Abstract
To determine the role of UCP1-mediated thermogenesis in controlling maternal metabolic adaptation to pregnancy, energy metabolism of C57BL/6 wild-type (WT) and Ucp1 gene knockout (Ucp1−/−) mice was studied during pregnancy. With the progression of pregnancy, maternal energy expenditure rates (EERs), expression of UCP1, and core body temperature steadily declined in WT dams. Despite no significant alterations in core body temperature and weight gain during pregnancy, Ucp1−/− dams exhibited lower rates in EER decline. High-fat (HF) feeding not only robustly increased maternal UCP1 expression and core body temperature but also abolished gestation-suppressed EER in WT dams. However, HF-increased EERs were significantly attenuated in Ucp1−/− dams. Significantly increased fetal body weights and fetal/placental weight ratio were detected in fetuses from Ucp1−/− dams compared with fetuses from WT dams. Markedly increased expression levels of glucose transporter 1 and amino acid transporters were also observed in placentas from Ucp1−/− dams. Furthermore, blood glucose concentrations of fetuses from Ucp1−/− dams were significantly higher than those of fetuses from WT dams, indicating that maternal UCP1 has an inhibitory effect on placental efficiency and fetal growth. Taken all together, this study demonstrated that maternal brown adipose tissue plays an important role in controlling maternal metabolic adaptation and placental nutrient transport.
Keywords: brown adipose tissue, fetal growth, maternal metabolic adaptation, placental nutrient transport, thermogenesis
INTRODUCTION
During pregnancy, maternal metabolism goes through a series of adaptations that affect placental function, the intrauterine metabolism, and fetal growth. Due to the obesity epidemic, maternal obesity has become a major risk factor for many adverse pregnancy outcomes (13, 26). Therefore, elucidating how maternal metabolism is adjusted under both normal and obese conditions should provide useful information to improve pregnancy outcome.
Although many factors are involved in controlling maternal metabolic adaptation, the status of maternal energy metabolism, including energy intake and expenditure, plays essential roles in this process. Brown adipose tissue (BAT) is specialized in nonshivering thermogenesis, which consumes a significant amount of chemical energy (19). The rediscovery of BAT in human adults has revealed the key role of BAT in maintaining energy homeostasis (5, 29). Studies have reported that the expression of uncoupling protein-1 (UCP1), the key protein of the thermogenic chain in the mitochondrial membrane, in maternal BAT is significantly suppressed during pregnancy (1, 16, 26). However, very limited information is available regarding the role of UCP1 and nonshivering thermogenesis in maternal metabolic adaptations.
Using pregnant mouse models, this study demonstrates that UCP1-mediated thermogenesis plays an important role in controlling maternal metabolic adaptation during both normal pregnancies and pregnancies complicated by high-fat (HF) diet-induced obesity. This study reveals that the reduction of maternal UCP1 expression during normal pregnancy not only preserves energy for anabolic metabolism but also evades the inhibitory effects of maternal UCP1/thermogenesis on placental nutrient transport and fetal growth.
MATERIAL AND METHODS
Materials.
Glucose and glucose oxidase were from Sigma-Aldrich (St. Louis, MO). Antibodies against UCP1 were from Abcam (Cambridge, MA). Antibodies against phospho-Akt (Ser473), phospho-mTOR (mammalian target of rapamycin), phospho-AMP-activated protein kinase (AMPK, Thr172), Akt, mTOR, and AMPK were from Cell Signaling (Danvers, MA). Anti-GAPDH and horseradish peroxidase (HRP)-linked secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Free fatty acid (FFA) and triglyceride (TG) assay kits were purchased from Wako Diagnostics (Richmond, VA). NuPAGE gels, SuperScript III reverse transcriptase, and oligo(dT)12–18 primer were from Invitrogen (Carlsbad, CA). The mouse diabetes multiplex assay kit was from Bio-Rad (Hercules, CA). HF diet (60 kcal% from fat, 20 kCal% from protein, 20 kCal% from carbohydrate; energy density, 5.24 kcal/g, catalog no. D12492) was from Research Diets (New Brunswick, NJ). Regular chow (17 kcal% from fat, 25 kcal% from protein, 58 kcal% from carbohydrate; energy density, 3.1 kcal/g, catalog no. 7912) was from Harlan Laboratories (Madison, WI).
Experimental animals.
Ucp1−/− and C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Ucp1−/− mice had been back-crossed to C57BL/6 (7). Ten- to twelve-week-old female mice were randomly selected for mating. For studies of maternal metabolic adaptation, C57BL/6 mice were mated. To study the effect of maternal UCP1 on metabolic adaptation, Ucp1−/− female mice were mated with WT males or WT female mice mated with Ucp1−/− sires (see Fig. 3A, cross-mating), which produced all fetuses that were Ucp1-/+. For maternal obesity studies, WT and Ucp1−/− female mice were fed HF diet 1 mo before mating and during pregnancy. Age-matched nonpregnant WT or Ucp1−/− female mice were fed HF or chow diet as controls. Pregnancy was determined by the presence of a vaginal plug and was assigned the embryonic day (E)0.5. Sires were fed regular chow. Placentas and fetal samples were collected at indicated gestational days through Caesarean section under maternal anesthesia (21, 22). Fetuses were immediately euthanized by decapitation, and blood was collected through the wound. Blood glucose concentration was measured using glucose oxidase (21, 22). Except for some studies with fasted mice (see details in figure legends), tissue samples were collected when the dams were in the fed state. Body composition was determined by using an Echo-MRI system (Houston, TX), which measures whole body fat without depot delineation. Experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the University of California San Diego Animal Care and Use Committee.
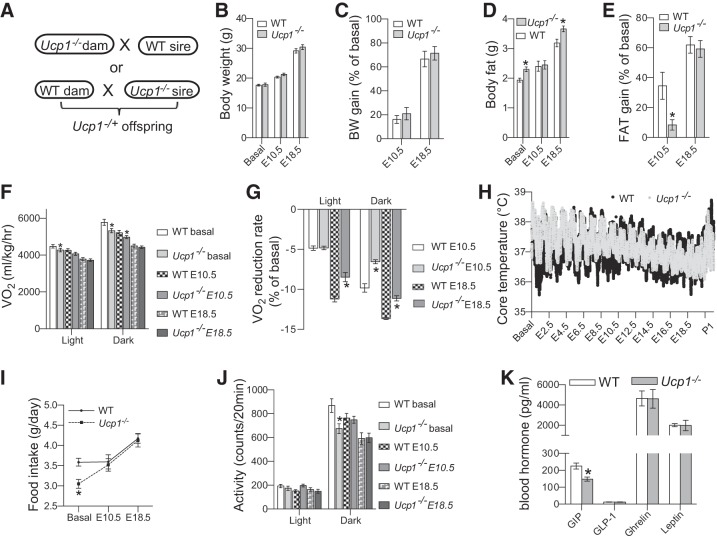
Fig. 3.
Maternal uncoupling protein-1 (UCP1) deficiency attenuated pregnancy-induced reduction of energy expenditure. A: Ucp1−/− and wild-type (WT) mice were cross-mated to produce fetuses with an identical genotype. B–E: body composition was longitudinally studied using Echo-MRI. Energy metabolism at indicated gestational ages was studied using indirect calorimetry (F, G, I, and J). H: core body temperatures of another set of mice were monitored using ip implanted emitter from 1 day before mating (Basal) to postpartum day 1. K: blood hormone levels were measured using a Bio-Rad mouse diabetes Luminex kit with nonpregnant female mouse blood samples. Data are presented as means ± SE; n = 10, except core body temperature study (n = 4). *P < 0.05 vs. WT at the same time point.
Indirect calorimetry and core body temperature monitoring.
Energy intake, oxygen consumption rate (V̇o2, also referred to as energy expenditure rate, EER), and locomotor activity were measured using the Oxymax/Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH) with a 24-h acclimatization as we previously described (31). The resting metabolic rate was the mean of the three lowest V̇o2 measurements during the light cycle. Core body temperature was wirelessly monitored using an intraperitoneally implanted emitter and the VitalView data acquisition system (Mini Mitter, Bend, OR) (24). The emitter was implanted into the peritoneal cavity through a 3- to 5-mm incision. The mice were allowed a 1- to 2-wk recovery before mating. Due to the prolonged continued monitoring, the emitter-implanted mice were dedicated for core body temperature assay.
Western blot and real-time PCR assays.
Protein samples were extracted from fat, livers, or placentas and separated using NuPAGE gels. Proteins were blotted with the indicated antibodies (see details in figure legends). The bands from Western blots were quantified using Quantity One software (Bio-Rad). Total RNA was prepared from tissues by use of TRIzol following the manufacturer’s protocol. cDNA was synthesized using SuperScript III Reverse Transcriptase and oligo(dT)12–18 primer. Real-time PCR was performed using a mx3000p Real-Time PCR system (Stratagene, San Diego, CA) and specific primers (Table 1). The levels of PCR product were calculated from standard curves established from each primer pair. Expression data were normalized to the amount of 18S rRNA.
Table 1.
Sequences for real-time PCR primers
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| 18S rRNA | CGAAAGCATTTGCCAAGAAT | AGTCGGCATCGTTTATGGTC |
| Igfbp1 | TGGTCAGGGAGCCTGTGTA | ACAGCAGCCTTTGCCTCTT |
| Igf2 | CGCTTCAGTTTGTCTGTTCG | GCAGCACTCTTCCACGATG |
| Glut1 | GACCCTGCACCTCATTGG | GATGCTCAGATAGGACATCCAAG |
| Snat1 | CTTCAGCCATAAAATCCCTCAT | CATCGACGTACCAGGCTGA |
| Snat2 | CAATGAGATCCGTGCAAAAG | TTGTGTACCCAATCCAAAACAA |
| Lat1 | CAAAGTGCCAAGAAAAAGAGC | CTGAGCAGGGAGGAACCAC |
| Lat2 | TTACTCTTATGTGAAGGACATCG | CCAGCACAGCAATCCACA |
| Lpl | GAAGTCTGACCAATAAGAAGGTCAA | TGTGTGTAAGACATCTACAAAATCAGC |
| Cd36 | TTGAAAAGTCTCGGACATTGAG | TCAGATCCGAACACAGCGTA |
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed using the Student t-test for two groups comparison or one-way ANOVA for repeated measurement or multiple comparison followed by Bonferroni posttests using Prism software. Differences were considered significant at P < 0.05.
RESULTS
Reduction of maternal energy expenditure during pregnancy.
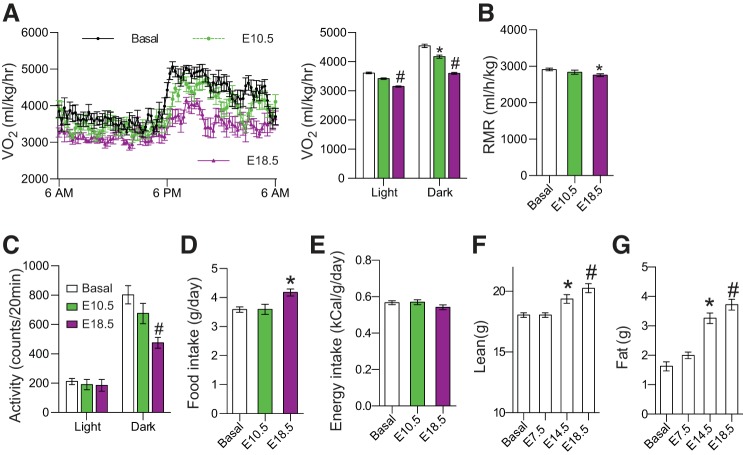
To determine the underlying mechanism of these metabolic adaptations, we longitudinally studied the energy metabolism of C57BL/6 mice before and during pregnancy, using the indirect calorimetry system. We found that the V̇o2 (or EER) and resting metabolic rates (RMRs) steadily declined with the progression of pregnancy (Fig. 1, A and B). For the note, the results of reduction in V̇o2 during pregnancy seems to contradict previous reports, in which most of the data were presented without any consideration of body weight (9, 12, 15) or fetal weight (17). Without normalization, our data also showed an increase in V̇o2 during pregnancy (data not shown). However, since energy metabolism is closely associated with body weight, V̇o2 is commonly normalized by body weight, especially in rodents (27). There was a significant reduction in locomotor activity during late pregnancy in the dark period (Fig. 1C). Interestingly, despite food intake being significantly increased during late pregnancy (Fig. 1D), energy intakes were slightly reduced at the late pregnancy after normalization of body weight (Fig. 1E; P > 0.05). Although these measurements could not separate energy metabolism between maternal and fetal compartments, the significant increase in maternal lean (Fig. 1F) and fat tissue masses (Fig. 1G) and fetal weight indicate an anabolic metabolism in both compartments. Since there is no significant increase in energy intake (Fig. 1E) and the reduction of physical activity is mainly in late pregnancy during the dark period (Fig. 1C), the reduction in energy expenditure should play an important role to ensure energy supply for the anabolic metabolism during pregnancy.
Fig. 1.
Pregnancy suppressed energy expenditure in C57BL/6 mice. Ten- to 12-wk-old C57BL/6 mice were randomly selected for mating. Energy metabolism was longitudinally studied before mating (basal), at embryonic day (E)10.5 and E18.5 using the indirect calorimetry Oxymax system. With the progress of pregnancy, rate of O2 consumption (V̇o2; A) and resting metabolic rate (RMR, B) were gradually reduced. C: a significant reduction of locomotor activity was observed only in the dark period at E18.5. Although food intake was significantly increased at late pregnancy (D), energy intake was stable during pregnancy (E), when body weight change was considered. Maternal lean (F) and fat mass (G) were measured by Echo-MRI with removal of the placentas and pups. Data are presented as means ± SE; n = 8. *P < 0.05; #P < 0.01 vs. Basal.
Reduction of UCP1 expression and thermogenesis during pregnancy.
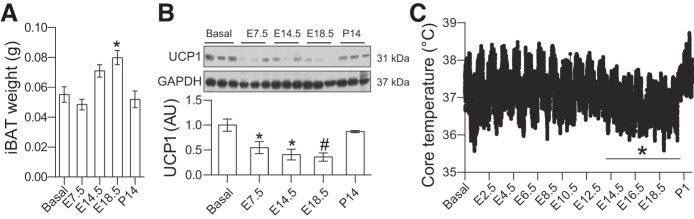
Interscapular BAT (iBAT) is the largest BAT in adult mice. There was a significant increase in iBAT mass during late pregnancy (Fig. 2A). However, the expansion of iBAT mass was mainly caused by lipid accumulation [tissue TG: 157 ± 25 (Basal) vs. 218 ± 34 (E18.5) mg/g, P < 0.05, n = 8). In contrast, UCP1 protein levels were robustly reduced during pregnancy (Fig. 2B). To study thermogenesis, we monitored core body temperature during pregnancy. As shown in Fig. 2C, core body temperatures were significantly reduced in late pregnancy. After delivery, core body temperature was rapidly restored to normal levels (Fig. 2C).
Fig. 2.
Pregnancy inhibited uncoupling protein-1 (UCP1) expression and thermogenesis. A and B: tissue samples were collected from nonpregnant (Basal) or pregnant C57BL/6 mice at indicated gestation ages or 14 days after parturition. A: tissue weights of interscapular brown adipose tissue (iBAT) were measured using a scale. B: expression levels of UCP1 were measured by Western blotting. C: emitters were transplanted (ip) into 10-wk-old C57BL/6 female mice. After 1–2 wk of recovery, core body temperatures were monitored every minute before mating and during gestation and parturition. Average core body temperature during a light and dark cycle (24 h) were compared with the mean core body temperature at Basal. Data are presented as means ± SE; n = 8–10 for A and B, n = 4 for C. *P < 0.05; #P < 0.01 vs. Basal.
Maternal Ucp1 gene deletion attenuated pregnancy-induced reduction of energy expenditure.
To further investigate the role of UCP1-mediated thermogenesis in maternal metabolic adaptation, Ucp1−/− mice were crossed with WT mice, which ensured an identical genotype of fetuses (Ucp1−/+) and allowed us to focus on the effects of Ucp1 gene on maternal compartment (Fig. 3A). Although there was no difference in body weight and weight gain during pregnancy (Fig. 3, B and C), tissue masses of maternal body fat were significant higher in Ucp1−/− dams at both basal and the end of pregnancy (Fig. 3D). Interestingly, maternal body fat gain rates of Ucp1−/− dams were remarkably lower in the middle of pregnancy (Fig. 3E). As expected, V̇o2 of nonpregnant Ucp1−/− female mice were significantly lower than those of WT female mice during both light and dark periods (Fig. 3F). The difference in V̇o2 between Ucp1−/− and WT mice was gradually reduced and eventually vanished at the end of pregnancy (Fig. 3F). Most importantly, the reduction rates of V̇o2 in Ucp1−/− dams were remarkably lower than those of WT dams (Fig. 3G). These results indicate that reduction of Ucp1 gene expression and thermogenesis during pregnancy contributes to the gestation-induced decrease of energy expenditure. Interestingly, similar core body temperatures were observed between WT and Ucp1−/− mice at basal and during gestation (Fig. 3H), suggesting that UCP1-mediated thermogenesis is compensated by other mechanisms that maintain core body temperature under regular housing conditions in Ucp1−/− mice. Noticeably low levels of food intake and physical activity were observed in Ucp1−/− mice at basal but not during pregnancy (Fig. 3, I and J). Futhermore, except for a significant reduction in blood gastric inhibitory polypeptide of the Ucp1−/− mice, the gut hormones GLP-1, insulin, and leptin were comparable between WT and Ucp1−/− mice (Fig. 3K). The underlying mechanisms of the reduction of fat-gaining rate, food intake, and physical activity of Ucp1−/− female mice is under investigation by a separate project.
Maternal Ucp1 gene deletion partially attenuated HF feeding-enhanced energy expenditure during pregnancy.
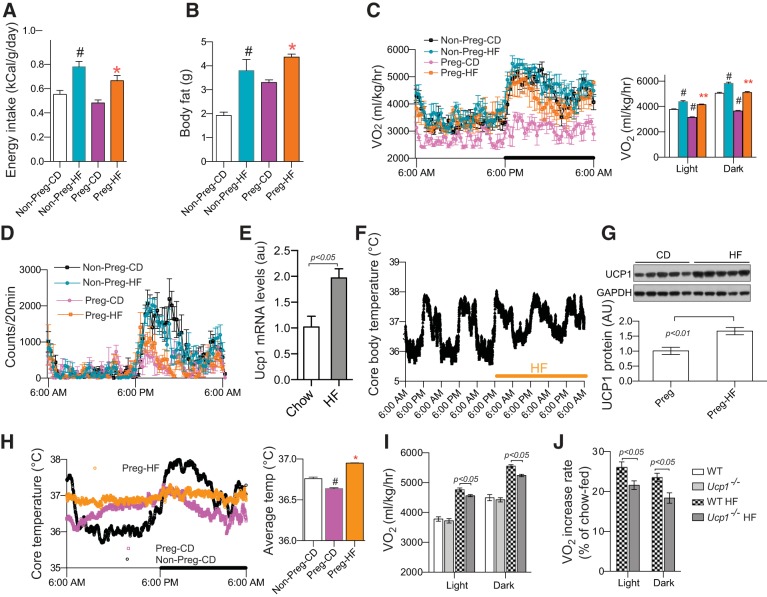
To mimic the common form of maternal obesity, we fed C57BL/6 female mice HF diet 1 mo before and during pregnancy. As expected, HF feeding significantly increased energy intake and maternal body fat (Fig. 4, A and B). V̇o2 of HF-fed pregnant mice (Preg-HF) were significantly increased and completely restored to the levels of nonpregnant chow-fed mice (Non-Preg-CD) (Fig. 4C), indicating that maternal HF feeding abolished pregnancy-induced reduction of energy expenditure. There was no significant change in locomotor activity of Preg-HF mice compared with chow-fed dams (Preg-CD) (Fig. 4D), ruling out any contribution of physical activity to the increased V̇o2. In nonpregnant WT mice, HF feeding robustly increased UCP1 expression and thermogenesis (Fig. 4, E and F), which has been considered as a mechanism to buffer energy overload (8, 10). Consistent with nonpregnant mice, increased UCP1 protein and core body temperature were detected in Preg-HF dams (Fig. 4, G and H). We then fed Ucp1−/− mice with HF diet as the above-described WT mice. Like WT mice, HF feeding increased V̇o2 of Ucp1−/− dams (Fig. 4I). However, compared with WT dams, HF diet-induced increases of V̇o2 of Ucp1−/− dams were significantly lower during both the light and dark periods (Fig. 4, I and J). These results indicate that increased UCP1 contributes to the HF-induced increase of energy expenditure during pregnancy.
Fig. 4.
Maternal uncoupling protein-1 (UCP1) deficiency attenuated high-fat (HF) feeding-increased energy expenditure in pregnant mice. Female C57BL/6 and Ucp1−/− mice were fed HF diet 1 mo before and during pregnancy (A–D, G–J, n = 10–12). HF feeding significantly increased energy intake (A), body fat (B), O2 consumption (V̇o2; C) but showed no effect on activity (D) at embryonic day (E)18.5. Two-day HF feeding increased UCP1 expression in interscapular brown adipose tissue (iBAT; E) and core body temperature (F) of nonpregnant C57BL/6 mice (n = 5). Significantly elevated UCP1 protein (G) core body temperatures (H) were observed in HF-fed pregnant mice at E18.5. However, HF-induced increase in V̇o2 rates were attenuated in Ucp1−/− dams at E18.5 (I and J). Nonpreg-CD, chow-fed nonpregnant mice; Nonpreg-HF, HF-fed nonpregnant mice; Preg-CD, chow-fed pregnant mice; Preg-HF, HF-fed pregnant mice. Data are presented as means ± SE. #P < 0.05 vs. Nonpreg-CD; *P < 0.05, **P < 0.01 vs. Preg-CD.
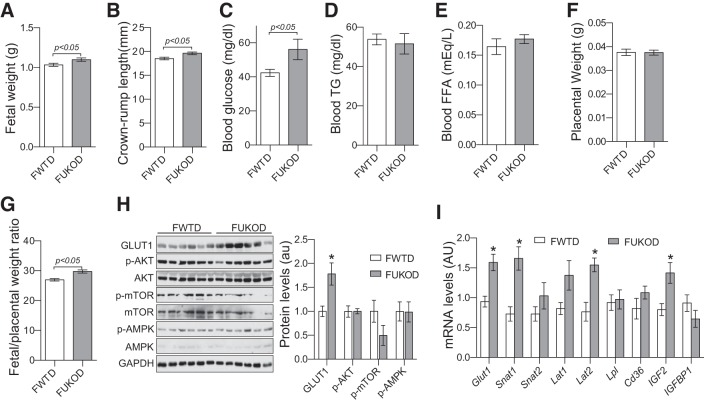
Maternal Ucp1 gene knockout enhanced fetal growth and placental efficiency.
To determine the importance and the regulatory effect of UCP1 on intrauterine metabolism, we measured fetal weight and main metabolites in fetal blood at E18.5 of the fetuses from the Ucp1−/− and WT cross-mated dams. There was no difference in litter size between Ucp1−/− and WT dams (data not shown). Surprisingly, fetal weight and crown-rump length of fetuses from Ucp1−/− dams (FUKOD) were significantly higher than those of fetuses from WT dams (FWTD)(Fig. 5, A and B). Blood glucose concentrations of FUKOD were also significantly elevated (Fig. 5C), whereas their blood TG and free fatty acid (FFA) concentrations were similar to those of FWTD (Fig. 5, D and E). These results indicate that maternal UCP1/BAT activation inhibits fetal growth and regulates intrauterine glucose metabolism. Since the core body temperatures of Ucp1−/− dams were similar to those of the WT dams during late pregnancy (Fig. 3H), these results suggest that maternal UCP1/BAT inhibits fetal growth through a temperature-independent mechanism(s).
Fig. 5.
Maternal uncoupling protein-1 (UCP1) deficiency increased fetal growth and placental efficiency. Fetuses and placentas were collected from Ucp1−/− and wild-type (WT) cross-mated dams at embryonic day (E)18.5 through C-section. Significantly increased fetal weight (A) and crown-rump length (B) were detected in fetuses of Ucp1−/− dams (FUKOD). Fetal blood glucose concentrations (C), but not triglycerides (TG) and free fatty acids (FFA) (pooled sample per litter, D and E), were remarkably elevated in FUKOD vs. fetuses from WT dams (FWTD). Despite no difference in placental weight (F), fetal/placental weight ratios were significantly increased in FUKOD (G). Expression levels of key nutrient transporters and signal pathways were measured by Western blotting and real-PCR using samples from placentas at E18.5 (H and I). p-Akt, phospho-protein kinase B; p-mTOR, phospho-mammalian target of rapamycin; p-AMPK, phospho-AMP-avtivated protein kinase. Data are presented as means ± SE. Per litter averages of body weight, length, glucose, placental weight, and fetal/placental weight ratio were used for statistical analysis; n = 7–9 for A–G, n = 12 for h H and I. *P < 0.05 vs. FWTD.
Placental nutrient transport plays an essential role in fetal growth. Although placental weights were comparable between FUKOD and FWTD (Fig. 5F), elevated fetal-to-placental weight ratios of FUKOD (Fig. 5G) indicated that maternal Ucp1 gene deletion increased placental efficiency. There are a series of proteins at the membranes of trophoblast cells that facilitate nutrient transport through the placental barrier. Glucose transporter 1 (GLUT1) is the main placental glucose transporter that transfers glucose from maternal blood to fetal circulation, driven by the transplacental glucose gradient (2). However, as with the nonpregnant Ucp1−/− mice (7), blood glucose concentrations of Ucp1−/− dams were similar to those of WT dams (data not shown), indicating a decrease in transplacental glucose gradient, which unlikely contributed to the increased fetal blood glucose of FUKOD. Interestingly, our results showed that GLUT1 protein and mRNA levels were significantly increased in the placentas from FUKOD (Fig. 5, H and I). In addition, expression of amino acid transporters, including Snat1 and Lat2, were also significantly increased in the placentas of FUKOD (Fig. 5I). These results suggest that increased nutrient transporters may be responsible for the increased placenta efficiency of FUKOD.
mTOR is a serine/threonine kinase that has been reported as a placental nutrient sensor and plays an important role in controlling placenta nutrient transport (25). To our surprise, instead of an increase, a trend toward a decrease in phosphorylation levels of mTOR was detected in placentas of Ucp1−/− dams (Fig. 5H), suggesting that maternal UCP1 deficiency increases placenta efficiency but not through mTOR signaling. In addition, phosphorylation of Akt and AMPK was not altered by maternal Ucp1 gene deletion (Fig. 5H). Insulin-like growth factor (IGF)-II is highly expressed in the placenta and plays a key role in controlling placental nutrient transport (4). Remarkably, increased IGF-II mRNA was detected in placentas of FUKOD (Fig. 5I). Expression levels of IGF-binding protein-1, which inhibits IGF signaling by reducing IGF bioavailability (22), were comparable between FWTD and FUKOD (Fig. 5I). Together, these results indicate that maternal UCP1/thermogenesis reduces placental efficiency and fetal growth.
DISCUSSION
The main goal of maternal metabolic adaptation is to sustain pregnancy and prepare for delivery and lactation. Although mice are altricial and have a gestation equivalent to the first and second trimesters of humans, there are many similarities in maternal metabolic adaptation (21, 23). The significantly increased maternal body weight and expansion in adipose tissue mass demonstrate an anabolic metabolism in the maternal compartment. By longitudinally monitoring energy metabolism of mice during pregnancy, the present study indicates that reduction in maternal energy expenditure is a key characteristic of maternal adaptation in energy metabolism. This study also demonstrates that reduction of maternal UCP1 expression and thermogenesis is an important mechanism underlying maternal metabolic adaptation during normal pregnancy.
Energy expenditure is a readout of many metabolic processes. Physical activity is a big component in these processes. We observed a reduction of physical movement of pregnant mice only during the dark period in late pregnancy. Apparently, the reduction of physical movement cannot explain the decreases of EER in midpregnancy and the light period of late pregnancy. Bioactive BAT has been “rediscovered” in adult humans (5, 14, 29). Human BAT mass and activity are inversely correlated with BMI, which indicates that BAT plays a key role in maintaining energy homeostasis (5, 6, 14, 29). Although women possess larger volumes of BAT than men (5, 20), little is known about the effect of BAT activation on maternal and intrauterine metabolism in humans. Consistent with previous studies (1, 16, 17, 26), our data of significantly reduced UCP1 expression and decreased core body temperature further demonstrate that BAT-mediated thermogenesis is inhibited during pregnancy.
To study the role of BAT in maternal metabolic adaptation, we cross-mated Ucp1−/− and WT mice to ensure the same genotype of fetuses and to avoid any effect from the fetal compartment. We found that pregnancy-induced reduction of EERs was significantly attenuated by maternal Ucp1 gene deletion (Fig. 3G). These results led us to propose that the reduction of maternal UCP1 expression and thermogenesis is a main component that contributes to pregnancy-induced decrease in energy expenditure. Since UCP1 deficiency partially attenuated pregnancy-reduced energy expenditure, we conclude that multiple mechanisms underlie gestation-reduced energy expenditure, and BAT-mediated thermogenesis is one of these factors. Regarding thermogenesis, our results showed that core body temperatures of Ucp1−/− dams were comparable to those of WT dams (Fig. 3H). Although UCP1-mediated thermogenesis is important in maintaining core body temperature, systemic Ucp1 gene deletion most likely induces compensatory mechanisms that maintain core body temperature in a normal range. The intact core body temperature of nonpregnant Ucp1−/− mice under regular housing conditions supports this compensation theory (7, 28). Our study also demonstrates that, despite no change in core body temperature, the impact of Ucp1 gene deletion on energy metabolism is illustrated by a decrease in EER of nonpregnant Ucp1−/− mice (Fig. 3F). Interestingly, EERs of Ucp1−/− dams were at the same level of WT dams at the end of pregnancy, suggesting that UCP1-derived energy expenditure is suppressed during normal pregnancy. Therefore, due to the decrease of UCP1 expression in WT dams, the difference in EER between WT and Ucp1−/− dams was gradually diminished and eventually disappeared. These results strongly indicate that reduction of UCP1 expression and BAT thermogenesis play an important role in controlling maternal metabolic adaptation during normal pregnancy. As in nonpregnant mice (3, 11, 18, 30), our study reveals that UCP1 expression and thermogenesis are enhanced when a pregnancy is complicated by HF-induced obesity. Using pregnant Ucp1−/− mice, our study further demonstrated that maternal Ucp1 gene deletion attenuated the HF feeding-induced increase of EER. Therefore, our study demonstrates that the increase in UCP1 expression and BAT thermogenesis is employed to dissipate overload energy when pregnant mice are fed HF diet. All taken together, this study demonstrates that BAT is an important player in controlling maternal metabolic adaptation to both normal and HF-induced obese pregnancy.
Maternal metabolic adaptation determines the intrauterine metabolic environment, which controls fetal growth. Although maternal blood glucose concentrations were not significantly altered by maternal Ucp1 gene deletion, a remarkable increase in fetal body weight and crown-rump length was observed in fetuses from Ucp1−/− dams. Surgical ablation of maternal iBAT before conception also increased fetal weight (17). Therefore, we propose that maternal BAT has an inhibitory effect on fetal growth. An important question is raised about how maternal BAT regulates fetal growth. The results of intact core body temperature rule out the contribution of cold stress. In addition, Ucp1 gene deletion did not alter maternal blood glucose and FFA levels, which almost rule out an increase in maternal nutrient supply. However, the increased fetal/placental weight ratio indicates an increase in placental efficiency by maternal Ucp1 gene deletion. Significantly increased fetal blood glucose levels also support the idea of increased placental nutrient transport. Maternal Ucp1 gene deficiency increased fetal blood glucose but did not alter maternal glucose, resulting in reduction of the transplacental glucose gradient. Our study also shows that fetal blood insulin levels and phosphorylation of Akt in fetal skeletal muscle were comparable between FUKOD and FWTD (data not shown). Therefore, it is logical to assume that, despite of the reduction in the transplacental glucose gradient, maternal UCP1 deficiency increases fetal glucose supply by enhancing placental glucose transport. Significantly elevated GLUT1 protein levels in placentas provide a mechanism for the increased fetal blood glucose of Ucp1−/− dams. Increased expression levels of amino acid transporters in placentas of Ucp1−/− dams should also enhance the efficiency of placental amino acid transport. Therefore, these data suggest that maternal Ucp1 gene deletion increases fetal growth by enhancing placental nutrient transport. Since UCP1 is a mitochondrial protein in brown adipocytes, the inhibitory effect of maternal UCP1/BAT on placental nutrient transport should be by an indirect mechanism. We found that IGF-II expression levels were significantly increased in placentas from Ucp1−/− dams. Due to the essential role of IGF-II in placental development and fetal nutrient supply (4), we hypothesize that increased IGF-II expression underlies maternal Ucp1 deficiency-enhanced placental nutrient transport. Future studies are warranted to investigate how maternal BAT regulates IGF-II expression and placental nutrient transport.
In summary, the current study demonstrates that UCP1-mediated thermogenesis plays an important role in controlling the adaptation of maternal energy metabolism to pregnancy. We propose that reduction of maternal UCP1 expression during normal pregnancy preserves energy for the anabolic metabolisms in both maternal and fetal compartments, whereas in obesity-complicated pregnancies elevated UCP1 expression dissipates energy to buffer energy overload. Our study also reveals that maternal UCP1/BAT exhibits an inhibitory effect on placental nutrient transport and fetal growth in mice. Due to the differences between rodent and human pregnancies, further studies are required to verify whether maternal BAT plays the same role in modulating energy metabolism and placental nutrient transport in humans.
GRANTS
This work was supported by NIH Grants HD-069634 (J. Shao), DK-095132 (J. Shao), DK-113007 (J. Shao) HD-007186 (W. W. Hay, Jr.), HD-068372 (W. W. Hay, Jr.), and UL1 TR-001082 (W. W. Hay, Jr.) and American Diabetes Association Grant 1-16-IBS-272 (J. Shao).
DISCLAIMERS
J. Shao is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Q. and J.S. conceived and designed research; L.Q., S.L., and A.N. performed experiments; L.Q. and J.S. analyzed data; W.W.H.Jr. and J.S. interpreted results of experiments; J.S. prepared figures; J.S. drafted manuscript; W.W.H.Jr. and J.S. edited and revised manuscript; J.S. approved final version of manuscript.
REFERENCES
- 1.Andrews JF, Richard D, Jennings G, Trayhurn P. Brown adipose tissue thermogenesis during pregnancy in mice. Ann Nutr Metab 30: 87–93, 1986. doi: 10.1159/000177180. [DOI] [PubMed] [Google Scholar]
- 2.Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine 19: 13–22, 2002. doi: 10.1385/ENDO:19:1:13. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SL, Rothwell NJ, Stock MJ, Goodbody AE, Trayhurn P. Increased proton conductance pathway in brown adipose tissue mitochondria of rats exhibiting diet-induced thermogenesis. Nature 286: 274–276, 1980. doi: 10.1038/286274a0. [DOI] [PubMed] [Google Scholar]
- 4.Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417: 945–948, 2002. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enerbäck S. Human brown adipose tissue. Cell Metab 11: 248–252, 2010. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 8.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Forsum E, Löf M. Energy metabolism during human pregnancy. Annu Rev Nutr 27: 277–292, 2007. doi: 10.1146/annurev.nutr.27.061406.093543. [DOI] [PubMed] [Google Scholar]
- 10.Fromme T, Klingenspor M. Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300: R1–R8, 2011. doi: 10.1152/ajpregu.00411.2010. [DOI] [PubMed] [Google Scholar]
- 11.García-Ruiz E, Reynés B, Díaz-Rúa R, Ceresi E, Oliver P, Palou A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int J Obes 39: 1619–1629, 2015. doi: 10.1038/ijo.2015.112. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg GR, Prentice AM, Coward WA, Davies HL, Murgatroyd PR, Wensing C, Black AE, Harding M, Sawyer M. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr 57: 494–505, 1993. doi: 10.1093/ajcn/57.4.494. [DOI] [PubMed] [Google Scholar]
- 13.Lecoutre S, Breton C. Maternal nutritional manipulations program adipose tissue dysfunction in offspring. Front Physiol 6: 158, 2015. doi: 10.3389/fphys.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 15.Lof M, Olausson H, Bostrom K, Janerot-Sjöberg B, Sohlstrom A, Forsum E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am J Clin Nutr 81: 678–685, 2005. doi: 10.1093/ajcn/81.3.678. [DOI] [PubMed] [Google Scholar]
- 16.Martin I, Giralt M, Viñas O, Iglesias R, Mampel T, Villarroya F. Adaptative decrease in expression of the mRNA for uncoupling protein and subunit II of cytochrome c oxidase in rat brown adipose tissue during pregnancy and lactation. Biochem J 263: 965–968, 1989. doi: 10.1042/bj2630965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlvride S, Mushtaq A, Papacleovoulou G, Hurling C, Steel J, Jansen E, Abu-Hayyeh S, Williamson C. A progesterone-brown fat axis is involved in regulating fetal growth. Sci Rep 7: 10671, 2017. doi: 10.1038/s41598-017-10979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer SW, Trayhurn P. Effect of high fat diets on the thermogenic activity of brown adipose tissue in cold-acclimated mice. J Nutr 114: 1151–1158, 1984. doi: 10.1093/jn/114.6.1151. [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab 13: 238–240, 2011. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Häring H-U, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59: 1789–1793, 2010. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao L, Guo Z, Bosco C, Guidotti S, Wang Y, Wang M, Parast M, Schaack J, Hay WW Jr, Moore TR, Shao J. Maternal high-fat feeding increases placental lipoprotein lipase activity by reducing SIRT1 expression in mice. Diabetes 64: 3111–3120, 2015. doi: 10.2337/db14-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao L, Wattez JS, Lee S, Guo Z, Schaack J, Hay WW Jr, Zita MM, Parast M, Shao J. Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia 59: 2417–2425, 2016. doi: 10.1007/s00125-016-4061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao L, Wattez JS, Lee S, Nguyen A, Schaack J, Hay WW Jr, Shao J. Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes 66: 1126–1135, 2017. doi: 10.2337/db16-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao L, Yoo H, Bosco C, Lee B, Feng G-S, Schaack J, Chi N-W, Shao J. Adiponectin reduces thermogenesis by inhibiting brown adipose tissue activation in mice. Diabetologia 57: 1027–1036, 2014. doi: 10.1007/s00125-014-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 297: C723–C731, 2009. doi: 10.1152/ajpcell.00191.2009. [DOI] [PubMed] [Google Scholar]
- 26.Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia 55: 1597–1606, 2012. doi: 10.1007/s00125-012-2505-5. [DOI] [PubMed] [Google Scholar]
- 27.Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ukropec J, Anunciado RVP, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147: 2468–2480, 2006. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- 29.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N-J, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 30.Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 289: 34129–34140, 2014. doi: 10.1074/jbc.M114.591008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo HS, Qiao L, Bosco C, Leong L-H, Lytle N, Feng G-S, Chi N-W, Shao J. Intermittent cold exposure enhances fat accumulation in mice. PLoS One 9: e96432, 2014. doi: 10.1371/journal.pone.0096432. [DOI] [PMC free article] [PubMed] [Google Scholar]