Abstract
Thrombospondin 1 (TSP1) is a multifunctional matricellular protein. Recent studies demonstrate that TSP1 is highly expressed in adipose tissue (AT) and positively associated with AT inflammation and insulin resistance (IR). In this study, the contribution of different cellular sources of TSP1 to obesity-induced metabolic complications is determined by using mice with either adipocyte or myeloid/macrophage-specific deletion of TSP1 in a diet-induced obese model. The results demonstrated that neither adipocyte nor myeloid/macrophage-specific deletion of TSP1 affected the development of long-term high-fat diet-induced obesity. Adipocyte-specific deletion of TSP1 did not protect mice from obesity-induced inflammation and IR. On the contrary, obese mice with myeloid/macrophage loss of TSP1 had reduced macrophage accumulation in AT, which was accompanied with reduced inflammation and improved glucose tolerance and insulin sensitivity compared with obese control mice. Reduced macrophage-derived-TGF-β1 signaling and adipose tissue fibrosis were also observed in long-term high-fat-fed mice with myeloid/macrophage-specific TSP1 deletion. Moreover, in vitro experiments demonstrated an autocrine effect of TSP1-mediated TGF-β activation in macrophages in obesity. Collectively this study highlights the critical contribution of myeloid/macrophage-derived TSP1 to obesity-associated chronic inflammation and IR, which may serve as a new therapeutic target for metabolic disease.
Keywords: inflammation, insulin resistance, macrophage, obesity, TSP1
INTRODUCTION
Obesity is associated with chronic inflammation that promotes the development of insulin resistance (IR) and metabolic complications (51). Increased accumulation of adipose tissue macrophages (ATMs) has been demonstrated in obese rodents and humans and is recognized as a significant contributor to inflammation in obesity and a key mediator of IR (18, 50, 51). Although there are advances in the study of ATMs in obesity (14–16, 25, 30–33, 42, 49, 50), the mechanisms underlying ATM recruitment and activation remain to be determined.
Thrombospondin 1 (TSP1) is a multifunctional matricellular protein (2–4, 6, 8, 10, 11, 13, 23, 26, 27, 36, 47, 46, 53). As a physiological activator of profibrotic factor TGF-β, TSP1 has been shown to play an important role in cardiovascular diseases and renal fibrotic changes (3, 8, 11, 13, 47, 46). Recently TSP1’s contribution to obesity and metabolic disease has been identified. TSP1 is highly expressed in visceral adipose tissue (AT) from obese and insulin-resistant humans or obese rodents (34, 43, 44). Both adipocytes and macrophages produce TSP1, contributing to obesity-induced TSP1 expression in AT (9, 43). The positive association of AT TSP1 with AT inflammation and IR has been observed in obese human subjects (43). Moreover, by using global TSP1-deficient mice, we revealed a novel role for TSP1 in stimulating macrophage accumulation and activation in AT that promotes inflammation and IR resulting from high-fat (HF) diet-induced obesity (DIO) (21). We found that feeding a HF diet to wild-type and global TSP1-deficient mice caused similar obesity, but only mice with TSP1 deficiency remained insulin sensitive. The protection of global TSP1-deficient mice against IR was associated with reduced ATMs and decreased adipose and systemic inflammation. In vitro data demonstrated that TSP1-deficient monocyte/macrophages had decreased chemotactic activity and a reduced proinflammatory phenotype (20). Based on these reports, the objective of the current study is to further determine the contribution of different cellular sources (adipocytes and/or macrophages) of TSP1 to obesity-induced inflammation and metabolic complications in a DIO paradigm.
In the current study, mice with either adipocyte or myeloid/macrophage-specific deletion of TSP1 are used. These mice were fed with low-fat (LF) diet or HF diet for up to 32 wk. The results demonstrated that TSP1 deletion in either adipocytes or myeloid/macrophages did not affect mice from HF DIO. Interestingly, only myeloid/macrophage-specific TSP1 deficiency protected mice from late-stage obesity-induced chronic inflammation and IR. This study highlights the in vivo importance of TSP1 in regulating macrophage function and its contribution to obesity-associated metabolic complications.
MATERIALS AND METHODS
Animal experimental protocol.
TSP1 floxed mice (TSP1fl/fl on 129/B6 background) were generated by using the service from inGenious Targeting Laboratory (Ronkonkoma, NY) and genotyped by PCR analysis of genomic DNA from tail of wild-type (WT, 366 bp) or TSP1fl/fl mice for the presence of LoxP sites (437 bp) using the primers listed in Table 1. TSP1fl/fl mice were bred with Adipoq-Cre or Lyz2-Cre mice (from Jackson Laboratory) for two generations to produce adipocyte (TSP1∆adipo) and myeloid/macrophage-specific TSP1 knockout mice (TSP1∆Mɸ), respectively. Because male mice are sensitive to DIO and its associated complications, to determine the protective effect of tissue-specific TSP1 deficiency on DIO and its complications, initially we chose to use the male mice. All experiments were performed on 8-wk-old male TSP1fl/fl, TSP1∆adipo, and TSP1∆Mɸ mice. Mice were given a HF [60% kcal from fat (D12492; Research Diets), a widely used diet to induce obesity and insulin resistance in mouse models] or LF diet (10% kcal from fat; D12450B; Research Diets) for up to 32 wk with standard laboratory water. Each group contained 10–15 mice. Body weight was measured weekly. Glucose tolerance tests were measured after 8, 16, 24, and 32 wk of HF feeding. This allowed us to determine the temporal effect of tissue-specific TSP1 deficiency on glucose homeostasis under HF feeding conditions. In addition, the long-term HF feeding regimen allowed us to determine obesity-induced chronic complications such as adipose tissue fibrosis that needs more time to develop. At the end of the study, mice were euthanized. Blood was collected, and adipose tissue depots and other organs were harvested for various analyses. All experiments involving mice conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Table 1.
Primer sequences for genotyping and qPCR
| Target | Forward | Reverse |
|---|---|---|
| Wild-type/Floxed gene | 5′-GGT GTT CTG GGT GCT TTC AGT ATG G-3′ | 5′-TCT GAG TTT GCT TGT GGT GAA CGC-3′ |
| Lyz mutant | 5′-CTT GGG CTG CCA GAA TTT CTC-3′ | 5′-CCC AGA AAT GCC AGA TTA CG-3′ |
| Lyz wild | 5′-CTT GGG CTG CCA GAA TTT CTC-3′ | 5′-TTA CAG TCG GCC AGG CTG AC-3′ |
| TSP1 | 5′-CTA GGT GTC CTG TTC CTG TTG-3′ | 5′-AAG GAA GCC AGG AAG ATG AAG-3′ |
| Arg1 | 5′-CTC CAA GCC AAA GTC CTT AGA G-3′ | 5′-AGG AGC TGT CAT TAG GGA CAT C-3′ |
| CD11c | 5′-CTG GAT AGC CTT TCT TCT GCT G-3′ | 5′-GCA CAC TGT GTC CGA ACT C-3′ |
| F4/80 | 5′-CTT TGG CTA TGG GCT TCC AGT C-3′ | 5′-GCA AGG AGG ACA GAG TTT ATC GTG-3′ |
| iNOS2 | 5′-AAG AGG AGC AAC TAC TG-3′ | 5′-GCT CTG TTG AGG TCT AA-3′ |
| IL-1β | 5′-TGG AGA GTG TGG ATC CCA AGC AAT-3′ | 5′-TGT CCT GAC CAC TGT TGT TTC CCA-3′ |
| IL-6 | 5′-CTG CAA GAG ACT TCC ATC CAG TT-3′ | 5′-GAA GTA GGG AAG GCC GTG G-3′ |
| TNF-α | 5′-AGC CGA TGG GTT GTA CCT-3′ | 5′-TGA GTT GGT CCC CCT TCT-3′ |
| MCP1 | 5′-CAG CCA GAT GCA GTT AAC GC-3′ | 5′-GCC TAC TCA TTG GGA TCA TCT TG-3′ |
| TGF-β1 | 5′-ACA ATT CCT GGC GTT ACC-3′ | 5′-GGC TGA TCC CGT TGA TTT-3′ |
TSP1, thrombospondin 1.
Indirect calorimetry and body composition.
Before the end of study (2 wk), mice were placed in TSE LabMaster chambers (TSE Systems) individually for 5 days for measurement of food intake, water intake, and indirect calorimetry. Body composition including lean and fat mass was measured by EchoMRI (Echo Medical System) basally and after 32 wk of HF/LF feeding.
Intraperitoneal glucose and insulin tolerance test.
Glucose tolerance and insulin sensitivity tests were analyzed basally and after different periods of LF or HF diet feeding. Mice were fasted 6 h before intraperitoneal injections of glucose (1 g/kg body wt) or insulin (0.5 U/kg body wt; Novolin R; Novo Nordisk). Blood glucose concentrations were measured using a glucometer at 0, 15, 30, 60, and 120 min postinjection.
Real-time quantitative PCR.
Total RNA from frozen tissues or cells were extracted using an RNeasy Mini Kit (Qiagen). RNA was reverse transcribed to cDNA by a High Capacity cDNA Reverse Transcription Kit (Invitrogen, Carlsband, CA). Real-time quantitative PCR was performed on a MyiQ Real-time PCR Thermal Cycler (Bio-Rad) with SYBR Green PCR Master Kit (Qiagen, Valencia, CA). Relative mRNA expression was calculated using the MyiQ system software as previously reported (21) and normalized to 18S RNA levels. All primer sequences used in this study are found in Table 1.
Western blotting.
Total protein was extracted from tissue or cultured cells. Protein (30 µg) from each sample was subjected to electrophoresis in SDS-PAGE gel and then transferred to nitrocellulose membrane. Protein expression was determined by immunoblotting with the following antibodies: anti-TSP1 (Abcam, Cambridge, MA) and anti-GAPDH (Santa Cruz). Membranes were blocked and incubated with primary antibodies at room temperature for 1 h or overnight at 4°C with gentle agitation, followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA). Labeled proteins were detected with an enhanced chemiluminescence system (Pierce).
Differentiation of bone marrow-derived cells to macrophages and treatment.
Bone marrow cells were collected from femurs and tibia of 8- to 10-wk-old male control (TSP1fl/fl) and TSP1∆MΦ mice and cultured in RPMI 1640 with 10% FBS, 15% L-929 condition medium (as a source of colony-stimulating factor-1), and 1% penicillin/strep for 7 days to differentiate into macrophages as described previously (7). After differentiation, cells were harvested for further analysis of TSP1 mRNA and protein levels by qPCR and immunoblotting, respectively.
In addition, differentiated cells were seeded in a 10-cm dish at a density of 5 × 106 and cultured in a hypoxia chamber (1% O2; Coy Laboratory Products) in RPMI media with 0.1–1% BSA in the presence or absence of LSKL peptide (TSP1 antagonist, 1 µM; Anaspec, Fremont, CA) or SLLK (control peptide, 1 µM) for 24 h. Normoxia condition (with 21% O2) was also included. After treatment, conditioned media were collected for analyzing TSP1 and TGF-β levels by ELISA and plasminogen activator inhibitor-1 (PAI-1)/luciferase assay, respectively.
TGF-β assay.
Total and active TGF-β levels in the condition media of cell culture or in fat tissue lysates were analyzed using the PAI-1/luciferase assay as previously described (46). For TGF-β bioassay, we used mink lung epithelial cells stably transfected with the TGF-β response element of the human PAI-1 gene promoter fused to firefly luciferase reporter gene [TMLCs, a generous gift from Dr. D. B. Rifkin (New York University Medical Center)]. Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, l-glutamine, and 200 μg/ml G418. First, the TGF-β standard curve was generated. To do it, TMLCs were plated in a 24-well tissue culture plate (at a density of 2 × 105 cells/well) and incubated for 3–4 h to allow optimal attachment. Next, different concentrations of human recombinant TGF-β1 (15–1,000 pg/ml; R&D Systems) in 500 µl DMEM/0.1% BSA media in triplicates were added to TMLCs and incubated overnight at 37°C. Cells were lysed by incubation with 100 µl 1× passive lysis buffer (Promega) at room temperature with rocking for 20 min. Lysates (20 µl) were analyzed for luciferase activity using a luminometer (with addition of 100 µl of luciferase assay reagent from Promega) recorded as the relative luciferase unit (RLU). Standard curved was plotted by using RLU and the relative TGF-β1 concentrations. Specificity of the assay had been proven before by neutralization of the TGF-β activity in conditioned media with an anti-TGF-β antibody (46). Next, TGF-β bioactivity in tissue lysate or cell culture conditioned media was similarly measured as described above. Briefly, to prepare fat tissue lysates, ~50 mg of epididymal fat (EAT) or subcutaneous fat (SAT) were homogenized and sonicated in 500 µl lysis buffer [50 mM Tris·HCl (pH 7.5) containing protease and phosphatase inhibitor cocktail] on ice. Samples were then centrifuged at 13,000 revolutions/min for 10–15 min at 4°C. Supernatant was collected and used for the TGF-β assay. For active TGF-β measurement, tissue lysates were diluted in DMEM/0.1% BSA media (20- to 25-fold dilution) before adding to TMLC cells. For total TGF-β levels, tissue lysates were heat activated for 3–5 min at 100°C and diluted in DMEM/0.1%BSA (50- to 100-fold dilution) before adding to TMLC. Next, TGF-β activity was similarly measured as described above. In addition, cell culture conditioned media was added to TMLC for active TGF-β levels or heat activated for 3–5 min at 100°C and diluted in DMEM/0.1%BSA (5-fold dilution) for total TGF-β measurement. The mean RLU values of duplicate samples were converted to concentrations of TGF-β (ng/mg tissue or ng/cell number) using a standard curve obtained with human recombinant TGF-β1 as described above.
Plasma parameters analysis.
Plasma insulin, IL-1β, IL-6, TNF-α, MCP1 (eBioscience, Waltham, MA), and TSP1 (TSZELISA, Framingham, MA) were measured by ELISA.
Immunohistochemical staining and Masson trichrome staining of fat tissue.
Epididymal adipose tissue was fixed and embedded in paraffin. Paraffin-fixed adipose tissues were cut into 4-µm sections and placed on slides. Sections were deparaffinized, rehydrated in graded mixtures of ethanol/water, and pretreated by boiling in citrate buffer (pH 6.0), and endogenous peroxidase activity was blocked with 3% H2O2 for 30 min at room temperature. The sections were incubated with a rat anti-mouse F4/80 antibody (AbD Serotec, Raleigh, NC) in blocking buffer for 1 h at room temperature and followed by washing and incubation with biotinylated secondary antibody for 30 min. Last, peroxidase substrate diaminobenzidine (Vector Laboratory) was applied and incubated for 30 min. The slides were rinsed, counterstained with hematoxylin, and mounted. Images were acquired with a Nikon Edipse 55i microscope. The positive F4/80 staining area in each slide (including 5 fields) was analyzed by using NIS-element software. In addition, Masson trichrome staining was performed in a paraffin-fixed fat tissue section by using the service provided by COBRE Pathology Core at the University of Kentucky.
Statistical analysis.
Data are expressed as mean values ± SE. Statistical analysis was performed with GraphPad Software and analyzed using ANOVA with the Bonferroni ad hoc test. A P value <0.05 was considered statistically significant.
RESULTS
Generation and characterization of mice with adipocyte or myeloid/macrophage-specific deletion of TSP1.
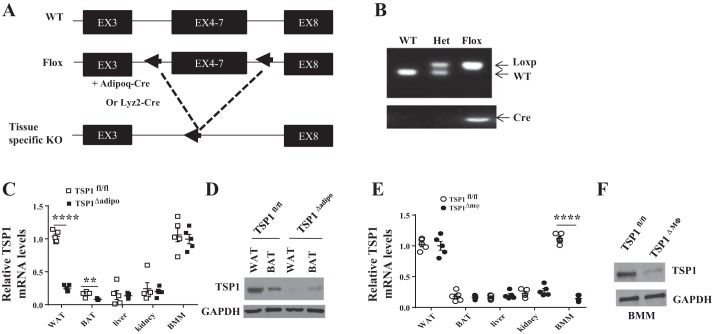
To determine the contribution of different cellular sources of TSP1 to obesity and its related inflammation and insulin resistance, we generated TSP1 floxed mice (TSP1fl/fl) using the service from inGenious Targeting Laboratory. TSP1fl/fl mice (on 129/B6 background) were constructed by homologous recombination in ES cells using standard methods. Lox P sites were inserted in introns 3 and 7. Cre-mediated excision resulted in the deletion of exons 4–7. TSP1fl/fl mice were bred with Adipoq-Cre and Lyz2-Cre mice (Jackson Laboratory) to generate adipocytes (TSP1∆adipo) and myeloid/macrophage-specific TSP1-deficient mice (TSP1∆Mɸ), respectively. PCR and Western blotting results confirmed the efficient adipocyte or macrophage-specific deletion of TSP1 in TSP1∆adipo and TSP1∆Mɸ mice, respectively (Fig. 1).
Fig. 1.
Characterization of tissue-specific thrombospondin 1 (TSP1) knockout mice. A: adipocyte or myeloid/macrophage-specific TSP1 gene knockout strategy. B: PCR analysis of genomic DNA from tail of wild-type (WT, 366 bp), heterozygous (Het), or TSP1fl/fl mice for the presence of LoxP sites (437 bp). C and D: TSP1fl/fl mice were bred with Adipoq-Cre mice to generate adipocyte-specific TSP1 knockout mice (TSP1∆adipo). Expression of TSP1 from different tissues was determined by qPCR and immunoblotting. E and F: TSP1fl/fl mice were bred with Lyz2-Cre mice to generate myeloid/macrophage-specific TSP1 knockout mice (TSP1∆Mɸ). Expression of TSP1 from different tissues and bone marrow-derived macrophages was determined by qPCR and immunoblotting. Data are presented as means ± SE (n = 5 mice/group). **P < 0.01 and ****P < 0.0001 (2-way ANOVA). WAT, white adipose tissue (from epididymal fat); BAT, brown adipose tissue; BMM, bone marrow-derived macrophages.
Adipocyte-specific deletion of TSP1 did not protect mice from DIO and its associated inflammation and insulin resistance.
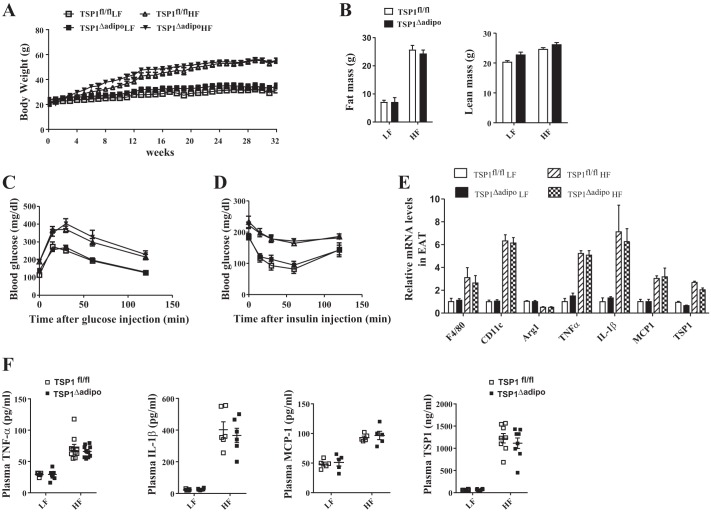
To assess the metabolic role of adipocyte-derived TSP1, mice with adipocyte-specific deletion of TSP1 (TSP1∆adipo) and control TSP1 floxed mice (TSP1fl/fl) were challenged with either a LF (10% kcal from fat) or HF (60% kcal from fat) diet for up to 32 wk. First, we determined whether adipocyte-specific deletion of TSP1 had any effect on obesity development. The body weight was measured weekly. Before the end of the study, body composition was analyzed using EchoMRI. Food intake and energy expenditure were evaluated by indirect calorimetric assay. The results showed that body weight, fat mass, and lean mass were similar between TSP1∆adipo and control mice under either LF or HF feeding conditions (Fig. 2, A and B). Food intake or energy expenditure was comparable between knockout and control groups (data not shown). These data suggest that adipocyte-specific deletion of TSP1 did not affect DIO.
Fig. 2.
Adipocyte-specific thrombospondin 1 (TSP1) deficiency did not protect mice from high-fat diet-induced obesity, inflammation, or insulin resistance. Male TSP1∆adipo mice and control TSP1fl/fl (8 wk old) were fed with low-fat (LF) or high-fat (HF) diet for 32 wk. A: weekly body weight. B: fat and lean mass of mice measured by EchoMRI before the end of the study. C and D: ip glucose tolerance test (C) and insulin sensitivity test (D) were performed in mice at the end of study. E: expression of proinflammatory cytokines, macrophage markers, and TSP1 in epididymal adipose tissue (EAT) was determined by real-time PCR and normalized to 18S RNA. F: plasma TNF-α, IL-1β, MCP-1, and TSP1 levels measured by ELISA. Data are presented as means ± SE (n = 5–9 mice/group).
Second, we determined the whole body glucose homeostasis in four groups of mice by measuring fasting plasma glucose and insulin levels and performing glucose tolerance and insulin sensitivity assay. The results demonstrated that both HF-fed control mice (TSP1fl/fl) and TSP1∆adipo mice developed similar levels of glucose intolerance and insulin resistance at the end of study (Fig. 2, C and D). Similar glucose intolerance was also seen between TSP1fl/fl and TSP1∆adipo mice after 2, 4, or 6 mo of HF feeding (data not shown), suggesting that adipocyte-specific deletion of TSP1 did not protect mice from obesity-associated impaired glucose homeostasis.
Third, adipose tissue and systemic inflammation were analyzed. Visceral adipose tissue has been suggested to be the primary source of cytokine and adipokine release within obesity-associated inflammation (45). Moreover, increased accumulation of adipose tissue macrophages is a significant contributor to obesity-induced chronic inflammation (18, 50, 51). Therefore, the expression of proinflammatory cytokines and macrophage marker F4/80 in epididymal adipose tissue (EAT) was determined. We found that both HF-fed control mice and HF-fed TSP1∆adipo mice had similarly increased mRNA levels of F4/80, CD11c, TNF-α, IL-1β, and MCP1 in EAT (Fig. 2E) and plasma levels of TNF-α, IL-1β, and MCP-1 (Fig. 2F). M2 macrophage marker-Arg1 (arginase 1) was similarly reduced in EAT from both HF-fed groups. No significant difference of TSP1 levels in EAT or plasma was revealed between TSP1∆adipo and control mice. Collectively, these data indicate that adipocyte-specific deletion of TSP1 did not protect mice from HF diet feeding-induced obesity and its associated inflammation and insulin resistance.
Myeloid/macrophage-specific deletion of TSP1 protected mice from late-stage obesity-associated inflammation and insulin resistance.
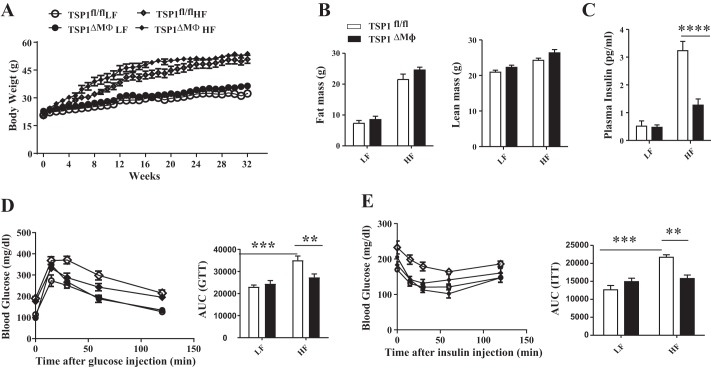
The effect of myeloid/macrophage-derived TSP1 on DIO and metabolic complication was determined. TSP1∆Mɸ and control mice (TSP1fl/fl) were fed with LF or HF diet for up to 32 wk. We found that TSP1∆Mɸ mice developed similar levels of obesity under HF diet feeding conditions compared with control mice (Fig. 3, A and B). Food intake and energy expenditure were also similar between TSP1∆mɸ and control mice (data not shown). These data suggest that myeloid/macrophage-specific deletion of TSP1 does not affect DIO.
Fig. 3.
Myeloid/macrophage-specific thrombospondin 1 (TSP1) deficiency did not protect mice from diet-induced obesity, but high-fat (HF)-fed TSP1∆ mɸ mice had improved glucose tolerance and insulin sensitivity. Male TSP1∆Mɸ mice and control TSP1fl/fl mice (8 wk old) were fed with low-fat (LF) or HF diet for 32 wk. A: weekly body weight. B: fat and lean mass of mice measured by EchoMRI before the end of the study. C: plasma insulin levels measured by ELISA. D and E: ip glucose tolerance test (D) and insulin sensitivity test (E) were performed before the end of the study, and area under curve (AUC) was calculated as described in materials and methods. Data are presented as means ± SE (n = 5–10 mice/group). **P < 0.01, ***P < 0.001, and ****P < 0.0001 (2-way ANOVA).
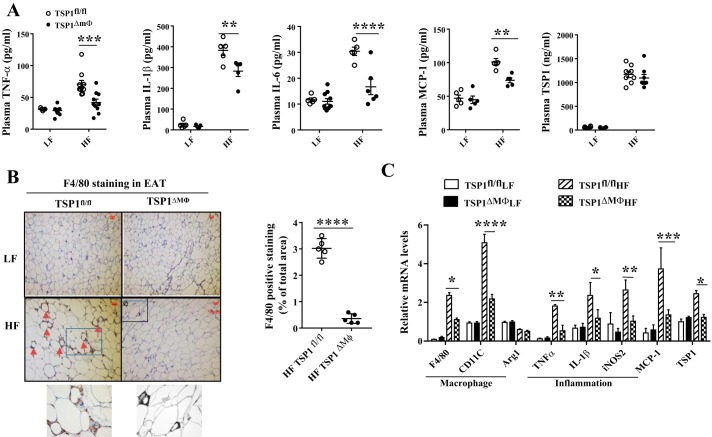
Next, we determined the effect of myeloid/macrophage-specific TSP1 deficiency on obesity-associated inflammation and impaired glucose homeostasis in four groups of mice. We found that obese TSP1∆Mɸ mice had improved glucose tolerance and insulin sensitivity at the end of the study after 8 mo of HF feeding (Fig. 3, C–E). This protection from TSP1∆Mɸ mice was not detected in the early stage of HF feeding (e.g., 2 or 4 mo) (data not shown), suggesting a time course-dependent effect of myeloid-derived TSP1 on HF-induced insulin resistance. In addition, plasma TNF-α, IL-1β, IL-6, and MCP-1 levels were significantly reduced in HF-fed TSP1∆Mɸ mice compared with HF-fed control mice (Fig. 4A). The reduced macrophage accumulation in EAT and decreased proinflammatory cytokine production were observed in HF-fed TSP1∆Mɸ mice (Fig. 4, B and C). However, the M2 macrophage marker Arg1 was comparable in two genotypes, suggesting that TSP1 is a major regulator of proinflammatory macrophages (F4/80+ CD11c+) seen in crown-like structures in obese adipose tissue (25). TSP1 expression in EAT was upregulated in HF-fed control mice but was significantly reduced in HF-fed TSP1∆Mɸ mice (Fig. 4C). In addition, there were no changes in angiogenesis-related genes (CD31 and vascular endothelial growth factor) in EAT between HF-fed TSP1∆Mɸ mice and HF-fed wild-type mice (data not shown). Together, these data indicate that myeloid/macrophage-specific deletion of TSP1 reduces proinflammatory macrophage accumulation in white fat tissue and protects mice from late-stage obesity-associated chronic inflammation and insulin resistance.
Fig. 4.
High-fat (HF)-fed TSP1∆Mɸ mice had reduced adipose tissue macrophage infiltration and inflammation. A: plasma TNF-α, IL-1β, IL-6, MCP-1, and thrombospondin 1 (TSP1) levels were measured by ELISA. B: macrophage accumulation in epididymal adipose tissue (EAT) was determined by anti-F4/80 staining. The positive staining showed brown color as indicated by the red arrowhead. Representative images with enlarged small portions are shown. Positive F4/80 staining area was determined by analyzing the images using NIS-element software. C: expression of macrophage markers, proinflammatory cytokines, and TSP1 in EAT was determined by qPCR and normalized to 18S RNA. LF, low fat. Data are presented as means ± SE (n = 5–9 mice/group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 (2-way ANOVA).
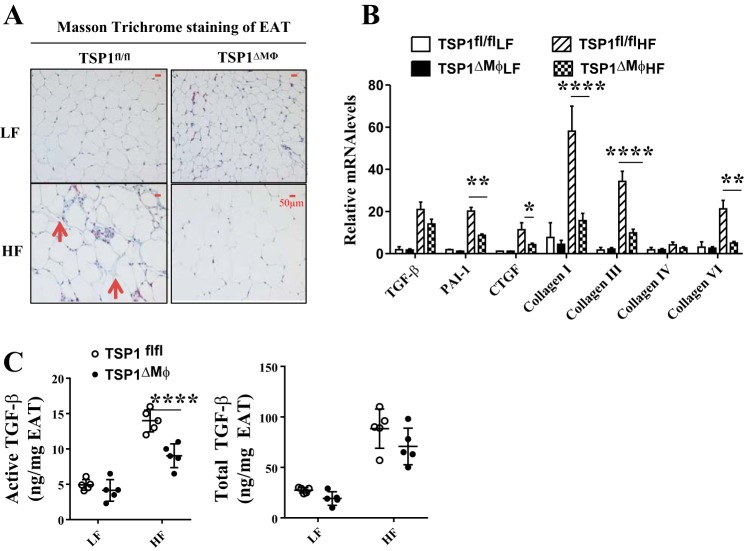
Mice with myeloid/macrophage-specific deletion of TSP1 had reduced active TGF-β levels and adipose tissue fibrosis under HF feeding conditions.
As a physiological activator of latent TGF-β (38), TSP1 has been shown to play an important role in tissue fibrosis (8, 11, 46). In adipose tissue, macrophages are a major cellular source of TGF-β (9, 29). Under obese conditions, enhanced TGF-β signaling has been observed in adipose tissue, contributing to adipose tissue fibrosis and the resultant impaired adipocyte function (39, 41, 52). Therefore, we determined whether macrophage-specific deletion of TSP1 could attenuate TGF-β signaling in obese EAT. As expected, we found that EAT fibrosis was significantly attenuated in HF-fed TSP1∆Mɸ mice as demonstrated by reduced positive Masson staining and expression of collagen I, III, and VI in adipose tissue compared with HF-fed control mice (Fig. 5, A and B). This phenotype was associated with the reduced active TGF-β levels [quantified by using the PAI-1/luciferase assay as previously described by our laboratory (46)] and reduced expression of TGF-β downstream molecules (PAI-1 and connective tissue growth factor) in EAT (Fig. 5, B and C). Total TGF-β levels were comparable between two genotypes. However, HF diet feeding did not increase active or total TGF-β levels in SAT that has less macrophage infiltration compared with EAT during obesity development (data not shown). Collectively, these data suggest that a TSP1-mediated TGF-β activation mechanism might be involved in obesity-induced adipose tissue fibrosis.
Fig. 5.
High-fat (HF)-fed TSP1∆Mɸ mice had reduced adipose tissue fibrosis compared with HF-fed control mice. A: adipose tissue fibrosis was determined by Masson trichrome staining. The positive staining showed blue color as indicated by the red arrowhead. Representative images are shown. B: expression of fibrosis-related genes in adipose tissue was determined by qPCR and normalized to 18S RNA. C: active and total TGF-β levels in epididymal adipose tissue (EAT) lysates were determined by plasminogen activator inhibitor-1 (PAI-1)/luciferase assay. TSP1, thrombospondin 1. Data are presented as means ± SE (n = 5 mice/group), *P < 0.05, **P < 0.01, and ****P < 0.0001 (2-way ANOVA).
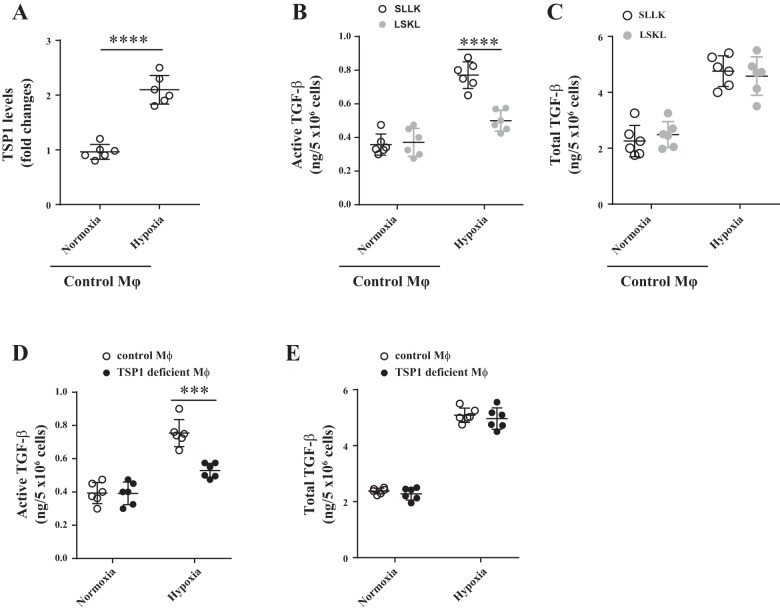
To further determine the autocrine mechanism of TSP1-mediated TGF-β activation in macrophages in the context of obese conditions, we cultured bone marrow-derived macrophages from TSP1fl/fl and TSP1∆mɸ mice under hypoxic conditions (1% O2). Normoxic conditions (21% O2) were included as controls. Wild-type macrophages (isolated from TSP1fl/fl mice) were treated with the TSP1 antagonist peptide LSKL [a peptide to specifically inhibit TSP1-mediated latent TGF-β activation (24, 35, 54)] or the control peptide SLLK for 24 h. TSP1 and active TGF-β levels in the conditioned media were determined by ELISA and PAI-1/luciferase assay, respectively. As shown in Fig. 6, hypoxia condition upregulated both TSP1 and active TGF-β levels in wild-type macrophages. With TSP1 deficiency or LSKL treatment, hypoxia-induced active TGF-β levels were significantly reduced. LSKL treatment did not affect total TGF-β levels. Taken together, these data suggest that macrophage-derived TSP1 activates latent TGF-β and promotes adipose tissue fibrosis under obese conditions.
Fig. 6.
Effect of thrombospondin 1 (TSP1) antagonist or TSP1 deficiency on macrophage-derived TGF-β levels (active and total) under hypoxia conditions. Bone marrow-derived macrophages from wild-type or TSP1∆Mɸ mice were cultured under normoxia (21% O2) or hypoxia (1% O2) conditions in the presence or absence of TSP1 antagonist peptide LSKL (1 µM) or control peptide SLLK (1 µM) for 24 h. Conditioned media (CM) were collected. A: TSP1 protein levels in CM were analyzed by ELISA. B–E: active or total TGF-β levels in CM were determined by plasminogen activator inhibitor-1 (PAI-1)/Luciferase assay. Data are presented as means ± SE (n = 6 samples from 3 separate experiments). ***P < 0.001 and ****P < 0.0001 (2-way ANOVA or t-test).
DISCUSSION
Recent human and rodent studies suggest that TSP1 plays an important role in obesity-associated inflammation and insulin resistance. In this study, by using tissue-specific TSP1 knockout mice, we determined the contribution of different cellular sources of TSP1 (adipocytes and/or macrophages) to obesity-induced metabolic complications in a DIO (DIO) model. The results demonstrated that only macrophage-specific TSP1 deletion (TSP1∆Mɸ) protected mice against obesity-associated ATM accumulation, AT fibrosis, inflammation, and insulin resistance. This protection was only observed in late-stage HF-induced obesity, suggesting a time-dependent effect of myeloid-derived TSP1 on obesity-associated adipose tissue inflammation, fibrosis, and insulin resistance. Mechanistically, through an autocrine effect, macrophage-derived TSP1 activated latent TGF-β in adipose tissue and promoted obesity-induced adipose tissue fibrosis and the resultant adipocyte dysfunction.
TSP1 is expressed by many cell types and is involved in various diseases such as kidney diseases and obesity-associated metabolic complications (12, 17, 21, 43). It is highly expressed in adipose tissue from obese and insulin-resistant humans or obese rodents (34, 43, 44). Adipocytes and the stromal vascular cell fraction (SVF) (e.g., macrophages) contribute to obesity-induced TSP1 expression in adipose tissue. An interaction between adipocytes and macrophages augmented TSP1 expression/secretion (9, 43). Moreover, our in vitro studies demonstrated that TSP1 stimulated macrophage migration and production of proinflammatory cytokines (20, 21), suggesting that obesity-induced TSP1 in adipose tissue may act as both a chemoattractant and proinflammatory activator for macrophages and promote obesity-associated inflammation and insulin resistance (20, 21). Based on these reports, we anticipate that specific deletion of TSP1 in adipocytes (TSP1∆adipo) or macrophages (TSP1∆mɸ) would show metabolic protection in a DIO model. Surprisingly, no metabolic protection was observed in TSP1∆adipo mice under either short-term or long-term HF diet feeding conditions. This might be explained by the following reasons. First, we found that adipocyte-specific TSP1 deficiency does not affect HF diet feeding-induced TSP1 levels either locally (adipose tissue) or systemically (circulation), suggesting that adipocyte-derived TSP1 does not contribute significantly to obesity-induced TSP1 in vivo (17). Therefore, this adipocyte-derived TSP1 might not have significant impact on adipose tissue function rather than help maintain its structural integrity. Second, the similar levels of MCP1 [an important macrophage chemoattractant signal and a major player in obesity-associated ATM accumulation and the development of inflammation and insulin resistance (14, 15, 49, 50)] were observed in adipose tissue or circulation between obese TSP1∆adipo mice and control mice. This would also help explain the phenotype of TSP1∆adipo mice. In contrast to adipocyte-specific TSP1 deficiency, our data demonstrated that macrophage-derived TSP1 is a major cellular source of TSP1 in obese adipose tissue and a significant contributor to chronic HF diet-fed induced adipose tissue inflammation and insulin resistance. Consistently, in one of our ongoing studies, we found that M1-like macrophages in white adipose tissue (WAT) from 4 mo of HF-fed wild-type mice expressed more TSP1 than that from M2-like macrophages (unpublished observations), suggesting that infiltrated but not resident macrophages might be key producers of TSP1 in obese WAT. This is supported by our finding that macrophage-specific TSP1 deficiency failed to protect mice from short-term (e.g., 2 mo) HF-fed induced insulin resistance when the M1 macrophage population was not significantly increased in WAT (19, 51). In addition, TSP1-deficient macrophages demonstrated reduced adhesion and migratory ability (21, 22) and decreased production of proinflammatory cytokines (20, 21, 40). These intrinsic effects might also contribute to the metabolic protection phenotype observed in TSP1∆mɸ mice. However, at this stage, the molecular mechanisms by which TSP1 is regulated in macrophages and why TSP1-deficient macrophages show defects in migration and cytokine production are unknown and warrant further investigation.
In this study, chronic HF diet feeding induced white adipose tissue (e.g., EAT) to undergo fibrotic changes in wild-type mice, which was significantly attenuated in HF-fed TSP1∆mɸ mice. A potential mechanism is through TGF-β signaling since we found that active TGF-β levels and its downstream signaling molecules were reduced in EAT of HF-fed TSP1∆mɸ mice. It is known that TGF-β is synthesized and secreted as a latent complex (latent TGF-β), which needs to be converted to the active state before binding to its receptors and eliciting cellular functions. Latent TGF-β can be activated by a number of factors, and one well-characterized mechanism is through TSP1 (38). Macrophage has been suggested to be an important cellular source of TGF-β in adipose tissue, which regulates adipose progenitor functions and contributes to adipose tissue remodeling and insulin sensitivity (5, 29). Blockade of TGF-β signaling has been shown to have beneficial effects on combating obesity and diabetes (52). In addition to TGF-β, TSP1 is increased in adipose tissue with obesity (1, 17, 28, 34, 37, 43, 44). Moreover, the current study demonstrates that macrophages are major cellular sources of TSP1 in obese adipose tissue. Through an autocrine mechanism, TSP1 induces latent TGF-β activation in macrophages, contributing to obesity-associated adipose tissue fibrosis and the resultant impaired adipocyte function. This concept is supported by our data showing that HF diet feeding did not increase active or total TGF-β levels in SAT that has less macrophage infiltration compared with EAT during obesity development. However, the in vivo importance of this mechanism in obesity-associated metabolic complications needs to be determined in future studies.
In summary, by utilization of tissue-specific TSP1 knockout mice in a DIO model, our studies highlight the critical role of macrophage-derived TSP1 in late-stage obesity-associated inflammation and insulin resistance. It suggests that macrophage-derived TSP1 may serve as a therapeutic target for obesity-associated comorbidities.
GRANTS
This work was supported by the Department of Veterans Affairs Merit Review Award (to S. Wang) and National Institutes of Health Grants DK-098176 (to S. Wang) and COBRE Grant P20-GM-103527-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.W. conceived and designed study; H.M., D.L., K.T., and S.W. performed experiments; H.M., D.L., and S.W. analyzed data; H.M. and S.W. interpreted results of experiments; H.M. and S.W. prepared figures; S.W. drafted manuscript; H.M., D.L., C.Z., Y.L., Y.W., and S.W. edited and revised manuscript; H.M., D.L., K.T., C.Z., Y.L., Y.W., and S.W. approved final version of manuscript.
REFERENCES
- 1.Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 46: 860–867, 1997. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- 2.Bige N, Shweke N, Benhassine S, Jouanneau C, Vandermeersch S, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int 81: 1226–1238, 2012. doi: 10.1038/ki.2012.21. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoy A, Moura R, Hoylaerts MF. The evolving role of thrombospondin-1 in hemostasis and vascular biology. Cell Mol Life Sci 65: 713–727, 2008. doi: 10.1007/s00018-007-7487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest 107: 929–934, 2001. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, Bouloumié A. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One 7: e31274, 2012. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjögren’s syndrome. PLoS One 8: e75937, 2013. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui W, Maimaitiyiming H, Zhou Q, Norman H, Zhou C, Wang S. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim Biophys Acta 1852: 1323–1333, 2015. doi: 10.1016/j.bbadis.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esemuede N, Lee T, Pierre-Paul D, Sumpio BE, Gahtan V. The role of thrombospondin-1 in human disease. J Surg Res 122: 135–142, 2004. doi: 10.1016/j.jss.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Finlin BS, Zhu B, Starnes CP, McGehee RE Jr, Peterson CA, Kern PA. Regulation of thrombospondin-1 expression in alternatively activated macrophages and adipocytes: role of cellular cross talk and omega-3 fatty acids. J Nutr Biochem 24: 1571–1579, 2013. doi: 10.1016/j.jnutbio.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiscott P, Armstrong D, Batterbury M, Kaye S. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histol Histopathol 14: 1309–1320, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Hugo C, Daniel C. Thrombospondin in renal disease. Nephron, Exp Nephrol 111: e61–e66, 2009. doi: 10.1159/000198235. [DOI] [PubMed] [Google Scholar]
- 12.Inoue M, Jiang Y, Barnes RH II, Tokunaga M, Martinez-Santibañez G, Geletka L, Lumeng CN, Buchner DA, Chun TH. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology 154: 4548–4559, 2013. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci 65: 728–742, 2008. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 15.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, Takamura T, Yamamoto H, Miyamoto K, Ginsberg HN, Mukaida N, Kaneko S, Ota T. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 61: 1680–1690, 2012. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab 305: E439–E450, 2013. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE, Lee H, Chen J, Carrasco E, Kishore P, Zhang K, Hawkins M. Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes 62: 1843–1854, 2013. doi: 10.2337/db12-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Qi X, Tong X, Wang S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol Immunol 10: 506–512, 2013. doi: 10.1038/cmi.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One 6: e26656, 2011. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res 117: 129–141, 2015. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm 2011: 296069, 2011. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol 178: 2573–2586, 2011. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMaken S, Exline MC, Mehta P, Piper M, Wang Y, Fischer SN, Newland CA, Schrader CA, Balser SR, Sarkar A, Baran CP, Marsh CB, Cook CH, Phillips GS, Ali NA. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One 6: e19654, 2011. doi: 10.1371/journal.pone.0019654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMorrow JP, Crean D, Gogarty M, Smyth A, Connolly M, Cummins E, Veale D, Fearon U, Tak PP, Fitzgerald O, Murphy EP. Tumor necrosis factor inhibition modulates thrombospondin-1 expression in human inflammatory joint disease through altered NR4A2 activity. Am J Pathol 183: 1243–1257, 2013. doi: 10.1016/j.ajpath.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes 30: 1308–1314, 2006. doi: 10.1038/sj.ijo.0803189. [DOI] [PubMed] [Google Scholar]
- 29.Nawaz A, Aminuddin A, Kado T, Takikawa A, Yamamoto S, Tsuneyama K, Igarashi Y, Ikutani M, Nishida Y, Nagai Y, Takatsu K, Imura J, Sasahara M, Okazaki Y, Ueki K, Okamura T, Tokuyama K, Ando A, Matsumoto M, Mori H, Nakagawa T, Kobayashi N, Saeki K, Usui I, Fujisaka S, Tobe K. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun 8: 286, 2017. doi: 10.1038/s41467-017-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 31.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 61: 346–354, 2012. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61: 2718–2727, 2012. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J 37: 165–172, 2013. doi: 10.4093/dmj.2013.37.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramis JM, Franssen-van Hal NL, Kramer E, Llado I, Bouillaud F, Palou A, Keijer J. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci 59: 1960–1971, 2002. doi: 10.1007/PL00012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem 274: 13586–13593, 1999. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 36.Rico MC, Manns JM, Driban JB, Uknis AB, Kunapuli SP, Dela Cadena RA. Thrombospondin-1 and transforming growth factor beta are pro-inflammatory molecules in rheumatoid arthritis. Transl Res 152: 95–98, 2008. doi: 10.1016/j.trsl.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med 3: 37–48, 1997. doi: 10.1007/BF03401666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem 270: 7304–7310, 1995. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 39.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 299: E1016–E1027, 2010. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein EV, Miller TW, Ivins-O’Keefe K, Kaur S, Roberts DD. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1β Production and Activation through CD47. Sci Rep 6: 19684, 2016. doi: 10.1038/srep19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tateya S, Kim F, Tamori Y. Recent advances in obesity-induced inflammation and insulin resistance. Front Endocrinol (Lausanne) 4: 93, 2013. doi: 10.3389/fendo.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ III, Lee MJ, Fried SK, McGehee RE Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57: 432–439, 2008. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 45.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem 279: 34311–34322, 2004. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem 277: 9880–9888, 2002. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- 49.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14: 67–79, 2011. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang K, Vega JL, Hadzipasic M, Schatzmann Peron JP, Zhu B, Carrier Y, Masli S, Rizzo LV, Weiner HL. Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. J Autoimmun 32: 94–103, 2009. doi: 10.1016/j.jaut.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem 279: 38032–38039. Epub 32004: 38018, 2004. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]