Abstract
The isolated saline-perfused heart is used extensively to study cardiac physiology. Previous isolated heart studies have demonstrated lower tissue oxygenation compared with in vivo hearts based on myoglobin oxygenation and the mitochondrial redox state. These data, consistent with small anoxic regions, suggest that the homeostatic balance between work and oxygen delivery is impaired. We hypothesized that these anoxic regions are caused by inadequate local perfusion due to a paradoxical arteriole constriction generated by a disrupted vasoregulatory network. We tested this hypothesis by applying two exogenous vasodilatory agents, adenosine and cromakalim, to relax vascular tone in an isolated, saline-perfused, working rabbit heart. Oxygenation was monitored using differential optical transmission spectroscopy and full spectral fitting. Increases in coronary flow over control with adenosine (27 ± 4 ml/min) or cromakalim (44 ± 4 ml/min) were associated with proportional spectral changes indicative of myoglobin oxygenation and cytochrome oxidase (COX) oxidation, consistent with a decrease in tissue anoxia. Quantitatively, adenosine decreased deoxymyoglobin optical density (OD) across the wall by 0.053 ± 0.008 OD, whereas the reduced form of COX was decreased by 0.039 ± 0.005 OD. Cromakalim was more potent, decreasing deoxymyoglobin and reducing the level of COX by 0.070 ± 0.019 OD and 0.062 ± 0.019 OD, respectively. These effects were not species specific, as Langendorff-perfused mouse hearts treated with adenosine demonstrated similar changes. These data are consistent with paradoxical arteriole constriction as a major source of regional anoxia during saline heart perfusion. We suggest that the vasoregulatory network is disrupted by the washout of interstitial vasoactive metabolites in vitro.
NEW & NOTEWORTHY Regional tissue anoxia is a common finding in the ubiquitous saline-perfused heart but is not found in vivo. Noninvasive optical techniques confirmed the presence of regional anoxia under control conditions and demonstrated that anoxia is diminished using exogenous vasodilators. These data are consistent with active arteriole constriction, occurring despite regional anoxia, generated by a disrupted vasoregulatory network. Washout of interstitial vasoactive metabolites may contribute to the disruption of normal vasoregulatory processes in vitro.
Keywords: coronary blood flow, cytochrome oxidase, myoglobin, spectral analysis, transmission optical spectroscopy
INTRODUCTION
The isolated saline-perfused heart is a commonly used, well-controlled preparation to study cardiac hemodynamics, mechanics, pathophysiology, pharmacology, and metabolism. However, many previous studies, using a variety of species, perfusion conditions, and technologies, have found evidence of regional anoxia (9, 31, 39, 46, 51, 66, 76), despite high phosphorylation potentials even in the rabbit (35), which has not been found in the heart in vivo using optical (3) and proton NMR techniques (5). The origin of these relatively small anoxic zones in the saline-perfused heart is unknown. Their existence complicates the interpretation of metabolic and functional data from the perfused heart.
A sensitive method of monitoring striated muscle oxygenation is optical spectroscopy, which permits the evaluation of net cytosolic oxygenation via myoglobin and mitochondrial oxygenation via cytochrome oxidase (COX) chromophores. The partial pressure of oxygen to achieve 50% saturation (P50) of myoglobin [2.39–2.75 mmHg (64, 68)] is higher than that of COX [0.05–0.15 mmHg (80)], facilitating the transfer of oxygen from the cytosol to the mitochondrion. It is important to note that the large differences in myoglobin and COX oxygen affinities imply that deoxygenation of myoglobin does not necessarily reflect an oxygen limitation for oxidative phosphorylation via COX. Thus, to establish whether oxygen is limiting for oxidative phosphorylation, the assessment of COX redox state is required.
Although many studies have evaluated the myoglobin oxygenation status of the perfused heart with spectroscopy (39, 42, 46, 47, 49, 58, 66, 67, 76), fewer studies have explored Po2 at the mitochondrion using the redox status of the cytochromes, including COX (31, 39, 46, 51, 57, 76). Prior optical studies of myoglobin and COX surprisingly found that both deoxygenated myoglobin and reduced COX were present in the isolated perfused hearts of various species (9, 31, 39, 46, 51, 66, 76). Because of the large difference in oxygen affinities between myoglobin and COX as well the small percentage of reduced COX, it was suggested that this observation represented small zones of total anoxia in the perfused heart (9, 31, 51, 76).
A preponderance of data support the notion that in vivo myoglobin is nearly completely oxygenated under control conditions (3, 5, 19, 69, 82). Additionally, in vivo hearts can tolerate modest changes in cardiac workload without changes in the oxygenation state (3, 5) or cytochrome c redox state (3). These data demonstrate that workload and flow are orchestrated, in vivo, to eliminate oxygen limitations to cardiac metabolism and function. In contrast, investigators have reported only 72–82% myoglobin oxygenation and a high reduction level of COX during retrograde Langendorff perfusion despite the fact that the workload of this preparation is quite low compared with in vivo conditions (4, 10, 12, 23). Furthermore, changes in workload, such as adrenergic stimulation with epinephrine (28) or isoproterenol (46), yield myoglobin deoxygenation in the isolated heart. These latter data suggest that the resting workload of the heart adjusts to available oxygen in the perfusate. This conclusion is supported by studies using saline perfusate supplemented with red blood cells (9, 12, 18, 27, 53, 61, 66) or perfluorocarbons (17, 51, 72) to improve oxygen carrying capacity. Improving oxygen delivery led to improved cardiac performance (9, 12, 17, 18, 27, 51, 53, 61, 66, 72) as well as increased myoglobin oxygenation (9, 51, 66) and cytochrome oxidation (51) in vitro.
Our laboratory developed a transmission optical spectroscopy system for the isolated rabbit heart that permits real-time monitoring of cardiac chromophore absorption, including myoglobin and heme aa3 groups of COX (31). Applying this approach, we confirmed the existence of deoxygenated myoglobin and reduced COX in the saline-perfused rabbit heart during both Langendorff perfusion (31, 42) and left working heart preparation (31, 51). These observations of myoglobin oxygenation and COX redox state agree with earlier spectroscopic studies suggesting saline perfusion propagates small regions of total anoxia.
The reasons for the regional anoxia commonly found in the saline-perfused heart are unknown. The normal hypoxia-driven vasoregulatory systems could be disrupted by a damaged vasculature, obstruction of vessels, or alterations in the interstitial signaling network limiting the ability of the heart to match flow with oxygen demand. We hypothesized small regions of the myocardium are not adequately perfused, and are consequently anoxic, because of a paradoxical arteriole constriction (PAC) generated by disrupted hypoxia-driven vasoregulatory mechanisms controlling arteriole tone. We focused on arteriole function because of the role of arterioles as the major source of vascular resistance and control in the heart (21), the relatively small size of the anoxic regions, and their consistent detection across many studies implying a distributed pattern. If arteriole tone is maintaining these anoxic zones, exogenously applied vasodilators should decrease this tone and result in a reduction in regional anoxia. To test the PAC hypothesis in the perfused, left ventricular working rabbit heart and the Langendorff-perfused mouse heart, we used transmission optical spectroscopy to examine the effects of different exogenous vasodilators on cytosolic and mitochondrial oxygenation simultaneously with conventional measures of cardiac function and metabolism.
METHODS
Heart Excision, Perfusion, and Physiological Monitoring
All animal protocols were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee and performed in accordance with the guidelines described in the Animal Care and Welfare Act (7 USC 2142 § 13).
Isolated rabbit hearts.
Male New Zealand White rabbits (2.5–3.25 kg) were preanesthetized using an intramuscular injection of ketamine-acepromazine (10:1). Fifteen minutes after preanesthetic administration, isoflurane (2–3%) was administered with a mixture of medical air-oxygen via inhalation. After a proper depth of anesthesia was confirmed, an intravenous line was placed in the marginal ear vein. Intravenous administration of 1.5 ml heparin (1,000 U/ml) was followed by a 5-min circulation period. The animal was euthanized with an intravenous bolus of 3 ml KCl (2 meq/ml). Immediately, a midline sternotomy was performed to expose the heart, after which the heart was excised rapidly and immersed in ice-cold saline buffer. The saline buffer used for all experiments contained (in mM) 10 mM HEPES, 137 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 1.0 Na2HPO4, 10 glucose, and 1 lactate. All buffer was filtered through a 1-µm pore membrane (Whatman) before use.
After the aorta was cannulated, the heart was retrograde perfused at constant pressure (62 mmHg) with buffer oxygenated with 100% O2, maintained at 37°C, and filtered through a 12-µm pore membrane (Whatman). Hearts were perfused on an extensively modified perfused heart system (120101BEZ, Radnoti), as the high flow resistance of the commercial system was not compatible with a working rabbit heart (51). Importantly, the internal diameter of all tubing was 5 mm or greater, standard three-way valves were replaced with wide-bore T connectors, and all of the inflow channels on the bubble traps were severely shortened to prevent bubbles entering the perfusion path at high flow. In the absence of three-way valves, flow was redirected using external tubing clamps. These additional modifications, required to assure that vasodilation was limited by the heart and not the perfusion apparatus, resulted in higher peak flow rates in the rabbit heart than our previous publications on this modified system (31, 51). The vena cava and pulmonary veins were then ligated, and the pulmonary artery was cannulated for coronary flow measurements. A small piece of the left atrial appendage was removed, and the left atrium was cannulated. If hearts were to be paced, electrodes were sutured onto the right atrial appendage at least 5 mm apart. Hearts were stimulated at 5–8 V with 1.5-ms pulses using a Grass S44 Stimulator (Grass Medical Instruments). Upon transition into the left working heart, a side-firing white light LED-tipped catheter, as previously reported by Femnou et al. (31), was fed into the perfusion system at a point superior to the aortic cannula and then through the aorta into the left ventricle.
In the left working mode, there was a left atrial preload pressure of 7 mmHg and an aortic afterload pressure of 55 mmHg, determined by the height of the preload and afterload chambers. All flows were measured using 4PXN flow-through sensors (Transonic) coupled to a TS410 Tubing Flow Module (Transonic). Heart rate was calculated as the frequency of coronary flow oscillations, as well as aortic output oscillations, for validation. Arterial oxygenation was measured using an optical oxygen sensor probe (Ocean Optics) situated in the left atrial preload chamber. Venous oxygenation was measured using a custom flow-through optical oxygen sensor (Ocean Optics) positioned past the pulmonary artery cannulation. Both sensors were connected to a NeoFox-GT phase fluorimeter (Ocean Optics). A calibration of perfusate oxygen saturation was performed during each experiment by either bubbling the perfusate with 100% nitrogen or 100% oxygen for 0% or 100% oxygen saturation, respectively. Temperature and pressures were acquired using an MP150 data acquisition system (BIOPAC). A Skin Temperature Amplifier (SKT100B, BIOPAC) was coupled to a temperature probe (RX202A, BIOPAC) secured in the perfusion system just before the left atrial cannulation for continuous measurement of temperature. A General Purpose Transducer Amplifier (DA100C, BIOPAC) was coupled to pressure transducers (TSD104A, BIOPAC) with disposable pressure sensors (RX104A, BIOPAC) for measurements of aortic and left atrial pressures. Aortic pressure was measured from a point superior to the aortic cannulation, and left atrial pressure was measured from a point in the perfusion line just before the left atrial cannulation. All functional signals were digitized via a PowerLab interface (AD Instruments). Functional signals were collected at 1,000 samples/s and then downsampled for a final sampling rate of 1 sample/s. Sixty samples at each steady-state event were averaged for functional measurements. For reference, steady states were reached within 1 min of any perturbation.
Isolated mouse hearts.
Male C57 BL/6N mice between 12 and 16 wk of age (Taconic Farms) were anesthetized with a mixture of pentobarbital sodium (50 mg/kg) and heparin (50 USP units) via intraperitoneal injection. A thoracotomy was performed, and the heart was excised rapidly and submerged in ice-cold saline buffer. The heart was then cannulated and perfused on a Langendorff perfusion apparatus at constant pressure (75 mmHg) with saline buffer composed as described above and passed through a 0.22-µm filter before use, oxygenated with 100% O2, and maintained at 37°C.
Myocardial Optical Absorbance Spectroscopy
Two different intraventricle light source catheters were used in the rabbit and mouse because of size differences. A previously published custom, side-firing white light LED-tipped catheter (31) was inserted into the left ventricle of the rabbit heart as described above. However, this catheter was too big to insert into the mouse heart, requiring a different intracavity light source. To accomplish this task, a commercially available side-firing fiber optic (FIP150165195, Polymicro Technologies Molex) was inserted through the mitral valve into the left ventricle of the mouse after removal of the left atrial appendage, as previously described by Femnou et al. (31) in the rabbit using the larger catheter. This fiber optic had an outer diameter of 195 µm with a fiber core of 150 µm, a numerical aperture of 0.22, and a length of 1.5 m. The fiber SMA905 connector was attached to a high-power white light source (A FCS 0000, MIGHTEX). This fiber is smaller than many Millar catheters used for mouse hearts around 1-Fr (333 μm) (1) and did not impact the observed function of the heart.
In both experimental setups, light transmitted through the left ventricular free wall was collected using a 2-mm fiber optic light guide (Thor Laboratories) positioned perpendicular to the epicardial surface of the left ventricle, ~1 cm away, at a point of maximum transmitted light intensity. Changes in transmural visible light absorbance were measured using a cooled, rapid-scanning spectrometer (QE65PRO, Ocean Optics) connected to the fiber optic. Light intensity was recorded at 1,044 points between 349–742 nm. Spectra were collected at 1 sample/s using a custom LabVIEW-based program (31) designed for spectral acquisition and analysis. No effort to compare systole with diastole was made, as a previous study has shown no differences in the heart chromophores through the cardiac cycle (51). The software permitted real-time monitoring of the raw transmitted light as well as the absorbance difference between a dynamically defined control period and current acquisition. In addition, the real-time linear least-squares regression fitting of the data with isolated reference spectra was displayed, as described below. Care was taken to eliminate any other sources of light and to position the catheter and detection fiber to collect maximum transmitted light. In a few circumstances, large motion artifacts due to the heart swinging prevented adequate light intensity collection.
Spectral Analysis
Analyses were performed in optical difference mode to minimize static absorbance and scattering effects. Spectral fitting was performed using chromophore reference standards and a previously described LabVIEW-based program (31). Reference spectra used were identical to previous publications (20, 31) and included chemically reduced myoglobin, flavin adenine dinucleotide (FAD)/flavin mononucleotide (FMN), 2 distinct cytochrome aa3 species (with maxima at 605 and 580 nm), and cytochrome c, cytochrome c1, cytochrome bH, and cytochrome bL in addition to a light sieving spectrum unique to each light source. In the present study, we used a bandwidth of 490–630 nm for spectral analysis. Two methods of analysis were conducted: 1) steady-state analysis was performed by averaging 50–150 spectra at each steady-state event, and optical density (OD) was calculated relative to the appropriate control as noted in results, and 2) time courses of chromophore absorbance were determined by averaging 50–150 spectra to generate an appropriate control spectrum and then calculating the optical difference at each time point relative to that control. Reported ODs were calculated using the steady-state methodology to improve signal to noise.
Chromophore contributions were quantified by reporting ∆ODnm of a characteristic peak wavelength (in nm) of each fitted chromophore reference. For myoglobin (∆OD582), the 582-nm peak of oxygenated myoglobin was used. Therefore, increases in ∆OD582 refer to an increase in myoglobin oxygenation. In the present study, which dealt primarily with alterations in tissue oxygenation, the species used primarily for COX fitting was hypoxia-reduced cytochrome aa3 (∆OD605) characterized by a 605-nm peak, which represents the fully reduced heme a and heme a3. Increases in ∆OD605 refer to an increase in overall reduction level of COX (20). Hypoxia-reduced cytochrome c (∆OD550) was characterized by a 550-nm peak. Increases in ∆OD550 refer to an increase in the reduction level of cytochrome c.
It is important to note that these single wavelength ∆OD values were extracted, for quantitation, from the total fit of all chromophores across all frequencies and should not be confused with single or dual wavelength measures (for examples, see Refs. 42 and 46) that are prone to error (31).
Statistics
Data are reported as means ± SE. Significant differences in functional and spectral outcomes between conditions were determined using unpaired or paired Student’s t-tests when appropriate and are indicated in the results. P values of <0.05 were considered statistically significant.
RESULTS
The major objective of the present study was to determine the effect of exogenous vasodilators on cellular oxygenation and mitochondrial redox state in the isolated working rabbit heart. A secondary objective was to adapt our transmission spectroscopy technique for use in the Langendorff-perfused mouse heart to confirm the effects of vasodilation on metabolism in this popular model of cardiac physiology. Two vasodilators were used in this study: 1) adenosine, a physiological, extracellular vasodilator working through adenosine receptors of the heart (10, 13, 26, 50, 59, 65), and 2) cromakalim, a pharmaceutical agent with a direct relaxation effect on vascular smooth muscle (15, 24, 73). Both of these agents, along with potentially secondary effects (40, 52), directly hyperpolarize the vascular smooth muscle via activation of K+ channels (15, 22, 24, 44, 73).
Adenosine
Adenosine causes vasodilation as well as a reduction in heart rate (11, 26). To separate chronotropic from vasodilatory effects, dose-response experiments of exogenous adenosine were performed in the isolated working rabbit heart over concentrations of 4.4, 16, 38, 72, and 117 µM with and without continuous pacing slightly above the intrinsic heart rate.
Before pacing, the average heart rate was 149 ± 8 beats/min, coronary flow was 36 ± 2 ml/min, and aortic output was 111 ± 8 ml/min. Additionally, average oxygen consumption was 6.1 ± 0.5 µmol O2·min−1·g−1. Average ventricular wet weight was 5.7 ± 0.1 g. To facilitate pacing capture, hearts were paced 3.7 ± 0.4% above their intrinsic rate (P < 0.005). This resulted in a 1.9 ± 0.8% increase in coronary flow (P < 0.025), a 2.5 ± 1.5% increase in aortic output (P < 0.05), and a 2.8 ± 0.9% increase in oxygen consumption (P < 0.005).
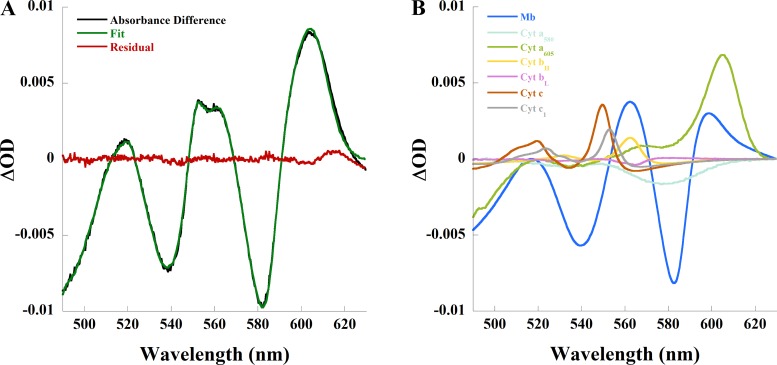
Modest increases in heart rate were accompanied by significant changes in the absorption spectrum of the heart. The absorbance difference spectrum between pacing and control is shown in Fig. 1A, with the individual chromophore contributions to the fit of this example are shown in Fig. 1B. The spectral analysis demonstrated a decrease in oxygenated myoglobin and an increase in reduced cytochrome aa3 and reduced cytochrome c consistent with increased tissue anoxia with pacing. Pacing decreased ∆OD582 of myoglobin by 0.008 ± 0.002 (P < 0.0005) consistent with deoxygenation, whereas ∆OD605 of cytochrome aa3 increased 0.006 ± 0.001 (P < 0.0005) and ∆OD550 of cytochrome c increased 0.002 ± 0.001 (P < 0.05), indicative of a cytochrome reduction. The worsened tissue anoxia, made evident by myoglobin deoxygenation and an increased COX reduction level, in response to small stimulus of exogenous work by pacing suggests that even oxygenated regions are on the brink of hypoxia and fail to elicit an appropriate regulatory increase in flow.
Fig. 1.
Changes in myocardial absorbance of the perfused rabbit heart due to pacing. In this representative study, heart rate increased from 107 to 113 beats/min, coronary flow increased from 25 to 26 ml/min, aortic output increased from 73 to 79 ml/min, and oxygen consumption increased from 4.8 to 5.1 µmol· min−1· g−1. A: absorbance difference (black) of pacing compared with control, linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. B: contribution of each chromophore to the spectral fit. Cyt, cytochrome; Mb, myoglobin; OD, optical density.
Adenosine caused dose-dependent vasodilation and changes in myocardial performance in both unpaced and paced conditions (Table 1). The half-maximal effective concentration of adenosine on coronary flow was not significantly different at 18 ± 4 and 15 ± 2 µM in unpaced and paced hearts, respectively.
Table 1.
Average changes in function and myocardial absorbance of the perfused rabbit heart as a result of adenosine
| Adenosine Concentration, μM |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 4.4 | 16 | 38 | 72 | 117 | n | |
| Coronary vascular resistance, mmHg·min·ml−1 | |||||||
| Unpaced | 1.29 ± 0.19 | 1.25 ± 0.23 | 1.06 ± 0.19 | 0.79 ± 0.11 | 0.72 ± 0.08* | 0.70 ± 0.07 | 6 |
| Paced | 1.11 ± 0.24 | 1.00 ± 0.19 | 0.85 ± 0.16 | 0.73 ± 0.13 | 0.68 ± 0.11* | 0.68 ± 0.11 | 3 |
| Coronary flow rate, ml/min | |||||||
| Unpaced | 42 ± 5 | 47 ± 6 | 54 ± 7 | 64 ± 5 | 67 ± 5* | 66 ± 4 | 8 |
| Paced | 39 ± 4 | 44 ± 4 | 55 ± 5 | 63 ± 5 | 66 ± 4* | 65 ± 4 | 8 |
| Aortic output, ml/min | |||||||
| Unpaced | 126 ± 9 | 137 ± 9 | 140 ± 8 | 139 ± 7 | 133 ± 6† | 130 ± 6 | 8 |
| Paced | 127 ± 11 | 146 ± 11 | 157 ± 10 | 157 ± 10 | 157 ± 9* | 156 ± 9 | 8 |
| Heart rate, beats/min | |||||||
| Unpaced | 153 ± 12 | 144 ± 12 | 139 ± 11 | 130 ± 9 | 124 ± 9*† | 122 ± 9 | 8 |
| Paced | 163 ± 14 | 163 ± 14 | 163 ± 14 | 163 ± 14 | 163 ± 14 | 163 ± 14 | 8 |
| Oxygen consumption, μmol O2·min−1·g−1 | |||||||
| Unpaced | 5.9 ± 0.7 | 6.1 ± 0.7 | 6.3 ± 0.6 | 6.2 ± 0.6 | 5.9 ± 0.6† | 5.7 ± 0.6 | 7 |
| Paced | 7.0 ± 0.7 | 7.8 ± 0.7 | 8.5 ± 0.8 | 8.4 ± 0.8 | 8.3 ± 0.8* | 8.2 ± 0.7 | 6 |
| Venous Po2, mmHg | |||||||
| Unpaced | 53 ± 12 | 95 ± 24 | 161 ± 29 | 271 ± 30 | 318 ± 33* | 331 ± 34 | 8 |
| Paced | 19 ± 8 | 35 ± 8 | 128 ± 29 | 218 ± 32 | 248 ± 30* | 249 ± 29 | 8 |
| Myoglobin (∆OD582) | |||||||
| Unpaced | 0.009 ± 0.001 | 0.021 ± 0.002 | 0.032 ± 0.003 | 0.042 ± 0.003*† | 0.048 ± 0.004 | 6 | |
| Paced | 0.010 ± 0.001 | 0.019 ± 0.001 | 0.026 ± 0.003 | 0.031 ± 0.004* | 0.034 ± 0.005 | 6 | |
| Cytochrome c, (∆OD550) | |||||||
| Unpaced | −0.005 ± 0.001 | −0.011 ± 0.002 | −0.017 ± 0.002 | −0.020 ± 0.003*† | −0.022 ± 0.004 | 6 | |
| Paced | −0.004 ± 0.001 | −0.008 ± 0.001 | −0.011 ± 0.001 | −0.013 ± 0.002* | −0.014 ± 0.002 | 6 | |
| Cytochrome aa3 (∆OD605) | |||||||
| Unpaced | −0.011 ± 0.001 | −0.022 ± 0.002 | −0.033 ± 0.003 | −0.040 ± 0.003*† | −0.045 ± 0.004 | 6 | |
| Paced | −0.009 ± 0.001 | −0.017 ± 0.002 | −0.022 ± 0.003 | −0.026 ± 0.004* | −0.029 ± 0.004 | 6 | |
Data are means ± SE. Adenosine resulted in dose-dependent changes in cardiac function and chromophore absorbance. OD, optical density.
Adenosine (72 µM) was significantly different compared with control (no adenosine) in either condition;
adenosine (72 µM) in unpaced hearts was significant from 72 µM adenosine in paced hearts.
Focusing on the near maximally effective dose of adenosine in terms of vasodilation, 72 µM adenosine decreased coronary vascular resistance (CVR) by 42 ± 5% (P < 0.005) in unpaced hearts and 38 ± 4% (P < 0.05) in paced hearts, whereas coronary flow doubled in both conditions. Decreases in CVR and accompanied increases in coronary flow brought about large increases in venous Po2 in both conditions. Aortic output did not change in unpaced hearts despite an 18 ± 3% reduction in heart rate (P < 0.001), whereas aortic output increased by 27 ± 6% (P < 0.0005) in paced hearts. These functional data suggest that the cardiac output, and associated oxygen consumption, per heart beat increased with adenosine vasodilation and increased oxygen delivery. Consistent with this notion, oxygen consumption paralleled the functional data: oxygen consumption was unchanged in unpaced hearts but increased by 19 ± 6% (P < 0.01) in paced hearts. Exogenous adenosine greatly improved coronary perfusion in both conditions but also increased cardiac work and oxygen consumption in paced hearts. These functional data support the notion that under control conditions, the heart maintains vascular tone that is apparently limiting cardiac function.
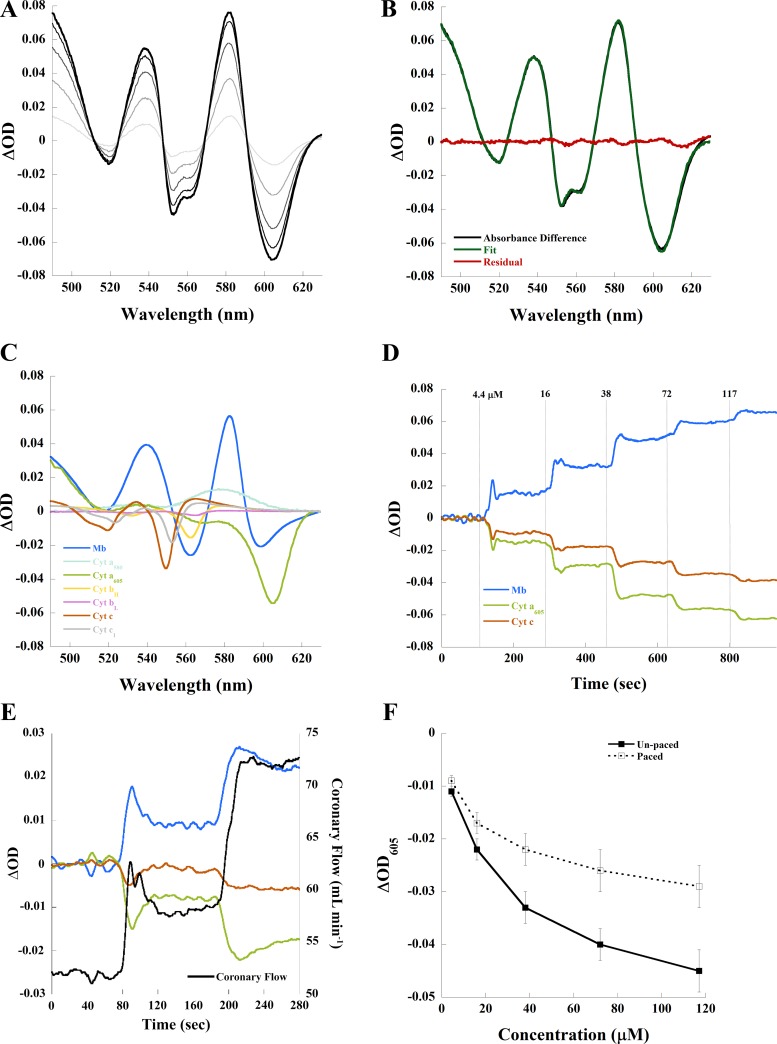
As shown by the unpaced example in Fig. 2A, adenosine caused dose-dependent changes in myocardial absorbance. Again, using 72 µM adenosine as an example, the difference spectrum of adenosine and control in the same unpaced heart was fit adequately (note minimal residuals from the fit) using our chromophore references (Fig. 2B). The individual chromophore contributions to the spectral fit are shown in Fig. 2C. Delineated in this fit, 72 μM adenosine caused a large increase in oxygenated myoglobin and oxidized cytochrome c and cytochrome aa3. Similar data were obtained for all adenosine doses and revealed a dose-dependent increase in myoglobin oxygenation and cytochrome c and aa3 oxidation. The time course of the fitted ΔOD582 (myoglobin), ΔOD550 (cytochrome c), and ΔOD605 (cytochrome aa3) from an adenosine dose-response experiment in an unpaced heart is shown in Fig. 2D. As seen in this time course, low doses of adenosine caused a transient change in absorbance. This occurred in both unpaced and paced hearts, as demonstrated in a paced example of the time course of the initial adenosine doses plotted against coronary flow (Fig. 2E). Even under these transient conditions, the optical absorbance changes tracked the coronary flow induced by adenosine in both unpaced and paced conditions. The reason for the transient effect of adenosine at low dose is unknown but may be related to changes in flow altering the effective concentration of adenosine, infused at a constant rate, or even endogenous vasoactive agents.
Fig. 2.
Changes in myocardial absorbance of the perfused rabbit heart due to adenosine. A: adenosine resulted in dose-dependent changes in absorbance difference spectrum compared with control in this unpaced example. Adenosine (4.4 µM) is represented by light grey, and 117 µM adenosine is represented by black. B: absorbance difference (black) between 72 µM adenosine and control, linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. C: contribution of each chromophore to the spectral fit. D: time course of myoglobin (Mb), cytochrome (Cyt) c, and Cyt aa3 absorbance compared with control in an unpaced experiment. E: time course of Mb, Cyt c, and Cyt aa3 absorbance compared with paced control and coronary flow for first two doses of adenosine in a paced experiment. F: effect of adenosine on steady-state ΔOD605 (Cyt aa3) with and without pacing. OD, optical density.
Optical data for adenosine titration studies are shown in Table 1 alongside functional measures emphasizing that dose-dependent changes in chromophore absorbance, quantified from spectral fitting, coincided with changes in CVR, coronary flow, and venous Po2 in the steady state. In unpaced hearts, 72 µM adenosine caused a significant increase in the ∆OD582 of myoglobin by 0.042 ± 0.003 (P < 0.0005), consistent with oxygenation, whereas the same concentration of adenosine caused a significantly smaller increase in the ∆OD582 of myoglobin, 0.031 ± 0.004 (P < 0.0005), in paced hearts. In unpaced hearts, the ∆OD605 of cytochrome aa3 (ΔOD605) was −0.040 ± 0.003 (P < 0.0005) and the ∆OD550 of cytochrome c was −0.020 ± 0.003 (P < 0.001), consistent with cytochrome oxidation. In paced hearts, the ∆OD of cytochrome aa3 (ΔOD605) was −0.026 ± 0.004 (P < 0.001), whereas that of cytochrome c was −0.013 ± 0.002 (P < 0.0005).
Despite differences in magnitude, myoglobin oxygenation, and COX oxidation by 72 µM adenosine occurred in both unpaced and paced conditions; however, the negative chronotropic effect of adenosine had a clear impact on the magnitude of change in tissue oxygenation. This is shown in Fig. 2F, in which cytochrome aa3 was maintained more reduced during pacing compared with the unpaced condition with decreasing heart rate. Parallel effects were also observed for deoxygenated myoglobin and reduced cytochrome c (Table 1). These data demonstrate that an imbalance between workload and flow continues to compromise tissue oxygenation in the paced condition even in the presence of exogenous vasodilation and increases in cardiac work output. Thus, exogenous vasodilation does not assure adequate tissue oxygenation over all possible workloads in the saline-perfused heart.
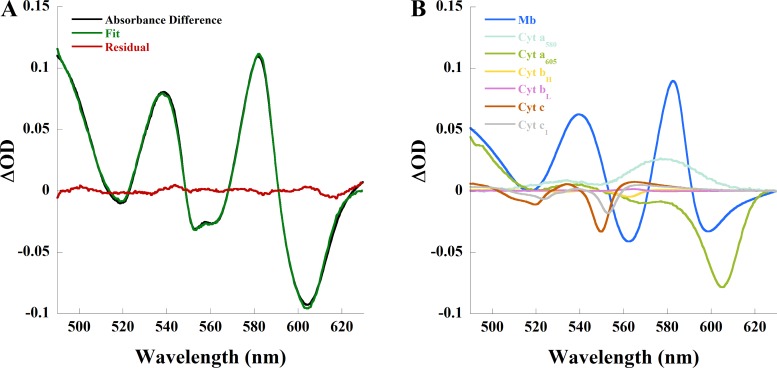
In the mouse heart, 20 µM adenosine [a saturating dose (32)] increased coronary flow from 1.8 ± 0.4 to 3.1 ± 0.4 ml/min (P < 0.005, n = 4), consistent with previous studies. This increase in flow was associated with myoglobin oxygenation and cytochrome oxidation as demonstrated by the absorbance difference spectrum between adenosine and control in the isolated mouse heart (Fig. 3A) and the individual chromophore contributions to the spectral fit of the difference spectrum (Fig. 3B). Adenosine caused a 0.066 ± 0.01 (n = 4, P < 0.01) increase in ∆OD582 of myoglobin and 0.038 ± 0.005 (n = 4, P < 0.005) and 0.063 ± 0.017 (n = 4, P < 0.025) decreases in ∆OD of cytochrome aa3 (ΔOD605) and cytochrome c (ΔOD550), respectively. An ischemia versus adenosine absorbance difference spectrum from the same heart (Fig. 3C) and the individual chromophore contributions to the spectral fit of that difference spectrum (Fig. 3D) are provided for reference. These results parallel those of the rabbit heart; adenosine increased flow and elicited myoglobin oxygenation and cytochrome oxidation, implying regional anoxia under control conditions.
Fig. 3.
Changes in myocardial absorbance of the Langendorff-perfused mouse heart due to adenosine. A: absorbance difference (black) between 20 µM adenosine and control, linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. B: contribution of each chromophore to the spectral fit in A. C: absorbance difference (black) between ischemia and 20 µM adenosine, linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. D: contribution of each chromophore to the spectral fit in C. Cyt, cytochrome; Mb, myoglobin; OD, optical density.
Cromakalim
Cromakalim was used to confirm the requirement of vasodilation to relieve tissue anoxia in the perfused rabbit heart. To match the paced adenosine condition, hearts administered 5 µM cromakalim were also paced. Cromakalim was apparently more effective in decreasing CVR, increasing coronary flow and venous Po2 (Table 2) compared with adenosine (Table 1). Cromakalim did not affect aortic output or oxygen consumption, despite enhanced perfusion and oxygenation, consistent with a net negative inotropic effect (55, 81). Indeed, we found that pacing was required to maintain a stable cardiac output with cromakalim.
Table 2.
Average changes in function and myocardial absorbance of the perfused rabbit heart due to cromakalim followed by adenosine
| Control | Cromakalim | Adenosine | n | |
|---|---|---|---|---|
| Coronary vascular resistance, mmHg·min·ml−1 | 1.05 ± 0.14 | 0.45 ± 0.01* | 0.45 ± 0.02 | 4 |
| Coronary flow rate, ml/min | 40 ± 4 | 84 ± 1* | 85 ± 3 | 4 |
| Aortic output, ml/min | 122 ± 13 | 104 ± 13 | 108 ± 13 | 4 |
| Oxygen consumption, μmol O2·min−1·g−1 | 6.7 ± 1.8 | 6.7 ± 0.3 | 6.5 ± 0.2 | 3 |
| Venous Po2, mmHg | 40 ± 15 | 423 ± 11* | 435 ± 13 | 3 |
| Myoglobin (∆OD582) | 0.070 ± 0.019* | 0.074 ± 0.020 | 4 | |
| Cytochrome c (∆OD550) | −0.032 ± 0.009* | −0.031 ± 0.008 | 4 | |
| Cytochrome aa3 (∆OD605) | −0.062 ± 0.019* | −0.064 ± 0.020 | 4 |
Data are means ± SE. Adenosine (80 µM) after cromakalim did not alter cardiac function or myocardial absorbance. OD, optical density.
Functional or absorbance measure as a result of cromakalim was significantly different from the paced control.
Vasodilation by cromakalim increased myoglobin oxygenation and cytochrome oxidation (Table 2) to a larger extent than adenosine, suggesting that adenosine did not fully oxygenate the myocardium as suggested from the earlier comparison with paced and unpaced adenosine functional effects. This is demonstrated in the absorbance difference spectrum between cromakalim and paced control (Fig. 4A) and individual chromophore contributions to the spectral fit (Fig. 4B). There was an increase in ∆OD582 of myoglobin by 0.070 ± 0.019 (P < 0.025) and decrease in ΔOD605 and ΔOD550 by 0.062 ± 0.019 (P < 0.025) and 0.032 ± 0.009 (P < 0.025), respectively. These data reaffirm that the isolated, saline-perfused working heart maintains vascular tone that upon attenuation by exogenous vasodilators relieves regional anoxia.
Fig. 4.
Changes in myocardial absorbance of the perfused rabbit heart as a result of vasodilation by cromakalim. A: absorbance difference (black) between 5 µM cromakalim and paced control, linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. B: contribution of each chromophore to the spectral fit. Cyt, cytochrome; Mb, myoglobin; OD, optical density.
To determine whether cromakalim caused maximal vasodilation, paced hearts administered 5 µM cromakalim were also administered 80 µM adenosine after functional stabilization. Adenosine did not cause any further vasodilation, nor did it improve myoglobin oxygenation or cytochrome oxidation (Table 2).
The order of cromakalim and adenosine administration was reversed for further comparison. Functional and optical data are shown in Table 3. Despite an already significant enhancement of coronary perfusion by 80 µM adenosine, 5 µM cromakalim decreased CVR by an additional 39 ± 7% (P < 0.025) and increased coronary flow by an additional 33 ± 6 ml/min (P < 0.005). Cromakalim increased venous Po2 by an additional 66 ± 16% (P < 0.005), confirming the earlier conclusion that cromakalim was more effective in generating vasodilation. Cromakalim eliminated increases in aortic output and oxygen consumption elicited by adenosine, returning these parameters to values not significantly different than control, again consistent with a net negative inotropic effect (55, 81).
Table 3.
Average changes in function and myocardial absorbance of the perfused rabbit heart due to adenosine followed by cromakalim
| Control | Adenosine | Cromakalim | n | |
|---|---|---|---|---|
| Coronary vascular resistance, mmHg·min·ml−1 | 1.48 ± 0.18 | 0.78 ± 0.11* | 0.44 ± 0.04*† | 4 |
| Coronary flow rate, ml/min | 30 ± 4 | 58 ± 7* | 91 ± 5*† | 4 |
| Aortic output, ml/min | 88 ± 15 | 111 ± 14* | 80 ± 16† | 4 |
| Oxygen consumption, μmol O2·min−1·g−1 | 5.2 ± 0.7 | 5.9 ± 0.8* | 5.3 ± 0.6† | 3 |
| Venous Po2, mmHg | 51 ± 12 | 328 ± 42* | 520 ± 21*† | 3 |
| Myoglobin (∆OD582) | 0.053 ± 0.008* | 0.100 ± 0.015† | 4 | |
| Cytochrome c (∆OD550) | −0.018 ± 0.004* | −0.032 ± 0.006† | 4 | |
| Cytochrome aa3 (∆OD605) | −0.039 ± 0.005* | −0.068 ± 0.011† | 4 |
Data are means ± SE. OD, optical density.
Functional or absorbance measure as a result of adenosine or adenosine and cromakalim was significantly different from the paced control;
functional or absorbance measure as a result of adenosine and cromakalim was significantly different from adenosine alone.
Cromakalim after adenosine caused a significant increase in myoglobin oxygenation and cytochrome oxidation. Adenosine induced an 0.053 ± 0.008 (P < 0.005) increase in ∆OD582 of myoglobin, which approximately doubled to 0.100 ± 0.015 (P < 0.025) with the addition of cromakalim. ∆OD605 of cytochrome aa3 was −0.039 ± 0.005 (P < 0.005) after adenosine. ∆OD605 after cromakalim was −0.068 ± 0.011 (P < 0.025). These changes paralleled the enhanced vasodilation by cromakalim; improved tissue perfusion increased cytosolic and mitochondrial oxygenation. Note that the magnitude of myoglobin oxygenation and cytochrome oxidation in response to either order of vasodilator was not statistically different.
Estimation of Myoglobin Oxygenation and the Redox State
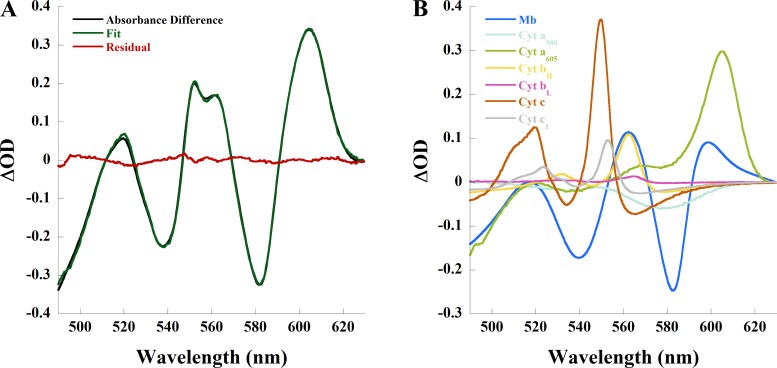
To attempt to quantitate cytosolic oxygenation and the oxidation/reduction state of the working rabbit heart, the full dynamic range of oxygenated and deoxygenated myoglobin as well as oxidized and reduced COX must be estimated. Estimating fully oxygenated myoglobin and oxidized COX is difficult in a working heart system, which relies on metabolically supported contraction for perfusion. Fully oxidized mitochondria, generated with drugs or conditions, cannot produce ATP, thereby preventing contraction and perfusion and, subsequently, generating ischemia. Thus, applying agents that oxidize COX by blocking electron flow, as used in a prior retrograde perfusion study (31), could not be used. For this estimate in the working heart, we used the steady-state maximum vasodilation condition in each heart (~85–90 ml/min) as an estimate of complete myoglobin oxygenation and COX oxidation. The steady state after ischemia was used to estimate complete myoglobin deoxygenation and COX reduction level (31). An absorbance difference spectrum between maximally vasodilated (adenosine and cromakalim) and ischemia (Fig. 5A) represents the difference between the most oxygenated/oxidized and deoxygenated/reduced states in this experimental series. Individual chromophore contributions to the fit of this example are shown in Fig. 5B.
Fig. 5.
Difference in myocardial absorbance between maximally vasodilated state and ischemia in the perfused rabbit heart. A: absorbance difference (black) between ischemia and maximal vasodilation (adenosine and cromakalim), linear least-squares regression fit (green) of the absorbance difference, and residual (red) of the spectral fit. B: contribution of each chromophore to the spectral fit. Cyt, cytochrome; Mb, myoglobin; OD, optical density.
The absorbance range (total ODnm), calculated per experiment as the difference between maximal increase and maximal decrease in ∆OD for each chromophore (Fig. 5B), was highly reproducible, yielding error close to 5%. For example, total OD582 (myoglobin) was 0.220 ± 0.012 and total OD605 (COX) was 0.247 ± 0.015. The high precision suggests that this approach provided consistent estimates of the fully oxygenated/oxidized and deoxygenated/reduced states of the heart, independent of starting conditions. However, it is unlikely the fully oxygenated and oxidized states were being achieved with this protocol. Using these approximations, we estimated percent myoglobin oxygenation and cytochrome reduction level per heart as follows:
Again, these calculations assume that cromakalim completely oxygenated myoglobin and oxidized COX. As shown in Table 4, myoglobin was 67.2 ± 5.0% oxygenated under control conditions, whereas cytochrome aa3 was 20.2 ± 3.1% reduced compared with the metabolic state after cromakalim. After pacing, there was less oxygenated myoglobin at 63.3 ± 4.9% oxygenated and more reduced cytochrome aa3 at 23.2 ± 3.0% reduced. Adenosine (80 µM) increased oxygenated myoglobin to 80.1 ± 7.1% and decreased the reduction level of cytochrome aa3 to 10.5 ± 3.5%, again relative to the metabolic state after cromakalim.
Table 4.
Estimation of myoglobin oxygenation and the cytochrome reduction level in perfused rabbit hearts
| Control | After Pacing | Adenosine | |
|---|---|---|---|
| Percent myoglobin oxygenated | 67 ± 5 | 63 ± 5 | 80 ± 7 |
| Percent cytochrome c reduced | 12 ± 2 | 15 ± 2 | 5.8 ± 1.9 |
| Percent cytochrome aa3 reduced | 20 ± 3 | 23 ± 3 | 10.5 ± 3.5 |
| n | 8 | 8 | 4 |
Myoglobin oxygenation and cytochrome reduction level were estimated based on the assumption that cromakalim fully oxygenated myoglobin and oxidized cytochromes c and aa3 and ischemia fully deoxygenated myoglobin and reduced cytochromes c and aa3.
Although the assumption that ischemia generated a fully deoxygenated and reduced condition is likely very reasonable, we believe that the absorbance steady state after maximal vasodilation for the fully oxygenated and oxidized state is likely an underestimate, especially for the mitochondrial redox state. However, the values calculated do provide a lower limit for the dynamic changes associated with the protocols used in this study.
Vascular Tone Contributes to Tissue Deoxygenation
To confirm that vasoconstriction in the isolated heart propagates tissue deoxygenation, 53 ± 23 nM arginine vasopressin (AVP), a potent coronary vasoconstrictor (60), was infused into paced, isolated working rabbit hearts. AVP caused a decrease in coronary flow from 35 ± 2 to 15 ± 5 ml/min (P < 0.05). Vasoconstriction was accompanied by decreased cardiac function. Aortic output decreased from 150 ± 10 to 67 ± 32 ml/min (P < 0.025), and oxygen consumption decreased from 6.3 ± 0.5 to 3.0 ± 1.0 µmol O2·min−1·g−1 (P < 0.025). Decreases in flow were accompanied by a decrease in ∆OD582 of myoglobin by 0.125 ± 0.032 (P < 0.05). Myoglobin deoxygenation due to AVP represented 70 ± 14% of the level of myoglobin deoxygenation after anoxia. There was an increase in ∆OD605 of cytochrome aa3 by 0.123 ± 0.033 (P < 0.05). Increases in the reduction level of COX represented 65 ± 13% of the reduction level of COX after anoxia. These data imply that vasoconstriction causes marked myoglobin deoxygenation and COX reduction and compromises cardiac function in the isolated perfused heart.
DISCUSSION
The present study demonstrates that exogenous vasodilators relax vascular tone and increase cytosolic and mitochondrial oxygenation in both the isolated, saline-perfused working rabbit heart and retrograde Langendorff-perfused mouse heart. In the rabbit heart, both vasodilators decreased CVR and increased coronary flow and venous Po2. More importantly, increases in perfusion led to proportional increases in myoglobin oxygenation and COX oxidation. Similarly, adenosine increased flow, myoglobin oxygenation, and COX oxidation in the mouse heart as well. The vasoconstriction by AVP demonstrates that vascular tone contributes markedly to myoglobin deoxygenation and cytochrome reduction. These data suggest that the presence of PAC prevents optimal perfusion and maintains regional anoxia in the isolated heart in contrast to prior speculation that vascular bed geometry alone (9) or irreversible damage and vessel blockages (74) generate these low oxygen regions. These data demonstrate that the isolated heart has the capacity to improve its net tissue oxygenation, and subsequently cardiac function, by modulating vascular tone and yet fails to do so. The homeostatic orchestration of metabolic demand and coronary flow in the isolated heart is apparently compromised during saline perfusion in vitro. This is further illustrated by the observation that, in the absence of vasodilator, very slight increases in work due to pacing increased regional anoxia in the rabbit heart rather than elicited an appropriate hemodynamic response to maintain oxygen delivery and metabolic homeostasis. Similar observations have been made in the perfused guinea pig heart with epinephrine (28) and in the perfused mouse heart with isoproterenol (46), again suggesting even with these agents that the balance between energy demand and flow is not being properly maintained in the perfused heart.
The correlation between deoxygenated myoglobin and reduced COX observed in early spectroscopy studies of isolated systems even at high venous Po2 (6, 74, 76) led to the hypothesis that detection of deoxymyoglobin does not imply a global reduction in Po2 but rather indicates the existence of small regions of complete anoxia leading to both myoglobin deoxygenation and COX reduction (9, 31, 51, 76). This concept of regional anoxia in isolated heart systems is supported by visualization of hypoxic tissue using mitochondrial NADH imaging of the perfused heart (4, 74, 79). As the heart approached hypoxia, discrete regions of increased mitochondrial NADH fluorescence, consistent with local hypoxic regions, appeared before global effects were observed (4, 74). Whether these regions exist to a lesser extent under control conditions has not been addressed using this technique other than the qualitative statement by Steenbergen et al. (74) that NADH fluorescence in the isolated heart under control conditions was “relatively homogenous . . . [with] a few small areas of high fluorescence.” Although NADH and FAD fluorescence imaging only looks at the subepicardial regions of the heart (33), transillumination of the heart used here samples the entire wall (31, 76), including the more vulnerable endocardium. For this reason, a direct correlation of the fluorescent imaging studies and transmission spectroscopy would be difficult. Yet data from numerous laboratories, preparations, and species using a variety of techniques, in addition to the present study, agree that discrete regions of anoxia exist in the isolated, saline-perfused heart under control conditions.
The present study implicates PAC in the development of regional anoxia, since vasodilation with exogenous agents improved myoglobin oxygenation and cytochrome oxidation from control. Vasoregulation requires a complex signaling network, including feedback and feedforward systems (30). This critical regulatory network has numerous elements, including many metabolites, many signaling molecules including O2, CO2, NO, and K+, intrinsic adrenergic signaling, pH, blood cell signaling, and mechanosensing, that can influence local CVR in response to different stimuli, including hypoxia and changes in workload (7, 25, 30, 45, 74, 77). Indeed, with the disruption of many vascular elements along with nonphysiological flow and arterial Po2, it is somewhat surprising that the vasoregulatory network in the isolated, saline-perfused heart works as well as it does. Mechanisms contributing to PAC in the saline-perfused heart are unresolved but might include altered shear signaling, high flow washout of interstitial vascular signaling factors, lack of red blood cell and platelet processing, and high tissue Po2.
Limited oxygen-carrying capacity along with low viscosity of saline buffer, compared with whole blood, is thought to drive a compensatory enhancement of coronary flow during in vitro perfusion that far exceeds normal physiological values (12, 18). Both low viscosity and high flow could lead to altered signaling between the tissue and vasculature, including shear effects on vascular endothelial cells. Shear stress can be calculated as the product of viscosity and shear rate [4 × flow/(π × r3)], where r is the inner radius of the vessel (62). The ratio of flow in the isolated rabbit heart during saline perfusion (~7 ml·min−1·g−1) to flow estimated in vivo [~2 ml·min−1·g−1 (63)] is similar to the ratio of saline [~0.8 cP (75)] to whole blood viscosity [~3.2 cP (48)]. Assuming vascular geometry remained the same during either perfusion condition, inversely proportional changes in flow and viscosity would not elicit large changes in shear stress. These estimates suggest that shear signaling is similar in vivo and during in vitro saline perfusion and therefore is not likely responsible for generating PAC.
Another possibility is that high flow alters vasoregulation by washing out signaling molecules in the interstitium. Affected regulatory molecules include adenosine, ATP, NO, CO2, H+, K+, Ca2+, and, possibly, yet to be identified agents. The concentration of vasoactive signaling molecules in the interstitium is a function of the rate of synthesis, rate of metabolic modification, and flow-dependent washout. With very high flow rates, it is conceivable that severe dilution of interstitial regulatory factors alters their control over vascular tone, potentially in a highly localized fashion depending on vascular anatomy or metabolic heterogeneity. It is interesting to compare interstitial metabolite measures in the in situ rabbit heart with the isolated, saline-perfused rabbit heart from the same laboratory. Matherne and colleagues reported in situ interstitial concentrations of adenosine (0.4 µM), inosine (1.8 µM), and hypoxanthine (3.6 µM) (41) to be much higher than in the saline-perfused rabbit heart [adenosine (0.2 µM), inosine (1.2 µM), and hypoxanthine (0.3 µM)] (54). These data are consistent with high flow washout of interstitial metabolites in the saline-perfused heart. Indeed, an argument could be made that the decrease in regional anoxia observed with exogenous adenosine in the current study implies that endogenous adenosine generation from these hypoxic zones was inadequate to support the appropriate regional vasodilation, potentially because of high flow washout.
Large differences in tissue Po2 between the in vivo and in vitro conditions may contribute to observed PAC. Arterial Po2 in our isolated rabbit heart preparation was ~760 mmHg, whereas in vivo arterial Po2 of the rabbit is only ~85–102 mmHg under control conditions (8). The high Po2 of saline perfusate is necessary to provide adequate oxygen to support cardiac function in vitro but potentially limits oxygen delivery through vasoconstrictive effects (7). However, the observation of reduced COX suggests that Po2 approaches zero in hypoxic regions. This implies, because of the rapid diffusion of oxygen in muscle [1.16 × 103 µm2/s (3)], that any impact of high Po2 on arteriole tone must occur remotely to hypoxic regions, since diffusion would tend to equilibrate the hypoxic and high Po2 regions. This consideration brings some doubt to the role of Po2 in generating the small hypoxic zones observed.
Because of the apparently small size of the hypoxic zones, we reasoned that PAC involves the feeding resistance arterioles (21). The direct interplay of arteriole tone and the metabolic state of the tissue surrounding dependent capillaries is a very controversial area, and it is still unclear if and how dependent capillary metabolic signals are transmitted upstream to the feeding arteriole (71). Direct electrical signaling via the endothelial cells, a feasible path for upstream signaling over these large distances, has been proposed (70). However, mechanisms for this propagation pathway remain obscure. Another possibility considers the heart as a functional syncytium with workload evenly distributed over large regions. This implies that appropriate arteriolar capillary feedback may occur via adjacent capillary beds rather than the dependent capillaries perfused by the arteriole. This latter possibility makes arteriole and capillary geometry a critical factor when considering the role of interstitial metabolite signaling, especially under high flow conditions.
Red blood cells have been implicated in vasoregulation through several signaling pathways, including the release of ATP in response to decreased blood Po2 at the level of hemoglobin (29, 36, 37, 45, 56). Thus, lack of red blood cells may be compromising cardiac function beyond simply limiting oxygen delivery in the isolated system. Unfortunately, understanding the role of red blood cell signaling in the isolated heart using current spectroscopy techniques is hindered by the optical absorbance of hemoglobin.
The mammalian heart has a large heterogeneous distribution of catecholamine stores found at intracardiac sympathetic nerve terminals as well as in chromaffin-like cells in the atria and intrinsic cardiac adrenergic (ICA) cells (34, 43). Epinephrine and norepinephrine are powerful vasoconstrictors that can override metabolic signaling on the vasculature (14, 38, 78) and are effective at concentrations below the metabolic stimulus threshold (35a). Interestingly, ICA cells are distributed throughout the ventricles in close proximity to the microvasculature (43). Although it is unresolved how these stores may be activated, a regional release of catecholamines, from either ICA or sympathetic nerves, in the deinnervated perfused heart could result in localized vasoconstriction without seriously impacting global cardiac function.
It is important to consider overcoming PAC before using the widely used isolated perfused heart preparation to study cardiac function and metabolism. Although it might be possible to pretreat with vasodilatory agents to reduce PAC and improve tissue oxygenation, it is not clear that PAC is completely eliminated with this strategy at all workloads (see Fig. 2F). In addition, off-target effects of these agents, such as chronotropic and inotropic effects, need to be considered. Finally, the disruption of the normal vascular regulatory system with exogenous agents limits the utility of these experiments in revealing physiological processes. In any event, the present study underscores the necessity of monitoring tissue oxygenation when interpreting work-related changes in cardiac function and metabolism in the saline-perfused heart.
In summary, the isolated saline-perfused heart partially lacks the homeostatic balance between cardiac work and oxygen delivery, via flow, resulting in small regions of total anoxia. Exogenous vasodilatory agents improve perfusion and diminish regional anoxia, supporting our hypothesis that PAC generates these zones. PAC likely results from the disruption of normal feedback and feedforward mechanisms involved in vasoregulation. Based on our analysis, we believe that the most likely source of PAC is washout of interstitial vasoactive agents complicated by the complex vascular anatomy. It is interesting to note that some aspects of cardiac function and metabolism in the isolated saline-perfused heart mimic characteristics of microvascular dysfunction observed in clinical studies (16, 23).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.V.G., J.S., S.K.-G., R.C., E.M., and R.S.B. conceived and designed research; A.V.G., A.N.F., S.K.-G., J.L.T., and R.S.B. performed experiments; A.V.G., J.S., A.N.F., R.C., E.M., and R.S.B. analyzed data; A.V.G., J.S., A.N.F., S.K.-G., R.C., E.M., and R.S.B. interpreted results of experiments; A.V.G. prepared figures; A.V.G. and R.S.B. drafted manuscript; A.V.G., J.S., A.N.F., S.K.-G., J.L.T., R.C., E.M., and R.S.B. edited and revised manuscript; A.V.G., J.S., A.N.F., S.K.-G., J.L.T., R.C., E.M., and R.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Xi Ye and Dr. Charles Steenbergen for the review of the manuscript and insightful guidance and comments.
REFERENCES
- 1.Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ, Martino TA. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res 114: 1713–1722, 2014. doi: 10.1161/CIRCRESAHA.114.302995. [DOI] [PubMed] [Google Scholar]
- 3.Arai AE, Kasserra CE, Territo PR, Gandjbakhche AH, Balaban RS. Myocardial oxygenation in vivo: optical spectroscopy of cytoplasmic myoglobin and mitochondrial cytochromes. Am J Physiol Heart Circ Physiol 277: H683–H697, 1999. doi: 10.1152/ajpheart.1999.277.2.H683. [DOI] [PubMed] [Google Scholar]
- 4.Ashruf JF, Coremans JMCC, Bruining HA, Ince C. Increase of cardiac work is associated with decrease of mitochondrial NADH. Am J Physiol Heart Circ Physiol 269: H856–H862, 1995. doi: 10.1152/ajpheart.1995.269.3.H856. [DOI] [PubMed] [Google Scholar]
- 5.Bache RJ, Zhang J, Murakami Y, Zhang Y, Cho YK, Merkle H, Gong G, From AH, Ugurbil K. Myocardial oxygenation at high workstates in hearts with left ventricular hypertrophy. Cardiovasc Res 42: 616–626, 1999. doi: 10.1016/S0008-6363(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 6.Barlow CH, Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science 193: 909–910, 1976. doi: 10.1126/science.181843. [DOI] [PubMed] [Google Scholar]
- 7.Baron JF, Vicaut E, Hou X, Duvelleroy M. Independent role of arterial O2 tension in local control of coronary blood flow. Am J Physiol Heart Circ Physiol 258: H1388–H1394, 1990. doi: 10.1152/ajpheart.1990.258.5.H1388. [DOI] [PubMed] [Google Scholar]
- 8.Barzago MM, Bortolotti A, Omarini D, Aramayona JJ, Bonati M. Monitoring of blood gas parameters and acid-base balance of pregnant and non-pregnant rabbits (Oryctolagus cuniculus) in routine experimental conditions. Lab Anim 26: 73–79, 1992. doi: 10.1258/002367792780745904. [DOI] [PubMed] [Google Scholar]
- 9.Beard DA, Schenkman KA, Feigl EO. Myocardial oxygenation in isolated hearts predicted by an anatomically realistic microvascular transport model. Am J Physiol Heart Circ Physiol 285: H1826–H1836, 2003. doi: 10.1152/ajpheart.00380.2003. [DOI] [PubMed] [Google Scholar]
- 10.Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis 32: 73–97, 1989. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- 11.Belardinelli L, West A, Crampton R, Berne RM. Chronotropic and dromotropic effects of adenosine. In: Regulatory Function of Adenosine: Proceedings of the International Symposium on Adenosine, Charlottesville, Virginia, June 7–11, 1982, edited by Berne RM, Rall TW, Rubio R. Boston, MA: Springer US, 1983, p. 377–398. doi: 10.1007/978-1-4613-3909-0_24. [DOI] [Google Scholar]
- 12.Bergmann SR, Clark RE, Sobel BE. An improved isolated heart preparation for external assessment of myocardial metabolism. Am J Physiol Heart Circ Physiol 236: H644–H661, 1979. doi: 10.1152/ajpheart.1979.236.4.H644. [DOI] [PubMed] [Google Scholar]
- 13.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 14.Berne RM. Effect of epinephrine and norepinephrine on coronary circulation. Circ Res 6: 644–655, 1958. doi: 10.1161/01.RES.6.5.644. [DOI] [PubMed] [Google Scholar]
- 15.Brayden JE, Quayle JM, Standen NB, Nelson MT. Role of potassium channels in the vascular response to endogenous and pharmacological vasodilators. Blood Vessels 28: 147–153, 1991. doi: 10.1159/000158854. [DOI] [PubMed] [Google Scholar]
- 16.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 356: 830–840, 2007. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 17.Chemnitius JM, Burger W, Bing RJ. Crystalloid and perfluorochemical perfusates in an isolated working rabbit heart preparation. Am J Physiol Heart Circ Physiol 249: H285–H292, 1985. doi: 10.1152/ajpheart.1985.249.2.H285. [DOI] [PubMed] [Google Scholar]
- 18.Chen V, Chen YH, Downing SE. An improved isolated working rabbit heart preparation using red cell enhanced perfusate. Yale J Biol Med 60: 209–219, 1987. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Zhang J, Eljgelshoven MH, Zhang Y, Zhu XH, Wang C, Cho Y, Merkle H, Uĝcurbil K. Determination of deoxymyoglobin changes during graded myocardial ischemia: an in vivo 1H NMR spectroscopy study. Magn Reson Med 38: 193–197, 1997. doi: 10.1002/mrm.1910380206. [DOI] [PubMed] [Google Scholar]
- 20.Chess DJ, Billings E, Covian R, Glancy B, French S, Taylor J, de Bari H, Murphy E, Balaban RS. Optical spectroscopy in turbid media using an integrating sphere: mitochondrial chromophore analysis during metabolic transitions. Anal Biochem 439: 161–172, 2013. doi: 10.1016/j.ab.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 22.Cooke JP, Rossitch E Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crea F, Lanza GA. Angina pectoris and normal coronary arteries: cardiac syndrome X. Heart 90: 457–463, 2004. doi: 10.1136/hrt.2003.020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Günther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 25.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 26.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol 68: 213–237, 1929. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duvelleroy MA, Duruble M, Martin JL, Teisseire B, Droulez J, Cain M. Blood-perfused working isolated rat heart. J Appl Physiol 41: 603–607, 1976. doi: 10.1152/jappl.1976.41.4.603. [DOI] [PubMed] [Google Scholar]
- 28.Ejike JC, Arakaki LSL, Beard DA, Ciesielski WA, Feigl EO, Schenkman KA. Myocardial oxygenation and adenosine release in isolated guinea pig hearts during changes in contractility. Am J Physiol Heart Circ Physiol 288: H2062–H2067, 2005. doi: 10.1152/ajpheart.00777.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone in regions of low Po2. Physiology (Bethesda) 24: 107–116, 2009. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Femnou AN, Kuzmiak-Glancy S, Covian R, Giles AV, Kay MW, Balaban RS. Intracardiac light catheter for rapid scanning transmural absorbance spectroscopy of perfused myocardium: measurement of myoglobin oxygenation and mitochondria redox state. Am J Physiol Heart Circ Physiol 313: H1199–H1208, 2017. doi: 10.1152/ajpheart.00306.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flood A, Headrick JP. Functional characterization of coronary vascular adenosine receptors in the mouse. Br J Pharmacol 133: 1063–1072, 2001. doi: 10.1038/sj.bjp.0704170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fralix TA, Heineman FW, Balaban RS. Effects of tissue absorbance on NAD(P)H and Indo-1 fluorescence from perfused rabbit hearts. FEBS Lett 262: 287–292, 1990. doi: 10.1016/0014-5793(90)80212-2. [DOI] [PubMed] [Google Scholar]
- 34.Friedman WF, Pool PE, Jacobowitz D, Seagren SC, Braunwald E. Sympathetic innervation of the developing rabbit heart. Biochemical and histochemical comparisons of fetal, neonatal, and adult myocardium. Circ Res 23: 25–32, 1968. doi: 10.1161/01.RES.23.1.25. [DOI] [PubMed] [Google Scholar]
- 35.Gard JK, Kichura GM, Ackerman JJH, Eisenberg JD, Billadello JJ, Sobel BE, Gross RW. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophys J 48: 803–813, 1985. doi: 10.1016/S0006-3495(85)83839-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Glomstein A, Hauge A, Oye I, Sinclair D. Effects of adrenaline on coronary flow in isolated perfused rat hearts. Acta Physiol Scand 69: 102–110, 1967. doi: 10.1111/j.1748-1716.1967.tb03495.x. [DOI] [PubMed] [Google Scholar]
- 36.González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol 572: 295–305, 2006. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. doi: 10.1161/01.RES.0000044939.73286.E2. [DOI] [PubMed] [Google Scholar]
- 38.Hardin RA, Scott JB, Haddy FJ. Effect of epinephrine and norepinephrine on coronary vascular resistance in dogs. Am J Physiol 201: 276–280, 1961. doi: 10.1152/ajplegacy.1961.201.2.276. [DOI] [PubMed] [Google Scholar]
- 39.Hassinen IE, Hiltunen JK, Takala TES. Reflectance spectrophotometric monitoring of the isolated perfused heart as a method of measuring the oxidation-reduction state of cytochromes and oxygenation of myoglobin. Cardiovasc Res 15: 86–91, 1981. doi: 10.1093/cvr/15.2.86. [DOI] [PubMed] [Google Scholar]
- 40.Headrick JP, Berne RM. Endothelium-dependent and -independent relaxations to adenosine in guinea pig aorta. Am J Physiol Heart Circ Physiol 259: H62–H67, 1990. doi: 10.1152/ajpheart.1990.259.1.H62. [DOI] [PubMed] [Google Scholar]
- 41.Headrick JP, Emerson CS, Berr SS, Berne RM, Matherne GP. Interstitial adenosine and cellular metabolism during β-adrenergic stimulation of the in situ rabbit heart. Cardiovasc Res 31: 699–710, 1996. [PubMed] [Google Scholar]
- 42.Heineman FW, Kupriyanov VV, Marshall R, Fralix TA, Balaban RS. Myocardial oxygenation in the isolated working rabbit heart as a function of work. Am J Physiol Heart Circ Physiol 262: H255–H267, 1992. doi: 10.1152/ajpheart.1992.262.1.H255. [DOI] [PubMed] [Google Scholar]
- 43.Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, Kelly RA, Smith TW. An intrinsic adrenergic system in mammalian heart. J Clin Invest 98: 1298–1303, 1996. doi: 10.1172/JCI118916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson WF, König A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 264: H238–H243, 1993. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- 45.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 46.Jilkina O, Kuzio B, Rendell J, Xiang B, Kupriyanov VV. K+ transport and energetics in Kir6.2(−/−) mouse hearts assessed by 87Rb and 31P magnetic resonance and optical spectroscopy. J Mol Cell Cardiol 41: 893–901, 2006. doi: 10.1016/j.yjmcc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Jue T, Anderson S. 1H NMR observation of tissue myoglobin: an indicator of cellular oxygenation in vivo. Magn Reson Med 13: 524–528, 1990. doi: 10.1002/mrm.1910130322. [DOI] [PubMed] [Google Scholar]
- 48.Kato T. Relation between blood viscosity and hematocrit in rabbits subjected to repeated treatment with endotoxin. Resuscitation 21: 61–66, 1991. doi: 10.1016/0300-9572(91)90079-E. [DOI] [PubMed] [Google Scholar]
- 49.Kreutzer U, Jue T. 1H-nuclear magnetic resonance deoxymyoglobin signal as indicator of intracellular oxygenation in myocardium. Am J Physiol Heart Circ Physiol 261: H2091–H2097, 1991. doi: 10.1152/ajpheart.1991.261.6.H2091. [DOI] [PubMed] [Google Scholar]
- 50.Kusachi S, Thompson RD, Olsson RA. Ligand selectivity of dog coronary adenosine receptor resembles that of adenylate cyclase stimulatory (Ra) receptors. J Pharmacol Exp Ther 227: 316–321, 1983. [PubMed] [Google Scholar]
- 51.Kuzmiak-Glancy S, Covian R, Femnou AN, Glancy B, Jaimes R III, Wengrowski AM, Garrott K, French SA, Balaban RS, Kay MW. Cardiac performance is limited by oxygen delivery to the mitochondria in the crystalloid-perfused working heart. Am J Physiol Heart Circ Physiol 314: H704–H715, 2018. doi: 10.1152/ajpheart.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lupi A, Buffon A, Finocchiaro ML, Conti E, Maseri A, Crea F. Mechanisms of adenosine-induced epicardial coronary artery dilatation. Eur Heart J 18: 614–617, 1997. doi: 10.1093/oxfordjournals.eurheartj.a015305. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald VW, Winslow RM. Oxygen delivery and myocardial function in rabbit hearts perfused with cell-free hemoglobin. J Appl Physiol 72: 476–483, 1992. doi: 10.1152/jappl.1992.72.2.476. [DOI] [PubMed] [Google Scholar]
- 54.Matherne GP, Headrick JP, Coleman SD, Berne RM. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res 28: 348–353, 1990. doi: 10.1203/00006450-199010000-00010. [DOI] [PubMed] [Google Scholar]
- 55.McCullough JR, Normandin DE, Conder ML, Sleph PG, Dzwonczyk S, Grover GJ. Specific block of the anti-ischemic actions of cromakalim by sodium 5-hydroxydecanoate. Circ Res 69: 949–958, 1991. doi: 10.1161/01.RES.69.4.949. [DOI] [PubMed] [Google Scholar]
- 56.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997. doi: 10.1152/ajpheart.1997.272.4.H1886. [DOI] [PubMed] [Google Scholar]
- 57.Moravec J, Moravec M, Hatt PY, Corsin A, Laplace M, Dronne MT. Rate of pyridine nucleotide oxidation and cytochrome oxidase interaction with intracellular oxygen in hearts from rats with compensated volume overload. Pflugers Arch 392: 106–114, 1981. doi: 10.1007/BF00581257. [DOI] [PubMed] [Google Scholar]
- 58.Nighswander-Rempel SP, Kupriyanov VV, Shaw RA. Relative contributions of hemoglobin and myoglobin to near-infrared spectroscopic images of cardiac tissue. Appl Spectrosc 59: 190–193, 2005. doi: 10.1366/0003702053085106. [DOI] [PubMed] [Google Scholar]
- 59.Olsson RA, Davis CJ, Khouri EM, Patterson RE. Evidence for an adenosine receptor on the surface of dog coronary myocytes. Circ Res 39: 93–98, 1976. doi: 10.1161/01.RES.39.1.93. [DOI] [PubMed] [Google Scholar]
- 60.Ouattara A, Landi M, Le Manach Y, Lecomte P, Leguen M, Boccara G, Coriat P, Riou B. Comparative cardiac effects of terlipressin, vasopressin, and norepinephrine on an isolated perfused rabbit heart. Anesthesiology 102: 85–92, 2005. doi: 10.1097/00000542-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 61.Podesser BK, Hallström S, Schima H, Huber L, Weisser J, Kröner A, Fürst W, Wolner E. The erythrocyte-perfused “working heart” model: hemodynamic and metabolic performance in comparison to crystalloid perfused hearts. J Pharmacol Toxicol Methods 41: 9–15, 1999. doi: 10.1016/S1056-8719(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 62.Pohl U, Herlan K, Huang A, Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol Heart Circ Physiol 261: H2016–H2023, 1991. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- 63.Rosolowsky M, Weiss HR. Effect of dopamine and blockade of dopaminergic and adrenergic receptors on coronary blood flow in ischemic rabbit myocardium. J Cardiovasc Pharmacol 7: 700–708, 1985. doi: 10.1097/00005344-198507000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Rossi-Fanelli A, Antonini E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys 77: 478–492, 1958. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- 65.Rubio R, Berne RM. Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ Res 25: 407–415, 1969. doi: 10.1161/01.RES.25.4.407. [DOI] [PubMed] [Google Scholar]
- 66.Schenkman KA, Beard DA, Ciesielski WA, Feigl EO. Comparison of buffer and red blood cell perfusion of guinea pig heart oxygenation. Am J Physiol Heart Circ Physiol 285: H1819–H1825, 2003. doi: 10.1152/ajpheart.00383.2003. [DOI] [PubMed] [Google Scholar]
- 67.Schenkman KA, Ciesielski WA. Improved myoglobin saturation measurement made by partial least-squares analysis of optical reflectance spectra. Appl Spectrosc 56: 1215–1221, 2002. doi: 10.1366/000370202760295476. [DOI] [Google Scholar]
- 68.Schenkman KA, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82: 86–92, 1997. doi: 10.1152/jappl.1997.82.1.86. [DOI] [PubMed] [Google Scholar]
- 69.Schenkman KA, Marble DR, Burns DH, Feigl EO. Optical spectroscopic method for in vivo measurement of cardiac myoglobin oxygen saturation. Appl Spectrosc 53: 332–338, 1999. doi: 10.1366/0003702991946541. [DOI] [Google Scholar]
- 70.Segal SS. Cell-to-cell communication coordinates blood flow control. Hypertension 23: 1113–1120, 1994. doi: 10.1161/01.HYP.23.6.1113. [DOI] [PubMed] [Google Scholar]
- 71.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 72.Segel LD, Rendig SV. Isolated working rat heart perfusion with perfluorochemical emulsion Fluosol-43. Am J Physiol Heart Circ Physiol 242: H485–H489, 1982. doi: 10.1152/ajpheart.1982.242.4.H485. [DOI] [PubMed] [Google Scholar]
- 73.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science 245: 177–180, 1989. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 74.Steenbergen C, Deleeuw G, Barlow C, Chance B, Williamson JR. Heterogeneity of the hypoxic state in perfused rat heart. Circ Res 41: 606–615, 1977. doi: 10.1161/01.RES.41.5.606. [DOI] [PubMed] [Google Scholar]
- 75.Sutera SP, Tilton RG, Larson KB, Kilo CJ, Williamson JR. Vascular flow resistance in rabbit hearts: “apparent viscosity” of RBC suspensions. Microvasc Res 36: 305–313, 1988. doi: 10.1016/0026-2862(88)90030-1. [DOI] [PubMed] [Google Scholar]
- 76.Tamura M, Oshino N, Chance B, Silver IA. Optical measurements of intracellular oxygen concentration of rat heart in vitro. Arch Biochem Biophys 191: 8–22, 1978. doi: 10.1016/0003-9861(78)90062-0. [DOI] [PubMed] [Google Scholar]
- 77.Tune JD, Richmond KN, Gorman MW, Feigl EO. channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868–H875, 2001. doi: 10.1152/ajpheart.2001.280.2.H868. [DOI] [PubMed] [Google Scholar]
- 78.Vatner SF, Higgins CB, Braunwald E. Effects of norepinephrine on coronary circulation and left ventricular dynamics in the conscious dog. Circ Res 34: 812–823, 1974. doi: 10.1161/01.RES.34.6.812. [DOI] [PubMed] [Google Scholar]
- 79.Wengrowski AM, Kuzmiak-Glancy S, Jaimes R III, Kay MW. NADH changes during hypoxia, ischemia, and increased work differ between isolated heart preparations. Am J Physiol Heart Circ Physiol 306: H529–H537, 2014. doi: 10.1152/ajpheart.00696.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wittenberg BA, Wittenberg JB. Oxygen pressure gradients in isolated cardiac myocytes. J Biol Chem 260: 6548–6554, 1985. [PubMed] [Google Scholar]
- 81.Yanagisawa T, Hashimoto H, Taira N. The negative inotropic effect of nicorandil is independent of cyclic GMP changes: a comparison with pinacidil and cromakalim in canine atrial muscle. Br J Pharmacol 95: 393–398, 1988. doi: 10.1111/j.1476-5381.1988.tb11658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Ugurbil K, From AH, Bache RJ. Myocardial oxygenation and high-energy phosphate levels during graded coronary hypoperfusion. Am J Physiol Heart Circ Physiol 280: H318–H326, 2001. doi: 10.1152/ajpheart.2001.280.1.H318. [DOI] [PubMed] [Google Scholar]