Abstract
Dehydroepiandrosterone (DHEA) is an adrenal steroid hormone, which has the highest serum concentration among steroid hormones with DHEA sulfate (DHEAS). DHEA possesses an inhibitory action on glucose-6-phosphate dehydrogenase (G6PD), the first pentose-phosphate pathway enzyme that reduces NADP+ to NADPH. DHEA induced relaxation of high K+-induced contraction in rat arterial strips, whereas DHEAS barely induced it. We studied the effects of DHEA on L-type Ca2+ current (ICa,L) of A7r5 arterial smooth muscle cells and compared the mechanism of inhibition with that produced by the 6-aminonicotinamide (6-AN) competitive inhibitor of G6PD. DHEA moderately inhibited ICa,L that was elicited from a holding potential (HP) of −80 mV [voltage-independent inhibition (VIDI)] and accelerated decay of ICa,L during the depolarization pulse [voltage-dependent inhibition (VDI)]. DHEA-induced VDI decreased peak ICa,L at depolarized HPs. By applying repetitive depolarization pulses from multiple HPs, novel HP-dependent steady-state inactivation curves (f∞-HP) were constructed. DHEA shifted f∞-HP to the left and inhibited the window current, which was recorded at depolarized HPs and obtained as a product of current-voltage relationship and f∞-HP. The IC50 value of ICa,L inhibition was much higher than serum concentration. DHEA-induced VDI was downregulated by the dialysis of guanosine 5′-O-(2-thiodiphosphate), which shifted f∞-voltage to the right before the application of DHEA. 6-AN gradually and irreversibly inhibited ICa,L by VIDI, suggesting that the inhibition of G6PD is involved in DHEA-induced VIDI. In 6-AN-pretreated cells, DHEA induced additional inhibition by increasing VIDI and generating VDI. The inhibition of G6PD underlies DHEA-induced VIDI, and DHEA additionally induces VDI as described for Ca2+ channel blockers.
NEW & NOTEWORTHY Dehydroepiandrosterone, the most abundantly released adrenal steroid hormone with dehydroepiandrosterone sulfate, inhibited L-type Ca2+ current and its window current in aortic smooth muscle cells. The IC50 value of inhibition decreased with the depolarization of holding potential to 15 µM at −20 mV. The inhibition occurred in a voltage-dependent manner as described for Ca2+ channel blockers and in a voltage-independent manner because of the inhibition of glucose-6-phosphate dehydrogenase.
Keywords: Ca2+ channel blocker, dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, L-type Ca2+ current, window current
INTRODUCTION
Dehydroepiandrosterone (DHEA) is an adrenal steroid hormone with the highest serum concentration among steroid hormones with its sulfate ester, DHEA sulfate (DHEAS). DHEA/DHEAS is regarded as the prohormone of sex steroids, such as estrogen and testosterone (25). Serum DHEAS concentration can be as high as 10 μM in young adults and decreases with age (25, 35). In a healthy aged 65- to 81-yr-old population, plasma DHEAS concentration is 2.2 µM in women and 3.3 µM in men (13). Serum DHEA concentration is around 20 nM in young adults and decreases with age to 7 nM (25). To compensate for the decrease in aged people, DHEA is available over the counter in the United States. DHEA and DHEAS are synthesized and have functional roles in the brain as neurosteroids (27a).
Among steroid hormones, DHEA and its metabolite epiandrosterone (EPI) are unique because they have potent inhibitory action on glucose-6-phosphate dehydrogenase (G6PD), the first pentose-phosphate pathway (PPP) enzyme (14, 28, 40, 41); the inhibition develops at a relatively high concentration in the micromole order, e.g., 80% inhibition occurs at 40 µM (28). DHEAS needs concentration of about two orders higher to inhibit G6PD (14) compared with DHEA. Since G6PD reduces NADP+ to NADPH and PPP generates ribose necessary for synthesis of nucleic acids, the inhibition of G6PD influences redox regulation and cell proliferation (41). Cancer cells use the ATP provided by glycolysis and ribose provided by PPP to proliferate (40, 41, 43, 45). 6-Aminonicotinamide (6-AN) is a competitive inhibitor of G6PD since its metabolite 6-AN adenine dinucleotide phosphate binds G6PD competitively with NADP+ to inhibit G6PD (24).
DHEA activates large-conductance Ca2+-activated K+ channel current in cultured ferret pulmonary artery smooth muscle cells and produces relaxation of hypoxia-induced contraction of the ferret pulmonary artery (10). DHEA-induced activation of large-conductance Ca2+-activated K+ channel current prevents and reverses chronic hypoxic pulmonary hypertension in rats (4). We found that DHEA, EPI, and 6-AN produce relaxation of high K+-induced contraction of arterial strips from the aorta and pulmonary artery of rats (16). EPI and 6-AN inhibit G6PD, decrease the NADPH-to-NADP+ ratio, and induce relaxation of high K+-induced contraction in bovine coronary artery smooth muscles (15). Furthermore, DHEA inhibits G6PD, decreases the NADPH-to-NADP ratio, and induces relaxation of high K+-induced contraction in bovine pulmonary airway smooth muscle (ASM) (36). We found that the DHEA metabolite EPI inhibits L-type Ca2+ current (ICa,L) in a voltage-dependent manner in ventricular myocytes from rabbits (17). DHEA inhibits recombinant T-type Ca2+ channel current (ICa,T) in a voltage-dependent manner in cultured neuroblastoma cells (5). Moreover, from the effects of pertussis toxin and by the dialysis of guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), it has been proposed that G protein-coupled receptor (GPCR) signaling is involved in the mechanism of DHEA-induced inhibition of ICa,T (5). 6-AN inhibits ICa,L in a voltage-independent manner in ventricular myocytes from rats (38).
ICa,L, through Cav1.2 channels [L-type Ca2+ channels (LCCs)], plays important roles in the regulation of blood pressure and blood flow that depend on the contraction of ASM cells (ASMCs) (20, 30). The resting membrane potential in ASMCs is relatively less and sustains low depolarization by the opening of transient receptor potential channels (9, 18, 46) and activates LCCs to produce window current (IWD) with a small amplitude and over a sustained time course (32), resulting in a sustained increase of the intracellular Ca2+ concentration to induce tonic contraction (11).
We studied dose- and voltage-dependent DHEA-induced inhibition of ICa,L and IWD and the effect of 6-AN on ICa,L in ASMCs. IWD was successfully recorded at depolarized holding potentials (HPs) and was simulated by a steady-state inactivation curve (f∞-HP) obtained by the depolarization of HP and current-voltage (I–V) relationship. DHEA inhibited ICa,L and IWD in a voltage-dependent manner [voltage-dependent inhibition (VDI)]. In addition, DHEA induced voltage-independent inhibition (VIDI) that was also produced by 6-AN.
MATERIALS AND METHODS
The A7r5 aortic smooth muscle cell line derived from the thoracic aorta of rat embryos (23) was used. Methods of culture of A7r5 cells, whole cell clamp, and data analysis have been described in detail in previous studies (33, 34). To examine the effects of DHEA and DHEAS on the contraction of ASM, the excised aorta and carotid artery from rats were used. The experimental protocols of using rats conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and had approval from the Committee of New York Medical College.
Solutions for patch clamp.
The normal Tyrode solution contained the following (in mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, and 5.5 glucose, adjusted to pH 7.4 with NaOH. The Ba2+ solution contained the following (in mM): 108 NaCl, 20 TEACl, 5.4 CsCl, 10 BaCl2, 1 MgCl2, 5 HEPES, and 5.5 glucose, adjusted to pH 7.4 with NaOH. The pipette solution contained the following (in mM): 115 Cs-aspartate, 20 TEA Cl, 1 MgCl2, 5 BAPTA, 3 Mg.ATP, 0.2 GTP, and 10 HEPES, adjusted to pH 7.2 using CsOH. DHEA and DHEAS were dissolved in DMSO to make 100 mM stock solutions. 6-AN was dissolved in DMSO as a 1 M stock solution. All salts and drugs were from Sigma-Aldrich (St. Louis, MO). Experiments were performed at room temperature.
Patch clamp.
Dissociated A7r5 cells were perfused with normal Tyrode solution for 20–30 min in a perfusion chamber on the stage of an inverted microscope. The pipette resistance was 8–15 MΩ. ICa,L for the I-V relationships was usually recorded by applying 500-ms depolarization steps in 10-mV increments from −50 or −40 to 50 mV at 0.2 Hz, starting from a HP of −80 mV, preceded by a short prepulse to −50 or −40 mV. To examine the dose-dependent effects of DHEA and 6-AN, constant depolarization steps to 0 or −10 mV were repeatedly applied at 1/20 s.
Data analysis.
ICa,L was analyzed after the low-pass filtering with a cutoff of 0.5 or 1 kHz, using pClamp software. ICa,L was quantified after subtracting Cd2+ (30 or 100 µM)-resistant current. I-V relationships were fitted to the following equation adapted from the Boltzmann equation: ICa,L = Gmax (V − Erev)/{1 + exp [(V0.5 − V)/k)]}, where Gmax is maximal conductance, Erev is reversal potential, V0.5 is the half-activation potential, and k is the slope factor. Steady-state inactivation curves (f∞-V and f∞-HP) were fitted with the following Boltzmann equation: f∞ = c0 + (c1 −c0)/[1 + exp (V − V0.5)/k], where V0.5 is the voltage that causes a 50% reduction in the amplitude of the inactivating component of ICa,L. The dose-inhibition relationship was obtained applying the Hill equation. Curve fitting was conducted using Igor (V.7, Wavemetrics, Portland, OR).
Statistics.
Data are presented as means ± SE in the figures and as means (SD) in the tables. Statistical analysis was performed by GraphPad Prism (V.7, GraphPad Software, San Diego, CA) and Igor.
Vascular tone experiments.
The thoracic aorta and left common carotid artery were taken from female Wistar-Kyoto rats. After vessels were excised from the animal, they were placed in normal Krebs bicarbonate buffer solution (pH 7.4) containing the following (in mM): 118 NaCl, 4.7 KCl, 1.5 CaCl2·2H2O, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, 5.6 glucose, and 10 HEPES. Isolated arteries were then cleaned of connective tissue, cut in rings, and studied for changes in isometric force. Arterial rings were mounted to force transducers in individual temperature-regulated (37°C) 10-ml baths (Metro Scientific) at an optimal passive tension of 1 g for the aorta and 0.5 g for the carotid artery in Krebs buffer solution. After 30 min of incubation, the arterial preparation viability and equilibration were assessed against the response to 60 mM KCl. A brief membrane depolarization of vascular preparations with 60 mM KCl increases the reproducibility of the subsequent vascular responses. All drugs and chemical compounds were sourced from Sigma-Aldrich (Milwaukee, WI) and Cayman Chemical (Ann Arbor, MI). For the registration of vascular ring contractile activity and its following analysis, AcqKnowledge 3.9.1.6 (BIOPAC Systems) software was used. Vascular tension is presented as a percentage of the constriction level obtained to the exposure to 60 mM KCl. Results are expressed as means ± SE of these measurements. A Student’s t-test for unpaired data was carried out to evaluate the statistical significance. Differences were assumed to reach statistical significance if the confidence range was P < 0.05.
RESULTS
Relaxation of high K+-induced contraction by DHEA.
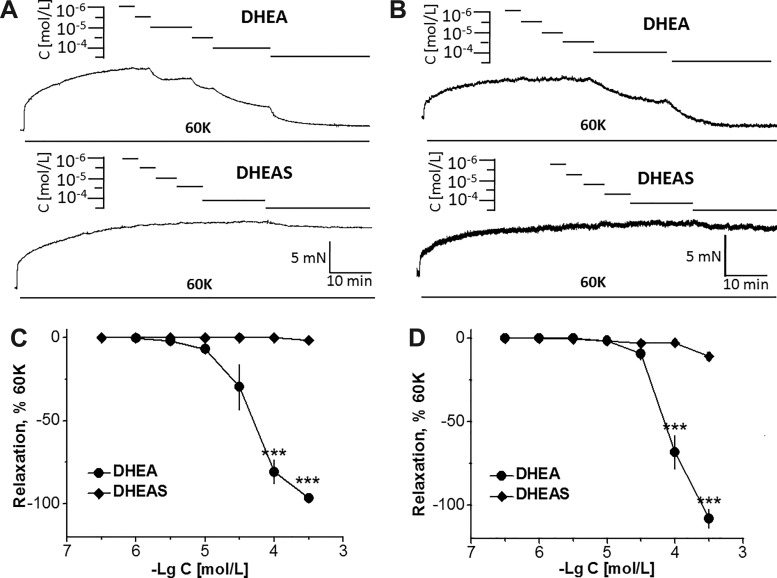
High K+ causes contraction via Ca2+ influx through Ca2+ channels. The effect of DHEA and DHEAS on 60 mM K+-induced contraction of rat arteries was examined. As noted from the original traces and summarized plots, DHEA dose dependently induced relaxation of arterial rings from the aorta and carotid artery (Fig. 1). The relaxation started at pC(−logC)5 (10 µM) in the aorta and at pC4.5 in the carotid artery, strongly increasing at 100 µM in both arteries, and full relaxation was attained at pC3.5 (316 µM) in the aorta. In the carotid artery, pC3.5 DHEA induced relaxation beyond the basal tone (Fig. 1, B and D). In contrast, DHEAS induced only negligible relaxations, even at the highest concentration of pC3.5 (Fig. 1D). The extent of relaxation was different from that produced by DHEA, with statistical significance at pC4 and 3.5.
Fig. 1.
Effect of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) on the tone of the intact aorta and left common carotid artery of Wistar-Kyoto rats. A: original traces illustrating the effect of DHEA and DHEAS on the tone of the aorta precontracted with 60 mM KCl (60K). B: original traces illustrating the effect of DHEA and DHEAS on the tone of the left common carotid artery precontracted with 60K. C: summarized data showing the effect of DHEA and DHEAS on the tone of the aorta precontracted with 60K (n = 5 from 5 animals). D: summarized data showing the effect of DHEA and DHEAS on the tone of the left common carotid artery precontracted with 60K (n = 5 from 5 animals). Each symbol represents the mean ± SE. ***P < 0.001 compared with relaxation produced by DHEAS.
DHEA-induced inhibition of ICa,L in the I-V relationship.
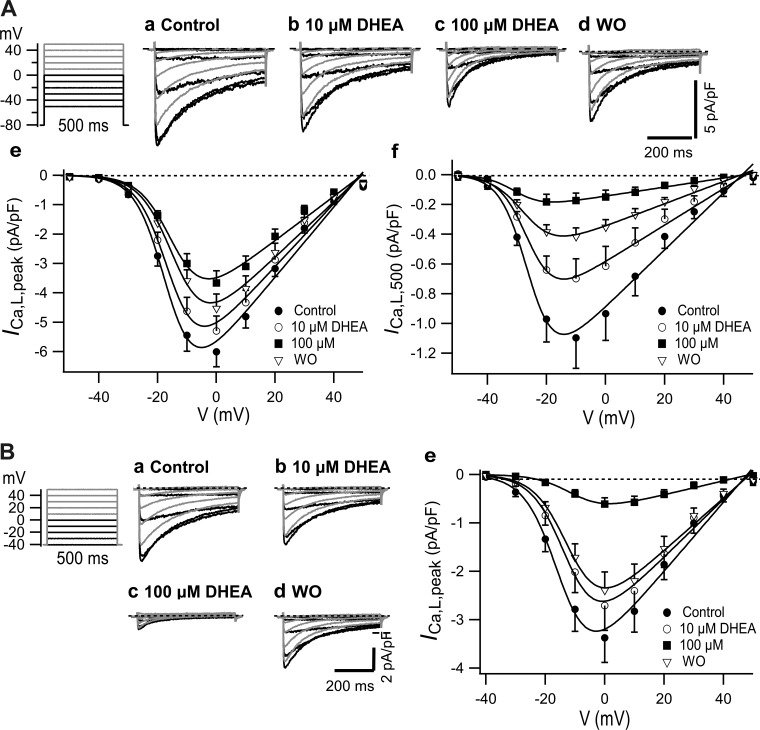
As shown in Fig. 2A, ICa,L was elicited by the depolarization pulses from a HP of −80 mV. A substantial ICa,L appeared at −30 mV, which increased to reach a maximum at around 0 mV, and gradually decreased with a further increase in depolarization to reach a reversal potential at nearly 50 mV. Application of 10 and 100 µM DHEA dose dependently inhibited ICa,L, and it was partially reversible through the washout of DHEA. DHEA decreased the current density of peak amplitude of ICa,L (ICa,L,peak; Fig. 2Ae). Curve fitting with Boltzmann’s equation revealed that the decrease was due to the decrease in Gmax of ICa,L,peak by 10% and 37% at 10 and 100 µM, respectively (Table 1). DHEA at 100 µM slightly shifted V0.5 for activation to the right. The I-V relationship of ICa,L,500, which is the amplitude at the end of the pulse, was maximal at −10 mV, and DHEA inhibited it more extensively than ICa,L,peak (Fig. 2Af) by decreasing Gmax by 36% and 84% at 10 and 100 µM, respectively (Table 1). The larger inhibition of ICa,L,500 compared with that of ICa,L,peak was due to the VDI that developed during the depolarization pulses. Since voltage-dependent inactivation is negligible at a HP of −80 mV, as shown later in Fig. 5B, the inhibition of ICa,L,peak elicited from −80 mV by DHEA was due to VIDI. DHEA-induced potent inhibition of ICa,L,500 materialized because of the VDI that developed during 500-ms depolarization in the presence of VIDI. ICa,L density at a HP of −40 mV was smaller than that at a HP of −80 mV by ~40% (Fig. 2B). At a HP of −40 mV, 10 and 100 µM DHEA inhibited ICa,L more greatly than that at a HP of −80 mV through the contribution of VDI, decreasing Gmax by 16% and 80%, respectively (Table 1). V0.5 required for activation was slightly shifted to the right by DHEA.
Fig. 2.
Effect of dehydroepiandrosterone (DHEA) on the current-voltage (I-V) relationship of L-type Ca2+ current (ICa,L). A: holding potential (HP) of −80 mV. a–d refer to typical current traces, e is the I-V of peak current density (ICa,L,peak) (n = 23), and f is the I-V of current density at 500 ms (ICa,L,500) (n = 18). B: HP of −40 mV. a–d show typical traces, and e is the I-V of ICa,L,peak when n = 9. I-V relationships were fitted with the Botzmann equation as per the parameters shown in Table 1. WO, washout.
Table 1.
Effect of DHEA, HP, and GDP-β-S dialysis on parameters of the I-V relationship shown in Figs. 2, 5, and 7
| n | Gmax, pS/pF | V0.5, mV | |
|---|---|---|---|
| Control (HP: −80 mV) | 23 | 119.2 (9.0) | −16.5 (1.1) |
| 10 µM DHEA | 23 | 107.4 (8.1)† | −15.5 (1.1) |
| 100 µM DHEA | 23 | 75.4 (6.0)‡ | −14.3 (1.2)‡ |
| Control I500 | 18 | 19.2 (1.3) | −25.7 (1.0) |
| 10 µM DHEA | 18 | 12.3 (0.7)‡ | −25.9 (0.8) |
| 100 µM DHEA | 12 | 3.0 (0.2)‡ | −30.0 (0.8)‡ |
| Control (HP: −40 mV) | 9 | 71.5 (6.1) | −14.5 (1.3) |
| 10 µM DHEA | 9 | 59.9 (4.7)‡ | −12.1 (1.7)‡ |
| 100 µM DHEA | 9 | 14.5 (1.0)‡ | −9.8 (1.1)‡ |
| Control (HP: −80 mV) | 21 | 129.6 (12.4) | −15.1 (1.4) |
| 30 µM DHEA | 21 | 83.2 (8.1)‡ | −14.6 (1.5) |
| Control (HP: −80 mV) | 22 | 137.0 (14.2) | −17.1 (1.6) |
| GDP-β-S (HP: −80 mV) | 17 | 140.8 (10.9) | −13.8 (1.2)‡ |
| Control (HP: −40 mV) | 22 | 80.0 (7.6) | −14.5 (1.5) |
| GDP-β-S (HP: −40 mV) | 17 | 110.4 (10.0)‡ | −11.8 (1.4)‡ |
Mean values of L-type Ca2+ current (ICa,L) density were plotted against the test potential and then applied to the Boltzmann equation of ICa,L activation given in materials and methods. Mean (SD) values were obtained by curve fitting of 10 or 11 points; n, number of cells. Gmax, maximal conductance; V0.5, half-activation potential; DHEA, dehydroepiandrosterone; HP, holding potential; GDP-β-S, guanosine 5′-O-(2-thiodiphosphate). Statistical comparisons of the values of control and test were performed using ordinary one-way ANOVA followed by Dunnet’s test.
P < 0.01;
P < 0.001.
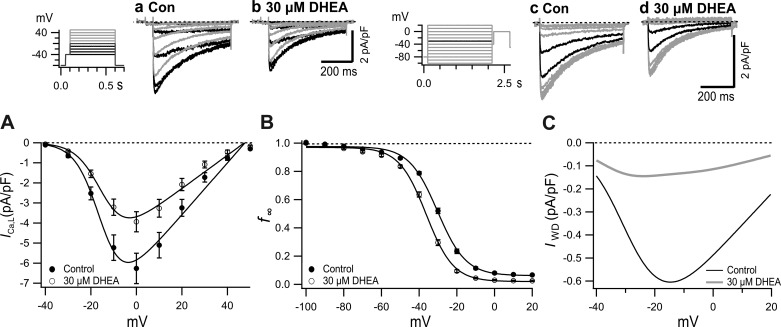
Fig. 5.
Effects of 30 µM dehydroepiandrosterone (DHEA) on current-voltage (I-V), quasi-steady-state inactivation curve (f∞-V), and window current (IWD)-V relationships. a and b: Typical current traces before (a) and after (b) application of 30 µM DHEA. Inset: pulse protocol. c and d: Typical records for control (c) and 30 µM DHEA (d). Inset: pulse protocol with 2-s conditioning pulses and 500-ms test pulse. Currents after the conditioning pulses to −40 mV and −30 mV are shown by black traces. A: I-V relationship of peak L-type Ca2+ current (ICa,L,peak) in the presence and absence of 30 µM DHEA with fitting by the Boltzmann’s equation. B: f∞-V relationship before and after application of 30 µM DHEA. Plots were fitted with the Boltzmann’s equation (Table 3). C: voltage-dependent changes of IWD obtained as a product of I-V (A) and f∞-V (B) in the absence and presence of 30 µM DHEA. Con, control.
Dose and HP dependence of DHEA-induced inhibition of ICa,L.
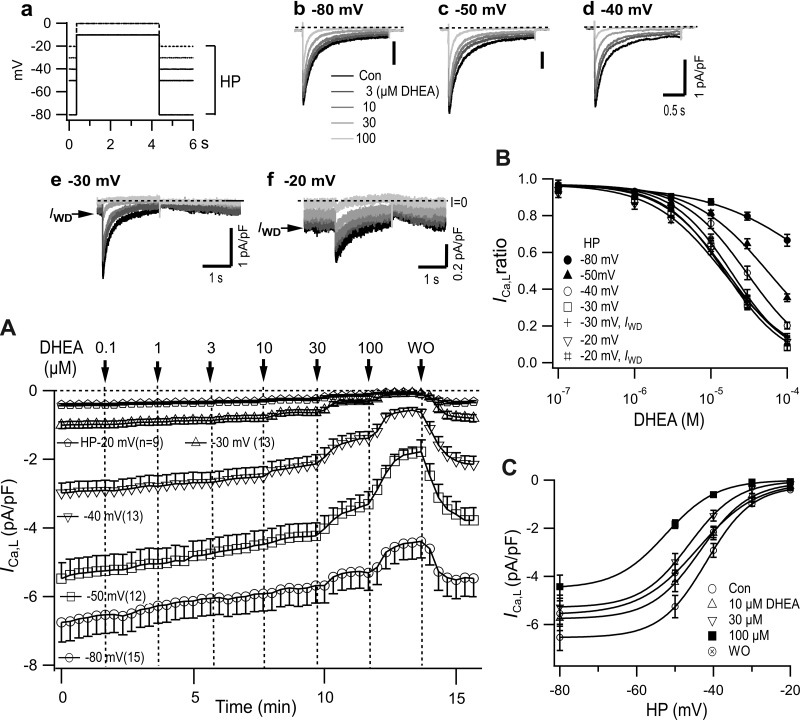
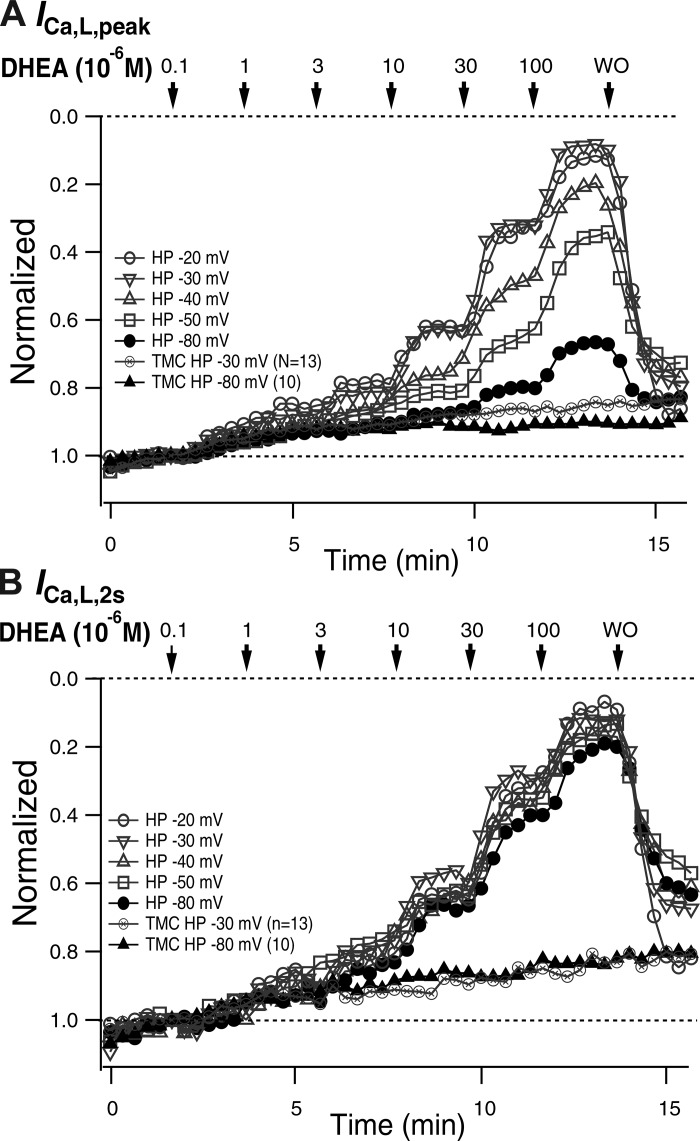
To study the dose and HP dependence of DHEA-induced inhibition of ICa,L, increasing concentrations of DHEA were applied at various HPs between −80 and −20 mV. First, over 10 depolarizing pulses, with a duration of 2 s in a 20-s interval, were applied to obtain ICa,L with a constant amplitude. Subsequently, 0.1 µM DHEA was introduced for 2 min, and the concentration was increased at 2-min intervals to 0.3, 1, 3, 10, 30, and 100 µM and DHEA was washed out (Fig. 3A). DHEA dose dependently decreased the amplitude and accelerated the time course of decay of ICa,L (Fig. 3, b–f). At each HP, ICa,L,peak exhibited a slight and slow decrease between 1 and 10 µM and a stepwise decrease when DHEA concentrations were increased to 10, 30, and 100 µM (Fig. 3A). The extent of inhibition was clearly increased by the depolarization of the HP. At HPs of −30 and −20 mV, IWD was detectable as a steady inward current before and after the depolarization steps (Fig. 3, e and f). Interestingly, IWD gradually increased to the amplitude before the pulse by the repolarization to HP, presenting the time course of recovery from the voltage-dependent inactivation, which developed during test pulses. IWD was inhibited by DHEA to the same extent as ICa,L,peak at HPs of −30 and −20 mV in a dose-dependent manner (Fig. 3, e and f).
Fig. 3.
Dose and holding potential (HP) dependence of dehydroepiandrosterone (DHEA)-induced inhibition of L-type Ca2+ current (ICa,L). Depolarization steps (2 s; a) to −10 or 0 mV (from HPs of −30 and −20 mV) were applied at 1/20 s, and the concentration of DHEA was changed stepwise at 2-min intervals. Representative traces at different HPs are shown in b–f. Window current (IWD) is indicated by an arrow (e and f). A: sequential plot of the peak current density (mean ± SE). B: dose-inhibition relationship obtained for each HP from A. Curves show fittings to the Hill equation with IC50 values shown in Table 2. The curve for the HP of −80 mV did not converge properly. C: density of ICa,L,peak plotted versus HP in the absence and presence of several concentrations of DHEA from A.
The inhibition of ICa,L,peak that was produced by increasing concentrations of DHEA was quantified by the amplitude immediately before the exchange of solutions and plotted versus concentration (Fig. 3B). IC50 values obtained by fitting the plot to the Hill equation are shown in Table 2. IC50 values decreased with depolarization of the HP to the minimum of −15.1 µM (SD 1.7) at a HP of −20 mV. The IC50 value of IWD was not much different from that of ICa,L. The density of ICa,L at each concentration of DHEA was also plotted versus HP (Fig. 3C). ICa,L in each of the concentrations of DHEA decreased with the depolarization of HP, presenting sigmoidal curves. DHEA decreased the amplitude in a dose-dependent manner and shifted the curves to the left.
Table 2.
IC50 values of dehydroepiandrosterone-induced inhibition of L-type Ca2+ current and window current in depolarized HPs
| HP, mV |
||||||
|---|---|---|---|---|---|---|
| −50 | −40 | −30 | −20 | −30 (IWD) | −20 (IWD) | |
| n | 12 | 13 | 13 | 9 | 13 | 9 |
| IC50, µM | 59.8 (6.1) | 29.9 (2.3) | 15.6 (1.4) | 15.1 (1.7) | 16.6 (1.5) | 19.0 (0.9) |
Values are means (SD); n, Number of cells. IC50 values were obtained by fitting mean values of six different concentrations to the Hill equation shown in Fig. 3B. HP, holding potential.
Figure 4 shows normalized values of ICa,L,peak and ICa,L,2s versus time. Depolarization of HP clearly increased DHEA-induced inhibition of ICa,L,peak (Fig. 4A). DHEA-induced inhibition of ICa,L at a HP of −80 mV (Fig. 4, thick line) reflects VIDI. DHEA-induced VIDI decreased ICa,L,peak by 33% at 100 µM. In contrast to ICa,L,peak, normalized ICa,L,2s values were almost equally inhibited by DHEA despite the difference of HPs, and the maximal inhibition by 100 µM amounted to nearly 80% (Fig. 4B). Inhibition at a HP of −80 mV was slightly less extensive than that obtained with depolarized HPs. The time-matched control of HPs of −80 and −30 mV showed that the time-dependent decrease could contribute 10% of the decrease of ICa,L,peak in 15 min and that the decrease was greater with ICa,L,2000.
Fig. 4.
Holding potential (HP)-, dose-, and time-dependent inhibitions of L-type Ca2+ current (ICa,L). A: ICa,L,peak. B: ICa,L,2s. Normalized values of the results partly shown in Fig. 2A and those of the time-matched control of ICa,L elicited from HPs of −80 (n = 9) and −30 mV (n = 13) are plotted. DHEA, dehydroepiandrosterone; TMC, time-matched control.
Window ICa,L (IWD) simulated using HP-induced inactivation.
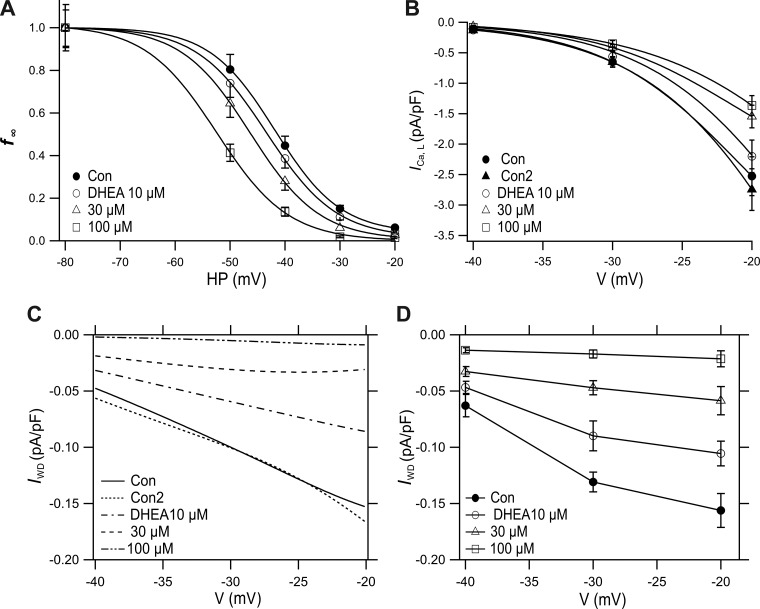
The I-V and f∞-V relationships of ICa,L were challenged by 30 µM DHEA to clarify the inhibitory action more quantitatively (Fig. 5). DHEA (30 µM) inhibited ICa,L by decreasing the peak amplitude and accelerating the time course of inactivation (Fig. 5, a, b, and A), thereby decreasing Gmax by 36% (Table 1). DHEA-induced VDI (30 µM) was quantified by generating a quasi-steady-state inactivation curve (f∞-V) with 2-s conditioning pulses followed by test pulses to 0 mV (Fig. 5, c, d, and B). When 30 µM DHEA was applied, the curve shifted leftward with a change of V0.5 from control −30.3 mV to −36.2 mV (Table 2). From the simulated curves shown in Fig. 5, A and B, IWD was tentatively calculated as the product of I-V and f∞-V (Fig. 5C). The maximal current density of the tentative IWD near −20 mV was −0.6 pA/pF, which was ~10% of the maximal density of ICa,L.
We obtained a novel f∞-HP relationship in the absence and presence of DHEA by plotting the normalized values from Fig. 3C in Fig. 6A. The plot was fitted by Boltzmann’s equation. In control, V0.5 was −41.8 mV, 11.5 mV more negative than that obtained by the 2-s prepulse method, and k was 6.0 mV, which was 1.5 mV steeper than that by the prepulse method (Table 3). IWD was obtained from the f∞-HP (Fig. 6A) and I-V relationships (Fig. 6B) in the absence and presence of 10, 30, and 100 µM DHEA (Fig. 6C). Control IWD density at −20 mV was 0.15 pA, a quarter of that calculated applying the f∞-V curve shown in Fig. 5C, reflecting a greater development of inactivation during HPs. Figure 6D shows the voltage dependence of IWD recorded at depolarized HPs in Fig. 3. DHEA dose dependently and strongly inhibited both the calculated and recorded IWD values in a concentration-dependent manner (Fig. 6, C and D). Both IWD curves were not substantially different. However, the recorded IWD was slightly greater than the simulated current in the presence of 30 and 100 µM DHEA (see discussion).
Fig. 6.
Effect of dehydroepiandrosterone (DHEA) on window current (IWD) obtained with changes of holding potential (HP). A: HP-dependent steady-state inactivation curves (f∞-HP) relationship in the absence and presence of 10, 30, and 100 µM DHEA from the results shown in Fig. 3C. Curves were obtained by fitting to the Boltzmann’s equation. B: part of current-voltage (I-V) relationship of peak L-type Ca2+ current (ICa,L,peak) from results shown in Fig. 2A and Fig. 5A (Con2, 30 µM DHEA). C: IWD obtained as a product of I-V (B) and f∞-HP (A) in the absence and presence of DHEA. D: voltage dependence of recorded IWD from experiments partly shown in Fig. 3. Values are means ± SE; n = 13 for HPs of −40 mV and −30 mV; n = 9 for HP of −20 mV.
Table 3.
Effect of DHEA and GDP-β-S on parameters of f∞-V and f∞-HP relationships
| n | V0.5, mV | k, mV | |
|---|---|---|---|
| Control, f∞-V | 15 | −30.3 (0.5) | 7.5 (0.4) |
| 30 µM DHEA | 15 | −36.2 (0.5)* | 7.2 (0.5) |
| Control, f∞-HP | 9~15 | −41.8 (0.1) | 6.0 (0.1) |
| 10 µM DHEA | 9~15 | −43.4 (0.5)* | 6.4 (0.4) |
| 30 µM DHEA | 9~15 | −46.3 (0.5)* | 6.3 (0.5) |
| 100 µM DHEA | 9~15 | −52.4 (0.7)* | 6.5 (0.9) |
| Control, f∞-V | 15 | −28.9 (0.5) | 7.7 (0.4) |
| GDP-β-S, f∞-V | 7 | −23.1 (0.5)* | 7.9 (0.4) |
Mean (SD) values were obtained by fitting plot with 5 points for holding potential (HP)-dependent steady-state inactivation curves (f∞-HP) and 13 points for quasi-steady-state inactivation curves (f∞-V); n, number of cells. V0.5, voltage that causes a 50% reduction in the amplitude of the inactivating component of L-type Ca2+ current; k, slope factor. Statistical comparison in the absence and presence of dehydroepiandrosterone (DHEA) and control versus guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S) was performed using ordinary one-way ANOVA followed by Dunnet’s test.
P < 0.001 compared with control.
Indirect modulation by GPCR signaling of DHEA-induced voltage-dependent inhibition.
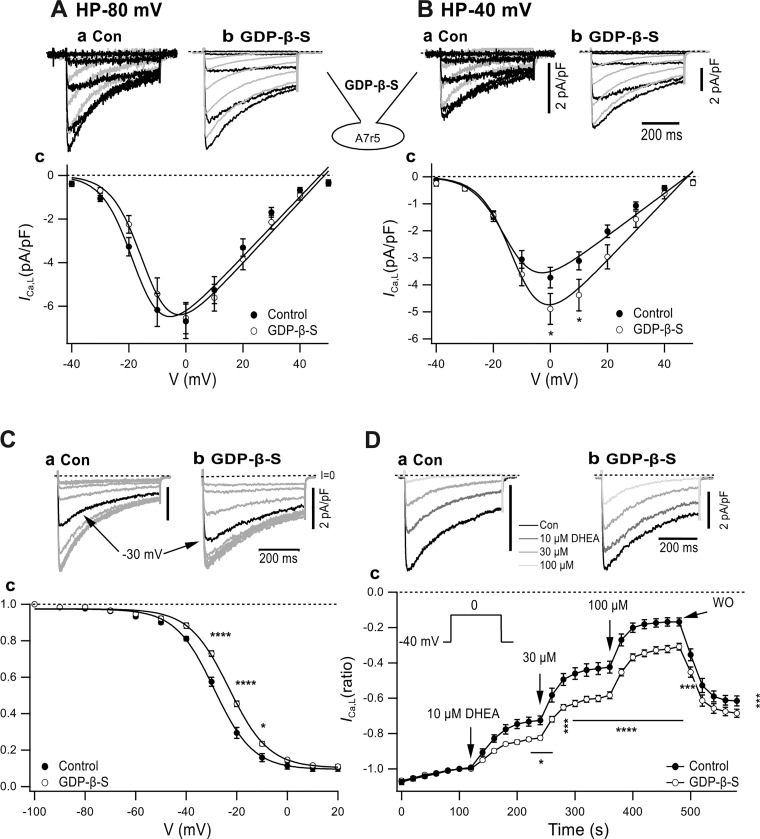
The voltage-dependent inhibitory action of DHEA on ICa,T is reduced by the inhibition of GPCR signaling through the dialysis of GDP-β-S in cultured neurons that express recombinant T channels (5). To examine the involvement of GPCR signaling in DHEA-induced inhibition of ICa,L, 1 mM GDP-β-S was dialyzed from the pipette solution into A7r5 cells. We discovered that dialysis of GDP-β-S significantly modulated the properties of basal ICa,L. At a HP of −80 mV, GDP-β-S dialysis shifted I-V to the right (Fig. 7Ac) with a rightward shift of V0.5 by 3.3 mV without affecting Gmax (Table 1). GDP-β-S induced a significant increase of ICa,L at a HP of −40 mV (Fig. 7Bc) with the increase of Gmax by 38% along with a shift of V0.5 to the right by 2.7 mV (Table 1). The effect of GDP-β-S dialysis on the quasi-steady-state inactivation curve was examined by 2-s prepulses (Fig. 7C). The representative records (Fig. 7C, a and b) show that inactivation by the −30-mV conditioning pulse was clearly diminished by GDP-β-S dialysis. The quasi-steady-state inactivation curve (f∞-V) was shifted to the right (Fig. 7Cc) with a shift of V0.5 by 5.8 mV from control −28.9 mV to −23.1 mV through dialysis (Table 3).
Fig. 7.
Effect of guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S) dialysis on current-voltage (I-V) and quasi-steady-state inactivation curve (f∞-V) relationships and dehydroepiandrosterone (DHEA)-induced inhibition of L-type Ca2+ current (ICa,L). A and B: effect of GDP-β-S on I-V obtained at HPs of −80 mV (A) and −40 mV (B). a and b: Typical records from a control cell (a) and GDP-β-S-dialyzed cell (b). Darker traces show depolarization pulses to −40–0 mV; lighter traces show 10–40 mV. Ac: I-V from a HP of −80 mV. Bc: I-V from a HP of −40 mV. *P < 0.05. I-V relationships were fitted with Boltzmann’s equation along with the parameters shown in Table 1. C: effect of GDP-β-S on f∞-V relationships obtained by 2-s prepulses. a and b: Typical traces. c: f∞-V. n and fitting parameters are shown in Table 3. *P < 0.05; ****P < 0.0001. D: effect of GDP-β-S on DHEA-induced inhibition of ICa,L. a and b: Typical records. c: Normalized ICa,L,peak plotted versus time. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, GDP-β-S compared with control. All statistical comparisons were done with two-way ANOVA followed by Sidak’s test.
The effect of GDP-β-S on DHEA-induced inhibition of ICa,L was examined by repeatedly applying constant depolarization steps from the depolarized HP of −40 mV (Fig. 7B). The averaged maximal current density at a HP of −40 mV was slightly greater in GDP-β-S-dialyzed cells [control cells: 2.40 ± 0.30 pA/pF (means ± SE, n = 13) versus GDP-β-S-dialyzed cells: 3.14 ± 0.43 pA/pF (means ± SE, n = 19), not significant]. As shown in Fig. 7D, the DHEA concentration was increased with an interval of 2 min in the order of 10, 30, and 100 µM. The typical data clearly show that DHEA-induced inhibition was smaller in GDP-β-S-dialyzed cells (Fig. 7D, a and b). Normalized ICa,L density decreased by DHEA in a staircase manner with the increase of the concentration, and its inhibition was partially reversible through washout (Fig. 7Dc). GDP-β-S dialysis decreased DHEA-induced inhibition with a statistically high significant difference, i.e., in the presence of 100 µM DHEA, normalized ICa,L,peak was 16.8 ± 2.4% in control cells (means ± SE, n = 13) and 31.4 ± 2.1% (n = 19) in GDP-β-S-dialyzed cells (P < 0.0001; Fig. 7Dc). The suppressive effect of GPCR signaling inhibition on DHEA-induced inhibition at the HP of −40 mV was considered to originate from the GDP-β-S-induced decrease of steady-state inactivation before the application of DHEA.
Inhibition of ICa,L by 6-AN.
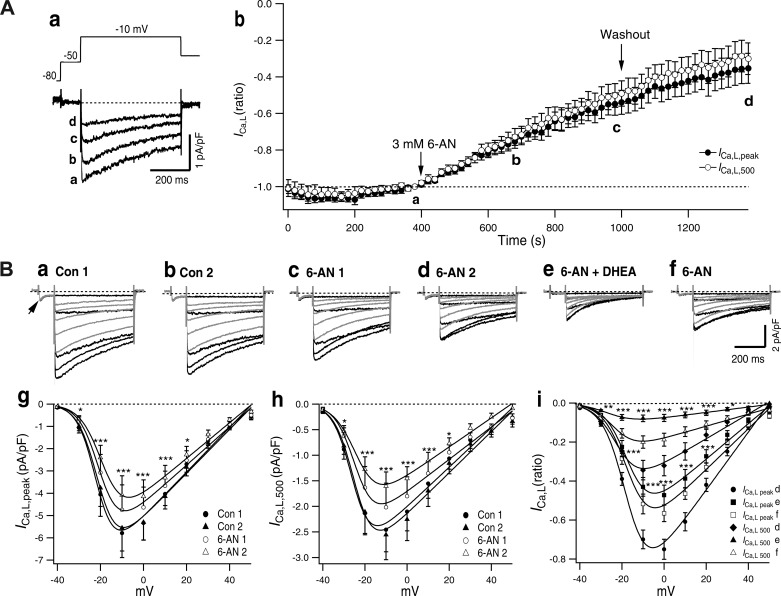
DHEA is a noncompetitive inhibitor of G6PD (28), and the cellular metabolite of 6-AN is a competitive inhibitor of G6PD (24). We have studied how inhibition of G6PD by 6-AN affects ICa,L in ASMCs to clarify the role of G6PD in the regulation of ICa,L. As shown in Fig. 8A, after ICa,L was recorded 20 times in the absence of 6-AN, 3 mM 6-AN was introduced to the chamber and ICa,L was continuously recorded. ICa,L gradually decreased in the presence of 6-AN in 30 pulses (10 min) to 55% with ICa,L,peak and to 50% with ICa,L,500. The 6-AN-induced inhibition did not reach a steady state in 10 min, and the inhibition continued to develop even after the washout of 6-AN. ICa,L,peak and ICa,L,500 decreased almost in parallel, i.e., 6-AN only slightly affected VDI. Figure 8B shows the effect of 3 mM 6-AN, which was examined by recording the I-V relationship. Control of the two I-V relationships recorded with a 5-min interval was superposable with both ICa,L,peak and ICa,L,500 (Fig. 8B, g and h). When 3 mM 6-AN was introduced, ICa,L was significantly inhibited in 5 min, and the inhibition was further increased in 10 min (Fig. 8B, c, d, g, and h). 6-AN (3 mM) decreased Gmax of ICa,L,peak by 14% in 5 min and 20% in 10 min and that of ICa,L,500 by 18% in 5 min and 27% in 10 min (Table 4). After application of 3 mM 6-AN for 10 min, 2-min application of 100 µM DHEA decreased ICa,L,peak and accelerated the decay of current resulting decrease ICa,L,500 (Fig. 8B, d, e, and i). These effects of DHEA were rapidly reversible by washout of DHEA in the presence of 6-AN (Fig. 8B, f and i). ICa,T (Fig. 8Ba, arrow) was little affected by 6-AN but was inhibited by DHEA.
Fig. 8.
6-Aminonicotinamide (6-AN)-induced inhibition of L-type Ca2+ current (ICa,L). A: time course of 3 mM 6-AN-induced inhibition of ICa,L. a: Typical record and test pulse. The test pulse was applied with 20-s intervals, 20 for control, 30 in the presence of 3 mM 6-AN, and 20 after washout of 6-AN. Traces in a–d were recorded just before application of 6-AN [control (a)] at a 5-min (b) and 10-min (c) application of 6-AN and washout for 400 s (d). b: Time-dependent changes of normalized ICa,L,peak and ICa,L,500. Values are means ± SE; n = 8. Control current density was −2.9 ± 0.3 pA/pF for ICa,L,peak and −1.6 ± 0.2 pA/pF for ICa,L,500. B: effect of 3 mM 6-AN and DHEA on the current-voltage (I-V) relationship of A7r5 cells. Typical current traces are shown; darker traces indicate currents by −40, −30, −20, −10, and 0 mV pulses and lighter traces indicate currents by depolarization to 10, 20, 30, 40, and 50 mV. The depolarization pulses were preceded by short step to −40 mV. In control 1 (Con 1), the arrow indicates T-type Ca2+ channel current (a). Control 2 (Con 2) was recorded after 5 min of Con 1 (b). c and d: 6-AN 1 after 5-min application (c) and 6-AN 2 after 10-min application (d) of 3 mM 6-AN. e and f: after 2-min application of 100 µM DHEA in the presence of 6-AN (e) and after 2-min washout of DHEA in the presence of 6-AN (f). g and h: I-V relationship of ICa,L,peak (g) and ICa,L,500 (h) before and after application of 3 mM 6-AN recorded at the times shown in a–d. Values are means ± SE; n = 8. i: Effect of 100 µM DHEA applied after 6-AN 2. Values are means ± SE; n = 7. I-V relationships of normalized values by maximal amplitude of Con 1 are shown. Curves were obtained by fitting to Boltzmann’s equation (Table 4). Statistical comparisons in g–i were done by two-way ANOVA followed by Tukey’s multiple-comparisons test. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 4.
Effect of 6-AN on some parameters of the current-voltage relationship of L-type Ca2+ current shown in Fig. 8
| n | Gmax, pS/pF | V0.5, mV | |
|---|---|---|---|
| Control 1 | 8 | 100.2 (7.5) | −21.5 (1.0) |
| Control 2 | 8 | 98.4 (6.6) | −20.7 (0.9) |
| 6-AN 1 | 8 | 86.0 (5.5)* | −20.0 (0.8)* |
| 6-AN 2 | 8 | 80.1 (5.3)† | −18.6 (0.9)† |
| Control 1500 | 8 | 38.7 (2.9) | −25.7 (1.1) |
| Control 2500 | 8 | 40.2 (3.2) | −24.6 (1.1) |
| 6-AN 1500 | 8 | 31.6 (2.4)† | −24.2 (1.0)* |
| 6-AN 2500 | 8 | 28.4 (1.6)† | −23.5 (0.8)† |
Mean (SD) values were obtained by curve fitting of 10 points; n, number of cells. 6-AN, 6-aminonicotinamide; Gmax, maximal conductance; V0.5, half-activation potential. Statistical comparisons of the values of control and test were performed using ordinary one-way ANOVA followed by Dunnet’s test.
P < 0.01 and
P < 0.001 compared with control 1 or control 1500.
DISCUSSION
DHEA was much more potent in inducing the relaxation of high K+-induced contraction compared with DHEAS. DHEA inhibited ICa,L and IWD in a voltage-dependent and -independent manner. These inhibitions occurred at concentrations that were several orders higher than the serum concentration. However, they are in the range of pharmacological concentrations that are used to inhibit G6PD and, thus, PPP. The fact that 6-AN, a competitive inhibitor of G6PD, inhibited ICa,L in a voltage-independent manner strongly suggests that DHEA induced VIDI by means of inhibition of G6PD.
DHEA-induced VDI of ICa,L.
DHEA-induced VDI has features common with VDI produced by dihydropyridine Ca2+ channel blockers (DHP-CCBs) (26, 29). DHP-CCBs preferentially bind to LCCs in the inactivated state, according to the modulated receptor hypothesis (3, 19, 39). However, it also binds with the channels in the opened state to produce open channel blockade (22, 26). DHP-CCBs bind pore regions in bacterial LCCs and allosterically induce conformation change of the selectivity filter to the resultant blockade (42). The dependence on HP of DHEA-induced VDI may depend on the progressive binding of DHEA to the opened channels during IWD. Since LCC Ca2+ sparklets are recorded even at −70 mV in rat ASMCs (1), LCCs could open at the hyperpolarized HP.
DHP-CCBs are used in antihypertensive treatment (2, 48). New generations of antihypertensive drugs have slow onset and long-lasting inhibitory actions that can minimize side effects (44), whereas DHEA and EPI induce inhibition of ICa,L with rapid onset and rapid reversibility (Fig. 3) (17). Angina pectoris, induced by coronary spasm, can be another target of DHP-CCBs (47, 48). Spastic contractions of ASMCs in the coronary and other arteries as well as visceral smooth muscle cells may be rapidly and reversibly abolished by DHEA.
In the present study, GDP-β-S, which interrupts GPCR signaling, shifted f∞-V rightward in the absence of DHEA and downregulated the following DHEA-induced VDI. Although the GDP-β-S-induced shift of the f∞-V curve was small at the prepotential of −40 mV, the shift could be more prominent in f∞-HP at a HP of −40 mV, at which the train pulse was applied. Thus, DHEA-induced inhibition of ICa,L could be secondarily modulated by inhibition of GPCR signaling via its prior shift of f∞-HP to the right. The downregulation of the DHEA-induced VDI of ICa,T by the inhibition of GPCR signaling (5) may be explicable by a similar secondary effect. The fact that GDP-β-S shifted f∞-V to a hyperpolarizing direction indicates that GPCR signaling regulates voltage-dependent inactivation of LCC in ASMCs.
VIDI produced by inhibition of G6PD.
G6PD is the first enzyme of PPP and reduces NADP+ to NADPH. DHEA is a noncompetitive inhibitor of G6PD along with EPI (14, 28). DHEA, EPI, and other steroids with an inhibitory action on G6PD possess a ketone group in the C17 or C20 positions, whereas progesterone, corticosteroids, estrogens, and testosterone have a hydroxyl rather than a ketone group at C17 or C20 and have little or no inhibitory effect on G6PD (28). 6-AN adenine dinucleotide phosphate, the cellular metabolite of 6-AN, inhibits G6PD by binding it competitively with NADP+ (24). 6-AN also inhibits NADH dehydrogenase (mitochondrial complex I), which reduces NAD+ to NADH, a vital step in the generation of ATP (8). However, in the present study, in the presence of ATP in the pipette solution, 6-AN decreased Gmax of LCCs and decreased ICa,L,peak to result in VIDI. DHEA applied in the presence of 6-AN induced further inhibition of ICa,L,peak and accelerated current decay rapidly and reversibly (Fig. 8B). The DHEA-induced decrease of ICa,L,peak in the presence of 6-AN indicates that the 6-AN-induced competitive inhibition of G6PD was still partial at 10 min and the noncompetitive inhibition by DHEA proceeded rapidly and reversibly. The DHEA-induced decrease of ICa,L,500 in the presence of 6-AN indicates that VDI proceeded independent of VIDI.
Inhibition of G6PD produced by 6-AN and DHEA increases NADP+, in turn, increasing oxidized glutathione (GSSG) that is associated with a decrease in the reduced form (GSH) (15, 21, 36). The resulting oxidative environment near the LCC could induce sulfhydryl modification of SH residues of the α-subunit of LCCs to decrease the open probability of LCCs to result in VIDI (6). The reversal of 6-AN-induced inhibition of ICa,L through dialysis of NADPH evidently shows that G6PD-regulated redox is involved in 6-AN-induced inhibition of ICa,L (38). Since G6PD is the first and rate-limiting enzyme of PPP, which supplies NADPH to maintain the normal redox status and generates the ribose necessary to synthesize nucleic acids, inhibition of G6PD has a profound influence on the proliferation of various cells (40, 41), which include cancer cells (9a, 43, 45). The inhibition of G6PD underlies the potent anticancer efficacy of DHEA (9a, 27, 37). We showed that when DHEA is applied to cancer cells at the concentration to inhibit PPP, it can inevitably inhibit ICa,L and IWD in ASMCs.
IWD of ASMCs and its inhibition by DHEA.
The Ca2+ influx necessary to induce contraction of ASMCs is supplied by IWD through LCCs during sustained depolarization (32), producing a sustained increase in intracellular Ca2+ concentration (11). IWD was observed at depolarized HPs. In principle, IWD is proportional to the product of the parameters for steady-state activation (d∞) as well as steady-state inactivation (f∞) (7, 29, 32). f∞ is usually obtained with prepulses of several seconds (Fig. 5). Here, we obtained the f∞-HP relationship by plotting the amplitude of ICa,L recorded at several HPs versus HP (Fig. 6). The f∞-HP relationship presented more negative V0.5 than that from the prepulse method and exhibited a steeper slope of decay with the increase in conditioning depolarization (Table 3). IWD, calculated as product of ICa,L,peak − V and f∞-HP, fit well with IWD recorded at HP. Since inhibition by DHEA is produced by VIDI reflected in ICa,L,peak − V and by VDI reflected in f∞-HP, DHEA-induced inhibition of IWD is produced both by VIDI and VDI. The recorded IWD was slightly greater than the calculated IWD in the presence of 30 and 100 µM DHEA, probably because of the increase of Cd2+-resistant current used for the subtraction of background current during the experiment. Since the simulated IWD is much less affected by the change of background current, as ICa,L is greater than the recorded IWD, the simulation method could be more accurate. f∞-V obtained by prepulse provides a quasi-voltage dependence; the IWD density at −20 mV calculated by f∞-HP was a quarter of that by f∞-V.
DHEA inhibited ICa,L and IWD at micromolar concentrations in ASMCs by VDI and VIDI mechanisms. DHEA-induced VDI developed in a mode of open channel blocks produced by DHP-CCBs. Since the competitive G6PD inhibitor 6-AN produces VIDI, we conclude that DHEA-induced VIDI is generated by DHEA-induced inhibition of G6PD. DHEA-induced VDI and VIDI inhibits ICa,L and induces relaxation of depolarization-induced contraction of ASM. In addition, DHEA-induced hyperpolarization by the activation of large-conductance Ca2+-activated K+ channel current indirectly decreases ICa,L to induce relaxation. DHEA is a candidate drug for several diseases, including cancer, based on its inhibitory action on PPP. However, since the use of DHEA in micromolar concentrations is accompanied by the inhibition of ICa,L, caution is required in its usage.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-085352 and RO1-HL-132574 (to S. A. Gupte).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.O. and S.A.G. conceived and designed research; R.O., S.C., and I.K. performed experiments; R.O. and I.K. analyzed data; R.O., I.K., and S.A.G. interpreted results of experiments; R.O. and I.K. prepared figures; R.O. drafted manuscript; R.O., S.C., and S.A.G. edited and revised manuscript; R.O., S.C., I.K., and S.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jing Huang for the preparation of cultured A7r5 cells.
REFERENCES
- 1.Amberg GC, Navedo MF, Nieves-Cintrón M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol 579: 187–201, 2007. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong C; Joint National Committee . JNC8 guidelines for the management of hypertension in adults. Am Fam Physician 90: 503–504, 2014. [PubMed] [Google Scholar]
- 3.Bean BP. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci USA 81: 6388–6392, 1984. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau J-P, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalier M, Gilbert G, Lory P, Marthan R, Quignard JF, Savineau JP. Dehydroepiandrosterone (DHEA) inhibits voltage-gated T-type calcium channels. Biochem Pharmacol 83: 1530–1539, 2012. doi: 10.1016/j.bcp.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Chiamvimonvat N, O’Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res 76: 325–334, 1995. doi: 10.1161/01.RES.76.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Cohen NM, Lederer WJ. Calcium current in isolated neonatal rat ventricular myocytes. J Physiol 391: 169–191, 1987. doi: 10.1113/jphysiol.1987.sp016732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich LS, Friedland IM, Kaplan LA. Pyridine nucleotide metabolism: mechanism of action of the niacin antagonist, 6-aminonicotinamide. J Biol Chem 233: 964–968, 1958. [PubMed] [Google Scholar]
- 9.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 9a.Fang Z, Jiang C, Feng Y, Chen R, Lin X, Zhang Z, Han L, Chen X, Li H, Guo Y, Jiang W. Effects of G6PD activity inhibition on the viability, ROS generation and mechanical properties of cervical cancer cells. Biochim Biophys Acta 1863: 2245–2254, 2016. doi: 10.1016/j.bbamcr.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca2+-activated K+-channel opener. Am J Physiol 274: L186–L195, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmann BK, Murray RK, Kotlikoff MI. Voltage window for sustained elevation of cytosolic calcium in smooth muscle cells. Proc Natl Acad Sci USA 91: 11914–11918, 1994. doi: 10.1073/pnas.91.25.11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisler C, Schweitzer L, Müller MJ. Functional correlates of detailed body composition in healthy elderly subjects. J Appl Physiol 124: 182–189, 2018. doi: 10.1152/japplphysiol.00162.2017. [DOI] [PubMed] [Google Scholar]
- 14.Gordon G, Mackow MC, Levy HR. On the mechanism of interaction of steroids with human glucose 6-phosphate dehydrogenase. Arch Biochem Biophys 318: 25–29, 1995. doi: 10.1006/abbi.1995.1199. [DOI] [PubMed] [Google Scholar]
- 15.Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 285: H2316–H2326, 2003. doi: 10.1152/ajpheart.00229.2003. [DOI] [PubMed] [Google Scholar]
- 16.Gupte SA, Li KX, Okada T, Sato K, Oka M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther 301: 299–305, 2002. doi: 10.1124/jpet.301.1.299. [DOI] [PubMed] [Google Scholar]
- 17.Gupte SA, Tateyama M, Okada T, Oka M, Ochi R. Epiandrosterone, a metabolite of testosterone precursor, blocks L-type calcium channels of ventricular myocytes and inhibits myocardial contractility. J Mol Cell Cardiol 34: 679–688, 2002. doi: 10.1006/jmcc.2002.2008. [DOI] [PubMed] [Google Scholar]
- 18.Hill-Eubanks DC, Gonzales AL, Sonkusare SK, Nelson MT. Vascular TRP channels: performing under pressure and going with the flow. Physiology (Bethesda) 29: 343–360, 2014. doi: 10.1152/physiol.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69: 497–515, 1977. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofmann F, Flockerzi V, Kahl S, Wegener JW. L-type Cav1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 94: 303–326, 2014. doi: 10.1152/physrev.00016.2013. [DOI] [PubMed] [Google Scholar]
- 21.Jain M, Brenner DA, Cui L, Lim CC, Wang B, Pimentel DR, Koh S, Sawyer DB, Leopold JA, Handy DE, Loscalzo J, Apstein CS, Liao R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ Res 93: e9–e16, 2003. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 22.Kawashima Y, Ochi R. Voltage-dependent decrease in the availability of single calcium channels by nitrendipine in guinea-pig ventricular cells. J Physiol 402: 219–235, 1988. doi: 10.1113/jphysiol.1988.sp017201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimes BW, Brandt BL. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res 98: 349–366, 1976. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- 24.Köhler E, Barrach H, Neubert D. Inhibition of NADP dependent oxidoreductases by the 6-aminonicotinamide analogue of NADP. FEBS Lett 6: 225–228, 1970. doi: 10.1016/0014-5793(70)80063-1. [DOI] [PubMed] [Google Scholar]
- 25.Labrie F, Luu-The V, Bélanger A, Lin S-X, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169–196, 2005. doi: 10.1677/joe.1.06264. [DOI] [PubMed] [Google Scholar]
- 26.Lee KS, Tsien RW. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature 302: 790–794, 1983. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Fath MA, Scarbrough PM, Watson WH, Spitz DR. Combined inhibition of glycolysis, the pentose cycle, and thioredoxin metabolism selectively increases cytotoxicity and oxidative stress in human breast and prostate cancer. Redox Biol 4: 127–135, 2015. doi: 10.1016/j.redox.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30: 65–91, 2009. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marks PA, Banks J. Inhibition of mammalian glucose-6-phosphate dehydrogenase by steroids. Proc Natl Acad Sci USA 46: 447–452, 1960. doi: 10.1073/pnas.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74: 365–507, 1994. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 30.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 259: C3–C18, 1990. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 33.Ochi R, Chettimada S, Gupte SA. Poly(ethylene glycol)-cholesterol inhibits L-type Ca2+ channel currents and augments voltage-dependent inactivation in A7r5 cells. PLoS One 9: e107049, 2014. doi: 10.1371/journal.pone.0107049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochi R, Dhagia V, Lakhkar A, Patel D, Wolin MS, Gupte SA. Rotenone-stimulated superoxide release from mitochondrial complex I acutely augments L-type Ca2+ current in A7r5 aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 310: H1118–H1128, 2016. doi: 10.1152/ajpheart.00889.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551–555, 1984. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 36.Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α. Am J Physiol Lung Cell Mol Physiol 306: L383–L391, 2014. doi: 10.1152/ajplung.00301.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratko TA, Detrisac CJ, Mehta RG, Kelloff GJ, Moon RC. Inhibition of rat mammary gland chemical carcinogenesis by dietary dehydroepiandrosterone or a fluorinated analogue of dehydroepiandrosterone. Cancer Res 51: 481–486, 1991. [PubMed] [Google Scholar]
- 38.Rawat DK, Hecker P, Watanabe M, Chettimada S, Levy RJ, Okada T, Edwards JG, Gupte SA. Glucose-6-phosphate dehydrogenase and NADPH redox regulates cardiac myocyte L-type calcium channel activity and myocardial contractile function. PLoS One 7: e45365, 2012. doi: 10.1371/journal.pone.0045365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanguinetti MC, Kass RS. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res 55: 336–348, 1984. doi: 10.1161/01.RES.55.3.336. [DOI] [PubMed] [Google Scholar]
- 40.Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 64: 362–369, 2012. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Grüning NM, Krüger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc 90: 927–963, 2015. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang L, Gamal El-Din TM, Swanson TM, Pryde DC, Scheuer T, Zheng N, Catterall WA. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature 537: 117–121, 2016. doi: 10.1038/nature19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang AL, Iadecola C, Wang G. New generations of dihydropyridines for treatment of hypertension. J Geriatr Cardiol 14: 67–72, 2017. doi: 10.11909/j.issn.1671-5411.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warburg O. On the origin of cancer cells. Science 123: 309–314, 1956. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 46.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 47.Yasue H, Nakagawa H, Itoh T, Harada E, Mizuno Y. Coronary artery spasm−clinical features, diagnosis, pathogenesis, and treatment. J Cardiol 51: 2–17, 2008. doi: 10.1016/j.jjcc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 67: 821–870, 2015. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]