Abstract
Pancreatic β-cell expansion is a highly regulated metabolic adaptation to increased somatic demands, including obesity and pregnancy; adult β cells otherwise rarely proliferate. We previously showed that high-fat diet (HFD) feeding induces mouse β-cell proliferation in less than 1 wk in the absence of insulin resistance. Here we metabolically profiled tissues from a short-term HFD β-cell expansion mouse model to identify pathways and metabolite changes associated with β-cell proliferation. Mice fed HFD vs. chow diet (CD) showed a 14.3% increase in body weight after 7 days; β-cell proliferation increased 1.75-fold without insulin resistance. Plasma from 1-wk HFD-fed mice induced β-cell proliferation ex vivo. The plasma, as well as liver, skeletal muscle, and bone, were assessed by LC and GC mass-spectrometry for global metabolite changes. Of the 1,283 metabolites detected, 159 showed significant changes [false discovery rate (FDR) < 0.1]. The majority of changes were in liver and muscle. Pathway enrichment analysis revealed key metabolic changes in steroid synthesis and lipid metabolism, including free fatty acids and other bioactive lipids. Other important enrichments included changes in the citric acid cycle and 1-carbon metabolism pathways implicated in DNA methylation. Although the minority of changes were observed in bone and plasma (<20), increased p-cresol sulfate was increased >4 fold in plasma (the largest increase in all tissues), and pantothenate (vitamin B5) decreased >2-fold. The results suggest that HFD-mediated β-cell expansion is associated with complex, global metabolite changes. The finding could be a significant insight into Type 2 diabetes pathogenesis and potential novel drug targets.
Keywords: β-cell proliferation, diabetes, high-fat diet, metabolomics
INTRODUCTION
β-Cell mass expansion in response to macronutrient stimuli, such as glucose (2, 66) or high fat diet (HFD), and/or insulin resistance is observed in rodent models (33, 55, 60) and is also thought to occur in humans; autopsy studies have revealed that nondiabetic obese individuals have 50% greater β-cell mass compared with lean individuals (71). Inadequate β-cell proliferation in the face of insulin resistance can lead to diabetes (47). Pancreata from Type 2 diabetes (T2D) patients have diminished β-cell mass compared with nondiabetic body mass index (BMI)-matched individuals (8), suggesting failed β-cell compensation in the face of insulin resistance. However, lack of reliable methodology to longitudinally measure β-cell mass in living humans makes it difficult to assess their true dynamics. It is possible that some individuals are born with less β-cell mass than others, thus making them more susceptible to metabolic stressors such as HFD later in life.
In adult mice, β-cell mass increases via proliferation in response to several stimuli including pregnancy (5, 64, 70, 76), obesity (8, 10, 65), and HFD (25, 57, 78). Evidence for proliferation in human β-cells in response to pregnancy or altered nutrition in vivo has been equivocal (9, 34) and β cells from human cadaver donor islets ex vivo often show inconsistent responsiveness to the same factors effective in mice (79). Importantly, several recently identified and widely dissimilar stimulators of adult mouse β-cell proliferation induce significant proliferation in adult human β cells ex vivo or when transplanted into mice. These include the serine protease serpin B1 (61), the DYRK1A inhibitor harmine (87), antagonists of the PGE2 receptor EP3, and agonists of the PGE2 receptor EP4 (11, 42), soluble factors released from CD4+/CD8+ T cells (24), and the bone-related decoy receptor osteoprotegerin and its related osteoporosis drug, denosumab (44). Thus identifying the key regulators of β-cell mass expansion in response to HFD-feeding in mice could help us understand regulation of compensatory β-cell proliferation in response to metabolic and nutritional cues, and develop new strategies for augmenting functional β-cell mass in T2D patients.
Whereas long-term HFD consumption induces glucose intolerance, insulin resistance, and enhanced β-cell mass and proliferation in rodents (1, 6, 52, 67, 85), it is clear that β-cell proliferation increases within 1 wk in response to HFD, in the absence of insulin resistance (57, 82). Metabolomic assessment of α-hydroxybutyrate (αHB), a plasma biomarker increased with insulin resistance in humans (18, 30, 32, 86), in conjunction with hyperinsulinemic-euglycemic clamps, confirmed the absence of insulin resistance in multiple tissues of 1 wk chow diet (CD)- and HFD-fed mice, thereby validating this biomarker in mouse models (57). Together, these results suggest that in the setting of HFD, β cells initially proliferate in response to a change in diet composition or an unknown acutely-upregulated HFD-induced factor. Thus adult pancreatic β cells respond robustly to proliferative cues without the complications of insulin resistance. These previous studies laid the groundwork for defining the early HFD-induced proliferative signals, the molecular nature of which are unknown. There has been a relative lack of information regarding changes in metabolite profiles following HFD feeding. Here we used metabolomic analysis on plasma and metabolically-important tissues to characterize the changes that occur within 1 wk of HFD feeding in mice. These studies reveal candidate factors that change during β-cell proliferation and mass expansion, which may correlate with promotion in β-cell compensation in response to overnutrition in the short term.
METHODS
Experimental animals.
Seven-week old male C57Bl/6J mice (Jackson Laboratory, Bar Harbor, ME) were delivered to the AAALAC accredited Vanderbilt Division of Animal Care and allowed to acclimatize for 1 wk. Mice were weighed and randomly assigned to one of two groups: 1) chow diet (CD; 11% kcal from fat, Laboratory Diet 5LJ5, Purina, St. Louis, MO) and 2) HFD (60% kcal from fat, BioServ F3282, Frenchtown, NJ). Mice were housed on a 12:12-h light-dark cycle receiving water and food ad libitum. Body weight was obtained at 8 wk of age, before starting the diets and at 1 wk after diet initiation. Euthanasia was performed at 9 wk of age using isoflurane until unconscious followed by cervical dislocation, consistent with the Panel on Euthanasia of the American Veterinary Medical Association. All procedures were conducted in accordance with Vanderbilt Division of Animal Care and Use Committee approved protocols and under the supervision of the Division of Animal Care.
Intraperitoneal glucose tolerance test (IP-GTT).
Animals were fasted for 16 h and fasting blood glucose measured from 2 μl of tail vein blood using an Accu-Chek Aviva glucometer and glucose test strips (Roche Diagnostics, Indianapolis, IN). Animals received an intraperitoneal (IP) injection of filter-sterilized glucose (2 mg dextrose/g body wt) and blood glucose was measured at 15-, 30-, 60-, 90-, and 120-min intervals following injection. Plasma insulin was not measured during the IP-GTT in this cohort. For historical data using this model the reader is referred to our previous publication (57).
Plasma collection.
Mice were placed under anesthesia following an IP-GTT. Whole blood was drawn from the vena cava. The blood was placed on ice before being centrifuged for 15 min at 13,000 rpm at 4°C. The plasma layer was collected and frozen. Other tissues used for metabolomics were also collected at this time. Use of isoflurane in healthy young female C57Bl/6 mice has been shown to impair glucose tolerance during an oral glucose tolerance test (OGTT), without affecting insulin secretion (89). Isoflurane produces a mild hyperglycemic effect in healthy young male C57Bl/6 mice, without affecting insulin secretion (19). Although all animals received the same treatment, and thus any effects of isoflurane would be expected to be present, we acknowledge the possibility that the response to anesthesia may be affected by diet.
Ex vivo β-cell proliferation assays.
The β-cell proliferation assay was performed as previously described (56). Islets were isolated from 8- to 10-wk-old wild-type C57Bl/6J male and female mice by collagenase digestion through the pancreatic duct and then hand-picked until free from acinar tissue. Islets from at least three different mice were combined and the isolated mouse islets were aliquoted at 40 islets per well into a 96-well plate and incubated with mouse proliferation media (56) supplemented 10% with plasma from CD- or HFD-fed mice collected from animals used for metabolomics studies. Separate cohorts of control islets were incubated with the following compounds: vehicle 1 [phosphate-buffered saline (PBS)], vehicle 2 [sodium bicarbonate (NaHCO3)], 0.5 μg/ml recombinant human placental lactogen (PL; Harbor-UCLA Research and Education Institute) in NaHCO3, 1–10 μM α-hydroxyisobutyrate (Sigma) in PBS, 50–250 μM p-cresol sulfate (ApexBiotech) in PBS. Islets were incubated for 3 days, with one media change after 36 h. For combined treatment 5 μM α-hydroxyisobutyrate and 50 μM p-cresol sulfate were used. Following incubation, islets were mildly dispersed using 0.025% trypsin and cells were cytospun onto charged slides. Slides were fixed and immunolabeled with guinea pig anti-insulin (1:400; Dako), rabbit anti-Ki67 (1:400; Abcam), Cy2-conjugated anti-guinea pig IgG (1:300; Jackson ImmunoResearch Laboratories), and Cy3-conjugated anti-rabbit IgG (1:300; Jackson ImmunoResearch Laboratories). Nuclei were visualized with 4′,6′-diamidino-2-phenylindole (DAPI, 1 μg/ml; Molecular Probes). Images were obtained using a ScanScope FL slide scanner (Aperio Technologies). β-Cell proliferation was determined by quantifying the number of insulin-Ki67 dual-positive cells using a macro generated with the CytoNuclearFL algorithm in eSlide Manager (Aperio Technologies). Data are represented as fold change in proliferation compared with vehicle-treated islets.
Metabolite profiling by mass spectrometry.
Whole liver, skeletal muscle (gastrocnemius, soleus, and plantaris muscles), bone (fibula and tibia), and plasma were collected from 9-wk-old C57Bl/6J mice fed either a HFD (n = 8) or maintained on a CD (n = 8) for 1 wk. Tissues were dissected, flash frozen in liquid nitrogen, crushed by mortar and pestle, and stored at −80°C before being sent to Metabolon (Durham, NC) for both GC-MS and LC-MS-based untargeted metabolic profiling. Sample preparation, instrument analysis, and data processing analysis performed by Metabolon are as detailed in previous publications (28, 59). Metabolites were identified by their Metabolon identification number, which were converted to Human Metabolome Database (HMDB) or Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolite numbers for subsequent analysis.
Metabolomic analysis and statistics.
Metabolite data sets from each tissue type were assessed separately. Metabolites undetected in >50% of samples within a tissue were filtered from analysis. Any remaining missing values were imputed with half the minimum detected value. Following imputation, metabolite intensities were mean centered but otherwise untransformed. Means for control and HFD groups were compared using either a 2-sample t-test with Welsh’s correction or nonparametric Mann-Whitney as appropriate following a test for equal variance using Metaboanalyst 3.0 (91). The false discovery rate (FDR, Q value) was calculated to account for multiple comparisons according to the Benjamini-Hochberg procedure (38). FDR values ≤ 0.1 were considered significant, as appropriate for large biological data sets (40). Multivariate analysis was carried out using partial least-squares discriminant analysis (PLS-DA) as a reductive method (29). The data were projected using Plotly for Python (https://plot.ly) with a modified 3-D plotting script (v2.7.13). Random Forest analysis was undertaken to better assess the importance of the metabolite changes to group differences (84). Analysis of relative enrichment of KEGG pathways was also carried out with Metaboanalyst 3.0. Enrichments were determined by analysis of covariance (ANCOVA) method. The outputted data files were matched for common pathway enrichments between tissues (FDR<0.1), and covarying metabolites contributing to enrichment matched to significantly changing metabolites (FDR<0.1) using custom python scripts. The results were then projected as a networked hive plot (46), using a modified common license HTML script, which makes use of the open source D3.js. Metabolites contributing to significant pathway enrichment were only plotted if they were also determined to be changing between groups (by mean comparison above). Complete enrichment data are included as a supplement (Supplemental Table S2, available at the Journal website with the online version of this article).
RESULTS
HFD feeding for as little as 1 wk leads to β-cell expansion.
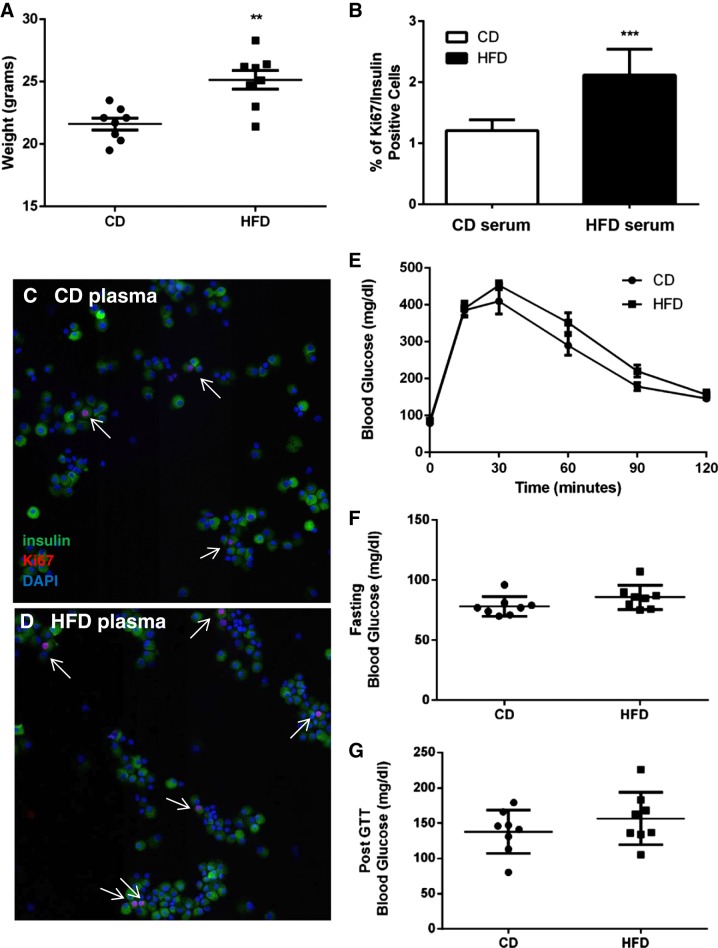
To determine the overall physiological metabolic impact of an acute switch to HFD, and to identify potential factors that promote β-cell proliferation in response to short-term HFD in the absence of insulin resistance, 8-wk-old male C57Bl/6J mice were either maintained on CD or fed HFD for 1 wk. The HFD-fed group weighed on average 3.6 g more than the CD-fed group at euthanasia (HFD = 25.2 ± 0.8 g, CD = 21.6 ± 0.5 g, P = 0.0014) (Fig. 1A). Plasma from short-term HFD-fed mice was assessed to determine whether it contained factors capable of stimulating β-cell proliferation. Intact islets isolated from wild-type CD-fed mice were cultured for 3 days as previously described with media containing plasma collected from mice fed either CD or HFD (56). Following treatment, islets were dissociated and β-cell proliferation quantified using insulin and Ki67, a nuclear marker of actively proliferating cells (Fig. 1, B–D). β-Cell proliferation was increased 1.75-fold in islets cultured with HFD-fed mouse plasma compared with islets cultured with CD-fed mouse plasma (Fig. 1B). Blood glucose levels were modestly, but not significantly, elevated in the HFD group relative to the CD group, at fasting, during the IP-GTT and 2 h post glucose injection (Fig. 1, E–G). Thus it is unlikely that elevations in blood glucose in the HFD-fed mice explain the observed increase in β-cell proliferation.
Fig. 1.
Significant β-cell proliferation occurs with high-fat diet (HFD) feeding for as little as 1 wk. A: mice fed HFD for 1 wk were significantly heavier compared with chow diet (CD)-fed mice. B: increased β-cell proliferation in control mouse islets cultured in the presence of HFD-fed (n = 6) mouse plasma compared with CD-fed plasma (n = 8). C and D: representative images used for B. Arrows mark proliferating β cells. E: blood glucose measurements during intraperitoneal glucose tolerance test (IP-GTT), at fasting (F) and at the 2-h time point (G). **P = 0.001, ***P = 0.0001.
Unique metabolite profiles of liver, muscle, bone, and plasma are altered in HFD.
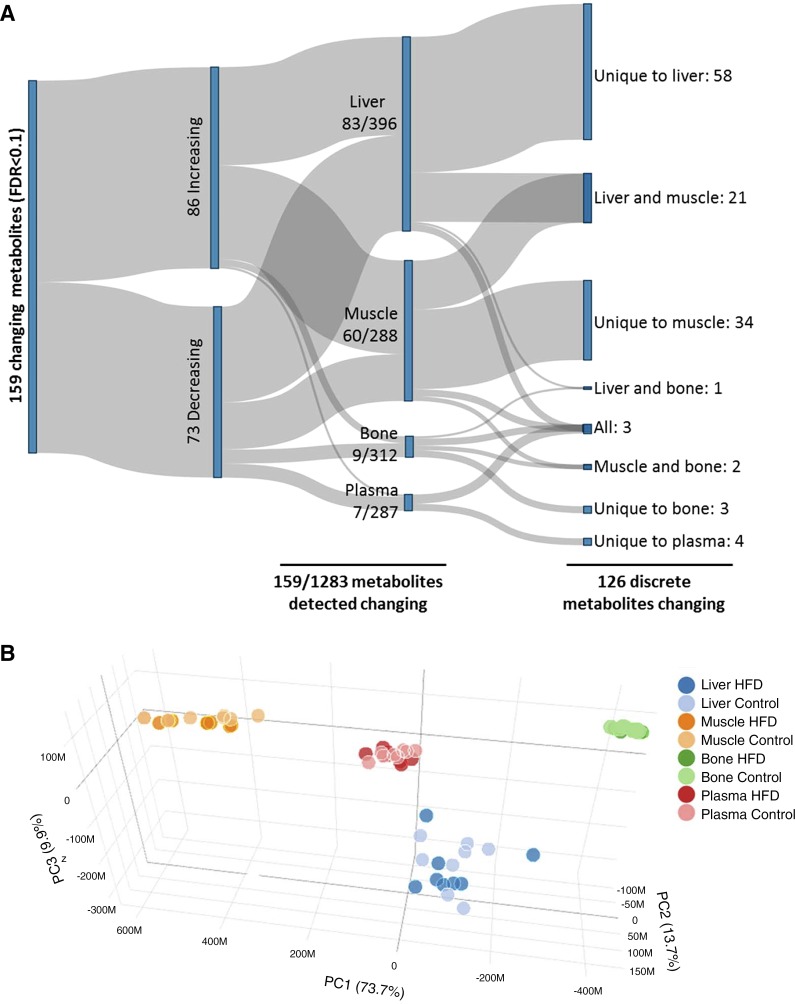
To identify metabolites and metabolic pathways associated with HFD-induced β-cell expansion, we performed global metabolic profiling on liver, muscle, bone, and plasma. The number of compounds identified in each tissue and the number of biochemical metabolites that were significantly elevated in each diet group are reported in Fig. 2. Overall plasma and bone had the fewest changes in response to 1-wk HFD, with a total of seven and nine statistically relevant changes, respectively (FDR < 0.1) (Fig. 2A). Liver and muscle were more metabolically responsive with a total of 83 and 60 statistically significant changes, respectively (FDR < 0.1) (Fig. 2A). The significant changes were split largely evenly between increasing and decreasing, indicating compensatory metabolic effects beyond just the detection of dietary fats and other diet-related components. Moreover, the changes were distinct for the different tissues, with 126 of 159 total significant changes being discrete metabolites, and 99 of the metabolite changes taking place in only one of the four tissues assessed (Fig. 2A).
Fig. 2.
Summary of metabolite profiles identified for liver, muscle, bone, and plasma from high-fat diet (HFD)-fed compared with chow diet (CD)-fed mice. A: overall, 159 significant changes were observed across all tissues to 126 discrete species of metabolite in HFD-fed animals compared with controls [false discovery rate (FDR) < 0.1]. Changes were primarily in highly metabolically active tissues (muscle and liver), whereas bone and plasma were relatively resistant to changes associated with high-fat diet. B: principal component (PC) analysis: sample profiles were assessed by partial least squares-discriminant analysis (PLS-DA) regression analysis of HMDB metabolites. Permutation analysis revealed significant differentiation of the tissue clusters (P < 0.001). Metabolites not detected in at least two tissues were removed from the plot. Liver and muscle exhibited the largest spread across PC1 which accounted for the majority of the variation (73.7%). When assessed individually by PLS-DA, there was no difference in overall profile by diet for the four tissues.
Overall the changes led to distinct separation of metabolite profiles by tissue type as assessed by multivariate PLS-DA reduction, which was significant by permutation analysis (P < 0.001), indicating strong tissue-specific metabolite level profiles (Fig. 2B). Separation of these metabolite profiles was due to differences in metabolites normally associated with the particular tissue as determined by loadings plot assessment (data not shown). For example, glucose level was highest in liver and plasma, whereas phosphate and lactic acid were highest in muscle. This verifies that the profiles are a true reflection of the metabolism of the various tissues, which we later subjected to pathway analysis for tissue-specific changes.
The variance observed as a result of the HFD for any given tissue was much smaller than the variance observed between tissue types as assessed by the spread of the clusters in Fig. 2B (light vs. dark vs. different colors). When separately assessed by PLS-DA, there was no difference between HFD and CD profiles for any of the tissues. Plasma exhibited the smallest amount of variance (Fig. 2B, green), while liver was the most variable (Fig. 2B, blue).
Although the PLS-DA did not differentiate CD or HFD metabolite profiles (Fig. 2B), when we assessed the data sets by RandomForest there were significant differences between the groups, with a classification accuracy of 100% for the liver and muscle, and 87.5% for bone and plasma. This indicates that HFD profiles were statistically distinguishable from CD profiles at least seven of eight times for all four tissues. An analysis of the metabolites that were most distinguishing between CD and HFD, as calculated by the mean decrease accuracy, are discussed, tissue-by-tissue, below, and are listed in Supplemental Table S1, available at the Journal website with the online version of this article.
Metabolite changes in liver, muscle, and bone associated with HFD.
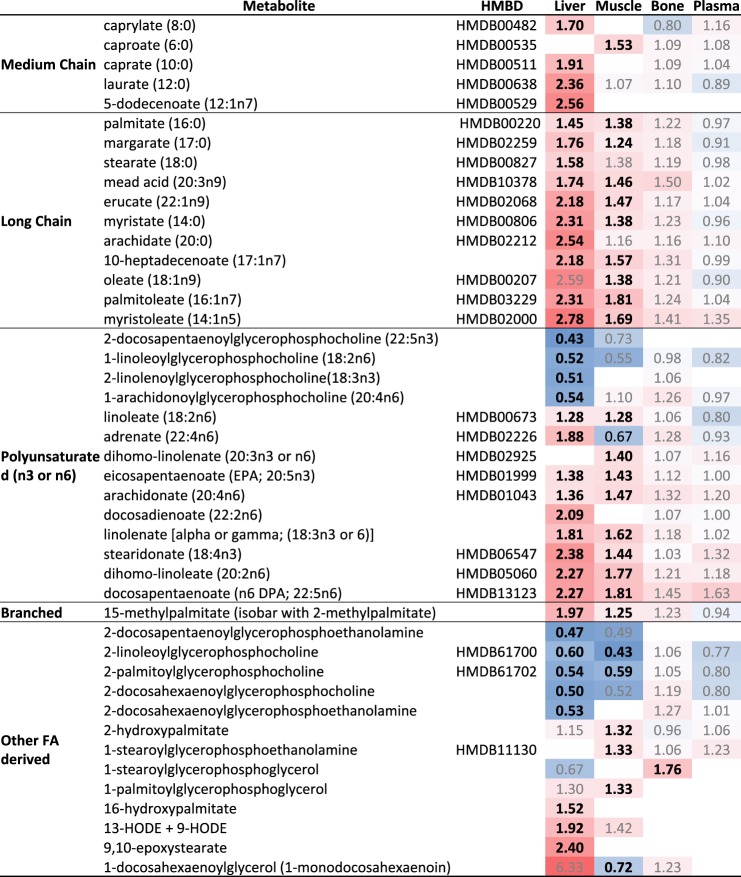
The overall changes of metabolites (FDR < 0.1) are indicated in Figs. 3–5 as fold change from CD organized by metabolite classification. For lipids, fatty acids, the principal diet composition difference in HFD compared with CD, were assessed separately (Fig. 3). Overwhelmingly, fatty acid metabolites were significantly increased in liver, except for the glycerolphosphocholine and glycerophosphoethanolamine lysophospholipids (Fig. 3). Significant fatty acid changes in the liver overlapped with the nine most important metabolite classifiers by RandomForest, inclusive of branched-chain, medium-chain, long-chain, and polyunsaturated fatty acids (Supplemental Table S1; Fig. 3); malonylcarnitine, a biomarker of disrupted fatty acid oxidation, appears as the 10th-most-changed metabolite in liver.
Fig. 3.
Heat map summary of fatty acid metabolite changes in high-fat diet (HFD)-fed mice compared with chow diet (CD)-fed controls. Fold change indicated with significant changes (P < 0.05, FDR<0.1) are designated by bold, whereas nonsignificant are annotated by gray. Heat map is colored by relative scale of change across all tissues and metabolites. Darker shades of red show increases while darker shades of blue show decreases.
Fig. 5.
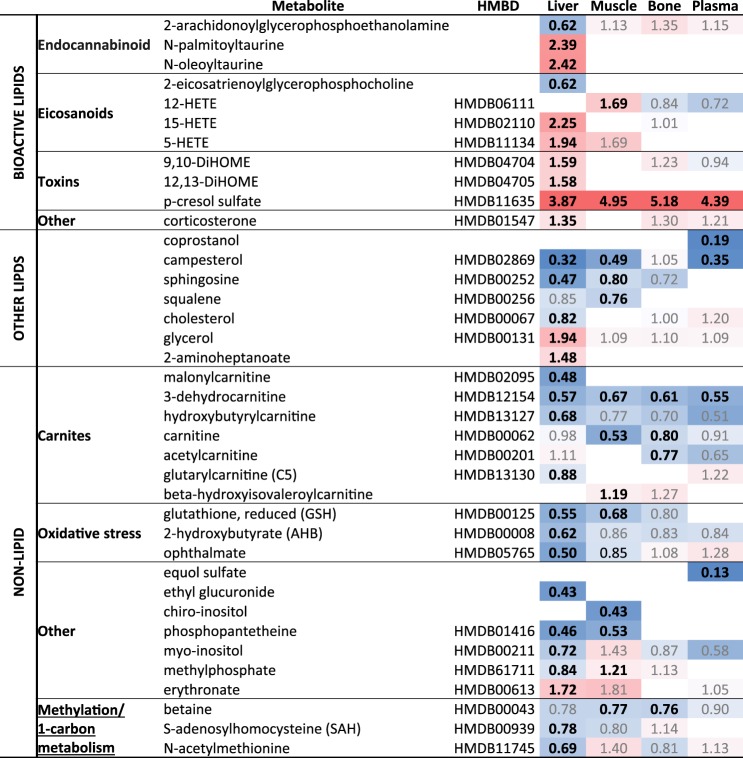
Heat map summary of other metabolite changes in high-fat diet (HFD)-fed mice compared with chow diet (CD)-fed controls. Fold change indicated with significant changes (P < 0.05, FDR < 0.1) are designated by bold, whereas nonsignificant are gray. Heat map is colored by relative scale of change across all tissues and metabolites. Darker shades of red show increases while darker shades of blue show decreases.
For muscle, 3-dehydrocarnitine, an intermediary in the production of carnitine (a molecule central to fatty acid oxidation), was the most important by mean decrease accuracy (MDA). This was followed by the fatty acids palmitoleate (16:1n7) and dihomo-linoleate (20:2n6) (Supplemental Table S1). In bone, p-cresol sulfate, the largest significantly changing metabolite in all tissues (Fig. 5), was the most important RandomForest classifier and was also the third most important in plasma (Supplemental Table S1). p-Cresol sulfate is a metabolite derived from the catalysis of tyrosine by the colonic microbiota. As with liver and muscle, fatty acids and carnitines demonstrated significant changes, including 3-dehydrocarnitine, acetylcarnitine, and hexanoylcarnitine as the 4th, 7th, and 8th highest MDA, respectively (Supplemental Table S1). Interestingly, although the greatest number of overall metabolite changes took place in the liver, the greatest amount of changes to metabolites associated with carbohydrate and energy metabolism took place in muscle (Fig. 4).
Fig. 4.
Heat map summary of metabolite changes associated with energy, DNA, and protein metabolism in high-fat diet (HFD)-fed mice compared with chow diet (CD)-fed controls. Fold change indicated with significant changes (P < 0.05, FDR<0.1) are designated by bold, whereas nonsignificant are gray. Heat map is colored by relative scale of change across all tissues and metabolites. Darker shades of red show increases while darker shades of blue show decreases.
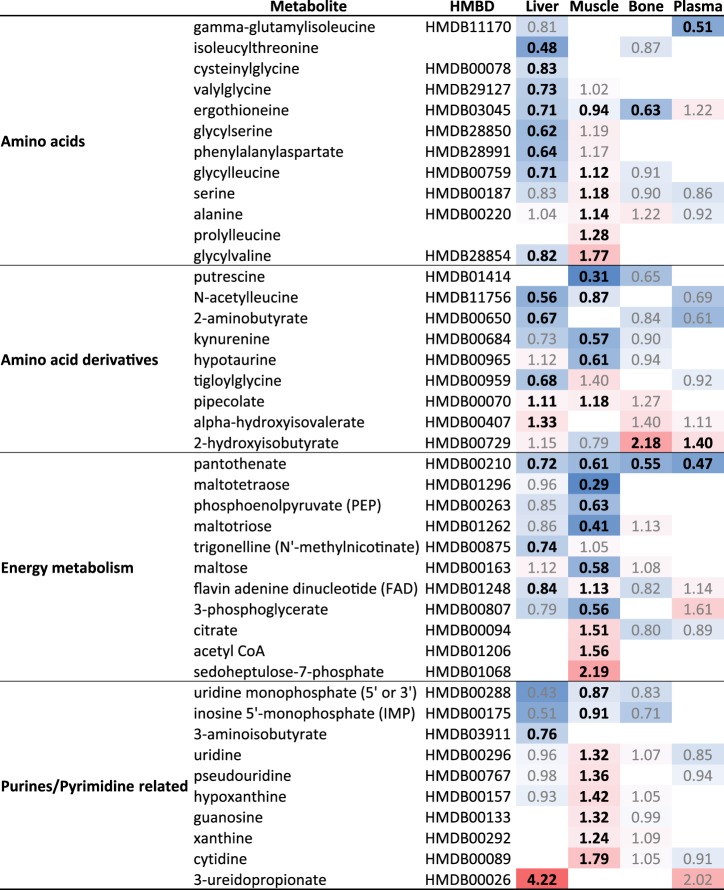
Other notable compounds that were altered in liver included metabolites implicated in one-carbon metabolism, as well as bioactive lipids including endocannabinoids, eicosanoids, and various toxins (Fig. 5). The sulfur-containing AAs, which are indicative of oxidative stress and redox homeostasis, exhibited large alterations when mice were placed on HFD for 1 wk (Fig. 4). Significant alterations in purine and pyrimidine metabolism were observed following 1 wk HFD-feeding, particularly in the muscle, and may indicate alterations in oxidative stress due to a short-term exposure to HFD (Fig. 4). Purine metabolites such as inosine, hypoxanthine, and xanthine were increased in short-term HFD-fed mouse muscle tissue by RandomForest analysis, and there was a striking increase in 3-ureidoproprionate in the liver (Fig. 4).
Analysis of metabolic pathway enrichment following HFD.
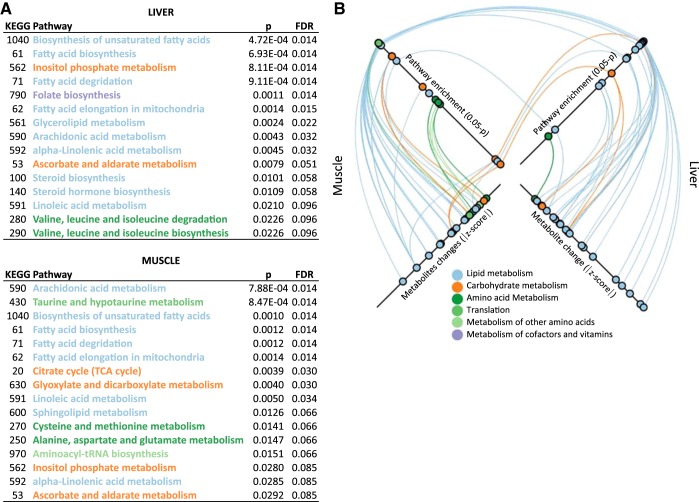
Changes in metabolically active tissues can have distal effects via plasma intermediaries. To determine which pathway changes may contribute to or associate with the β-cell mass expansion, we conducted a pathway enrichment analysis. We assessed tissues individually for covarying metabolites (ANCOVA) within KEGG pathways. Due to the small number of changes and low variance in the bone and plasma in HFD compared with CD (Fig. 2), significant enrichment was not detected for these tissues. However, a total of 15 pathways in liver and 17 pathways in muscle exhibited significant enrichment, including nine that were common to both tissues, for a total of 23 distinct pathways (Fig. 6A). The pathways are colored by their KEGG classification. A complete list of the covarying metabolites within these pathways is included in Supplemental Table S2.
Fig. 6.
Summary of enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways with 1-wk high-fat diet (HFD). A: pathway analysis performed based on global analysis of covariance (ANCOVA) of metabolites revealed 31 separate significant enrichments of 23 discrete Mus musculus KEGG pathways in either liver or muscle (FDR < 0.1). Pathways are colored according to their metabolic classification in KEGG Orthology. B: graphical representation of the significance of the pathway enrichments as well as the significantly changed metabolites within those enriched pathways for muscle (left axes) and liver (right axes). Top axes: pathways in the table are plotted by increasing significance of enrichment (P < 0.05) from the center outward. Pathways enriched in both muscle and liver are linked. Bottom axes: metabolites within these KEGG pathways that were significantly changed with HFD are plotted by increasing magnitude of change (absolute fold change) from the center outward. Links are colored by KEGG pathway classification. A list of the covarying metabolites associated with the pathway enrichment are included as Supplemental Table S2).
Pathways were plotted by increasing significance of their enrichment (outermost plotted values corresponding to lowest P) (top two axes, Fig. 6B). The bottom two axes of Fig. 6B show the metabolites identified by ANCOVA as covarying metabolites within the linked pathways and plotted by the magnitude of that change (outermost plotted values corresponding to largest fold changes) (Fig. 6B). The data highlight the profound changes in fatty acid and lipid metabolism that are associated with processing the extra lipids in the HFD, with 18 enriched lipid pathways (Fig. 6, blue). These pathways are among the most significantly enriched (blue plots, Fig. 6B, top axes), due to the large number of covering metabolite changes that are mostly fatty acids (blue plots, Fig. 6B bottom axes; and Supplemental Table S2).
Enrichment of lipid pathways was common to both muscle and liver, likely as a result of increased fatty acid uptake and processing. Notably, however, for non-lipid pathway enrichments, only two carbohydrate pathways were common to both tissues (orange links, Fig. 6B, top axes). In other words, shared pathway enrichments were almost exclusively lipid pathways, whereas nonshared enrichments were almost exclusively non-lipid pathways (Fig. 3, Supplemental Table S2). First, this implies that there are profound regulatory effects in both muscle and liver that far exceed the pathways necessary to directly process these lipids. Second, muscle and liver adapt and respond metabolically very differently to a HFD. Significant non-lipid pathways enriched include important regulatory pathways such as inositol phosphate metabolism (Fig. 6), central to which is myo-inositol and associated compound changes, which are known to be both a marker of, and a treatment for, metabolic disease (17, 22, 60). Other pathways of interest that could potentially affect distal β-cell proliferation include steroid hormone biosynthesis, and folate biosynthesis, which has been implicated in DNA methylation and one-carbon metabolism. Broad metabolic rearrangements may influence β-cell expansion through intermediaries from these tissues secreted into the plasma.
Altered plasma metabolites as factors for β-cell proliferation.
Muscle and liver are active secretors of bioactive metabolites into plasma. Since plasma from 1-wk HFD-fed mice stimulated β-cell proliferation, we anticipated that peripheral tissues might be a source of circulating stimulatory metabolites in HFD plasma. Most metabolites were decreased in plasma, with only two showing a significant increase: 2(α)-hydroxyisobutyrate (Fig. 4) and p-cresol sulfate (Fig. 5). We exposed isolated mouse islets to each of these compounds independently and in combination ex vivo but did not detect a significant increase in β-cell proliferation (not shown). Plasma serves as a reservoir of metabolite biomarkers, and thus it was surprising to find that the top ten RandomForest classifiers in plasma were not primarily fatty acids or acylcarnitines (Supplemental Table S2). In fact, no statistically significant fatty acid changes were observed in plasma (Fig. 3), which demonstrates the homeostatic resilience of plasma compared with highly metabolic tissues (Fig. 2). The only amino acid (AA) altered exclusively in HFD plasma was γ-glutamylisoleucine, a catabolic product of γ-glutamyltransferase, which was decreased (Fig. 4).
When assessed by Random Forest the most important classifier was α-hydroxyisovalerate (Supplemental Table S1), a marker for ketosis, followed by coprostanol, a gut microbial-derived derivative of cholesterol that was detected exclusively in plasma and demonstrated a 5-fold decrease with HFD (Fig. 5). Additionally among them, a structurally similar plant-derived phytosterol, campesterol, which is also a stoichiometric competitor to cholesterol, was decreasing, as was 3-dehydrocarnitione, a gut microbial metabolite (Fig. 5). Equol sulfate, another metabolite that is gut-derived, significantly changed, was exclusively detected in plasma, and was also among the most important classifiers (Fig. 5). It is metabolized from the soy compound daidzein, and is an estrogen receptor β agonist.
Other important RandomForest classifiers included chenodeoxycholate, a primary human bile acid that is less common in mice, and tauroursodeoxycholate (Supplemental Table S1), which is also gut microbially derived. These bile acid changes are consistent with increased demand for gut absorption of dietary fats via plasma in the enterohepatic circulation. Pantothenate (vitamin B5), essential for coenzyme A synthesis and fat metabolism, was reduced in plasma (as well as muscle and liver) with HFD (Fig. 4). Derivatives of pantothenate, including phosphopantetheine (Fig. 5), were diminished in the liver and not detected in the other tissues. Others are phenol sulfate, another gut microbial-derived compound, and myoinositol (Fig. 5), which was significantly decreased in liver, and is known to have insulin signaling cross-talk and to be associated with metabolic disease. These metabolites were also important RandomForest classifiers (12th and 20th greatest MDA, respectively) (see Supplemental Table S1).
DISCUSSION
The main findings of the current study were to demonstrate for the first time an overall broad reshaping of metabolites and metabolism in mice after only 1-wk exposure to high-fat diet. Changes in metabolically active tissues, liver and muscle in particular, demonstrate broad rearrangement of fatty acid-associated pathways to accommodate the enriched diet. Beyond direct effects there were significant changes associated with carbohydrate metabolites in all tissues, but particularly muscle. Enrichment of other metabolites and pathways included purines and pyrimidines, AAs, and derivatives, as well as cofactors and vitamins. These changes, together with several alterations that could be derived only from gut microbiota, demonstrate the breadth and universality of metabolic change associated with even a short exposure to HFD. Changes in particular to metabolites implicated in DNA methylation and 1-carbon metabolism, oxidative stress, toxicity, and bioactive lipids, also show the enormous potential for regulatory change in diffuse tissues associated with diet. These changes could underpin broader more permanent biological changes in β-cell proliferation and/or long-term metabolic health.
The majority of changes seen after acute HFD-diet feeding, and in the absence of insulin resistance, align with what is known in the literature, specifically being an overall decrease in carbohydrate metabolism within muscle tissue, and an increase in lipid utilization and metabolism within the liver (23, 41, 43). Similarly, while widely reported and statistically significant, the metabolite αHB did not rank among the highest changing metabolites in this analysis. This is likely explained by the lack of accompanying insulin resistance at this early stage (57). Pantothenate (vitamin B5) was uniformly decreased in all tissues examined following HFD-feeding, supporting similar findings in a model of diet-induced non-alcoholic steatohepatitis (NASH) (50), and which may be affected further by compensatory ketogenesis (20). Conversely, the uremic toxin p-cresol sulfate (pCS) was equally increased across all tissues, aligning with known elevations in insulin-resistant states, such as chronic kidney disease (CKD) (36, 45, 77). Thus elevations in p-cresol sulfate may precede and predict insulin resistance. Interestingly, alterations in pCS have been shown in urine and plasma of children with Type 1 diabetes, who otherwise demonstrated good glycemic control and were taking insulin (4). This suggests that pCS may be a general biomarker of metabolic function and may warrant further examination, although we could not directly attribute these changes to increases in β-cell proliferation following acute HFD exposure in the present study.
These data also support the involvement of other metabolites including glucose, mannose, unsaturated fatty acids, and lactate, which were shown to decrease in serum significantly following Roux-en-Y gastric bypass (RYGB) surgery, improving glycemic profiles and insulin sensitivity in both obese and Type 2 diabetes patients (58). That previous study also showed an elevation in lipid metabolites including sphingosine following RYGB, as well as increased p-cresol sulfate in obese and diabetic subjects (58). Our data reveal a decrease in sphingosine presence in the liver and muscle following HFD-feeding; interestingly, sphingosine-1-phosphate (S1P) phosphatase 2 (Sgpp2) has been reported to be a specific regulator of β-cell mass and endoplasmic reticulum stress (ER) homeostasis. Sgpp2−/− mice exhibit ER stress, and show defects in β-cell proliferation and function following long-term HFD feeding (80). The metabolism of S1P is critically required for regulating relative levels of three related molecules in the sphingolipid pathway, S1P, sphingosine, and ceramide, and alterations in the sphingolipid pathway have been implicated in HFD models of disease (15). We also observed a decrease in the 1-carbon metabolite pathway following HFD-feeding. Previous studies have demonstrated that supplementation along the 1-carbon pathway can alter DNA methylation, gene expression, and heritability of epigenetic traits in rodent models, including insulin resistance and metabolic syndrome (35, 74).
Within the liver specifically, the purine/pyrimidine related metabolite 3-ureidopropionate, a urea derivative of β-alanine and intermediate in uracil metabolism, was significantly increased. In disparate disease etiologies, HFD feeding shares characteristics with the parasitic infection schistosomiasis, causing disruption in AA metabolism (90), and broadly mimicking other liver diseases, including hepatitis (14). In a separate study, gene mutations in UBP1 coding for β-ureidopropionase gathered from plasma proteomic assessment of patients at risk for cardiovascular disease demonstrated inborn errors in metabolism (69). Thus ureidopropionate may be a novel factor to investigate the impetus for β-cell proliferation in response to HFD. Indeed, decades-old evidence has implicated the liver as the mechanistic driver by which high-fat diets, even briefly, impair glucose tolerance (13), and which has been reinforced by more recent and elegant genetic knockout studies (31, 39, 53, 61, 62, 75). One purported hepatic target includes insulin-like growth factor-1 (IGF-1), with one study using an inducible liver-specific insulin receptor knockout model (iLIRKO). The authors report IGF-1 to be acting through the insulin-receptor A isoform (27), putatively via mTORC1/p70S6kinase signaling on β-cells in response to insulin resistance (26).
Pharmacological evidence supports the impact of glycemic perturbation resulting from altered lipid profiles within the liver. Dimethyl biguanide (metformin) is a first-line treatment for Type 2 diabetes, particularly in overweight individuals (51), affecting blood glucose reduction by inhibiting hepatic glucose generation and promoting adipose- and muscle-glucose uptake (3). Metformin has been shown to inhibit β-cell proliferation induced by 1-wk HFD feeding (82), independent of its effects on insulin resistance, and putatively acting by reduction of mTOR signaling, via the activation of AMPK (82). Previous work from a different group showed that the addition of a dipeptidyl peptidase-IV (DPP-IV) inhibitor, TS-021, with metformin treatment had the opposite effect, increasing β-cell mass and lowering glucose, although β-cell proliferation directly was not examined (81). Furthermore, the incretin-effect of DPP-IV inhibition on mouse β-cell mass expansion following HFD feeding has not been validated by other studies (21). Similarly, although in an entirely different class of drugs, β-hydroxy-β-methylglutaryl (HMG)-CoA reductase inhibitors (statins), which act by lowering endogenous cholesterol synthesis in the liver, have been linked with a 9% increased risk of Type 2 diabetes, particularly in older patients (73). The authors’ explanations of this link was not causal, however, suggesting confounding factors; furthermore, there is no consensus regarding the effect of statins on insulin resistance or sensitivity in humans (33, 63) or rats (48). Although one report suggested that atorvastatin increased mouse “pancreatic proliferation,” this conclusion was reached by assessment of β-cell area, and not direct quantitation of proliferation (12), and studies performed by others were either inconclusive in human islet ex vivo conditions (7) or showed no direct effect by the addition of a statin on rat β-cell proliferation following HFD feeding (54). However, one recent report seemed to reconcile these findings, indicating a dual (and opposing) effect of improved insulin sensitivity with impaired β-cell function in mice following HFD feeding (72). Clearly, the widely ranging outcomes on β-cell mass and proliferation following treatment with hepatic-targeted drugs warrant further investigation, and reinforce the liver-endocrine-pancreas link.
In this study we sought to assess molecules that are possible stimulators of β-cell proliferation, which could be plasma-borne, even if their origins are elsewhere. Considering the total number of observed changes that were higher in liver but low in plasma (Fig. 2A), the data reflect a highly homeostatic plasma compared with a very dynamic metabolic response in liver with HFD challenge. Plasma is often considered to be a rich repository of biomarkers and biological effectors reflective of tissue changes. Given the homeostatic resilience observed here, the small list of metabolites that were able to very significantly overcome these mechanisms was of high interest. Neither α-hydroxyisobutyrate nor p-cresol sulfate stimulated β-cell proliferation in mouse islets ex vivo at the concentrations tested here. It is possible that either or both compounds would stimulate β-cell proliferation if tested at different concentrations. Alternatively, it may be that one of the compounds that was decreased in plasma from HFD-treated animals (there were 5) may be an inhibitor of β-cell proliferation. This possibility remains to be tested in future experiments.
Our data demonstrate that the liver primarily, and muscle to a lesser degree, derive the greatest amount of metabolic changes in response to short-term HFD, but that many changes are common to different tissues. Of the rare specific changes in plasma, α-hydroxyisobutyrate elevation has been previously associated with protein ingestion-induced insulin resistance (37), and shares similarities with neonatal murine undernutrition including muscle and fat catabolism, liver injury, and oxidative stress (68). Furthermore, although metabolomic analyses in the Framingham Offspring study showed a positive relationship between elevated plasma branched-chain and aromatic AA presence and the future risk of Type 2 diabetes (88), we found no such AA elevations following HFD feeding. In fact, within the liver, this AA metric was decreased, but ostensibly could be explained in our study by the brief (1 wk) HFD exposure in a model free of insulin resistance.
Forces driving pancreatic β-cell proliferation and its consequent mass expansion have been the subject of intensive scrutiny, both to understand normal physiological changes during response to injury and development, as well as to target pharmacological agents for intervention in diabetes therapy. A limitation of our strategy is that circulating factors with unchanged abundance, but increased or decreased flux, would be missed. Despite that, to our knowledge, this is the first report to use an unbiased platform and describe HFD-induced metabolite changes in metabolically important organs such as liver and muscle. Some of these metabolites are tissue-inherent whereas others are derived from the gut microbiome, which has been shown to be changed by HFD feeding (16, 49). The effects of a Western diet high in fat on these metabolites and their potential correlating impact on the endocrine pancreas warrant further investigation. Given the universality and magnitude of diverse pathway changes, particularly from liver, our findings are consistent with the theory that β cells initially proliferate in response to a HFD-induced factor potentially released from the liver. These data further reveal numerous avenues for interrogation to elucidate the mechanisms affecting β-cell proliferation, as well as potential novel drug targets.
GRANTS
V. Poitout holds the Canada Research Chair in Diabetes and Pancreatic Beta Cell Function and was supported by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grant R01-DK-58096), the Canadian Institutes of Health Research (MOP77686), and the Juvenile Diabetes Research Foundation (17-2012-26). S. E. Townsend was supported by the Vanderbilt Molecular Endocrinology Training Grant (5-T32-DK-07563). M. Gannon was supported by grants from the NIH/NIDDK (Grant 2R24-DK-090964-06), Department of Veterans Affairs (1I01 BX003744-01), and the Juvenile Diabetes Research Foundation (17-2012-26). K. M. Aagaard was supported by the NIH/NIDDK (Grant 2R24-DK-090964-06).
DISCLOSURES
K. Pappan is an employee and stockholder of Metabolon, Inc., where some of the experiments were performed. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
R.E.M., S.E.T., V.P., and M.G. conceived and designed research; R.E.M., S.E.T., and K.P. performed experiments; M.D.S., C.A.B., R.E.M., S.E.T., K.P., K.A., and M.G. analyzed data; M.D.S., C.A.B., S.E.T., K.P., K.A., and M.G. interpreted results of experiments; M.D.S., C.A.B., S.E.T., and K.P. prepared figures; M.D.S., C.A.B., and M.G. drafted manuscript; M.D.S., C.A.B., R.E.M., K.P., V.P., K.A., and M.G. edited and revised manuscript; M.D.S., C.A.B., R.E.M., K.P., V.P., K.A., and M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Bethany Carboneau for assistance in sample collection.
REFERENCES
- 1.Ahrén B, Pacini G. Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283: E738–E744, 2002. doi: 10.1152/ajpendo.00199.2002. [DOI] [PubMed] [Google Scholar]
- 2.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O’Donnell CP, Garcia-Ocaña A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 56: 1792–1801, 2007. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CJ, Turner RC. Metformin. N Engl J Med 334: 574–579, 1996. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 4.Balderas C, Rupérez FJ, Ibañez E, Señorans J, Guerrero-Fernández J, Casado IG, Gracia-Bouthelier R, García A, Barbas C. Plasma and urine metabolic fingerprinting of type 1 diabetic children. Electrophoresis 34: 2882–2890, 2013. doi: 10.1002/elps.201300062. [DOI] [PubMed] [Google Scholar]
- 5.Beamish CA, Zhang L, Szlapinski SK, Strutt BJ, Hill DJ. An increase in immature β-cells lacking Glut2 precedes the expansion of β-cell mass in the pregnant mouse. PLoS One 12: e0182256, 2017. doi: 10.1371/journal.pone.0182256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, Jewell MM, Powers AC, Wasserman DH. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57: 1790–1799, 2008. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugliani M, Syed F, Masini M, Marselli L, Suleiman M, Novelli M, Filipponi F, Boggi U, Masiello P, De Tata V, Marchetti P. Direct effects of rosuvastatin on pancreatic human beta cells. Acta Diabetol 50: 983–985, 2013. doi: 10.1007/s00592-013-0465-y. [DOI] [PubMed] [Google Scholar]
- 8.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 53: 2167–2176, 2010. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52: 2304–2314, 2003. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 11.Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M. Opposing effects of prostaglandin E2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation. Mol Metab 6: 548–559, 2017. doi: 10.1016/j.molmet.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZY, Liu SN, Li CN, Sun SJ, Liu Q, Lei L, Gao LH, Shen ZF. Atorvastatin helps preserve pancreatic β cell function in obese C57BL/6 J mice and the effect is related to increased pancreas proliferation and amelioration of endoplasmic-reticulum stress. Lipids Health Dis 13: 98, 2014. doi: 10.1186/1476-511X-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisholm KW, O’Dea K. Effect of short-term consumption of a high fat diet on glucose tolerance and insulin sensitivity in the rat. J Nutr Sci Vitaminol (Tokyo) 33: 377–390, 1987. doi: 10.3177/jnsv.33.377. [DOI] [PubMed] [Google Scholar]
- 14.Cho SG, Kim MY, Kim HJ, Kim YS, Choi W, Shin SH, Hong KC, Kim YB, Lee JH, Suh CH. Chronic hepatitis: in vivo proton MR spectroscopic evaluation of the liver and correlation with histopathologic findings. Radiology 221: 740–746, 2001. doi: 10.1148/radiol.2213010106. [DOI] [PubMed] [Google Scholar]
- 15.Choi S, Snider AJ. Sphingolipids in High Fat Diet and Obesity-Related Diseases. Mediators Inflamm 2015: 520618, 2015. doi: 10.1155/2015/520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 8: 77, 2016. doi: 10.1186/s13073-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements RS Jr, Reynertson R. Myoinositol metabolism in diabetes mellitus. Effect of insulin treatment. Diabetes 26: 215–221, 1977. doi: 10.2337/diab.26.3.215. [DOI] [PubMed] [Google Scholar]
- 18.Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. Alpha-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Diabetes Care 39: 988–995, 2016. doi: 10.2337/dc15-2752. [DOI] [PubMed] [Google Scholar]
- 19.Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52: e21–e31, 2011. [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 124: 5175–5190, 2014. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox AR, Lam CJ, Rankin MM, Rios JS, Chavez J, Bonnyman CW, King KB, Wells RA, Anthony D, Tu JX, Kim JJ, Li C, Kushner JA. Incretin therapies do not expand β-cell mass or alter pancreatic histology in young male mice. Endocrinology 158: 1701–1714, 2017. doi: 10.1210/en.2017-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, Corrado F, Di Benedetto A. myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 36: 854–857, 2013. doi: 10.2337/dc12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devarshi PP, McNabney SM, Henagan TM. Skeletal muscle nucleo-mitochondrial crosstalk in obesity and type 2 diabetes. Int J Mol Sci 18: E831, 2017. doi: 10.3390/ijms18040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirice E, Kahraman S, Jiang W, El Ouaamari A, De Jesus DF, Teo AKK, Hu J, Kawamori D, Gaglia JL, Mathis D, Kulkarni RN. Soluble factors secreted by T cells promote β-cell proliferation. Diabetes 63: 188–202, 2014. doi: 10.2337/db13-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellenbroek JH, Töns HA, de Graaf N, Loomans CJ, Engelse MA, Vrolijk H, Voshol PJ, Rabelink TJ, Carlotti F, de Koning EJ. Topologically heterogeneous beta cell adaptation in response to high-fat diet in mice. PLoS One 8: e56922, 2013. doi: 10.1371/journal.pone.0056922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Escribano O, Gómez-Hernández A, Díaz-Castroverde S, Nevado C, García G, Otero YF, Perdomo L, Beneit N, Benito M. Insulin receptor isoform A confers a higher proliferative capability to pancreatic beta cells enabling glucose availability and IGF-I signaling. Mol Cell Endocrinol 409: 82–91, 2015. doi: 10.1016/j.mce.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Escribano O, Guillén C, Nevado C, Gómez-Hernández A, Kahn CR, Benito M. Beta-Cell hyperplasia induced by hepatic insulin resistance: role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes 58: 820–828, 2009. doi: 10.2337/db08-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81: 6656–6667, 2009. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 29.Favretto D, Cosmi E, Ragazzi E, Visentin S, Tucci M, Fais P, Cecchetto G, Zanardo V, Viel G, Ferrara SD. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem 402: 1109–1121, 2012. doi: 10.1007/s00216-011-5540-z. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62: 1730–1737, 2013. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA 98: 7475–7480, 2001. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E; RISC Study Group . alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5: e10883, 2010. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gannagé-Yared MH, Azar RR, Amm-Azar M, Khalifé S, Germanos-Haddad M, Neemtallah R, Halaby G. Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metabolism 54: 947–951, 2005. doi: 10.1016/j.metabol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Genevay M, Pontes H, Meda P. Beta cell adaptation in pregnancy: a major difference between human and rodents? Diabetologia 53: 2089–2092, 2010. doi: 10.1007/s00125-010-1848-z. [DOI] [PubMed] [Google Scholar]
- 35.Goodspeed D, Seferovic MD, Holland W, Mcknight RA, Summers SA, Branch DW, Lane RH, Aagaard KM. Essential nutrient supplementation prevents heritable metabolic disease in multigenerational intrauterine growth-restricted rats. FASEB J 29: 807–819, 2015. doi: 10.1096/fj.14-259614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-Cresyl sulfate. Toxins (Basel) 9: E52, 2017. doi: 10.3390/toxins9020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris LLS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, Kelly SC, Porter LC, Klein S, Yoshino J, Mittendorfer B. Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Diabetes 66: 1871–1878, 2017. doi: 10.2337/db16-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 9: 811–818, 1990. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 39.Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science 322: 1250–1254, 2008. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- 40.Iterson M van, ’t Hoen PAC, Pedotti P, Hooiveld GJEJ, den Dunnen JT, van Ommen GJB, Boer JM, Menezes RX. Relative power and sample size analysis on gene expression profiling data. BMC Genomics 10: 439, 2009. doi: 10.1186/1471-2164-10-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakimoto PA, Kowaltowski AJ. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol 8: 216–225, 2016. doi: 10.1016/j.redox.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, Brar HK, Attie AD. Prostaglandin E2 receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes 62: 1904–1912, 2013. doi: 10.2337/db12-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knudsen JG, Joensen E, Bertholdt L, Jessen H, van Hauen L, Hidalgo J, Pilegaard H. Skeletal muscle IL-6 and regulation of liver metabolism during high-fat diet and exercise training. Physiol Rep 4: e12788, 2016. doi: 10.14814/phy2.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondegowda NG, Fenutria R, Pollack IR, Orthofer M, Garcia-Ocaña A, Penninger JM, Vasavada RC. Osteoprotegerin and Denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-κB ligand pathway. Cell Metab 22: 77–85, 2015. doi: 10.1016/j.cmet.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 24: 88–99, 2013. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krzywinski M, Birol I, Jones SJ, Marra MA. Hive plots–rational approach to visualizing networks. Brief Bioinform 13: 627–644, 2012. doi: 10.1093/bib/bbr069. [DOI] [PubMed] [Google Scholar]
- 47.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114: 828–836, 2004. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lalli CA, Pauli JR, Prada PO, Cintra DE, Ropelle ER, Velloso LA, Saad MJA. Statin modulates insulin signaling and insulin resistance in liver and muscle of rats fed a high-fat diet. Metabolism 57: 57–65, 2008. doi: 10.1016/j.metabol.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 49.Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Alan Harris R, Frias AE, Grove KL, Aagaard KM. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 5: 3889, 2014. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machado MV, Kruger L, Jewell ML, Michelotti GA, Pereira TA, Xie G, Moylan CA, Diehl AM. Vitamin B5 and N-acetylcysteine in nonalcoholic steatohepatitis: a pre-clinical study in a dietary mouse model. Dig Dis Sci 61: 137–148, 2016. doi: 10.1007/s10620-015-3871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB, Bolen S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med 164: 740–751, 2016. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 52.McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. NIH experiment in centralized mouse phenotyping: the Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab 297: E849–E855, 2009. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97, 2000. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 54.Mizukami H, Inaba W, Takahashi K, Inoue K, Sawanobori K, Yagihashi S. Augmented reduction of islet β-cell mass in Goto-Kakizaki rats fed high-fat diet and its suppression by pitavastatin treatment. J Diabetes Investig 3: 235–244, 2012. doi: 10.1111/j.2040-1124.2011.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest 117: 2860–2868, 2007. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosser RE, Gannon M. An assay for small scale screening of candidate β cell proliferative factors using intact islets. Biotechniques 55: 310–312, 2013. doi: 10.2144/000114115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab 308: E573–E582, 2015. doi: 10.1152/ajpendo.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutch DM, Fuhrmann JC, Rein D, Wiemer JC, Bouillot JL, Poitou C, Clément K. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS One 4: e7905, 2009. doi: 10.1371/journal.pone.0007905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nieman DC, Shanely RA, Gillitt ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res 12: 4577–4584, 2013. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 60.Nissen PM, Nebel C, Oksbjerg N, Bertram HC. Metabolomics reveals relationship between plasma inositols and birth weight: possible markers for fetal programming of type 2 diabetes. J Biomed Biotechnol 2011: 378268, 2011. doi: 10.1155/2011/378268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouaamari A El, Dirice E, Gedeon N, Hu J, Zhou J-Y, Shirakawa J, Hou L, Goodman J, Karampelias C, Qiang G, Boucher J, Martinez R, Gritsenko MA, De Jesus DF, Kahraman S, Bhatt S, Smith RD, Beer H-D, Jungtrakoon P, Gong Y, Goldfine AB, Liew CW, Doria A, Andersson O, Qian W-J, Remold-O’Donnell E, Kulkarni RN. SerpinB1 Promotes Pancreatic β Cell Proliferation. Cell Metab 23: 194–205, 2016. doi: 10.1016/j.cmet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouaamari A El, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, Katsuta H, Hollister-Lock J, Qian WJ, Wagers AJ, Kulkarni RN. Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states. Cell Reports 3: 401–410, 2013. [Erratum published in Cell Rep 3: 969, 2013]. doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paniagua JA, López-Miranda J, Escribano A, Berral FJ, Marín C, Bravo D, Paz-Rojas E, Gómez P, Barcos M, Moreno JA, Pérez-Jiménez F. Cerivastatin improves insulin sensitivity and insulin secretion in early-state obese type 2 diabetes. Diabetes 51: 2596–2603, 2002. doi: 10.2337/diabetes.51.8.2596. [DOI] [PubMed] [Google Scholar]
- 64.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130: 1459–1466, 1992. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 65.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat Diabetes 47: 358–364, 1998. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- 66.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, Girard CA, Karni R, Kaestner KH, Ashcroft FM, Magnuson MA, Saada A, Grimsby J, Glaser B, Dor Y. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 13: 440–449, 2011. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab 296: E1003–E1012, 2009. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preidis GA, Keaton MA, Campeau PM, Bessard BC, Conner ME, Hotez PJ. The undernourished neonatal mouse metabolome reveals evidence of liver and biliary dysfunction, inflammation, and oxidative stress. J Nutr 144: 273–281, 2014. doi: 10.3945/jn.113.183731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhee EP, Yang Q, Yu B, Liu X, Cheng S, Deik A, Pierce KA, Bullock K, Ho JE, Levy D, Florez JC, Kathiresan S, Larson MG, Vasan RS, Clish CB, Wang TJ, Boerwinkle E, O’Donnell CJ, Gerszten RE. An exome array study of the plasma metabolome. Nat Commun 7: 12360, 2016. doi: 10.1038/ncomms12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, Attie AD, Kaestner KH. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 23: 1702–1712, 2009. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 36: 111–117, 2013. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salunkhe VA, Mollet IG, Ofori JK, Malm HA, Esguerra JLS, Reinbothe TM, Stenkula KG, Wendt A, Eliasson L, Vikman J. Dual effect of rosuvastatin on glucose homeostasis through improved insulin sensitivity and reduced insulin secretion. EBioMedicine 10: 185–194, 2016. doi: 10.1016/j.ebiom.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375: 735–742, 2010. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 74.Seferovic MD, Goodspeed DM, Chu DM, Krannich LA, Gonzalez-Rodriguez PJ, Cox JE, Aagaard KM. Heritable IUGR and adult metabolic syndrome are reversible and associated with alterations in the metabolome following dietary supplementation of 1-carbon intermediates. FASEB J 29: 2640–2652, 2015. doi: 10.1096/fj.14-266387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seyer P, Vallois D, Poitry-Yamate C, Schütz F, Metref S, Tarussio D, Maechler P, Staels B, Lanz B, Grueter R, Decaris J, Turner S, da Costa A, Preitner F, Minehira K, Foretz M, Thorens B. Hepatic glucose sensing is required to preserve β cell glucose competence. J Clin Invest 123: 1662–1676, 2013. doi: 10.1172/JCI65538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29: 301–307, 1997. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 77.Soulage CO, Koppe L, Fouque D. Protein-bound uremic toxins…new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J Ren Nutr 23: 464–466, 2013. doi: 10.1053/j.jrn.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 78.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab 305: E149–E159, 2013. doi: 10.1152/ajpendo.00040.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart AF, Hussain MA, Garcia-Ocana A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN. Human beta cell proliferation and intracellular signaling: part 3. Diabetes 64: 1872–1885, 2015. doi: 10.2337/db14-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taguchi Y, Allende ML, Mizukami H, Cook EK, Gavrilova O, Tuymetova G, Clarke BA, Chen W, Olivera A, Proia RL. Sphingosine-1-phosphate phosphatase 2 regulates pancreatic islet β-cell endoplasmic reticulum stress and proliferation. J Biol Chem 291: 12029–12038, 2016. doi: 10.1074/jbc.M116.728170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tajima A, Hirata T, Taniguchi K, Kondo Y, Kato S, Saito-Hori M, Ishimoto T, Yamamoto K. Combination of TS-021 with metformin improves hyperglycemia and synergistically increases pancreatic β-cell mass in a mouse model of type 2 diabetes. Life Sci 89: 662–670, 2011. doi: 10.1016/j.lfs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Tajima K, Shirakawa J, Okuyama T, Kyohara M, Yamazaki S, Togashi Y, Terauchi Y. Effects of metformin on compensatory pancreatic β-cell hyperplasia in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 313: E367–E380, 2017. doi: 10.1152/ajpendo.00447.2016. [DOI] [PubMed] [Google Scholar]
- 84.Touw WG, Bayjanov JR, Overmars L, Backus L, Boekhorst J, Wels M, van Hijum SAFT. Data mining in the Life Sciences with Random Forest: a walk in the park or lost in the jungle? Brief Bioinform 14: 315–326, 2013. doi: 10.1093/bib/bbs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 86.Varvel SA, Pottala JV, Thiselton DL, Caffrey R, Dall T, Sasinowski M, McConnell JP, Warnick GR, Voros S, Graham TE. Serum α-hydroxybutyrate (α-HB) predicts elevated 1 h glucose levels and early-phase β-cell dysfunction during OGTT. BMJ Open Diabetes Res Care 2: e000038, 2014. doi: 10.1136/bmjdrc-2014-000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang P, Alvarez-Perez J-C, Felsenfeld DP, Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK, Garcia-Ocaña A, Stewart AF. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat Med 21: 383–388, 2015. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Windeløv JA, Pedersen J, Holst JJ. Use of anesthesia dramatically alters the oral glucose tolerance and insulin secretion in C57Bl/6 mice. Physiol Rep 4: e12824, 2016. doi: 10.14814/phy2.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Xu W, Ming Z, Dong H, Tang H, Wang Y. Metabolic changes reveal the development of schistosomiasis in mice. PLoS Negl Trop Dis 4: e807, 2010. doi: 10.1371/journal.pntd.0000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res 43, Suppl W1: W251–W257, 2015. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]