Abstract
The IGF system has an important role in growth and development. IGF-II is a recognized fetal growth promoter. However, its physiological postnatal role remains uncertain, although it is maintained in the circulation at a substantially high level throughout life. IGF-II has been strongly linked to obesity in genetic studies, and more recent evidence suggests a metabolic role. We examined fat depot differences in IGF-II’s action on differentiation and metabolism. We speculate a specific effect on visceral adipocytes in relation to the differential distribution of insulin receptors between visceral and subcutaneous fat depots. We used a previously established adipocyte, cell culture system of matched pairs of visceral and subcutaneous fat biopsies from 20 normal weight children undergoing routine surgery for nonmalignant, nonseptic conditions. Preadipocytes were differentiated for 14 days in the presence or absence of IGF-II. Oil Red O staining, Western blotting, and reverse transcription polymerase chain reaction techniques were employed to assess levels of adipogenesis markers and levels of the insulin receptor and insulin receptor isoforms. Our data indicate that IGF-II promotes preadipocyte differentiation in subcutaneous preadipocytes but showed a protective, opposing effect restricting visceral preadipocyte differentiation, confirmed by reductions in the differentiation markers peroxisome proliferator-activated receptor gamma and adiponectin and in triglyceride staining. Additionally, IGF-II reduced mRNA expression of the insulin receptor in adipocytes and downregulated insulin receptor isoform A and glucose transporter 4 abundance and corresponding glucose uptake in visceral adipocytes. In conclusion, IGF-II is a regulator of preadipocyte differentiation and metabolism by acting as a differential modulator of fat accumulation favoring less visceral fat deposition in children.
Keywords: adipocytes, childhood, IGFs, subcutaneous fat, visceral fat
INTRODUCTION
Childhood obesity is a global health issue with more than 42 million overweight children worldwide (46a). It is of particular concern because 25% of obese adults tend to be overweight as children, and early overweight onset is associated with greater negative adult health consequences (12). In addition, overweight children are at increased risk of developing obesity-related systemic and psychological problems (30).
Humans have evolved for an active lifestyle with significantly more intermittent feeding compared with lower mammals, whose activity is closely tied to their feeding frequency. Adipocytes are the only cells specifically designed to store sufficient energy to sustain such a lifestyle. Concurrent with this, visceral and subcutaneous adipocytes in humans have evolved with clear functional distinctions and different pathogenic significance; in contrast, such depot-specific distinctions in adipocyte function are much less marked in rodents (1). Although the majority of body fat is subcutaneous, visceral fat has attracted more attention because of its association with metabolic syndrome, type II diabetes, and cardiovascular risk (7). Subcutaneous fat is less of a metabolic risk and is considered to be protective against metabolic abnormalities (43). There are multiple intrinsic characteristic differences between fat depots in humans (1, 23). The distribution of insulin receptors (IRs) in mature adipocytes differs between the fat depots with a higher abundance of IRs in visceral compared with subcutaneous fat. The majority of this increase is due to IR isoform A (IR-A); the IR comes in two isoforms, IR-A and IR isoform B (IR-B), according to the presence or absence of exon 11, and a major functional difference is the high affinity of IR-A for insulin-like growth factor-II (IGF-II) (6). These site-specific differences in adipose tissue structure and metabolism are important to consider in relation to the pathogenesis of obesity.
The IGF system has an established role in adipose tissue growth and metabolism. IGF-I is a potent promoter of preadipocyte growth and adipogenesis (15, 47), and fat depot differences in IGF-I responses have been reported (16). IGF-II is recognized for being an embryonic and placental growth factor, but its physiological role postnatally is still to be determined. The reason for this might be because IGF-II is not expressed postnatally in mouse models, whereas humans maintain extremely high levels of IGF-II, averaging around 700 ng/ml (20, 22, 24), with concentrations considerably higher than those of IGF-I (4). Most of the circulating IGF-II is present in a ternary complex with IGF binding protein-3 that has limited access to the tissues, and therefore tissue IGF-II concentrations to which adipocytes are exposed are much lower (1%–10% of circulating levels) but still considerably higher than those of insulin (19).
IGF-II expression has been strongly related to weight and adiposity; the level of IGF-II gene methylation is associated with birth weight (3), and the expression of IGF-II in utero promotes adipogenesis and fat storage during pregnancy (45). Additionally, methylation status of the IGF-II gene at birth has been linked to early childhood weight (27), and the level of IGF-II in the circulation during childhood has been closely related to fat mass (35). In adults, polymorphic genetic differences in IGF-II expression are correlated with weight gain: homozygous individuals with Apal AA have a significant increase in IGF-II levels, and this has been associated with lower body weight and lower risk of pathological body mass index in comparison to those with Apal GG, who had lower levels of IGF-II (14, 33). Furthermore, IGF-II level has been proposed as a prognostic marker to predict future weight gain because lower baseline circulating levels of IGF-II are associated with a higher risk of obesity and future weight gain (40). With a limited understanding of IGF-II’s role postnatally and the lack of data on children, we aimed to study the physiological role of IGF-II by conducting a series of experiments on primary cultures of matched pairs of subcutaneous and visceral adipocytes from children to test the hypothesis that IGF-II is an important regulator of adipocyte physiology with specific effects on visceral adipocytes. We hypothesized that the differential distribution of IR isoforms between visceral and subcutaneous adipocytes may enable IGF-II to exert distinct effects on fat cells in different fat depots.
MATERIALS AND METHODS
Subjects.
Samples were obtained from young children admitted to a regional children’s hospital for elective surgery. The study was approved by the National Research Ethics Service (NRES) Committee South West–Exeter [Research Ethics Committee (REC) reference: 14/SW/0109]. An invitation to the study and a written information sheet were sent by post to all potential subjects along with the surgery admission letter. Parents/legal guardians were approached with a verbal explanation of the study on the morning of admission for surgery, and written, informed consent was obtained from the parent/legal guardian of each of the 20 participants. Children recruited were of normal weight and were admitted to the Bristol Royal Hospital for Children for routine renal surgery (nonmalignant, nonseptic operations). Fat tissue samples were collected by specialist pediatric surgeons during the operation. Subcutaneous and intra-abdominal perinephric (visceral) fat biopsies (0.2–0.5 g) were collected and transferred immediately to the laboratory. Clinical data for the participants are shown in Table 1.
Table 1.
Clinical data of the 20 children who participated in fat biopsy collection
| Biopsy No. | Sex | Age, yr | Type of Operation | BMI, kg/m2 | BMI SDS |
|---|---|---|---|---|---|
| 1 | Male | 6 | Pyeloplasty | 16.1 | −0.2 |
| 2 | Male | 0 | Pyeloplasty | 14.1 | −1.6 |
| 3 | Female | 4 | Heminephrectomy | 16.7 | +0.14 |
| 4 | Female | 2 | Pyeloplasty | 15.3 | −0.8 |
| 5 | Male | 3 | Pyeloplasty | 15.1 | −0.9 |
| 6 | Male | 1 | Pyeloplasty | 16.6 | +0.07 |
| 7 | Male | 3 | Nephrectomy | 16.2 | −0.2 |
| 8 | Female | 0 | Heminephrectomy | 18.3 | +1.2 |
| 9 | Male | 0 | Pyeloplasty | 16.6 | +0.07 |
| 10 | Female | 0 | Pyeloplasty | 18.9 | +1.6 |
| 11 | Male | 4 | Pyeloplasty | 16 | −0.3 |
| 12 | Male | 5 | Pyeloplasty | 16.5 | 0 |
| 13 | Male | 7 | Pyeloplasty | 16.5 | 0 |
| 14 | Female | 2 | Heminephrectomy | 17.2 | +0.4 |
| 15 | Male | 2 | Pyeloplasty | 15.2 | −0.9 |
| 16 | Female | 7 | Heminephrectomy | 17.2 | +0.4 |
| 17 | Male | 2 | Nephrectomy | 16.7 | +0.1 |
| 18 | Female | 0 | Nephrectomy | 18.8 | +1.4 |
| 19 | Male | 3 | Pyeloplasty | 17 | +0.3 |
| 20 | Female | 1 | Nephrectomy | 18.4 | +1.3 |
BMI, body mass index; SDS, standard deviation score.
Reagents.
All reagents were obtained from Sigma-Aldrich (Gillingham, UK) unless stated otherwise. Recombinant, human IGF-II peptide was purchased from Gropep (Adelaide, South Australia, Australia). Dulbecco's Modified Eagle Medium/Ham’s F-12 (DMEM/Ham’s F-12), FBS, and Hank’s balanced salt solution (HBSS) were obtained from GIBCO (Paisley, UK), penicillin/streptomycin and l-glutamine from Lonza (Berkshire, UK), fungizone from Fisher Scientific (Paisley, UK), and insulin from Novo Nordisk (West Sussex, UK). Tissue culture plastic materials were purchased from Greiner Labortechnik Ltd (Tyne and Wear, UK) and phosphate buffered saline (PBS) was acquired from Oxoid (Basingstoke, UK). Nitrocellulose and enhanced chemiluminescence Western blotting reagents and 2-deoxy-d-[3H]glucose (2DG) were purchased from Amersham Pharmacia Biotech (Little Chalfont, UK), and the bicinchoninic acid protein assay and SuperSignal West-Dura chemiluminescence reagents were obtained from Pierce (Rockford, IL).
Isolation, culture, and differentiation of adipocyte precursor cells.
The techniques of preadipocyte isolation, culture, differentiation, and characterization have been described previously (16). In brief, small sections (0.2–0.5 g) of adipose tissue collected during surgery under sterile conditions were transported immediately to the laboratory. The tissue was washed three times in 10 ml of HBSS and then cut into 1-mm3 pieces and digested with 10 ml of type II collagenase (1 mg/ml) in HBSS for 60 min at 37°C in a shaking water bath (150 cycles/min). Adipocytes were separated from the stromal-vascular cells by centrifugation at 80 g for 3 min. The pellet of sedimented stromal-vascular cells, including fibroblasts, endothelial cells, preadipocytes etc. (but excluding mature adipocytes), was suspended in preadipocyte growth media (DMEM/Ham's F12) medium (1:1 vol/vol) supplemented with 20% FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin and seeded into T75 flasks coated with 0.2% gelatin; these were maintained at 37°C in a humidified atmosphere of 5% CO2. The medium was changed every 72 h until cells became confluent. For differentiation, preadipocytes obtained from the visceral and subcutaneous biopsies were cultured in gelatin-coated 6-well plates at a seeding density of 0.2 × 106, and after 16 h they were induced to differentiate using a technique established previously (16). In brief, preadipocytes were washed with PBS twice and cultured for 14 days with a chemically defined medium (DMEM/Ham’s F12) (1:1 vol/vol) supplemented with 15 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 15 mmol/l sodium bicarbonate (NaHCO3), 10 g/ml apotransferrin, 33 mol/l biotin, 100 nmol/l insulin (for the first 10 days only), 0.2 nmol/l 3,3,5-triiodothyronine, 17 mol/l pantothenate, and, for the initial 3 days of culture, 10 M rosiglitazone and 25 mol/l 3-isomethyl-l-methylxanthine in the absence or presence of a continuous exposure to 7.5 ng/ml or 62.5 ng/ml IGF-II. To maintain consistency, experiments were only performed using cell passages 3–5.
Western blotting.
Protein cell lysates (50 μg), as estimated by bicinchoninic acid protein assay (23225; Thermo Fisher Scientific), were separated using sodium dodecyl-polyacrylamide electrophoresis 10% gels. Following transfer to nitrocellulose membranes (RPN119B; Amersham), nonspecific binding sites were blocked using 5% nonfat dried milk for IR β subunit (IRβ); glucose transporter 4 (GLUT4), glyceraldehyde-3P-dehydrogenase (GAPDH), and β-actin or 5% bovine serum albumin for peroxisome proliferator-activated receptor gamma (PPARγ); and adiponectin or 3% bovine serum albumin for fatty acid synthase (FASN) in Tris-buffered saline (10 mM Tris·HCl, pH 7.8, 150 mM NaCl) 0.1% Tween 20 for 1 h at room temperature before overnight probing (4°C) with the following primary antibodies: adiponectin (1:500, ab22554; abcam), PPARγ (1:1,000, E-8: sc-7273; Santa Cruz Biotechnology), anti-IRβ (1:1,000 C-19: sc-711; Santa Cruz Biotechnology), GLUT4 (1:1,000, ab654; abcam), FASN (1:5,000, 610963; Biosciences), GAPDH (1:5,000, MAB 374; Millipore), and β-actin (1:10,000, A5441; Sigma-Aldrich). After washing, membranes were incubated for 1 h at room temperature with horseradish-peroxidase conjugated secondary antibodies: anti-mouse for PPARγ (1:2,000); anti-rabbit (1:2,000) for adiponectin; IRβ, anti-goat antibody, for GLUT4 (1:2,000); and anti-mouse antibody (1:2,000) for FASN and (1:5,000) for β-actin or GAPDH. Proteins were visualized using enhanced chemiluminescence and detected using the ChemiDoc XRS+ System and Image Laboratory Software (170–8265; Bio-Rad, Hercules, CA). Quantification of western immunoblots was performed using Image J 1.46r software.
Quantitative RT-PCR.
Cells were seeded (0.2 × 106) in 6-well plates and at day 14 of differentiation, total RNA was extracted using TRIzol reagent (Invitrogen). Two micrograms of total RNA were used for cDNA synthesis with Green JumpStart SYBR (Sigma, H5041). Real-time PCR was carried out using StepOne plus the Realtime PCR (qPCR) System (Applied Biosystems, 4376600). qPCR reactions were run in duplicate in three independent experiments and to control the variability in expression, data were normalized to the geometric mean of a housekeeping gene (GAPDH) and were analyzed using the 2−∆∆CT method. PCR primers were designed using OligoPerfect online software from Qiagen under consideration of the special design criteria for real-time RT-PCR primers, spanning the junction between exons. Primers were purchased from Thermo Scientific and primer sequences were as follows: PPARγ forward: 5′-GGTGGCCATCCGCATCT-3′, reverse: 5′-TGCTTTTGGCATACTCTGTGATCT-3′; adiponectin forward: 5′-TCAGCATTCAGTGTGGGATTG-3′, reverse: 5′-GGTAAAGCGAATGGGCATGT-3′; IR forward: 5′-TGACAACGACCAGTGTGGAG-3′, reverse: 5′-GCAGCCGTGTGACTTACAGA-3′; IR-A forward: 5′- TTCGGCCGGCGAATGCTGCT-3′and reverse: 5′-CCGAGTGGCCTGGGGACGA-3′; IR-B forward: 5′-AAAACCTCTTCAGGCACTGG-3′, reverse: 5′-GAGGAAGTGTTGGGGAAAGC-3′; GAPDH forward: 5′-GATCATCAGCAATGCCTCCT-3′, reverse: 5′-TGTGGTCATGAGTCCTTCCA-3′.
Oil Red O triglyceride staining.
To evaluate the level of preadipocyte differentiation, cells were stained using Oil Red O (ORO). Stock was made up by dissolving 0.25 g ORO stain powder (O0625; Sigma) in 50 ml of isopropanol, and a working solution of ORO was made by adding 10 ml of ORO stock solution to 6.67 ml of distilled water. Fully differentiated cells were washed twice in PBS before being fixed in 10% formalin for 10 min and then stained with ORO for 10 min. Following stain removal, cells were washed with 60% isopropanol to eliminate any excess stain. The cells were then washed with distilled water and viewed under light microscopy. Following image capture, the level of staining was quantified by leaching the stain with 100% isopropanol (1 ml/well) followed by spectrophotometry (FLUOstar OPTIMA, BMG LABTECH) at 490 nm.
Glucose uptake assay.
Differentiated adipocytes at day 14 were washed with PBS twice and serum-starved for 5 h in serum-free media before incubation with 900 µl of glucose-free Krebs-Ringer phosphate (KRP)/well at 37°C for 15 min. Following this incubation, cells were transferred to a water bath, also at 37°C, and stimulated with 60 ng/ml insulin or IGF-II per well for 15 min. After stimulation, 100 µl of radiolabeled glucose solution (2DG) in KRP buffer was added to each well for 10 min. Glucose transport was terminated by transferring the dishes to ice, removing the KRP buffer, and washing the cells gently three times with ice-cold PBS before disruption with 1% Triton X-100 PBS. The cell-Triton X solution was then added to 10 ml of ultima gold scintillation fluid (Perkin Elmer, Bucks, UK); the cell-associated radioactivity was counted with a liquid scintillation counter. Basal glucose uptake was measured in the absence of insulin or IGF-II.
Statistical analysis.
SPSS 12.0.1 for Windows using one-way ANOVA was used to analyze data, followed by least significant difference post hoc test, with a significant statistical difference at P < 0.05.
RESULTS
Characterization of subcutaneous and visceral preadipocyte differentiation.
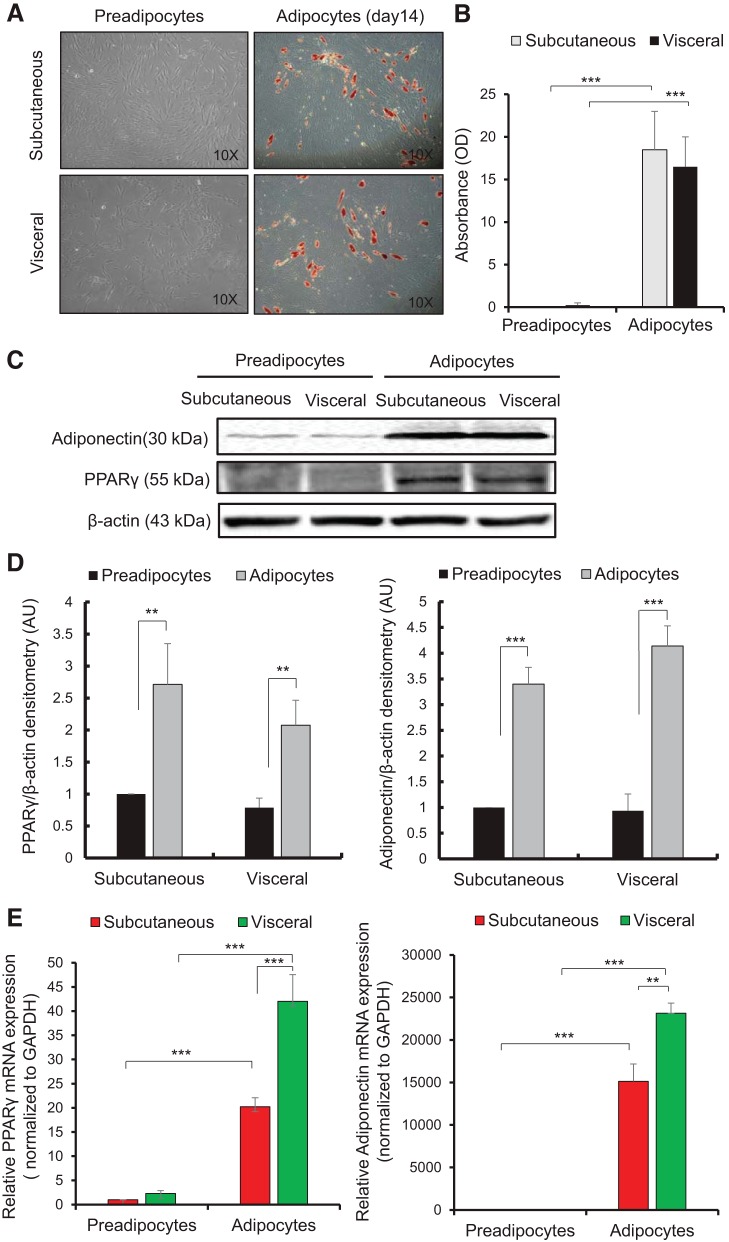
Paired biopsies from subcutaneous and visceral fat were prepared as described in Materials and Methods (16). Figure 1A shows representative phase contrast micrographs of subcutaneous and visceral preadipocytes on day 0 that display a fibroblastic morphology and differentiated adipocytes on day 14 stained with ORO stain for triglycerides. Figure 1B represents the corresponding spectrophotometric absorbance analysis of ORO stain that shows a significant increase in fat deposition with differentiation; the staining was undetectable for subcutaneous and visceral preadipocytes but there was an 18.5-fold increase for subcutaneous adipocytes (P < 0.001) and a 16.5-fold increase for visceral adipocytes (P < 0.001) in ORO staining absorbance versus corresponding preadipocytes. To confirm further successful differentiation, protein levels of two differentiation markers (PPARγ and adiponectin), which are known to be increased during adipogenesis (13), were analyzed using Western blotting (Fig. 1C). A significant increase in protein abundance of both differentiation markers, PPARγ (P < 0.01) and adiponectin (P < 0.001), was seen in adipocytes from both cell types versus corresponding preadipocytes indicating differentiation (Fig. 1D). A similar increase was seen for mRNA expression of these markers using qPCR; PPARγ expression significantly increased for subcutaneous and visceral adipocytes in comparison to preadipocytes (P < 0.001); the adiponectin marker also showed a significant increase in both adipocytes compared with preadipocytes (P < 0.001) (Fig. 1E). A difference in adiponectin and PPARy mRNA expression in visceral adipocytes in comparison to subcutaneous adipocytes was seen at day 14 of differentiation. Of the stromal-vascular cell fraction isolated, ~20%–30% of the cells differentiated into mature adipocytes (presumably reflecting the proportion of preadipocytes in the crude mixed-cell fraction), and there were no differences in this between subcutaneous and visceral cultures as assessed by fat deposition or protein markers of differentiation (Fig. 1).
Fig. 1.
Characterization of subcutaneous and visceral preadipocyte fat biopsies from prepubertal children. A: photomicrographs of human subcutaneous and visceral preadipocytes (day 0) displaying a fibroblastic morphology and differentiated adipocytes (day 14) stained with Oil Red O. Magnification at (×10). B: quantitative absorbance analysis of Oil Red O staining showing a significant increase in fat deposition in mature adipocytes in comparison with preadipocytes. C: Western blotting of the differentiation markers (PPAR-γ, adiponectin) in subcutaneous and visceral preadipocytes and in differentiated adipocytes (day 14). β-Actin was used as a loading control. D: quantitative densitometry analysis of Western blot indicating an increase in differentiation marker expression. E: relative mRNA expression of differentiation markers using qPCR, indicating an increase in differentiation. GAPDH used as reference gene. Data expressed as the means ± SE of duplicate runs for absorbance analysis and qPCR; each Western blot densitometry is representative of experiments performed in triplicate from four individual biopsies (n = 4). Statistical analysis performed using one-way ANOVA (**P < 0.01, ***P < 0.001). PPAR-γ, peroxisome proliferator-activated receptor gamma.
IGF-II promoted differentiation of subcutaneous but not visceral preadipocytes.
After characterization of preadipocyte differentiation, we studied the role of IGF-II in preadipocyte differentiation and adipogenesis. We examined the effect of IGF-II treatment at a low (7.5 ng/ml) and high (62.5 ng/ml) dose following a preliminary dose-response assessing IGF-II-induced preadipocyte proliferation (data not shown).
Visceral and subcutaneous preadipocytes were differentiated for 14 days with normal glucose (5 mM/l) differentiation media, or differentiation media supplemented with the IGF-II concentrations as described above.
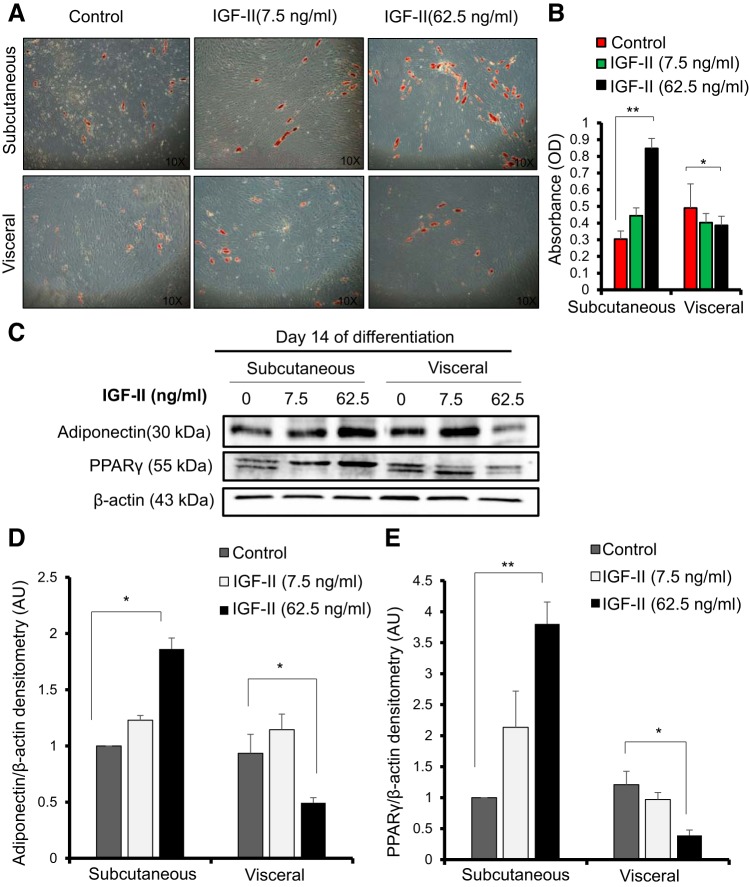
IGF-II (62.5 ng/ml) treatment enhanced fat deposition in subcutaneous fat in comparison to the control; this was observed under the microscope (Fig. 2A) and by ORO staining, as the absorbance analysis showed a significant increase in comparison to the control (P < 0.01) (Fig. 2B). In addition, the protein abundance of the differentiation markers PPARγ and adiponectin showed a significant increase in relative fold change in comparison to the control (1.86 vs. 1.0 for adiponectin; P < 0.05 and 3.8 vs. 1.0 for PPARγ: P < 0.01). This IGF-II-induced increase in PPARγ in subcutaneous adipocytes was mainly due to an increase in PPARγ2 protein (Fig. 2, C–E). Interestingly, in terms of visceral preadipocyte differentiation, IGF-II reduced the amount of preadipocyte differentiation as seen under the microscope (Fig. 2A). ORO absorbance analysis showed a reduction in triglycerides with 62.5 ng/ml IGF-II treatment (P < 0.05) (Fig. 2B). A decrease in the relative fold change of protein abundance of the differentiation markers in comparison to the control was also observed for adiponectin (0.4 vs. 0.9; P < 0.05) and PPARγ (0.3 vs. 1.2; P < 0.05) (Fig. 2, C–E).
Fig. 2.
IGF-II promoted differentiation of subcutaneous but not visceral preadipocytes. Subcutaneous and visceral preadipocytes were differentiated for 14 days in the presence or absence of IGF-II at 7.5 ng/ml or 62.5 ng/ml. A: micrograph of differentiated preadipocytes stained in Oil Red O at day 14 of differentiation. Magnification at (×10). B: quantitative Oil Red O staining absorbance analysis of A showing a reduction in Oil Red O absorbance in visceral adipocytes (P < 0.05) and an increase in absorbance in subcutaneous differentiated adipocytes (P < 0.01) with IGF-II treatment (62.5 ng/ml). C: Western blot of the differentiation markers PPARγ and adiponectin. D: densitometry of adiponectin. E: densitometry of PPARγ Western blot showing protein abundance after normalization to the reference protein β-actin. Data expressed as means ± SE of duplicate runs for absorbance analysis; each Western blot densitometry is representative of experiments performed in triplicate from three individual biopsies (n = 3). Statistical analysis performed using one-way ANOVA on day 14 of differentiation (*P < 0.05, **P < 0.01).
Differentiation with IGF-II decreased fat deposition in visceral preadipocytes: effect on the abundance of the IR, GLUT4, and FASN.
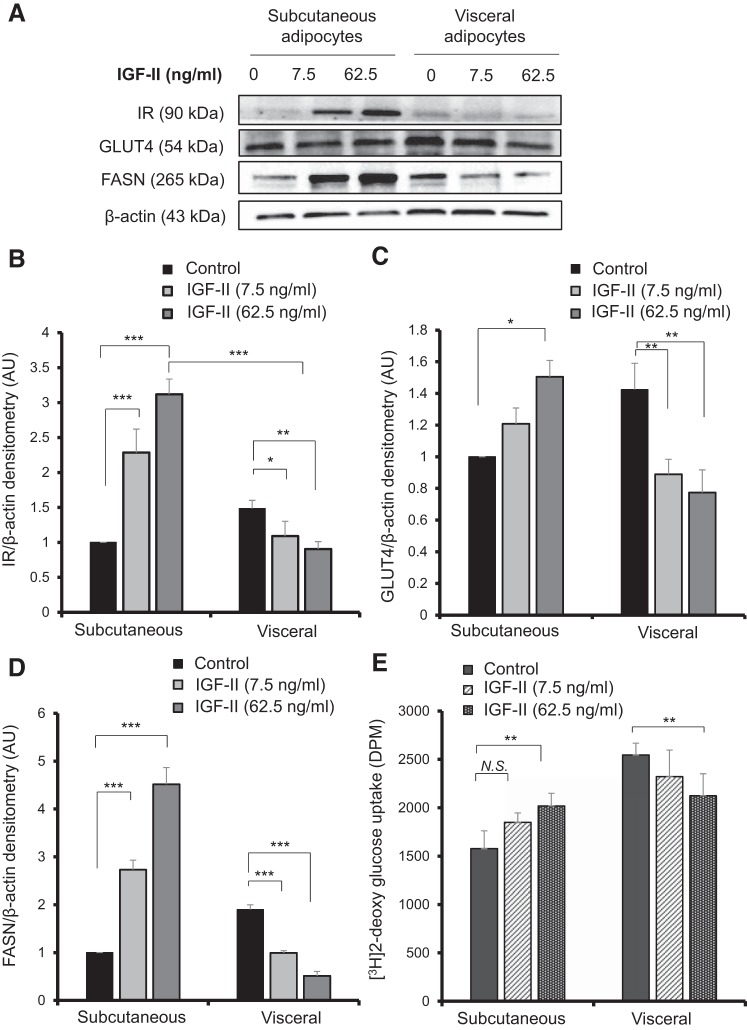
To confirm further that IGF-II reduced differentiation and fat deposition in visceral preadipocytes while enhancing it in subcutaneous preadipocytes, we examined the protein abundance of the IR, GLUT4, and FASN using Western blotting after 14 days of differentiation of paired subcutaneous and visceral preadipocytes with IGF-II in two doses (7.5 ng/ml and 62.5 ng/ml). With visceral adipocytes, there was a significant IGF-II-induced reduction in IR abundance (Fig. 3, A and B) in comparison to the control; the relative fold change reduction was significant for both doses, 7.5 ng/ml (1.0 vs. 1.4; P < 0.05) and 62.5 ng/ml (0.9 vs.1.4; P < 0.01). A consistent decrease in GLUT4 protein abundance (Fig. 3, A and C) was also seen in visceral adipocytes when differentiated with IGF-II at 7.5 ng/ml (0.8 vs. 1.4; P < 0.01) and at 62.5 ng/ml (0.7 vs. 1.4; P < 0.01). FASN was also reduced in visceral adipocytes (Fig. 3, A and D) for 7.5 ng/ml IGF-II (0.9 vs.1.8; P < 0.001) and for 62.5 ng/ml IGF-II (0.5 vs. 1.8; P < 0.001). This was reflected in a 16% reduction in insulin-stimulated radioactive 2DG glucose uptake into visceral adipocytes in comparison to the control (P < 0.01) (Fig. 3E) following IGF-II (62.5 ng/ml) treatment. In contrast, for the subcutaneous differentiated adipocytes, IGF-II enhanced IR abundance and the relative fold change in comparison to the control was 2.2 vs. 1.0 (P < 0.001) for 7.5 ng/ml IGF-II and 3.1 vs. 1.0 (P < 0.001) for 62.5 ng/ml IGF-II. When contrasting fat depots, there was a significant difference in IR protein abundance, with IGF-II (62.5 ng/ml) increasing IR abundance 2.2-fold in subcutaneous adipocytes in comparison to visceral adipocytes (P < 0.001) (Fig. 3B). GLUT4 relative protein abundance increased in subcutaneous adipocytes with IGF-II (62.5 ng/ml) (1.5 vs. 1.0; P < 0.05) in comparison to the control (Fig. 3C). Similarly, FASN protein abundance was enhanced in subcutaneous adipocytes with both doses of IGF-II: 7.5 ng/ml (2.7 vs. 1.0; P < 0.001) and 62.5 ng/ml (4.5 vs. 1.0; P < 0.001) (Fig. 3D). Insulin-stimulated glucose uptake was significantly increased using the higher dose of IGF-II (P < 0.01) (Fig. 3E), which further indicates a depot-specific action of IGF-II.
Fig. 3.
IGF-II treatment reduced fat deposition in visceral adipocytes after 14 days of differentiation. Western blotting of paired subcutaneous and visceral preadipocytes differentiated for 14 days with IGF-II (7.5 ng/ml or 62.5 ng/ml) showing insulin receptor, GLUT4, and FASN protein abundance (A). Densitometry analysis of insulin receptor protein abundance (B) GLUT4 (C), and FASN (D). β-Actin was used to ensure equal loading of samples. Densitometry represents the means ± SE of three experimental repeats from three individual biopsies (n = 3). Insulin stimulated H3 −2deoxy glucose uptake for visceral and subcutaneous adipocytes following differentiation with IGF-II (n = 3) (E). Statistical analysis performed using one-way ANOVA on day 14 of differentiation (*P < 0.05, **P < 0.01, ***P < 0.001). FASN, fatty acid synthase; GLUT, glucose transporter; IR, insulin receptor.
IGF-II treatment for 24-h downregulated total IR, IR-A, and GLUT4 mRNA expression and glucose uptake in visceral adipocytes.
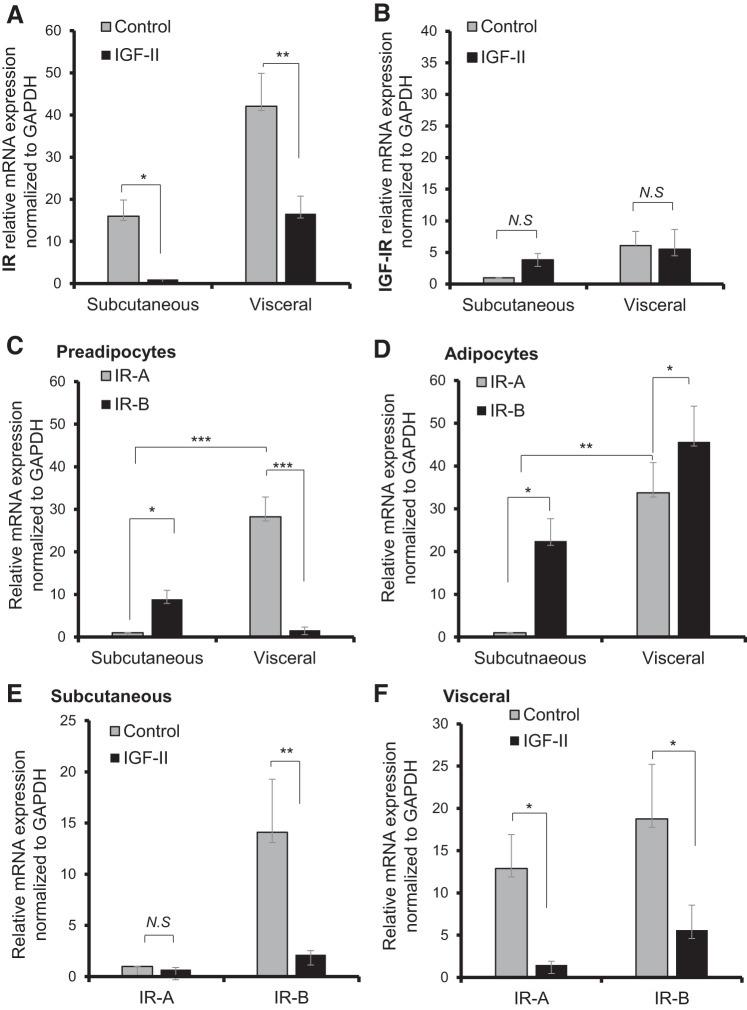
After investigating the role of IGF-II in differentiation, we examined the acute effect of IGF-II on mature differentiated adipocytes, these cells being differentiated with normal glucose (5 mM/l) differentiation media and collected on day 14. These adipocytes were serum-starved for 24 h followed by 24-h treatment with IGF-II (62.5 ng/ml). IGF-II treatment downregulated the mRNA expression of total IR levels in visceral adipocytes; the expression was normalized to GAPDH reference gene and relative fold change versus control was 16.5 versus 42.07 (P < 0.01). Furthermore, there was a reduction in total levels of the IR mRNA in subcutaneous adipocytes (15.9 vs. 1.0; P < 0.05) (Fig. 4A). There were no significant changes in IGF-IR mRNA expression after IGF-II treatment for subcutaneous and visceral adipocytes (Fig. 4B). When looking specifically at the IR isoforms, we initially characterized the IR isoform distribution in preadipocytes (Fig. 4C) and adipocytes (Fig. 4D): visceral preadipocytes show a predominance of IR-A/IR-B (28.2 vs. 1.5; P < 0.001), whereas subcutaneous preadipocytes have higher IR-B in comparison to IR-A (8.9 vs. 1.0; P < 0.05). With differentiation, the IR-B ratio has increased significantly in both fat depots. The relatively high expression of IR-A in visceral compared with subcutaneous preadipocytes was maintained with differentiation to mature adipocytes.
Fig. 4.
Effect of IGF-II treatment on receptor expression in differentiated adipocytes. A: relative mRNA expression of the insulin receptor (IR). B: IGF-IR following 24 h IGF-II (62.5 ng/ml) treatment was performed using qPCR showing downregulation of the IR in visceral adipocytes. Insulin receptor isoform distribution in preadipocytes (C) and adipocytes (D). Relative mRNA expression of the insulin receptor isoforms IR-A and IR-B in subcutaneous (E) and visceral (F) adipocytes showing downregulation of IR-A and IR-B with IGF-II treatment in visceral adipocytes and in IR-B expression in subcutaneous adipocytes. In all experiments, expression was normalized to the reference gene GAPDH; data represented as the means ± SE of experiments performed in duplicate runs from individual biopsies (n = 6–4). Statistical analysis performed using one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001).
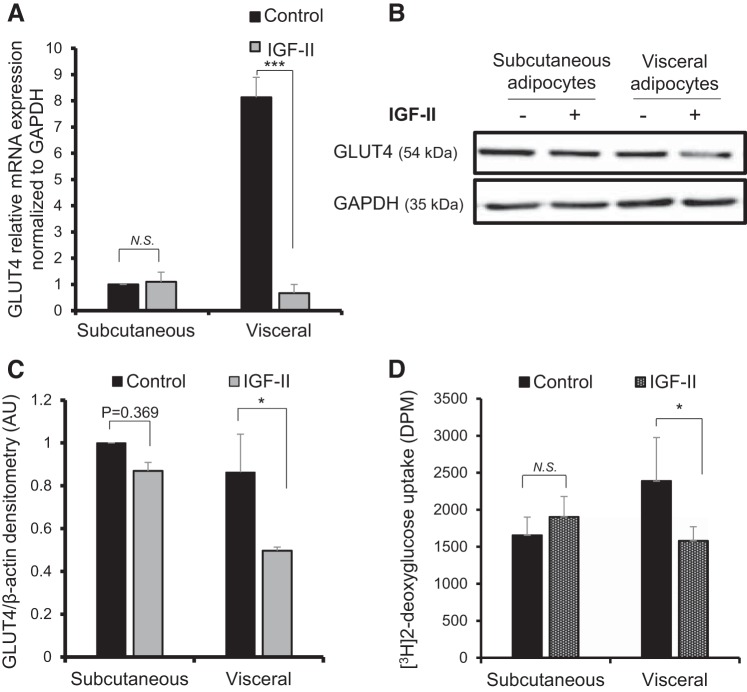
With IGF-II treatment, there was an IGF-II-induced downregulation of IR-B in both visceral and subcutaneous adipocytes versus control (visceral 5.5 vs. 18.7: P < 0.05; subcutaneous 2.1 vs. 14.0: P < 0.01) (Fig. 4, E and F). However, IR-A was only significantly downregulated in visceral adipocytes (1.4 vs. 12.8; P < 0.05), consistent with the maintenance of IR-A and maintained sensitivity to IGF-II. IGF-II treatment downregulated GLUT4 mRNA expression in visceral adipocytes with a fold change in comparison to the control (0.66 vs. 8.13; P < 0.001) (Fig. 5A); this was also reflected in IGF-II-induced reduction in visceral GLUT4 protein abundance (0.4 vs. 0.8; P < 0.05) (Fig. 5, B and C). The downregulation of GLUT4 was associated with a 33.8% reduction in 2DG glucose uptake (1,580.5 vs. 2,388.8 disintegrations/min (DPM); P < 0.05) (Fig. 5D). There were no significant changes in GLUT4 or glucose uptake with IGF-II treatment in subcutaneous adipocytes.
Fig. 5.
Effect of IGF-II treatment on GLUT 4 and glucose uptake in differentiated adipocytes. A: relative mRNA expression levels of GLUT4 in subcutaneous and visceral adipocytes measured by qPCR and normalized to GAPDH reference gene. B: Western blot showing GLUT4 protein abundance; GAPDH used a loading control. C: densitometry analysis of B indicating a reduction in GLUT4 protein abundance in visceral adipocytes. D: [3H]2-Deoxy glucose uptake following IGF-II treatment in visceral and subcutaneous adipocytes. Data expressed as the means ± SE of duplicate runs for qPCR; each Western blot densitometry is representative of experiments performed in triplicate from three individual biopsies (n = 3). Statistical analysis performed using one-way ANOVA (*P < 0.05, ***P < 0.001). GLUT, glucose transporter.
DISCUSSION
The IGF system has recognized effects on adipose tissue. IGF-I promotes preadipocyte proliferation and differentiation, as seen in the 3T3-L1 cell line (22) and in primary cultures (4) and, as we previously reported, in primary cultures obtained from children (16). However, IGF-II’s role has been poorly investigated despite its abundance in the human circulation and its predominant local production from adipose tissue, where it exceeds that of IGF-I (17).
A potential role of IGF-II in adipose tissue regulation has emerged because of its association with weight, obesity, and a number of cancers (9). With the increasing prevalence of obesity overall and in childhood, and the association of IGF-II genetic variations with body weight and obesity (8, 33, 39), further understating of IGF-II’s role in adipose tissue is needed.
Because visceral fat accumulation is strongly related to metabolic risk, we investigated IGF-II’s actions using paired visceral and subcutaneous fat biopsies, our data suggesting that IGF-II has depot-specific actions in terms of promoting preadipocyte differentiation. IGF-II treatment with physiological concentrations enhances subcutaneous preadipocyte growth to mature adipocytes and fat deposition but, in contrast, has a restricting effect on visceral preadipocyte maturation. This effect was seen with an increase in the differentiation markers PPARγ and adiponectin in subcutaneous preadipocytes. Interestingly, IGF-II specifically increased PPARγ2 expression, one of the two alternatively spliced forms of PPARγ, indicating that IGF-II alters the splicing preferences of another key adipocyte protein. This may be important, because although PPARγ1 and PPARγ2 are involved in adipogenesis in vitro, PPARγ2 is more related to the nutritional status and maintenance of insulin sensitivity (26). It would be interesting to examine whether proteins involved in alternative splicing are differentially regulated in subcutaneous and visceral adipocytes. Differentiation with IGF-II also increased IR, GLUT4, FASN, and insulin-stimulated glucose uptake in subcutaneous preadipocytes with an opposing effect on visceral preadipocytes.
IGF-II stimulated differentiation of adipose tissue isolated from eyelids in humans in vitro (25), but there is limited literature pointing to any fat depot-specific effects. However, in mammals, similar findings on fat depot differences caused by the actions of IGF-II have been reported in fetal baboons, with higher lipid deposition being seen in subcutaneous fat in comparison to visceral fat (42). The depot-specific pattern of action of IGF-II has been described in human genetic studies, with higher methylation of IGF2/H19 imprinting control region in young adults at the age of 17 being associated with higher IGF-II expression and an increase in subcutaneous fat but not increased waist circumference or visceral fat accumulation (21). Furthermore, analysis of the East Hertfordshire cohort study reported that individual variance in the genetic region IGF2-INS-TH is related to body weight. Individuals with an IGF2-INS-TH *5 haplotype, which involves the IGF2 Apal A allele together with allele 9 of TH01 and a subset of class I alleles of INS VNTR as a gene cluster, and who are known to have higher IGF-II levels (33), had lower associated waist circumference, hip-to-waist ratio, and body mass index in comparison to non *5 haplotype individuals (37, 38). Controversially, other studies have suggested that increased IGF-II levels are positively associated with central adiposity (29) and overall weight gain (39). However, ethnic differences may be important in IGF-II weight-related effects (10, 36).
We considered that receptor distribution differences might contribute to these IGF-II depot-specific actions (28). IGF-IR abundance decreases with preadipocyte differentiation (48), while IR abundance increases and visceral fat has higher IR abundance than subcutaneous fat (28). Insulin receptor isoform distribution, which can markedly alter tissue-specific biological responses to IGF-II, shows a higher distribution of IR-B in tissues primarily responsive to insulin action, such as adipocytes, muscle, and liver, whereas IR-A is more abundant in fetal and some cancer cells where mitogenic responses are important (11, 32), consistent with the reported mainly mitogenic signaling activated by IR-A. Our data indicate that IR-A is the predominant isoform in visceral preadipocytes, whereas IR-B predominates in subcutaneous preadipocytes. Although the IR-B/IR-A ratio increased with differentiation, IR-A remains significantly higher in visceral adipocytes in comparison to subcutaneous adipocytes, rendering them potentially more IGF-II responsive.
Additionally, we investigated how acute IGF-II treatment affected mRNA expression of the IGF-IR, IR, and its isoforms in adipocytes. As predicted, our results indicate that IGF-II exposure has a minimal effect on the IGF-IR in adipocytes. However, IGF-II caused downregulation of total IR mRNA levels, particularly with respect to IR-A in visceral adipocytes. This downregulation was associated with a lowering of GLUT4 (insulin-sensitive glucose transporter) and glucose uptake into the cell. The reason that IGF-II actions were more profound in visceral adipocytes may be the higher ratio of IR-A in visceral adipocytes in comparison to subcutaneous fat, the higher affinity for IGF-II binding to the IR-A (6), and the fact that IR-A has a higher internalization rate than IR-B (44). It has previously been reported that IGF-II can activate the IR in human adipocytes at physiological levels (2) and that the ability of IGF-II to modulate glucose uptake is mediated through IRs (41).
The ability of IGF-II to regulate IR-A expression in visceral fat might be of clinical importance, because insulin resistance has been linked to higher mRNA expression of IR-A (34). Furthermore, IGF-II has been reported to have an anti-inflammatory effect by reducing TNF-α, associated with obesity and insulin resistance (31). However, the relationship between obesity and IGF-II is far from clear (5). Although a high-fat diet has been reported to cause downregulation of IGF-II expression from adipocytes and upregulation of IGF-IIR expression, leading to a decrease in tissue availability with obesity (31), another conflicting study has indicated that IGF-II secretion and bioavailability are increased in obese individuals, particularly from visceral fat (18). However, whether this obesity-related increase in IGF-II is a compensatory mechanism associated with obesity or a cause of obesity requires further investigation and understanding.
Overall, our results indicate that IGF-II might have a role as a physiological regulator of preadipocyte growth and metabolism and may play a protective role in regulating body fat composition. Interestingly, IGF-II’s actions are not only limited to fat, with IGF-II also previously reported to be a regulator of muscle mass and skeletal muscle cell differentiation (46). IGF-II could also restrain metabolic risk by favoring muscle formation over that of fat. In conclusion, our data suggest a novel role for IGF-II in adipocyte regulation in childhood because it acts in a depot-specific manner to buffer excess visceral fat accumulation.
GRANTS
This work was supported by Imam Abdulrahman Bin Faisal University. C. M. Perks, J. M. P. Holly, and J. P. Hamilton-Shield are supported by the National Institute for Health Research, Bristol Nutritional Biomedical Research Centre, based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
This study was supported by and presents an opinion independent from a Biomedical Research Centre in the National Institute for Health Research Biomedical Research Centre scheme. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
AUTHOR CONTRIBUTIONS
M.N.A., C.M.P., J.P.H.-S., and J.M.P.H. conceived and designed research; M.N.A. performed experiments; M.N.A. analyzed data; M.N.A., C.M.P., J.P.H.-S., and J.M.P.H. interpreted results of experiments; M.N.A. prepared figures; M.N.A. drafted manuscript; M.N.A., C.M.P., J.P.H.-S., and J.M.P.H. edited and revised manuscript; M.N.A., C.M.P., J.P.H.-S., and J.M.P.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the urology surgeons M. Shalaby, G. Nicholls, and M. Woodward, and the theater staff at the Bristol Royal Hospital for Children, for help in collecting the biopsies. We acknowledge the General Surgery and Urology waiting list coordinators, Matthew Price and Edward Appleton, for help in the recruitment process.
REFERENCES
- 1.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19: 471–482, 2005. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Bäck K, Brännmark C, Strålfors P, Arnqvist HJ. Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes–role of insulin and IGF-I receptors. Mol Cell Endocrinol 339: 130–135, 2011. doi: 10.1016/j.mce.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Bennett A, Wilson DM, Liu F, Nagashima R, Rosenfeld RG, Hintz RL. Levels of insulin-like growth factors I and II in human cord blood. J Clin Endocrinol Metab 57: 609–612, 1983. doi: 10.1210/jcem-57-3-609. [DOI] [PubMed] [Google Scholar]
- 4.Birnie K, Ben-Shlomo Y, Holly JM, Gunnell D, Ebrahim S, Bayer A, Gallacher J, Martin RM. Associations of insulin and insulin-like growth factors with physical performance in old age in the Boyd Orr and Caerphilly studies. PLoS One 7: e30096, 2012. doi: 10.1371/journal.pone.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev 19: 111–120, 2008. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denley A, Bonython ER, Booker GW, Cosgrove LJ, Forbes BE, Ward CW, Wallace JC. Structural determinants for high-affinity binding of insulin-like growth factor II to insulin receptor (IR)-A, the exon 11 minus isoform of the IR. Mol Endocrinol 18: 2502–2512, 2004. doi: 10.1210/me.2004-0183. [DOI] [PubMed] [Google Scholar]
- 7.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881–887, 2006. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 8.Faienza MF, Santoro N, Lauciello R, Calabrò R, Giordani L, Di Salvo G, Ventura A, Delvecchio M, Perrone L, Del Giudice EM, Cavallo L. IGF2 gene variants and risk of hypertension in obese children and adolescents. Pediatr Res 67: 340–344, 2010. doi: 10.1203/PDR.0b013e3181d22757. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AP, Koldobskiy MA, Göndör A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat Rev Genet 17: 284–299, 2016. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowke JH, Matthews CE, Yu H, Cai Q, Cohen S, Buchowski MS, Zheng W, Blot WJ. Racial differences in the association between body mass index and serum IGF1, IGF2, and IGFBP3. Endocr Relat Cancer 17: 51–60, 2010. doi: 10.1677/ERC-09-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol 19: 3278–3288, 1999. doi: 10.1128/MCB.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics 115: 22–27, 2005. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46: 1369–1379, 2005. doi: 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Gaunt TR, Cooper JA, Miller GJ, Day IN, O’Dell SD. Positive associations between single nucleotide polymorphisms in the IGF2 gene region and body mass index in adult males. Hum Mol Genet 10: 1491–1501, 2001. doi: 10.1093/hmg/10.14.1491. [DOI] [PubMed] [Google Scholar]
- 15.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev 78: 783–809, 1998. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 16.Grohmann M, Sabin M, Holly J, Shield J, Crowne E, Stewart C. Characterization of differentiated subcutaneous and visceral adipose tissue from children: the influences of TNF-α and IGF-I. J Lipid Res 46: 93–103, 2005. doi: 10.1194/jlr.M400295-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Gude MF, Frystyk J, Flyvbjerg A, Bruun JM, Richelsen B, Pedersen SB. The production and regulation of IGF and IGFBPs in human adipose tissue cultures. Growth Horm IGF Res 22: 200–205, 2012. doi: 10.1016/j.ghir.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Gude MF, Hjortebjerg R, Oxvig C, Thyø AA, Magnusson NE, Bjerre M, Pedersen SB, Frystyk J. PAPP-A, IGFBP-4 and IGF-II are secreted by human adipose tissue cultures in a depot-specific manner. Eur J Endocrinol 175: 509–519, 2016. doi: 10.1530/EJE-16-0569. [DOI] [PubMed] [Google Scholar]
- 19.Holly JM, Perks CM. Insulin-like growth factor physiology: what we have learned from human studies. Endocrinol Metab Clin North Am 41: 249–263, 2012. doi: 10.1016/j.ecl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins KD, Lehmann ED, Jones RL, Holly JM, Cwyfan-Hughes SC, Turay RC, Teale JD, Gosling RG. Ethnicity affects IGFBP-3 and IGF-II in normal healthy young adult subjects. Clin Endocrinol (Oxf) 45: 327–331, 1996. doi: 10.1046/j.1365-2265.1996.00815.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang RC, Galati JC, Burrows S, Beilin LJ, Li X, Pennell CE, van Eekelen J, Mori TA, Adams LA, Craig JM. DNA methylation of the IGF2/H19 imprinting control region and adiposity distribution in young adults. Clin Epigenetics 4: 21, 2012. doi: 10.1186/1868-7083-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humbel RE. Insulin-like growth factors I and II. Eur J Biochem 190: 445–462, 1990. doi: 10.1111/j.1432-1033.1990.tb15595.x. [DOI] [PubMed] [Google Scholar]
- 23.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93, Suppl 1: S57–S63, 2008. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juul A, Dalgaard P, Blum WF, Bang P, Hall K, Michaelsen KF, Müller J, Skakkebaek NE. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab 80: 2534–2542, 1995. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- 25.Kang HM, Park S, Kim H. Insulin-like growth factor 2 enhances insulinogenic differentiation of human eyelid adipose stem cells via the insulin receptor. Cell Prolif 44: 254–263, 2011. doi: 10.1111/j.1365-2184.2011.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE. The ubiquitin ligase Siah2 regulates PPARγ activity in adipocytes. Endocrinology 153: 1206–1218, 2012. doi: 10.1210/en.2011-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Stunff C, Fallin D, Bougnères P. Paternal transmission of the very common class I INS VNTR alleles predisposes to childhood obesity. Nat Genet 29: 96–99, 2001. doi: 10.1038/ng707. [DOI] [PubMed] [Google Scholar]
- 28.Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, Vidal H. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 47: 98–103, 1998. doi: 10.2337/diab.47.1.98. [DOI] [PubMed] [Google Scholar]
- 29.Martin RM, Holly JM, Davey Smith G, Gunnell D. Associations of adiposity from childhood into adulthood with insulin resistance and the insulin-like growth factor system: 65-year follow-up of the Boyd Orr Cohort. J Clin Endocrinol Metab 91: 3287–3295, 2006. doi: 10.1210/jc.2006-0745. [DOI] [PubMed] [Google Scholar]
- 30.Miller J, Rosenbloom A, Silverstein J. Childhood obesity. J Clin Endocrinol Metab 89: 4211–4218, 2004. doi: 10.1210/jc.2004-0284. [DOI] [PubMed] [Google Scholar]
- 31.Morita S, Horii T, Kimura M, Arai Y, Kamei Y, Ogawa Y, Hatada I. Paternal allele influences high fat diet-induced obesity. PLoS One 9: e85477, 2014. doi: 10.1371/journal.pone.0085477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosthaf L, Grako K, Dull TJ, Coussens L, Ullrich A, McClain DA. Functionally distinct insulin receptors generated by tissue-specific alternative splicing. EMBO J 9: 2409–2413, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Dell S, Miller G, Cooper J, Hindmarsh P, Pringle P, Ford H, Humphries S, Day I. Apal polymorphism in insulin-like growth factor II (IGF2) gene and weight in middle-aged males. Int J Obesity Relat Metab Disord 21: 822–825, 1997. doi: 10.1038/sj.ijo.0800483. [DOI] [PubMed] [Google Scholar]
- 34.Okabayashi Y, Maddux BA, McDonald AR, Logsdon CD, Williams JA, Goldfine ID. Mechanisms of insulin-induced insulin-receptor downregulation. Decrease of receptor biosynthesis and mRNA levels. Diabetes 38: 182–187, 1989. doi: 10.2337/diab.38.2.182. [DOI] [PubMed] [Google Scholar]
- 35.Ong K, Kratzsch J, Kiess W, Dunger D, Team AS; ALSPAC Study Team . Circulating IGF-I levels in childhood are related to both current body composition and early postnatal growth rate. J Clin Endocrinol Metab 87: 1041–1044, 2002. doi: 10.1210/jcem.87.3.8342. [DOI] [PubMed] [Google Scholar]
- 36.Perkins E, Murphy SK, Murtha AP, Schildkraut J, Jirtle RL, Demark-Wahnefried W, Forman MR, Kurtzberg J, Overcash F, Huang Z, Hoyo C. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J Pediatr 161: 31–39, 2012. doi: 10.1016/j.jpeds.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodríguez S, Gaunt TR, Dennison E, Chen XH, Syddall HE, Phillips DI, Cooper C, Day IN. Replication of IGF2-INS-TH*5 haplotype effect on obesity in older men and study of related phenotypes. Eur J Hum Genet 14: 109–116, 2006. doi: 10.1038/sj.ejhg.5201505. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez S, Gaunt TR, O’Dell SD, Chen XH, Gu D, Hawe E, Miller GJ, Humphries SE, Day IN. Haplotypic analyses of the IGF2-INS-TH gene cluster in relation to cardiovascular risk traits. Hum Mol Genet 13: 715–725, 2004. doi: 10.1093/hmg/ddh070. [DOI] [PubMed] [Google Scholar]
- 39.Roth SM, Schrager MA, Metter EJ, Riechman SE, Fleg JL, Hurley BF, Ferrell RE. IGF2 genotype and obesity in men and women across the adult age span. Int J Obes Relat Metab Disord 26: 585–587, 2002. doi: 10.1038/sj.ijo.0801927. [DOI] [PubMed] [Google Scholar]
- 40.Sandhu MS, Gibson JM, Heald AH, Dunger DB, Wareham NJ. Low circulating IGF-II concentrations predict weight gain and obesity in humans. Diabetes 52: 1403–1408, 2003. doi: 10.2337/diabetes.52.6.1403. [DOI] [PubMed] [Google Scholar]
- 41.Sinha MK, Buchanan C, Raineri-Maldonado C, Khazanie P, Atkinson S, DiMarchi R, Caro JF. IGF-II receptors and IGF-II-stimulated glucose transport in human fat cells. Am J Physiol Endocrinol Metabl 258: E534–E542, 1990. doi: 10.1152/ajpendo.1990.258.3.E534. [DOI] [PubMed] [Google Scholar]
- 42.Tchoukalova YD, Nathanielsz PW, Conover CA, Smith SR, Ravussin E. Regional variation in adipogenesis and IGF regulatory proteins in the fetal baboon. Biochem Biophys Res Commun 380: 679–683, 2009. doi: 10.1016/j.bbrc.2009.01.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7: 410–420, 2008. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt B, Carrascosa JM, Ermel B, Ullrich A, Häring H-U. The two isotypes of the human insulin receptor (HIR-A and HIR-B) follow different internalization kinetics. Biochem Biophys Res Commun 177: 1013–1018, 1991. doi: 10.1016/0006-291X(91)90639-O. [DOI] [PubMed] [Google Scholar]
- 45.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr 132: 768–776, 1998. doi: 10.1016/S0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- 46.Wilson EM, Rotwein P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. J Biol Chem 281: 29962–29971, 2006. doi: 10.1074/jbc.M605445200. [DOI] [PubMed] [Google Scholar]
- 46a.World Health Organization. Obesity and overweight: fact sheet no. 311. 2015. http://wedocs.unep.org/bitstream/handle/20.500.11822/18767/WHO_Obesity_and_overweight.pdf. 2015. [Google Scholar]
- 47.Wright JT, Hausman GJ. Insulinlike growth factor-1 (IGF-1)-induced stimulation of porcine preadipocyte replication. In Vitro Cell Dev Biol Anim 31: 404–408, 1995. doi: 10.1007/BF02634290. [DOI] [PubMed] [Google Scholar]
- 48.Zizola CF, Balañá ME, Sandoval M, Calvo JC. Changes in IGF-I receptor and IGF-I mRNA during differentiation of 3T3-L1 preadipocytes. Biochimie 84: 975–980, 2002. doi: 10.1016/S0300-9084(02)00009-3. [DOI] [PubMed] [Google Scholar]