Abstract
The extracellular matrix (ECM) actively participates in diverse aspects of cardiovascular development and physiology as well as during disease development and progression. ECM roles are determined by its physical and mechanical properties and by its capacity to both release bioactive signals and activate cell signaling pathways. The ECM serves as a storage depot for a wide variety of molecules released in response to injury or with aging. Indeed, there is a plethora of examples describing how cells react to or modify ECM stiffness, how cells initiate intracellular signaling pathways, and how cells respond to the ECM. This Perspectives article reviews the contributions of 21 articles published in the American Journal of Physiology-Heart and Circulatory Physiology in response to a Call for Papers on this topic. Here, we summarize the contributions of these studies focused on the cardiac and vascular ECM. We highlight the translational importance of these studies and conclude that the ECM is a critical component of both the heart and vasculature. Readers are urged to examine and learn from this special Call for Papers.
Keywords: cardiac, cardiovascular, collagen, extracellular matrix, matrix metalloproteinases, myocardial, vascular
The cardiovascular extracellular matrix (ECM) is defined as the hundreds of proteins that comprise the complex scaffold that surrounds cells of the heart and vasculature. In response to a recent Call for Papers in the American Journal of Physiology-Heart and Circulatory Physiology, 21 reviews and original research articles provide additional evidence for the extensive roles that the ECM plays in cardiovascular physiology and pathophysiology. Some articles address ECM in cardiac and vascular cell development and differentiation. Others are focused on the mechanisms by which ECM contributes to heart and blood vessel physiological and pathological responses to aging, stress, or injury. Most address ECM contributions in a diverse array of pathologies, implicating the ECM as a logical translational target.
Cocciolone et al. (5) reviewed elastin formation and maturation as well as the role that elastin plays in the mechanical properties of the cardiovascular system with an emphasis on conduit arteries. There is a nice summary of the roles that deficiencies in elastin fibers have on specific human vascular diseases and how animal models of elastin insufficiency provide avenues to evaluate human disease. Klein et al. (10) reported that culturing mouse resistance arteries without tone caused cannulated and pressurized vessels to undergo outward ECM remodeling. Culture in the presence of vasoconstrictors prevented arterial outward remodeling, indicating that the ECM can be structurally modified by tone. In line with this, Knutsen et al. (11) showed that mice with elastin haploinsufficiency have stiffer arteries and reduced cerebral blood flow. When the haploinsufficient animals were treated with the vasodilator Minoxidil, cerebral blood flow was restored and carotid vascular diameter increased to wild-type amounts. The changes in vascular mechanics and function persisted for weeks after the treatment ended and were accompanied by differential expression of 127 ECM genes.
Parker et al. (20) performed an unbiased proteomics screen on the ascending aortas from wild-type mice and mice with abnormal fibrillin 1 expression that serve as a model for Marfan syndrome. Transforming growth factor (TGF)-β was increased in both young and aged mice with Marfan syndrome, whereas the rapamycin-independent component of target of rapamycin (RICTOR) was increased only in aged mice with Marfan syndrome. TGF-β signaling occurred in cultured vascular smooth muscle cells through a RICTOR-dependent pathway, affected by β3-integrin and integrin-linked kinase, similar to that found in vivo in blood vessels from subjects with Marfan syndrome. These results provide new targets for the potential treatment of aneurysms.
Collagen is a major component of the vascular wall and myocardium. Steffensen and Rasmussen (24) summarized current knowledge on single-nucleotide polymorphisms in the 13q34 locus that harbors the genes for the α1- and α2-subunits of collagen type IV, which control collagen type IV expression in the vascular wall, and reported how alterations in these genes are linked to the risk of coronary artery disease, vascular stiffening and atherosclerosis. Holzapfel and Ogden (9) described the microstructural characteristics of collagen, focusing on its relationship with smooth muscle cells as primary components influencing vascular strain and stress. Additional considerations are given to the changes in microstructure of these passive and active components that take place in diseases such as atherosclerosis and aneurysm formation, and the authors provided evidence for the need of computational modeling to better understand ECM roles in vascular diseases.
Caggiano and colleagues (4) tested the effect of changing the local mechanical environment on scar collagen turnover, accumulation, and alignment in Sprague-Dawley rats at 1, 2, 3 and 6 wk after myocardial infarction (MI) by sewing a Dacron patch to the epicardium to eliminate circumferential strain while permitting continued longitudinal stretching with each heartbeat. They found that collagen in healing infarcts aligned parallel to regional strain and perpendicular to the pre-MI muscle and collagen fiber direction, indicating that the mechanical environment is a primary determinant of scar alignment.
There were several articles focused on antifibrotic and anti-matrix metalloproteinase (MMP) strategies for the prevention of adverse remodeling after injury. Using a hydrogel that released tissue inhibitor of metalloproteinase (TIMP)-3 after MI, Purcell et al. (21) found that localized delivery of TIMP-3 interrupted adverse post-MI remodeling, underlining the translational importance of this strategy. Li et al. (12) treated diabetic rats with the natural alkaloid berberine, which downregulated insulin growth factor 1 receptor expression in cardiac fibroblasts and subsequently reduced MMP-2, MMP-9, smooth muscle α-actin, and collagen type I expression in diabetic hearts. McDonald et al. (17) examined the role of macrophage-derived secreted protein acidic and rich in cysteine (SPARC) on pressure overload. They found that macrophages were important for time-dependent increases in SPARC, which enhanced postsynthetic collagen processing, insoluble collagen content, and myocardial stiffness.
The ECM is an important component of blood vessel remodeling during pregnancy. Ren and colleagues (23) investigated the balance between signaling from soluble fms-like tyrosine kinase-1 and placental growth factor on the expression and activity of MMPs and collagen types I and IV. The addition of soluble fms-like tyrosine kinase-1 to pregnant rats simulated preeclampsia, and infusion of placental growth factor reversed the effects.
The extent of ECM cross-linking determines the stiffness of blood vessels and cardiac tissue. Craighead et al. (7) demonstrated that the ECM cross-linking enzyme lysyl oxidase-like 2 was greater in skin biopsies from middle-aged human subjects with essential hypertension compared with young or normotensive control subjects, whereas soluble lysyl oxidase (LOX) expression was diminished with hypertension. Brief in vivo pharmacological inhibition of LOX in the cutaneous microvasculature affected the vasomotor responses to vasoconstrictor and endothelium-independent vasodilator agonists only in normotensive subjects, suggesting that LOX activity plays an important role in the acute control of vascular tone under normal physiological conditions. In response to volume overload in the heart, inhibition of LOX prevented the left ventricular wall stress increase, partially attenuated cardiac hypertrophy, completely blocked increases in fibrotic proteins, including collagens, MMPs, and TIMPs, and prevented the decline in cardiac function (8).
Changes to ECM structure translate the changes in physiology. Corporan et al. (6) found that in a rat model of severe mitral regurgitation, an imbalance in the MMP-to-TIMP ratio in the myocardium occurred early and contributed to the increase in dilation. Su and colleagues (25) provided evidence of ECM changes in the proximal portion of the pulmonary artery in response to chronic hypoxia, with downstream effects on the mechanical properties of blood vessels in vivo and ex vivo. A decrease in the elastin-to-collagen ratio was the most prominent structural change and was accompanied by increased measures of pulmonary artery stiffness including pulse wave velocity and moduli of elasticity, changes not observed in the aorta. A better understanding of altered dynamic afterload parameters in pulmonary arterial hypertension should provide new targets for therapy.
Cardiac fibroblasts are the predominant source of ECM, and Trial and Cieslik (26) reviewed our current understanding of how this cell type transitions over the course of aging. Defective TGF-β signaling enhances activation of the ERK1/2 pathway to activate fibroblasts and stimulate interstitial fibrosis. Al-Hattab et al. (1) investigated how scleraxis transactivates Twist1 and Snai1 to stimulate epithelial-to-mesenchymal transition during development. Scleraxis was a critical controller of fibroblast genesis and fate in the myocardium, implicating this transcription factor in wound healing and fibrosis after injury. Qin et al. (22) chronically supplemented exogenous humanin in middle-aged mice and found that this can prevent and reverse age-dependent cardiac fibrosis. Aging mice with macrophage overexpression of MMP-9 have increased macrophages at 7 days after MI, which improves diastolic physiology and cardiac remodeling by altering cardiac wound healing (18).
Ariyasinghe et al. (2) reviewed how microphysiological systems are being engineered to establish direct relationships between distinct features in the ECM and myocardial function with unprecedented in vitro control and resolution. This technology will continue to unravel important ECM features that control myocardial cell and tissue physiology. Likewise, Liem et al. (13) used phrase mining to determine the association between ECM and six cardiovascular pathologies, namely, ischemic heart disease, cardiomyopathy, cerebrovascular accident, congenital heart disease, arrhythmias, and valve disease. This bioinformatics approach using 1 million research publication abstracts indicated that 82 ECM proteins associate with all 6 pathologies. A recent review on MI remodeling has also highlighted the importance of the ECM, and text mining strategies will uncover previously unidentified or underappreciated targets (19).
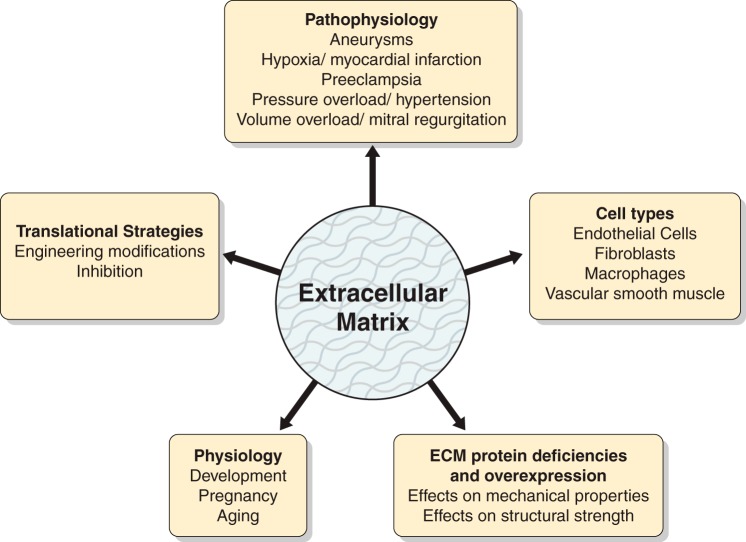
In conclusion, this compendium of ECM articles displays the complexity of this research field and highlights the translational relevance of understanding ECM roles in both normal conditions and during pathophysiological processes (Fig. 1). In conjunction with recent guidelines published by the American Journal of Physiology-Heart and Circulatory Physiology on myocardial ischemia and infarction models, measuring cardiac physiology, antibody use, and statistical reporting, this ECM collection will aid investigators new to the field in developing experiments that have strong rigor and reproducibility potential (3, 14–16). We hope that you enjoy reading these articles as much as we did editing this Call for Papers.
Fig. 1.
The articles covered in the extracellular matrix (ECM) Call of Papers span both physiological and pathophysiological processes and a number of cell types, involve mechanistic studies using deficient and overexpression approaches, and evaluate translational strategies to define ECM roles in the cardiovascular system.
GRANTS
This work was supported by the University of Southern Denmark, National Institutes of Health Grants GM-104357, GM-114833, GM-115428, HL-051971, HL-075360, HL-088105, and HL-129823, and Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Grant 5I01BX000505.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.B., M.L.L., and L.A.M.-L. drafted manuscript; M.B., M.L.L., and L.A.M.-L. edited and revised manuscript; M.B., M.L.L., and L.A.M.-L. approved final version of manuscript.
REFERENCES
- 1.Al-Hattab DS, Safi HA, Nagalingam RS, Bagchi RA, Stecy MT, Czubryt MP. Scleraxis regulates Twist1 and Snai1 expression in the epithelial-to-mesenchymal transition. Am J Physiol Heart Circ Physiol 315: H658–H668, 2018. doi: 10.1152/ajpheart.00092.2018. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasinghe NR, Lyra-Leite DM, McCain ML. Engineering cardiac microphysiological systems to model pathological extracellular matrix remodeling. Am J Physiol Heart Circ Physiol 315: H771–H789, 2018. doi: 10.1152/ajpheart.00110.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caggiano LR, Lee JJ, Holmes JW. Surgical reinforcement alters collagen alignment and turnover in healing myocardial infarcts. Am J Physiol Heart Circ Physiol 315: H1041–H1050, 2018. doi: 10.1152/ajpheart.00088.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocciolone AJ, Hawes JZ, Staiculescu MC, Johnson EO, Murshed M, Wagenseil JE. Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol 315: H189–H205, 2018. doi: 10.1152/ajpheart.00087.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corporan D, Onohara D, Hernandez-Merlo R, Sielicka A, Padala M. Temporal changes in myocardial collagen, matrix metalloproteinases and their inhibitors in experimental chronic mitral regurgitation in rodents. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craighead DH, Wang H, Santhanam L, Alexander LM. Acute lysyl oxidase inhibition alters microvascular function in normotensive but not hypertensive men and women. Am J Physiol Heart Circ Physiol 314: H424–H433, 2018. doi: 10.1152/ajpheart.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Hajj EC, El Hajj MC, Ninh VK, Gardner JD. Inhibitor of lysyl oxidase improves cardiac function and the collagen/MMP profile in response to volume overload. Am J Physiol Heart Circ Physiol 315: H463–H473, 2018. doi: 10.1152/ajpheart.00086.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzapfel GA, Ogden RW. Biomechanical relevance of the microstructure in artery walls with a focus on passive and active components. Am J Physiol Heart Circ Physiol 315: H540–H549, 2018. doi: 10.1152/ajpheart.00117.2018. [DOI] [PubMed] [Google Scholar]
- 10.Klein A, Joseph PD, Christensen VG, Jensen LJ, Jacobsen JCB. Lack of tone in mouse small mesenteric arteries leads to outward remodeling, which can be prevented by prolonged agonist-induced vasoconstriction. Am J Physiol Heart Circ Physiol 315: H644–H657, 2018. doi: 10.1152/ajpheart.00111.2018. [DOI] [PubMed] [Google Scholar]
- 11.Knutsen RH, Beeman SC, Broekelmann TJ, Liu D, Tsang KM, Kovacs A, Ye L, Danback JR, Watson A, Wardlaw A, Wagenseil JE, Garbow JR, Shoykhet M, Kozel BA. Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am J Physiol Heart Circ Physiol 315: H18–H32, 2018. doi: 10.1152/ajpheart.00683.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Xing W, Zhang M, Geng FH, Yang H, Zhang H, Zhang X, Li J, Dong L, Gao F. Anti-fibrotic cardioprotection of berberine via down-regulating myocardial IGF-1 receptor-regulated MMP-2/9 expression in diabetic rats. Am J Physiol Heart Circ Physiol 315: H802–H813, 2018. doi: 10.1152/ajpheart.00093.2018. [DOI] [PubMed] [Google Scholar]
- 13.Liem DA, Murali S, Sigdel D, Shi Y, Wang X, Shen J, Choi H, Caufield JH, Wang W, Ping P, Han J. Phrase mining of textual data to analyze extracellular matrix protein patterns across cardiovascular disease. Am J Physiol Heart Circ Physiol 315: H910–H924, 2018. doi: 10.1152/ajpheart.00175.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald LT, Zile MR, Zhang Y, Van Laer AO, Baicu CF, Stroud RE, Jones JA, LaRue AC, Bradshaw AD. Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis. Am J Physiol Heart Circ Physiol 315: H92–H100, 2018. doi: 10.1152/ajpheart.00719.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meschiari CA, Jung M, Iyer RP, Yabluchanskiy A, Toba H, Garrett MR, Lindsey ML. Macrophage overexpression of matrix metalloproteinase-9 in aged mice improves diastolic physiology and cardiac wound healing after myocardial infarction. Am J Physiol Heart Circ Physiol 314: H224–H235, 2018. doi: 10.1152/ajpheart.00453.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouton AJ, Rivera OJ, Lindsey ML. Myocardial infarction remodeling that progresses to heart failure: a signaling misunderstanding. Am J Physiol Heart Circ Physiol 315: H71–H79, 2018. doi: 10.1152/ajpheart.00131.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker SJP, Stotland A, MacFarlane E, Wilson N, Orosco A, Venkatraman V, Madrid K, Gottlieb R, Dietz HC, Van Eyk JE. Proteomics reveals Rictor as a noncanonical TGF-β signaling target during aneurysm progression in Marfan mice. Am J Physiol Heart Circ Physiol 315: H1112–H1126, 2018. doi: 10.1152/ajpheart.00089.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell BP, Barlow SC, Perreault PE, Freeburg L, Doviak H, Jacobs J, Hoenes A, Zellars KN, Khakoo AY, Lee T, Burdick JA, Spinale FG. Delivery of a matrix metalloproteinase-responsive hydrogel releasing TIMP-3 after myocardial infarction: effects on left ventricular remodeling. Am J Physiol Heart Circ Physiol 315: H814–H825, 2018. doi: 10.1152/ajpheart.00076.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Q, Mehta H, Yen K, Navarrete G, Brandhorst S, Wan J, Delrio S, Zhang X, Lerman LO, Cohen P, Lerman A. Chronic treatment with the mitochondrial peptide humanin prevents age-related myocardial fibrosis in mice. Am J Physiol Heart Circ Physiol 315: H1127–H1136, 2018. doi: 10.1152/ajpheart.00685.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Z, Cui N, Zhu M, Khalil RA. Placental growth factor reverses decreased vascular and uteroplacental MMP-2 and MMP-9 and increased MMP-1 and MMP-7 and collagen types I and IV in hypertensive pregnancy. Am J Physiol Heart Circ Physiol 315: H33–H47, 2018. doi: 10.1152/ajpheart.00045.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffensen LB, Rasmussen LM. A role for collagen type IV in cardiovascular disease? Am J Physiol Heart Circ Physiol 315: H610–H625, 2018. doi: 10.1152/ajpheart.00070.2018. [DOI] [PubMed] [Google Scholar]
- 25.Su J, Logan CC, Hughes AD, Parker KH, Dhutia NM, Danielsen CC, Simonsen U. Impact of chronic hypoxia on proximal pulmonary artery wave propagation and mechanical properties in rats. Am J Physiol Heart Circ Physiol 314: H1264–H1278, 2018. doi: 10.1152/ajpheart.00695.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trial J, Cieslik KA. Changes in cardiac resident fibroblast physiology and phenotype in aging. Am J Physiol Heart Circ Physiol 315: H745–H755, 2018. doi: 10.1152/ajpheart.00237.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]