Notch signaling is central to a number of vital developmental processes, as evidenced by the lethality of global knockout of a number of Notch proteins (5). Notch signaling requires both a membrane-bound ligand [e.g., Jagger1, Jagger2, delta-like protein (Dll)1, Dll3, or Dll4] and membrane-bound receptor (e.g., Notch1, Notch2, Notch3, or Notch4) on a neighboring cell. The role of these signaling cascades has long been understood to regulate the proliferation, migration, and differentiation of these cells during both development and pathophysiological states, such as injury-induced angiogenesis. Indeed, angiogenesis can be alternatively regulated, depending on whether Jagger1 or DII4 is the dominant ligand (7). However, we are just beginning to understand the vital role for Notch signaling independent of developmental requirements, e.g., in vascular tone of mature arteries. In arteries, Notch signaling between smooth muscle cells (SMCs) and endothelial cells (ECs), where ligands and receptors from homotypic and heterotypic cells can interact and induce both canonical and noncanonical Notch-dependent signaling cascades, may augment or limit the contractility of SMCs (1). Once more, the expression patterns of Notch receptors and ligands differ between SMCs and ECs, enabling unique signaling mechanisms in a directional manner. This could conceivably allow for specific pathways to constrict or dilate an artery.
The vasoreactivity of arteries has been found to be altered in mice with defective Notch signaling. In Notch3 knockout mice, myogenic tone was impaired in numerous vascular beds, with a concomitant decrease in RhoA/Rho kinase-dependent phosphorylation of myosin light chain (MLC) (4, 6). Interestingly, global loss of Notch3 imparts resistance to increases in vascular resistance and blood pressure, including angiotensin II-induced hypertension and hypoxia-induced pulmonary arterial hypertension (5, 8). These data suggest a potential role of Notch3 signaling in regulating vascular resistance and thus blood pressure. Although global Notch3 knockout is one of the few nonconditional Notch protein knockout mice that are not lethal, developmental effects such as changes in structural composition of arteries may contribute to the blood pressure phenotypes. To examine the role of smooth muscle Notch signaling in vascular tone regulation, expression of dominant negative MAML1 (DNMAML1) protein was used to inhibit CSL-dependent canonical Notch signaling (SMC-DNMAML1+ mice) (3). Arteries from these mice failed to vasodilate or vasoconstrict in response to receptor- and nonreceptor-mediated vasoactive agents. Although baseline blood pressure was unaffected, in vivo pressor responses were blunted when canonical Notch signaling was inhibited.
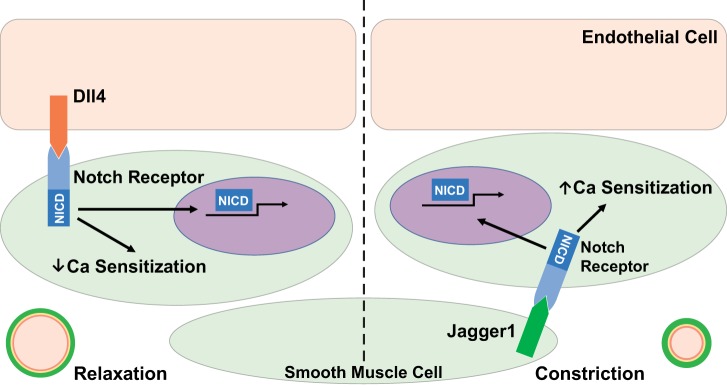
Notch signaling can be induced by ligands expressed in either ECs or SMCs and initiate canonical and noncanonical signaling mechanisms. In a study recently published in the American Journal of Physiology-Heart and Circulatory Physiology, Basu et al. (2) have provided convincing evidence for differential regulation of vascular tone by Jagged1 and Dll4 through the use of cell type-specific and temporal conditional deletion mice. Smooth muscle cell-specific deletion of Jagged1 resulted in an overall decrease in myosin light chain kinase (MLCK) protein content, with no changes in other proteins vital to regulation and development of SMC constriction [including myosin phosphatase target subunit 1 (MYPT1), smooth muscle myosin heavy chain, and smooth muscle actin]. This decrease in MLCK, which phosphorylates MLC (p-MLC), resulted in a reduced agonist and depolarization-induced vasoconstriction due to blunted p-MLC levels. Alternatively, deletion of Dll4 from ECs resulted in reduced MYPT1 protein levels, the myosin phosphatase regulatory protein, which led to both increased vasoconstriction and impaired vasodilation. As predicted with the changes in regulatory protein levels in each of these mice, Ca2+ sensitivity levels were differentially altered, with loss of SMC-Jagged1 reducing and loss of EC-Dll4 increasing Ca2+ sensitivity. In isolated SMCs, Jagged1 induced a Rho kinase-dependent increase in phosphorylation of MYPT1 at sites that enable inhibition of myosin phosphatase, which shifted the sensitivity of SMCs to more vasoconstriction. Conversely, Dll4 treatment on isolated SMCs increased total levels of MYPT1 without increasing myosin phosphatase-inhibitory phosphorylation sites, thus shifting the cells toward a less vasoconstrictive status. These data indicate a possible “Yin and Yang” role for Jagged1 and Dll4 on SMC function in vascular function independent of their roles in development (Fig. 1).
Fig. 1.
Summary of the potential “Yin and Yang” in Notch signaling according to Basu et al. (2). NICD, Notch intracelleular domain.
SMC-DNMAML1+ mice, which have impaired Notch signaling in SMCs, have no reported change in overall baseline blood pressure (3). Basu et al. (2) showed that, indeed, both MLCK and MYPT1 protein levels were reduced in SMCs from SMC-DNMAML1+ mice. The question remains as to whether the loss of just one of the Notch ligands results in changes in baseline blood pressure. Based on the blunted pressure changes observed in SMC-DNMAML1+ mice after treatment with vasoconstrictors and changes in vasoreactivity, it would be predicted that loss of Jagged1 would result in a hypotensive baseline blood pressure, whereas loss of Dll4 would be predicted to cause an increase in baseline blood pressure. This regulation of blood pressure may represent a novel mechanism of hypertension or pulmonary hypertension development. Indeed, the protection against development of hypertension and pulmonary hypertension observed in Notch3 knockout mice may be due to an inability of SMC-Jagged1 to shift SMCs toward a more vasoconstrictive status (5). However, the Notch receptors that are important for the differential response to SMC-Jagged1 and EC-Dll4 signaling remain unknown.
The signaling mechanisms after initiation of Notch signaling remain elusive. Although clearly a canonical signaling mechanism is initiated by both Jagged1 and Dll4 via alterations in expression of MLCK and MYPT1, respectively, the mechanism by which these ligands alter Ca2+ sensitivity is unclear (2). As Basu et al. (2) demonstrated, Jagged1 shifts the phosphorylation status of MYPT1 toward a more active state in a Rho kinase-dependent mechanism. It is unclear how Notch signaling activates Rho kinase activation, but this suggests a potentially noncanonical mechanism by which Notch can differentially alter SMC Ca2+ sensitivity in a ligand-dependent manner. Interestingly, the presence of EC-Jagged1 was not sufficient to maintain Jagged1-dependent SMC Notch signaling, indicating a unique signaling mechanism activated via homotypic versus heterotypic Jagged1-Notch signaling activation in SMCs. Furthermore, the regulation of expression and membrane localization of Notch ligands in vascular cells postdevelopment will need to be explored. Changes in expression of Notch ligands could occur after a perturbation of the system, such as an alteration in the renin-angiotensin system or a change in the sympathetic drive, and result in changes in vascular resistance. Of particular note, in genome-wide association studies of hypertension, the Jagged1 locus was identified as a potential player in patients with hypertension, indicating a potential role for Notch signaling in human disease states (9). The work highlighted here by Basu et al. (2) provides a rational basis for these genetic data.
In summary, novel Notch-mediated signaling mechanisms within the vasculature may regulate vascular tone via differential roles of SMC-Jagged1 and EC-Dll4, and an imbalance in these signaling pathways can lead to a shift in vascular resistance. As we begin to understand the regulation and role of Notch signaling postdevelopment, we can begin to understand how these signaling mechanisms contribute to human diseases such as systemic and pulmonary hypertension.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-088554 (to B. E. Isakson) and HL-143165 (to M. E.Good).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.G. and B.E.I. prepared figures; M.E.G. and B.E.I. drafted manuscript; M.E.G. and B.E.I. edited and revised manuscript; M.E.G. and B.E.I. approved final version of manuscript.
REFERENCES
- 1.Baeten JT, Lilly B. Notch signaling in vascular smooth muscle cells. Adv Pharmacol 78: 351–382, 2017. doi: 10.1016/bs.apha.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu S, Barbur I, Calderon A, Banerjee S, Proweller A. Notch signaling regulates arterial vasoreactivity through opposing functions of Jagged1 and Dll4 in the vessel wall. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00293.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S, Srinivasan DK, Yang K, Raina H, Banerjee S, Zhang R, Fisher SA, Proweller A. Notch transcriptional control of vascular smooth muscle regulatory gene expression and function. J Biol Chem 288: 11191–11202, 2013. doi: 10.1074/jbc.M112.442996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belin de Chantemèle EJ, Retailleau K, Pinaud F, Vessières E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, Joutel A, Henrion D. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol 28: 2216–2224, 2008. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher J, Gridley T, Liaw L. Molecular pathways of notch signaling in vascular smooth muscle cells. Front Physiol 3: 81, 2012. doi: 10.3389/fphys.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulos N, Helle F, Dussaule JC, Placier S, Milliez P, Djudjaj S, Guerrot D, Joutel A, Ronco P, Boffa JJ, Chatziantoniou C. Notch3 is essential for regulation of the renal vascular tone. Hypertension 57: 1176–1182, 2011. doi: 10.1161/HYPERTENSIONAHA.111.170746. [DOI] [PubMed] [Google Scholar]
- 7.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 27: 5132–5137, 2008. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 15: 1289–1297, 2009. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munroe PB, Barnes MR, Caulfield MJ. Advances in blood pressure genomics. Circ Res 112: 1365–1379, 2013. doi: 10.1161/CIRCRESAHA.112.300387. [DOI] [PubMed] [Google Scholar]