Abstract
Cadherin-11 (CDH11) is upregulated in a variety of fibrotic diseases, including arthritis and calcific aortic valve disease. Our recent work has identified CDH11 as a potential therapeutic target and shown that treatment with a CDH11 functional blocking antibody can prevent hallmarks of calcific aortic valve disease in mice. The present study investigated the role of CDH11 in regulating the mechanobiological behavior of valvular interstitial cells believed to cause calcification. Aortic valve interstitial cells were harvested from Cdh11+/+, Cdh11+/−, and Cdh11−/− immortomice. Cells were subjected to inflammatory cytokines transforming growth factor (TGF)-β1 and IL-6 to characterize the molecular mechanisms by which CDH11 regulates their mechanobiological changes. Histology was performed on aortic valves from Cdh11+/+, Cdh11+/−, and Cdh11−/− mice to identify key responses to CDH11 deletion in vivo. We showed that CDH11 influences cell behavior through its regulation of contractility and its ability to bind substrates via focal adhesions. We also show that transforming growth factor-β1 overrides the normal relationship between CDH11 and smooth muscle α-actin to exacerbate the myofibroblast disease phenotype. This phenotypic switch is potentiated through the IL-6 signaling axis and could act as a paracrine mechanism of myofibroblast activation in neighboring aortic valve interstitial cells in a positive feedback loop. These data suggest CDH11 is an important mediator of the myofibroblast phenotype and identify several mechanisms by which it modulates cell behavior.
NEW & NOTEWORTHY Cadherin-11 influences valvular interstitial cell contractility by regulating focal adhesions and inflammatory cytokine secretion. Transforming growth factor-β1 overrides the normal balance between cadherin-11 and smooth muscle α-actin expression to promote a myofibroblast phenotype. Cadherin-11 is necessary for IL-6 and chitinase-3-like protein 1 secretion, and IL-6 promotes contractility. Targeting cadherin-11 could therapeutically influence valvular interstitial cell phenotypes in a multifaceted manner.
Keywords: cadherin-11, heart valve, interstitial cell, mechanobiology, myofibroblast
INTRODUCTION
Calcific aortic valve disease (CAVD) is a condition that affects 25% of people over 65 yr old and progresses from aortic sclerosis, characterized by valve thickening, to aortic stenosis, defined as obstruction of blood flow (2). CAVD is the primary cause of aortic stenosis, which necessitates total valve replacement either through open-chest surgery or transcatheter aortic valve replacement, and is the main indication for the >100,000 valve replacements performed annually in the United States (20). Unfortunately, given the advanced age of many patients with CAVD, such intervention carries a high rate of morbidity and mortality and is often a procedure of last resort, leaving many to suffer the ill effects of worsening heart function as CAVD progresses (30). With the number of calcific aortic stenosis patients expected to double by 2050, and possibly triple by 2060 (12, 40), there is a need for a better understanding of the biological mechanisms driving CAVD to develop well-tolerated, noninvasive pharmacological therapies for valvular calcification (23).
Once believed to be a passive process of degeneration, aortic valve calcification is now thought to be an active process of valvular remodeling mediated largely by resident aortic valve interstitial cells (AVICs) (34). AVICs are a heterogeneous population of fibroblast-like cells present in all three layers of the aortic valve and are important in the structural maintenance of the valve (9, 31). When activated by transforming growth factor (TGF)-β1, AVICs transition to myofibroblasts characterized by increased contractility, collagen deposition, and expression of smooth muscle α-actin (α-SMA) and cadherin-11 (CDH11). The resulting increases in intracellular and intercellular tensions can cause membrane tearing and subsequent apoptosis-mediated cell death, leading to calcific nodule formation. TGF-β1 is upregulated in diseased human valves and, when given exogenously in vitro, exacerbates calcific nodule formation (14, 28). TGF-β1 has also been shown to activate myofibroblasts in valves, leading to increased α-SMA expression (25, 54). As these myofibroblasts become more contractile, they likely activate latent TGF-β1 from the extracellular matrix (11). This positive feedback loop provides a strong potential mechanism for disease progression in vivo. In a study of excised human aortic valves, 83% showed evidence of this process, termed dystrophic calcification (38), which is believed to be the most prevalent mechanism of CAVD.
CDH11 was identified as a potential therapeutic target when it was found to be enriched in calcified human aortic valves and necessary for calcific nodule formation in vitro (24). Investigation of the mode of action of CDH11 in CAVD is further motivated by observations that the Notch1+/− mutation results in a twofold increase in CDH11 expression in murine aortic valves (8, 10, 24). Notch1 mutations have been correlated with an increased risk of aortic stenosis in humans (15), and the long noncoding RNA H19 has recently been shown to silence NOTCH1 in cases of idiopathic CAVD (16). Additionally, Notch1+/− mice on a high-fat/high-cholesterol diet treated with a functional blocking antibody against CDH11 had a higher ejection fraction velocity ratio than the isotype control, indicative of preserved heart function (10). Genetic ablation of CDH11 yielded similar results, yet the mechanism underlying how decreased CDH11 expression or activity leads to disease prevention remains poorly understood.
CDH11 is a mechanosensitive transmembrane protein involved in Ca2+-dependent cell-cell adhesion. The intracellular region of CDH11 is subdivided into a cytoplasmic binding domain and a juxtamembrane domain and is known to complex with β-catenin, p120-catenin, γ-catenin, and angiomotin. These proteins mediate connections to the cytoskeleton through α-catenin and are required for full-strength homotypic bonds to persist. Homotypic bonds formed by CDH11 are twofold stronger than those formed by cadherin-2 (CDH2), another mesenchymal cadherin, and far stronger than bonds formed by cadherin-1 and cadherin-5 (42). CDH11 is also the only cadherin known to participate in focal adhesions (32). CDH11 engagement is known to increase the secretion of IL-6, suggesting an inflammatory role of CDH11 as well. IL-6 is a complex ~25-kDa cytokine, acting in both pro- and anti-inflammatory capacities. Typically secreted by macrophages, fibroblasts, and endothelial cells, IL-6 can also be secreted by osteoblasts, smooth muscle cells, and adipose tissue (41). IL-6 complexes with IL-6Rα and glycoprotein 130 (GP130), resulting in a signaling cascade through JNKs and STATs. Chitinase-3-like protein 1 (Chi3l1) is a secreted inflammatory glycoprotein downstream of STAT3 with unclear function but has been associated with a variety of cardiovascular diseases (5, 29). Together, these characteristics make CDH11 a unique mechanosensitive protein with the potential to sense cell-cell and cell-substrate interactions and influence inflammation (49). To understand the intrinsic role of CDH11 in valvular calcification, we examined how CDH11 influences AVIC behavior in the context of mechanical and inflammatory cytokine cues relevant to the heart valve environment.
METHODS
Isolation of murine aortic valves and interstitial cells.
CDH11+/+ [wild-type (WT)], CDH11+/−, and CDH11−/− murine AVICs were isolated from Cdh11+/+, Cdh11+/−, and Cdh11−/− immortomice generated by crossing Cdh11 transgenic mice (provided by Roberto Civitelli) with the “Immortomouse” (Charles River, 237 HO, 238 HE). In our experience, it is necessary to have immortalized AVICs when isolating because the murine valves yield very low cell numbers (8). Although immortalized cell lines can theoretically be expanded and used indefinitely, we limited our passage number to under passage 30. AVICs were harvested from littermates, and genotyping was performed by PCR analysis using the following primer pairs for the Cdh11 WT allele (forward: 5′-GTGTATTGGTTGCACCATGGGG-3′ and reverse: 5′-TCTATCGCCTTCTTGACGAGTTC-3′), Cdh11 nonfunctional allele (forward: 5′-TTCAGTCGGCAGAAGCAGGAC-3′ and reverse: 5′-TCTATCGCCTTCTTGACGAGTTC-3′), and immorto allele (forward: 5′-CCTCTGAGCTATTCCAGAAGTAGTG-3′ and reverse: 5′-TTAGAGCTTTAAATCTCTGTAGGTAG-3′).
Immediately after euthanasia, hearts were excised, and aortic valve leaflets were either embedded in OCT for histological analysis (12-mo-old, nonimmortomice) (Table 1) or processed to isolate AVICs (8-wk-old, immortomice) (Table 2). For cell isolation, valves were digested in 2 mg/ml collagenase for 30 min at room temperature and then put into DMEM supplemented with 10% FBS, 1% penicillin-streptomycin antibiotic, and 10 μg/ml recombinant murine interferon-γ (immortomedia). Cells were allowed to adhere to 0.1% gelatin-coated six-well tissue culture-treated plates. To allow for sustained immortal growth by activating the simian virus 40 T antigen, cells were cultured at 33°C and 5% CO2 in immortomedia when not plated for experiments. When plated for experiments, AVICs were incubated at 37°C and 5% CO2 in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin antibiotic (complete media). All breeding and experimental procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Table 1.
Animals
| Species/Strain | Number and Genotype | Vendor or Source | Background Strain | Sex |
|---|---|---|---|---|
| Mouse | 138 Cdh11+/+ | Roberto Civitelli | Originally C57Bl/6 (22) | Male |
| 195 Cdh11+/+ | Male | |||
| 197 Cdh11+/+ | Male | |||
| 198 Cdh11+/+ | Male | |||
| 207 Cdh11+/− | Male | |||
| 211 Cdh11+/− | Female | |||
| 214 Cdh11−/− | Female | |||
| 838 Cdh11−/− | Male | |||
| 1204 Cdh11−/− | Female | |||
| 1205 Cdh11+/− | Female |
Cdh11, cadherin 11 gene.
Table 2.
Cultured cells
| Name | Vendor or Source | Sex |
|---|---|---|
| WT AVIC | Cdh11+/+ Immortomouse | Female |
| CDH11+/− AVIC | Cdh11+/− Immortomouse | Male |
| CDH11−/− AVIC | Cdh11−/− Immortomouse | Male |
WT, wild-type; AVIC, aortic valve interstitial cell; CDH11, cadherin-11.
Collagen gel contraction.
Collagen type I (no. 5005 Advanced Biomatrix), 10× PBS without Ca2+ or Mg2+ (PBS−/−), and 0.1 M NaOH at an 8:1:1 ratio was mixed and adjusted to pH 7.4 with 0.1 M HCl. This solution (200 µl) was carefully pipetted into the center of 0.5-in.-diameter Teflon rings (no. 5612-303-62, Seastrom Manufacturing) that had been sterilized with ethanol and ultraviolet light. After polymerization at 37°C for 1 h, 40,000 cells in 200 µl of complete media were seeded on top of each gel and allowed to adhere at 37°C for 30 min. Complete media with or without 1 ng/ml recombinant murine TGF-β1 (no. 7666MB005, Fisher Scientific) or 100 ng/ml IL-6 (no. 78052, StemCell Technologies) was then added, and gels were detached from the rings and the bottom of the untreated tissue culture plate. After equilibration for 30 min, free-floating gels were imaged on a Leica dissection microscope. Gels were imaged at least every 24 h, and media were changed every 48 h for up to 4 days. The gel area was quantified via ImageJ (48), and data are reported as 1 – A/A0, where A is the gel area at the given time and A0 is the gel area at time 0.
Western blot analysis.
After treatment in complete media with or without 5 ng/ml recombinant murine TGF-β1 or 100 ng/ml IL-6 for 24 h, cells were lysed in RIPA buffer and frozen at −80°C. Protein lysate was linearized by the addition of β-mercaptoethanol and heat (5 min at 100°C) and run on an 8% or 12% polyacrylamide gel to separate proteins by size. Proteins were transferred to a nitrocellulose membrane (no. 926, LI-COR) and blocked with Odyssey Blocking Buffer (no. 927, LI-COR) to prevent nonspecific antibody binding. Membranes were incubated serially in primary antibody against proteins of interest followed by secondary antibodies conjugated to a fluorescent tag (Table 3). Membranes were scanned on a LI-COR Odyssey fluorescent scanner. Proteins were quantified with densitometry (Image Studio Lite) and normalized to α-tubulin, unless otherwise indicated, and then to WT. Antibodies were validated according to standardized guidelines (4).
Table 3.
Antibodies
| Target Antigen | Vendor or Source | Catalog Number | Working Concentration |
|---|---|---|---|
| α-SMA | Abcam | 5694 | WB: 1:1000 |
| α-SMA-Cy3 | Sigma | 6198 | IF: 1:400 |
| α-SMA-647 | Novus | NBP2-34522AF647 | FACS: 1:500 |
| α-Tubulin | Vanderbilt MCBR Core | Lot no. 12 | WB: 1:1000 |
| Cadherin-2 | Sigma | 2542 (GC-4) | IF: 1:100; WB: 1:1000 |
| Cadherin-11 | Cell Signaling Technologies | 4442BF | IF: 1:50; WB: 1:1000 |
| Cadherin-11 | Michael Brenner (Mouse IgG1) | 23C6 | FACS: 1.25 µg/ml |
| IgG1-PE | BioLegend | 406607 | FACS: 1.25 µg/ml |
| IL-6 | Abcam | 6672 | IF: 1:50 |
| Phospho-STAT3 (Tyr705) | Cell Signaling Technologies | 9145 | IF: 1:100; WB: 1:1000 |
| STAT3 | Cell Signaling Technologies | 9139 | IF: 1:800; WB: 1:1000 |
| Syndecan-4 | Abcam | 24511 | WB: 1:250 |
| Vinculin | Sigma | V9131 (human VIN-1) | IF: 1:400 |
| 488 Anti-mouse | Jackson ImmunoResearch | 715-545-150 | IF: 1:400 |
| 647 Anti-mouse | Jackson ImmunoResearch | 715-605-150 | IF: 1:400 |
| 647 Anti-rabbit | ThermoFisher | A21245 | IF: 1:400 |
| 680 Anti-rabbit | ThermoFisher | A10043 | WB: 1:20,000 |
| 790 Anti-mouse | Jackson ImmunoResearch | 115-655-146 | WB: 1:10,000 |
α-SMA, smooth muscle α-actin; MCBR, Molecular Cell Biology Resource; WB, Western blot; IF, immunofluorescence.
Picrosirius red assay.
Cells were plated at 10,000 cells/cm2 and grown in complete media with or without 5 ng/ml recombinant murine TGF-β1 or 100 ng/ml IL-6 for 96 h with media changes every 48 h. Cells were rinsed with PBS−/−, fixed in 90% methanol at −20°C overnight, washed in PBS−/−, and incubated with picrosirius red stain (no. 26357-02, Electron Microscopy Sciences) for 1 h. To elute the stain for quantification, cells were rinsed in acidified water (0.5% acetic acid) and incubated with 0.1 M NaOH for 1 h. Each sample (200 μl/sample) was transferred to a 96-well plate, and optical densities were read at 540 nm. Optical densities were normalized to proliferation measured from multiple cell counts at 96 h.
ELISAs.
Cells were plated at 10,000 cells/cm2 in complete media, and conditioned media were harvested after 48 h. Media was centrifuged at 1,500 rpm for 10 min at 4°C. ELISAs for IL-6 (DY406, R&D Systems) and Chi3l1 (DY2649, R&D Systems) were performed on the supernatant from each sample. Optical densities were read at 450 nm with densities at 562 nm subtracted to correct for optical imperfections in the plate. Standards were fit to a four-parameter logistic model and were used to calculate sample concentrations using MyAssays (38a).
Traction force microscopy.
High-resolution traction force microscopy (TFM) was used to quantify the cell-matrix traction forces generated by each cell line (n > 72 for single cells and n > 25 for cell pairs). TFM was performed using experimental and computational methods similar to those previously described (6, 13, 18, 26, 47).
Briefly, 35-mm glass-bottom dishes (P35G-0-14-C, MatTek) were coated with 0.1 N NaOH for 5 min before treatment with 3-aminopropyltrimethoxysilane (no. 281778, Sigma-Aldrich) for 10 min at room temperature. After multiple washes with ultrapure water, dishes were treated with 0.5% glutaraldehyde solution (0.5% in 1× PBS, no. 01909, Polysciences) for 30 min at room temperature. A polyacrylamide solution consisting of 169 µl ultrapure water, 300 µl of 40% acrylamide, and 300 µl of 2% bis-acrylamide was combined with 230 µl human plasma fibronectin (1 mg/ml in ultrapure water, no. 33016-015, Life Technologies) and 8 µl of 200-nm red fluorescent microspheres (excitation/emission of 580/605 nm, F8810, ThermoFisher). To ensure even polymerization, the solution was then mixed with 1 µl acrylic acid N-hydroxysuccinimide ester (10 mg/ml in ultrapure water, A8060, Sigma-Aldrich), 1 µl ammonium persulfate (100 mg/ml in DMSO, D8418, Sigma-Aldrich), and 2 µl tetramethylethylenediamine (no. 161-0800, Bio-Rad) before placement on the glass-bottom dishes (8.48 µl for ~75-µm thickness gel). This formulation results in a rigid polyacrylamide gel (12% acrylamide and 0.6% bis-acrylamide) with an elastic modulus of ~23 kPa (1, 26). AVICs were plated sparsely and allowed to adhere for 14 h in complete media (27). The media were changed to L-15 media for at least 1 h before imaging to prevent sensitivity to CO2 levels.
Comparisons of the bead distributions below each cell before and after detachment were used to compute gel displacement fields via a subpixel correlation by image interpolation (SCII) approach (18). To reduce the mechanical effects from neighboring cells and the underlying glass coverslip, image acquisition was restricted to flat, isolated cells on a smooth section of the polyacrylamide gel (without inclusions or wrinkling) that had a thickness of >25 µm. Combined with polyacrylamide gel material properties, the computed displacements were used to reconstruct traction forces on the surface of the polyacrylamide gel using a regularized Fourier transform traction cytometry framework (19, 43, 50). Here, the regularization parameter λ was chosen based on an optimal L1-regularization of the L-curve (18). Values of λ were independently determined for each cell with an average value of λ = 2.89 × 10−4 over all cells.
Given the reconstructed traction fields, total force generated by each cell () was calculated as the integral of the traction magnitude within the cell boundary (44) as follows:
| (1) |
where Tx(x,y) and Ty(x,y) are components of the local reconstructed traction vector [T(x)] at any spatial location x = (x,y) contained with the cell boundary Ω and dx = dy = 0.162 µm/pixel. Cell-cell interaction forces (Fcc) were estimated from the traction imbalance that arises when partitioning the reconstructed traction field at the cell contact border. Values were computed as , where F1 and F2 are the resultant traction force vectors in each cell of the doublet (37).
Histology and immunofluorescence.
Tissue was embedded in OCT within 30 min of dissection and sectioned at 7 µm. For assessment of calcification, 12-mo-old Cdh11+/+, Cdh11+/−, and Cdh11−/− murine aortic valves were stained with alizarin red (A5533, Sigma-Aldrich) and von Kossa (no. 24633, Polysciences). Briefly, tissue was cleared of OCT in PBS−/−, rinsed in deionized H2O, and incubated with 14 mM alizarin red stain (after being filtered through a 0.45-μm filter) for 30 min. After being rinsed with distilled H2O, slides were dehydrated with acetone and xylenes and preserved using Permount (SP15, Fisher Scientific). Tissue stained with von Kossa was processed and mounted similarly but incubated in 3% silver nitrate for 40 min under a 60-W bulb, rinsed in distilled H2O, incubated in 5% sodium thiosulfate for 2 min, rinsed in distilled H2O, and then stained with filtered nuclear fast red for 10 min. Stained slides were dehydrated in ethanol and xylenes before being mounted. Custom image processing analyses based on hue, saturation, and lightness (HSL) color segmentation (3) were developed to quantify the percentage of aortic valve leaflets occupied by calcification. Calcification fractions were calculated as the ratio of positive calcification pixels (for alizarin red: between hue = 0–360°, saturation = 0–1, and light color segmentation = 0–0.3 and for von Kossa: between hue = 0–360°, saturation = 0–1, and light color segmentation = 0–0.45) to the total number of valve leaflet pixels; data were normalized to WT expression.
For immunostaining of AVICs, cells were plated on fibronectin-coated coverslips, incubated in complete media for 48 h, fixed and permeabilized in 4% paraformaldehyde + 0.1% Triton X-100 for 10 min, blocked in 5% BSA for 1 h, and incubated with primary antibody in 1% BSA overnight at 4°C. Coverslips were washed and incubated for 90 min in secondary antibody in 1% BSA before mounting in ProLong Gold with DAPI (P36931, Invitrogen) (Table 3). Vinculin-stained coverslip images were analyzed using custom image processing analyses to quantify focal adhesion length and number. Briefly, after background removal and contrast enhancement, images were blurred by Gaussian filtering with a standard deviation of σ = 3 and adaptively thresholded to generate a binary mask of potential focal adhesion locations. Focal adhesion boundaries were extracted from binary masks, and boundaries in contact with image borders, nuclei borders, or with low median intensity values (based on Otsu’s method) were excluded from further analysis. Candidate boundaries were then assessed for potential contacting adhesions and, when applicable, were separated using a previously described object separation algorithm based on the convex hull and watershed transform (53). Finally, individual boundaries were extracted, and focal adhesion lengths were calculated as the major axis length of the corresponding best-fit ellipse to each boundary. At least 250 adhesions/image and at least 6 images/group were quantified.
Using the same immunostaining procedures, the average fluorescent intensity of α-SMA, CDH2, IL-6, STAT3, and phosphorylated STAT3 was quantified from 12-mo-old Cdh11+/+, Cdh11+/−, and Cdh11−/− murine aortic valves using custom image processing analyses. Grayscale fluorescence images of cell nuclei (DAPI) were preprocessed to remove background intensities and used as a mask approximating the total leaflet area. Average fluorescent intensities within the masked aortic valve leaflets were computed from at least four histological sections per mouse and three to four mice from each genotype; data were normalized to WT expression. For all image quantification, sample sizes were the numbers of mice. To allow for comparison across genotypes for a given stain, all sections were stained under the same conditions and imaged using the same microscope settings.
Cell adhesion.
AVICs were seeded at 10,000 cells/cm2 on tissue culture treated plates in complete media and allowed to adhere for 1 or 3.5 h. Wells were then gently washed with PBS−/−, and the remaining cells were trypsinized and counted.
Fluorescence-activated cell sorting (FACS).
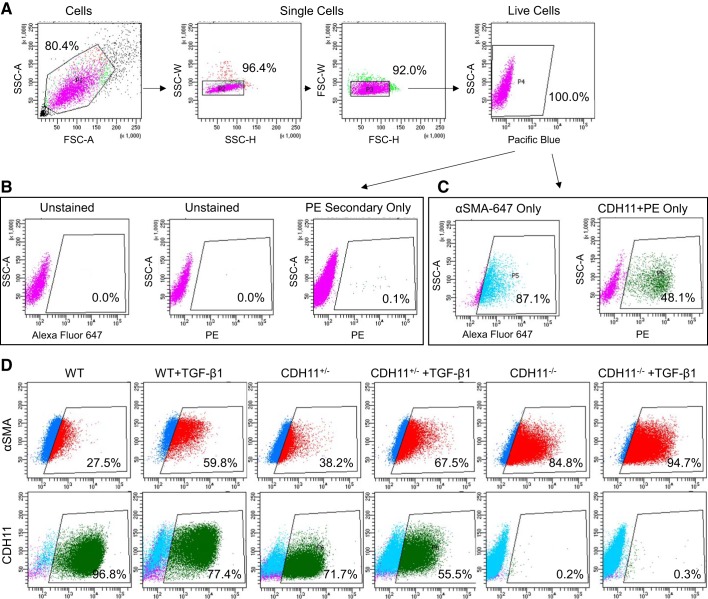
Cells were plated at 10,000 cells/cm2 and grown in complete media with or without 5 ng/ml recombinant murine TGF-β1 for 48 h. AVICs were lifted with 0.05% trypsin for no more than 10 min and resuspended in HEPES-buffered saline (HBS) FACS buffer (20 mM HEPES + 137 mM NaCl + 3 mM KCl + 1 mM CaCl2 + 2% FBS in MilliQ). Cells were centrifuged at 350 g for 10 min at 4°C, strained through a 100-µm filter (CLS431752, Sigma-Aldrich), and aliquoted at 500,000 live cells/tube. Cells were centrifuged at 350 g for 10 min at 4°C and resuspended in 100 µl of primary antibody against CDH11 (1.25 µg/ml 23C6, gift from Michael Brenner) for 1 h. Cells were washed in 1 ml HBS FACS buffer with 1:20,000 DAPI (D1306, Life Technologies) and centrifuged at 350 g for 10 min at 4°C. Samples were then incubated with 100 µl of secondary antibody (1.25 µg/ml IgG1-PE, no. 406607, BioLegend) for 30 min in the dark. Cells were washed in HBS FACS buffer and incubated in Transcription Factor Fixation/Permeabilization working solution (TNB-0607-KIT, Tonbo Biosciences) for 30 min in the dark. After this, all washes and incubations were performed in permeabilization buffer (TNB-0607-KIT, Tonbo Biosciences) to facilitate diffusion through the fixed cells. Cells were centrifuged at 350 g for 10 min at 4°C and resuspended in 100 µl of conjugated antibody against α-SMA (1:500 α-SMA-647, NBP2-34522AF647, Novus Biologicals) for 30 min in the dark. Cells were washed in permeabilization buffer and centrifuged at 350 g for 10 min at 4°C before being resuspended in 0.5 ml HBS FACS buffer and analyzed. Briefly, the gating strategy involved isolating single live cells by shape and DAPI staining (Fig. 4A), setting gates in the PE and 647 channels such that unstained or secondary only samples had very few or no positive events (Fig. 4B), and validating that single-stain controls displayed a marked increase in signal (Fig. 4C).
Fig. 4.
FACS gating strategy. Representative dot plots are shown for the FACS gating strategy used to evaluate cadherin (CDH11) stained with PE and smooth muscle α-actin (α-SMA) conjugated to Alexa Fluor 647. Live single cells were isolated by shape and DAPI staining (Pacific blue) (A). Negative controls of unstained and secondary only were used to set gates with few to no false positive events (B). Stained cells showed a 100- or 1,000-fold increase in fluorescence intensity (C). Representative FACS dot plots are shown for α-SMA and CDH11 with positive cells inside the gate (D). TGF-β1, transforming growth factor-β1; WT, wild-type; SSC-A/H/W, side scatter-area/height/width; FSC-A/H/W; forward scatter-area/height/width.
Statistics.
Differences were analyzed with SigmaPlot (version 11.0) via one-way ANOVA with pairwise multiple comparisons made using the Holm-Sidak post hoc testing method. Non-normal data sets were analyzed via Kruskal-Wallis one-way ANOVA on ranks with pairwise multiple comparisons made using Dunn’s post hoc testing method. Data are presented as means ± SE. P values of <0.05 were considered statistically significant. The numbers of independent samples are reported in all figures. For cultured cells, the independent samples are the numbers of wells that were harvested.
RESULTS
CDH11 regulates α-SMA, collagen deposition, cadherin switching, and inflammation.
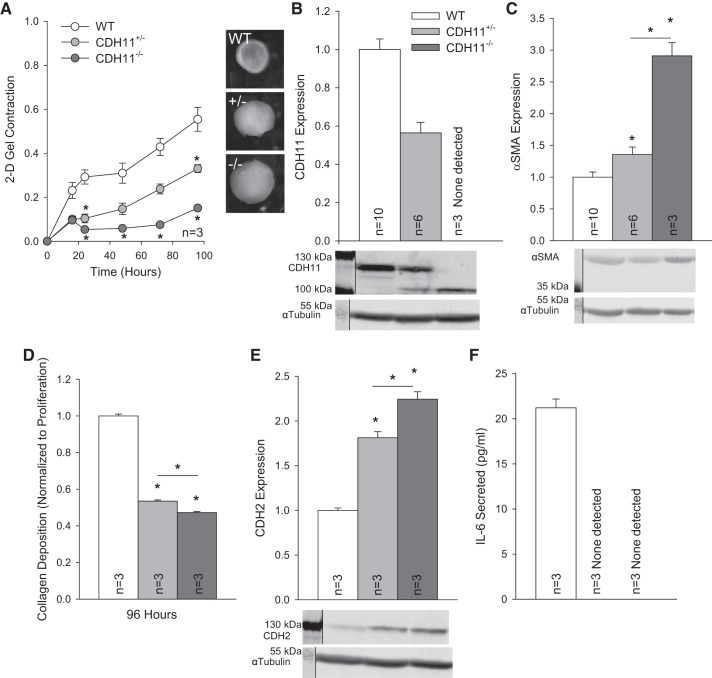
We first examined the impact of CDH11 expression on AVIC function by comparing phenotypes from WT, CDH11+/−, and CDH11−/− cell lines. Collagen gel compaction revealed that a loss of CDH11 results in a proportional decrease in contraction at the population level (Fig. 1A). Furthermore, we observed that CDH11 expression (Fig. 1B) was inversely correlated with α-SMA expression (Fig. 1C), a myofibroblast marker often associated with contractility, and positively correlated with collagen deposition (Fig. 1D), a measure of valve remodeling potential. Cadherin switching is a defining feature of cell phenotypes. By inspection of expression of multiple cadherins we found an inverse relationship between CDH11 and CDH2 (Fig. 1E). IL-6 secretion was undetected in AVICs with CDH11 deletion (Fig. 1F).
Fig. 1.
Cadherin 11 (CDH11) regulates the aortic valve interstitial cell (AVIC) phenotype through smooth muscle α-actin (α-SMA), collagen deposition, cadherin switching, and inflammation. Loss of CDH11 reduced population-level contractility (A) despite an inverse relationship with α-SMA (B and C). Collagen deposition was correlated with CDH11 expression (D). Loss of CDH11 resulted in an upregulation of CDH2 (E), and IL-6 secretion was only measurable in wild-type (WT) AVICs (F). For clarity, representative Western blots have been cropped to display relevant staining. *P < 0.05 compared with WT cells unless otherwise indicated. 2-D, two dimensional.
CDH11 regulates intercellular tension and cell-substrate force transmission.
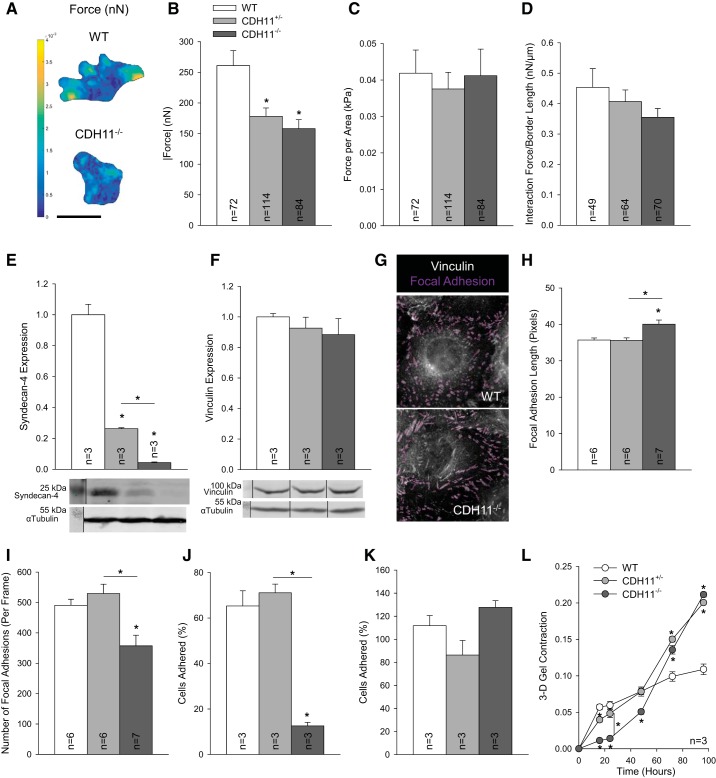
CDH11 is distinct from other cadherins in that it can directly interact with the substrate and participate in focal adhesion complexes by binding with syndecan-4 (32). To investigate cell-substrate interactions at the single cell level and isolate the mechanical effects of cell-substrate interactions from cell-cell interactions, we used TFM on single cells and cell doublets. Similar to the collagen gel contraction, deletion of CDH11 resulted in reduced total traction force generation in single cells (Fig. 2, A and B). However, we also observed that CDH11+/− and CDH11−/− cells tended to be smaller than WT cells (6,000.87 ± 305.57, 4,844.22 ± 159.84, and 4,078.00 ± 239.72 µm2 for WT, CDH11+/−, and CDH11−/− cells, respectively). Interestingly, when total force was normalized to cell area (Fig. 2C), the differences between groups were eliminated. Unsurprisingly, the normalized interaction forces between cell doublets trended with CDH11 expression (Fig. 2D).
Fig. 2.
Cadherin 11 (CDH11) regulates contractility through focal adhesions. Traction force microscopy was used to quantify traction force maps (in nN) based on gel displacement (A). Deletion of CDH11 reduced total force generation by single cells (A and B). Differences between single cells disappeared when normalized to cell area (C). Analysis of cell doublets revealed a decrease in cell-cell interaction force with loss of CDH11 (D). Syndecan-4 expression was CDH11 dependent (E), but vinculin expression was unaffected (F). Lack of CDH11 resulted in longer focal adhesion lengths (G and H) but fewer focal adhesions overall (G and I; shown in magenta), leading to reduced adhesion at 1 h (J), which resolved by 3.5 h (K). When embedded in a three-dimensional (3-D) gel, CDH11 expression had an inverse relationship with contractility by 96 h (L). For clarity, representative Western blots have been cropped to display relevant staining. Scale bar = 50 µm. *P < 0.05 compared with wild-type (WT) cells unless otherwise indicated.
To determine if CDH11 regulates cell size through focal adhesions, we first evaluated syndecan-4 expression. Similar to the differences in cell area, CDH11+/− and CDH11−/− lines produced significantly less syndecan-4 than WT AVICs (Fig. 2E). In addition to syndecan-4, the focal adhesion protein vinculin has been shown to participate in focal adhesions with CDH11 (32). Although total vinculin expression was consistent across cell lines (Fig. 2F), CDH11−/− AVICs had longer and fewer adhesions than WT or CDH11+/− AVICs (Fig. 2, G–I). An adhesion assay further revealed that lack of CDH11 reduced adhesion by ~80% at 1 h (Fig. 2J). However, at 3.5 h, these adhesion differences were insignificant (Fig. 2K). To test the importance of cells’ initial adhesion to the substrate, we performed another gel contraction but prepared the experiment with AVICs embedded within the gel [three dimensional (3-D)] as opposed to on top [two dimensional (2-D)]. While the first 24 h of the 3-D gel contraction followed the same trends as the 2-D gel contraction, by 48 h, CDH11+/− and CDH11−/− AVICs overtook WT AVICs (Fig. 2L).
TGF-β1 overrides normal regulation of interstitial cell phenotypes.
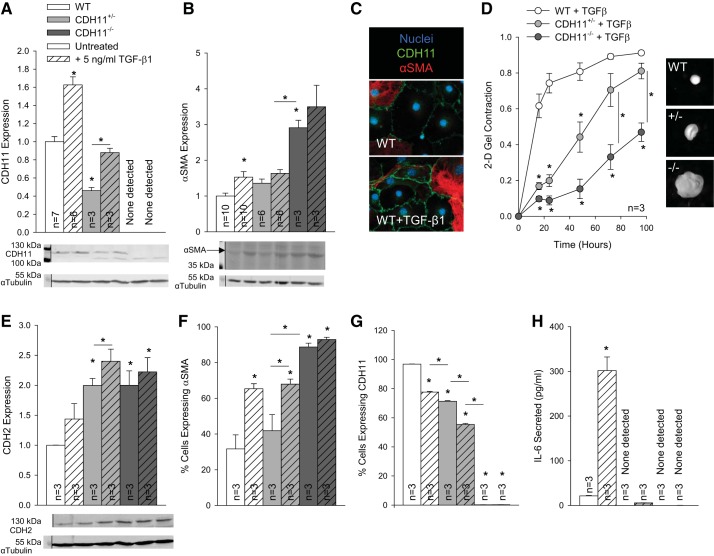
Having established the intrinsic effect of CDH11 on the AVIC phenotype, we next wanted to clarify its role in mediating common disease initiators. In particular, TGF-β1 treatment increased both CDH11 and α-SMA expression in all cell lines, consistent with the myofibroblast disease phenotype (Fig. 3, A–C). Despite an overall increase in contractility because of TGF-β1 treatment (cf. Fig. 1A), loss of CDH11 still resulted in a graded decrease in contractility, relative to WT cells (Fig. 3D). CDH2 expression also trended up with TGF-β1 treatment (Fig. 3E). After 24 h of TGF-β1 treatment, the percentage of cells that expressed α-SMA increased (Figs. 3F and 4D), whereas the percentage of cells that expressed CDH11 slightly decreased (Figs. 3G and 4D), as measured by FACS (Fig. 4). IL-6 secretion increased dramatically in TGF-β1-treated WT AVICs but only slightly in CDH11+/− AVICs and remained undetectable in CDH11−/− AVICs (Fig. 3H).
Fig. 3.
Transforming growth factor (TGF)-β1 increases myofibroblast markers. TGF-β1 treatment increased both cadherin 11 (CDH11) and smooth muscle α-actin (α-SMA) expression, overriding their normally inverse relationship (A–C). Overall contractility was increased with TGF-β1 but was still dependent on CDH11 deletion (D). TGF-β1 slightly increased CDH2 expression (E) while increasing the percentage of cells expressing α-SMA (F), despite decreasing percentages of aortic valve interstitial cells (AVICs) expressing CDH11 (G), as measured by FACS. IL-6 secretion was increased with TGF-β1 treatment but remained barely detectable in CDH11+/− and CDH11−/− AVICs (H). *P < 0.05 compared with wild-type (WT) cells unless otherwise indicated. 2-D, two dimensional.
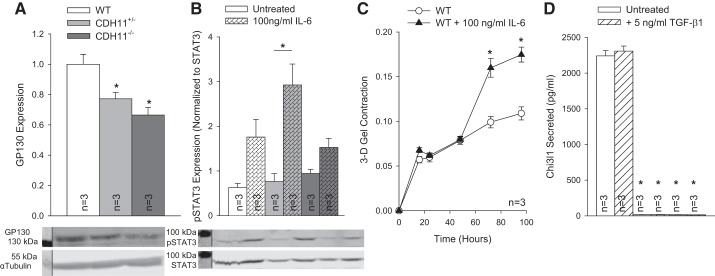
Interstitial cell inflammation is dependent on CDH11.
Having demonstrated that IL-6 secretion correlates with CDH11 expression, we proceeded to determine if IL-6 could be part of a positive feedback mechanism driving the myofibroblast phenotype. Expression of the IL-6 receptor GP130 correlated with CDH11 expression (Fig. 5A), and we observed an increase in STAT3 phosphorylation with IL-6 treatment (Fig. 5B). As a more functional readout, we treated 3-D collagen gels with IL-6 and observed an increase in AVIC contractility (Fig. 5C). In addition, Chi3l1, like IL-6, was only secreted at a detectable level in WT AVICs, although it was unaffected by TGF-β1 treatment (Fig. 5D).
Fig. 5.
IL-6 signaling activates aortic valve interstitial cells (AVICs). Expression of glycoprotein 130 (GP130) correlated with cadherin 11 (CDH11) (A), and IL-6 treatment caused an increase in both STAT3 phosphorylation (B) and AVIC contractility (C). Chitinase-3-like protein 1 (Chi3l1) secretion correlated with IL-6 secretion but was unaffected by transforming growth factor-β1 treatment (D). For clarity, representative Western blots have been cropped to display relevant staining. *P < 0.05 compared with wild-type (WT) cells unless otherwise indicated. 3-D, three dimensional.
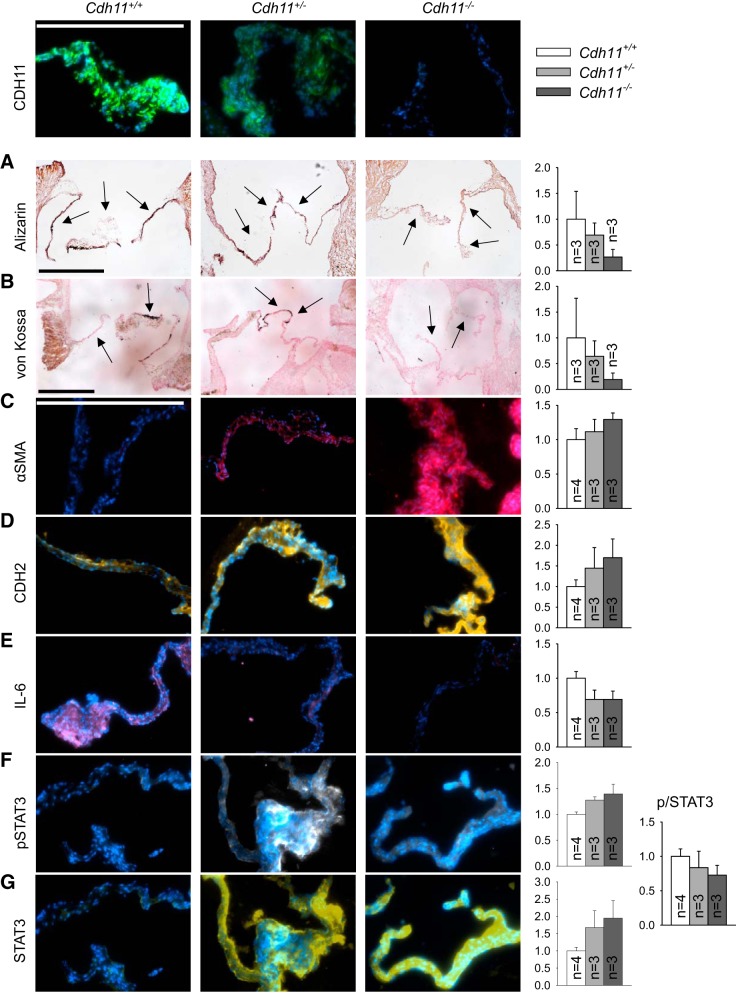
Calcification is associated with increased myofibroblasts and IL-6 signaling.
Positive alizarin red and von Kossa (i.e., calcification) staining in murine leaflets decreased from Cdh11+/+ to Cdh11+/− to Cdh11−/− murine leaflets, although not significantly (Fig. 6, A and B). α-SMA expression (Fig. 6C) and CDH2 expression (Fig. 6D) were inversely correlated with CDH11 in vivo, consistent with our observations in vitro. Expression of both IL-6 (Fig. 6E) and the fraction of phosphorylated STAT3 (Fig. 6, F and G), its downstream transcription factor, was correlated with CDH11.
Fig. 6.
Aortic valves from cadherin 11 (Cdh11) transgenic mice have reduced calcification and similar trends in myofibroblast and inflammatory markers as observed in vitro. A−G: histology (A and B) and immunofluorescence (C–G) of Cdh11+/+, Cdh11+/−, and Cdh11−/− murine aortic valves. Calcification was reduced with loss of CDH11 (A and B). CDH11 expression was inversely correlated with smooth muscle α-actin (α-SMA; C) and CDH2 (D) but positively correlated with IL-6 (E) and the fraction of phosphorylated STAT3 (F and G). Arrows denote leaflets. Blue indicates DAPI-stained nuclei. Scale bars = 500 µm.
DISCUSSION
Previous in vivo work has shown that targeting CDH11 prevents CAVD (10) and that global overexpression leads to disease (51). Given that AVICs are the primary mediators of CAVD, we were interested in understanding the intrinsic role of CDH11 in the mechanobiological regulation of the AVIC phenotype. Myofibroblasts are the activated, disease-driving phenotype of AVICs and are characterized by increased contractility, collagen deposition, α-SMA expression, and, more recently, CDH11 expression. We expected that CDH11 and α-SMA would work synergistically to promote disease, or contraction, in AVICs. Upon evaluation of collagen gel contraction, we found that loss of CDH11 resulted in decreased contractility and was inversely correlated with α-SMA expression (Fig. 1, A–C). This was unexpected but has been previously observed in porcine cells (52). The inverse relationship between CDH11 and α-SMA can be understood by considering the forces on AVICs. CDH11 forms bonds stronger than any other cadherin and stronger than the integrity of the cell membrane (55). If the intracellular tension is increasing with α-SMA, the cells may be downregulating CDH11 to reduce intercellular tension and maintain a homeostatic mechanical environment. Given this inverse regulation of myofibroblast markers, we next looked at collagen deposition to further characterize the AVIC phenotype. Unsurprisingly, collagen deposition correlated with collagen gel contraction (Fig. 1D), supporting the finding that WT AVICs are the most active or myofibroblastic phenotype, although they have the least α-SMA.
We were also interested in whether other cadherins were compensating for the loss in CDH11, as cadherin switching is a well-studied phenomenon that can define the myofibroblast phenotype (21). Indeed, we found that CDH2 has an inverse relationship with CDH11 (Fig. 1E). The switching between these two mesenchymal cadherins is likely instrumental in the formation of calcific nodules. In the dystrophic calcification hypothesis, cells upregulate CDH11 and are thus very strongly bound to their neighbors. Thus, when subjected to increased strain, cells could tear their neighbors’ membranes before releasing their homotypic CDH11 bond. Perhaps there is normally a protective cadherin switching from CDH11 to CDH2 under these circumstances, allowing cells to disengage from their neighbors before membrane tearing and eventual calcific nodule formation. Indeed, immunostaining confirmed this switching from CDH11 to CDH2 in vivo (Fig. 6D), which may counteract the increased intracellular tensions provided by α-SMA (Fig. 6C). IL-6 secretion is absent in AVICs (Fig. 1F) and murine aortic valves (Fig. 6E) with reduced CDH11 expression, indicating that CDH11 promotes a robust inflammatory response in the aortic valve.
CDH11 is unique in that it is the only known cadherin that participates in focal adhesions (32), thereby allowing it to directly sense both the substrate and neighboring cells. In the focal adhesion, CDH11 complexes with syndecan-4, a plasma membrane proteoglycan that binds fibronectin. To isolate the cell-substrate interactions from all CDH11-mediated binding, we performed TFM on single cells and cell doublets. The interaction forces between pairs of cells resulted in an expected decreasing trend with loss of CDH11 (Fig. 2D). Like in the population-level gel contraction assay, single cell TFM revealed reduced total force generation with loss of CDH11 expression (Fig. 2, A and B). When forces were normalized to cell area, however, the differences washed out (Fig. 2C). This suggests that cell spreading, and thereby available surface area for focal adhesion attachment, determines how much force the cell exerts. When we quantified the expression of CDH11’s focal adhesion partner, syndecan-4, we observed the same trends as gel contraction and force generation; the loss of CDH11 reduces syndecan-4 expression (Fig. 2E).
To probe the maturity of these focal adhesions, we quantified focal adhesion length from vinculin staining using custom image analysis tools (Fig. 2G). Although total vinculin was unchanged (Fig. 2F), CDH11−/− AVICs had significantly longer (Fig. 2H) but fewer focal adhesions (Fig. 2I). While consistent vinculin expression implies an ability of the cells to anchor the actin cytoskeleton to the cell membrane, changes in focal adhesion morphology suggest that CDH11 plays an important role in focal adhesion initiation and maturation, which may dictate cell spreading and migration. Using an adhesion assay, we observed a dramatic reduction in the ability of CDH11−/− AVICs to adhere at 1 h (Fig. 2J), supporting the hypothesis that initiation of focal adhesions is largely dependent on CDH11. To control for the cells’ ability to initially bind to the gel, we embedded AVICs in collagen gels and performed a 3-D contraction assay. The first 48 h look almost identical to the 2-D contraction assay and TFM force measurements, with WT AVICs most contractile; however, by 72 h, CDH11+/− and CDH11−/− AVICs overtook WT AVICs, and, by 96 h, the contractility correlated with α-SMA expression, as originally expected (Fig. 2L). This finding provides an explanation for why the two-dimensional contractility and α-SMA expression are inversely correlated (Fig. 1, A and C). Initially, gel contraction is likely mediated by the ability of AVICs to form robust focal adhesions and is therefore dominated by WT AVICs with the highest syndecan-4 expression. As time progresses, however, CDH11-depleted AVICs are able to form enough focal adhesions to sufficiently anchor to the gel and are thus more contractile than WT AVICs because of their significantly increased α-SMA expression. Of note, there is no difference in adhesion between cell lines at 3.5 h (Fig. 2K). All other experimental assays provided sufficient time for initial seeding (>3.5 h) and can thus be interpreted without this confounding factor.
Having characterized CDH11-dependent changes under unstimulated conditions, we asked how CDH11 would regulate the AVIC phenotype in the presence of TGF-β1. This is thought to be a biochemical model of disease initiation, as TGF-β1 induces myofibroblast differentiation. Treatment induced upregulation of both CDH11 and α-SMA, overriding their usual inverse relationship, to create a more contractile myofibroblastic phenotype (Fig. 3, A–C). Although CDH11 and α-SMA are increased, and there is increased collagen gel contraction overall, differences in contractility are still dependent on CDH11 expression (Fig. 3D). TGF-β1 also slightly increases CDH2 expression, indicating an overriding of the normal cadherin switching as well (Fig. 3E). FACS analysis of AVICs (Fig. 4) showed that the percentage of cells expressing α-SMA, an indicator of myofibroblast differentiation, increased with TGF-β1 treatment in a CDH11-dependent manner (Figs. 3F and 4D). In particular, WT AVICs had a large increase in the percentage of α-SMA-expressing cells, whereas CDH11−/− AVICs had a small increase, likely because of differences in α-SMA expression in unstimulated conditions. However, there were slight decreases in percentage of CDH11-expressing AVICs (Figs. 3G and 4D), indicating that TGF-β1 treatment increases CDH11 expression in a smaller population of cells. It is also possible that TGF-β1 induces shedding of the extracellular domain of CDH11. The FACS antibody recognizes the extracellular portion of CDH11, and the Western blot antibody recognizes an intracellular domain. CDH11 is known to shed when subjected to TNF-α, providing some precedent for shedding as a result of inflammatory signaling (39). The shift in α-SMA expression demonstrates that TGF-β1 treatment induces myofibroblast differentiation of AVICs as opposed to promoting the activity of resident myofibroblasts within each population. The addition of TGF-β1 also causes a dramatic, CDH11-dependent increase in IL-6 secretion that mirrors 2-D gel contraction and TFM force generation trends (Fig. 3H).
To determine whether this secreted IL-6 is regulating the AVICs themselves or signaling to neighboring cells, we probed its receptor, GP130. Interestingly, GP130 expression correlated very well with contractility trends (Fig. 5A). This suggests that secreted IL-6 could be acting on nearby AVICs in a positive feedback or paracrine signaling fashion, noting that evaluation of STAT3 phosphorylation confirms that all lines are responsive to IL-6 (Fig. 5B). While total STAT3 levels increase with loss of CDH11 (Fig. 6G), the fraction of activated phosphorylated STAT3 to STAT3 decreases (Fig. 6, F and G). A possible explanation could be that there is a compensatory increase in STAT3 transcription by other inflammatory cytokines, such as IL-23 (33, 35). When 3-D collagen gels were treated with IL-6, they were more contractile (Fig. 5C), suggesting a regulatory mechanism between CDH11 and AVIC contractility. Specifically, engagement of CDH11 causes secretion of IL-6, which signals through GP130 to phosphorylate STAT3 and results in AVIC contraction. This could even be a mechanism of myofibroblast differentiation wherein CDH11-expressing cells secreting IL-6 can activate the contractility of neighboring cells.
Downstream of STAT3 is Chi3l1, a secreted inflammatory glycoprotein with unclear function that has been clinically associated with multiple cardiovascular diseases (5, 29). Chi3l1 secretion appears to require full CDH11 expression and thereby presents an interesting future direction of study (Fig. 5D). Unlike IL-6, Chi3l1 is not acutely sensitive to TGF-β1 treatment, which could mean it is more tightly regulated by CDH11 than by proinflammatory cytokine signaling. Serum levels of YKL-40 (the human analog of Chi3l1) have been associated with thromboembolic stroke but not myocardial infarction, indicating a specificity of YKL-40 to certain adverse cardiac events (45). Evaluation of YKL-40 could be a quick and inexpensive method for tracking or initially screening patients with CAVD, provided that a robust signal in the systemic circulation can be detected. Although it is not likely a disease initiator (29), it could be important in the progression of calcification and therefore a potential therapeutic target. Thus, further study of Chi3l1 and its human analog, YKL-40, is needed. Additionally, its secretory pattern matches IL-6 secretion, indicating that CDH11 suppression may attenuate broader secretory mechanisms. Cell-cell adhesion (7, 46) and focal adhesions (17) have been shown to regulate various secretory mechanisms, and the role of CDH11 in the secretion of other cytokines warrants further study.
Taken together, our data suggest that CDH11 regulates AVIC contractility through focal adhesions and modulates the AVIC phenotype through IL-6 signaling. Thus, functionally blocking CDH11 could provide a therapeutic alternative to aortic valve replacement by modulating the mechanobiological and inflammatory cues driving the induction of a myofibroblastic phenotype and ultimately exacerbating CAVD progression.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants R35-HL-135790, R01-HL-115103, and T32-HL-007411, National Science Foundation (NSF) CAREER Award 1055384, and NSF Graduate Research Fellowship 2013170175.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.B. and W.D.M. conceived and designed research; M.A.B. and R.J.J. performed experiments; M.A.B. and M.R.B. analyzed data; M.A.B., M.R.B., L.M.R., and W.D.M. interpreted results of experiments; M.A.B. prepared figures; M.A.B. and M.R.B. drafted manuscript; M.A.B., M.R.B., L.M.R., and W.D.M. edited and revised manuscript; M.A.B., M.R.B., L.M.R., R.J.J., A.P., and W.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Christine Scott, Camryn L. Johnson, Alison K. Schroer, Joseph Chen, and Cyndi R. Clark for providing mice and helping with murine dissections. We are grateful to Albert B. Reynolds for discussions surrounding p120 catenin and for providing various antibodies. We thank Michael B. Brenner as well for providing the CDH11 antibody used for FACS and Elizabeth L. Reynolds for the work optimizing FACS antibodies.
REFERENCES
- 1.Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol 18: 1295–1299, 2008. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 3.Bersi MR, Khosravi R, Wujciak AJ, Harrison DG, Humphrey JD. Differential cell-matrix mechanoadaptations and inflammation drive regional propensities to aortic fibrosis, aneurysm or dissection in hypertension. J R Soc Interface 14: 20170327, 2017. doi: 10.1098/rsif.2017.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgmaier M, Hoppe S, Krüger T, Mahnken AH, Ketteler M, Reith S, Mühlenbruch G, Marx N, Brandenburg V. Serum levels of C-peptide are associated with coronary artery calcification in patients with rheumatoid arthritis. Rheumatol Int 35: 1541–1547, 2015. doi: 10.1007/s00296-015-3244-y. [DOI] [PubMed] [Google Scholar]
- 6.Butler JP, Tolić-Nørrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol 282: C595–C605, 2002. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 7.Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, Leon L, Lee SY, Lee DM, Brenner MB. Cadherin-11 regulates fibroblast inflammation. Proc Natl Acad Sci USA 108: 8402–8407, 2011. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Ryzhova LM, Sewell-Loftin MK, Brown CB, Huppert SS, Baldwin HS, Merryman WD. Notch1 mutation leads to valvular calcification through enhanced myofibroblast mechanotransduction. Arterioscler Thromb Vasc Biol 35: 1597–1605, 2015. doi: 10.1161/ATVBAHA.114.305095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chester AH, Taylor PM. Molecular and functional characteristics of heart-valve interstitial cells. Philos Trans R Soc Lond B Biol Sci 362: 1437–1443, 2007. doi: 10.1098/rstb.2007.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CR, Bowler MA, Snider JC, Merryman WD. Targeting cadherin-11 prevents notch1-mediated calcific aortic valve disease. Circulation 135: 2448–2450, 2017. doi: 10.1161/CIRCULATIONAHA.117.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J 22: 1769–1777, 2008. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danielsen R, Aspelund T, Harris TB, Gudnason V. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: the AGES-Reykjavík study. Int J Cardiol 176: 916–922, 2014. doi: 10.1016/j.ijcard.2014.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76: 2307–2316, 1999. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol 12: 5–17, 2013. doi: 10.1007/s10237-012-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274, 2005. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 16.Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, Bouchareb R, Marchand JT, Nsaibia MJ, Guauque-Olarte S, Pibarot P, Bouchard L, Bossé Y, Mathieu P. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation 134: 1848–1862, 2016. doi: 10.1161/CIRCULATIONAHA.116.023116. [DOI] [PubMed] [Google Scholar]
- 17.Hagiwara M, Kokubu E, Sugiura S, Komatsu T, Tada H, Isoda R, Tanigawa N, Kato Y, Ishida N, Kobayashi K, Nakashima M, Ishihara K, Matsushita K. Vinculin and Rab5 complex is required for uptake of Staphylococcus aureus and interleukin-6 expression. PLoS One 9: e87373, 2014. doi: 10.1371/journal.pone.0087373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han SJ, Oak Y, Groisman A, Danuser G. Traction microscopy to identify force modulation in subresolution adhesions. Nat Methods 12: 653–656, 2015. doi: 10.1038/nmeth.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen PC. Analysis of discrete Ill-posed problems by means of the L-curve. SIAM Rev 34: 561–580, 1992. doi: 10.1137/1034115. [DOI] [Google Scholar]
- 20.Hermans H, Herijgers P, Holvoet P, Verbeken E, Meuris B, Flameng W, Herregods MC. Statins for calcific aortic valve stenosis: into oblivion after SALTIRE and SEAS? An extensive review from bench to bedside. Curr Probl Cardiol 35: 284–306, 2010. doi: 10.1016/j.cpcardiol.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15: 4310–4320, 2004. doi: 10.1091/mbc.e04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horikawa K, Radice G, Takeichi M, Chisaka O. Adhesive subdivisions intrinsic to the epithelial somites. Dev Biol 215: 182–189, 1999. doi: 10.1006/dbio.1999.9463. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol 11: 218–231, 2014. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutcheson JD, Chen J, Sewell-Loftin MK, Ryzhova LM, Fisher CI, Su YR, Merryman WD. Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol 33: 114–120, 2013. doi: 10.1161/ATVBAHA.112.300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. 5-HT(2B) antagonism arrests non-canonical TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol 53: 707–714, 2012. doi: 10.1016/j.yjmcc.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerrell RJ, Parekh A. Cellular traction stresses mediate extracellular matrix degradation by invadopodia. Acta Biomater 10: 1886–1896, 2014. doi: 10.1016/j.actbio.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerrell RJ, Parekh A. Polyacrylamide gels for invadopodia and traction force assays on cancer cells. J Vis Exp 2015: 52343, 2015. doi: 10.3791/52343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian B, Narula N, Li QY, Mohler ER III, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75: 457–465, 2003. doi: 10.1016/S0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 29.Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Role of inflammatory marker YKL-40 in the diagnosis, prognosis and cause of cardiovascular and liver diseases. Crit Rev Clin Lab Sci 53: 396–408, 2016. doi: 10.1080/10408363.2016.1190683. [DOI] [PubMed] [Google Scholar]
- 30.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB; PARTNER Trial Investigators . Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 366: 1686–1695, 2012. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 31.Ku CH, Johnson PH, Batten P, Sarathchandra P, Chambers RC, Taylor PM, Yacoub MH, Chester AH. Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res 71: 548–556, 2006. doi: 10.1016/j.cardiores.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Langhe RP, Gudzenko T, Bachmann M, Becker SF, Gonnermann C, Winter C, Abbruzzese G, Alfandari D, Kratzer MC, Franz CM, Kashef J. Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat Commun 7: 10909, 2016. doi: 10.1038/ncomms10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PW, Smith AJ, Yang Y, Selhorst AJ, Liu Y, Racke MK, Lovett-Racke AE. IL-23R-activated STAT3/STAT4 is essential for Th1/Th17-mediated CNS autoimmunity. JCI Insight 2: e91663, 2017. doi: 10.1172/jci.insight.91663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Xu S, Gotlieb AI. The progression of calcific aortic valve disease through injury, cell dysfunction, and disruptive biologic and physical force feedback loops. Cardiovasc Pathol 22: 1–8, 2013. doi: 10.1016/j.carpath.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Kim KW, Cho ML, Ju JH, Kang CM, Oh HJ, Min JK, Lee SH, Park SH, Kim HY. IL-23 induces receptor activator of NF-kappaB ligand expression in fibroblast-like synoviocytes via STAT3 and NF-kappaB signal pathways. Immunol Lett 127: 100–107, 2010. doi: 10.1016/j.imlet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci USA 108: 4708–4713, 2011. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohler ER III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 103: 1522–1528, 2001. doi: 10.1161/01.CIR.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 38a.MyAssays, Limited Four Parameter Logistic Curve [Online]. https://www.myassays.com/four-parameter-logistic-curve.assay [22 January 2018].
- 39.Noss EH, Watts GF, Zocco D, Keller TL, Whitman M, Blobel CP, Lee DM, Brenner MB. Evidence for cadherin-11 cleavage in the synovium and partial characterization of its mechanism. Arthritis Res Ther 17: 126, 2015. doi: 10.1186/s13075-015-0647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 62: 1002–1012, 2013. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol 536: 329–337, 2001. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittet P, Lee K, Kulik AJ, Meister JJ, Hinz B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J Cell Sci 121: 877–886, 2008. doi: 10.1242/jcs.024877. [DOI] [PubMed] [Google Scholar]
- 43.Plotnikov SV, Sabass B, Schwarz US, Waterman CM. High-resolution traction force microscopy. Methods Cell Biol 123: 367–394, 2014. doi: 10.1016/B978-0-12-420138-5.00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinhart-King CA, Dembo M, Hammer DA. The dynamics and mechanics of endothelial cell spreading. Biophys J 89: 676–689, 2005. doi: 10.1529/biophysj.104.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker PM, Chasman DI, Rose L, Loscalzo J, Elias JA. Plasma levels of the proinflammatory chitin-binding glycoprotein YKL-40, variation in the chitinase 3-like 1 gene (CHI3L1), and incident cardiovascular events. J Am Heart Assoc 3: e000897, 2014. doi: 10.1161/JAHA.114.000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubinek T, Yu R, Hadani M, Barkai G, Nass D, Melmed S, Shimon I. The cell adhesion molecules N-cadherin and neural cell adhesion molecule regulate human growth hormone: a novel mechanism for regulating pituitary hormone secretion. J Clin Endocrinol Metab 88: 3724–3730, 2003. doi: 10.1210/jc.2003-030090. [DOI] [PubMed] [Google Scholar]
- 47.Sabass B, Gardel ML, Waterman CM, Schwarz US. High resolution traction force microscopy based on experimental and computational advances. Biophys J 94: 207–220, 2008. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroer AK, Merryman WD. Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J Cell Sci 128: 1865–1875, 2015. doi: 10.1242/jcs.162891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz US, Balaban NQ, Riveline D, Bershadsky A, Geiger B, Safran SA. Calculation of forces at focal adhesions from elastic substrate data: the effect of localized force and the need for regularization. Biophys J 83: 1380–1394, 2002. doi: 10.1016/S0006-3495(02)73909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sung DC, Bowen CJ, Vaidya KA, Zhou J, Chapurin N, Recknagel A, Zhou B, Chen J, Kotlikoff M, Butcher JT. Cadherin-11 overexpression induces extracellular matrix remodeling and calcification in mature aortic valves. Arterioscler Thromb Vasc Biol 36: 1627–1637, 2016. doi: 10.1161/ATVBAHA.116.307812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Leinwand LA, Anseth KS. Roles of transforming growth factor-β1 and OB-cadherin in porcine cardiac valve myofibroblast differentiation. FASEB J 28: 4551–4562, 2014. doi: 10.1096/fj.14-254623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wienert S, Heim D, Saeger K, Stenzinger A, Beil M, Hufnagl P, Dietel M, Denkert C, Klauschen F. Detection and segmentation of cell nuclei in virtual microscopy images: a minimum-model approach. Sci Rep 2: 503, 2012. doi: 10.1038/srep00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokokawa M, Takeyasu K, Yoshimura SH. Mechanical properties of plasma membrane and nuclear envelope measured by scanning probe microscope. J Microsc 232: 82–90, 2008. doi: 10.1111/j.1365-2818.2008.02071.x. [DOI] [PubMed] [Google Scholar]