Abstract
Little is known about vascular mitochondrial respiratory function and the impact of age. Therefore, skeletal muscle feed arteries were harvested from young (33 ± 7 yr, n = 10), middle-aged (54 ± 5 yr, n = 10), and old (70 ± 7 yr, n = 10) subjects, and mitochondrial respiration as well as citrate synthase (CS) activity were assessed. Complex I (CI) and complex I + II (CI+II) state 3 respiration were greater in young (CI: 10.4 ± 0.8 pmol·s−1·mg−1 and CI+II: 12.4 ± 0.8 pmol·s−1·mg−1, P < 0.05) than middle-aged (CI: 7 ± 0.6 pmol·s−1·mg−1 and CI+II: 8.3 ± 0.5 pmol·s−1·mg−1) and old (CI: 7.2 ± 0.4 pmol·s−1·mg−1 and CI+II: 7.6 ± 0.5 pmol·s−1·mg−1) subjects and, as in the case of complex II (CII) state 3 respiration, were inversely correlated with age [r = −0.56 (CI), r = −0.7 (CI+II), and r = 0.4 (CII), P < 0.05]. In contrast, state 4 respiration and mitochondria-specific superoxide levels were not different across groups. The respiratory control ratio was greater in young (2.2 ± 0.2, P < 0.05) than middle-aged and old (1.4 ± 0.1 and 1.1 ± 0.1, respectively) subjects and inversely correlated with age (r = −0.71, P < 0.05). As CS activity was inversely correlated with age (r = −0.54, P < 0.05), when normalized for mitochondrial content, the age-related differences and relationships with state 3 respiration were ablated. In contrast, mitochondrion-specific state 4 respiration was now lower in young (15 ± 1.4 pmol·s−1·mg−1·U CS−1, P < 0.05) than middle-aged and old (23.4 ± 3.6 and 27.9 ± 3.4 pmol·s−1·mg−1·U CS−1, respectively) subjects and correlated with age (r = 0.46, P < 0.05). Similarly, superoxide/CS levels were lower in young (0.07 ± 0.01) than old (0.19 ± 0.41) subjects and correlated with age (r = 0.44, P < 0.05). Therefore, with aging, vascular mitochondrial respiratory function declines, predominantly as a consequence of falling mitochondrial content. However, per mitochondrion, aging likely results in greater mitochondrion-derived oxidative stress, which may contribute to age-related vascular dysfunction.
NEW & NOTEWORTHY This study determined, for the first time, that vascular mitochondrial oxidative respiratory capacity, oxidative coupling efficiency, and mitochondrial content fell progressively with advancing age. In terms of single mitochondrion-specific respiration, the age-related differences were completely ablated and the likelihood of free radical production increased progressively with advancing age. This study reveals that vascular mitochondrial respiratory capacity declines with advancing age, as a consequence of falling mitochondrial content, as does oxidative coupling efficiency.
Keywords: mitochondrial content, proton leak, respiratory control ratio, skeletal muscle feed arteries
INTRODUCTION
Aging is recognized as a potent independent predictor of cardiovascular risk and is associated with structural and functional vascular changes, such as progressive arterial stiffening, atherosclerotic plaque, and endothelial dysfunction (4, 18, 19, 35). One of the foremost consequences of such age-related vascular alterations is decreased vascular conductance to the periphery, specifically to skeletal muscle (6, 31). This diminished capacity to increase vascular conductance results in impaired blood flow and O2 delivery to skeletal muscle with advancing age (6, 13, 18, 31). Indeed, recently, it was reported that skeletal muscle feed arteries (SMFAs), the vascular inlet to skeletal muscle, which are recognized to have regulatory potential (16), are negatively impacted by advancing age (1, 16, 26). However, the precise mechanism underlying SMFA dysfunction with advancing age is not well understood.
Although the exact role of vascular mitochondria is not clear, it is likely that mitochondrial respiratory function has an impact on vessel function. Interestingly, mitochondria from cardiac, skeletal, and smooth muscle have recently been documented to have equivalent respiration rates per mitochondrion (25). However, it was also determined that mitochondria from these three different types of muscle exhibit intrinsic differences in mitochondrial respiratory function, which likely influence the efficiency of oxidative phosphorylation and could, potentially, alter free radical production (25). The respiratory control ratio (RCR) was lower and proton leak was higher per mitochondrion in the vasculature, harvested from SMFAs, than in skeletal and cardiac muscle mitochondria in this prior study, signifying attenuated oxidative coupling efficiency and, likely, augmented free radical production. In terms of aging, it has been documented that, in cardiac and skeletal muscle, tissue with relatively high metabolic demand, RCR declines with advancing age (2, 32). However, little is known about age-related changes in vascular mitochondrial respiratory function and the aging process.
Therefore, this study sought to determine the effect of advancing age on vascular mitochondrial respiratory function. Specifically, we harvested SMFAs from young, middle-aged, and old subjects and comprehensively assessed mitochondrial respiration in these vessels. We tested the hypothesis that mitochondrial respiratory function would be progressively attenuated across the young, middle-aged, and old groups. Furthermore, we examined the relationships between the measured indexes of respiratory function and age, with the expectation that the hypothesized decline in respiratory function would be linearly related to age.
METHODS
Subjects.
A heterogeneous group of 30 subjects (16 men and 14 women) was studied in the main mitochondria-focused investigation; 16 additional subjects (10 men and 6 women) were studied in the preliminary investigation, which included both mitochondria and vessel function assessments. All subjects agreed to have their vessels harvested during surgery and used in this study (Table 1). None of the subjects was taking medications recognized to alter mitochondrial function, and all subjects were predominantly free from overt cardiovascular disease (Table 1). All protocols were approved by the Institutional Review Boards of the University of Utah and Salt Lake City Veterans Affairs Medical Center, and written informed consent was obtained from all subjects before surgery.
Table 1.
Subject characteristics
| Main Investigation (Mitochondrial Function) |
Preliminary Investigation (Mitochondrial and Vascular Function) |
|||||
|---|---|---|---|---|---|---|
| Young | Middle-aged | Old | Young | Middle-aged | Old | |
| n | 10 | 10 | 10 | 5 | 2 | 5 |
| Age, yr | 33 ± 7* | 54 ± 5† | 70 ± 7 | 30 ± 6* | 53 ± 7 | 63 ± 4 |
| Sex (men/women), n | 3/7 | 6/4 | 7/3 | 3/2 | 2/0 | 3/2 |
| Height, cm | 171 ± 10 | 173 ± 8 | 170 ± 8 | 168 ± 9 | 178 ± 8 | 172 ± 4 |
| Body mass, kg | 81 ± 22 | 94 ± 21 | 87 ± 22 | 89 ± 14 | 85 ± 1 | 83 ± 13 |
| Body mass index, kg/m2 | 28 ± 7 | 31 ± 7 | 30 ± 7 | 30 ± 4 | 26 ± 1 | 27 ± 3 |
| Systolic blood pressure, mmHg | 116 ± 11 | 126 ± 13 | 134 ± 21 | 132 ± 12 | 131 ± 5 | 132 ± 14 |
| Diastolic blood pressure, mmHg | 70 ± 16 | 76 ± 10 | 75 ± 10 | 81 ± 12 | 87 ± 9 | 78 ± 9 |
| Medication history | ||||||
| Cardiovascular | ||||||
| Statin | 1/10 | 2/10 | 0/10 | |||
| Cholesterol medication | 1/10 | 1/10 | 0/10 | 0/5 | 0/2 | 1/5 |
| Anticoagulant | 0/10 | 0/10 | 0/10 | 0/5 | 0/2 | 1/5 |
| Angiotensin-converting enzyme inhibitor | 0/10 | 0/10 | 0/10 | |||
| Metabolic | ||||||
| Antidiabetes medication | 0/10 | 0/10 | 3/10 | |||
| Analgesic | ||||||
| Nonsteroidal anti-inflammatory drug | 3/10 | 2/10 | 3/10 | 0/5 | 0/2 | 2/5 |
| Hormonal | ||||||
| Melatonin | 0/10 | 0/10 | 2/10 | 0/5 | 0/2 | 1/5 |
| Thyroid | 0/10 | 1/10 | 1/10 | |||
Values are means ± SD; n, number of subjects.
Significant difference, young vs. middle-aged and old groups;
significant difference, middle-aged vs. old groups.
Skeletal muscle feed arteries.
Vessel samples were harvested during surgery, as previously reported (14, 15). Subjects were anesthetized using a standard protocol that included propofol, fentanyl, benzodiazepines, and succinylcholine, all delivered intravenously. Human SMFAs (~7 mg/wet wt) supplying the axillary (e.g., serratus anterior or latissimus dorsi) and inguinal (e.g., quadriceps femoris or hip adductors) regions were obtained during melanoma-related node dissection surgery. The vessels were excised and immediately stored in precooled physiological saline solution and brought to the laboratory for analysis within 15 min of being harvested.
Vessel permeabilization and mitochondrial respiration.
Vessel samples were prepared and permeabilized for mitochondrial respiration analysis, as previously described by Park et al. (25). Briefly, adipose and connective tissues around the SMFAs were removed under a microscope (model SZX10, Olympus, Center Valley, PA) and placed in 4°C physiological salt solution [containing (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 3.0 MOPS with 10 g/l BSA, pH 7.4). Vessels were trimmed, weighed to minimize variation in sample mass, and then stored in ice-cold biopsy preservation fluid [BIOPS; containing (in mM) 2.77 CaK2EGTA, 7.23 K2EGTA, 6.56 MgCl2, 0.5 DTT, 50 K-MES, 20 imidazole, 20 taurine, 5.77 Na2ATP, and 15 phosphocreatine, pH 7.1 at 4°C] for 30 min before the permeabilization procedure (17). BIOPS-immersed vessels were carefully separated with fine-tip forceps and subsequently bathed and shaken in BIOPS-saponin solution (50 mg/ml) for 40 min. After saponin treatment, vessels were rinsed twice in MIR05 solution [containing (in mM) 2.77 CaK2EGTA, 7.23 K2EGTA, 6.56 MgCl2, 0.5 DTT, 20 imidazole, 5.77 ATP, 15 phosphocreatine, 50 K-MES, and 20 taurine, pH 7.0] for 20 min.
Vessel samples were placed in a temperature-controlled Clark-type high-resolution Oxygraph respirometer (Hansatech, Kings Lynn, UK) in 2 ml of MIR05 solution and continuously stirred at 37°C. After the vessel samples were allowed to equilibrate for10 min, mitochondrial respiratory function was assessed using the protocol shown in Table 2. To measure the function of each mitochondrial complex, O2 consumption was assessed with the addition of a series of respiratory substrates and inhibitors in the following order and final concentrations in the chamber: 2 mM glutamate-10 mM malate, 5 mM ADP, 10 mM succinate, 0.5 μM rotenone, 10 μM cytochrome c, 2.5 μM antimycin A, and 2 g/ml oligomycin. This allowed determination of 1) complex I (CI) state 3 respiration, the ADP-activated state of oxidative phosphorylation, assessed in the presence of glutamate, malate, and ADP, 2) complex I + II (CI+II) state 3 respiration, assessed in the presence of glutamate, malate, ADP, and succinate, 3) complex II (CII) state 3 respiration, assessed in the presence of glutamate, malate, ADP, succinate, and rotenone, 4) mitochondrial membrane integrity, assessed in the presence of cytochrome c, and 5) state 4 respiration, assessed by blocking ATP synthase (oligomycin). After the respiration assessments, each vessel sample assessed for respiratory function was weighed and then frozen; these vessel weights were used for the normalization of respiration rate.
Table 2.
Mitochondrial respiration protocol
| Step Number | Chemical | Site of Action | Respiration State |
|---|---|---|---|
| 1 | Malate (2 mM)-glutamate (10 mM) | + Complex I | Complex I state 3 |
| ADP (5 mM) | + Complex V | ||
| 2 | Succinate (10 mM) | + Complex II | Complex I + II state 3 |
| 3 | Rotenone (0.5 μM) | − Complex I | Complex II state 3 |
| 4 | Cytochrome c (10 μM) | Test of mitochondrial membrane integrity | |
| 5 | Oligomycin (2 g/ml) | − Complex V | State 4 |
Shown is a description of the protocol used to assess mitochondrial respiratory function, the site of action of each chemical introduced to the preparation [substrate (+) or inhibitor (−)], and respiration state associated with each step. The duration of each step was ~3 min.
In each condition, the respiration rate was recorded for 3 min. The rate of O2 consumption was measured as picomoles of O2 per second and then expressed relative to vessel sample mass (pmol·s−1·mg wet wt−1). These indexes of respiration, except RCR (state 3-to-state 4 ratio), were further normalized for citrate synthase (CS) activity to obtain mitochondrion-specific respiration. Because RCR is a ratio, normalization for CS activity would not alter the interpretation of changes in this variable.
Mitochondria-specific superoxide measurements.
Frozen SMFA segments were used to measure mitochondria-specific superoxide with electron paramagnetic resonance (EPR) spectroscopy (EMX X-band spectrometer, Bruker, Billerica, MA). Each individual vessel sample was placed in a microcentrifuge tube containing 150 μl of the mitochondria-specific superoxide spin trap 1-hydroxy-4[2-(triphenylphosphoino)-acetamido]-2,2,6,6-tetramethylpiperidine (mitoTempo-H; 0.5 mmol/l, Enzo Life Sciences, San Diego, CA) and incubated for 60 min at 37°C. Samples were placed on ice, and 50 μl of the solution were loaded into a quartz capillary tube. The EPR spectroscopy scan was performed, and the area under the curve of the spectra was calculated by double integration.
CS activity.
After the mitochondrial respiration measurements, vessel samples (6.0 ± 1.5 mg wet wt) were homogenized with homogenization buffer [containing (in mM) 250 sucrose, 40 KCl, 2 EGTA, and 20 Tris·HCl]. CS activity was assayed and then analyzed utilizing a spectrophotometer (BioTek Instruments), as previously described (29).
Flow-mediated vasodilatory function assessment.
SMFAs were cannulated at both ends of micropipette tips and placed in the chamber of a pressure myograph system (model 110p, DMT Systems, Aarhus, Denmark). SMFAs were preincubated for 1 h in 37°C physiological salt solution. The outer diameter of the vessels was then recorded using an inverted microscope fitted with a video camera (Eclipse TS100, Nikon, Melville, NY), and data were streamed, in real time, to edge-detection software (VAS, v0.2.0, DMT Systems). Arteries free from fluid leaks, assessed during the pressurizing process (60 mmHg), were then used to assess vasodilatory function. After preconstriction with phenylephrine (10−6–10−4 M, Sigma-Aldrich) to ~70% of the maximum phenylephrine response, vasodilatory function was assessed in response to flow-induced shear stress at a 45-mmHg pressure difference across the vessel.
Statistical analysis.
One-way ANOVA was performed using SPSS (version 22, SPSS, Chicago, IL). If significance was detected, Tukey’s post hoc test was used to identify the significant difference. Correlations between variables were assessed with Pearson’s product-moment correlation. For all analyses, P = 0.05 was considered significantly different. Values are means ± SE unless otherwise noted.
RESULTS
Subject and vessel characteristics.
Thirty human SMFAs from the inguinal or axial region were successfully harvested in young (33 ± 7 yr, n = 10), middle-aged (54 ± 5 yr, n = 10), and old (70 ± 7 yr, n = 10) subjects (Table 1). Each SMFA was weighed after the respiration assessments (5.9 ± 1.5 mg wet wt) to normalize respiration rates for wet weight. In addition, as in our previous studies (14, 16, 26), there was no measurable effect of anatomic origin (n = 10 inguinal and 20 axial) or sex on mitochondrial respiration. Thus, there was no interaction between sex, anatomic origin, and age. An additional 16 subjects were studied in the preliminary investigation, which included both mitochondrial and vessel function assessments, for which subject characteristics are available for 12 of 16 subjects (Table 1).
State 3 and 4 respiration with advancing age.
As shown in Fig. 1, CI state 3 respiration was significantly higher in young (10.38 ± 0.78 pmol·s−1·mg−1) than middle-aged and old (7.03 ± 0.55 and 7.23 ± 0.39 pmol·s−1·mg−1, respectively) subjects. In addition, CI+II state 3 respiration, which is a maximal O2 consumption rate, was significantly higher in young (12.38 ± 0.81 pmol·s−1·mg−1) than middle-aged and old (8.32 ± 0.52 and 7.62 ± 0.5 pmol·s−1·mg−1, respectively) subjects (Fig. 1). In contrast, there was no significant difference between the age groups for CII state 3 respiration and nonphosphorylating state 4 respiration (Fig. 1).
Fig. 1.
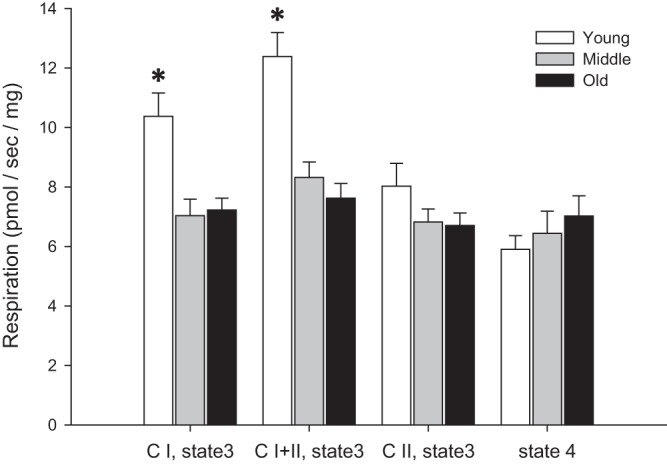
Mitochondrial state 3 and state 4 respiration in skeletal muscle feed arteries from young, middle-aged, and old subjects. CI, complex I; CI+II, complex I + II, CII, complex II. Values are means ± SE; n = 10 young, 10 middle-aged, and 10 old subjects. *Significantly different from middle-aged and old (P < 0.05).
Relationship between state 3 and 4 respiration with age.
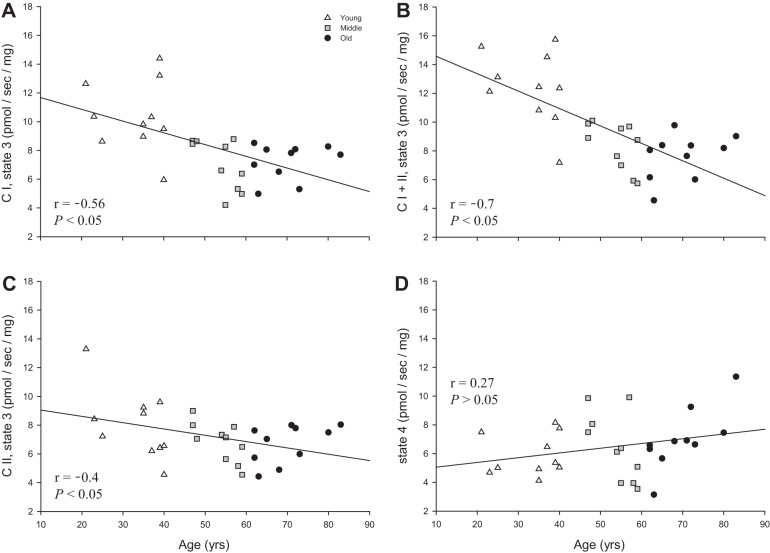
CI, CI+II, and CII state 3 respiration exhibited a significant inverse relationship with advancing age (Fig. 2, A–C). However, state 4 respiration was not significantly correlated with advancing age (Fig. 2D).
Fig. 2.
Relationships between mitochondrial state 3 and state 4 respiration in skeletal muscle feed arteries and age. A: complex I (CI) state 3 respiration and age. B: complex I + II (CI+II) state 3 respiration and age. C: complex II (CII) state 3 respiration and age. D: state 4 respiration and age.
RCR and the relationship with advancing age.
As shown in Fig. 3A, CI state 3 respiration was significantly higher in young (2.16 ± 0.15) than middle-aged and old (1.38 ± 0.09 and 1.14 ± 0.08, respectively) subjects. Furthermore, RCR exhibited a significant inverse relationship with advancing age (Fig. 3B).
Fig. 3.
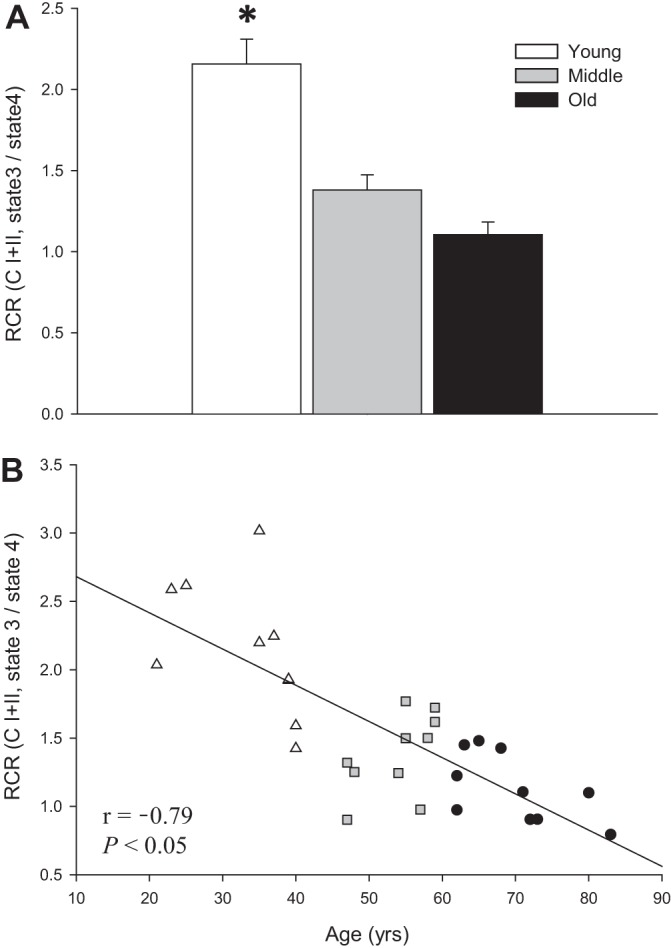
Respiratory control ratio (RCR) in skeletal muscle feed arteries from young, middle-aged, and old subjects and relationship between RCR and age. A: RCR (state 3-to-state 4 ratio) in young, middle-aged, and old subjects. CI + II, complex I + II. Values are means ± SE; n = 10 young, 10 middle-aged, and 10 old subjects. *Significantly different from middle-aged and old (P < 0.05). B: RCR and age.
Mitochondria-specific superoxide and the relationship with advancing age.
As shown in Fig. 4A, there was no difference in mitochondria-specific superoxide levels between the three age groups. However, the mitochondria-specific superoxide level was significantly correlated with advancing age (Fig. 4B).
Fig. 4.

Mitochondria-specific superoxide level in skeletal muscle feed arteries from young, middle-aged, and old subjects and relationship between mitochondria-specific superoxide levels and age. A: mitochondria-specific superoxide levels in young, middle-aged, and old subjects. AU, arbitrary units. Values are means ± SE; n = 9 young, 8 middle-aged, and 10 old subjects. B: mitochondria-specific superoxide levels and age.
CS activity with advancing age.
As shown in Fig. 5A, CS activity was significantly higher in young (9.87 ± 0.94 μmol·min−1·g−1) than middle-aged and old (6.97 ± 0.62 and 6.37 ± 0.55 μmol·min−1·g−1, respectively) subjects. Furthermore, CS activity exhibited a significant inverse relationship with advancing age (Fig. 5B).
Fig. 5.
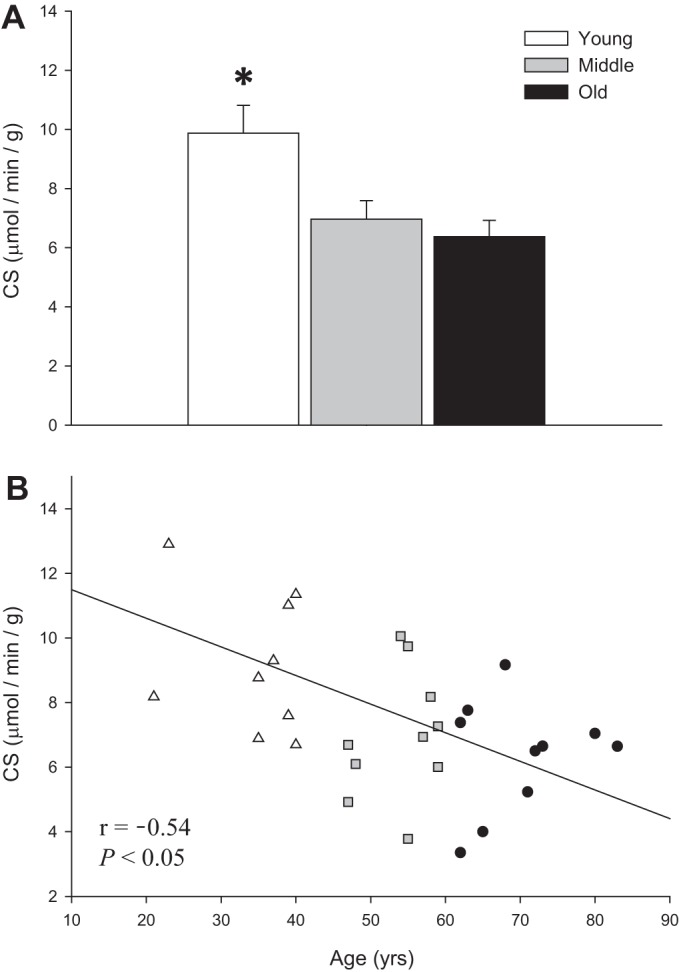
Citrate synthase (CS) activity (normalized for vessel wet weight) in skeletal muscle feed arteries from young, middle-aged, and old subjects and relationship between CS activity and age. A: CS activity in young, middle-aged, and old subjects. Values are means ± SE; n = 10 young, 10 middle-aged, and 10 old subjects. *Significantly different from middle-aged and old (P < 0.05). B: CS activity and age.
Mitochondrion state 3 and 4 respiration with advancing age.
The significant difference between young and both middle-aged and old subjects in CI and CI+II state 3 respiration (Fig. 1) was negated by normalization for CS activity (Fig. 6). CII state 3 respiration was not different between groups when normalized for CS activity (Fig. 6). Interestingly, when normalized for CS activity, state 4 respiration, which was previously not different between age groups, was significantly lower in young than old subjects (Fig. 6).
Fig. 6.
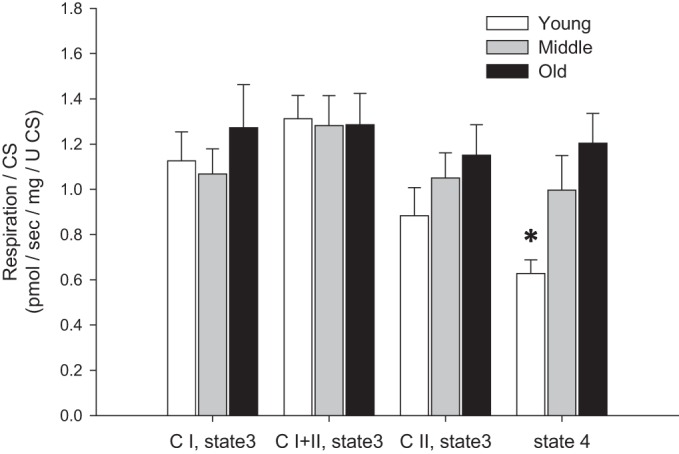
Mitochondrion-specific state 3 and state 4 respiration [normalized by citrate synthase (CS) activity] in skeletal muscle feed arteries from young, middle-aged, and old subjects. Values are means ± SE; n = 8 young, 10 middle-aged, and 10 old subjects. *Significantly different from old (P < 0.05).
Relationship of mitochondrion state 3 and 4 respiration with advancing age.
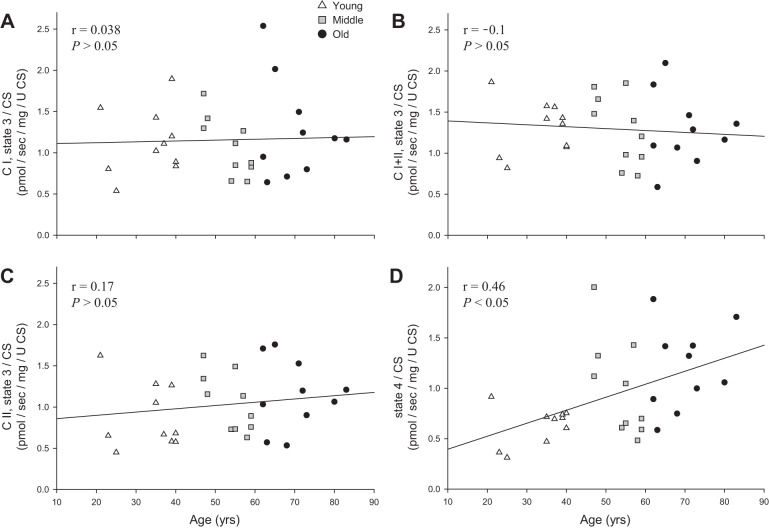
The previously significant inverse relationships of CI, CI+II, and CII state 3 respiration with advancing age (Fig. 2, A–C) were negated by normalization for CS activity (Fig. 7, A–C). Interestingly, the previously nonsignificant relationship between state 4 respiration and advancing age was enhanced by normalization for CS activity, resulting in a significant positive relationship between mitochondrion-specific state 4 respiration and advancing age (Fig. 7D).
Fig. 7.
Relationship between mitochondrion-specific state 3 and state 4 respiration and age in skeletal muscle feed arteries from young, middle-aged, and old subjects. A: mitochondrion-specific complex I (CI) state 3 respiration and age. B: mitochondrion-specific complex I + II (CI+II) state 3 respiration and age. C: mitochondrion-specific complex II (CII) state 3 respiration and age. D: mitochondrion-specific state 4 respiration and age. U CS, units of citrate synthase (CS) activity.
Mitochondrion-specific superoxide and the relationship with advancing age.
As shown in Fig. 8A, when normalized for CS activity, the previously nonsignificant difference in mitochondrion-specific superoxide levels between the three age groups was now significantly different between the young (0.07 ± 0.01) and old (0.19 ± 0.41) subjects. In addition, the previously significant relationship between mitochondrion-specific superoxide levels and advancing age was somewhat improved by normalization for CS activity (Fig. 8B).
Fig. 8.
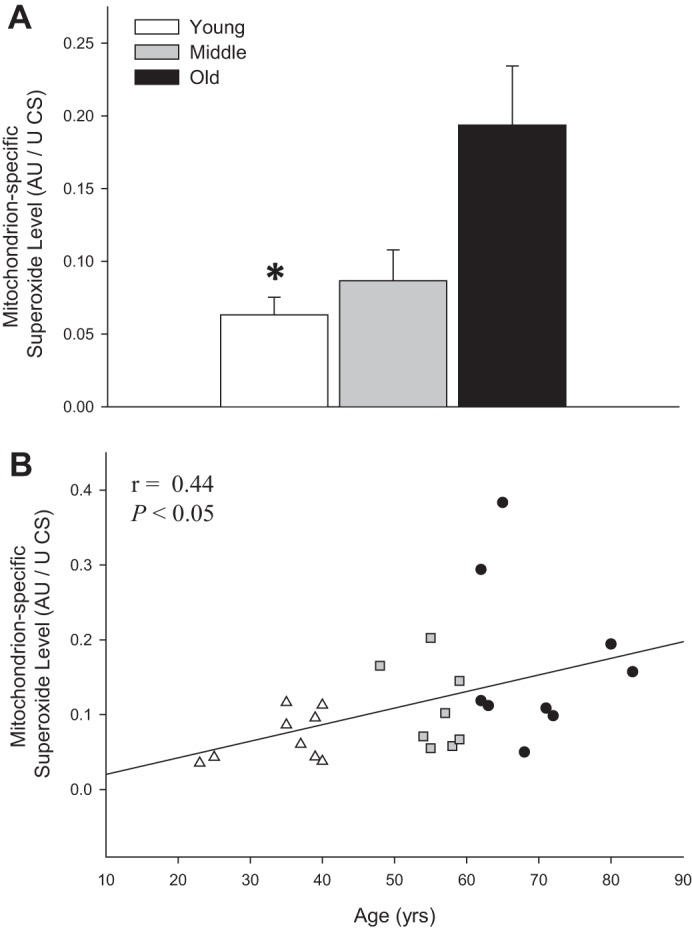
Mitochondrion-specific superoxide levels in skeletal muscle feed arteries from young, middle-aged, and old subjects and relationship between mitochondrion-specific superoxide levels and age. A: mitochondrion-specific superoxide levels in young, middle-aged, and old subjects. AU, arbitrary units. Values are means ± SE; n = 9 young, 8 middle-aged, and 10 old subjects. *Significantly different from old (P < 0.05). B: mitochondrion-specific superoxide levels and age.
Linking vascular mitochondrial respiration and vasodilatory function.
A preliminary, and certainly not comprehensive, assessment of mitochondrial (state 3) and vascular (flow-mediated vasodilation) function was catalyzed by the results of this study (subject characteristics for 12 of 16 subjects are shown in Table 1). Interestingly, as shown in Fig. 9, the age-related mitochondrial dysfunction in CI+II state 3 respiration exhibited a significant negative correlation with endothelium-dependent flow-mediated vasodilation (r = 0.78, P < 0.05). With the inclusion of only the 12 subjects for whom subject characteristics were available, the relationship was similarly strong (r = 0.76, P < 0.05).
Fig. 9.
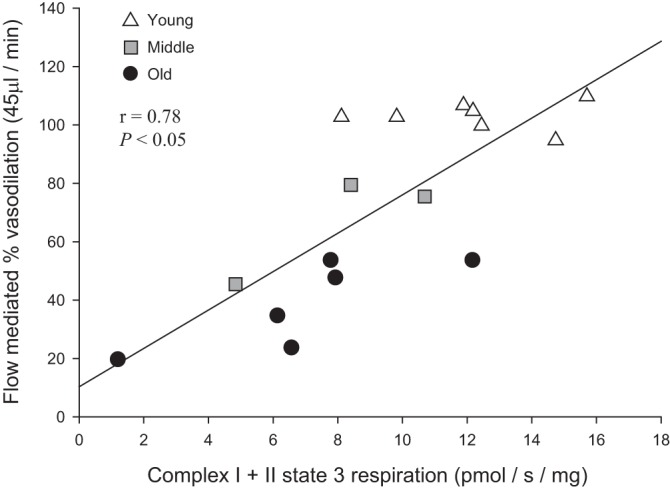
Relationship between vascular mitochondrial respiration and vasodilatory function: complex I + II state 3 respiration and flow-mediated vasodilation in human skeletal muscle feed arteries from young, middle-aged, and old subjects.
DISCUSSION
In general, progressive mitochondrial dysfunction has been described with advancing age in tissue such as skeletal muscle, heart, brain, and liver (11, 22, 24, 28). However, in terms of advancing age, such alterations in peripheral vascular mitochondrial respiratory capacity in humans have yet to be established. This study sought to determine, for the first time, the effect of aging on vascular mitochondrial respiratory function. This work resulted in several novel findings. First, vascular mitochondrial oxidative respiratory capacity, measured as state 3 respiration, fell progressively from the young to the old group and was inversely correlated with age. In contrast, state 4 respiration, an index of mitochondria-derived free radical production, was not different across age groups, nor was it related to age. Therefore, RCR, an indicator of oxidative coupling efficiency, was greater in the young group than the two older groups and inversely correlated with age. Interestingly, although mitochondria-specific superoxide levels were positively correlated with age, this age effect was not clearly evident across age groups. Second, vascular mitochondrial content, as estimated by CS activity, fell progressively from the young to old group and was also inversely correlated with age. Thus, when normalized for mitochondrial content, the age-related differences and relationships in state 3 respiration were completely ablated. In contrast, the mitochondrion-specific state 4 respiration was now lower in the young group than the two older groups and positively correlated with age. Similarly, superoxide/CS levels, which were lower in the young than old group, were correlated with age. Finally, our preliminary data on flow-mediated vasodilatory function exhibited a strong correlation with the age-related attenuation of maximal mitochondrial respiration. Therefore, this study reveals that vascular mitochondrial respiratory capacity and oxidative coupling decline with advancing age, the former predominantly as a consequence of falling mitochondrial content. However, per mitochondrion, aging results in greater mitochondria-derived free radicals, which may play a role in the vascular dysfunction exhibited by the elderly.
Vascular mitochondrial respiratory function and aging.
Due to the different physiological roles of cardiac, skeletal, and smooth muscle, as well as the mechanisms responsible for contraction and relaxation in the vasculature, vascular smooth muscle requires far less ATP than other muscle. However, despite this difference in intrinsic metabolic requirements, it has recently been documented that mitochondria in cardiac, skeletal, and smooth muscle have equivalent respiration rates per mitochondrion (25). With respect to aging, in cardiac and skeletal muscle it is clear that mitochondrial oxidative respiratory capacity and oxidative coupling decline with advancing age (2, 32). The present study reveals a similar pattern of altered respiration in the vasculature with age. CI and CI+II state 3 respiration were significantly attenuated in middle-aged and old subjects compared with young subjects (Fig. 1), with a strong inverse correlation across all subjects with advancing age (Fig. 2, A and B). Interestingly, in terms of vascular damage, which is associated with aging, such attenuated mitochondrial oxidative respiratory capacity has previously been documented. Specifically, CI and CII respiration were markedly diminished in vascular smooth muscle cells collected from areas of the aorta with and without atherosclerotic plaque (41). In combination, these data support the premise that mitochondrial oxidative respiratory capacity in the vasculature is attenuated by age-related structural and functional changes, as has been documented in cardiac and skeletal muscle.
Electrons entering the electron transport chain at CII require more O2 to resynthesize an equal quantity of ATP than electrons entering at CI. ATP production from the electron transport chain system relies more on CI than CII respiration, and CII respiration is recognized to be less efficient than CI respiration. Therefore, CI- and CII-driven O2 consumption are not equal in skeletal muscle: respiration preferentially originates at CI (12). There seems to be no published evidence of the ratio of CI to CII in the mitochondria of the vasculature, but certainly there is no evidence of the impact of the aging process on this ratio. However, interestingly, CI, but not CII, respiration is attenuated in the skeletal muscle of patients with chronic obstructive pulmonary disease compared with healthy controls (9). This suggests a greater metabolic reliance on CII than CI respiration (reduced CI-to-CII ratio) and, therefore, a lower metabolic efficiency in this disease state (9). Likewise, in mouse vascular smooth muscle cells, severe and longer exposure to atherosclerotic risk factors attenuates CI respiration and CI, but not CII, protein expression (41). In agreement with these changes in mitochondrial complex utilization with disease state, the present study documented changes in the function of CI and CII of the respiratory chain in SMFAs with advancing age. Specifically, despite a significant correlation with age across all subjects (Fig. 2C), CII state 3 respiration was not statistically different between the three age groups, whereas CI state 3 respiration was higher in young than middle-aged and old subjects (Fig. 1). Therefore, the ratio of CI to CII state 3 respiration was significantly greater in the young than middle-aged and old subjects (P < 0.05; data not shown). Thus, in agreement with the previous skeletal muscle study (9) in patients with chronic obstructive pulmonary disease, which highlighted this finding as a negative effect of the disease state, the present study provides evidence of a reduction of the ratio of CI to CII state 3 respiration with advancing age that was also likely driven by attenuated CI state 3 respiration. This suggests a decrement in vascular mitochondrial respiratory efficiency with advancing age, which may have implications for vascular health/function.
RCR, as an index of oxidative coupling efficiency, provides a gauge of how effectively mitochondria consume O2 to produce ATP in response to a given metabolic demand (3). In tissue with a high metabolic demand, such as cardiac and skeletal muscle, RCR declines with age-related functional loss (2, 32). In the present study, although state 4 respiration did not change across age groups, RCR (state 3-to-state 4 ratio) was clearly attenuated with advancing age (Fig. 3, A and B), as is vascular function (1, 6, 13, 16, 18, 23, 26, 31), implying that oxidative coupling likely has a functional role in the vasculature. Indeed, this implication is supported by the somewhat preliminary results presented here. Specifically, vascular function, assessed by flow-mediated vasodilation, was significantly correlated with maximal mitochondrial respiration rate (CI+II state 3 respiration) (Fig. 9). Although not comprehensive, this finding implies that attenuated mitochondrial respiratory function with advancing age likely plays a role in vascular dysfunction. Thus, further investigations of this concept are warranted. Consequently, although it is not clear exactly how mitochondrial respiration contributes to vascular function, it appears that vascular mitochondrial respiratory function (i.e., oxidative capacity, CI-to-CII ratio, and oxidative coupling) falls with advancing age, implying that the mitochondrial respiratory function may, indeed, be mechanistically linked to the vascular dysfunction that, typically, is also attenuated by aging.
Vascular mitochondrial content, mitochondrion-specific respiratory function, and aging.
Typically, in cardiac and skeletal muscle, age-related alterations are often attributed, at least in part, to a fall in mitochondrial content (5, 30, 34), which contributes to the recognized decline in mitochondrial respiratory function with advancing age (32). A commonly used approach to assess mitochondrial content is measurement of CS activity (25, 32). This method was used in a recent animal study (10) that revealed lower CS activity in skeletal muscle resistance arteries from old sedentary rats than young rats with a similar activity level. Additionally, utilizing mitochondrial complex protein expression, rather than CS activity, our group recently documented that old human SMFAs exhibited both attenuated mitochondrial content and diminished vasodilatory function compared with their young counterparts (27). In agreement with these prior investigations, the present study also reveals greater CS activity in the young than two older groups (Fig. 5A), with a significant inverse correlation with age (Fig. 5B). Thus, in combination, these findings support the concept that vascular mitochondrial content, which diminishes with advancing age, may, as in the case of cardiac and skeletal muscle, be linked to function, specifically, vascular function. However, a comprehensive assessment of the link between vascular mitochondrial content and vascular function has yet to be performed.
Interestingly, in terms of mitochondrial respiration, when each state 3 respiration rate was normalized for CS activity and, therefore, mitochondrial content, all significant age-related differences and correlations were completely ablated (Figs. 6 and 7, A–C). This finding suggests invariant mitochondrion-specific respiratory capacity across age groups or across the whole age range studied here (Figs. 6 and 7, A–C). In contrast, after this normalization for mitochondrial content, mitochondrion-specific state 4 respiration was greater in middle-aged and old than young subjects (Fig. 6), and there is now evidence of a significant positive correlation with advancing age (Fig. 7D). This suggests that mitochondrion-specific nonphosphorylating proton leak increases with advancing age. In line with the mitochondrial respiratory capacity findings, in skeletal muscle it has been documented that a decline in mitochondrial respiration with aging was ablated when normalized for CS activity (32). Interestingly, when two different age groups were matched for maximum O2 consumption, as assessed during an incremental exercise test, mitochondrial respiration was similar, but respiration per mitochondrion was significantly lower in the old than young group. This indicates that skeletal muscle requires a greater mitochondrial content in the old than the young group for a given metabolic demand (20). In contrast, nothing is known regarding changes in vascular mitochondrial content in relation to overall physical capacity. Thus, in future investigations, it would be both fascinating and novel to characterize the relationship between physical capacity and vascular mitochondrial function in different age groups. In summary, the present study revealed that the age-related decrement in mitochondrial respiratory function does not seem to be the result of abnormal mitochondrial respiratory capacity but, rather, decreased mitochondrial content with advancing age. However, based on the increase in mitochondrion-specific state 4 respiration with advancing age and the decreased oxidative coupling efficiency, aging likely results in greater mitochondrion-specific oxidative stress.
State 4 respiration in the vasculature: implications for vascular dysfunction with aging.
The mitochondrial respiratory complexes are considered a primary source of free radicals with advancing age (8, 21). This study documents that aging results in mitochondrion-specific nonphosphorylating proton leak in the vasculature, indicating unnecessary O2 consumption that likely results in free radicals (36). In a prior study (27), we documented elevated mitochondria-derived free radicals, as assessed by EPR spectroscopy, in SMFAs of old compared with young subjects. Although in the present study there was a nonsignificant difference in mitochondria-specific superoxide levels between the three age groups (Fig. 4A), there was a significant positive correlation between mitochondria-specific superoxide levels and advancing age (Fig. 4B). Interestingly, it was also evident that, in terms of the mitochondrion, superoxide levels were the lowest in the young group, with a somewhat improved correlation with advancing age (Fig. 8, A and B). These data are consistent with the state 4 respiration results in terms of mitochondria and the single mitochondrion. Therefore, it is probable that a primary mechanism responsible for development of vascular dysfunction with age may be excessive mitochondria-derived free radical production in the vasculature. Interestingly, in an animal study, elevated inflammatory risk factors in aged cultured vascular cells were not recovered by catalase, a general H2O2 scavenger, but, rather, by mitigation of mitochondria-derived H2O2 (37). Furthermore, in the vasculature, physiological interventions attenuating vascular mitochondria-derived free radical production have recently been acknowledged as a viable approach to rejuvenate vascular dysfunction with age (7, 27, 33, 36, 39, 40). In addition, inhibition of cytoplasmic NADPH oxidase, one of the most accepted sources of free radicals, does not always prevent or overcome age-related vascular dysfunction, which supports the existence of another important source of free radicals, vascular mitochondria, that is responsible for vessel dysfunction (37, 38). Thus, the findings of this study support the supposition that vascular aging is likely associated with mitochondria-derived free radical production and that targeting these free radicals may be beneficial for the amelioration of vascular dysfunction with advancing age.
Summary.
This study assessed vascular mitochondrial respiration in human SMFAs by comparing respiratory function in SMFAs from young, middle-aged, and old subjects. Although state 3 respiration and RCR were greatest in the young subjects with no difference in state 4 respiration with advancing age, when normalized for mitochondrial content, the significant difference in state 3 respiration was ablated and mitochondrion-specific state 4 respiration was now greater in the two older groups than the young group and was significantly correlated with age. Therefore, this study demonstrated that vascular mitochondrial respiration declines with advancing age and that, per mitochondrion, aging likely results in greater mitochondria-derived oxidative stress, which may play a role in age-related vascular dysfunction.
GRANTS
This work was funded, in part, by National Heart, Lung, and Blood Institute Grant PO1-HL-1091830 and Veterans Administration Rehabilitation Research and Development Service Grants E6910-R, E1697-R, E1433-P, E9275-L, and E1572-P.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.H.P., O.-S.K., S.-Y.P., J.C.W., R.H.A., J.R.H., V.R.R., and R.S.R. conceived and designed research; S.H.P., O.-S.K., S.-Y.P., J.C.W., R.H.A., J.R.H., V.R.R., and R.S.R. performed experiments; S.H.P., O.-S.K., and R.S.R. analyzed data; S.H.P., O.-S.K., S.-Y.P., J.C.W., R.H.A., J.R.H., and R.S.R. interpreted results of experiments; S.H.P., O.-S.K., and R.S.R. prepared figures; S.H.P., O.-S.K., and R.S.R. drafted manuscript; S.H.P., O.-S.K., S.-Y.P., J.C.W., R.H.A., J.R.H., and R.S.R. edited and revised manuscript; S.H.P., O.-S.K., S.-Y.P., J.C.W., R.H.A., J.R.H., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Behnke BJ, Prisby RD, Lesniewski LA, Donato AJ, Olin HM, Delp MD. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol 575: 617–626, 2006. doi: 10.1113/jphysiol.2006.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen TS, Rolim NP, Fischer T, Baekkerud FH, Medeiros A, Werner S, Brønstad E, Rognmo O, Mangner N, Linke A, Schuler G, Silva GJ, Wisløff U, Adams V; Optimex Study Group . Heart failure with preserved ejection fraction induces molecular, mitochondrial, histological, and functional alterations in rat respiratory and limb skeletal muscle. Eur J Heart Fail 17: 263–272, 2015. doi: 10.1002/ejhf.239. [DOI] [PubMed] [Google Scholar]
- 3.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312, 2011. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Corsetti G, Pasini E, D’Antona G, Nisoli E, Flati V, Assanelli D, Dioguardi FS, Bianchi R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am J Cardiol 101: S26–S34, 2008. doi: 10.1016/j.amjcard.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 6.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 7.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 75: 95–126, 2013. doi: 10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- 8.Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol 45: 563–572, 2010. doi: 10.1016/j.exger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Gifford JR, Trinity JD, Layec G, Garten RS, Park SY, Rossman MJ, Larsen S, Dela F, Richardson RS. Quadriceps exercise intolerance in patients with chronic obstructive pulmonary disease: the potential role of altered skeletal muscle mitochondrial respiration. J Appl Physiol (1985) 119: 882–888, 2015. doi: 10.1152/japplphysiol.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gittemeier EM, Ericson T, Ghosh P, Copp SW, Opoku-Acheampong AB, Behnke BJ. Effects of aging and exercise training on the dynamics of vasoconstriction in skeletal muscle resistance vessels. Eur J Appl Physiol 117: 397–407, 2017. doi: 10.1007/s00421-017-3541-0. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Freire M, de Cabo R, Bernier M, Sollott SJ, Fabbri E, Navas P, Ferrucci L. Reconsidering the role of mitochondria in aging. J Gerontol A Biol Sci Med Sci 70: 1334–1342, 2015. doi: 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinkle PC. P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1706: 1–11, 2005. doi: 10.1016/j.bbabio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol 42: 660–665, 1987. doi: 10.1093/geronj/42.6.660. [DOI] [PubMed] [Google Scholar]
- 14.Ives SJ, Andtbacka RH, Noyes RD, McDaniel J, Amann M, Witman MA, Symons JD, Wray DW, Richardson RS. Human skeletal muscle feed arteries studied in vitro: the effect of temperature on α1-adrenergic responsiveness. Exp Physiol 96: 907–918, 2011. doi: 10.1113/expphysiol.2011.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS. α1-Adrenergic responsiveness in human skeletal muscle feed arteries: the impact of reducing extracellular pH. Exp Physiol 98: 256–267, 2013. doi: 10.1113/expphysiol.2012.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ives SJ, Andtbacka RH, Park SY, Donato AJ, Gifford JR, Noyes RD, Lesniewski LA, Richardson RS. Human skeletal muscle feed arteries: evidence of regulatory potential. Acta Physiol (Oxf) 206: 135–141, 2012. doi: 10.1111/j.1748-1716.2012.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3: 965–976, 2008. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 18.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III. Cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 19.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. I. Aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 20.Larsen S, Hey-Mogensen M, Rabøl R, Stride N, Helge JW, Dela F. The influence of age and aerobic fitness: effects on mitochondrial respiration in skeletal muscle. Acta Physiol (Oxf) 205: 423–432, 2012. doi: 10.1111/j.1748-1716.2012.02408.x. [DOI] [PubMed] [Google Scholar]
- 21.Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19: 1469–1480, 2013. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martín-Fernández B, Gredilla R. Mitochondria and oxidative stress in heart aging. Age (Dordr) 38: 225–238, 2016. doi: 10.1007/s11357-016-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- 24.Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 287: R1244–R1249, 2004. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Gifford JR, Andtbacka RH, Trinity JD, Hyngstrom JR, Garten RS, Diakos NA, Ives SJ, Dela F, Larsen S, Drakos S, Richardson RS. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol 307: H346–H352, 2014. doi: 10.1152/ajpheart.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Ives SJ, Gifford JR, Andtbacka RH, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD, Richardson RS. Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol 310: H217–H225, 2016. doi: 10.1152/ajpheart.00716.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SY, Kwon OS, Andtbacka RHI, Hyngstrom JR, Reese V, Murphy MP, Richardson RS. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol (Oxf), 222: e12947, 2018. doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 28.Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res 2012: 194821, 2012. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol 295: R659–R668, 2008. doi: 10.1152/ajpregu.90357.2008. [DOI] [PubMed] [Google Scholar]
- 30.Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 9: 1032–1046, 2010. doi: 10.1111/j.1474-9726.2010.00628.x. [DOI] [PubMed] [Google Scholar]
- 31.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS.. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 32.Porter C, Hurren NM, Cotter MV, Bhattarai N, Reidy PT, Dillon EL, Durham WJ, Tuvdendorj D, Sheffield-Moore M, Volpi E, Sidossis LS, Rasmussen BB, Børsheim E. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab 309: E224–E232, 2015. doi: 10.1152/ajpendo.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71: 1056–1063, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One 5: e10778, 2010. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scuteri A, Najjar SS, Morrell CH, Lakatta EG; Cardiovascular Health Study . The metabolic syndrome in older individuals: prevalence and prediction of cardiovascular events: the Cardiovascular Health Study. Diabetes Care 28: 882–887, 2005. doi: 10.2337/diacare.28.4.882. [DOI] [PubMed] [Google Scholar]
- 36.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 37.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 38.Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, Madamanchi NR. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, Xie L, Jin K. Recent progress in vascular aging: mechanisms and its role in age-related diseases. Aging Dis 8: 486–505, 2017. doi: 10.14336/AD.2017.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu E, Mercer J, Bennett M. Mitochondria in vascular disease. Cardiovasc Res 95: 173–182, 2012. doi: 10.1093/cvr/cvs111. [DOI] [PubMed] [Google Scholar]
- 41.Yu EPK, Reinhold J, Yu H, Starks L, Uryga AK, Foote K, Finigan A, Figg N, Pung YF, Logan A, Murphy MP, Bennett M. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler Thromb Vasc Biol 37: 2322–2332, 2017. [Erratum in Arterioscler Thromb Vasc Biol 38: e135, 2018.] doi: 10.1161/ATVBAHA.117.310042. [DOI] [PMC free article] [PubMed] [Google Scholar]