Abstract
Gain-of-function (GOF) mutations in the ATP-sensitive potassium (KATP) channels cause neonatal diabetes. Despite the well-established genetic root of the disease, pathways modulating disease severity and treatment effectiveness remain poorly understood. Patient phenotypes can vary from severe diabetes to remission, even in individuals with the same mutation and within the same family, suggesting that subtle modifiers can influence disease outcome. We have tested the underlying mechanism of transient vs. permanent neonatal diabetes in KATP-GOF mice treated for 14 days with glibenclamide. Some KATP-GOF mice show remission of diabetes and enhanced insulin sensitivity long after diabetes treatment has ended, while others maintain severe insulin-resistance. However, insulin sensitivity is not different between the two groups before or during diabetes induction, suggesting that improved sensitivity is a consequence, rather than the cause of, remission, implicating other factors modulating glucose early in diabetes progression. Leptin, glucagon, insulin, and glucagon-like peptide-1 are not different between remitters and nonremitters. However, liver glucose production is significantly reduced before transgene induction in remitter, relative to nonremitter and nontreated, mice. Surprisingly, while subsequent remitter animals exhibited normal serum cytokines, nonremitter mice showed increased cytokines, which paralleled the divergence in blood glucose. Together, these results suggest that systemic inflammation may play a role in the remitting versus non-remitting outcome. Supporting this conclusion, treatment with the anti-inflammatory meloxicam significantly increased the fraction of remitting animals. Beyond neonatal diabetes, the potential for inflammation and glucose production to exacerbate other forms of diabetes from a compensated state to a glucotoxic state should be considered.
Keywords: diabetes, glibenclamide, inflammation, insulin, KATP, mice, permanent, remission, sulfonylurea, transient, treatment
INTRODUCTION
ATP-sensitive potassium (KATP) channels play a critical role in many tissues by linking metabolism to electrical activity, including pancreatic islet β-cells, where they control glucose-dependent insulin secretion (29, 36). As blood glucose rises, β-cell KATP channels close, leading to plasma membrane depolarization, consequent opening of voltage-dependent calcium channels, and calcium influx, which, in turn, triggers insulin secretion. Gain-of-function (GOF) mutations in the KATP channel have been identified as the main cause of human neonatal diabetes (NDM) (10). In this case, KATP channels remain open despite rising blood glucose, and these patients develop severe diabetes. KATP variants have also been linked to development of type 2 diabetes (38, 50).
We have previously generated inducible KATP-GOF transgenic mice by insertion of an ATP-insensitive pore-forming Kir6.2 subunit construct in the Rosa26 locus, under Cre-recombinase control (37). By crossing these mice with tamoxifen-inducible PDX1-CreER (PDX-Cre) mice, we generate inducible pancreatic β-cell-specific KATP-GOF mice. As predicted by the mutation, all KATP-GOF mice develop severe diabetes within 2 wk after tamoxifen induction, as a result of loss of glucose-dependent insulin secretion (37), reiterating the features of human NDM resulting from KATP-GOF mutations. Fed and fasting blood glucose rise to >500 mg/dl in all KATP-GOF mice within ~15 days after tamoxifen induction of transgene expression and remain high thereafter.
NDM patients with KATP-GOF mutations are now treated with sulfonylurea (SU) drugs, also widely used to treat type 2 diabetic patients, directly inhibiting KATP channels to restore endogenous insulin secretion (25, 33). One challenge to both understanding the underlying pathophysiology of NDM and increasing the effectiveness of available treatments is the significant variability of disease severity between patients (7, 8, 11, 25, 33, 36, 46, 49, 51), which occurs even in individuals within the same family with the same KATP mutation (25, 51). Some individuals presenting with NDM will enter remission within the first few months of life (transient NDM), whereas others require lifelong treatment (permanent NDM), but underlying mechanisms remain elusive (21, 32). Without treatment, all KATP-GOF mice develop severe diabetes; however, if treated for 6 days with the SU glibenclamide, a subset of KATP-GOF mice regain control of blood glucose that lasts long after the SU treatment has ended (remitters) although the remainder become, as expected, severely diabetic (nonremitters) (35). With this differential outcome, these mice offer the exciting possibility to study mechanisms underlying transient vs. permanent NDM.
We previously demonstrated improved peripheral insulin sensitivity in remitter animals long after blood glucose separation (35), but whether this was a cause or consequence of the differential outcome was not studied. To identify potential mechanisms and markers of diabetes remission, we have studied KATP-GOF mice before and after disease induction. We show here that large differences in insulin sensitivity between remitted and nonremitted animals are a consequence, rather than the cause, of the differences in blood glucose. We further show that various potential candidates which could be involved in diabetes remission do not change with disease progression. Importantly, however, alterations in inflammatory markers, particularly interleukin-6 (IL-6), correlate with the separation in glucose between the groups early in disease progression and may represent the underlying mechanism of the remission phenotype.
MATERIALS AND METHODS
Animal lines and maintenance.
Rosa26-Kir6.2[K185Q, ΔN30] mice were previously generated (Fig. 1A) and crossed with PDX1-CreER mice to generate β-cell-specific KATP-GOF mice (37). It is important to note that K185Q mutations have been identified in human NDM (45). These mice will not express the KATP-GOF transgene until being treated with tamoxifen. All procedures were approved by Washington University Institutional Animal Care. To generate pure remitter and nonremitting lines, remitted KATP-GOF mice (blood glucose <350 mg/dl following tamoxifen induction and SU treatment) were in-crossed with other remitted KATP-GOF mice over 13 generations, and littermate single transgenic (PDX1-Cre and Rosa26-Kir6.2[K185Q, ΔN30]) mice from crosses generating nonremitting double-transgenic animals were in-crossed for more than 13 generations, respectively (Fig. 1B).
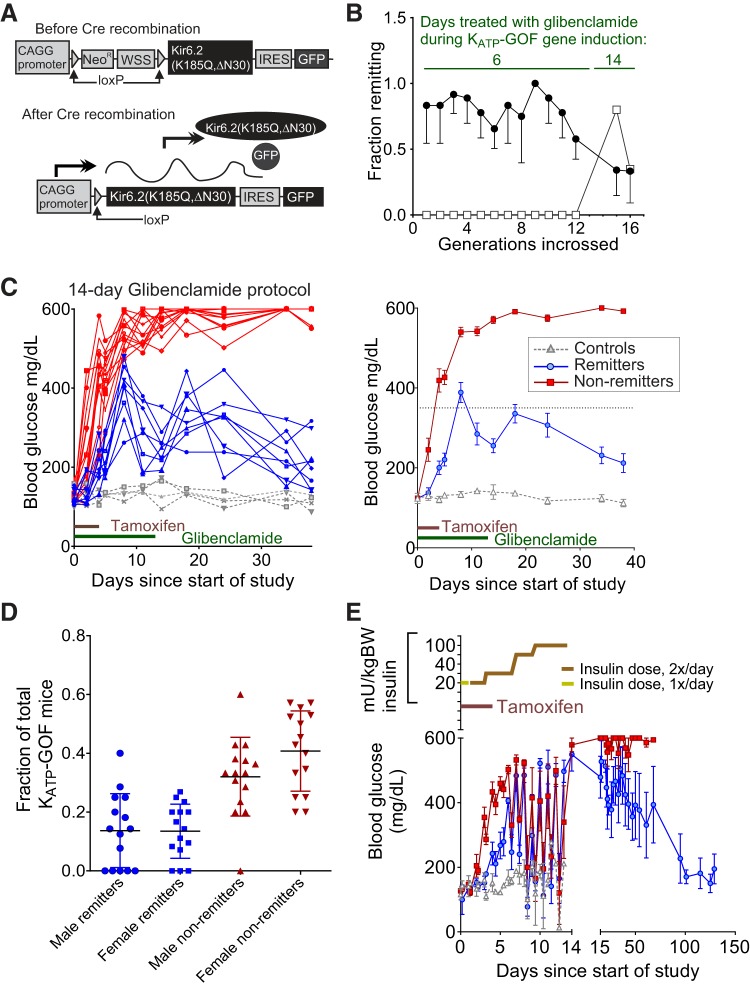
Fig. 1.
The remitter phenotype is not purely genetic. A: transgene structure of previously generated mice (37). These mice (Rosa-Kir6.2) have a construct inserted at the Rosa26 locus containing a CAGG promoter, Neo/WSS cassette (a stop sequence) flanked by LoxP sequences, a mutant pore-forming subunit of ATP-sensitive potassium (KATP) channels [Kir6.2(K185QΔN30)], an internal ribosomal entry sequence, and GFP. Without Cre recombination (top), the stop sequence blocks transgene expression. With Cre recombination (bottom), the stop sequence is removed, and the mutant Kir6.2 is expressed. B: multiple generations of backcrossing tamoxifen-induced remitting animals failed to consistently reach 100% remission (3–4 separate branches/generation; filled circles, remitter; open squares, nonremitter). C: increasing the duration of glibenclamide injections to 14 days shows emergence of remission phenotype in nonremitting line (empty squares in A), which had not shown remission previously using the original 6 days of treatment protocol [8 remitters (blue), 4 controls (gray), and 13 nonremitters (red) compiled from several inductions]; left, individual traces; right, averages. Animals were 9–12 wk old at the time of induction. D: proportion of remitting and nonremitting males and females over the various cohorts studied. No. of animals in each sex and group are expressed as a fraction of total KATP-GOF mice for their respective cohorts. Data are means ± SD. E: short-term injections of insulin (varied in dose in an attempt to sustain lower blood glucose; dosage switched from 1×/day to 2×/day after the 2nd day; dosing indicated on top) can result in remission [6 nonremitters (red), 3 remitters (blue), and 3 controls (gray) from two cohorts]. Animals in the insulin experiment were 20–25 wk old.
Gene induction.
KATP-GOF mice were induced by five daily injections of 50 mg/kg body wt tamoxifen (T5648; Sigma) dissolved at 5 mg/ml in corn oil (C8267; Sigma). Blood glucose measurements were made from the tail vein using OneTouch Ultra glucometers. Groups treated were injected with 50 mg/kg body wt glibenclamide (20 mg/ml stock in DMSO, final concentration 5 mg/ml in PBS; Sigma) for 14 days beginning at day 0. For the insulin-treated groups, the Novolin R dose was adjusted as indicated in Fig. 1C. Initial injections of one time per day were insufficient to provide equivalent glucose control to the glibenclamide-treated groups, and the dose was adjusted to two times per day as indicated for all animals in the group until the final injection on day 14. A schematic of the gene construct used in the mice, the induction protocol, and the resulting glucose curves for an example set of mice treated with tamoxifen and either the SU glibenclamide or vehicle is shown in Figs. 1A and 3A, respectively.
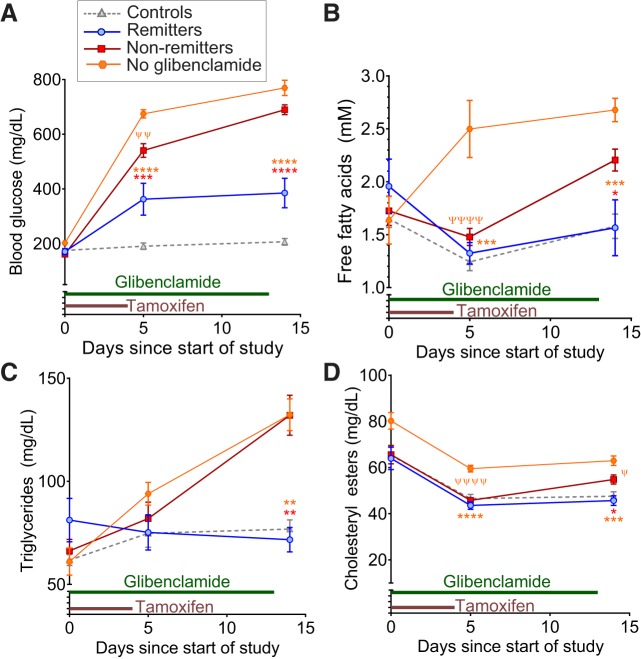
Fig. 3.
Dysregulation of lipid metabolism follows changes in blood glucose. Blood glucose (A), plasma free fatty acids (FFA, B), triglycerides (C), and cholesteryl esters (D) measured at days 0, 5, and 14 posttamoxifen induction in groups treated with glibenclamide or vehicle. Animals are 9–12 wk old at time of induction. Significant differences (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001) are shown comparing remitters and nonremitters (red asterisks) or remitters and vehicle-treated ATP-sensitive potassium (KATP)-gain-of-function (GOF) mice (orange asterisks) via posttests following ANOVA at the indicated time points. Differences between nonremitters and vehicle-treated KATP-GOF mice are indicated by ψP < 0.05, ψψP < 0.01, and ψψψψP < 0.0001 for posttests following ANOVA at the indicated time points. n = 6 Remitters (blue), 20 nonremitters (red), and 14 controls (gray triangles) treated with glibenclamide in A–D. Some KATP-GOF mice (n = 10, orange hexagons) were also treated with tamoxifen and the vehicle (10% DMSO in 1× PBS) instead of glibenclamide.
Insulin tolerance tests.
Insulin tolerance tests were performed as described previously (35). Briefly, mice were fasted for 6 h before the study. Mice were intraperitoneally injected with 0.5 mU/g body wt insulin (Novolin R) diluted in saline solution, and blood glucose was measured in tail vein samples at 0, 15, 30, 45, 60, 90, and 120 min postinjection using OneTouch Ultra glucometers.
Glucose tolerance tests.
Glucose tolerance tests were performed as previously described (35). Briefly, mice were fasted overnight before the study. Mice were injected with 1.5 g/kg body wt glucose dissolved in saline solution. Blood glucose was measured in tail vein samples at 0, 15, 30, 45, 60, 90, and 120 min postinjection using OneTouch Ultra glucometers.
Fasting-refeeding study and fasting insulin measurement.
For the fasting and refeeding study, KATP-GOF mice at day 5 after the start of injections were fasted overnight (16 h), and blood glucose was measured before (time 0) and 1 h after being provided ad libitum access to chow food. Fasting plasma insulin was measured at days 0, 5, and 14 using an ELISA kit (catalog no. 90080; Crystal Chem).
Metabolic profiling.
Comprehensive metabolic, behavioral, and physiological variables were determined by TSE/PhenoMaster at the Washington University Diabetes Models Phenotyping Core (https://diabetesresearchcenter.dom.wustl.edu/diabetes-models-phenotyping-core/). Animals were induced as described above. Fourteen days after the first dose of tamoxifen, mice were placed in individual PhenoMaster cage units for measurement of O2 consumption, CO2 generation, physical activity, and food intake. They were allowed to equilibrate for 2 days, and then data were collected for 4 days and analyzed. Food intake was considered as the amount of food consumed per gram of mouse lean body mass per 12 h. The respiratory exchange ratio was calculated as the ratio between the amount of CO2 produced in metabolism and the O2 used. Metabolic efficiency was calculated as the ratio between body weight and food consumption.
Hyperinsulinemic-euglycemic clamp.
Hyperinsulinemic-euglycemic clamp was performed as previously described (35). Briefly, 5 days before the study, mice underwent surgeries to implant catheters in the jugular vein (for infusion) and femoral artery (for blood sampling). Surgeries were performed in the Hope Center Animal Surgery Core at Washington University. Insulin (Novolin R) was dissolved in saline solution and administered at 4 mU·kg body wt−1·min−1 (2 µl/min). Glucose (50% dextrose) was administered at a variable rate to maintain blood glucose at 100 mg/dl, as measured using OneTouch Ultra glucometers from the femoral artery sampling line. Plasma samples were taken at 0, 120, and 145 min for hormone and metabolite analyses.
Sequential plasma hormone and lipid measurements.
Blood samples were taken from the tail vein at 0, 5, and 14 days posttamoxifen induction for measurement of glucose, lipids, and plasma hormones. Blood was collected in heparinized tubes with protease inhibitor cocktail containing 1 µl each of benzamidine-hydrochloride (3.6 mg/ml), aprotinin (1 mg/ml; Sigma), sitagliptin (1 mM in DMSO), and EDTA (25 mM). Plasma lipids were measured at the Diabetes Models Phenotyping Core, Washington University (https://diabetesresearchcenter.dom.wustl.edu/diabetes-models-phenotyping-core/). Plasma hormones were measured using the Luminex-Milliplex Mouse Metabolic Hormone panel at the Vanderbilt Hormone Assay Core (http://hormone.mc.vanderbilt.edu/) and the Andrew and Jane Burskey Center for Human Immunology and Immunotherapy Program at Washington University (http://chiips.wustl.edu/iml-core-lab.html).
Anti-inflammatory treatment with meloxicam.
Two independent cohorts of KATP-GOF mice were treated with vehicle or meloxicam for 7 days, beginning 1 day before the 6-day tamoxifen and glibenclamide injection. Twenty-four KATP-GOF mice were treated with meloxicam (20 mg/g body wt) and 17 with vehicle (DMSO, 1 µl/g body wt). Blood glucose was followed over time, and pyruvate tolerance test was performed at day 0 (before treatment) and at day 7 after meloxicam/vehicle treatment.
Statistics.
Samples were analyzed by Tukey’s posttest following one-way ANOVA at the indicated time points, except where specifically indicated in legends for Figs. 1–8. Significant differences of P < 0.0001, 0.001, 0.01, and 0.05 are indicated in Figs. 1–8, and nonsignificant differences are annotated with their specific values where noted. All data are presented as means ± SE, except where specifically noted.
RESULTS
Aggressive sulfonylurea therapy in KATP-GOF mice can cause remission of diabetes.
KATP-GOF and littermate control mice (2–3 mo old) were injected for 5 consecutive days with tamoxifen to induce transgene expression (37). Within 2 wk after induction, untreated KATP-GOF mice develop severe diabetes, whereas littermate control mice are unaffected. We previously demonstrated that a short period of glibenclamide treatment (6 days) initiated at the same time as tamoxifen injection results in two distinct outcomes; while some KATP-GOF mice develop severe diabetes (blood glucose >350 mg/dl, nonremitters), others remained with blood glucose concentration of <350 mg/dl (remitters) (35). In initial breedings, KATP-GOF offspring of induced remitted mice showed a higher percentage of remitters when induced and treated with glibenclamide, whereas KATP-GOF offspring generated from single transgenics from a nonremitter line produced nonremitting mice, which suggested a potential background modifier gene as a basis for the divergent outcomes. We therefore repeatedly in-crossed remitters, as well as littermates of nonremitting animals (since nonremitters did not breed well), in an attempt to generate lines with consistently remitting or nonremitting mice, respectively. Nonremitter lines did indeed breed true by this protocol (Fig. 1B), and remitters generated a higher proportion of remitters in later (13+) generations compared with randomly generated KATP-GOF animals (35). It is important to note that the remitter and nonremitter phenotype showed no sex differences (Fig. 1D) and appeared even in older animals (Fig. 1E). Importantly, 100% remittance was never achieved, and increasing the treatment with glibenclamide from 6 to 14 days (as in this paper) did not increase remission rate, but instead resulted in the appearance of remitting animals in the “nonremitter” line (Fig. 1C). Failure of convergence of the phenotypes after many in-cross generations is inconsistent with a simple or purely genetic underlying basis.
Remission is a consequence of lowering of blood glucose.
Because SU drugs can have additional off-target effects than inhibition of KATP channels (54), which could be responsible for the remitting phenotype, a cohort of KATP-GOF animals was treated with insulin injections (doses as indicated in Fig. 1E, top). Insulin was injected at the same time as tamoxifen injections, in a parallel protocol to the 14-day glibenclamide treatment. Insulin treatment, despite providing less precise control of blood glucose, resulted in a similar proportion of remitting and nonremitting animals to glibenclamide treatment (Fig. 1E), indicating that indeed the remission process was a result of blood glucose lowering rather than any off-target effects of glibenclamide.
Changes in insulin sensitivity alone do not cause remission.
Our earlier studies (35) indicated lower insulin sensitivity in remitted animals compared with animals that remained diabetic: insulin tolerance and hyperinsulinemic-euglycemic clamp tests demonstrated that remitted animals were more insulin sensitive than control mice, and nonremitters were very insulin resistant. These findings raise the possibility that preexisting differences in insulin sensitivity between individuals might underlie the remitter vs. nonremitter outcomes, but, because the insulin sensitivity tests were conducted long after blood glucose separation, the observed differences in insulin sensitivity might be a consequence of the prolonged differences in blood glucose, rather than an underlying cause. To test the possibility of preexisting differences in insulin sensitivity, we have assessed insulin sensitivity before (day 0), near onset of (day 14), and late after (day 48) the blood glucose separation following glibenclamide treatment in outbred cohorts (Fig. 2A). It is extremely important to note that, before blood glucose separation, we could not predict which phenotype (remitter or nonremitter) KATP-GOF mice would exhibit; therefore, we conducted tests in cohorts of mice that were later regrouped as remitters or nonremitters according to the subsequent phenotype. Because we cannot prospectively group the mice, all studies done before glucose separation were survivable procedures.
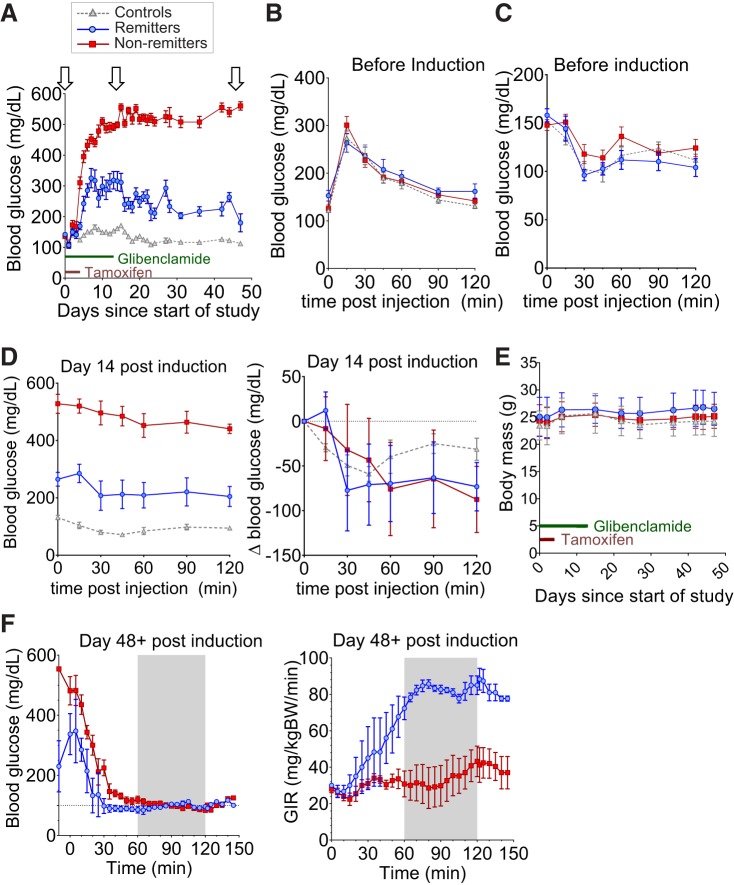
Fig. 2.
Insulin sensitivity does not solely drive remission. A: blood glucose curve for remitter (blue, n = 9), nonremitter (red, n = 15), and control (gray, n = 14) mice. Arrows indicate points at which glucose tolerance tests (GTTs), insulin tolerance tests (ITTs), or clamps were performed. All animals are 9–12 wk old at the time of induction. B and C: ip glucose tolerance test (IP-GTT, B) and ip insulin tolerance test (IP-ITT, C) performed on remitters (blue, n = 8), nonremitters (red, n = 10), and controls (gray, n = 14) before tamoxifen induction of transgene expression. D: raw blood glucose curve for IP-ITT performed at day 14 postinduction (left) and ITT glucose curves normalized for basal glucose level at day 14 postinduction (right). E: body mass (g) curves do not show significant differences between groups in the early induction phase (days 1–14) for any animal set. Data are means ± SD. F: hyperinsulinemic-euglycemic clamps at day 48 posttamoxifen induction; left, blood glucose levels; right, glucose infusion rate (GIR) during the clamp. The clamps were performed on 3 remitters (blue) and 5 nonremitters (red) of the original cohort in A.
Glucose tolerance (Fig. 2B) and insulin tolerance (Fig. 2C) tests at baseline (day 0) demonstrated no significant differences between subsequent remitting and nonremitting and control mice. Blood glucose levels during insulin tolerance tests at day 14 demonstrate that remitters are more insulin sensitive than nonremitters (Fig. 2D, left) but, when normalized to the very different baseline glucose between groups, these differences disappear (Fig. 2D, right). Body weights also do not differ significantly between groups in the early disease phase (Fig. 2E). At day 48, hyperinsulinemic-euglycemic clamp tests (Fig. 2F) demonstrate that, over the period when blood glucose levels were matched (Fig. 2F, left), the glucose infusion rate was much higher in remitters than in nonremitters (Fig. 2F, right), confirming that remitters are significantly more sensitive to insulin than nonremitters, but only long after diabetes induction (35).
Because early differences in basal glucose levels confound the interpretation of standard insulin tolerance tests, we sought an assay for insulin sensitivity that did not measure glucose. During fasting, adipose tissue triglycerides (TG) are hydrolyzed into free fatty acids (FFA) and glycerol, for oxidation and gluconeogenesis in the liver (41, 42). Plasma FFA and TG will increase in conditions of insulin resistance; therefore, we measured plasma lipids at baseline (day 0), day 5 (1 day after tamoxifen ends), and day 14 (the day after glibenclamide treatment ends). Although blood glucose already differs significantly between the groups at day 5 (Fig. 3A), differences in FFA, TG, and cholesteryl esters (Fig. 3, B, C, and D) appear only at day 14. As expected, mice treated with vehicle alone show a more rapid rise in blood glucose upon tamoxifen induction (>600 mg/dl by day 5; Fig. 3A), accompanied by an increase in FFA (>2.5 mM; Fig. 3B), compared with glibenclamide-treated mice. Together, these results suggest that the differences in insulin sensitivity between remitters and nonremitters are the consequence, rather than the cause, of the differential outcome in these animals.
Other glucoregulatory hormones do not differ between remitter and nonremitter animals.
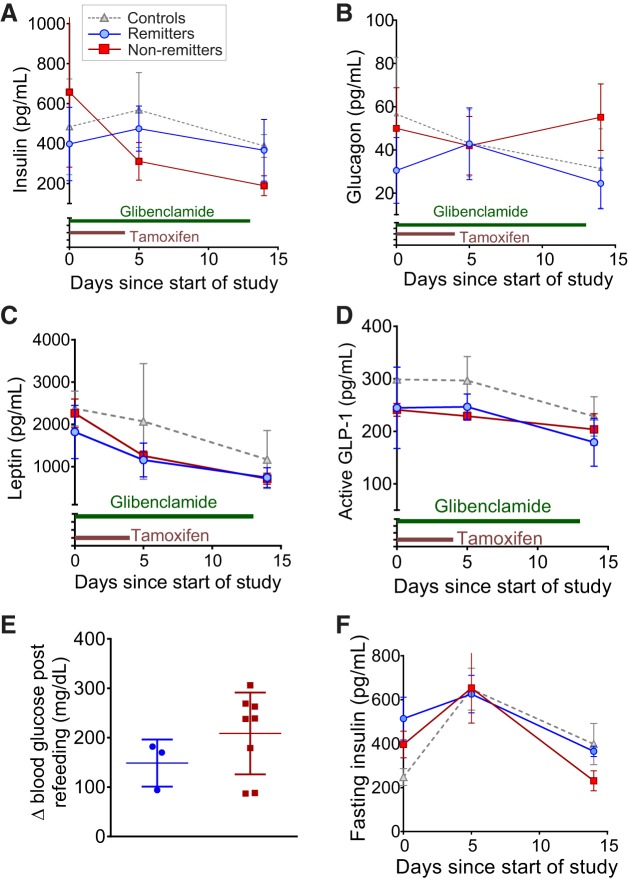
Randomly sampled insulin levels (Fig. 4A) were not significantly different between groups at day 0, and insulin sampled during the inactive light period (as well fasted insulin, Fig. 4F) remained similar across the early phase of disease (days 5 and 14) in controls and subsequent remitters and nonremitters, as previously observed using the 6-day glibenclamide protocol (35). One possible explanation for the emergence of the remitter phenotype is the involvement of other hormones that control gluconeogenesis, glucose uptake, and baseline metabolism. Glucagon is a key regulator of gluconeogenesis, and lowering of glucagon or antagonism of its receptor is sufficient to prevent diabetes in animal models of insulin insufficiency, showing promise as a potential therapy in early clinical trials (2, 52). Additionally, leptin produced by adipose tissue can alter activity, feeding behavior, energy expenditure, and insulin action, thereby affecting glucose tolerance (28). The incretin glucagon-like peptide-1 (GLP-1) can potentiate insulin secretion and regulate metabolic state in many tissues, including the brain (24). We tested the levels of these hormones in samples taken from subsequent remitter and nonremitter animals on days 0, 5, and 14 post-tamoxifen induction. Glucagon, leptin, and active GLP-1 plasma levels were not significantly different between groups (Fig. 4, B–D), indicating that differential counterregulatory or modulatory hormone levels are not inducing the differential disease outcome. These data are consistent with an absence of significant changes in body weight (Fig. 2E) during the early phase (days 0–14) of the study, suggesting that major fluctuations in body composition are also unlikely to underlie the differential phenotype. Additionally, there were no differences in food intake, energy expenditure, movement, respiratory exchange ratio, or oxygen consumption between groups at day 16 after initiation of the study (data not shown).
Fig. 4.
Changes in plasma metabolic hormones during induction process. Randomly sampled plasma at days 0, 5, and 14 posttamoxifen induction was analyzed to determine insulin (A), glucagon (B), leptin (C), and active glucagon-like peptide-1 (GLP-1, D). All animals were 9–12 wk old at the time of induction. n = 8 Remitters (blue), 9 nonremitters (red), and 7 controls for A; 21 remitters (blue), 24 nonremitters (red), and 15 controls (gray) for B; and 3 remitters (blue), 4 nonremitters (red), and 4 controls (gray) for C and D. E: change in blood glucose resulting from 1 h of ad libitum refeeding in ATP-sensitive potassium (KATP)-gain-of-function (GOF) mice following an overnight fast at day 6 after the start of induction. Animals are 9–12 wk old at the time of induction (n = 2 remitters and 6 nonremitters). F: fasting plasma insulin in 9- to 12-wk-old subsequent remitter (n = 4, blue), nonremitter (n = 3, red), and control (n = 4, gray) mice. Data are means ± SD.
Systemic inflammation in diabetes remission.
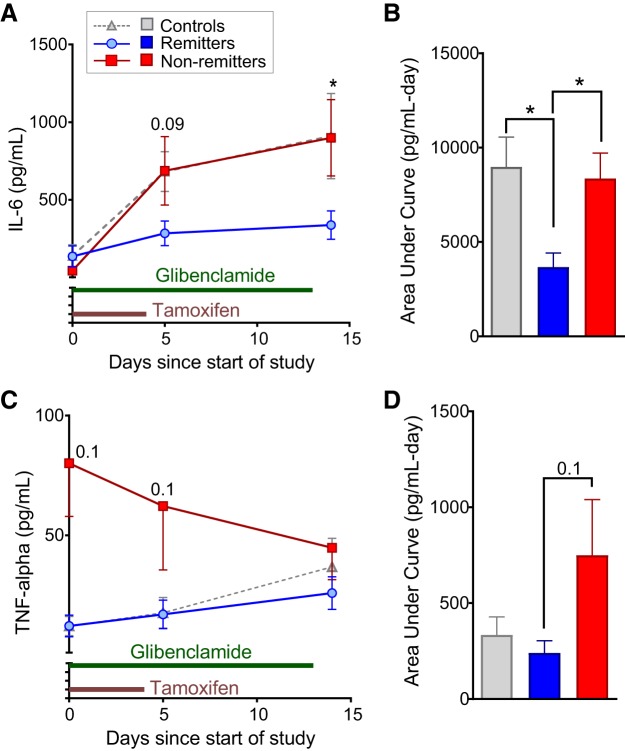
Systemic inflammation [such as elevated tumor necrosis factor-α (TNF-α) and IL-6] is known to affect islet function, insulin sensitivity, and liver glucose production and is associated with an increased risk of type 2 diabetes (3, 6, 34, 47). Notably, IL-6 was markedly lower at day 5 posttamoxifen induction in remitter mice compared with nonremitter and control mice (Fig. 5, A and B), a difference that persisted at day 14. The differences in IL-6 between the groups temporally correlated with the changes in plasma glucose, consistent with a potentially causal role in the separation of blood glucose between remitter and nonremitter animals. Nonremitter mice also showed higher levels of TNF-α at day 0, which remained higher at day 5, compared with remitter and control mice (Fig. 5, C and D). Importantly, KATP-GOF mice treated with vehicle instead of glibenclamide show rises in blood glucose and IL-6, similar to nonremitting animals, again pointing to inflammation as a cause of blood glucose separation.
Fig. 5.
Changes in inflammation correlate with separation in glucose. Randomly sampled plasma at days 0, 5, and 14 posttamoxifen induction was analyzed to determine tumor necrosis factor-α (TNF-α, A and B) and interleukin-6 (IL-6, C and D) in remitters (n = 15, blue), nonremitters (n = 19, red), and controls (n = 17, gray). Raw concentration traces are shown in A and C for IL-6 and TNF-α, respectively. Area under the curve (AUC) analyses for IL-6 and TNF-α are shown in B and D, respectively. Specific P values for comparisons between remitters and nonremitters are indicated at the time points on the curve. *P < 0.05, significant differences for posttest following ANOVA. All animals were 9–12 wk old at the time of induction.
Differential glucose production between remitters and nonremitters.
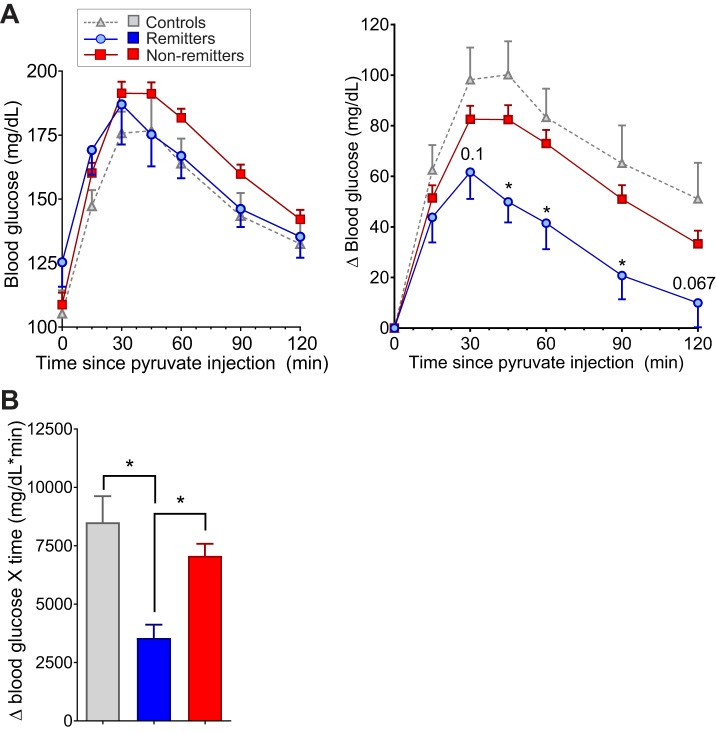
Because insulin-driven glucose disposal is not significantly different between the groups early in disease progression, another possible mechanism for divergence of blood glucose between the groups is basal glucose production by the liver. Animals with uncontrolled liver glucose production become hyperglycemic even though basal and postprandial insulin are significantly elevated (26). Therapies that target basal glucose production can significantly improve diabetic complications in humans and animals and even eliminate diabetes in some animal models (39, 52). To determine whether basal glucose production might differ between remitter and nonremitter animals, mice were given an intraperitoneal injection of sodium pyruvate, which is converted in the liver to glucose via gluconeogenesis. The change in glucose (Fig. 6, A and B) produced by the pyruvate injection was significantly reduced in the remitting animals, indicating an intrinsically lower basal rate of glucose production by the liver in remitting mice than in nonremitter and control mice.
Fig. 6.
Basal glucose production before separation in glucose. A: raw glucose traces (left) and change in blood glucose levels (right) following ip injection of sodium pyruvate in remitter (n = 14, blue), nonremitter (n = 70, red), and control (n = 10, gray) animals before transgene induction. Differences at indicated time points by Student’s t-test between remitter and nonremitter animals are indicated. B: area under the curve (AUC) analyses of the change in blood glucose levels in the ip pyruvate tolerance test (IP-PTT) data in A. Significance was evaluated by ANOVA with Tukey’s posttests. Significant differences: *P < 0.05. All animals were 9–12 wk old at the time of induction.
Anti-inflammatory treatment with meloxicam induces diabetes remission.
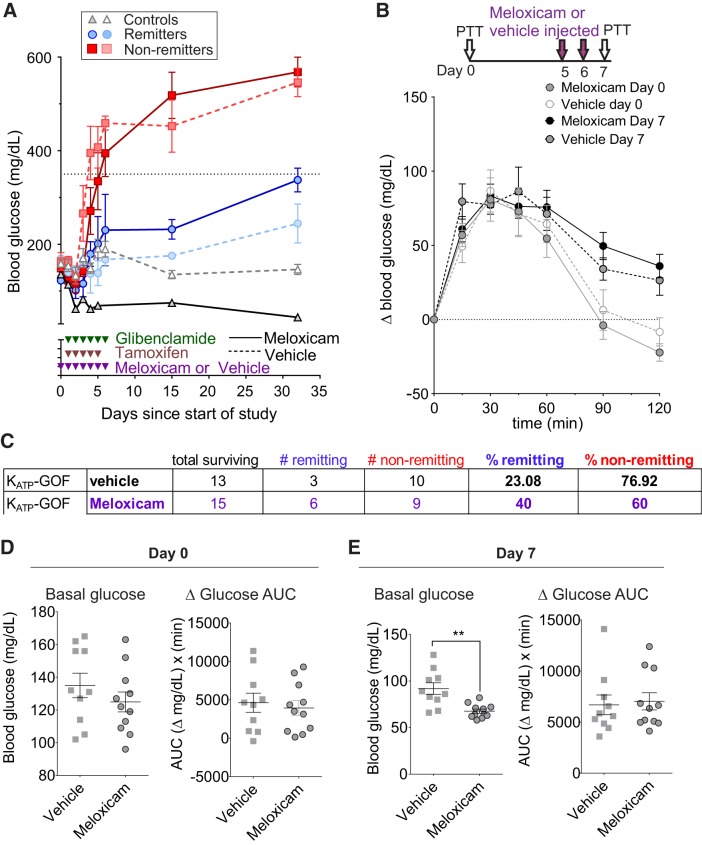
To further examine the role of inflammation on diabetes outcome, we treated mice with high levels of the anti-inflammatory agent meloxicam, hypothesizing that lowering inflammation in vivo might induce more animals to remit. The dose used (20 mg/g body wt) has been used in other studies of inflammation in mice (1, 16, 18, 40) and, although at such a dose meloxicam may have toxic effects in addition to its anti-inflammatory action, there were deaths in both meloxicam (9/24)- and vehicle (4/17)-treated mice, suggesting that death was primarily a consequence of multiple injection treatments rather than drug-specific toxicity. Importantly, in the surviving animals, the percentage of remitting animals almost doubled, from 23% in vehicle-treated to 40% in meloxicam-treated mice (Fig. 7, A and C).
Fig. 7.
Meloxicam treatment increase the no. of remitting animals. A: blood glucose over time on ATP-sensitive potassium (KATP)-gain-of-function (GOF) mice treated with meloxicam or vehicle. Red, nonremitters; blue, remitters; broken lines, vehicle-treated mice; solid lines, meloxicam-treated mice. B: pyruvate tolerance test (PTT) performed at days 0 and 7 after meloxicam treatment. C: table showing %remitter and nonremitter mice after meloxicam or vehicle treatment. D and E: blood glucose values and area under the curve during the PTT at day 0 (D) or 7 (E) after meloxicam or vehicle treatment. n = 17 KATP-GOF mice for vehicle and 24 for meloxicam. Significant differences: ** P < 0.01.
Finally, to examine potential interplay of hepatic glucose production and inflammation, we performed pyruvate tolerance tests in mice before (day 0) and at day 7 after meloxicam or vehicle treatment. Although meloxicam lowers fasting blood glucose, the area under the curve for the test was not significantly different between vehicle- and meloxicam-treated animals (Fig. 7, B and D). This is consistent with meloxicam keeping blood glucose low by reducing the glucose-mobilizing consequences of inflammation while having no off-target effects on liver glucose production directly.
DISCUSSION
Progressive consequences of KATP-GOF-induced diabetes.
Introduction of KATP channel GOF in pancreatic β-cells leads to loss of glucose-dependent excitation and insulin secretion, and therefore development of diabetes (9, 20, 37), mimicking human NDM. In addition to the immediate loss of glucose-dependent insulin secretion, we have demonstrated loss of insulin content and β-cell mass as disease progresses (37, 53). Loss of β-cell mass is promoted by loss of β-cell identity and dedifferentiation to islet-like progenitor cells (53), rather than β-cell death, as frequently assumed. Similar outcomes have been reported as secondary consequences of diabetes in other rodent models (4, 22, 48). Strikingly, these features can be prevented by lowering of blood glucose with SU therapy, syngenic islet transplantation, or with caloric restriction by pair feeding (17, 35, 37, 53), and even reversed by intensive insulin therapy (4, 53). Importantly, loss of islet β-cell identity has been demonstrated in pancreas from human diabetic organ donors (5, 13, 15), and even dedifferentiation of β-cells has been observed in some cases (5).
Early treatment and precise glucose control can prevent progressive loss of islet function in NDM.
KATP-dependent NDM can present as a permanent form, or transient form in which diabetes in infancy is followed by an unexplained remission that may or may not revert to frank diabetes in maturity (21, 32). Our previous studies highlight the significance of early intervention in both mice and human NDM: tight glucose control, provided by pharmacological inhibition of KATP, can lead to long-term remission (25, 35, 51). We also demonstrated that maintenance of blood glucose below an apparent threshold around 350 mg/dl is sufficient to maintain insulin content and to preserve markers of differentiated β-cell fate in KATP-GOF mouse islets (37, 53). Although the islets expressing the KATP-GOF themselves remain glucose unresponsive, basal levels of plasma insulin, as well as responsivity to SU stimulation, are maintained (37, 53). Our studies here show that remitting animals retain a high sensitivity to insulin, whereas nonremitting animals rapidly become insulin resistant. Avoidance of severe hyperglycemia and loss of insulin content, and hence prevention of glucotoxic insulin resistance, in KATP-GOF mice allow maintenance of islet responses to pharmacological secretagogues, consistent with our previous findings (35, 37). Together, these findings suggest that interventions early in the disease progression will maintain SU-responsive islets in NDM and thereby lessen dose requirements for SU or insulin therapies.
Role of inflammation in insulin resistance and disease progression.
We show here that remitter animals have reduced circulating IL-6 and strong trends toward reduced systemic TNF-α compared with nonremitters. The differences in IL-6 between remitters and nonremitters appear at the onset of disease, suggesting that the initial level of inflammation may play a role in the divergence of blood glucose. These results are supported by the increase in the percentage of remitting animals after treatment with the anti-inflammatory drug meloxicam. Increased tissue inflammation is found in prediabetic obese and diabetic individuals, and elevated systemic inflammation has been associated with increased risk of developing type 2 diabetes in a number of studies (3, 6, 12, 19). Inflammation influences a number of different tissues, including fat, muscle, liver, brain, and islets (6, 19). Elevated FFA, endoplasmic reticulum stress, and increased reactive oxygen species are some of the factors present in hyperglycemic insulin-resistant or insulin-deficient states that can trigger activation of the inflammasome (via NOD-, LRR-, and pyrin domain-containing 3 NLRP3 or caspase 1), eventually resulting in elevation of TNF-α, IL-6, and other cytokines (6). Elevated cytokines in turn can recruit additional immune cells to tissues and cause resident macrophages to adopt a more proinflammatory phenotype. TNF-α signaling has been suggested to cause insulin resistance that accelerates the development of type 2 diabetes (3, 23), and inhibition of TNF-α signaling can prevent or reverse insulin resistance in animals (3) and reduce risk of type 2 diabetes in humans (3, 6). The reported relationship between IL-6 levels and glucose metabolism is conflicting. Acute elevations of IL-6 (such as in exercise) are associated with increased glucose metabolism in muscle in animals (12). However, this seems dependent on increased energy expenditure (exercise), since IL-6 infusions in the absence of exercise are associated with increased muscle accumulation of fatty acyl-CoA, suggesting that chronic IL-6 may impair muscle function (12). Chronic elevations in IL-6 are associated with increased insulin resistance (19), and extreme elevations in IL-6 in some animal models can induce diabetic phenotypes, effects that can be reversed with anti-IL-6 antibodies. This mirrors findings in human patients taking IL-6 antagonists, who show improved metabolic parameters, including significantly reduced HOMA-IR (44).
Glucose production and inflammation in NDM outcomes.
Retrospective analysis reveals that KATP-GOF mice which remit after early SU treatment exhibit significantly lower basal glucose production rates before disease onset than those mice that do not remit. One possible explanation is that differences in basal glucose production reflect different levels of inflammation, since chronic elevations in cytokines can alter insulin signaling and increase liver glucose production (14, 31, 43).
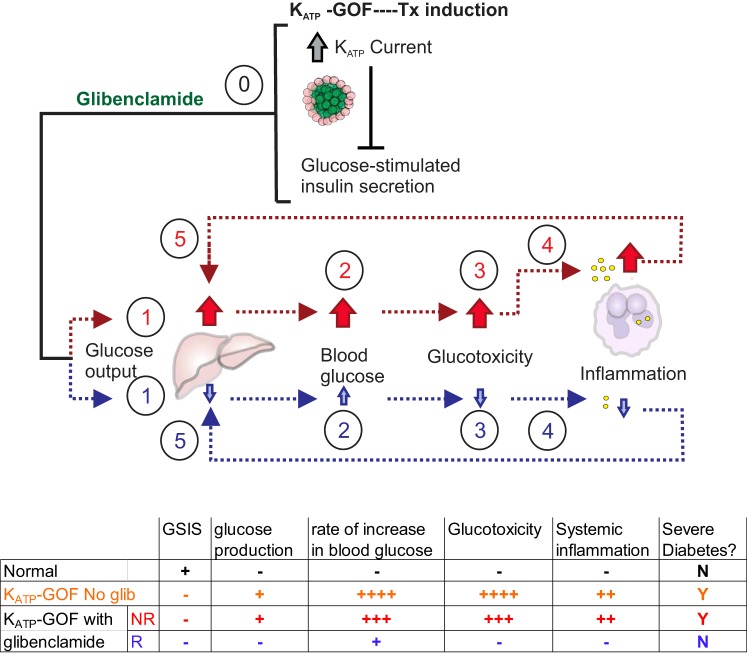
In Fig. 8, we propose a model to explain the differential outcome in KATP-GOF mice in response to early SU treatment. KATP-GOF mutations block insulin secretion in islets of both remitters and nonremitters (0), resulting in a rise in blood glucose in both groups. In the early period of the disease, nonremitting animals have increased basal glucose production which, in the absence of glucose-stimulated insulin secretion, causes a rapid rise in glucose levels. As a result, glucose levels quickly pass a glucotoxic threshold, and glucotoxicity increases systemic inflammation (27, 47). This systemic inflammation drives further hepatic glucose output (31) and β-cell dysfunction (30) in a feedforward cycle that results in persistent severe diabetes. By contrast, in the early period of the disease, remitting animals present with reduced levels of inflammation and decreased basal glucose production. As a consequence, glucose rises more slowly, avoiding the glucotoxic threshold and increased inflammation. The absence of inflammation prevents increases in hepatic glucose output and allows increased sensitivity to basal insulin and thereby avoidance of severe diabetes. In agreement with a causative role for inflammation in the glucotoxic process, anti-inflammatory meloxicam treatment, by reducing inflammation, prevents elevated glucose production and increases the number of remitting animals.
Fig. 8.
Proposed model of remission in ATP-sensitive potassium (KATP)-gain-of-function (GOF) mice. In uninduced KATP-GOF mice, any differences at baseline regarding glucose production and inflammation between subsequent remitter (blue arrows) and nonremitter (red arrows) animals are countered by normal glucose-stimulated insulin secretion, and blood glucose levels are kept in the normal range. In the early phase of induction, KATP-GOF blocks insulin secretion in both remitter and nonremitter mice (0). In nonremitters (red nos. and arrows), increased basal glucose production (1) before induction causes a more rapid increase in glucose (2). This results in early glucotoxicity (3) that then drives increases in systemic inflammation (4). This inflammation then inhibits glucose disposal and enhances hepatic glucose production (5) in a feedforward manner, resulting in persistent severe diabetes. By contrast, in remitter mice (blue arrows and nos.), basal glucose production and inflammation are low (1), and glucose rises more slowly (2), thereby allowing the mice to avoid early glucotoxicity (3), the subsequent increase in inflammation (4), and inflammatory-mediated increases in glucose production (5). Avoidance of the feedforward loop between glucotoxicity, inflammation, and glucose production enables later alterations in sensitivity to basal insulin to compensate for gross insulin deficiency. Broken lines indicate proposed links in the model. A summary table indicating relative changes in each group is indicated on bottom.
Conclusions.
Although the underlying basis of remission after early therapy in experimental NDM remains incompletely understood, our studies clearly demonstrate that early pharmacological intervention in combination with reduced inflammation can induce maintenance of low blood glucose, which can lead to long-term remission. In a clear parallel, we have previously reported a human family in which the mother and older sibling both carrying the same KATP-GOF mutation suffered permanent nonremitting NDM that was, in each case, treated for many years with insulin, but then both achieved subsequent glucose control after switching to high-dose sulfonylureas (25, 51). In striking contrast, a younger sibling, carrying the same mutation, was treated with glibenclamide from the 3rd day of life and immediately achieved tight glucose control, allowing the glibenclamide dose to be markedly decreased and subsequently removed (N. White and B. Marshall, personal communication). A straightforward explanation is that the younger child has entered remission after tight glucose control in early onset diabetes. With similar observations in other reports, the differential outcomes we identify in mice are likely to inform the mechanistic basis of transient vs. permanent outcomes in human NDM. Beyond NDM, the potential for inflammation and glucose production to exacerbate other forms of diabetes, or to “tip the balance” from a compensated to a glucotoxic state, should be considered. Manipulation of the factors that affect insulin sensitivity and insulin-independent pathways of glucose control may provide an alternative approach to lowering blood glucose in individuals with other forms of diabetes for whom insulin secretagogues are not sufficiently effective. Understanding these pathways will therefore not only inform the basic mechanisms regulating metabolism but may also provide novel therapeutic targets for other metabolic disorders.
GRANTS
This work was supported by the following grants from the National Institute of Diabetes and Digestive and Kidney Diseases: R01-DK-098584 to M. S. Remedi, R01-DK-109407 to C. G. Nichols, and T32-DK-108742 (Fellowship to C. H. Emfinger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.H.E., C.G.N., and M.S.R. conceived and designed research; C.H.E., Z.Y., A.W., P.H., W.M., and M.S.R. performed experiments; C.H.E., Z.Y., A.W., P.H., W.M., and M.S.R. analyzed data; C.H.E., Z.Y., W.M., P.W.H., C.G.N., and M.S.R. interpreted results of experiments; C.H.E. and M.S.R. prepared figures; C.H.E. drafted manuscript; C.H.E., Z.Y., A.W., P.H., W.M., P.W.H., C.G.N., and M.S.R. approved final version of manuscript; C.G.N. and M.S.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank the Vanderbilt Hormone Assay Core (http://hormone.mc.vanderbilt.edu/), Andrew M. and Jane M. Burskey Center for Human Immunology and Immunotherapy Programs Immunomonitoring Laboratory (http://chiips.wustl.edu/iml-core-lab.html), and Diabetes Models Phenotyping Core at Washington University in St. Louis (https://diabetesresearchcenter.dom.wustl.edu/diabetes-models-phenotyping-core/) for assistance with specific assays.
REFERENCES
- 1.Arantes-Rodrigues R, Pinto-Leite R, Ferreira R, Neuparth MJ, Pires MJ, Gaivão I, Palmeira C, Santos L, Colaço A, Oliveira P. Meloxicam in the treatment of in vitro and in vivo models of urinary bladder cancer. Biomed Pharmacother 67: 277–284, 2013. doi: 10.1016/j.biopha.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Bagger JI, Knop FK, Holst JJ, Vilsbøll T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab 13: 965–971, 2011. doi: 10.1111/j.1463-1326.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 3.Borst SE. The role of TNF-α in insulin resistance. Endocrine 23: 177–182, 2004. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- 4.Brereton MF, Iberl M, Shimomura K, Zhang Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK, Ramracheya R, Gribble FM, Reimann F, Clark A, Rorsman P, Ashcroft FM. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. 5: 4639, 2014. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, Marselli L, Suleiman M, Ratner LE, Marchetti P, Accili D. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 101: 1044–1054, 2016. doi: 10.1210/jc.2015-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 13: 465–476, 2014. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 7.Ellard S, Flanagan SE, Girard CA, Patch A-M, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 81: 375–382, 2007. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT. Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia 49: 1190–1197, 2006. doi: 10.1007/s00125-006-0246-z. [DOI] [PubMed] [Google Scholar]
- 9.Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, Abdulkader F, Clark A, Ball V, Zubcevic L, Bentley L, Clark R, Church C, Hugill A, Galvanovskis J, Cox R, Rorsman P, Brüning JC, Ashcroft FM. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest 119: 80–90, 2009. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njølstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350: 1838–1849, 2004. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 11.Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJG, Shield JPH, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet 14: 925–934, 2005. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 12.Glund S, Krook A. Role of interleukin-6 signalling in glucose and lipid metabolism. Acta Physiol (Oxf) 192: 37–48, 2008. doi: 10.1111/j.1748-1716.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- 13.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 123: 3305–3316, 2013. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 15.Hunter CS, Stein RW. Evidence for loss in identity, de-differentiation, and trans-differentiation of islet β-cells in type 2 diabetes. Front Genet 8: 35, 2017. doi: 10.3389/fgene.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingrao JC, Johnson R, Tor E, Gu Y, Litman M, Turner PV. Aqueous stability and oral pharmacokinetics of meloxicam and carprofen in male C57BL/6 mice. J Am Assoc Lab Anim Sci 52: 553–559, 2013. [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida E, Kim-Muller JY, Accili D. Pair-feeding, but not insulin, phloridzin, or rosiglitazone treatment curtails markers of beta-cell dedifferentiation in db/db mice. Diabetes 66: 2092–2101, 2017. doi: 10.2337/db16-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Li H, Qiao H, Jiang H, Xu R, Sun X. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci 100: 2226–2233, 2009. doi: 10.1111/j.1349-7006.2009.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm 80: 613–633, 2009. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 20.Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 100: 645–654, 2000. doi: 10.1016/S0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 21.Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 54: 2645–2654, 2005. doi: 10.2337/diabetes.54.9.2645. [DOI] [PubMed] [Google Scholar]
- 22.Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. Critical reduction in β-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem 278: 2997–3005, 2003. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci 86, Suppl 14: E94–E104, 2008. doi: 10.2527/jas.2007-0462. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 51, Suppl 3: S434–S442, 2002. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 25.Marshall BA, Green RP, Wambach J, White NH, Remedi MS, Nichols CG. Remission of severe neonatal diabetes with very early sulfonylurea treatment. Diabetes Care 38: e38–e39, 2015. doi: 10.2337/dc14-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6: 87–97, 2000. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 27.Montane J, Cadavez L, Novials A. Stress and the inflammatory process: a major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab Syndr Obes 7: 25–34, 2014. doi: 10.2147/DMSO.S37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton GJ, Schwartz MW. Leptin and the CNS control of glucose metabolism. Physiol Rev 91: 389–411, 2011. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 30.Nordmann TM, Dror E, Schulze F, Traub S, Berishvili E, Barbieux C, Böni-Schnetzler M, Donath MY. The role of inflammation in β-cell dedifferentiation. Sci Rep 7: 6285, 2017. doi: 10.1038/s41598-017-06731-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okin D, Medzhitov R. The effect of sustained inflammation on hepatic mevalonate pathway results in hyperglycemia. Cell 165: 343–356, 2016. doi: 10.1016/j.cell.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patch AM, Flanagan SE, Boustred C, Hattersley AT, Ellard S. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab 9, Suppl 2: 28–39, 2007. doi: 10.1111/j.1463-1326.2007.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson ER, Flechtner I, Njølstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Søvik O, Polak M, Hattersley AT; Neonatal Diabetes International Collaborative Group . Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355: 467–477, 2006. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 34.Pitsavos C, Tampourlou M, Panagiotakos DB, Skoumas Y, Chrysohoou C, Nomikos T, Stefanadis C. Association between low-grade systemic inflammation and type 2 diabetes mellitus among men and women from the ATTICA study. Rev Diabet Stud 4: 98–104, 2007. doi: 10.1900/RDS.2007.4.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remedi MS, Agapova SE, Vyas AK, Hruz PW, Nichols CG. Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes. Diabetes 60: 2515–2522, 2011. doi: 10.2337/db11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remedi MS, Koster JC. K(ATP) channelopathies in the pancreas. Pflugers Arch 460: 307–320, 2010. doi: 10.1007/s00424-009-0756-x. [DOI] [PubMed] [Google Scholar]
- 37.Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, Tong A, Brüning JC, Koster JC, Nichols CG. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab 9: 140–151, 2009. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet 116: 133–145, 2005. doi: 10.1007/s00439-004-1216-5. [DOI] [PubMed] [Google Scholar]
- 39.Rines AK, Sharabi K, Tavares CDJ, Puigserver P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat Rev Drug Discov 15: 786–804, 2016. doi: 10.1038/nrd.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roughan JV, Bertrand HGMJ, Isles HM. Meloxicam prevents COX-2-mediated post-surgical inflammation but not pain following laparotomy in mice. Eur J Pain 20: 231–240, 2016. doi: 10.1002/ejp.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806, 2001. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 42.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87: 507–520, 2007. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol 64: 1403–1415, 2016. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, Laudes M. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One 5: e14328, 2010. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimomura K, de Nanclares GP, Foutinou C, Caimari M, Castaño L, Ashcroft FM. The first clinical case of a mutation at residue K185 of Kir6.2 (KCNJ11): a major ATP-binding residue. Diabet Med 27: 225–229, 2010. doi: 10.1111/j.1464-5491.2009.02901.x. [DOI] [PubMed] [Google Scholar]
- 46.Shimomura K, Girard CAJ, Proks P, Nazim J, Lippiat JD, Cerutti F, Lorini R, Ellard S, Hattersley AT, Barbetti F, Ashcroft FM. Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects. Diabetes 55: 1705–1712, 2006. doi: 10.2337/db05-1640. [DOI] [PubMed] [Google Scholar]
- 47.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150: 1223–1234, 2012. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaxillaire M, Dechaume A, Busiah K, Cavé H, Pereira S, Scharfmann R, de Nanclares GP, Castano L, Froguel P, Polak M; SUR1-Neonatal Diabetes Study Group . New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes 56: 1737–1741, 2007. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- 50.Villareal DT, Koster JC, Robertson H, Akrouh A, Miyake K, Bell GI, Patterson BW, Nichols CG, Polonsky KS. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes 58: 1869–1878, 2009. doi: 10.2337/db09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes 11: 286–288, 2010. doi: 10.1111/j.1399-5448.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M-Y, Yan H, Shi Z, Evans MR, Yu X, Lee Y, Chen S, Williams A, Philippe J, Roth MG, Unger RH. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci USA 112: 2503–2508, 2015. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 19: 872–882, 2014. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C-L, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325: 607–610, 2009. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]