Abstract
Functional interactions between endothelial cells (ECs) and smooth muscle cells (SMCs) in the arterial wall are necessary for controlling vasoreactivity that underlies vascular resistance and tone. Key signaling pathways converge on the phosphorylation of myosin light chain (p-MLC), the molecular signature of force production in SMCs, through coordinating the relative activities of myosin light chain kinase (MLCK) and myosin phosphatase (MP). Notch signaling in the vessel wall serves critical roles in arterial formation and maturation and has been implicated in arterial vasoregulation. In this report, we hypothesized that Notch signaling through ligands Jagged1 (in SMCs) and delta-like protein-4 (Dll4; in ECs) regulates vasoreactivity via homotypic (SMC-SMC) and heterotypic (EC-SMC) cell interactions. Using ligand induction assays, we demonstrated that Jagged1 selectively induced smooth muscle MLCK gene expression and p-MLC content while inhibiting MP function (i.e., increased Ca2+ sensitization) in a Rho kinase II-dependent manner. Likewise, selective deficiency of smooth muscle Jagged1 in mice resulted in MLCK and p-MLC loss, reduced Ca2+ sensitization, and impaired arterial force generation measured by myography. In contrast, smooth muscle Notch signaling triggered by Dll4 increased expression of MP-targeting subunit 1 (MYPT1; the MP regulatory subunit), whereas arteries from endothelial Dll4-deficient mice featured reduced MYPT1 levels, enhanced force production, and impaired relaxation independent of endothelium-derived nitric oxide signaling. Taken together, this study identifies novel opposing vasoregulatory functions for ligand-specific Notch signaling in the vessel wall, underscoring instructional signaling between ECs and SMCs and suggesting that Notch signals might behave as a “rheostat” in arterial tone control.
NEW & NOTEWORTHY The present study unveils novel roles for ligand-specific Notch signaling in arterial function. Smooth muscle Jagged1 and endothelial cell delta-like protein-4 ligands exhibit selective regulation of myosin light chain kinase and myosin phosphatase-targeting subunit 1/myosin phosphatase, respectively, providing a mechanistic link through which Notch signals modulate contractile activities in vascular smooth muscle. These findings may inform vascular derangements observed in human syndromes of Notch signaling deficiency while offering fundamental molecular insights into arterial physiological function.
Keywords: delta-like protein-4, Jagged1, Notch signaling, smooth muscle, vasoreactivity
INTRODUCTION
Smooth muscle cells (SMCs) within the arterial wall are the recipients of numerous instructional inputs, both endothelial cell (EC) dependent and independent, that establish a physiological balance of constrictor/relaxant behavior. Dysfunctional arterial SMCs are a feature of systemic and pulmonary hypertensive diseases, with hypertension serving as a major risk factor for stroke, heart failure, and myocardial infarction (38). Remarkably, modifying arterial constrictor behavior to enable a 10-mmHg reduction in systolic blood pressure is clinically sufficient to reduce myocardial infarction risk by 11% (42, 43).
In SMCs, phosphorylation of myosin light chain (p-MLC) triggers actin-myosin cross-bridging, and its abundance is determined by the relative activities of myosin light chain kinase (MLCK) and myosin phosphatase (MP) (20, 36). Vasoregulatory stimuli therefore produce constrictive or dilatory effects through selective regulation of MLCK and/or MP. MLCK activation is Ca2+/calmodulin dependent, triggered by Ca2+ influx through depolarized voltage-sensitive Ca2+ channels or via inositol 1,4,5-trisphosphate release of sarcoplasmic Ca2+ by G protein-coupled agonists (51). Constrictors also inactivate MP (i.e., Ca2+ sensitization) through phosphorylation of the MP regulatory subunit, MP-targeting subunit 1 (MYPT1), by Rho kinase II (ROCKII) at amino acid residues Thr696 and/or Thr853 (11, 41, 50). In contrast, lower p-MLC content is achieved in part through constrictor stimulus withdrawal and/or restoration of MP activity by endothelium-dependent nitric oxide (NO) signaling. In SMCs, NO binding to its cytosolic receptor guanylate cyclase results in cGMP production and activation of PKG. Among many functions of PKG is its phosphorylation of MYPT1 at Ser695, allosterically blocking Thr696 phosphorylation (13).
Despite the established aforementioned regulatory mechanisms of MLCK and MP activity, much less is known of other molecular cascades within the vessel wall affecting their activity or gene expression. Recent work from our laboratory unveiled novel vasoregulatory roles for Notch signaling in SMCs (1, 2). Notch is an evolutionarily conserved pathway operational in both ECs and SMCs and features multiple transmembrane receptors (Notch1–Notch4) and membrane-bound ligands [Jagged1 and Jagged2 as well as delta-like protein (Dll)1, Dll3, and Dll4] interacting in trans through homotypic or heterotypic cell contacts (23). Canonical Notch signaling initiates when ligand interacts with receptors triggering γ-secretase cleavage and release of the Notch receptor intracellular domain (NICD). Thereafter, NICD translocates to the nucleus, where it forms a transcription initiation complex with coactivator Mastermind (MAM) and DNA-binding partner CSL (CBF-1, suppressor of Hairless, Lag-2; also known as RBPJκ), promoting targeted gene expression (23, 55).
In our prior study, smooth muscle protein (SM)22-Cre+/dominant negative MAM-like 1 (DNMAML1)+ mice engineered to express a dominant negative MAM to suppress canonical signaling through all Notch receptors in smooth muscle featured impaired arterial vasoconstriction and vasorelaxation and blunted pressor responses in vivo (2). SM22Cre+/DNMAML1+ animals displayed reductions in MLCK and p-MLC content in parallel with impaired vasoconstriction. From other reports, characterization of Notch3 knockout mice revealed poor myogenic function in tail arteries and impaired cerebrovascular autoregulation, yet agonist constrictor and pressor responses remained intact (3). Taken together, these findings suggested that beyond a myogenic role, signaling through Notch family member receptors other than Notch3 in smooth muscle might serve a significant vasoregulatory function possibly dependent on distinct Notch ligand interactions. Indeed, we identified functional Jagged1-induced Notch1 complexes in the MLCK promoter identifying MLCK as a direct Notch target gene (1, 2). Hence, there exists a significant knowledge gap in our understanding of single and/or combinatorial roles of Notch receptors and ligands and their decisive impact on arterial physiological function.
In the present study, for the first time, we demonstrate a Notch ligand specificity that guides arterial vasoconstrictor versus dilator behavior in part through selective modulation of MLCK and MYPT1 in smooth muscle. SMCs exposed to Jagged1 ligand display increased MLCK and p-MLC content and ROCKII-dependent MYPT1 phosphorylation. Mice deficient in smooth muscle Jagged1 exhibit impaired arterial force production associated with reductions in MLCK content and RhoA-dependent Ca2+ sensitization. In contrast, SMCs stimulated by Dll4 ligand reveal increased MYPT1 expression, and arteries deficient in Dll4 demonstrate impaired relaxation and superior force generation, implicating a promotional role for Dll4 in vasorelaxation. As Dll4 is selectively expressed in the endothelial layer of the arterial wall (8, 34, 49, 56), its function might serve as a novel endothelium-dependent relaxation signal, which we show is independent of EC NO activity. Overall, arterial Notch signaling appears to function in a heterotypic and homotypic cell manner, fine tuning vessel tone by regulating vasoconstriction through smooth muscle Jagged1 stimulation and vasorelaxation through Dll4-triggered Notch activation from neighboring ECs in the vessel wall.
MATERIALS AND METHODS
Animals.
To generate a conditional and smooth muscle-restricted Jagged1-deficient mouse model, we interbred the well-established tamoxifen (TM)-inducible smooth muscle myosin heavy chain-CreERT2 (SMMHC-CreERT2) line (kindly provided by Dr. Stephen Offermanns) with conditional Jagged1 flox/flox (Jag1f/f) mice (stock no. 01068, The Jackson Laboratory) to generate SMMHC-CreERT2/Jag1f/f animals (14, 21, 44, 45, 58). Of note, the SMMHC-CreERT2 transgene is linked to the Y chromosome, and therefore only male offspring can be studied (58). Male mice with genotype SMMHC-CreERT2/Jag1f/f were phenotypically normal and viable. At an age of 8–12 wk, animals were injected with TM (1 mg/day ip) or sunflower oil (vehicle) for 7 days. Experimental/functional analyses including myography commenced 14 days after the first TM injection. To generate EC-specific Dll4 deficiency in adult arteries, TM-inducible Dll4-deficient mice were generated by interbreeding previously described mouse lines, vascular endothelial cadherin-CreERT2 [VeCad-CreERT2 (Ve)] (52) and Dll4 flox/flox (Dll4f/f) (22), kindly provided by Dr. Antonio Duarte. Cohorts of both male and female Ve−Dll4f/f (control) and Dll4-deficient (Ve+Dll4def, study) mice all received TM (1 mg ip) daily for 5 days with experimental procedures initiated 14 days after the first TM injection. The SM22Cre+DNMAML1+ mouse line has been previously described (2, 46). All parental lines in this study were backcrossed >10 times and maintained on a C57BL/6 background. Animal experimentation was performed under the approved protocols of the Case Western Reserve University Animal Care and Use Committee and National Institutes of Health guidelines.
Primary aortic SMC isolation and SMC culture.
Mouse primary aortic SMCs were isolated as previously described (46). For these experiments, adult mice (age 8–12 wk) were euthanized by CO2 inhalation. Primary SMCs were maintained in DMEM/F-12/GlutaMAX media (GIBCO/ThermoFisher Scientific) containing 10% FBS (Gemini), 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells from passages 3–8 were used in all experiments. Rat A10 aortic SMCs (catalog no. CRL-1476, American Type Culture Collection) were maintained in DMEM/GlutaMAX media (GIBCO/ThermoFisher Scientific) supplemented with 10% FBS (Sigma), 100 U/ml penicillin, and 100 µg/ml streptomycin.
Notch ligand stimulation assay.
Notch signaling was induced in primary aortic SMCs cultured in dishes displaying immobilized Jagged1 ligand (Fc-Jag1), Dll4 ligand (Fc-Dll4), or control (Fc) as previously described (2, 46). Where indicated, ROCK inhibitor Y27632 (catalog no. Y-0503, Sigma; 10 μM) or DMSO (vehicle control) was added to the growth media.
Myography-isometric tension measurements.
Adult mice (age 10–12 wk) were euthanized by CO2 inhalation. Segments of the thoracic aorta and third-order mesenteric arteries (MAs) were harvested, cut into 2-mm rings, and wire mounted in a four-chamber isometric tension myograph (model 620-M, DMT, Aarhus, Denmark). A minimum of two rings from each vessel per animal were examined, and treatments were performed in triplicate. Each chamber contained physiological saline solution (PSS; 112 mM NaCl, 25.7 mM NaHCO3, 4.9 mM KCl, 2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 11.5 mM glucose, and 10.0 mM HEPES, pH 7.4) equilibrated with a gas mixture of 95% O2 and 5% CO2 at 37°C as previously described (2). All chemicals were purchased from Sigma unless otherwise specified. For each MA, the resting tension-internal circumference relation was determined by the method of Mulvany and Halpern (39). Baseline aortic ring tension was preset at 4 mN before force induction by vasoconstrictors. Dose-response curves were generated for agonist constrictors [phenylephrine (PE) and angiotensin II (ANG II); 10−9–10−5 M]. Vessels were treated with KCl (60 mM) to achieve depolarization-induced constriction. Where indicated, constrictor experiments were performed with 100 µM Nω-nitro-l-arginine methyl ester (l-NAME; Sigma) pretreatment (10 min) to inhibit NO production. For relaxation experiments, vessels were preconstricted (10−5 M PE) followed by escalating doses of either sodium nitroprusside (SNP; 10−9–10−5 M) or acetylcholine (Ach; 10−8–10−5 M). Data are presented as active tension (in mN) for contractions and percentages of maximum tension for SNP- and ACh-induced relaxation.
Ca2+ permeabilization and force generation experiments were performed as previously detailed by our group (2). Briefly, MA rings were mounted in the wire myograph, incubated in high relaxing solution (53.28 mM KCl, 6.81 mM MgCl2, 0.025 mM CaCl2, 10.0 mM EGTA, 5.4 mM Na2ATP, and 12.0 mM creatine phosphate) for 20 min, and permeabilized for 1 h with addition of 1,000 U/ml α-toxin (Sigma). pCa solutions were generated using high relaxing solution and buffered EGTA as determined by the MAXC computer program based on the Bathe algorithm for free Ca2+ concentrations between pCa 9 and pCa 4.5. The force value (in mN) at pCa 9 was set as baseline. pCa response curves were repeated in triplicate per ring with active tension measured for 5 min at each concentration.
Agonist and depolarization treatment of aortic rings.
Thoracic aorta segments were harvested from smooth muscle Jagged1-deficient, EC Dll4-deficient, and respective littermate control mice and cut into 2-mm-long rings, each equilibrated in PSS buffer containing 1× cOmplete Mini protease inhibitor (Roche Applied Science) at 37°C. Thereafter, rings were treated with either KCl (60 mM) or ANG II (10−5 M; Sigma) for 10–15 min at 37°C, washed once in PSS, and immediately frozen in liquid nitrogen. Where indicated, aortic rings were pretreated for 10 min with 100 µM l-NAME (Sigma) to inhibit NO production. Frozen segments were homogenized in RIPA lysis buffer using TissueLyser II (2 × 2 min at 30 Hz, Qiagen) followed by centrifugation at 14,000 g and 4°C. Supernatants were subjected to immunoblot detection of p-MLC and total MLC.
Biochemical analysis.
Arterial segments were dissected from mice and snap frozen in liquid nitrogen. Whole tissue protein lysates were prepared by homogenization in RIPA buffer [50 mM Tris·Cl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM PMSF, and 1× Roche cOmplete Mini protease inhibitor] using TissueLyser II (2 × 2 min at 30 Hz, Qiagen) followed by clarification at 14,000 g and 4°C. Alternatively, A10 and primary aortic SMCs from either control or mutant mice were directly lysed in RIPA buffer followed by centrifugation at 10,000 g and 4°C. Protein concentrations were determined by the Bradford assay (Bio-Rad). Lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblots were performed using semidry methods following the manufacturer’s directions (Bio-Rad) with antibodies against MLCK (mouse monoclonal, catalog no. M-7906, Sigma; 1:5,000), MLC (rabbit polyclonal, catalog no. 3672, Cell Signaling Technology; 1:1,000), p-MLC (rabbit polyclonal, catalog no. 3671, Cell Signaling Technology; 1:1,000), MYPT1 (rabbit polyclonal, catalog no. 2634, Cell Signaling Technology; 1:1,000), p-MYPT1 (Thr696) (rabbit polyclonal, catalog no. 5163, Cell Signaling Technology; 1:1,000), p-MYPT1 (Thr853) (rabbit polyclonal, catalog no. 4563, Cell Signaling Technology; 1:1,000), Dll4 (rabbit polyclonal, catalog no. 7280, Abcam; 1:800), Jagged1 (rabbit monoclonal, catalog no. 28H8, Cell Signaling Technology; 1:1,000), β-actin (rabbit polyclonal, catalog no. 4967, Cell Signaling Technology; 1:10,000), SMMHC (rabbit polyclonal, catalog no. 562, Biological Technologies; 1:2,500), smooth muscle α-actin (SMA; mouse monoclonal, catalog no. F-3777, Sigma; 1:4,000), and GAPDH (mouse monoclonal, catalog no. sc-365062, Santa Cruz Biotechnology; 1:5,000). Protein bands were visualized with enhanced chemiluminescence (horseradish peroxidase) according to the manufacturer’s instructions (ThermoFisher Scientific) and quantified by densitometry (ImageJ, National Institutes of Health).
Quantitative PCR analysis.
For quantitative analysis of endogenous gene expression, total RNA was harvested from either primary aortic SMCs or from whole aortic tissue using QIAzol reagent (Qiagen). A QuantiTect reverse transcription kit (Qiagen) was used for cDNA synthesis. Real-time PCR analyses were performed on a StepOnePlus quantitative PCR system (Applied Biosystems). cDNA was added to each reaction mixture containing forward and reverse gene-specific primers and SYBR Green 2× master mix (FastStart Universal, Roche Applied Biosystems). Expression levels of target genes were normalized to GAPDH mRNA levels. Reactions were performed in triplicate, and mRNA quantification was calculated according to the method, where Ct is threshold cycle (29). Gene-specific primer sequences included the following: murine MLCK, forward 5′-AGAAGTCAAGGAGGTAAAGAATGATGT-3′ and reverse 5′-CGGGTCGCTTTTCATTGC-3′; murine MYPT1, forward 5′-AAAGCGACGGTCTACTGGAG-3′ and reverse 5′-AACAGAATCCGTCTGCGTT-3′; murine Hairy/enhancer-of-split related with YRPW motif protein (HEY)1, forward 5′-GAAGCCCCGACGAGACCGAATCAA-3′ and reverse 5′-CAGGGCGTGCGCGTCAAAATAACC-3′; murine HEY2, forward 5′-CGACGTGGGGAGCGAGAACAAT-3′ and reverse 5′-GGCAAGAGCATGGGCATCAAAGTA-3′; and murine GAPDH, forward 5′-GTGGCAAAGTGGAGATTGTTGCC-3′ and reverse 5′-GATGATGACCCGTTTGGCTCC-3′.
Immunohistochemistry.
To assess vessel morphology, aortic tissue cross sections were subjected to elastin staining according to the manufacturer’s instructions (Elastic Stain Kit, catalog no. HT-25, Sigma).
Statistical analysis.
All data are presented as means ± SE unless indicated in the figures. Statistical analyses were performed as indicated within each figure. One-way ANOVA with Tukey’s post hoc test was used for multiple comparisons. For all myography dose-response experiments, two-way ANOVA with Bonferroni’s post hoc test was used for multiple comparisons with repeated measures. Statistical analyses and data graphics were generated using GraphPad Prism software. P values of <0.05 were considered statistically significant.
RESULTS
Notch ligands trigger selective alterations in regulatory contractile protein content.
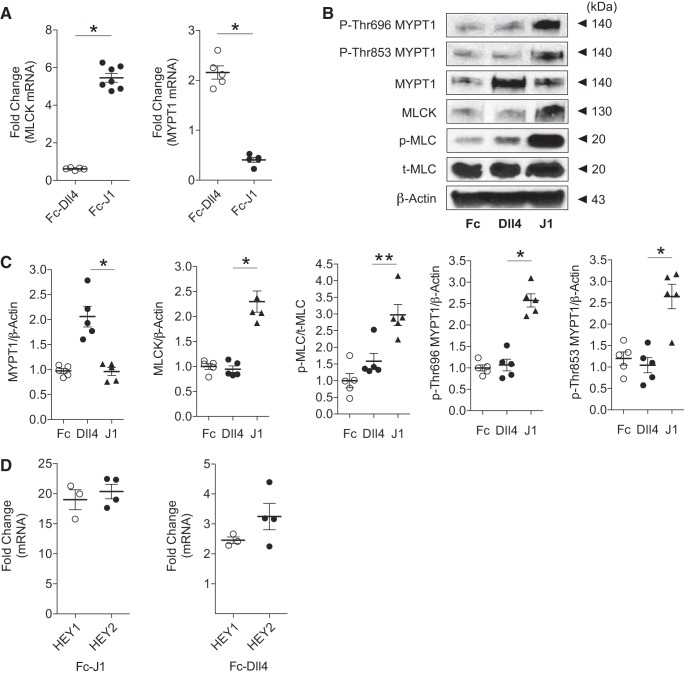
In a prior animal study (2), we showed that inhibition of canonical Notch signaling in smooth muscle by DNMAML1 resulted in MLCK loss with concomitant impairments in arterial constriction and relaxation. Since DNMAML1 functions as a downstream inhibitor of Notch signaling irrespective of ligand or receptor utilization, we sought to determine whether specific ligand activity is linked to these opposing vascular functions. We initially performed gain-of-function experiments to determine regulatory contractile gene expression changes associated with ligand stimulation. Primary aortic SMCs were cultured on plates displaying fixed Jagged1 or Dll4 ligand. Consistent with our prior report (2), Jagged1 specifically induced MLCK mRNA and protein levels with associated increase in p-MLC content (Fig. 1, A–C). In contrast, Dll4 triggered a selective increase in MYPT1 gene and protein expression without perturbation in MLCK content (Fig. 1, A–C). In this system, both ligands induced established Notch target genes HEY1 and HEY2 (Fig. 1D). Therefore, these findings provided the initial framework for ascertaining ligand-specific roles for smooth muscle Notch signaling in vasoregulation. Moreover, as Dll4 is exclusively expressed in the endothelium of the arterial wall (8, 34, 49, 56), these experiments suggested a possible in vivo mechanism featuring instructional Notch signaling between ECs and SMCs within the arterial wall.
Fig. 1.
Notch ligand-specific induction of regulatory contractile gene expression. A: wild-type primary mouse aortic smooth muscle cells (SMCs) induced by Jagged1 (Fc-J1) or delta-like protein-4 (Dll4; Fc-Dll4). Relative myosin light chain kinase (MLCK) and myosin phosphatase-targeting subunit 1 (MYPT1) transcript levels are shown. Real-time quantitative PCR data were normalized to control (Fc) treatment and represent means ± SE (n = 7 for MLCK; n = 5 for MYPT1). Data were analyzed using a t-test, *P < 0.001. B: immunoblots showing Jagged1 induction of MLCK, phosphorylated myosin light chain (p-MLC), and p-Thr696/853 MYPT1 in contrast to Dll4 induction of MYPT1. T-MLC, total myosin light chain. C: densitometric quantitation of representative immunoblots in B. Data were normalized to Fc treatment and represent means ± SE (n = 5). Data were analyzed using a one-way ANOVA with Tukey’s post hoc test, *P < 0.001 and **P < 0.01 (Dll4 vs. J1). p-MLC/T-MLC, p-MLC-to-total MLC ratio. D: Hairy/enhancer-of-split related with YRPW motif protein (HEY) target gene expression in Jagged1- and Dll4-stimulated wild-type primary aortic SMCs. Relative to unstimulating control ligand (Fc), both Jagged1 (Fc-J1) and Dll4 (Fc-Dll4) induced HEY1 and HEY2 transcripts. Real-time quantitative PCR data were normalized to control treatment and represent means ± SE (n = 3 for HEY1; n = 4 for HEY2). Data were analyzed using a t-test, P = not significant (HEY1 vs. HEY2 per ligand stimulation).
Smooth muscle Jagged1 deficiency impairs arterial contractility.
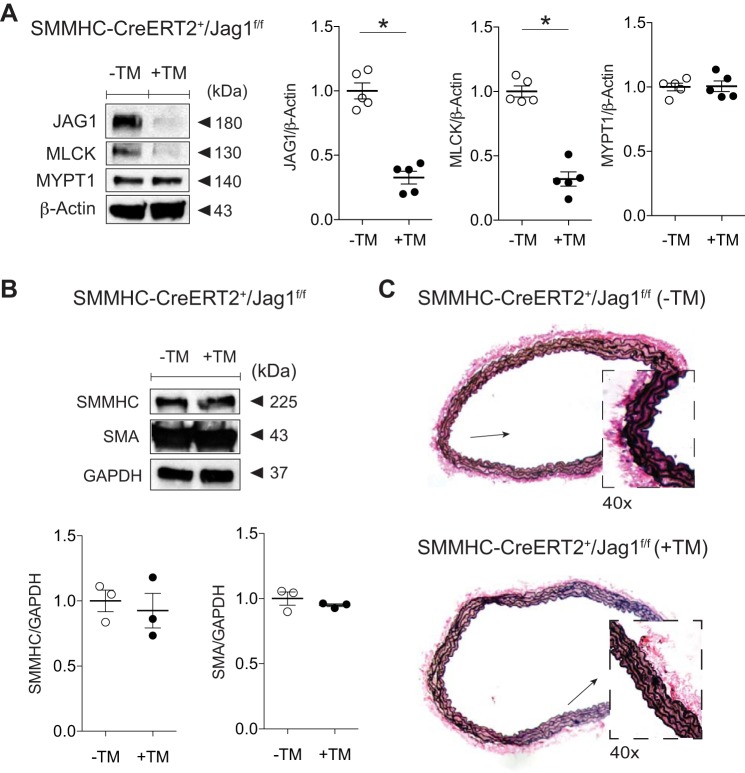
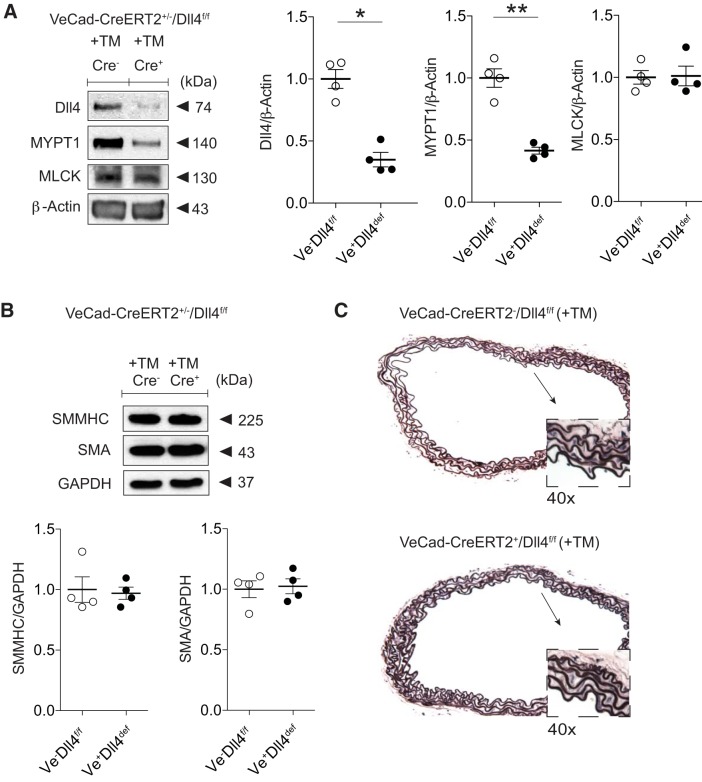
Since Jagged1 stimulation leads to increased MLCK and molecular indexes favoring force production [increased p-MLC-to-total MLC ratio (p-MLC/total MLC)], we designed a mouse model of smooth muscle Jagged1 deficiency to determine a reliance of this ligand for arterial contractile function in the adult animal. To circumvent the perinatal lethality of smooth muscle Jagged1 deficiency (12), we generated a conditional and smooth muscle-specific mouse model in which TM treatment (+TM) led to Jagged1 deficiency in adult animals (+TM, SMMHC-CreERT2/Jag1f/f). Our treatment protocol resulted in ~70–75% reductions in both Jagged1 and MLCK in the aorta with no change in MYPT1 levels (Fig. 2A). Notably, there was no measurable change in either aortic SMMHC or SMA content (Fig. 2B). There were also no gross phenotypic differences between control and smooth muscle Jagged1-deficient animals at the time of experimental analyses. Furthermore, elastin staining did not reveal features of structural derangements in the Jagged1-deficient aorta, as evidenced by preserved SMC layering and intact elastic lamina (Fig. 2C).
Fig. 2.
Smooth muscle Jagged1 deficiency selectively reduces myosin light chain kinase (MLCK) levels. A: aortas from tamoxifen (TM)-treated (+TM) versus control sunflower oil-treated (−TM) smooth muscle myosin heavy chain-CreERT2 (SMMHC-CreERT2)/Jagged1 flox/flox (Jag1f/f) mice reveal Jagged1 and MLCK loss with preserved myosin phosphatase-targeting subunit 1 (MYPT1) content as shown in representative immunoblots. Data represent means ± SE (n = 5) and were analyzed using a t-test, *P < 0.0001. B: representative immunoblots displaying preserved SMMHC and smooth muscle α-actin (SMA) content in Jagged1-deficient vessels. Data are means (SD) (n = 3) normalized to GAPDH and control (−TM) lane and were analyzed using a t-test, P = not significant (−TM vs. +TM). C: representative elastin-stained control and smooth muscle Jagged1-deficient aortic cross sections. Vessels were morphologically indistinguishable with intact elastic lamina; boxes are at ×40 magnification of low-power (×10) views (arrows).
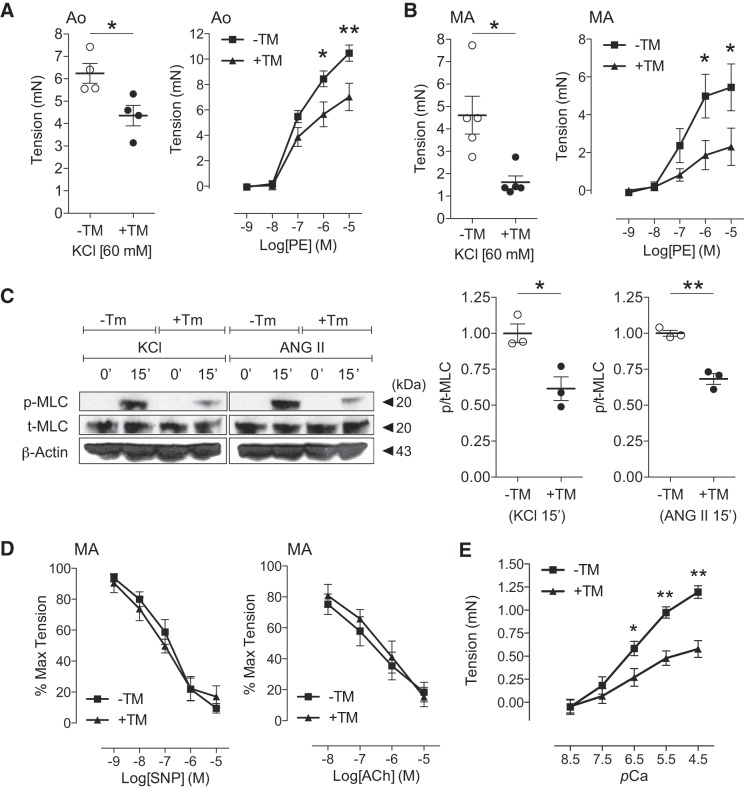
To measure arterial contractile function in smooth muscle Jagged1-deficient vessels, we performed wire-mounted myography using aortic rings stimulated by agonist or depolarization. Treatment with the depolarizing agent KCl revealed impaired force production in Jagged1-deficient segments (Fig. 3A, left). Similarly, reduced force in mutant rings was observed in response to the agonists PE and ANG II (Fig. 3A, right, and data not shown). In addition to studying the vasoreactive performance of a conductance vessel (i.e., the aorta), we extended our analyses to include resistance MAs. Myographic experiments of third-order MAs from smooth muscle Jagged1-deficient mice revealed parallel impairments in the constrictor response to KCl depolarization or PE (Fig. 3B). To determine parallel changes in the molecular signature of force production, we measured p-MLC/total MLC in constricted aortic segments. Both KCl and ANG II treatment raised p-MLC content in control vessels; however, constrictor responses were blunted in Jagged1-deficient (+TM) rings (Fig. 3C). To determine whether smooth muscle Jagged1 deficiency might also result in defective vasorelaxation, wire-mounted MAs were preconstricted with PE and treated with either SNP (EC independent) or ACh (EC dependent) to facilitate NO-mediated relaxation. Vasorelaxant dose-response curves were similar in both controls and Jagged1-deficient MAs (Fig. 3D). Moreover, we performed Ca2+ permeability and force generation experiments to further assess underlying myofilament function in Jagged1-deficient MAs. Permeabilized vessel segments were mounted on the wire myograph, and the Ca2+-force relationship was assessed with escalating pCa solutions. Similar to their responses to agonist constrictors, Jagged1-deficient arteries produced less force at submaximal (pCa 6.5–5.5) and maximal (pCa 4.5) Ca2+ concentrations (Fig. 3E). Coupled with preserved SMMHC and SMA levels, these data underscore impairment in myofilament force production by Ca2+ activation that is consistent with regulatory contractile defects in Jagged1-deficient smooth muscle. These results further imply that defective force production in Jagged1-deficient arteries is unlikely due to altered Ca2+ availability in the smooth muscle compartment. Taken together, Jagged1 loss in smooth muscle appears to recapitulate the MLCK reduction and arterial constriction defects observed across a spectrum of conductance and resistance vessels reported in our prior animal model of global Notch signaling deficiency [i.e., SM22Cre+DNMAML1+; Fig. 7A and Basu et al. (2)].
Fig. 3.
Smooth muscle Jagged1 deficiency blunts arterial force production without altering vasorelaxation. A: impaired aortic (Ao) constrictor response to depolarization (KCl, left) or agonist constrictor [phenylephrine (PE), right] with Jagged1 deficiency [tamoxifen (TM) treatment (+TM)] versus controls (−TM). Myography data represent means ± SE (n = 4). Data for KCl were analyzed using a t-test, *P = 0.015; data for PE were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05 and **P < 0.01. B: impaired mesenteric artery (MA) force production in response to depolarization (KCl, left) or agonist (PE, right) with Jagged1 deficiency (+TM) versus controls (−TM). Myography data represent means ± SE (n = 5). Data for KCl were analyzed using a t-test, *P = 0.014; data for PE were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05. C: representative immunoblots showing blunted phosphorylated myosin light chain (p-MLC) production in aortic segments from Jagged1-deficient animals (+TM) versus controls (−TM) treated for 15 min with constrictor [KCl or angiotensin II (ANG II)]. Data represent means ± SE (n = 3) normalized to −TM and were analyzed using a t-test, *P = 0.002 and **P = 0.017. T-MLC, total MLC; P/T-MLC, p-MLC-to-total MLC ratio. D: preserved mesenteric artery (MA) relaxation responses to sodium nitroprusside (SNP, left) and acetylcholine (ACh, right) with Jagged1 deficiency (+TM) versus controls (−TM). Myography data represent means ± SE (n = 5) and were analyzed using two-way ANOVA with Bonferroni’s post hoc test. Max, maximum. E: Ca2+ permeabilization and force generation experiments. MAs from control and TM-treated smooth muscle myosin heavy chain-CreERT2 (SMMHC-CreERT2)/Jagged1 flox/flox (Jag1f/f) mice were permeabilized with α-toxin and exposed to incremental Ca2+ concentrations. Myograph data are expressed as tension generated above set baseline at pCa 9. Data represent means ± SE (n = 4 per group) and were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05 and **P < 0.001.
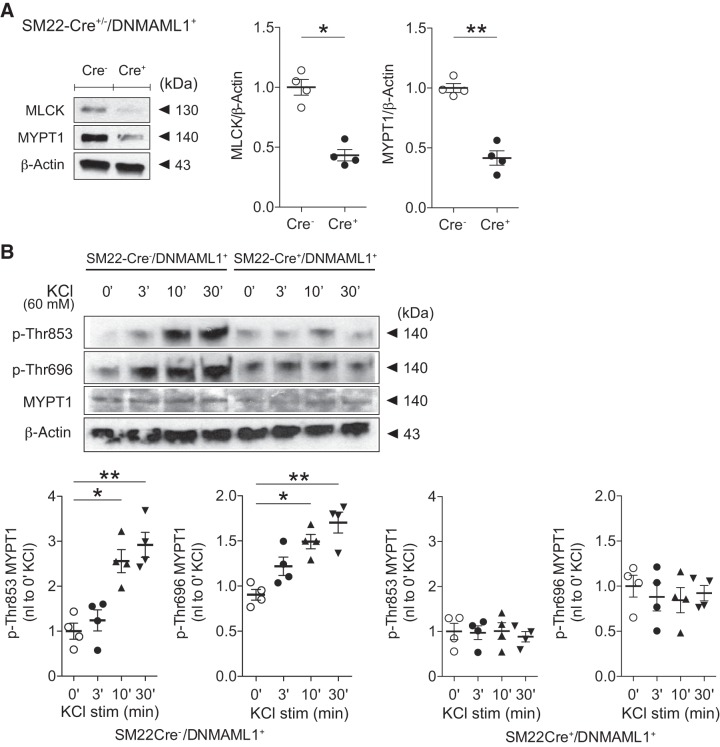
Fig. 7.
Regulatory contractile gene expression in Notch signaling-deficient smooth muscle. A: smooth muscle protein (SM)22-Cre+/dominant negative Mastermind-like 1 (DNMAML1)+ (SM22Cre+/DNMAML1+; Cre+) primary aortic smooth muscle cells displayed reduced myosin light chain kinase (MLCK) and myosin phosphatase-targeting subunit 1 (MYPT1) levels relative to SM22Cre−/DNMAML1+ (Cre−) controls. Representative immunoblots are shown with data normalized to β-actin and controls. Data represent means (SD) (n = 4) and were analyzed using a t-test, *P = 0.0004 and **P = 0.0002. B: Notch signaling inhibition in smooth muscle impairs Ca2+ sensitization. Representative immunoblots displayed blunted KCl-induced phosphorylation of MYPT1 (p-Thr696 and p-Thr853) in SM22Cre+/DNMAML1+ aortic SMCs exposed to 60 mM KCl for 0–30 min (0–30′). Data represent means ± SE (n = 4) and were analyzed using one-way ANOVA with Tukey’s post hoc test, *P < 0.01 and **P < 0.001 (vs. 0 min). KCl stim, KCl stimulation.
Endothelial Dll4 deficiency promotes superior arterial force generation and impaired relaxation.
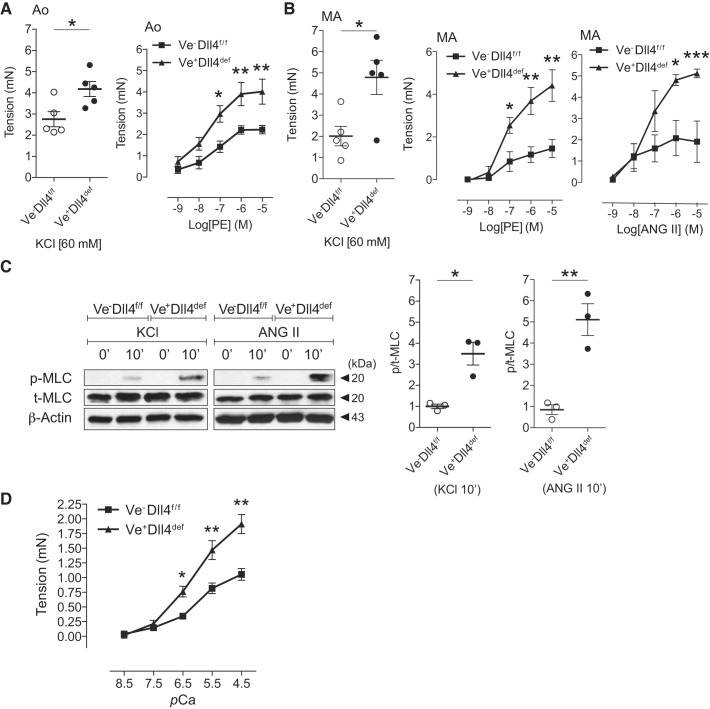
Our ligand induction experiments revealed a positive link between Dll4 stimulation and MYPT1 levels in SMCs (Fig. 1). To ascertain a role for Dll4-mediated Notch signaling in arterial function, we generated a conditional EC-specific Dll4-deficient mouse model to uncouple potential Dll4 ligation with smooth muscle Notch receptors. TM treatment of VeCad-CreERT2/Dll4f/f mice resulted in an ~70% loss in Dll4 and 60% reduction in MYPT1 with preserved MLCK levels (Fig. 4A). As observed in Jagged1-deficient animals, EC Dll4-deficient mice appeared indistinguishable from their control littermates at time of analyses. Furthermore, SMMHC and SMA content remained unaltered (Fig. 4B), and there were no reproducible differences in arterial vessel morphology or wall integrity as determined by elastin staining (Fig. 4C). To assess vasoreactivity function, EC Dll4-deficient aortic rings were subjected to myography and displayed enhanced force in response to both depolarizing and agonist constrictors KCl and PE, respectively (Fig. 5A). Dll4-deficient MAs similarly revealed heightened force responses to KCl depolarization and agonist (PE and ANG II) stimulation (Fig. 5B). At the molecular level, KCl and ANG II treatment of EC Dll4-deficient aortic rings resulted in greater p-MLC content than in control segments (Fig. 5C). Finally, we assessed the Ca2+-force relationship in permeabilized EC Dll4-deficient MAs. Similar to their respective responses to depolarization or agonist constrictors, Dll4-deficient arteries produced greater force at submaximal (pCa 6.5–5.5) and maximal (pCa 4.5) Ca2+ concentrations (Fig. 5D). These data suggest that the augmented Ca2+-induced force production is consistent with regulatory myofilament deficits present in Dll4-deficient vessels.
Fig. 4.
Endothelial cell delta-like protein-4 (Dll4) deficiency selectively reduces myosin phosphatase-targeting subunit 1 (MYPT1) content. A: representative immunoblot showing reduced aortic Dll4 and MYPT1 content with preserved myosin light chain kinase (MLCK) levels in tamoxifen (TM)-treated (+TM) vascular endothelial cadherin-CreERT2 [VeCad-CreERT2 (Ve)]/Dll4 flox/flox (Dll4f/f) mice. Data were normalized to Ve−Dll4f/f (control or Cre−) and represent means ± SE (n = 4). Data were analyzed using a t-test, *P = 0.0005 and **P = 0.0026. B: representative immunoblots displaying preserved smooth muscle myosin heavy chain (SMMHC) and smooth muscle α-actin (SMA) content in Dll4-deficient (Ve+Dll4def) vessels. Data are means (SD) (n = 4) normalized to GAPDH and control (Cre−) lane and were analyzed using a t-test, P = not significant (Cre− vs. Cre+). C: representative elastin-stained control and Dll4-deficient aortic cross sections. Vessels are morphologically indistinguishable with intact elastic lamina; boxes are ×40 magnification of low-power (×10) views (arrows).
Fig. 5.
Endothelial cell delta-like protein-4 (Dll4) deficiency augments myosin light chain (MLC) phosphorylation and arterial force generation. A: aortic (Ao) segments from tamoxifen-treated control [vascular endothelial cadherin-CreERT2 [VeCad-CreERT2 (Ve)]−/Dll4 flox/flox (Dll4f/f)] and Dll4-deficient (Ve+Dll4def) mice subjected to myography. Graphs display constrictor responses to KCl (left) and phenylephrine (PE, right). Data represent means ± SE (n = 5). Data for KCl were analyzed using a t-test, *P = 0.0126; data for PE were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05 and **P < 0.01. B: augmented mesenteric artery (MA) force production in response to depolarization (KCl, left) or agonists [phenylephrine (PE, middle) and angiotensin II (ANG II, right)] with Dll4 deficiency (Ve+Dll4def) versus controls (Ve−Dll4f/f). Myography data represent means ± SE. Data for KCl (n = 5) were analyzed using a t-test, *P = 0.0125; data for PE (n = 5) and ANG II (n = 4) were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05, **P < 0.001, and ***P < 0.01. C: aortic segments treated with KCl or ANG II for 10 min (10′). Representative protein blots demonstrated enrichment of phosphorylated MLC (p-MLC) relative to total MLC (T-MLC) and β-actin in Dll4-deficient rings (Ve+Dll4def) versus controls (Ve−Dll4f/f). Densitometric data represent means ± SE (n = 3) normalized to Ve−Dll4f/f and were analyzed using a t-test, *P = 0.0224 and **P = 0.0164. D: Ca2+ permeabilization and force generation experiments. MAs from control and tamoxifen-treated VeCad-CreERT2+/Dll4f/f (Ve+Dll4def) mice were permeabilized with α-toxin and exposed to incremental Ca2+ concentrations. Myograph data are expressed as tension generated above set baseline at pCa 9. Data represent means ± SE (n = 4 per group) and were analyzed using a two-way ANOVA with Bonferroni’s post hoc test, *P < 0.005 and **P < 0.001.
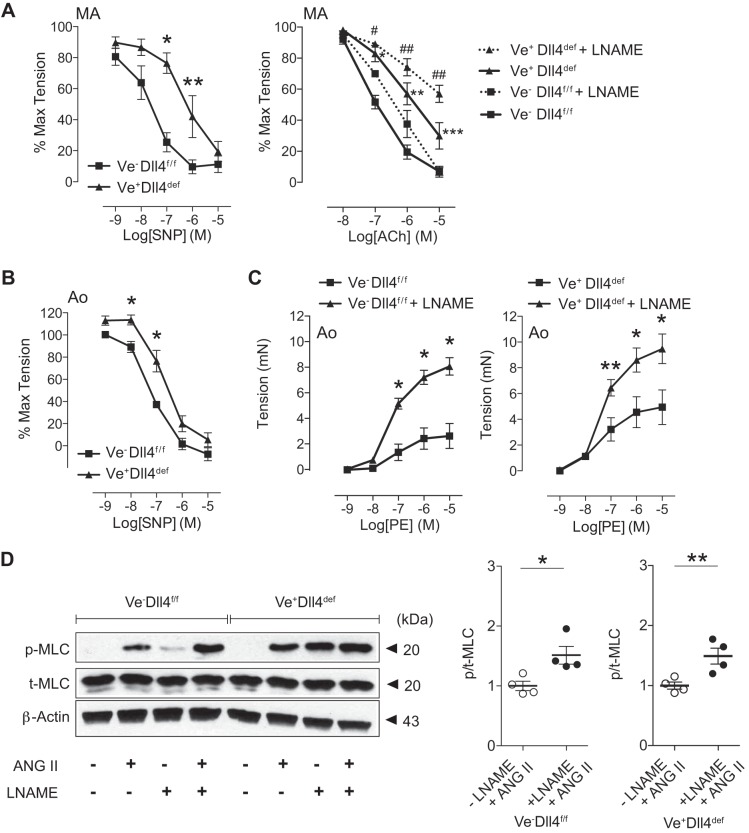
Given the concomitant reduction in smooth muscle MYPT1 content with EC Dll4 deficiency, we speculated that Dll4 might normally provide a vasorelaxant function. To test this assertion, EC Dll4-deficient MAs were mounted on the myograph, preconstricted with PE, and treated with vasodilators SNP or ACh. Compared with controls, Dll4-deficient MA segments demonstrated significantly impaired relaxation responses to both SNP and ACh (Fig. 6A, left and right, respectively, solid lines). Moreover, Dll4-deficient aortic rings similarly displayed reduced sensitivity to SNP (Fig. 6B). Together, these Dll4-dependent defects appear to recapitulate the intrinsic relaxation deficits observed in arteries from SM22Cre+DNMAML1+ animals (2).
Fig. 6.
Endothelial cell delta-like protein-4 (Dll4)-deficient arterial segments exhibit impaired relaxation. A: mesenteric artery (MA) segments from control [vascular endothelial cadherin-CreERT2 (Ve)]−/Dll4 flox/flox (Dll4f/f) and Dll4-deficient (Ve+Dll4def) mice were preconstricted with phenylephrine (PE) and subjected to sodium nitroprusside (SNP, left) and acetylcholine (ACh, right) dose-response experiments. MA segments were subjected to ACh in the presence (dotted lines) or absence (solid lines) of the endothelial NO synthase (eNOS) inhibitor Nω-nitro-l-arginine methyl ester (l-NAME). Myographic data are displayed as percentages of maximal (max) tension (PE preconstriction) and represent means ± SE (n = 5). Data were analyzed using two-way ANOVA with Bonferroni’s post hoc test [SNP (left): *P < 0.001 and **P < 0.05; ACh (right): solid line comparison, *P < 0.01, **P < 0.001, and ***P < 0.05, and dotted line comparison, #P < 0.05 and ##P < 0.001]. B: wire-mounted aortic (Ao) segments from control (Ve−Dll4f/f) and Dll4-deficient (Ve+Dll4def) mice were preconstricted with PE and subjected to SNP dose-response experiments. Myographic data are displayed as percentages of maximal tension (PE) and represent means ± SE (n = 5). Data were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.05. C: control (Ve−Dll4f/f) and Dll4-deficient (Ve+Dll4def) aortas were subjected to arterial myography in the presence or absence of l-NAME. Graphs display PE dose-response curves. Data represent means ± SE (n = 6) and were analyzed using two-way ANOVA with Bonferroni’s post hoc test, *P < 0.001 and **P < 0.01. D: effect of eNOS inhibition on phosphorylated myosin light chain (MLC) levels in constrictor-stimulated aortic rings. Aortic segments from Ve−Dll4f/f and Ve+Dll4def animals were treated with ANG II for 15 min in the presence (+) or absence (−) of l-NAME. Representative immunoblots display phosphorylated MLC (p_MLC) relative to total MLC (T-MLC) and β-actin. Data represent means ± SE of the p-MLC-to-total MLC ratio (n = 4) normalized to (−)l-NAME/(+)ANG II treatment groups. Data were analyzed using a t-test, *P = 0.0187 and **P = 0.0138. Note that in the combined absence of ANG II and l-NAME, no p-MLC signal was detected.
To determine whether Dll4-deficient impairments in vasoreactivity were independent of endothelium-derived NO relaxant function, we performed ACh dose-response experiments in MAs pretreated with the NO synthase inhibitor l-NAME. In the presence of l-NAME, both control and Dll4-deficient vessels incurred a similar reduction in relaxation, maintaining the significant difference in relaxation dependent on Dll4 (Fig. 6A, right, dotted lines). Moreover, the findings of higher force capacity in Dll4-deficient arterial segments led us to speculate that the augmented constrictor response might be in part a consequence of reduced relaxant function (i.e., unopposed vasoconstriction) also independent of NO activity. We therefore performed constrictor myography on Dll4-deficient arterial segments in the presence of l-NAME. In control vessels, l-NAME pretreatment increased force production throughout the PE dose-response curve, consistent with expected abolishment of NO signaling in ECs and unopposed vasoconstriction (Fig. 6C, left). Importantly, l-NAME treatment of Dll4-deficient vessels uncovered a similar augmentation of PE-generated force above the Dll4-deficient baseline (Fig. 6C, right). In parallel at the molecular level, l-NAME effectively and similarly raised p-MLC/total MLC content in ANG II-stimulated aortic rings from either control or Dll4-deficient vessels (Fig. 6D). Finally, we assessed the levels of soluble guanylyl cyclase, the NO receptor, in Dll4-deficient aortas and demonstrated no change in protein content of either subunit, GUCY1A3 or GUCY1B3 (data not shown). Taken together, the increased constrictor response and vasorelaxant impairments associated with intrinsic Dll4 deficiency appear unrelated to the status of endothelial NO synthase/NO activity. These findings suggest the intriguing possibility of a novel endothelial-dependent, and NO-independent, relaxation pathway through Dll4 ligand engagement of arterial smooth muscle Notch receptors.
Notch signaling deficiency in smooth muscle impairs Ca2+ sensitization.
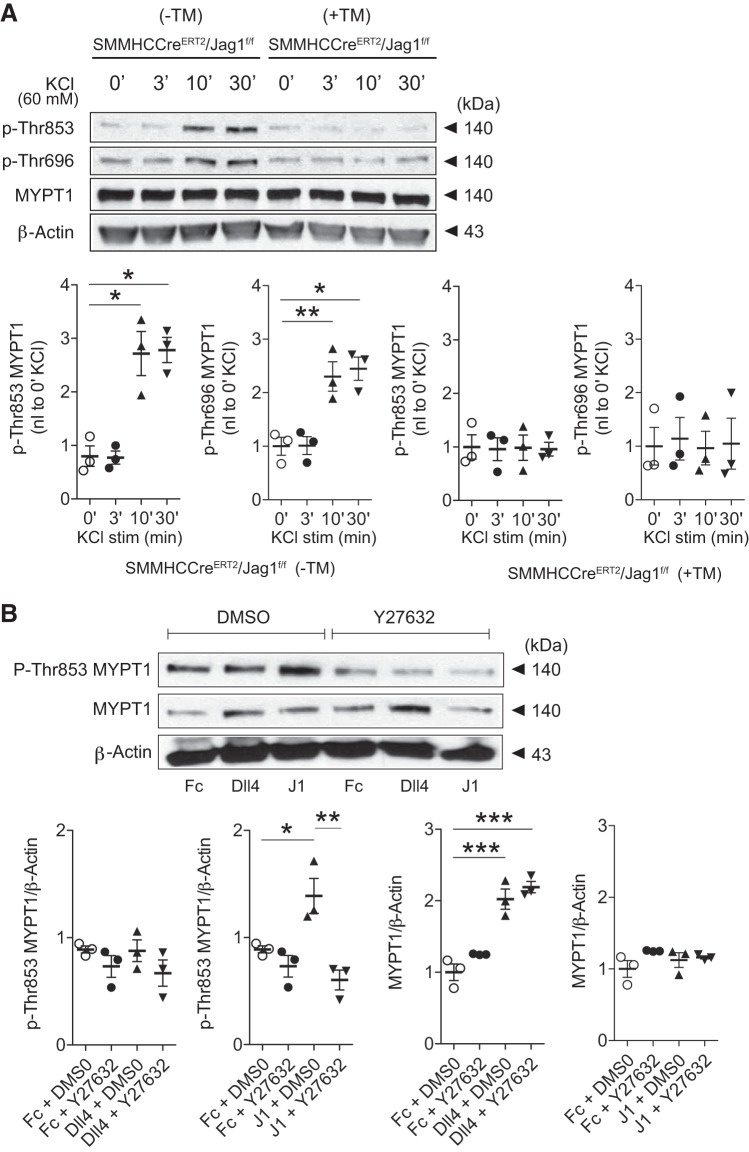
The activity of smooth muscle MP is largely dependent on the content and phosphorylation status of its regulatory subunit, MYPT1. Agonist- or depolarization-induced smooth muscle contractility is achieved not only through increasing cytosolic Ca2+ but also via coupling RhoA-ROCKII-dependent phosphorylation of MYPT1 at amino acids Thr853 and Thr696 leading to MP inhibition (13). The combined loss of enzymatic dephosphorylation of p-MLC with Ca2+/calmodulin-activated MLCK leads to greater p-MLC and force generation at a given Ca2+ concentration (i.e., Ca2+ sensitization) (50). Toward a further mechanistic understanding of vasoregulatory impairments coexistent in Notch signaling-deficient SM22Cre+DNMAML1+ mice, we identified not only a reduction in SMC MYPT1 content (Fig. 7A) but also abrogation of KCl-induced smooth muscle MYPT1 phosphorylation at Thr853 and Thr696 (Fig. 7B). Remarkably, this pattern was recapitulated in Jagged1-deficient SMCs exhibiting blunted Thr853 and Thr696 phosphorylation in response to KCl depolarization (Fig. 8A). Therefore, Jagged1 in SMCs appears necessary for optimal constrictor-induced Ca2+ sensitization.
Fig. 8.
Jagged1 deficiency impairs Ca2+ sensitization. A: representative immunoblots displaying impaired KCl-induced phosphorylation (P) of myosin phosphatase-targeting subunit 1 (MYPT1; residues Thr696 and Thr853) in Jagged1-deficient [tamoxifen (TM)-treated (+TM) smooth muscle myosin heavy chain-CreERT2 (SMMHC-CreERT2)/Jagged1 flox/flox (Jag1f/f)] aortic smooth muscle cells (SMCs). Cultured SMCs were exposed to 60 mM KCl for 0–30 min (0–30′). Data represent means ± SE (n = 3) and were analyzed using one-way ANOVA with Tukey’s post hoc test, *P < 0.01 and **P < 0.05 (vs. 0 min). KCl stim, KCl stimulation; −TM, sunflower oil control. B: Jagged1-stimulated MYPT1 phosphorylation was Rho kinase dependent. A10 aortic SMCs were treated for 5 h with DMSO or the Rho kinase inhibitor Y27632 followed by fixed-ligand [Fc, delta-like protein-4 (Dll4), or Jagged1 (J1)] stimulation for 24 h. Representative immunoblots are shown. Data represent means ± SE (n = 3) and were analyzed using one-way ANOVA with a Tukey’s post hoc test, *P < 0.05, **P < 0.01, and ***P < 0.001.
To further characterize a functional linkage between Jagged1 and Ca2+ sensitization, we returned to gain-of-function experiments using fixed ligand to determine a Jagged1 effect on MYPT1 phosphorylation. Surprisingly, Jagged1, but not Dll4, increased MYPT1 Thr853 and Thr696 phosphorylation in aortic SMCs (Fig. 1, B and C). Notably, this response occurred in the absence of exogenous constrictor. Given the established role of ROCKII on MYPT1 phosphorylation, we further examined whether Jagged1-mediated phosphorylation was ROCKII dependent. Indeed, in the ligand stimulation assay, SMCs treated with ROCK inhibitor (Y27632) were refractory to Jagged1-induced MYPT1 phosphorylation (Fig. 8B). Importantly, Y27632 had no effect on total MYPT1 levels, which were dependent on Dll4 (but not Jagged1) stimulation (Fig. 8B). Taken together, Jagged1-induced Notch signaling in smooth muscle appears to modulate ROCKII-mediated Ca2+ sensitization in a manner independent of constrictor stimulation.
DISCUSSION
This report uncovers novel mechanistic insights into selective Notch ligand control of vascular function. Arterial segments uncoupled from the circulatory system and subjected to ex vivo measures of force capacity revealed contrasting roles for EC Dll4 and smooth muscle Jagged1 in force production. Jagged1-triggered Notch signaling selectively augmented MLCK expression, p-MLC accumulation, and Ca2+ sensitization, all features associated with increasing arterial force. In contrast, Dll4 stimulation of smooth muscle led to biased accumulation of MYPT1, and its deficiency in arteries revealed impaired relaxation, consistent with a NO-independent vasorelaxant role for Dll4. These novel observations provide an explanation for the concomitant reductions in MLCK and MYPT1 and the arterial constrictive and relaxation defects in SMCs and arteries, respectively, from SM22Cre+DNMAML1+ animals harboring downstream suppression of Notch signaling [Fig. 7A and Basu et al. (2)].
In the vascular wall, Dll4 is exclusively expressed in the endothelium, whereas Jagged1 is present in both endothelial and smooth muscle compartments (8, 28, 34, 49, 56); Notch receptors 1 and 4 are present in endothelium, whereas Notch receptors 1, 2, and 3 are expressed in smooth muscle (18, 19, 56). The inherent juxtaposition of endothelial and smooth muscle layers in the arterial wall provides an anatomic basis for direct EC-SMC interactions via myoendothelial junctions permitting instructional signaling between cell types (5, 15, 26, 48, 53). Within the arterial wall, restriction of Dll4 expression in the endothelium therefore argues for directional signaling to the smooth muscle compartment, or heterotypic cell interaction, permitting Dll4-induced SMC changes in contractile function. In contrast, reduced force production observed in smooth muscle Jagged1-deficient arteries suggests that endothelial Jagged1 cannot effectively compensate, promoting the view that for its contractile function, Jagged1 signals to neighboring SMCs in a homotypic manner (24). The present study does not identify the receptor(s) through which ligand-specific signaling occurs. However, we have previously shown that Jagged1-stimulated SMCs accumulate Notch1-containing transcription complexes in the MLCK promoter, and preliminary studies from our group suggest a Notch1 dependency for arterial force production [Basu and Proweller (1), Basu et al. (2), and S. Basu and A. Proweller, unpublished observations]. Determination of unique Notch ligand-receptor pairings that subserve opposing vasoregulatory functions requires further investigation.
The importance of Jagged1-mediated signaling for arterial development and function has been reinforced using mouse models with constitutive ablation of Jagged1 in cardiac neural crest, endothelial, or smooth muscle lineages (12, 17, 24, 35). Notably, embryonic loss of Jagged1 in smooth muscle results in early, postgestational lethality due to failed closure of the ductus arteriosus with associated reduction in SMA myofilaments (12). Our inducible smooth muscle Jagged1-deficient mouse model allowed us to circumvent embryonic lethality associated with Jagged1 ablation, permitting examination of Jagged1-deficient arterial function in the adult. Interestingly, we did not observe measurable differences in contractile myofilament levels including SMA and SMMHC. Unlike developmental/perinatal contexts in which Jagged1 appears to enforce smooth muscle myofilament gene expression, our data in adult animals suggest novel roles for Jagged1 in supporting regulatory contractile gene expression (i.e., MLCK) and Ca2+ sensitization (see below) necessary for arterial force generation.
Dll4-initiated Notch signaling in ECs provides a well-established and critical role for this ligand in developmental sprouting angiogenesis (16, 31, 54). More recent investigations have begun to elucidate novel postnatal physiological functions for Dll4. In a study of retinal angiogenesis and arterial remodeling in mice, acute inhibition of Dll4 signaling prevented pathologic blood vessel regression by improving capillary perfusion associated with increased retinal expression of vasodilators (30). However, in this work, the molecular mechanisms linking Dll4/Notch signaling with vasoactive peptide production and identification of involved cell types (homotypic or heterotypic cell signaling interactions) were unclear. In contrast, heterozygous Dll4 mice displayed diminished perfusion recovery in a model of induced hindlimb ischemia (6). As previously reported, Dll4+/− resistance arteries appeared morphologically normal but exhibited impaired dilatory responses to shear stress and excessive force production when stimulated by constrictor agonists or depolarization (6). Interestingly, NO-dependent vasorelaxant responses were preserved. How Dll4 loss leads to heightened arterial contractility in this model remains uncertain. Our work suggests a regulatory link between Dll4 ligand with smooth muscle p-MLC content and arterial force production. In our view, the selective reduction in smooth muscle MYPT1 levels associated with EC Dll4 deficiency leads to diminished MP activity (as measured by p-MLC/total MLC) and therefore superior force production in response to vasoconstrictors. Interestingly, arteries from our EC Dll4-deficient mice exhibited intrinsic vasorelaxation defects not observed in vessels from Dll4 heterozygotes. This discrepancy may be in part a reflection of differences in EC Dll4 content reduction or acute versus chronic loss of Dll4 with potential for functional compensation in the chronic model. Finally, in the absence of its EC context, the ability of Dll4 ligand to alter MYPT1 levels in cultured SMCs further implies that an EC-derived vasomodulator is not required for this function.
Our in vitro ligand stimulation experiments associated Dll4 with induced MYPT1 transcript and protein levels suggesting that MYPT1 could be a direct transcriptional target of Dll4-activated Notch signaling as we have analogously found for Jagged1-mediated targeting of the MLCK promoter (1, 2). Less is known of the transcriptional regulation of MYPT1, whose promoter lacks a typical TATA box sequence (33). We have identified a conserved CSL-binding element within the MYPT1 promoter that requires functional testing (S. Basu and A. Proweller, unpublished observations). Alternatively, Dll4-dependent Notch signals could alter MYPT1 stability that is normally dependent on the proteasome (59).
An intriguing observation from our study is the Jagged1 influence on Ca2+ sensitization in addition to MLCK gene expression. Both of these effects collaboratively enhance smooth muscle contractility (i.e., increase in p-MLC/total MLC) by simultaneously inhibiting MP and promoting MLCK content, respectively. We showed that smooth muscle Jagged1 deficiency blunted constrictor-induced MYPT1 phosphorylation. Surprisingly, Jagged1 also stimulated smooth muscle-induced MYPT1 Thr696 and Thr853 phosphorylation in the absence of exogenous constrictor and in a ROCK-dependent manner. How Jagged1 recruits ROCK in this context will require further investigation but may represent a novel constrictor-independent mechanism for Ca2+ sensitization. Several additional serine and threonine residues in MYPT1 can serve as substrates for ROCK, integrin-linked kinase, Zipper-interacting protein kinase, PKA, and/or PKG with variable functional consequences though concern exists for specificity of site-specific p-MYPT1 antibodies (32). Future studies remain to determine whether Jagged1 exhibits a broader influence on MYPT1 phosphorylation and/or on other complex mechanisms that regulate Ca2+ sensitization and MP activity including the inhibitory function of PKC-potentiated inhibitor protein of 17 kDa (10, 27, 47). Jagged1-dependent proteomic analyses will likely be informative in this regard.
In humans, heritable mutations in Jagged1 give rise to Alagille syndrome, which features multiorgan system pathologies in the liver and bone, growth retardation, and a plethora of cardiovascular manifestations including tetralogy of Fallot, pulmonary artery stenosis, and patent ductus arteriosus (25, 37). The molecular mechanism(s) linking Jagged1 status to the vascular abnormalities remains uncertain but is likely related to the dependence on this ligand for arterial wall development and function, as we and others have suggested (12, 17, 35).
In this report, we used wire myography to study arterial force production in response to depolarization and agonist constrictors and relaxants. This methodology has yielded striking differences in pharmacologic responses in both conduit and resistance arteries lacking Notch ligands Dll4 or Jagged1. Complementary studies using pressure myography are needed to extend analyses to determine reliance of Notch ligands for myogenic function, whose mechanisms also feature Ca2+-induced MLCK and Ca2+ sensitization activities contributing to basal arterial tone and flow-dependent autoregulative properties (7, 57). Hemodynamic analyses are needed to further aid characterization of homeostatic and stress-responsive functions contributed by Dll4 and Jagged1 in vivo.
In summary, we have uncovered dual and opposing roles for Dll4 and Jagged1 ligands in arterial vasoreactivity. Precedent exists for coantagonistic functions of Dll4 and Jagged1 among ECs during sprouting angiogenesis through a mechanism dictated by the glycosylation status of the Notch1 receptor (4). Whether such ligand-discriminating mechanism is operational in arterial vasoreactivity is unknown, but it will be important to determine the proximal stimuli regulating Dll4 and Jagged1 activity in this context. Our data suggest a model in which Notch signaling instructions via heterotypic (EC-SMC) and homotypic (SMC-SMC) cell interactions provide modulatory input for arterial vasoregulation (Fig. 9). In our view, smooth muscle Jagged1 stimulation of Notch receptors (Notch1 and possibly other family members) leads to force enhancement, whereas EC Dll4 stimulates smooth muscle Notch receptors to promote vasorelaxation. These conclusions are derived from in vitro and ex vivo analyses linking ligand, intrinsic arterial force production, and the molecular signature of force (p-MLC/total MLC). The mechanisms coupling Dll4 signaling with MYPT1 content remain uncertain but may include transcriptional regulation of MYPT1 or posttranscriptional/translational modifications that favor Ca2+ desensitization. Our work suggests that Notch signaling might play a dynamic role in vasoregulation through influencing regulatory MLCK and MP activities, thereby functioning as a rheostat for arterial tone control. Pharmacological manipulation of Dll4 or Jagged1 in the vascular compartment may therefore be useful in disorders associated with arterial contractile dysfunction (for example, arterial hypertension, vasospasm, patent ductus arteriosus, portal hypertension, and heart failure). Interestingly, the Jagged1 locus has been identified in a genome-wide association study of hypertension (9, 40). Investigations in animal models that recapitulate human vascular disease syndromes should further highlight important roles for ligand-specific Notch signaling in the healthy and diseased vasculature.
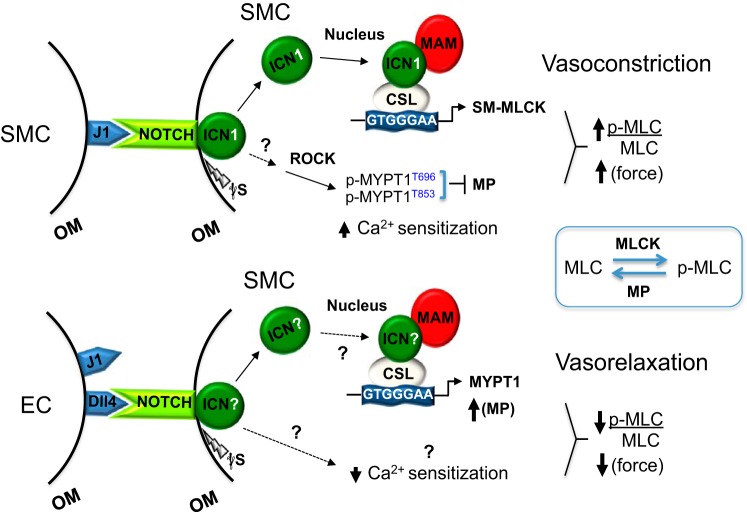
Fig. 9.
Model of Notch ligand-directed modulation of arterial vasoreactivity. Smooth muscle (SM) Jagged1 (J1) ligand engages Notch receptors on neighboring smooth muscle cells (SMCs) in the tunica media. These homotypic cell interactions trigger γ-secretase (γS) cleavage, release of intracellular Notch1 (ICN), and its translocation to the nucleus, where it targets and transcriptionally activates the myosin light chain kinase (MLCK) promoter. Jagged1 also stimulates Rho kinase (ROCK) phosphorylation of myosin phosphatase regulatory subunit 1 (MYPT1) residues Thr696 and Thr853 to inactivate myosin phosphatase (MP; i.e., Ca2+ sensitization). These combined effects result in a high phosphorylated myosin light chain (p-MLC)-to-total MLC ratio and force production. Though our prior report and data herein implicate the Notch1 receptor in transducing Jagged1-mediated vasoconstriction, it is possible that other Notch receptors may be used. Furthermore, how Notch signaling activates ROCK remains under investigation. Impairments in force production due to smooth muscle Jagged1 deficiency suggest that endothelial cell (EC) Jagged1 may be unable to confer comparable vasoconstrictor signals. In contrast to Jagged1, delta-like protein-4 (Dll4) is only expressed in the endothelium of arteries, which therefore suggests engagement of smooth muscle Notch receptors (as yet unidentified) through heterotypic cell interactions. Dll4 increases smooth muscle MYPT1 transcript and protein levels, suggesting that MYPT1 may be a target of Dll4-Notch activation. The heightened levels of MYPT1 support increased MP activity and vasorelaxation as reflected in relatively low p-MLC-to-total MLC ratios. The possibility that Dll4 alters Ca2+ sensitization remains to be determined. CSL, DNA-binding protein (CBF-1, suppressor of Hairless, Lag-2; also known as RBPJκ); MAM, Mastermind; OM, outer membrane; p-MYPT1, phosphorylated MYPT1 (residues Thr696 and Thr853). See text for further details.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grants HL-096603 and HL-128281-01 (to A. Proweller) and the Visconsi Scholars fund (to A. Proweller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S. Basu and A.P. conceived and designed research; S. Basu, I.B., A.C., and S. Banerjee performed experiments; S. Basu, I.B., A.C., S. Banerjee, and A.P. analyzed data; S. Basu, I.B., A.C., S. Banerjee, and A.P. interpreted results of experiments; S. Basu and A.P. prepared figures; S. Basu and A.P. drafted manuscript; S. Basu and A.P. edited and revised manuscript; S. Basu and A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Sergei Merkulov for technical assistance.
REFERENCES
- 1.Basu S, Proweller A. Autoregulatory control of smooth muscle myosin light chain kinase promoter by Notch signaling. J Biol Chem 291: 2988–2999, 2016. doi: 10.1074/jbc.M115.679803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Srinivasan DK, Yang K, Raina H, Banerjee S, Zhang R, Fisher SA, Proweller A. Notch transcriptional control of vascular smooth muscle regulatory gene expression and function. J Biol Chem 288: 11191–11202, 2013. doi: 10.1074/jbc.M112.442996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belin de Chantemèle EJ, Retailleau K, Pinaud F, Vessières E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, Joutel A, Henrion D. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol 28: 2216–2224, 2008. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135, 2009. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Billaud M, Lohman AW, Straub AC, Parpaite T, Johnstone SR, Isakson BE. Characterization of the thoracodorsal artery: morphology and reactivity. Microcirculation 19: 360–372, 2012. doi: 10.1111/j.1549-8719.2012.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cristofaro B, Shi Y, Faria M, Suchting S, Leroyer AS, Trindade A, Duarte A, Zovein AC, Iruela-Arispe ML, Nih LR, Kubis N, Henrion D, Loufrani L, Todiras M, Schleifenbaum J, Gollasch M, Zhuang ZW, Simons M, Eichmann A, le Noble F. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development 140: 1720–1729, 2013. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18: 2474–2478, 2004. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ et al.; International Consortium for Blood Pressure Genome-Wide Association Studies Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eto M, Kitazawa T. Diversity and plasticity in signaling pathways that regulate smooth muscle responsiveness: paradigms and paradoxes for the myosin phosphatase, the master regulator of smooth muscle contraction. J Smooth Muscle Res 53: 1–19, 2017. doi: 10.1540/jsmr.53.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 274: 37385–37390, 1999. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 12.Feng X, Krebs LT, Gridley T. Patent ductus arteriosus in mice with smooth muscle-specific Jag1 deletion. Development 137: 4191–4199, 2010. doi: 10.1242/dev.052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassie ME, Sutherland C, Ulke-Lemée A, Chappellaz M, Kiss E, Walsh MP, MacDonald JA. Cross-talk between Rho-associated kinase and cyclic nucleotide-dependent kinase signaling pathways in the regulation of smooth muscle myosin light chain phosphatase. J Biol Chem 287: 36356–36369, 2012. doi: 10.1074/jbc.M112.398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groneberg D, König P, Wirth A, Offermanns S, Koesling D, Friebe A. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation 121: 401–409, 2010. doi: 10.1161/CIRCULATIONAHA.109.890962. [DOI] [PubMed] [Google Scholar]
- 15.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix? Microcirculation 16: 307–322, 2009. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780, 2007. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 17.High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci USA 105: 1955–1959, 2008. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res 100: 1556–1568, 2007. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann JJ, Iruela-Arispe ML. Notch expression patterns in the retina: an eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns 7: 461–470, 2007. doi: 10.1016/j.modgep.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 21.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet 2: e4, 2006. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, Radtke F. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 205: 2515–2523, 2008. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233, 2009. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krebs LT, Norton CR, Gridley T. Notch signal reception is required in vascular smooth muscle cells for ductus arteriosus closure. Genesis 54: 86–90, 2016. doi: 10.1002/dvg.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16: 243–251, 1997. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 26.Lilly B. We have contact: endothelial cell-smooth muscle cell interactions. Physiology (Bethesda) 29: 234–241, 2014. doi: 10.1152/physiol.00047.2013. [DOI] [PubMed] [Google Scholar]
- 27.Lincoln TM. Myosin phosphatase regulatory pathways: different functions or redundant functions? Circ Res 100: 10–12, 2007. doi: 10.1161/01.RES.0000255894.25293.82. [DOI] [PubMed] [Google Scholar]
- 28.Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol 159: 875–883, 2001. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood 117: 6728–6737, 2011. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 31.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104: 3219–3224, 2007. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald JA, Walsh MP. Regulation of smooth muscle myosin light chain phosphatase by multisite phosphorylation of the myosin targeting subunit, MYPT1. Cardiovasc Hematol Disord Drug Targets 18: 4–13, 2018. doi: 10.2174/1871529X18666180326120638. [DOI] [PubMed] [Google Scholar]
- 33.Machida H, Ito M, Okamoto R, Shiraki K, Isaka N, Hartshorne DJ, Nakano T. Molecular cloning and analysis of the 5′-flanking region of the human MYPT1 gene. Biochim Biophys Acta 1517: 424–429, 2001. doi: 10.1016/S0167-4781(00)00285-2. [DOI] [PubMed] [Google Scholar]
- 34.Mailhos C, Lewis J, Ish-Horowicz D, Modlich U, Harris A, Bicknell R. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation 69: 135–144, 2001. doi: 10.1046/j.1432-0436.2001.690207.x. [DOI] [PubMed] [Google Scholar]
- 35.Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation 125: 314–323, 2012. doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun 369: 149–156, 2008. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, Goldmuntz E. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation 106: 2567–2574, 2002. doi: 10.1161/01.CIR.0000037221.45902.69. [DOI] [PubMed] [Google Scholar]
- 38.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics−2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. [Errata in Circulation 131: e535, 2015, and Circulation 133: e417, 2016.] 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 39.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977. doi: 10.1161/01.RES.41.1.19. [DOI] [PubMed] [Google Scholar]
- 40.Munroe PB, Barnes MR, Caulfield MJ. Advances in blood pressure genomics. Circ Res 112: 1365–1379, 2013. doi: 10.1161/CIRCRESAHA.112.300387. [DOI] [PubMed] [Google Scholar]
- 41.Murányi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett 579: 6611–6615, 2005. doi: 10.1016/j.febslet.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 42.Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet 356: 1955–1964, 2000. doi: 10.1016/S0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 43.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension 35: 539–543, 2000. doi: 10.1161/01.HYP.35.2.539. [DOI] [PubMed] [Google Scholar]
- 44.Osei-Owusu P, Sabharwal R, Kaltenbronn KM, Rhee MH, Chapleau MW, Dietrich HH, Blumer KJ. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. J Biol Chem 287: 12541–12549, 2012. doi: 10.1074/jbc.M111.332130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci USA 107: 15798–15803, 2010. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proweller A, Wright AC, Horng D, Cheng L, Lu MM, Lepore JJ, Pear WS, Parmacek MS. Notch signaling in vascular smooth muscle cells is required to pattern the cerebral vasculature. Proc Natl Acad Sci USA 104: 16275–16280, 2007. doi: 10.1073/pnas.0707950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao YN, He WQ, Chen CP, Zhang CH, Zhao W, Wang P, Zhang L, Wu YZ, Yang X, Peng YJ, Gao JM, Kamm KE, Stull JT, Zhu MS. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem 289: 22512–22523, 2014. doi: 10.1074/jbc.M113.525444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol 36: 67–76, 2009. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 49.Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev 14: 1313–1318, 2000. [PMC free article] [PubMed] [Google Scholar]
- 50.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 51.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sörensen I, Adams RH, Gossler A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113: 5680–5688, 2009. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 53.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491: 473–477, 2012. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A. The Notch ligand delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104: 3225–3230, 2007. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasquez-Del Carpio R, Kaplan FM, Weaver KL, VanWye JD, Alves-Guerra MC, Robbins DJ, Capobianco AJ. Assembly of a Notch transcriptional activation complex requires multimerization. Mol Cell Biol 31: 1396–1408, 2011. doi: 10.1128/MCB.00360-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev 108: 161–164, 2001. doi: 10.1016/S0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 57.Walsh MP, Cole WC. The role of actin filament dynamics in the myogenic response of cerebral resistance arteries. J Cereb Blood Flow Metab 33: 1–12, 2013. doi: 10.1038/jcbfm.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horváth B, Maser-Gluth C, Greiner E, Lemmer B, Schütz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008. [Erratum in Nat Med 14: 222, 2008. 10.1038/nm0208-222.] doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737, 2007. doi: 10.1161/01.RES.0000260189.38975.35. [DOI] [PubMed] [Google Scholar]