forward Primer | Reverse Primer |
|---|---|---|---|
| 1 | mCollagen-1 | TGTTCAGCTTTGTGGACCTC | GGTTTCCACGTCTCACCATT |
| 2 | mCollagen-3 | CAGGATCTGTCCTTTGCGAT | CCCACTCCAGACTTGACATC |
| 3 | mFibronectin | CGAAGAGCCCTTACAGTTCC | CCGTGTAAGGGTCAAAGCAT |
| 4 | GAPDH | ACCCAGAAGACTGTGGATGG | CACATTGGGGGTAGGAACAC |
Pancreatic primary cell isolation.
Pancreatic primary cells were isolated following removal of the complete pancreas from mice, washed two times with HBSS, and sliced into small pieces followed by enzymatic digestion with collagenase 1A (Sigma-Aldrich), 200 U/ml in HBSS with 10 mM HEPES for 15 min at 37°C. During the digestion period, mechanical dissociation was performed using a sterile pipette until the single cell suspension was obtained and filtered through a 100-μm cell strainer. The isolated primary pancreatic cells were examined for IL-15 receptor (IL-15R) and signal transducer and activator of transcription (STAT) signaling molecule activation by performing flow cytometer and Western Blot analysis.
Western blot analysis.
The pancreas tissue and primary pancreatic cells were washed with cold PBS, homogenized, and solubilized in M-PER Mammalian Protein extraction reagent (Thermo Scientific) containing protease inhibitor cocktail and phosphatase inhibitor (Sigma-Aldrich). Proteins (30 μg) were resolved on NuPAGE 4–12% Bis-Tris gel (Invitrogen, Waltham, MA) and transferred to PVDF membranes (Millipore, Billerica, MA) (30). TGF-β1, collagen-1, GAPDH, and β-actin were detected by Western blotting using a mouse anti-TGF-β1 antibody (1:500), anti-collagen-1 antibody (1:500; Santa Cruz), GAPDH (1:1,000; Cell Signaling Technology), STAT-5 and phosphorylated (p) STAT5 (1:1,000; Cell Signaling Technology), and anti-β-actin antibody (1:1,000; Cell Signaling Technology). Semiquantitative densitometric analysis was performed by Image J software.
Flow cytometer analysis.
Blood leukocytes were analyzed for NK and NKT cells in an IL-15-treated mouse model of chronic experimental pancreatitis. The blood cells were lysed with red blood cell lysis buffer for 10 min on ice and then stained with different fluorescence-tagged anti-CD3-APC (for T cells; Biolegend) and anti-CD49b-PE (NK cells; Biolegend) antibodies. The primary acinar cells were analyzed for the presence of IL-15R by using anti-IL-15R-APC (Biolegend) compared with the isotype-matched anti-IgG. The IL-15R-positive cells were analyzed using a FACS Calibur (BD Biosciences, San Diego, CA) and FlowJo software.
Statistical analysis.
The nonparametric Mann-Whitney U-test was performed for comparison of data between two groups and Krustal-Wallis for comparison of more than two groups. Parametric data were compared using t-tests or analysis of variance. Values are reported as means ± SD. P values <0.05 were considered statistically significant.
RESULTS
IL-15 deficiency is associated with atrophy of acinar cells, induced perivascular collagen deposition,and T cell and macrophage infiltration in the pancreas of mice.
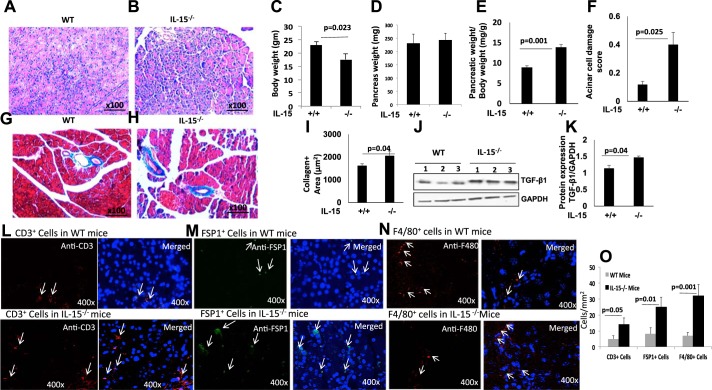
We tested the hypothesis that IL-15 deficiency promotes pancreatic tissue remodeling, including fibrosis. The H&E-stained tissue sections of wild-type and IL-15−/− mice indicate atrophy of acinar cells and possible edema in IL-15−/− mice (Fig. 1, A and B). A significantly decreased body weight and comparable pancreas weight were observed in IL-15−/− mice compared with wild-type mice (Fig. 1, C and D). However, the ratio of pancreas/body weight (mg/g) and acinar cell atrophy were found increased in IL-15−/− mice compared with the wild-type mice (Fig. 1, E and F). The increased pancreas-to-body weight (mg/g) ratio indicated induced pancreatic edema in IL-15−/− mice compared with wild-type mice. Furthermore, Masson’s trichrome tissue staining showed induced perivascular collagen accumulation in IL-15−/− mice compared with wild-type mice (Fig. 1, G and H). A semiquantitative morphometric analysis indicated significantly increased collagen-positive area in tissue sections of IL-15−/− mice compared with wild-type mice (Fig. 1I). Western blot analysis showed induced protein level of profibrotic cytokine TGF-β1 in pancreas of IL-15−/− mice compared with wild-type mice that was further quantitated by densitometry analysis of the protein bands (Fig. 1, J and K). This baseline induction of pancreatitis may not be an indicator of the developing spontaneous chronic pancreatitis in IL-15-deficient mice. Furthermore, significantly induced baseline T cells (anti-CD3+) and inflammatory macrophages [anti-FSP+ (37) and anti-F4/80+] were observed in IL-15−/− compared with wild-type mice (Fig. 1, L–N). The quantitative numbers of CD3+, FSP1,+ and F4/80+ cells showed a significant increase in IL-15 gene-deficient mice compared with wild-type mice (Fig. 1O). The induced T cells and inflammatory macrophages are characteristics of chronic pancreatitis.
Fig. 1.
Interleukin (IL)-15-deficient mice display pancreas atrophy, increased collagen deposition, and T cell and macrophage infiltration. A–F: representative photomicrograph of hematoxylin and eosin (H&E)-stained pancreas tissue section of wild-type (WT, A) and IL-15-deficient (IL-15−/−, B) mice, body weight (C), pancreas weight (D), the ratio of body and pancreas weight (E), and acinar cell damage (F). G and H: induced basal level of collagen in IL-15−/− mice compared with WT mice. I: morphometric analysis of tissue collagen-positive area measured in tissue sections presented for both WT and IL-15−/− mice. J and K: immunoblot analysis of induced level of transforming growth factor (TGF)-β1 and loading control GAPDH (J) and densitometric analysis of TGF-β1 bands normalized with GAPDH (K) in IL-15−/− mice compared with WT mice. L–M: CD3+ T cells (L), fibroblast-specific protein 1-positive (FSP1+, M), and F4/80+ inflammatory macrophages (N) in pancreas of WT and IL-15−/− mice. O: morphometric quantitation of each cell type. Data are presented as means ± SD; n = 6–8 mice/group. All photomicrographs shown are the original magnification ×100.
IL-15 pretreatment improves acinar cell atrophy and reduces accumulation of inflammatory cells in cerulein-induced chronic pancreatitis.
The cerulein-induced mouse model is the well-established experimental model of chronic pancreatitis (30, 50). Because we observed pancreatitis-like symptoms in IL-15-deficient mice, we next examined whether IL-15 pretreatment ameliorates cerulein-induced chronic pancreatitis. We induced chronic pancreatitis by intraperitoneal injection of cerulein and intravenously delivered rIL-15 in mice as per the protocol shown in Fig. 2A. IL-15R on stellate cells (PSCs) was analyzed by performing double-immunofluorescence analysis using anti-desmin and anti-IL-15R on pancreas tissue sections of cerulein-treated mice. Our analysis indicated PSCs express IL-15R (Fig. 2B). Next, light microscopic histopathological analysis detected an increased number of inflammatory cells and marked acinar cell injury in cerulein-induced chronic pancreatitis compared with saline-treated mice (Fig. 2, C and D). Interestingly, the IL-15-treated cerulein-injected mice revealed significantly improved acinar cell architecture and reduced accumulation of inflammatory cells compared with mice treated with cerulein alone (Fig. 2E). The saline + rIL-15 control mice tissue section photomicrograph shows no detrimental effect on the mouse pancreas (Fig. 2F). The semiquantitative analysis on the scale of 0–3 was performed to measure the level of acinar cell damage and the accumulation of inflammatory cells in the pancreas of saline-, cerulein-, and cerulein with rIL-15-treated mice. The ratio of pancreas/mouse body weight (mg/g), acinar cell damage, and inflammatory cells were examined in the pancreatic tissue sections. A significantly reduced pancreas-to-mouse ratio of body weight, induced acinar cell damage, and inflammatory cell accumulation were observed in cerulein-treated mice compared with saline-treated mice but was improved in rIL-15-injected cerulein-treated mice (Fig. 2, G–I). These data indicate that IL-15 has a protective role in chronic pancreatitis pathogenesis.
IL-15 pretreatment reduces cerulein-induced perivascular collagen deposition and regulates pancreatic remodeling.
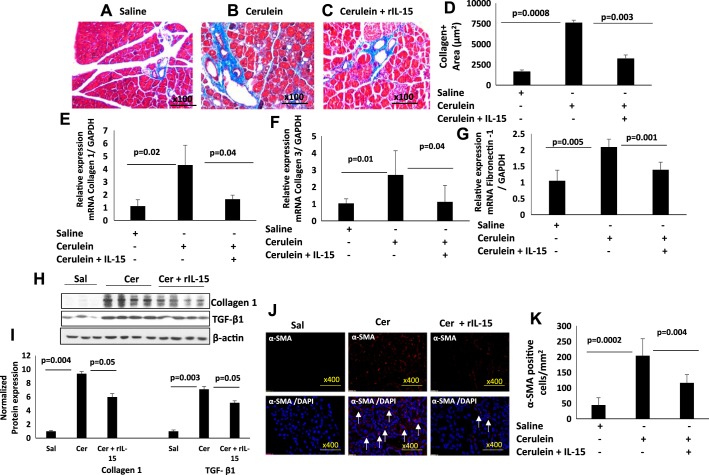
Because we observed improved pathology in cerulein-induced IL-15-treated pancreatitis in mice, we next examined whether IL-15 treatment attenuates pancreatic tissue remodeling in cerulein-induced chronic pancreatitis. Accordingly, we performed Masson’s trichrome staining for collagen accumulation in the pancreatic tissue sections. Our analysis showed highly increased perivascular collagen deposition in the pancreas of cerulein-treated mice compared with saline-treated mice (Fig. 3, A and B); however, reduced collagen accumulation was observed in the pancreatic tissue sections of cerulein with rIL-15-treated mice compared with cerulein-treated mice (Fig. 3C). The improved collagen accumulation by IL-15 treatment was confirmed by performing a morphometric analysis of collagen-positive area on multiple tissue sections of different fields in cerulein with rIL-15-treated mice compared with cerulein-treated mice (Fig. 3D). Furthermore, the reduction of tissue collagen in IL-15-injected cerulein-treated mice is also confirmed by performing qPCR transcript analysis of collagen-1, collagen-3, and fibronectin-1. The qPCR data indicate significantly upregulated mRNA levels of collagen-1, collagen 3, and fibronectin-1 in the pancreas of cerulein-treated mice compared with saline-treated mice, which is significantly reduced in cerulein with IL-15-treated mice compared with cerulein alone-treated mice (Fig. 3, E–G). Additionally, we have shown protein level expression of collagen-1 and profibrotic cytokines TGF-β1 with the GAPDH normalized densitometry. The analysis indicates an induced protein level of collagen-1 and TGF-β1 in cerulein-treated mice compared with saline mice that improved in cerulein and rIL-15-treated mice compared with cerulein-treated mice (Fig. 3, H and I). α-SMA+ cells were analyzed by performing immunofluorescence analysis of saline-, cerulein-, and cerulein with rIL-15-treated pancreatic tissue sections of mice. Immunofluorescence analysis of α-SMA demonstrated a significantly induced number of α-SMA+ cells in pancreatic tissue sections of cerulein-treated mice compared with saline-treated mice that significantly decreased in rIL-15-treated cerulein-treated mice (Fig. 3J). Furthermore, semiquantitative analysis of α-SMA+ cells validated significantly reduced α-SMA+ cells/mm2 in tissue sections of rIL-15- and cerulein-treated mice compared with cerulein alone-treated mice (Fig. 3K). These data indicate that IL-15 has the potential to downregulate profibrotic cytokines TGF-β1 and α-SMA+ cells, indicating the role of IL-15 in improving fibrosis in chronic pancreatitis.
Fig. 3.
Interleukin (IL)-15 pretreatment reduces in the levels of fibrosis-associated genes and collagen accumulation in cerulein-induced chronic pancreatitis. A–D: tissue collagen accumulation in saline (A)-, cerulein (B)-, and cerulein with IL-15 (C)-treated mice and the thickness of tissue collagen (D). E–G: mRNA of collagen 1 (E), collagen 3 (F), and fibronectin-1 (G) levels in saline-, cerulein-, and cerulein with IL-15-treated mice. H and I: immunoblot analysis of collagen-1, transforming growth factor (TGF)-β1 (H), and GAPDH normalized densitometry (I). J: immunofluorescence analysis of α-smooth muscle actin (α-SMA) in pancreas of saline-, cerulein-, and cerulein with IL-15-treated mice. Arrows indicate α-SMA-positive cells. K: morphometric analysis performed to quantitate α-SMA-positive cells in pancreatic sections of saline-, cerulein-, and cerulein with IL-15-treated mice presented as cells/mm2. Data are presented as means ± SD; n = 6–8 mice/group. A representative photomicrograph has been shown, and all photomicrographs are of the original magnification ×400.
IL-15 pretreatment induces NKT cells and IFN-γ in cerulein-induced chronic pancreatitis.
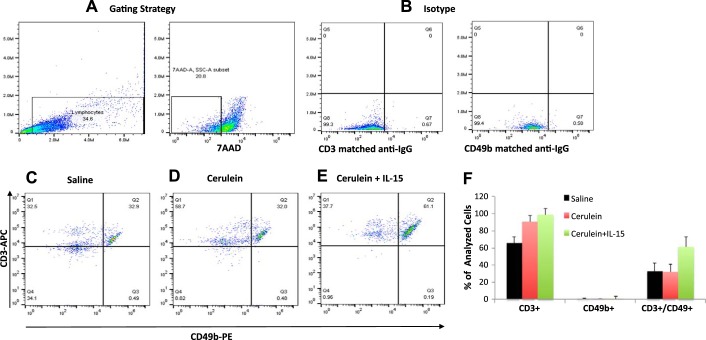
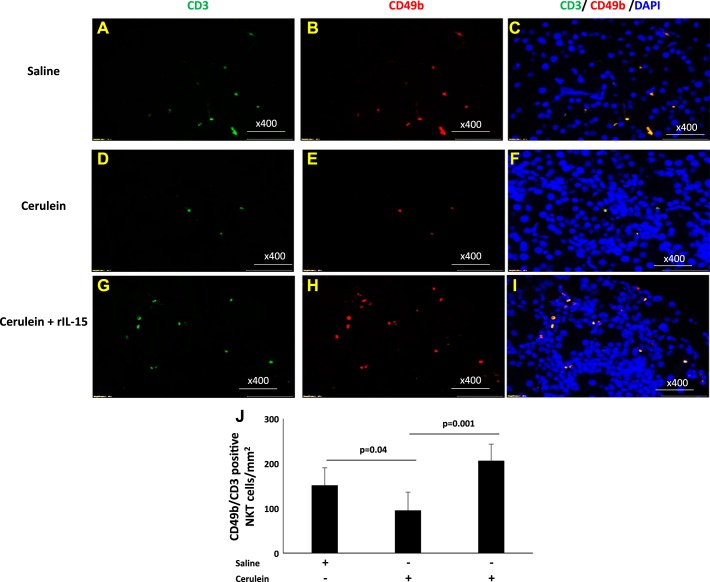
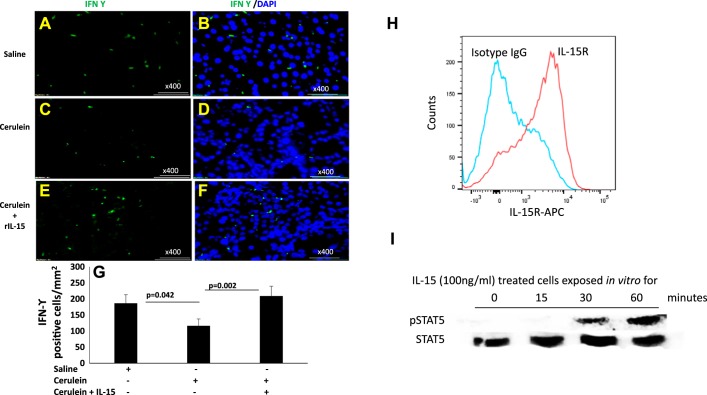
Earlier reported studies have indicated that IL-15 delivery induces tissue accumulation of iNKT cells (39). Therefore, we first analyzed the level of CD3 (T cells), CD49b (NK cells), and CD3 CD49b double positive as NKT cells in the blood of an IL-15-treated chronic pancreatitis mouse model. We observed no significant change in CD3+CD49b+ NKT cells in the blood of saline (32.9%) (Fig. 4, C and F)- and cerulein (32%)-treated mice (Fig. 4, D and F); however, mice treated with rIL-15 along with cerulein showed significantly induced number of CD3+CD49b+ NKT cells (61%) in blood compared with saline or cerulein only treated mice (Fig. 4, E and F). The analysis was performed using the gating strategy shown in Fig. 4A and matched anti-IgG for CD3 and CD49b (Fig. 4B). Furthermore, we extended our in vivo study to analyze tissue accumulation of NKT cells in pharmacologically administered IL-15 in cerulein-induced chronic pancreatitis. Immunofluorescence tissue staining showed that IL-15 treatment induces a number of NKT cells in experimental models of chronic pancreatitis. We performed double-immunofluorescence staining by using anti-CD3 and anti-CD49b to analyze NKT cells in pancreatic tissue sections of saline-, cerulein-, and cerulein + rIL15-treated mice (Fig. 5, A–I). Our analysis indicated the induced number of CD49b+CD3+ NKT cells in the pancreas of IL-15-treated cerulein-induced chronic pancreatitis compared with cerulein alone-treated mice (Fig. 5, F and I). Notably, the numbers of CD49b+CD3+ NKT cells were significantly reduced in cerulein-induced chronic pancreatitis compared with saline-treated mice (Fig. 5, C and F). The morphometric quantitation of NKT cells in multiple sections of each group of mice was statistically analyzed (Fig. 5J). Because NKT cells are the source of IFN-γ, we further performed intracellular immunofluorescence staining for IFN-γ in all stated groups of mice. We observed a significant increase in the number of IFN-γ+ cells in pancreas of IL-15-treated with cerulein-induced mice compared with cerulein alone-treated mice (Fig. 6, D and F). However, decreased numbers of IFN-γ+ cells were noticed in cerulein-induced chronic pancreatitis compared with saline (Fig. 6, B and D) These quantitative data show that IL-15 treatment induces and activates NKT cells (Fig. 5J), which are the source of IFN-γ (Fig. 6G) that improve cerulein-induced chronic pancreatitis in mice. Furthermore, we also examined the mechanistic link in the IL-15-mediated signaling pathway by examining the family members of STAT. IL-15-exposed pancreatic primary cells isolated from wild-type mice following collagenase digestion were tested for IL-15R, STAT5, and STAT6 activation by performing flow cytometer and Western blot analysis. The flow cytometer analysis using anti-IL-15R antibody detected IL-15 receptor on pancreatic primary acinar cells isolated from wild-type mice compared with isotype-matched anti-IgG-stained cells (Fig. 6H). Furthermore, pancreas primary cells were treated with rIL-15 (100 ng/ml) for 0, 15, 30, and 60 min and harvested to examine STAT5 and STAT6 activation (phosphorylation) using anti-pSTAT5 and anti-pSTAT6 antibodies. Western blot analysis indicated STAT5 phosphorylation after treating the primary pancreatic cells for 0, 15, 30, and 60 min compared with nontreated cells. rIL-15-treated cells showed maximum activation between 30 and 60 min posttreatment compared with 0 and 15 min posttreated cells (Fig. 6I). STAT6 showed no response to IL-15 exposure at any time point tested (data not shown).
Fig. 4.
Interleukin (IL)-15 pretreatment induces natural killer T (NKT) cells in blood of cerulein-induced chronic pancreatitis in mice. Flow cytometric analysis was performed to analyze CD3+CD49b+ NKT cells in an IL-15-treated mouse model of chronic pancreatitis. A and B: gating strategy (A) and matched anti-IgG for CD3 and CD49b (B). C and D: no significant change in CD3+CD49b+ NKT cells in the blood of saline (C)- and cerulein (D)-treated mice. C–E: mice treated with recombinant (r) IL-15 with cerulein (E) detected induced number of CD3+CD49b+ NKT cells in the blood compared with saline (C)- and cerulein (D)-treated mice. F: %NKT cells out of total live lymphocytes in saline-, cerulein-, and IL-15 with cerulein-treated mice. Data are presented as means ± SD; n = 6–8 mice/group.
Fig. 5.
Immunofluorescence analysis detected increased natural killer T (NKT) (CD3+CD49b+) cells following interleukin (IL)-15 treatment in cerulein-induced chronic pancreatitis. Double-immunofluorescence analysis was performed to detect CD3+CD49b+ (NKT) cells using anti-CD3-FITC- and anti-CD49b-PE-labeled antibody and mounting with DAPI in the pancreas tissue sections of saline (A–C)-, cerulein (D–F)-, and cerulein with IL-15 (G–I)-treated mice. Morphometric analysis quantitates CD3+CD49b+ (NKT) cells in these tissue sections expressed as NKT cells /mm2 (J). Data are presented as means ± SD; n = 6–8 mice/group. All photomicrographs are shown here with the original magnification ×400. A representative photomicrograph was presented in each group analyzed.
Fig. 6.
Immunofluorescence analysis indicated increased interferon (IFN)-γ+ cells in an interleukin (IL)-15-treated mouse model of cerulein-induced chronic pancreatitis. Pancreatic tissue sections were immunostained for intracellular IFN-γ using anti-IFN-γ FITC-labeled antibody followed by DAPI mounting on saline (A and B)-, cerulein (C and D)-, and cerulein with IL-15 (E and F)-treated mice. G: IFN-γ positive cells were quantitated in these tissue sections and expressed as IFN-γ-positive cells/mm2. H and I: the presence of IL-15 receptor (IL-15R) on primary isolated pancreatic acinar cells (H) and IL-15-mediated activation of signaling molecule signal transducer and activator of transcription (STAT) 5 (I). Data are presented as means ± SD; n = 6–8 mice/group. All photomicrographs are shown with the original magnification ×400. A representative photomicrograph was presented in each group analyzed.
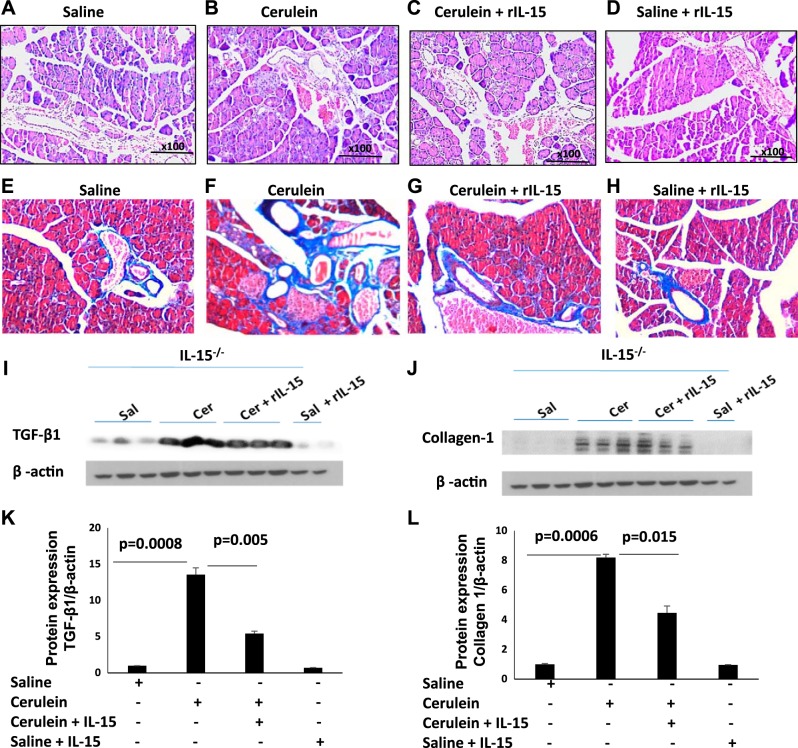
IL-15 pretreatment downregulates TGF-β1 and collagen-1 in cerulein-induced chronic pancreatitis in IL-15-deficient mice.
We tested the hypothesis that pharmacological delivery of rIL-15 in IL-15−/− protects from cerulein-induced chronic pancreatitis-associated fibrosis. First, we stained mouse pancreas with H&E to analyze the histopathological changes in pancreatic tissue sections of saline-, cerulein-, cerulein + rIL-15-, and saline + rIL-15-treated IL-15−/− mice. H&E-stained representative tissue sections show improved pancreatic histological features such as inflammation and acinar cell damage in cerulein + rIL-15-treated mice compared with cerulein alone-treated IL-15−/− mice (Fig. 7, B and C). However, no change was noticed in saline and saline + rIL-15-treated IL-15−/− mice (Fig. 7, A–D). Additionally, we also show that Masson’s trichrome stained representative tissue sections of saline (Fig. 7E)-, cerulein (Fig. 7F)-, cerulein + rIL-15 (Fig. 7G)-, and saline + rIL-15 (Fig. 7H)-treated IL-15−/− mice. The results indicate induced collagen accumulation in cerulein-treated mice that improved following IL-15 treatment in cerulein-induced IL-15−/− mice (Fig. 7, F and G). Immunoblot analysis indicates the significantly induced expression of fibrosis-associated proteins, i.e., TGF-β1 (Fig. 7, I and K) and collagen-1 (Fig. 7, J and L) in cerulein-treated IL-15−/− mice compared with saline-treated IL-15−/− mice. Notably, rIL-15 treatment in cerulein-injected IL-15−/− mice show significantly reduced level of TGF-β1 and collagen-1 compared with cerulein-treated IL-15−/− mice (Fig. 7, I–L). However, even baseline TGF-β1 in saline samples is downregulated following IL-15 treatment (saline vs. saline + IL-15) between saline- and saline + rIL-15-treated IL-15−/− mice (Fig. 7, I–L). Moreover, negligible collagen was detected in the saline- and saline + rIL-15-treated group (Fig. 7, J–L).
Fig. 7.
Interleukin (IL)-15 pretreatment downregulates transforming growth factor (TGF)-β1 and collagen-1 in cerulein-induced chronic pancreatitis in IL-15−/− mice. A–H: tissue histopathological changes in pancreatitis and the recovery following IL-15 treatment in hematoxylin and eosin (H&E, A–D)- and Masson’s trichrome (E–H)-stained tissue sections in IL-15−/− mice (n = 8 mice/group were analyzed, and representative photomicrograph of each group was presented). I and J: immunoblot analysis of TGF-β1 (I) and collagen 1 (J) in saline-treated, cerulein-treated, and recombinant (r) IL-15-injected cerulein-treated mice. The same immunoblot was restriped with β-actin, which serves as a loading control. K and L: densitometry analysis for TGF-β1 (K) and collagen 1 (L). Data are presented as means ± SD; n = 8 mice/group.
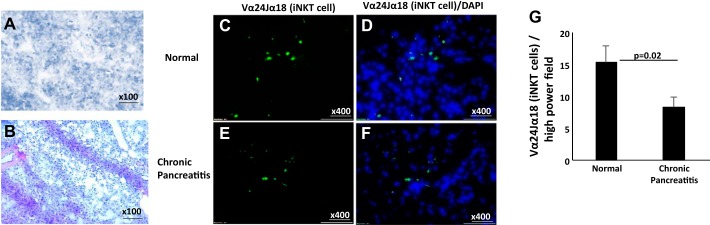
Detection and identification of iNKT cells in human pancreatitis.
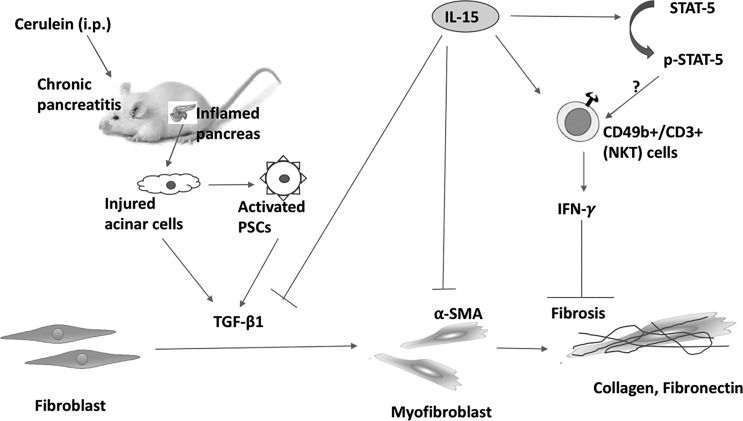
We analyzed H&E-stained human pancreatic tissue from normal and chronic pancreatitis patients for histopathological changes. Histopathology of these H&E-stained pancreatic tissue sections revealed increased acinar cell damage in chronic pancreatitis (Fig. 8B) compared with normal individuals (Fig. 8A). Furthermore, we validated our experimental finding on the significance of the role of iNKT cells in human pancreatitis. Immunofluorescence analysis has been performed to examine iNKT cells in tissue sections of human chronic pancreatitis and normal individual tissue biopsies using FITC-conjugated anti-human Vα24Jα18 antibody along with DAPI mounting material. Our analysis detected significantly reduced Vα24Jα18+ iNKT cells in patients with chronic pancreatitis (Fig. 8, C and D) compared with normal individuals (Fig. 8, E and F). A quantitative analysis of multiple tissue sections from both groups has been shown (Fig. 8F) as Vα24Jα18+ iNKT cells counted/high-power field and expressed as means ± SD; n = 3 in chronic pancreatitis, and 3 normal. We provided a summarized schematic diagram presenting a better understanding of the protective role of IL-15 in chronic pancreatitis and pancreatic fibrosis in Fig. 9.
Fig. 8.
Reduced number of invariant natural killer T (iNKT) cells was noticed in human chronic pancreatitis. A and B: representative hematoxylin and eosin (H&E) staining showing acinar cell atrophy in normal (A) and chronic pancreatitis (B) tissue. C–F: immunofluorescence staining was performed by using FITC-conjugated anti-human Vα24Jα18 antibody in human pancreatic biopsies of normal (C and D) and chronic pancreatitis (E and F) tissue along with DAPI mounting material for nuclear staining. A representative photomicrograph has been presented in each group analyzed. G: quantitation of Vα24Jα18+ expressed as cells/high-power field. _Means ± SD; n = 3 patients/group.
Fig. 9.
Schematic presentation of the protective role of interleukin (IL)-15 in cerulein-induced chronic pancreatitis and associated fibrosis. Repeated injections of cerulein (i.e., an analog of cholecystokinin receptors present on acinar cells) cause acinar cell injury that leads to activation of pancreatic stellate cells (PSCs); onset of chronic pancreatitis; induction of fibrosis-associated genes such as transforming growth factor (TGF)-β1, α-smooth muscle actin (α-SMA), collagen, and fibronectin; and fibrosis. Treatment of IL-15 causes induction of CD49+CD3+ [natural killer T (NKT)] cells that are the source of interferon (IFN)-γ and have a protective role in pancreatic fibrosis. IL-15 also inhibits TGF-β1, α-SMA, collagen, and fibronectin expression to restrict the progression of pancreatic fibrosis. IL-15 activates phosphorylation of signal transducer and activator of transcription (STAT) 5 but how phosphorylated (p) STAT5 regulates NKT cells in chronic pancreatic tissue needs to be explored further.
DISCUSSION
Chronic pancreatitis demonstrates an increased number of inflammatory cells, including neutrophils, eosinophils, monocytes, and macrophages with induced expression of inflammatory cell-associated cytokines and chemokines (29, 31, 50, 55). These induced inflammatory cells and cytokines instigate the activation of PSCs and promote disease pathogenesis (6, 31). PSCs are the major cells involved in the progression of pancreatic fibrosis (13, 48). Pancreatic fibrosis is a major concern in the successful treatment of chronic pancreatitis. Hence, the preventive progression of the fibrogenesis would be a promising approach for the treatment of pancreatic fibrosis. Earlier, the role of NK and NKT cell-derived IFN-γ has been implicated in the therapy of acute pancreatitis (2, 14, 36, 45). An in vitro study revealed that IL-15-activated NK cells have an important role to target human primary PSCs, which indicates the significance of IL-15 in the treatment of pancreatitis and possibly associated malignancy (44). An in vivo study has revealed that IL-15 improves antitumor activity of tumor-reactive CD8+ T cells (23) and tethered IL-15 enhances antitumor activity via promoting stem cell memory subsets of tumor-specific T cells (17). IL-15 is a cytokine implicated in innate and acquired immunity and has a critical role in the growth and survival of NK and NKT cells (8, 15, 32, 34, 35). IL-15 functions through its specific receptor IL-15Rα and also binds to the common cytokine γ-chain (CD132) IL-2/IL-15 receptor that shares receptor β (CD122) (11, 41). The findings on the potential role of IL-15-activated NK cells in killing human PSCs are in the agreement with other reports that show NK cells from pancreatic ductal adenocarcinoma (PDAC) patients are capable of eliminating PSCs (5, 44). Additionally, a report indicates that IL-15-producing PDAC cells induced a tumor-shrinking effect via NK cells (54). This report was consistent with another finding that IL-15 derived from human umbilical cord blood-derived mesenchymal stem cells are capable of eradicating pancreatic tumors in mice (19). Accordingly, we further investigated the preventive role of IL-15 in cerulein-induced chronic pancreatitis in mice. In support of the critical role of IL-15, we first showed the role of IL-15 in promoting tissue fibrosis by using IL-15-deficient mice. Notably, IL-15-deficient mice showed induced pancreatic remodeling, including collagen and profibrotic cytokines, i.e., TGF-β. Furthermore, our investigation revealed that pharmacological treatment of rIL-15 ameliorates the atrophy of pancreatic acinar cells and pancreatic fibrosis in cerulein-induced chronic pancreatitis in wild-type mice. These investigations provide the first in vivo evidence that regulation of IL-15 may be critical in promoting pancreatic fibrosis. Therefore, we next delivered rIL-15 in mice treated with cerulein and observed improved cerulein-induced chronic pancreatitis-associated fibrosis remodeling compared with the only cerulein-treated mice. These findings were consistent with a previously reported in vitro study (44). Furthermore, we mechanistically demonstrated the novel role of IL-15-responsive NKT cells and derived cytokines INF-γ in the pathogenesis of cerulein-induced chronic pancreatitis in mice. We showed that NKT cells were decreased in cerulein-induced chronic pancreatitis, whereas rIL-15 treatment in cerulein-treated mice showed increased numbers of NKT cells and may improve the pancreatitis pathogenesis in mice. Mechanistically, we identified that STAT5 signaling may regulate IL-15-mediated protection in cerulein-induced pancreatitis via activating NKT cell-induced innate responses. A similar observation was noted in human pancreatitis. Although a small number of chronic pancreatitis patients develop malignancy, no documentary evidence is available regarding the association of chronic pancreatitis to the development of pancreatic malignancy. The current study provided the association of reduced iNKT cells in human chronic pancreatitis. The limitation of this current study is the limited access of human pancreas biopsies at our facility. Taken together, the current findings indicate that decrease of IL-15-responsive IFN-γ-producing NK or NKT cells may be critical in promoting pancreatitis pathogenesis, since we show that IL-15 treatment reverses pancreatic fibrosis following the induction of INF-γ producing NK and NKT cells in cerulein-induced mouse model of chronic pancreatitis. Notably, INF-γ-producing NK and NKT cells require IL-15 for its survival (10, 22, 26). The reduced numbers of NK (3) and iNKT (18) cells have been reported in pancreatic malignancy. NK and iNKT cells maintain the innate immunity of the tissue/organ, and normal liver is the source of these cells, where they reside in large numbers (9, 28).The induced collagen and profibrotic cytokines in IL-15−/− mice further validate our findings, since these mice are deficient in NK and NKT cells (8, 35). We may assume that IL-15 deficiency or downregulation may promote spontaneous pancreatitis; however, to establish spontaneous pancreatitis, there is a need to examine further different-aged IL-15-deficient mice.
In conclusion, we provide evidence that rIL-15 may be a possible and potential therapeutic target strategy for human pancreatic fibrosis during chronic pancreatitis. Furthermore, to validate a critical role of IL-15 in regulating pancreatitis, other types, including l-arginine or alcohol-induced pancreatitis, need to be studied. The current study proposes rIL-15 or its agonist multicentral clinical trial may restrict or reverse fibrosis in chronic pancreatitis patients.
GRANTS
This work was supported in part by an National Institute of Allergy and Infectious Diseases Grant R01-AI-080581 (to A. Mishra). We thank the Edward G. Schlieder Educational Foundation and Tulane University Bridge funding for their support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M. conceived and designed research; M.M. and H.K.K. performed experiments; M.M. and H.K.K. analyzed data; M.M. and A.M. interpreted results of experiments; M.M., H.K.K., and A.K.V. prepared figures; M.M. and A.M. drafted manuscript; M.M., A.K.V., and A.M. edited and revised manuscript; A.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for M. Manohar: School of Medicine, Gastrointestinal and Hepatology Division, Stanford University, CA 94304.
REFERENCES
- 1.Afghani E. Introduction to pancreatic disease: acute pancreatitis. Pancreapedia. Exocrine Pancreas Knowledge Base 2014. doi: 10.3998/panc.2014.14. [DOI] [Google Scholar]
- 2.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med 183: 2391–2396, 1996. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang S, Kim HS, Choo YS, Park SW, Chung JB, Song SY. Differences in immune cells engaged in cell-mediated immunity after chemotherapy for far advanced pancreatic cancer. Pancreas 32: 29–36, 2006. doi: 10.1097/01.mpa.0000191651.32420.41. [DOI] [PubMed] [Google Scholar]
- 4.Banks PA, Conwell DL, Toskes PP. The management of acute and chronic pancreatitis. Gastroenterol Hepatol (N Y) 6, Suppl 3: 1–16, 2010. [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells–enhancement by therapeutic antibodies. PLoS One 2: e326, 2007. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204–7218, 2017. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 100: 3633–3638, 2002. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 8.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy 7: 23–35, 2005. doi: 10.1016/S1465-3249(05)70786-6. [DOI] [PubMed] [Google Scholar]
- 9.Felices M, Lenvik AJ, McElmurry R, Chu S, Hinderlie P, Bendzick L, Geller MA, Tolar J, Blazar BR, Miller JS. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3: 96219, 2018. doi: 10.1172/jci.insight.96219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 113: 1490–1497, 2004. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol 57: 763–766, 1995. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 12.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol 31: 395–399, 2015. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Ma J, Duan W, Zhang L, Yu S, Xu Q, Lei J, Li X, Wang Z, Wu Z, Huang JH, Wu E, Ma Q, Ma Z. Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 7: 18146–18158, 2016. doi: 10.18632/oncotarget.7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi T, Ishida Y, Kimura A, Iwakura Y, Mukaida N, Kondo T. IFN-gamma protects cerulein-induced acute pancreatitis by repressing NF-kappa B activation. J Immunol 178: 7385–7394, 2007. doi: 10.4049/jimmunol.178.11.7385. [DOI] [PubMed] [Google Scholar]
- 15.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol 92: 210–213, 2014. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 16.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 206: 25–34, 2009. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, Olivares S, Rabinovich B, Huls H, Forget MA, Datar V, Kebriaei P, Lee DA, Champlin RE, Cooper LJ. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci USA 113: E7788–E7797, 2016. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janakiram NB, Mohammed A, Bryant T, Ritchie R, Stratton N, Jackson L, Lightfoot S, Benbrook DM, Asch AS, Lang ML, Rao CV. Loss of natural killer T cells promotes pancreatic cancer in LSL-KrasG12D/+ mice. Immunology 152: 36–51, 2017. doi: 10.1111/imm.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing W, Chen Y, Lu L, Hu X, Shao C, Zhang Y, Zhou X, Zhou Y, Wu L, Liu R, Fan K, Jin G. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther 13: 2127–2137, 2014. doi: 10.1158/1535-7163.MCT-14-0175. [DOI] [PubMed] [Google Scholar]
- 20.Kamei K, Yasuda T, Ueda T, Qiang F, Shiozaki H, Ohyanagi H, and Takeyama Y. Significant expression of interleukin 15 in rat experimental severe acute pancreatitis. Eur Surg Res 44: 159–169, 2010. doi: 10.1159/000283241. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med 191: 771–780, 2000. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HY, Kim HJ, Min HS, Kim S, Park WS, Park SH, Chung DH. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor beta1 production. J Exp Med 201: 41–47, 2005. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA 101: 1969–1974, 2004. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koga T, Duan H, Furue M. Immunohistochemical detection of interferon-gamma-producing cells in granuloma formation of sporotrichosis. Med Mycol 40: 111–114, 2002. doi: 10.1080/mmy.40.2.111.114. [DOI] [PubMed] [Google Scholar]
- 25.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 386: 85–96, 2015. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- 26.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, Besra GS, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA 105: 8339–8344, 2008. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9: 669–676, 1998. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 28.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360: 360, 2018. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manohar MVA, Venkateshaiah SU, Mishra A. Immunological responses involved in promoting acute and chronic pancreatitis. J Clin Immunol Res 1: 1–8, 2017. [Google Scholar]
- 30.Manohar M, Verma AK, Venkateshaiah SU, Mishra A. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. Am J Physiol Gastrointest Liver Physiol 314: G211–G222, 2018. doi: 10.1152/ajpgi.00210.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 8: 10–25, 2017. doi: 10.4292/wjgpt.v8.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res 20: 2044–2050, 2014. doi: 10.1158/1078-0432.CCR-12-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, Blanchard C, Putnam PE, Rothenberg ME. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology 134: 204–214, 2008. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson SE, Keating N, Belz GT. Natural killer cells and anti-tumor immunity. Mol Immunol S0161-5890(17)30595-3, 2017. doi: 10.1016/j.molimm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Ohteki T. Critical role for IL-15 in innate immunity. Curr Mol Med 2: 371–380, 2002. doi: 10.2174/1566524023362519. [DOI] [PubMed] [Google Scholar]
- 36.Olson CM Jr, Bates TC, Izadi H, Radolf JD, Huber SA, Boyson JE, Anguita J. Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J Immunol 182: 3728–3734, 2009. doi: 10.4049/jimmunol.0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Österreicher CH, Penz-Österreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci USA 108: 308–313, 2011. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci USA 100: 2663–2668, 2003. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayapudi M, Rajavelu P, Zhu X, Kaul A, Niranjan R, Dynda S, Mishra A, Mattner J, Zaidi A, Dutt P, Mishra A. Invariant natural killer T-cell neutralization is a possible novel therapy for human eosinophilic esophagitis. Clin Transl Immunology 3: e9, 2014. doi: 10.1038/cti.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt J, Compton CC, Rattner DW, Lewandrowski K, Warshaw AL. Late histopathologic changes and healing in an improved rodent model of acute necrotizing pancreatitis. Digestion 56: 246–252, 1995. doi: 10.1159/000201251. [DOI] [PubMed] [Google Scholar]
- 41.Tamzalit F, Barbieux I, Plet A, Heim J, Nedellec S, Morisseau S, Jacques Y, Mortier E. IL-15.IL-15Rα complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci USA 111: 8565–8570, 2014. doi: 10.1073/pnas.1405514111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ueda T. Serum interleukin-15 level in patients with severe acute pancreatitis. Surgery 143: 695–696, 2008. doi: 10.1016/j.surg.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, Takase K, Matsumoto I, Fujita T, Ajiki T, Fujino Y, Kuroda Y. Serum interleukin-15 level is a useful predictor of the complications and mortality in severe acute pancreatitis. Surgery 142: 319–326, 2007. doi: 10.1016/j.surg.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Van Audenaerde JRM, De Waele J, Marcq E, Van Loenhout J, Lion E, Van den Bergh JMJ, Jesenofsky R, Masamune A, Roeyen G, Pauwels P, Lardon F, Peeters M, Smits ELJ. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget 8: 56968–56979, 2017. doi: 10.18632/oncotarget.18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol 91: 299–309, 2012. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 132: 1557–1573, 2007. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Mulatibieke T, Ni J, Han X, Li B, Zeng Y, Wan R, Wang X, Hu G. Dichotomy between receptor-interacting protein 1- and receptor-interacting protein 3-mediated necroptosis in experimental pancreatitis. Am J Pathol 187: 1035–1048, 2017. doi: 10.1016/j.ajpath.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Wu Q, Tian Y, Zhang J, Zhang H, Gu F, Lu Y, Zou S, Chen Y, Sun P, Xu M, Sun X, Xia C, Chi H, Ying Zhu A, Tang D, Wang D. Functions of pancreatic stellate cell-derived soluble factors in the microenvironment of pancreatic ductal carcinoma. Oncotarget 8: 102721–102738, 2017. doi: 10.18632/oncotarget.21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue J, Sharma V, Habtezion A. Immune cells and immune-based therapy in pancreatitis. Immunol Res 58: 378–386, 2014. doi: 10.1007/s12026-014-8504-5. [DOI] [PubMed] [Google Scholar]
- 50.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, Habtezion A. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nat Commun 6: 7158, 2015. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 144: 1252–1261, 2013. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav D, Timmons L, Benson JT, Dierkhising RA, Chari ST. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 106: 2192–2199, 2011. doi: 10.1038/ajg.2011.328. [DOI] [PubMed] [Google Scholar]
- 53.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med 168: 649–656, 2008. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida Y, Tasaki K, Miyauchi M, Narita M, Takenaga K, Yamamoto H, Yaaguchi T, Saisho H, Sakiyama S, Tagawa M. Impaired tumorigenicity of human pancreatic cancer cells retrovirally transduced with interleukin-12 or interleukin-15 gene. Cancer Gene Ther 7: 324–331, 2000. doi: 10.1038/sj.cgt.7700118. [DOI] [PubMed] [Google Scholar]
- 55.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 144: 1230–1240, 2013. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]