Abstract
We investigated structural and functional differences in primary and pial collateral circulations in adult normotensive male and female Wistar rats. Male (n = 10) and female (n = 7) rats were subjected to middle cerebral artery (MCA) occlusion and changes in relative cerebral blood flow in MCA and pial collateral territories were measured by multisite laser-Doppler flowmetry. Rats were then transcardially perfused with a mixture of carbon black and latex, perfusion fixed, and imaged to compare primary and pial collateral structure between male (n = 4) and female (n = 3) rats, including lumen diameters and number. To study pial collateral function, leptomeningeal anastomoses (LMAs) were isolated and pressurized from male (n = 7) and female (n = 6) rats. Myogenic tone and reactivity to pressure, vascular function to pharmacological activator, or inhibitor of ion channels was measured and compared. There was no difference between relative cerebral blood flow in both MCA and pial collateral territories during occlusion and reperfusion between groups. Compared with male LMAs, female LMAs had similar myogenic tone (24.0 ± 7.3% vs. 16.0 ± 3.7%, P > 0.05) and reactivity to increased pressure and similar vascular responses to vasoconstrictive and vasodilatory stimuli. Additionally, compared with female LMAs, male LMAs had similar numbers (21 ± 1 vs. 20 ± 2, P > 0.05) and diameters (30.5 ± 2.0 vs. 26.2 ± 0.6 μm, P > 0.05), and no sex difference was detected in the diameter of arterial segments of circle of Willis. Together, our data establish no sex difference of cerebral collateral structure or function, suggesting that the reduced severity of stroke outcome in female rats is not likely due to differences in the cerebral collateral circulation.
NEW & NOTEWORTHY Our work compared the function of leptomeningeal anastomoses between male and female adult normotensive rats with no sex difference found. We also confirmed no sex difference in primary and pial collateral structure in Wistar rats. Our findings suggest that the reduced severity of stroke in premenopausal women and reproductively intact female rodents is not likely due to improved primary and pial collateral circulations.
Keywords: ion channel, ischemic stroke, leptomeningeal anastomoses, pial collateral circulation
INTRODUCTION
Stroke is an enormous burden on global health, causing devastating disability (49), but few therapeutic advances have been accomplished despite decades of effort (26). On average, someone in the United States has a stroke every 40 s (8). Someone dies of stroke every 4 min, making it the fifth leading cause of death in the United States (8). Stroke is a sexually dimorphic disease with the recognition that men and women have different incidence, prevalence, and outcome (61). The average stroke onset for women is ~4 yr older than men (75 yr compared with 71 yr) (39); however, compared with men, higher incidence rates have been reported for women at 74–85 yr (43) and above 85 yr (55), which has been suggested to be related with a decline in sex hormones after menopause. Women are at a significantly greater risk for atrial fibrillation than men, a major cause of stroke (2a). Moreover, women account for more stroke deaths, consistently suffer from worse stroke outcomes, worse functional recovery, and poorer quality of life at 3, 6, and 12 mo after stroke (25, 50).
Sex differences are not only a consideration in human stroke but also are relevant in animal studies. Alkayed et al. (1) showed that reproductively intact female rats demonstrated a smaller infarct size after middle cerebral artery (MCA) occlusion (MCAO) in both cerebral cortex and caudoputamen compared with age-matched male rats, an effect that was mainly attributed to endogenous estrogen. However, it is unlikely that hormonal effects account fully for the sex differences in stroke incidence since women are protected until the age of 75–85 yr, well past the menopausal years (50). Several other factors have been shown to contribute to the sex differences in stroke outcome, including age, prestroke function, and comorbidities such as depression and social isolation (29, 52, 53, 58). Interestingly, adjustment for these factors did not sufficiently explain the sex differences observed in stroke outcome (21, 32). One possibility for sexually dimorphic outcome of stroke may relate to perfusion differences. In addition to showing a higher cerebral perfusion during rest, women also have a higher cerebral perfusion during cognitive activities, such as solving verbal analogies and a spatial line orientation task (28). Thus, intrinsic biological sex differences in cerebral vasculature may underlie the sex differences in stroke outcome.
The cerebral collateral circulation is closely associated with the occurrence, development, and outcome of acute ischemic stroke (64). The anatomy of the collateral circulation consists of extracranial and intracranial sources that can be divided into primary and secondary collateral circulations (41). Primary collaterals include the arterial segments of the circle of Willis and provide immediate redistribution of cerebral blood flow (CBF) during an occlusion, especially the occlusion of carotid arteries (35). Studies have shown that patients with incomplete circle of Willis suffered worse stroke outcome, whereas complete collateral circulation effectively reduced cerebral infarction (31, 56). Rapid establishment of collateral circulation after cerebral artery occlusion could significantly diminish the extent of brain injury (13, 14, 31, 56).
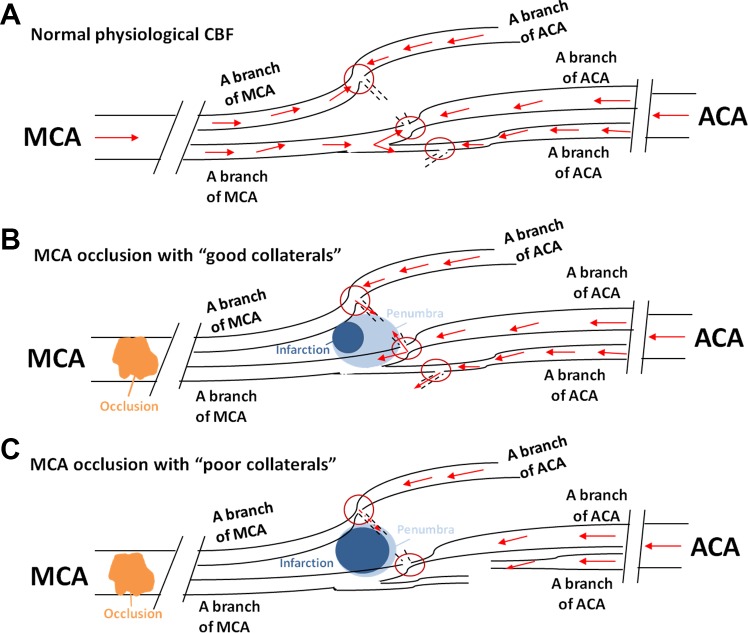
Secondary collaterals consist of the ophthalmic artery that originates from the internal carotid artery (ICA) and leptomeningeal anastomoses (LMAs), also known as leptomeningeal collaterals or pial collaterals (41). In acute ischemic stroke, the commonly engaged secondary collaterals are LMAs. LMAs are small vessels connecting the distal branches of major (anterior, middle, and posterior) cerebral arteries along the surface of the brain (41). LMAs importantly redirect CBF from nonoccluded territories to the occluded area to perfuse the penumbra and limit infarct expansion (3). The compensatory function of LMAs will not be a factor unless one of the major cerebral arteries is acutely occluded (59). From animal studies, LMAs have been determined to have an important role in the microvascular perfusion of the penumbra (11, 44). The ischemic penumbra refers to the peri-infarct region of the brain during focal occlusion with metabolic impairment but retained cellular integrity that can be salvaged if reperfusion occurs (2). Using computed tomography angiography to identify the penumbral tissue in stroke patients, clinical studies have demonstrated that good pial collateral status was a critical determinant of a smaller infarct and favorable stroke outcome (40, 48). See Fig. 1 for a schematic demonstration of the role of LMAs in stroke outcome.
Fig. 1.
Schematic picture showing the arteries and arterioles on the dorsal surface of the brain and the function of leptomeningeal anastomoses (LMAs) during ischemic stroke. A: under normal physiological conditions, abundant cerebral blood flow (CBF) is maintained by the anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA; not shown) and their distal branches that are connected by LMAs (red circles). Distal connections under these conditions have low and bidirectional flow because of similar pressure differential across the small vessels. B: in patients with “good pial collaterals,” i.e., LMAs that are larger and/or greater in number (red circles), when the proximal MCA is occluded, retrograde blood flow from nonoccluded territories, for example, the ACA territory, can sustain blood flow and limit infarct expansion. These patients have larger penumbra and smaller infarction. C: in patients with “poor pial collaterals,” i.e., smaller and fewer LMAs (red circles), when the proximal MCA is occluded, retrograde blood flow that is provided by nonoccluded territories is limited, which leads to less salvageable tissue and larger infarction.
The structure and function of LMAs have been investigated between C57BL/6J and BALB/cByj mouse strains, and no sex difference between groups has been reported (23). In the same study, the structure of posterior communicating arteries and primary cerebral arteries did not vary with sex either (23). In addition, the function of LMAs in male hypertensive rats was studied and compared with normotensive rats (12). This study on the function of pial collaterals showed that LMAs were vasoconstricted in young and aged male spontaneously hypertensive rats to a greater degree than young and aged Wistar-Kyoto rats. Additionally, LMAs from spontaneously hypertensive rats responded with significant myogenic vasoconstriction to increased intravascular pressure and had impaired vasodilatory responses compared with Wistar-Kyoto rats (12). Thus, hypertension appears to negatively affect LMA function; however, sex differences in LMA structure and function have yet to be investigated and may be an important contributor to sex-related differences in stroke outcome.
In the present study, we compared the vasoactive properties of isolated and pressurized LMAs that could actively influence vascular resistance and collateral flow between adult male and female Wistar rats. We also examined if the structure of LMAs, including the number and lumen diameter, varied with sex. Given the findings that premenopausal women and intact female rodents sustained more favorable outcome after acute ischemic brain injury compared with age-matched male subjects (1, 62), we hypothesized that female rats would have less perfusion deficit in both MCA and pial collateral territories during MCAO and that LMAs would have less myogenic tone and less vascular reactivity compared with male rats. We further hypothesized that there would be more anastomotic connections with larger lumen diameter in female rats and that female rats would have greater diameter of arterial segments of circle of Willis and greater primary collateral flow.
MATERIALS AND METHODS
Animals.
All experiments were conducted using adult male (10–11 wk) and female (15–17 wk) Wistar rats. Animal groups were matched by body weights and not age to avoid potential influence of varied brain sizes on vessel lumen diameter. Animals were housed in the Animal Care Facility, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Animals had access to food and water ad libitum and followed a 12:12-h light/dark cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont and complied with National Institutes of Health guidelines for care and usage of laboratory animals.
Middle cerebral artery occlusion.
Transient proximal MCAO was performed using the filament technique, as previously described (17, 18). Briefly, animals were anesthetized with isoflurane (1.5% in oxygen) and then tapered onto pentobarbital (50 mg/kg iv) for the remainder of the experiment. A midline neck incision was made to expose the right common carotid artery. A monofilament coated with silicon was inserted into the external carotid artery and advanced into the ICA until it occluded the MCA, as verified by laser-Doppler flowmetry. Animals were mechanically ventilated to maintain blood gases within a normal physiological range (Table 1), and a heating pad was used to maintain body temperature at ~37°C. The MCA was occluded, and ischemia was induced for 2 h. After this, the filament was removed and reperfusion allowed for 2 h. The order of male or female rat experiments was randomized using an online randomization tool (https://www.random.org/lists/).
Table 1.
Body weights and arterial blood gases of all animals during middle cerebral artery occulusion surgeries
| Male Rats | Female Rats | |
|---|---|---|
| Number of rats/group | 10 | 7 |
| Weight, g | 361 ± 6 | 307 ± 4 |
| Arterial blood gases | ||
| 15 min postocclusion | ||
| pH | 7.43 ± 0.02 | 7.48 ± 0.02 |
| Pco2, mmHg | 38 ± 3 | 32 ± 2 |
| Po2, mmHg | 113 ± 8 | 111 ± 6 |
| 1 h postocclusion | ||
| pH | 7.42 ± 0.01 | 7.42 ± 0.01 |
| Pco2, mmHg | 42 ± 2 | 41 ± 2 |
| Po2, mmHg | 79 ± 5 | 76 ± 5 |
| 15 min postreperfusion | ||
| pH | 7.45 ± 0.01 | 7.41 ± 0.02 |
| Pco2, mmHg | 40 ± 2 | 41 ± 3 |
| Po2, mmHg | 69 ± 2 | 69 ± 5 |
Values are presented as means ± SE.
Multisite laser-Doppler probes for core MCA and pial collateral flow measurements.
Changes in CBF in both MCA and pial collateral territories where LMAs are located were measured using laser-Doppler flowmetry (Perimed, Ardmore, PA), as previously described (16). Briefly, the skull was thinned and probes were secured using surgical glue at specific coordinates determined from the rat brain atlas (probe 1 to measure the CBF change in MCA territory: 4 mm lateral and 2 mm posterior of bregma; probe 2 to measure the CBF change in pial collateral territory: 3 mm lateral and 2 mm anterior of bregma). CBF was measured continuously during the entire procedure (~240 min). Measurements of CBF were normalized to the flow at baseline to calculate the percent change of CBF in both MCA and pial collateral territories. The following equation was used:
| %Change = (CBFmeasured – CBFbaseline)/CBFbaseline |
where CBFmeasured is the flow in laser-Doppler units at the measured time points and CBFbaseline is the flow in laser-Doppler units before occlusion.
Structural measurements in primary and pial collaterals.
At the end of the MCAO experiment (2-h ischemia and 2-h reperfusion), papaverine (45 mg/kg) was injected intravenously to standardize all vessels at full relaxation and for animal euthanasia, and a thoracotomy was performed. An 18-gauge cannula was inserted into the ascending aorta, and a warmed mixture of carbon black and latex (10%/90%) was injected to perfuse the cerebral circulation and allow visualization and characterization of primary and secondary collaterals. Rats were then placed on ice for 15 min to allow the carbon black and latex to solidify, and brains were then dissected and fixed in 10% neutral buffered formalin at 4°C overnight. All brains were imaged in a blinded manner using an Olympus SZX12 dissecting microscope at ×10, ×20, and ×40 magnifications; 2 images from each animal were taken at ×10, 8 images on average were taken from each animal at ×20, and 18 images on average were taken from every animal at ×40 magnification. Brain images were imported to ImageJ (National Institutes of Health, Bethesda, MD), and arterioles that connected distal anterior cerebral artery (ACA) and MCA were identified as LMAs to match the isolated LMA functional experiments. The total number of LMAs in the surface of brain ipsilateral to the ischemic stroke was quantified as well as the number of LMAs with or without penetrators. LMA diameters were individually assessed by averaging three diameter measurements along the wall of the collateral vessels. Arterial segments [ACA, ICA, posterior communicating artery, and posterior cerebral artery (PCA)] of the circle of Willis were identified, and diameter for each artery was determined by averaging three diameter measurements along the wall of collateral vessels.
In vitro isolated LMA experiments.
Isolated LMAs were identified as arterioles connecting the distal MCA and ACA and then dissected and mounted in an arteriograph chamber. LMAs were perfused with physiological saline solution (PSS), pressurized at 20 mmHg, and then equilibrated for 1 h to allow the development of spontaneous myogenic tone. After equilibration, pressure was decreased to 5 mmHg and then increased to 10, 20, 40, 60, and 80 mmHg in a stepwise manner. The lumen diameter at each pressure was recorded once stable. Pressure was returned to 40 mmHg for the remainder of the experiments. The thromboxane A2 agonist U46619 (10−7 M) was administered to preconstrict all LMAs in preparation for subsequent vessel dilation (19). NS309, a small- and intermediate-conductance (SK/IK) Ca2+-activated K+ channel agonist (63), was cumulatively added to the bath (10−8−10−5 M), and lumen diameter was recorded at each concentration once stable. After NS309 was washed from the bath, U46619 (10−7 M) was used to preconstrict LMAs again. PSS in the bath was then replaced with 13 mM KCl, a concentration that activates the inward rectifier K+ (Kir) channel (46), and the change in lumen diameter was recorded. Next, 13 mM KCl PSS was replaced with 30 mM KCl, a concentration that activates voltage-operated Ca2+ channels (37), and the change in lumen diameter was recorded. The bath was then replaced with PSS and 4.7 mM KCl, and NG-nitro-l-arginine methyl ester (l-NAME; 10−3 M), a nitric oxide (NO) synthase (NOS) inhibitor, was added to the bath, and the change in lumen diameter was recorded. In the presence of L-NAME, sodium nitroprusside (SNP), a NO donor, was cumulatively added to the bath (10−8−10−5 M), and lumen diameter was recorded at each concentration once stable. After a wash with Ca2+-free PSS, papaverine (10−4 M), a nonspecific inhibitor of phosphodiesterase (30), and diltiazem (10−5 M), an L-type Ca2+ channel inhibitor (47), were added to inhibit vascular smooth muscle contraction and obtain LMA measurements under passive conditions. Finally, pressure was decreased from 80 to 5 mmHg (80, 60, 40, 20, 10, and 5 mmHg), and lumen diameter and wall thickness were measured at each pressure.
Drugs and solutions.
Isolated vessel experiments were performed using PSS, the ionic composition of which was (in mM) 119.0 NaCl, 24.0 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4·7H2O, 1.6 CaCl2, 0.026 EDTA, and 5.5 glucose. PSS with a higher concentration of KCl (13 and 30 mM) was made with a reduced amount of NaCl to maintain a constant osmolarity. PSS was made each week and stored at 4°C. Glucose was added to PSS before each experiment. PSS was aerated with 5% CO2, 10% O2, and 85% N2 to maintain a constant pH of 7.4 ± 0.05. U46619, NS309, l-NAME, SNP, and papaverine were purchased from Sigma (St. Louis, MO). Diltiazem was purchased from MP Biomedicals (Santa Ana, CA). Stock solutions for l-NAME, SNP, diltiazem, and papaverine were made weekly and stored at 4°C. Aliquots of U46619 and NS309 were made and stored in the −20°C freezer until use.
Data calculations.
Myogenic tone was calculated by the following formula: [1 − (ϕactive/ϕpassive)] × 100%, where ϕactive is the lumen diameter of the vessel under active conditions and ϕpassive is the lumen diameter of the fully relaxed vessel under passive conditions. Percent constriction to l-NAME and 30 mM KCl was calculated using the following formula: [1 − (ϕdrug/ϕbaseline)] × 100%, where ϕdrug is the lumen diameter of the vessel after drug treatment and ϕbaseline is the lumen diameter before the drug was given. Percent dilation to 13 mM KCl was calculated by the following formula: [(ϕdrug/ϕbaseline) − 1] × 100%. Percent reactivity to NS309 and SNP was calculated using the following formula: [(ϕdose − ϕbaseline)/(ϕpassive − ϕbaseline)] × 100%, where ϕdose is the lumen diameter at a specific concentration of drug.
Statistical analysis.
Data are presented as means ± SE. A normality test was performed using SPSS (version 24.0, IBM SPSS, Chicago, IL). For normally distributed data, differences between the groups were determined using an unpaired Student’s t-test. For data not normally distributed, comparisons were made between groups using the Wilcoxon-Mann-Whitney test (GraphPad Prism, version 7.0, La Jolla, CA). Differences were considered statistically significant at P < 0.05.
RESULTS
Effect of sex on relative CBF changes during MCAO in MCA and pial collateral territories.
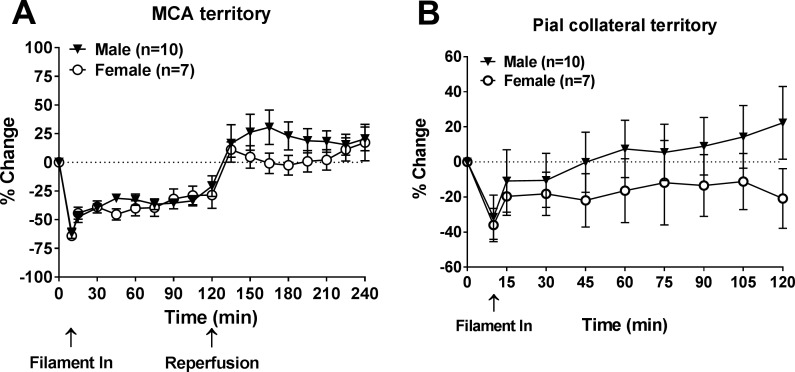
Figure 2A shows the percent change of relative CBF (rCBF) from baseline in the MCA territory during 2-h ischemia. Upon ischemia (filament in), a rapid and robust decrease in CBF was detected and was similar between male and female rats (61.9 ± 4.4% vs. 64.1 ± 2.9% of baseline); rCBF then increased after 10 min. The average change of rCBF over 120 min during ischemia was −37.6 ± 3.3% in male rats compared with −40.4 ± 5.6% in female rats (P > 0.05). After removal of the filament, rCBF was higher than baseline in male rats, demonstrating hyperperfusion; in female rats, rCBF went back to baseline. Similarly, Fig. 2B shows the percent change of rCBF of baseline in the pial collateral territory during 2-h ischemia only. An initial drop in rCBF occurred in the pial collateral territory upon placement of filament but was to a lesser degree than in the MCA territory. The rCBF change between male and female groups was −31.5 ± 12.7% versus −36.0 ± 9.5%. Recovery of rCBF was also observed in the pial collateral territory from both male and female rats, and no sex-dependent difference was found. The average change of rCBF over 120 min in pial collateral territory during ischemia was 33.7 ± 35.9% in male rats compared with −23.0 ± 14.4% in female rats (P > 0.05).
Fig. 2.
Relative cerebral blood flow (CBF) changes in middle cerebral artery (MCA) and collateral perfusion territories during ischemia in male and female Wistar rats. A: percent change of relative CBF in the MCA territory from baseline during 2-h ischemia and reperfusion. B: percent change of relative CBF in the pial collateral territory from baseline during 2-h ischemia. There was no sex difference in relative changes in CBF during ischemia or reperfusion.
Effect of sex on LMA myogenic reactivity and tone.
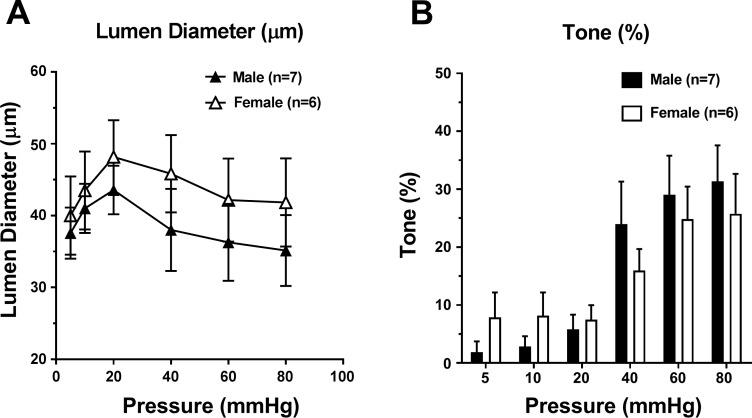
To determine if LMAs from male and female rats had different myogenic reactivity, diameter changes in response to pressure were measured. Figure 3A shows that LMAs from male and female rats had similar changes in diameter with increased pressure, indicating that LMAs from male and female rats had a similar myogenic response. As shown in Fig. 3B, LMAs from male and female rats had increased myogenic tone at 40, 60, and 80 mmHg compared with that at low pressures, and the tone was similar at various intravascular pressures.
Fig. 3.
Myogenic reactivity and percent myogenic tone to increased intravascular pressure in leptomeningeal anastomoses (LMAs) isolated from male and female Wistar rats. A: change in active lumen diameter in response to increased intravascular pressure of LMAs from male and female Wistar rats. B: change in percent tone in response to increased intravascular pressure of LMAs from male and female Wistar rats. There was no sex difference in myogenic reactivity or tone.
Effect of sex on LMA reactivity.
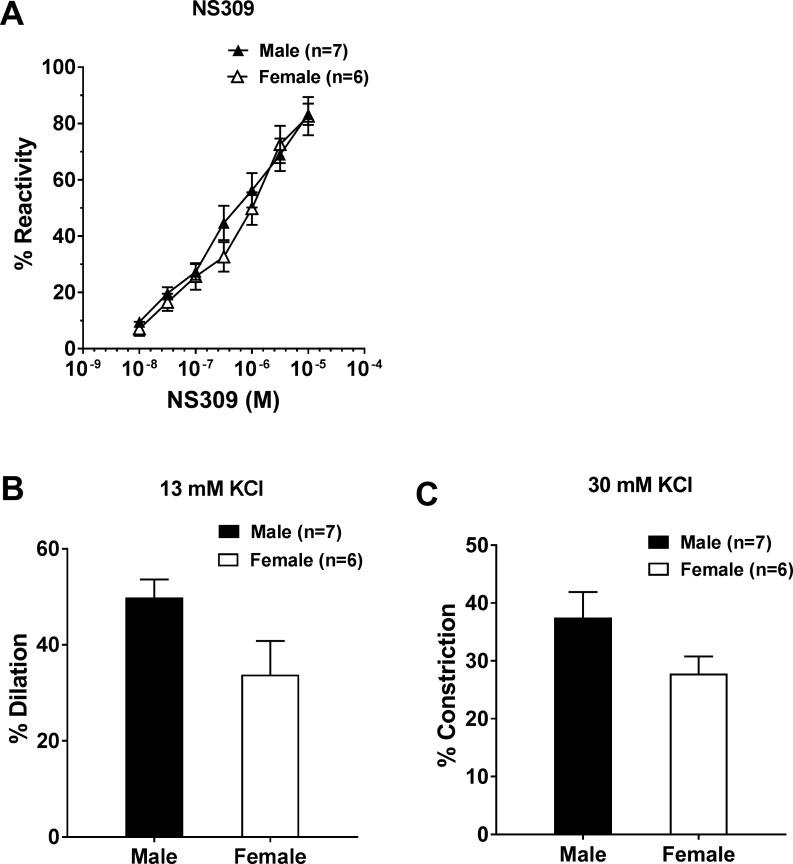
To better understand the potential sex differences in LMAs, we investigated the lumen diameter change and myogenic responses to vasoconstrictive and vasodilatory stimuli using pharmacological approaches. Vascular reactivity of LMAs to various ion channel activators and inhibitors was compared between male and female rats. Figure 4A shows the diameter response of LMAs to increased concentrations of the SK/IK channel activator NS309, demonstrating the presence of these channels in both LMAs from male and female rats. Additionally, LMAs from male and female rats showed similar reactivity to the activation of SK/IK channel.
Fig. 4.
Vascular reactivity to ion channel activators and inhibitors in leptomeningeal anastomoses (LMAs) from male and female Wistar rats. A: percent reactivity to NS309 (small-conductance/intermediate-conductance Ca2+-activated K+ channel activation and vessel dilation) in LMAs from male and female Wistar rats. B: percent dilation to 13 mM KCl (inward rectifier K+ channel activation) in LMAs from male and female Wistar rats. C: percent constriction to 30 mM KCl in LMAs from male and female Wistar rats. There was no sex difference in the response to NS309 or KCl.
KCl can produce vasodilation through the activation of Kir channels at concentrations of <15 mM (12, 24), after which it causes constriction because of depolarization and subsequent activation of voltage-dependent L-type Ca2+ channels (34). LMAs from both male and female rats dilated to 13 mM KCl (Fig. 4B), suggesting that Kir channels were present in LMAs from both male and female rats. In addition, LMAs from both groups showed similar reactivity to Kir channel activation. After a switch to 30 mM KCl, as shown in Fig. 4C, LMAs from both male and female rats constricted to 30 mM KCl, which was not different between groups.
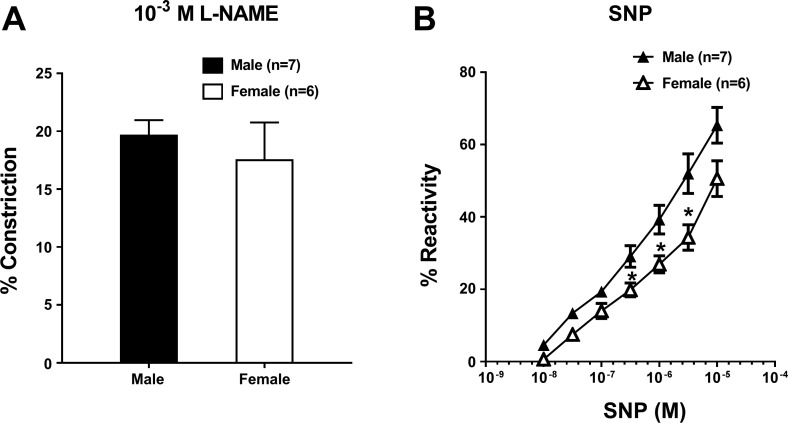
To study the vasodilatory influence of NO on LMA tone, constriction in response to a single concentration of the NOS inhibitor l-NAME was measured. Figure 5A shows that LMAs from both male and female rats constricted to NOS inhibition, and there was no difference between male and female rats. Figure 5B shows the vasodilatory response to the NO donor SNP. At lower concentrations, LMAs had similar reactivity to SNP. However, at higher concentrations, LMAs from female rats had a lower reactivity to SNP compared with male LMAs.
Fig. 5.
Reactivity of leptomeningeal anastomoses (LMAs) from male and female Wistar rats to nitric oxide (NO). A: percent constriction to the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 10−3 M) in LMAs isolated from male and female Wistar rats. LMAs from female rats had similar vascular reactivity to l-NAME as LMAs from male rats. B: percent reactivity to the NO donor sodium nitroprusside (SNP) in LMAs isolated from male and female Wistar rats. LMAs from female rats had less vascular reactivity to SNP at higher concentrations compared with LMAs from male rats.
Effect of sex on pial collateral structure.
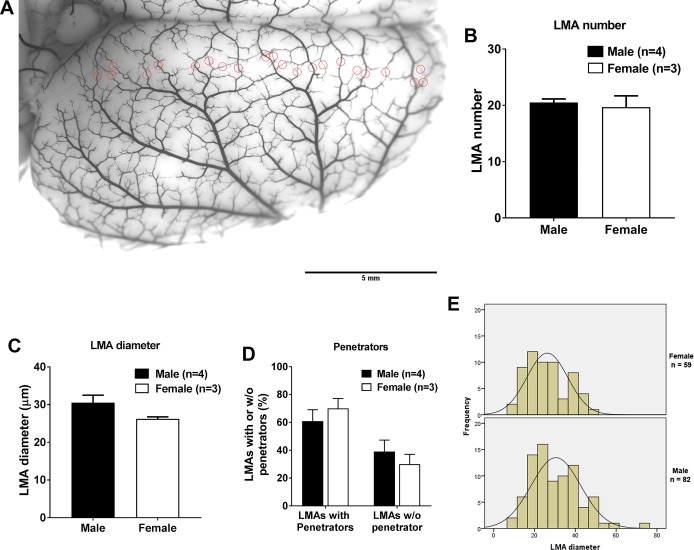
To determine if the structure of LMAs differed between male and female Wistar rats, a mixture of carbon black and latex was perfused to visualize and measure LMAs. As shown in Fig. 6A, identified LMAs were circled in the image and counted. The average LMA number in the right hemisphere was 21 ± 1 for male rats and 20 ± 2 for female rats (P > 0.05; Fig. 6B). Figure 6C shows that the average LMA diameter was not significantly different between male and female rats. Next, LMAs with and without penetrators were quantified and compared. As shown in Fig. 6D, male and female rats had similar percentage of LMAs with penetrators and without penetrators. Based on the diameter, LMAs were categorized and the frequency of the diameter range was assessed. The histogram shown in Fig. 6E demonstrates that male and female rats had a similar distribution of diameter of LMAs.
Fig. 6.
Comparison of the structure of leptomeningeal anastomoses (LMAs) from the right rat brain hemisphere from male and female Wistar rats. A: representative image showing the right rat brain hemisphere perfused with carbon black and latex. All identified LMAs were circled in red circles and counted. Scale bar = 5 mm. B: average number of LMAs did not differ between male and female Wistar rats. C: graph showing that average LMA diameter did not vary between male and female Wistar rats. D: graph showing that the percentage of LMAs with or without penetrators did not vary between male and female Wistar rats. E: histogram showing the LMA diameter distribution frequency between male and female rats. No significant difference was observed between male and female Wistar rats.
Effect of sex on primary collateral structure.
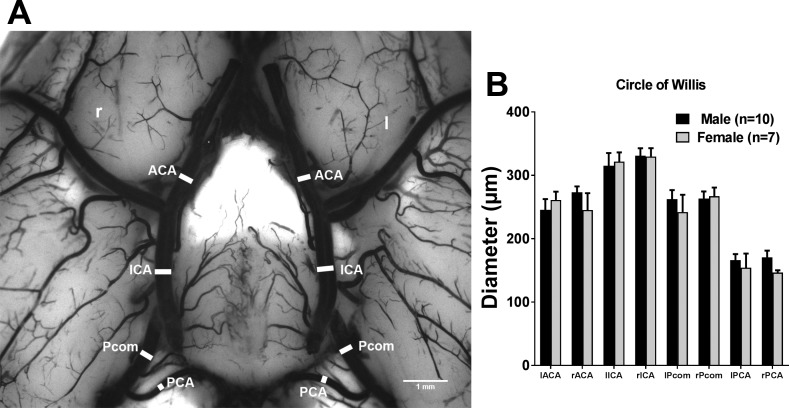
Artery segments that make up the circle of Willis were identified and diameters were measured, as shown in Fig. 7A. Figure 7B shows the average diameter of these artery segments, and no significant difference was found between male and female rats.
Fig. 7.
Comparison of diameter of artery segments that consisted of the circle of Willis between male and female Wistar rats. A: representative image showing the diameter of artery segments in the circle of Willis. B: graph showing the average diameter of artery segments of the circle of Willis between male and female Wistar rats. There was no difference between male and female Wistar rats. Scale bar = 1 mm. ACA, anterior cerebral artery; ICA, internal carotid artery; l, left; Pcom, posterior communicating artery; PCA, posterior cerebral artery; r, right.
DISCUSSION
In the present study, our data showed that mean rCBF drops in both MCA and pial collateral territories during MCAO were similar between male and female rats. In addition, compared with male LMAs, female LMAs had similar myogenic reactivity and tone at different intravascular pressures and similar reactivity to vasoconstrictive and vasodilatory stimuli. Furthermore, no sex differences of pial collateral structure were detected from male and female rats, as measured by LMA number, diameter, and percentage of LMAs with or without penetrators. Primary collateral structure, as measured by the diameter of arterial segments of the circle of Willis, was similar between groups. These results suggest no sex differences in primary or pial collateral circulation in adult normotensive Wistar rats.
In this study, we investigated whether male and female Wistar rats had a different rCBF drop in MCA and pial collateral territories during ischemia. Despite the incomplete occlusion of MCA and hyperperfusion, no sex difference was detected in either territory. For adult normotensive Wistar rats, a previous study reported a significantly lower CBF drop in intact female rats compared with male rats that was measured over the ipsilateral parietal cortex (1). However, Dziennis et al. (22) reported a similar CBF drop in MCA territory during MCAO between adult male and female Wistar rats, and our results are in support of this. The contradiction could be partly explained by the different position of the laser-Doppler probe. Nonetheless, our results suggest no sex difference in CBF drop in both MCA and pial collateral territories in adult normotensive Wistar rats.
Clinical studies have shown an important role for LMAs in stroke functional outcome. Miteff et al. (48) graded collateral status based on the extent of contrast visualized distal to the occlusion on computed tomography angiography. They found that good collateral status was significantly associated with a larger baseline penumbra and also appeared to maintain penumbral flow and limit infarct expansion (48). Additionally, patients with a better graded pial collateral flow had a lower rate of hemorrhagic transformation after endovascular therapy after acute ischemic stroke (4). An animal study also demonstrated that after occlusion of a major cerebral artery, LMAs allowed retrograde blood flow to the occluded artery territory (20). Thus, understanding the function of LMAs could provide us with a potential pharmacological approach to open pial collateral vessels, limit the cerebral infarct, and eventually improve stroke outcome. The findings in the present study suggest that LMAs from both male and female rats had similar myogenic tone and myogenic reactivity. LMAs from female rats had moderately decreased vascular reactivity to the NO donor SNP as well as in response to ion channel activation and inhibition compared with male rats. These results suggest that the favorable stroke outcomes in premenopausal women and reproductively intact female rodents are less likely to be due to the functional difference of LMAs.
Several pathophysiological factors have been identified that can improve or decease the efficiency of pial collateral flow, including the variability in number and diameter of LMAs. Anatomic studies have shown that the presence of LMAs was not similar, and some patients had anastomotic vessels that were larger and more numerous (27, 54). Brozici et al. (11) concluded that the efficiency of the pial collateral flow depends on the individual variability, mainly in the number, and lumen diameter of LMAs. The importance of the variability was also stated by Tariq and Khatri (59), who demonstrated that not only lumen diameter and number of LMAs but also the age of patients, duration of ischemia, and associated comorbidities may influence the efficacy of pial collateral flow. To determine if there were sex differences in native pial collaterals, we compared the number and diameter of LMAs between adult normotensive rats. Faber et al. (23) had previously concluded that there were no sex differences in the LMA number and lumen diameter in young adult, middle-aged, and old mice or in strains of mice with genetically determined differences in native collaterals. Our results were consistent in the way that adult male and female Wistar rats had similar numbers of LMAs that were similar in lumen diameter and distribution pattern.
The percentage of LMAs with or without penetrating arterioles was quantified. LMAs give rise to smaller vessels that eventually penetrate into the brain tissue (15), known as penetrating arterioles. These small arterioles deliver blood from LMAs to capillaries in the cerebral cortex and are considered crucial for the subsequent neuronal survival within the penumbra region (6). Occlusion of penetrating vessels can cause a direct flow change in downstream capillaries and lead to neuronal death, local inflammatory response, and cognitive decline (10, 57). Using rats as the animal model, studies have shown that the occlusion of only a single penetrating arteriole led to cognitive dysfunction in behavioral tasks (57). In the same study, the accumulation of multiple microinfarcts led to extended cortical tissue damage (57). Thus, LMAs with more penetrators may provide a protective mechanism by preserving collateral flow during ischemia. In the present study, we compared the percentage of LMAs with or without penetrators and found no sex-dependent difference.
In the present study, the architecture of the primary collateral circulation in the circle of Willis was investigated and compared between male and female rats. We found no sex-dependent difference in the diameter of arterial segments that construct the circle of Willis. These results were consistent with the previous study (23) showing that the diameter of primary cerebral arteries did not vary with sex. Horikoshi et al. (33) demonstrated sex-linked differences in anatomic variations of circle of Willis and reported that a nonvisualized unilateral PCA was more common in women, whereas a nonvisualized unilateral ACA occurred more commonly in men. However, only the structural completeness was investigated, and few studies have measured the sex differences in diameter of each arterial segment of the circle of Willis. Our results add to the knowledge base that there was no sex difference in diameter of each arterial segment of the circle of Willis in adult normotensive Wistar rats.
The present study has some limitations. First, only young adult normotensive rats were investigated, without comorbidities that are common in ischemic stroke patients such as hypertension, advanced age, or obesity (38). To closely resemble the pathophysiology of stroke patients, further investigations are needed in animal models to determine if there are sex differences in the cerebral circulation and perfusion in the settings of such comorbidities. Second, stroke outcomes between groups were not investigated and compared. After completion of MCAO, rats were perfused with the mixture of carbon black and latex for vessel visualization, and thus the stroke outcome measurement could not be processed. However, numerous studies have shown a favorable stroke outcome in reproductively intact female rats compared with male rats (1, 23, 42, 45). For example, Baskerville et al. (7) have shown that female rats had a significantly smaller infarct volume from 0.5 to 4 h after permanent MCAO compared with age-matched male rats. Third, some animals were excluded from the results because of either technical difficulties or inability to visualize collaterals with carbon black. Thus, some experiments may have been underpowered. Fourth, during the MCAO surgery, rCBF increased after the initial drop, suggesting incomplete occlusion of the MCA. This could be due to immediate collateral blood flow from PCA territory, effective pial collateral perfusion, or incomplete filament occlusion of the MCA. Finally, papaverine was injected to fully dilate and standardize all cerebral vessels. However, whether papaverine crosses the blood-brain barrier has not been tested. Papaverine has a limited molecular weight (339 Da), which might allow it to cross the blood-brain barrier (5). Moreover, papaverine has been shown to damage the blood-brain barrier (9, 36, 51), which may add to its perfusion through the blood-brain barrier.
In summary, our data show that LMAs from male and female Wistar rats had similar myogenic tone and reactivity. LMAs from female Wistar rats had moderately decreased vascular reactivity to an NO donor and similar vascular reactivity to ion channel activators and inhibitors compared with male rats. With regard to the structural analyses, pial collateral number, diameter, and percentage of penetrators did not suggest sex differences between male and female rats. Primary collateral circulation, indicated by the circle of Willis, did not vary with sex either. These findings indicate that factors other than function and structure of primary and pial collaterals may underlie the reduced severity of stroke in premenopausal female subjects.
GRANTS
This work was generously supported by National Institute of Neurologic Disorders and Stroke Grant R01-NS-093289, the Cardiovascular Research Institute of Vermont, and the Totman Medical Research Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.L. and M.J.C. conceived and designed research; Z.L. and S.M.T. performed experiments; Z.L., S.M.T., and M.J.C. analyzed data; Z.L., S.M.T., and M.J.C. interpreted results of experiments; Z.L. and S.M.T. prepared figures; Z.L. and M.J.C. drafted manuscript; Z.L., S.M.T., and M.J.C. edited and revised manuscript; Z.L., S.M.T., and M.J.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Abbie C. Johnson (Department of Neurological Sciences, University of Vermont) for reading and editing the manuscript. We also thank the Microscopy Imaging Center, Larner College of Medicine, University of Vermont, for help with brain images with carbon black and latex perfusion.
REFERENCES
- 1.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke 29: 159–165, 1998. doi: 10.1161/01.STR.29.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia−the ischemic penumbra. Stroke 12: 723–725, 1981. doi: 10.1161/01.STR.12.6.723. [DOI] [PubMed] [Google Scholar]
- 2a.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA 307: 1952–1958, 2012. doi: 10.1001/jama.2012.3490. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Viñuela F, Liebeskind DS; UCLA Collateral Investigators . Impact of collateral flow on tissue fate in acute ischemic stroke. J Neurol Neurosurg Psychiatry 79: 625–629, 2008. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS; UCLA-Samsung Stroke Collaborators . Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 42: 2235–2239, 2011. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol 9, Suppl 1: S3, 2009. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baran U, Li Y, Wang RK. Vasodynamics of pial and penetrating arterioles in relation to arteriolo-arteriolar anastomosis after focal stroke. Neurophotonics 2: 025006, 2015. doi: 10.1117/1.NPh.2.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baskerville TA, Macrae IM, Holmes WM, McCabe C. The influence of gender on ‘tissue at risk’ in acute stroke: a diffusion-weighted magnetic resonance imaging study in a rat model of focal cerebral ischaemia. J Cereb Blood Flow Metab 36: 381–386, 2016. doi: 10.1177/0271678X15606137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics−2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Errata in Circulation 135: e646, 2017, and Circulation 136: e196, 2017]. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee AK, Kondoh T, Nagashima T, Ikeda M, Ehara K, Tamaki N. Quantitative analysis of papaverine-mediated blood-brain barrier disruption in rats. Biochem Biophys Res Commun 289: 548–552, 2001. doi: 10.1006/bbrc.2001.6029. [DOI] [PubMed] [Google Scholar]
- 10.Blinder P, Shih AY, Rafie C, Kleinfeld D. Topological basis for the robust distribution of blood to rodent neocortex. Proc Natl Acad Sci USA 107: 12670–12675, 2010. doi: 10.1073/pnas.1007239107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 34: 2750–2762, 2003. doi: 10.1161/01.STR.0000095791.85737.65. [DOI] [PubMed] [Google Scholar]
- 12.Chan S-L, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke 47: 1618–1625, 2016. doi: 10.1161/STROKEAHA.116.013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 26: 1789–1797, 2005. [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang Y-M, Chan L, Lai Y-J, Kuo K-H, Chiou Y-H, Huang L-W, Kwok Y-T, Lai T-H, Lee S-P, Wu H-M, Yeh YC. Configuration of the circle of Willis is associated with less symptomatic intracerebral hemorrhage in ischemic stroke patients treated with intravenous thrombolysis. J Crit Care 28: 166–172, 2013. doi: 10.1016/j.jcrc.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Cipolla MJ. The cerebral circulation. Integrated Systems Physiology: From Molecule to Function 1: 1–59, 2009. 21452434 [Google Scholar]
- 16.Cipolla MJ, Linfante I, Abuchowski A, Jubin R, Chan SL. Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate™) in spontaneously hypertensive rats. J Cereb Blood Flow Metab 38: 755–766, 2017. doi: 10.1177/0271678X17705567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cipolla MJ, Sweet JG, Chan SL. Effect of hypertension and peroxynitrite decomposition with FeTMPyP on CBF and stroke outcome. J Cereb Blood Flow Metab 37: 1276–1285, 2017. doi: 10.1177/0271678X16654158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab 33: 1486–1492, 2013. doi: 10.1038/jcbfm.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocks TM, Kemp BK, Pruneau D, Angus JA. Comparison of contractile responses to 5-hydroxytryptamine and sumatriptan in human isolated coronary artery: synergy with the thromboxane A2-receptor agonist, U46619. Br J Pharmacol 110: 360–368, 1993. doi: 10.1111/j.1476-5381.1993.tb13818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuccione E, Padovano G, Versace A, Ferrarese C, Beretta S. Cerebral collateral circulation in experimental ischemic stroke. Exp Transl Stroke Med 8: 2, 2016. doi: 10.1186/s13231-016-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D; European BIOMED Study of Stroke Care Group . Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 34: 1114–1119, 2003. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 22.Dziennis S, Yang D, Cheng J, Anderson KA, Alkayed NJ, Hurn PD, Lein PJ. Developmental exposure to polychlorinated biphenyls influences stroke outcome in adult rats. Environ Health Perspect 116: 474–480, 2008. doi: 10.1289/ehp.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faber JE, Moore SM, Lucitti JL, Aghajanian A, Zhang H. Sex differences in the cerebral collateral circulation. Transl Stroke Res 8: 273–283, 2017. doi: 10.1007/s12975-016-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faraci FM, Sobey CG. Potassium channels and the cerebral circulation. Clin Exp Pharmacol Physiol 23: 1091–1095, 1996. doi: 10.1111/j.1440-1681.1996.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 25.Gargano JW, Reeves MJ; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators . Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke 38: 2541–2548, 2007. doi: 10.1161/STROKEAHA.107.485482. [DOI] [PubMed] [Google Scholar]
- 26.Gavins FN. Current treatments and development of modern therapies for stroke. Stroke 13: 57, 2018. [Google Scholar]
- 27.Gros C, Minvielle J, Vlahovitch B. Anastomoses artérielles intracraniennes; étude artériographique et clinique. Neurochirurgie 2: 281–302, 1956. [PubMed] [Google Scholar]
- 28.Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 217: 659–661, 1982. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke 29: 618–624, 1998. doi: 10.1161/01.STR.29.3.618. [DOI] [PubMed] [Google Scholar]
- 30.Hocking KM, Putumbaka G, Wise ES, Cheung-Flynn J, Brophy CM, Komalavilas P. Papaverine prevents vasospasm by regulation of myosin light chain phosphorylation and actin polymerization in human saphenous vein. PLoS One 11: e0154460, 2016. doi: 10.1371/journal.pone.0154460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoksbergen AW, Legemate DA, Csiba L, Csáti G, Síró P, Fülesdi B. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis 16: 191–198, 2003. doi: 10.1159/000071115. [DOI] [PubMed] [Google Scholar]
- 32.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke 31: 1833–1837, 2000. doi: 10.1161/01.STR.31.8.1833. [DOI] [PubMed] [Google Scholar]
- 33.Horikoshi T, Akiyama I, Yamagata Z, Sugita M, Nukui H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of willis and the occurrence of cerebral aneurysms. J Neurosurg 96: 697–703, 2002. doi: 10.3171/jns.2002.96.4.0697. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi T, Dietrich HH, Hongo K, Dacey RG Jr. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke 33: 2692–2699, 2002. doi: 10.1161/01.STR.0000034791.52151.6B. [DOI] [PubMed] [Google Scholar]
- 35.Hossmann K-A. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol 26: 1057–1083, 2006. doi: 10.1007/s10571-006-9008-1. [DOI] [PubMed] [Google Scholar]
- 36.Jin Y, Sagher O, Thai QA, Kassell NF, Lee KS. The effects of papaverine on phorbol dibutyrate-induced vasoconstriction in brain slice microvessels. J Neurosurg 81: 574–578, 1994. doi: 10.3171/jns.1994.81.4.0574. [DOI] [PubMed] [Google Scholar]
- 37.Katsura M, Mohri Y, Shuto K, Hai-Du Y, Amano T, Tsujimura A, Sasa M, Ohkuma S. Up-regulation of L-type voltage-dependent calcium channels after long term exposure to nicotine in cerebral cortical neurons. J Biol Chem 277: 7979–7988, 2002. doi: 10.1074/jbc.M109466200. [DOI] [PubMed] [Google Scholar]
- 38.Kesarwani M, Perez A, Lopez VA, Wong ND, Franklin SS. Cardiovascular comorbidities and blood pressure control in stroke survivors. J Hypertens 27: 1056–1063, 2009. doi: 10.1097/HJH.0b013e32832935ce. [DOI] [PubMed] [Google Scholar]
- 39.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, Broderick JP, Kleindorfer DO. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 79: 1781–1787, 2012. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucinski T, Koch C, Eckert B, Becker V, Krömer H, Heesen C, Grzyska U, Freitag HJ, Röther J, Zeumer H. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 45: 11–18, 2003. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 41.Liebeskind DS. Collateral circulation. Stroke 34: 2279–2284, 2003. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 29: 792–802, 2009. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Löfmark U, Hammarström A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Results from a community-based incidence study in northern Sweden. Neuroepidemiology 28: 135–141, 2007. doi: 10.1159/000102141. [DOI] [PubMed] [Google Scholar]
- 44.Maeda K, Hata R, Bader M, Walther T, Hossmann KA. Larger anastomoses in angiotensinogen-knockout mice attenuate early metabolic disturbances after middle cerebral artery occlusion. J Cereb Blood Flow Metab 19: 1092–1098, 1999. doi: 10.1097/00004647-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond) 7: 319–339, 2011. doi: 10.2217/WHE.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM Jr. Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke 29: 1469–1474, 1998. doi: 10.1161/01.STR.29.7.1469. [DOI] [PubMed] [Google Scholar]
- 47.Mieth A, Revermann M, Babelova A, Weigert A, Schermuly RT, Brandes RP. L-type calcium channel inhibitor diltiazem prevents aneurysm formation by blood pressure-independent anti-inflammatory effects. Hypertension 62: 1098–1104, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01986. [DOI] [PubMed] [Google Scholar]
- 48.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 132: 2231–2238, 2009. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 49.Paul SL, Srikanth VK, Thrift AG. The large and growing burden of stroke. Curr Drug Targets 8: 786–793, 2007. doi: 10.2174/138945007781077418. [DOI] [PubMed] [Google Scholar]
- 50.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40: 1032–1037, 2009. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Platz J, Baráth K, Keller E, Valavanis A. Disruption of the blood-brain barrier by intra-arterial administration of papaverine: a technical note. Neuroradiology 50: 1035–1039, 2008. doi: 10.1007/s00234-008-0455-x. [DOI] [PubMed] [Google Scholar]
- 52.Ramasubbu R, Robinson RG, Flint AJ, Kosier T, Price TR. Functional impairment associated with acute poststroke depression: the Stroke Data Bank Study. J Neuropsychiatry Clin Neurosci 10: 26–33, 1998. doi: 10.1176/jnp.10.1.26. [DOI] [PubMed] [Google Scholar]
- 53.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7: 915–926, 2008. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosegay H, Welch K. Peripheral collateral circulation between cerebral arteries; a demonstration by angiography of the meningeal arterial anastomoses. J Neurosurg 11: 363–377, 1954. doi: 10.3171/jns.1954.11.4.0363. [DOI] [PubMed] [Google Scholar]
- 55.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z; Oxford Vascular Study . Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 366: 1773–1783, 2005. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 56.Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 330: 1565–1570, 1994. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- 57.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16: 55–63, 2013. doi: 10.1038/nn.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sue-Min L, Duncan PW, Dew P, Keighley J. Sex differences in stroke recovery. Prev Chronic Dis 2: A13, 2005. [PMC free article] [PubMed] [Google Scholar]
- 59.Tariq N, Khatri R. Leptomeningeal collaterals in acute ischemic stroke. J Vasc Interv Neurol 1: 91–95, 2008. [PMC free article] [PubMed] [Google Scholar]
- 61.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis 26: 462–474, 2008. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson ME. Stroke: understanding the differences between males and females. Pflugers Arch 465: 595–600, 2013. doi: 10.1007/s00424-013-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Pascal JM, Schumann M, Armen RS, Zhang JF. Identification of the functional binding pocket for compounds targeting small-conductance Ca2+-activated potassium channels. Nat Commun 3: 1021, 2012. doi: 10.1038/ncomms2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou H, Sun J, Ji X, Lin J, Tang S, Zeng J, Fan YH. Correlation between the integrity of the circle of Willis and the severity of initial noncardiac cerebral infarction and clinical prognosis. Medicine (Baltimore) 95: e2892, 2016. doi: 10.1097/MD.0000000000002892. [DOI] [PMC free article] [PubMed] [Google Scholar]