Abstract
The incidence and severity of acute kidney injury is increased in patients with diabetes and with aging. However, the mechanisms involved have not been clearly established. The present study examined the effects of aging and diabetes on the severity of renal ischemia-reperfusion (IR) injury in Sprague-Dawley (SD) and type 2 diabetic (T2DN) rats. T2DN rats develop diabetes at 3 mo of age and progressive proteinuria and diabetic nephropathy as they age from 6 to 18 mo. Plasma creatinine levels after bilateral IR were significantly higher (3.4 ± 0.1 mg/dl) in 18-mo-old elderly T2DN rats than in middle-aged (12 mo) T2DN rats with less severe diabetic nephropathy or young (3 mo) and elderly (18 mo) control SD rats (1.5 ± 0.2, 1.8 ± 0.1, and 1.7 ± 0.1 mg/dl, respectively). Elderly T2DN rats exhibited a greater fall in medullary blood flow 2 h following renal IR and a more severe and prolonged decline in glomerular filtration rate than middle-aged T2DN and young or elderly SD rats. The basal expression of the adhesion molecules ICAM-1 and E-selectin and the number of infiltrating immune cells was higher in the kidney of elderly T2DN than age-matched SD rats or young and middle-aged T2DN rats before renal IR. These results indicate that elderly T2DN rats with diabetic nephropathy are more susceptible to renal IR injury than diabetic animals with mild injury or age-matched control animals. This is associated with increased expression of ICAM-1, E-selectin and immune cell infiltration, renal medullary vasocongestion, and more prolonged renal medullary ischemia.
Keywords: AKI, diabetes, intrarenal blood flow, renal ischemia-reperfusion injury
INTRODUCTION
Diabetes mellitus (DM) is one of the leading causes of chronic kidney disease (CKD) and a major risk factor for the development of acute kidney injury (AKI) (5, 9, 16, 43, 49). Renal ischemia-reperfusion (IR) injury is the most frequent cause of AKI (1, 39). The relative risk for end-stage renal disease (ESRD) in patients with CKD after AKI is 13 times greater than in patients with normal renal function (13, 46). Numerous mechanisms have been reported to enhance the susceptibility to AKI in elderly patients with CKD. These include decreased renal perfusion, increased oxidative stress, tubular apoptosis and autophagy, inflammation, reduced responsiveness to nitric oxide, and impaired tubular repair (46).
In contrast, there is a lack of consensus on the influence of diabetes on the severity of AKI. The susceptibility of the kidney to ischemic insults has been reported to be elevated in patients with diabetes and in experimental models of diabetes (22, 35). Studies in streptozotocin-treated diabetic rodents indicated that the kidney does not recover well following IR, and these animals develop progressive CKD (22, 23). Type 2 diabetic db/db mice also exhibit more severe IR injury than non-diabetic controls (35). This was associated with a delayed recovery of renal cortical and medullary flow for ~10 min following renal ischemia. On the other hand, Johnson et al. (14) reported that patients with DM develop less severe AKI compared with non-diabetic controls, and short-term recovery of renal function was greater in patients with diabetes. Moreover, the proportion of patients with diabetes that developed CKD tended to be lower in diabetic than non-diabetic controls (9).
The mechanisms by which DM influences the severity of renal IR injury remain to be determined. Recent studies indicate that a delayed fall in medullary blood flow (MBF) following reperfusion that leads to prolonged medullary vasocongestion and hypoxia contributes to strain differences in the severity of renal IR injury (27, 31, 32). The renoprotective effect of 20-hydroxyeicosatetraenoic acid following bilateral renal ischemia is also associated with preservation of MBF after reperfusion (27, 31, 32). Therefore, in the present study, we hypothesized that elderly type 2 diabetic (T2DN) rats with diabetic nephropathy might be more susceptible to renal IR injury, perhaps related to increased expression of adhesion molecules and increased renal inflammation. To test this hypothesis, we compared IR injury in middle-aged (12 mo) and elderly (18 mo) T2DN rats with mild and severe diabetic nephropathy versus young (3 mo) and age-matched, elderly, non-diabetic Sprague-Dawley (SD) rats following 30 min of bilateral renal IR.
MATERIALS AND METHODS
General.
Experiments were performed on young (3 mo old; 3M) and elderly (18 mo old; 18M) non-diabetic male SD rats and middle-aged (12 mo old; 12M) and elderly (18 mo old; 18M) male T2DN rats with mild and severe diabetic nephropathy. The T2DN rat is a strain in which the mitochondrial genome of the FHH rat that is susceptible to renal disease has been introgressed into the type II GK rat. T2DN rats develop diabetes at 3 mo of age and progressive proteinuria and diabetic nephropathy as they increase in age from 6 to 18 mo. This model has been shown to develop most of the features of diabetic nephropathy, including progressive proteinuria and renal fibrosis and a decrease in glomerular filtration rate (GFR) with nodular focal glomerular sclerosis (18, 19, 29).
The rats were maintained in colonies maintained at the University of Mississippi Medical Center. The rats were fed a low-salt diet (0.3% NaCl) to minimize the development of hypertension (37). They had free access to food and water during the course of the experiments. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Baseline measurements.
Blood samples were collected before renal IR from the subclavian vein in conscious rats for baseline measurement of blood glucose, glycosylated hemoglobin (HbA1c), and creatinine levels. These samples were collected with ad libitum feeding for 3 h into the lights-on cycle. Twenty-four-hour urine samples were also collected in metabolic cages to measure protein excretion. Plasma glucose and HbA1c levels were measured using a glucometer (Bayer HealthCare, Mishawaka, IN) and a rapid HbA1c device (Bayer HealthCare, Sunnyvale, CA). Plasma creatinine concentrations were determined by using creatinine assay kit (Wako Pure Chemical, Osaka, Japan). Urinary protein concentration was determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA). Blood pressure was measured using a tail-cuff device (Hatteras Instruments, Cary, NC).
Intraperitoneal glucose tolerance test.
Fasting (12 h) glucose levels were determined. The rats then received an intraperitoneal injection of 1 g/kg glucose solution. This low dose was chosen as it produced a large increase in plasma glucose concentration in the glucose intolerant T2DN rats but was not sufficient to elicit much of a response in the SD rats. Additional experiments were also performed using a higher 5 g/kg ip glucose challenge. Blood samples were then collected from the tail vein at 30, 60, 90, and 120 min after the glucose load. Plasma glucose levels were measured using a glucometer (Bayer HealthCare).
Renal IR injury.
Anesthesia was induced by inhalation of 3%–4% of isoflurane, and that rats were maintained using 1.5%–2.5% of isoflurane. The rats were placed on a heated surgical table to maintain body temperature at 37°C. The kidneys were exposed using a midline abdominal incision, followed by occlusion of both renal arteries and veins by using microvascular clamps for 30 min. The rats were allowed to recover from anesthesia after removal of clamps and closure of the abdominal incision. Sham rats received the same surgery, but the renal arteries were not clamped. Some rats in each group received bilateral nephrectomy as a complete renal failure control group. Twenty-four hours after renal IR, the rats were re-anesthetized with isoflurane, blood samples were collected for measurement of plasma creatinine concentrations, and the kidneys were flushed with phosphate-buffered saline (PBS) and collected for histological analysis.
Assessment of renal injury.
The kidneys were fixed by immersion in 10% formalin. Paraffin sections (3- μm) were prepared and stained with Masson’s trichrome to evaluate the degree of glomerular injury and renal fibrosis or hematoxylin-eosin to determine the degree of tubular necrosis. Images were captured using a Nikon Eclipse 55i microscope equipped with a Nikon DS-Fi1 color camera (Nikon Instruments Inc., Melville, NY). Thirty glomeruli per section were scored in a blinded fashion on a 0–4+ scale, with 0 representing a normal glomerulus; 1+ representing loss of 1%–25% of glomerular capillary area; 2+ representing a 26%–50% loss; 3+ representing a 51%–75% loss; and 4+ representing >75% loss of the capillaries in the glomerular tuft. Renal fibrosis was assessed by measuring the percentage of blue staining of collagen and fibronectin in trichrome-stained sections after thresholding using the NIS Elements D 3.0 software (Nikon Instruments Inc.).
Eosin fluorescence was used to quantify the degree of tubular necrosis (42). The sections stained with hematoxylin-eosin were examined using a Nikon Eclipse 55i epifluorescent microscope (excitation 540 nm and emission 590 nm). Ten randomly selected fields of corticomedullary and outermedullary were analyzed, and the percentage area of the necrotic tubular epithelium was quantified using the NIS Elements D 3.0 software.
Immunohistochemistry.
The slides were deparaffinized in xylene and rehydrated using decreasing concentrations (100% to 70%) of ethanol. The slides were permeabilized by incubation with proteinase K (0.1 mg/ml; S3020, Dako, Carpinteria, CA) for 10 min. The slides were then incubated with a serum-free blocking solution (X0909, Dako) for 30 min at room temperature. They were incubated with a 1:200 dilution of antibodies to either caspase-3 (ab-13847, Abcam, Cambridge, MA), ED1 (MCA-341-R, AbD Serotec, Raleigh, NC), or anti-neutrophil antibody (NIMP-R14; ab-2557, Abcam) overnight at 4°C. They were rinsed with PBS and then incubated for 1 h with Alexa Fluor 488 conjugated secondary antibodies (1:200, Jackson Immuno Research, West Grove, PA), washed with PBS, and counterstained with 0.001% Evans Blue (Sigma, St. Louis, MO) for 1 min to quench autofluorescence. The slides were then rinsed with PBS and coverslipped with a fluorescent mounting medium with DAPI (H-1200, Vector Laboratories, Burlingame, CA). Images were taken using a Nikon Eclipse 55i microscope equipped with a DS-Fi1 color camera (Nikon Instruments Inc.)
For ICAM-1 immunohistochemistry, 3.0-μm-thick paraffin-embedded renal tissues were deparaffinized with xylene and rehydrated by exposure to graded concentrations (100% to 70%) of ethanol. The antigen was retrieved by microwave heating for 5 min in 10 mmol/l citrate buffer (pH 6.0). The sections were blocked using a serum-free reagent (X0909, Dako, Glostrup, Denmark) and incubated with a mouse monoclonal anti-ICAM-1 antibody (1:100, ab-2213, Abcam) overnight at 4°C. After washing, the sections were incubated with a biotinylated goat anti-mouse IgG (1:500, BA-9200, Vector Laboratories) for 30 min at room temperature, followed by a horseradish peroxidase conjugated streptavidin (1:2,000, 7100-05, SouthernBiotech, Birmingham, AL) for 30 min at room temperature. The slides were developed using 3,3′-diaminobenzidine (Dojindo, Kumamoto, Japan), counterstained with hematoxylin, and photographed by a light microscope (DMi8, Leica, Wetzlar, Germany).
Western blot.
The renal cortical tissues of SD and T2DN rats were homogenized in an ice-cold radioimmunoprecipitation assay buffer (R0278; Sigma-Aldrich, St. Louis, MO) containing protease and phosphatase inhibitors (88663, Thermo Scientific). Thirty micrograms of protein were separated on a 4%–20% polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk in Tris-buffered saline-Tween 20 at room temperature for 1 h and then incubated with either a rabbit anti-ICAM-1 antibody (1:10,000, ab-206398, Abcam), a mouse anti-E-selectin (1:100, sc-137054, Santa Cruz Biotechnology, Santa Cruz, CA), or anti-β-actin antibody (1:10,000, ab-6276, Abcam) overnight at 4°C, followed by incubation with a horseradish peroxidase conjugated goat anti-rabbit (1:10,000, ab-6721, Abcam) or rabbit anti-mouse secondary antibody (1:10,000, ab-97046, Abcam) for 1 h at room temperature. The membranes were developed using an ECL and the intensities of the bands determined using a ChemiDoc Imager system (Bio-Rad).
Immune cell infiltration.
The rats were anesthetized with isoflurane, and the kidneys were perfused with 300 ml of 0.9% saline solution via the left ventricle at 150 mmHg. The kidneys were collected and pressed through a 250-µm sieve. The collected tissue was digested in 10 ml RPMI 1640 containing 100 U/ml collagenase IV and 200 U/ml DNAse I in PBS for 60 min at 37°C. The cell suspension was filtered through a 70-µm cell strainer and spun at 300 g for 10 min at 4°C. The pellet was resuspended in PBS and passed through a 40-µm cell strainer. The filtrate was collected, layered on a Ficoll-Paque solution (density 1.084), and centrifuged at 450 g for 30 min. The suspended layers were collected and mixed with Dulbecco's phosphate-buffered saline. The cell suspension was spun at 300 g for 10 min at 4°C. The immune cells in the pellet were resuspended in 1 ml of fluorescence-activated cell sorting (FACS) buffer and counted.
Immunofluorescence staining and FACS.
The leukocytes were spun down and the supernatant discarded. The cell surface was blocked with FACS buffer plus 0.5% mouse and goat serum for 10 min at 4°C. The cells were spun down and labeled with the following fluorescence conjugated antibodies (Miltenyi, Auburn, CA): VioBlue-CD4 (130–107–622), PE-Vio770-CD3 (130–103–773), VioBright-FITC-CD45R (130–106–778), PE-CD68 (130–102–724), PerCP-Vio700-CD45 (130–107–793), and APC-CD8a (130–108–882). FACS was conducted using a MACSQuant Analyzer (Miltenyi) and analyzed with MACSQuantify software (Miltenyi).
Assessment of renal hypoxia.
Hypoxic regions of the kidney were labeled in vivo using 2-pimonidizole, Hypoxyprobe-1 Plus Kit (Hypoxyprobe Inc., Burlington, MA), as previously described (6, 27, 50). The rats received a 60 mg/kg bw iv injection of Hypoxyprobe-TM-1 one hour before euthanasia. After euthanasia, the kidneys were collected and fixed overnight in 10% formalin, and paraffin sections were prepared. The slides were immunostained with an FITC-conjugated Hypoxyprobe-1 monoclonal antibody as described above.
Intrarenal hemodynamics.
These experiments were performed to determine if the increased susceptibility to renal IR was associated with changes in intrarenal hemodynamics. Rats were anesthetized with isoflurane and placed on a warming table to maintain body temperature at 37°C. Catheters were placed in the femoral artery for measurement of mean arterial pressure (MAP). The left kidney was exposed, and two optical fibers were implanted 4 mm into the kidney for measurement of outer MBF using a dual channel laser Doppler flowmeter (LDF; model 5000, Perimed Inc., Ardmore, PA) as previously described (51). Cortical blood flow (CBF) was measured at two sites using a second instrument equipped with 1-mm probes held in static position with micromanipulators 1 mm above the renal cortex. After surgery and a 30-min stabilization period, baseline CBF and MBF LDF signals were recorded. Then the blood supply to both kidneys was occluded for 30 min. CBF and MBF LDF signals were then recorded during the ischemic period and for 3 h following reperfusion.
Measurement of GFR following IR.
Rats were anesthetized with isoflurane, and catheters were placed in the right femoral artery and vein for measurement of MAP and intravenous infusions. The rats received an infusion of 0.9% NaCl solution containing 2% bovine serum albumin and 2 mg/ml fluorescein isothiocyanate-labeled inulin at a rate of 6 ml/h throughout the experiment for measurement of GFR. After surgery and a 30-min equilibration period, blood and urine samples were collected every 30 min following renal IR. The concentrations of inulin in urine and plasma samples were determined using a fluorescent microplate reader (BioTek Instruments).
Statistics.
All data are presented as mean values ± 1 SE. The significance of differences in mean values was determined using an unpaired t-test for two groups, or one- or two-way analysis of variance for repeated measures for multiple groups followed by a Holm-Sidak test for preplanned comparisons. A value of P < 0.05 using a two-tailed test was considered to be statistically significant.
RESULTS
Baseline blood pressure and plasma glucose concentrations in SD and T2DN rats.
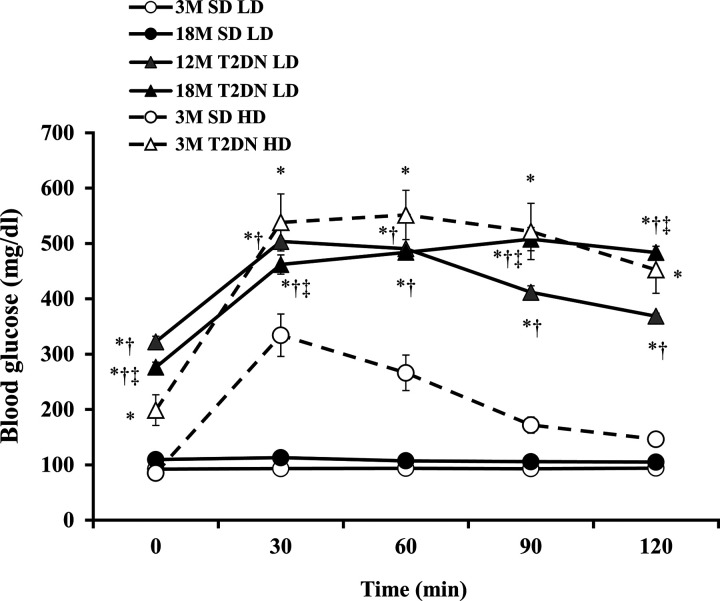
MAP was similar in young and elderly (3M and 18M) SD rats and averaged approximately 110 mmHg. Blood pressure was significantly higher in 12M and 18M T2DN rats and averaged ~140 mmHg. (Table 1). Fasting glucose (>250 mg/dl) and HbA1c levels (>10%) were significantly elevated in 12M and 18M T2DN rats in comparison with 3M and 18M SD control rats. Both 12M and 18M T2DN rats exhibited glucose intolerance as indicated by an increased area under the curve of the intraperitoneal glucose tolerance test relative to the values seen in 3M and 18M SD rats following administration of 1 g/kg glucose challenge. (Fig. 1). The degree of glucose intolerance was significantly greater in 18M than in 12M T2DN rats, but there was no significant difference in the area under the curves in 3M and 18M SD rats (Fig. 1). These results were also confirmed in T2DN and SD rats following administration of a higher 5 g/kg glucose load.
Table 1.
Baseline of general parameters
| 3M SD | 18M SD | 12M T2DN | 18M T2DN | |
|---|---|---|---|---|
| Body weight, g | 316.7 ± 5.6 | 567.5 ± 13.6* | 418.3 ± 10.9*† | 424.2 ± 9.8*† |
| Kidney weight, g | 1.33 ± 0.03 | 2.28 ± 0.10* | 1.92 ± 0.06*† | 2.01 ± 0.06*† |
| Blood glucose, mg/dl | 92.3 ± 2.7 | 109.5 ± 1.9* | 322.3 ± 10.1*† | 292.0 ± 3.8*†‡ |
| HbA1c, % | 4.6 ± 0.2 | 5.1 ± 0.1* | 10.6 ± 0.1*† | 8.3 ± 0.2*†‡ |
| Systolic blood pressure, mmHg | 111.0 ± 3.4 | 108.3 ± 2.8 | 142.8 ± 1.5*† | 146.8 ± 2.0*† |
| Urine flow rate, ml/day | 11.7 ± 0.4 | 10.3 ± 0.6 | 22.9 ± 1.7*† | 21.7 ± 1.0*† |
| Proteinuria, mg/day | 13.8 ± 1.5 | 292.9 ± 46.6* | 294.9 ± 36.0* | 1,143.8 ± 202.2*†‡ |
| Plasma creatinine, mg/dl | 0.46 ± 0.03 | 0.50 ± 0.02 | 0.53 ± 0.01 | 1.01 ± 0.10*†‡ |
Values are means ± SE; n = 6 rats per group. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
P < 0.05 vs. corresponding 3M SD,
P < 0.05 vs. corresponding 18M SD,
P < 0.05 vs. corresponding 12M T2DN.
Fig. 1.
Comparison of glucose tolerance in 3- and 18-mo-old Sprague-Dawley (SD) and T2DN rats. Glycemic profiles of male young adult (3 mo old; 3M) and elderly (18 mo old; 18M) SD rats, and middle-aged (12 mo old; 12M) and elderly (18M) T2DN rats are compared after intraperitoneal administration of glucose (LD) at time 0. These results were confirmed in T2DN and SD rats following administration of a higher 5 g/kg glucose load (HD). Data are means ± 1 SE; n = 6 rats per group. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. LD, low dose; HD, high dose; T2DN, type 2 diabetic nephropathy rat.
Baseline renal injury in SD and T2DN rats.
Daily urine output was higher in T2DN than in SD rats. Proteinuria increased from 14 ± 2 to 293 ± 47 mg/day in SD rats as they increased in age from 3 to 18 mo old. The degree of proteinuria was greater in T2DN than young SD rats, reaching 295 ± 36 mg/day in the 12M and 1,144 ± 202 mg/day in the 18M rats. Baseline plasma creatinine concentration was in the normal range (<0.5 mg/dl) and similar in 3M and 18M SD and in 12M T2DN rats (Table 1). However, it was significantly elevated in 18M T2DN rats (1.01 ± 0.1 mg/dl).
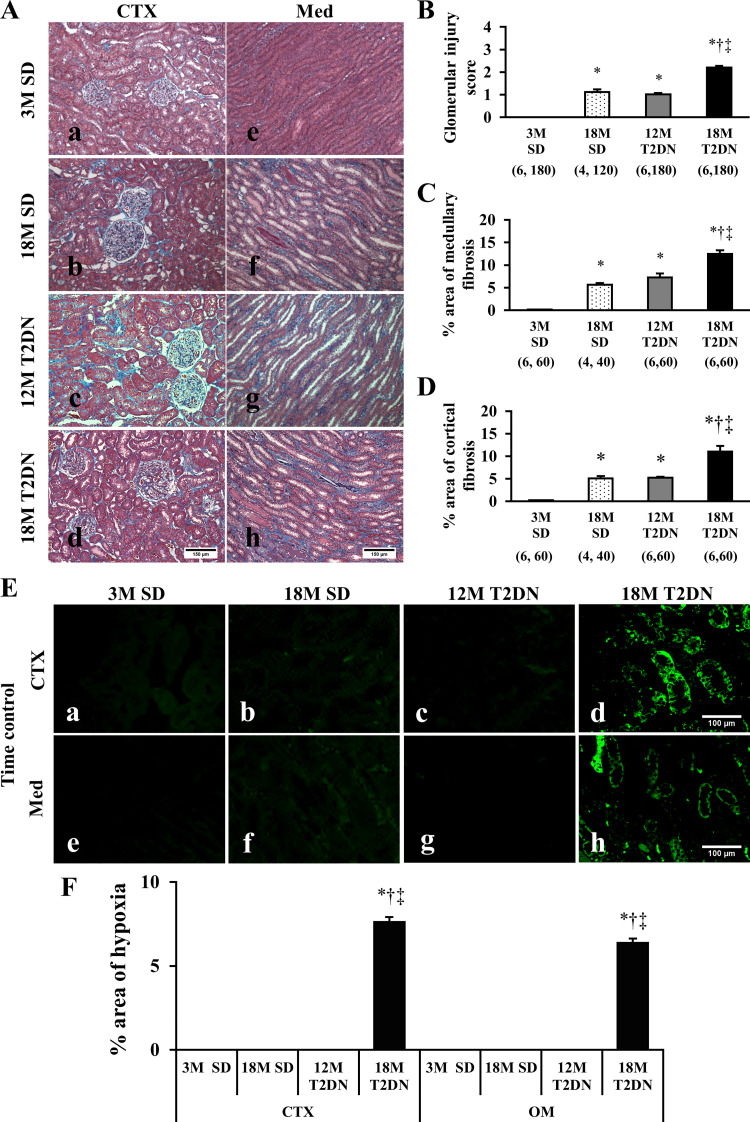
The kidneys of young SD rats did not exhibit any renal injury (Fig. 2A). The kidneys of 18M SD and 12M T2DN rats exhibited mild glomerular mesangial matrix expansion and renal interstitial fibrosis. Elderly 18M T2DN rats exhibited the classic characteristics of diabetic nephropathy, including thickening of the glomerular basement membrane, focal glomerulosclerosis, and renal interstitial fibrosis (Fig. 2A). Glomerular injury scores were significantly elevated in 18M T2DN rats relative to the levels seen in 3M and 18M SD and 12M T2DN rats (Fig. 2B). The percentage renal cortical and medullary fibrosis was also significantly higher in 18M T2DN rats than in 3M and 18M SD or 12M T2DN rats (Fig. 2, C and D). Hypoxyprobe staining indicated that there are regions of hypoxia (<10 mmHg) in the renal cortex and outer medulla of 18M T2DN rats, but not in 3M or 18M SD or 12M T2DN rats (Fig. 2, E and F).
Fig. 2.
Baseline of renal tissue damage and hypoxia in SD and T2DN rats. A–D: representative microphotographs of the renal cortex (CTX) and medulla (Med) stained with Masson’s trichrome in SD and T2DN rats (A). Magnification ×200. Scale bars = 150 μm. Degree of renal fibrosis was determined by measuring the percentage of the area stained blue on 10 random, nonoverlapping fields at ×200 magnification in the CTX and Med of each animal. Mean values ± 1 SE are presented. Numbers in parentheses indicate the number of glomeruli or areas scored per number of rats studied in each group. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. E and F: area of renal hypoxia was determined by Hypoxyprobe staining (E). Hypoxic tissue was observed only in CTX and Med of elderly 18M T2DN rats. Scale bar = 100 μm. Analysis of the percentage of the area stained with Hypoxyprobe was performed on 10 random, nonoverlapping fields in the CTX and Med of each animal. Mean values ± 1 SE from 4–6 rats per group are presented. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; OM, outer medulla; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
IR injury in SD and T2DN rats.
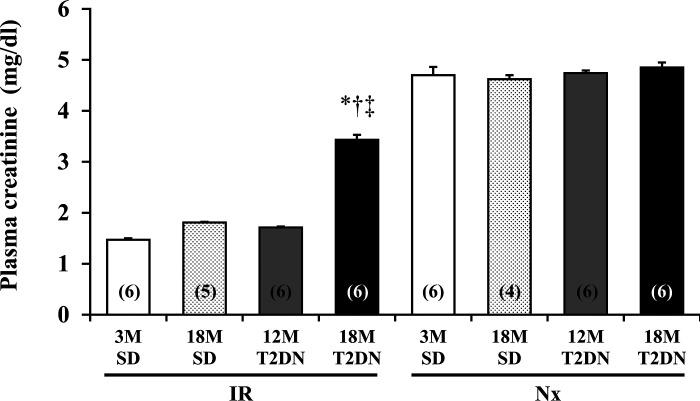
Twenty-four hours after bilateral renal IR, the plasma creatinine level was significantly higher in 18M T2DN rats (3.43 ± 0.10 mg/dl) than in 3M and 18M SD or 12M T2DN rats (Fig. 3). There was no statistical difference in plasma creatinine levels in 3M and18M SD rats or 12M T2DN rats after renal IR.
Fig. 3.
Comparison of the degree of renal injury in SD and T2DN rats 24 h following 30 min of bilateral renal ischemia. Bilaterally nephrectomized (Nx) rats served as an additional complete renal failure control within each group. Mean values ± 1 SE are presented. Numbers in parentheses indicate the number of rats studied per group. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; IR, ischemia-reperfusion; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
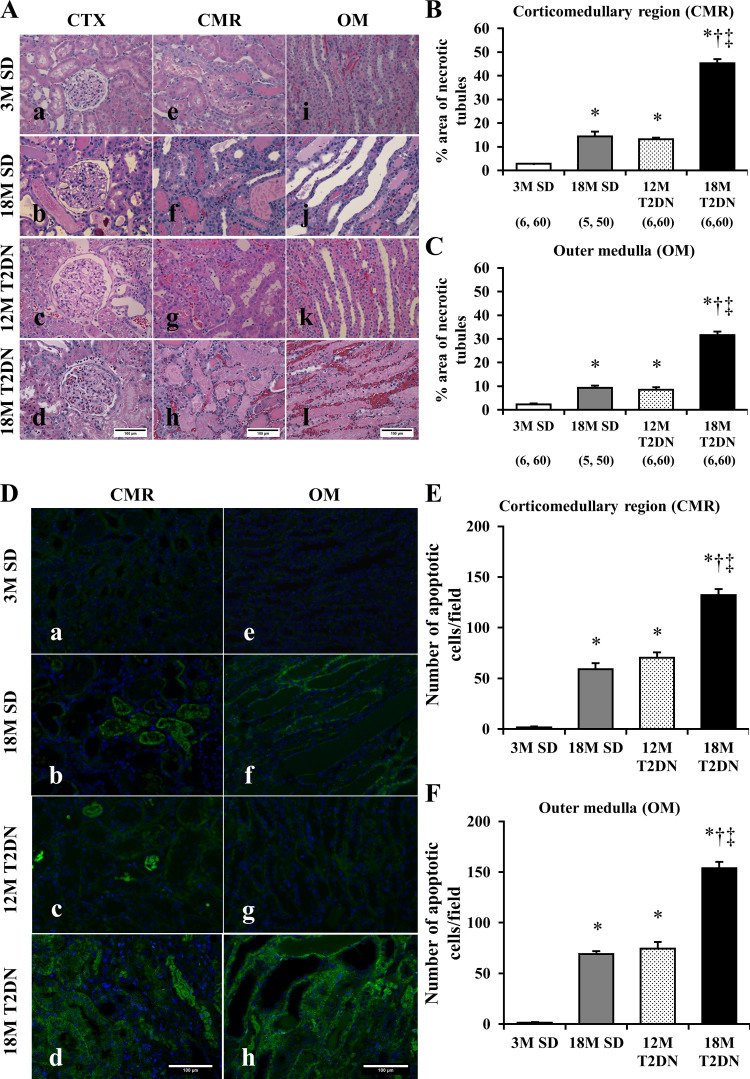
A comparison of the histology of the kidney after IR injury in T2DN and SD rats is presented in Fig. 4. Diffuse tubular cell denudation, tubular cell necrosis, intratubular debris, and tubular casts were present in the corticomedullary region and outer medulla 24 h after IR in 12M and 18M T2DN and in 18M SD rats (Fig. 4A). In contrast, the degree of renal injury was significantly less in 3M SD rats. The area of necrotic tubules was significantly greater in the corticomedullary region and outer medulla of 12M T2DN and 18M SD rats relative to 3M SD rats (Fig. 4, B and C). The degree of renal injury was significantly greater in the 18M T2DN rats than in all the other groups.
Fig. 4.
Comparison of tubular injury and apoptotic cells in SD and T2DN rats 24 h following 30 min of bilateral renal ischemia. A: sections were stained with hematoxylin-eosin. Severity of the renal injury was greater in 18M T2DN rats with severe tubular necrosis and exfoliation of tubular cells (d, h, and l). Renal tubular necrosis was quantified by detecting eosin red fluorescence of necrotic tubular cells. B and C: areas of renal tubular injury in the corticomedullary region (CMR) and outer medulla (OM) were significantly greater in 18M T2DN rats compared with the values seen in 3M SD, 18M SD, or 12M T2DN rats. Scale bar = 100 μm. D–F: numbers of apoptotic tubular cells in CMR and OM were significantly larger in 18M T2DN rats compared with the values seen in 3M SD, 18M SD, or 12M T2DN rats. Scale bar = 100 μm. Quantitative analysis was performed on 10 random, nonoverlapping fields in the CMR and the OM of each rat. Mean values ± 1 SE are presented. Numbers in parentheses indicate the number of areas scored/number of rats studied per group. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; CTX, renal cortex; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
The number of caspase-3 positive cells in the corticomedullary region and outer medulla in 18M SD and 12M T2DM rats was significantly higher than that seen in 3M SD rats following IR injury (Fig. 4, D–F). The number of apoptotic cells was significantly greater in 18M T2DN rats than in all the other groups.
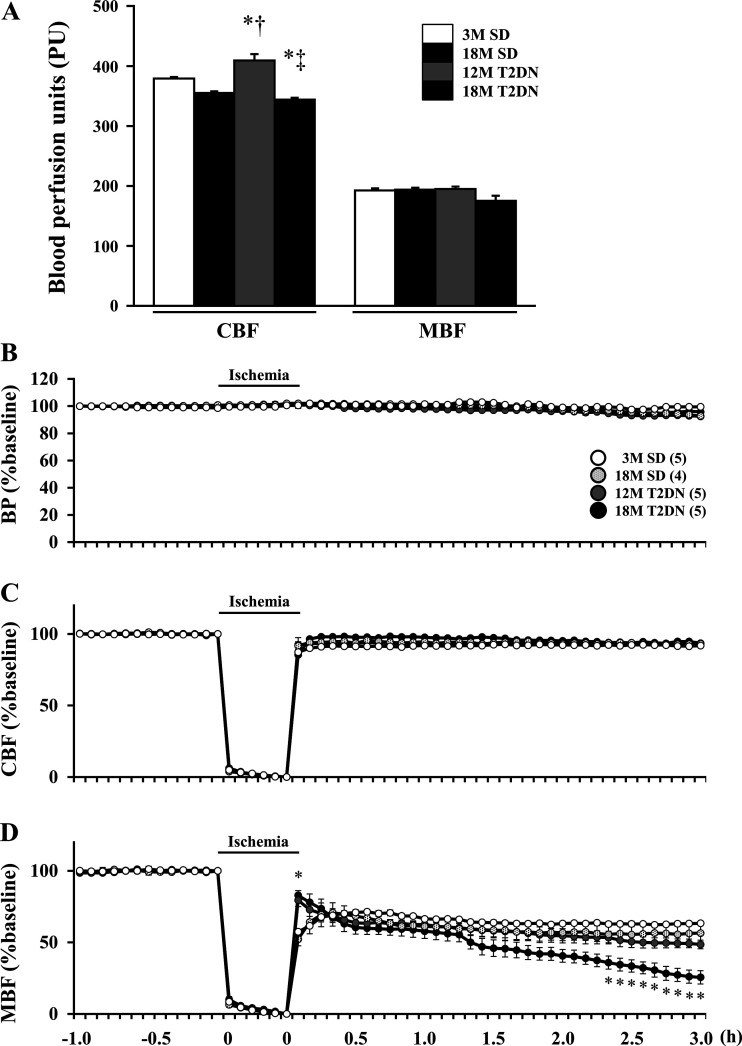
Intrarenal blood flow following IR injury in SD and T2DN rats.
Baseline CBF LDF signal was higher in 12M T2DN rats than in both young and aged SD rats (Fig. 5A), and it was significantly lower in 18M T2DN rats relative to the other groups. In contrast, baseline MBF was similar in 3M and 18M SD rats and 12M and 18M T2DN rats. CBF fell during the ischemic period and rapidly returned to control in 3M and 18M SD rats, as well as in 12M and 18M T2DN rats (Fig. 5C). MBF also recovered to 65%–70% of control following ischemia in all groups (Fig. 5D). MBF remained unchanged at 65% of control in 3M SD rats but fell to 50% of baseline in 18M SD and 12M T2DN rats and to 25% of baseline in 18M T2DN rats (Fig. 5D). MAP was stable during the ischemic period and for 3 h following reperfusion in all of the groups (Fig. 5B).
Fig. 5.
Comparison of baseline cortical and medullary blood flow (CBF and MBF) signals and time course of changes in CBF and MBF following IR in SD and T2DN rats. A–D: CBF and MBF were measured by laser Doppler flowmetry and mean arterial pressure (BP) was measured in SD and T2DN rats during a control period, during a 30 min bilateral ischemia period, and for 3 h of reperfusion. Mean values ± 1 SE from 4–5 rats per group are presented. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; IR, ischemia-reperfusion; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
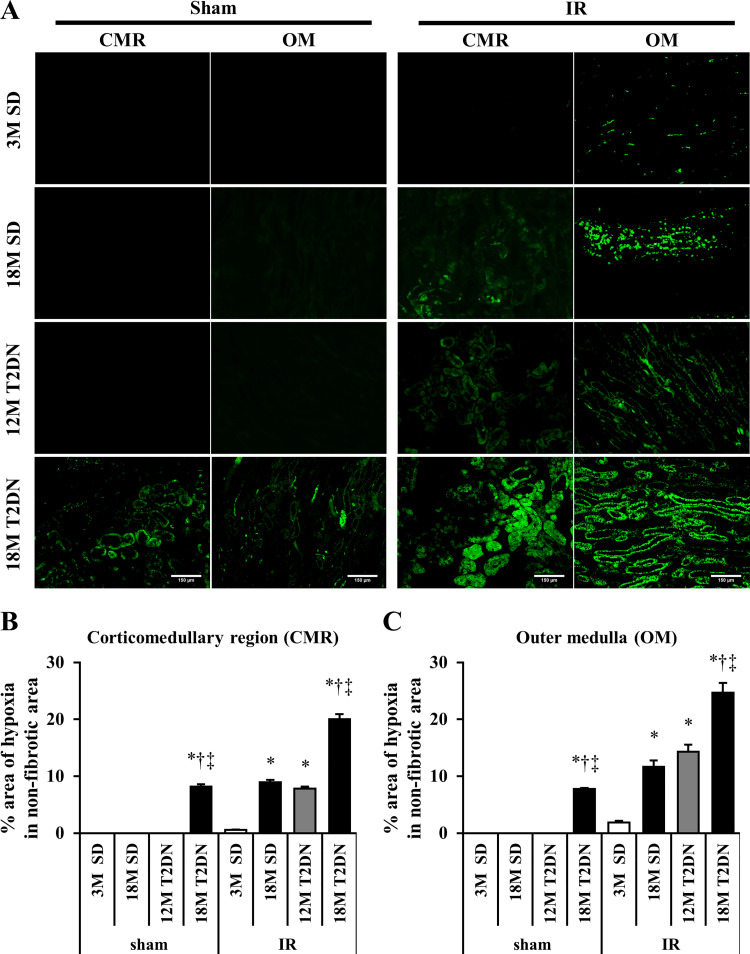
A comparison of the degree of hypoxia in the corticomedullary region and outer medulla of 3M and 18M SD and 12M and 18M T2DN rats 24 h following renal IR injury is presented in Fig. 6. The hypoxic area was greater in the outer medulla of 12M T2DN and 18M SD rats than in young 3M SD control rats (Fig. 6). Moreover, the hypoxic area in both the corticomedullary region and the outer medulla was much greater in 18M T2DN rats than in all other groups (Fig. 6, B and C).
Fig. 6.
Comparison of tissue hypoxia 24 h following 30 min of bilateral renal ischemia in SD and T2DN rats. A: area of renal hypoxia was determined by Hypoxyprobe staining. Percentage area of hypoxic tissue was significantly larger in the cortical medullary region (CMR) and outer medulla (OM) of 18M T2DN rats than that seen in 3M SD, 18M SD, or 12M T2DN rats. Scale bar = 150 μm. B and C: quantitative analysis of the percentage area stained with Hypoxyprobe was performed on 10 random nonoverlapping fields in the renal CMR and OM in each rat. Mean values ± 1 SE from 5 rats per group are presented. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; IR, ischemia-reperfusion; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
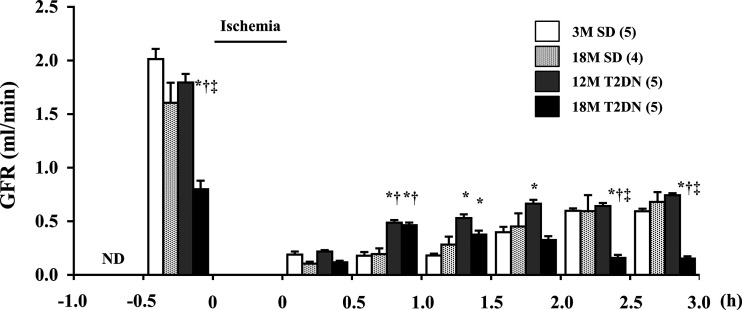
Time course of changes in GFR following IR injury in SD and T2DN rats.
Baseline GFR was significantly lower in 12M T2DN and 18M SD rats in comparison with the level seen in 3M SD control rats (Fig. 7). Baseline GFR was 50% lower in 18M T2DN rats relative to all other groups. GFR fell by more than 90% immediately after reperfusion in all groups. It gradually recovered to ~25% of control over the next 3 h in the 3M and 18M SD rats. GFR was greater in T2DN rats than in the SD controls during the first hour after reperfusion of the kidney. GFR remained at 25% of control in the 12M T2DN rats over the next 2 h, but it fell to 10% of control over this period in the 18M T2DN rats. The secondary fall in GFR in the 18M T2DN rats corresponds with the delayed fall MBF seen in this group.
Fig. 7.
Time course of changes in glomerular filtration rate (GFR) following 30 min of bilateral renal ischemia in SD and T2DN rats. GFR, measured by the clearance of inulin, was measured in SD and T2DN rats before, during, and for 3 h after reperfusion. Mean values ± 1 SE from 5 rats per group are presented. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat; ND, not determined.
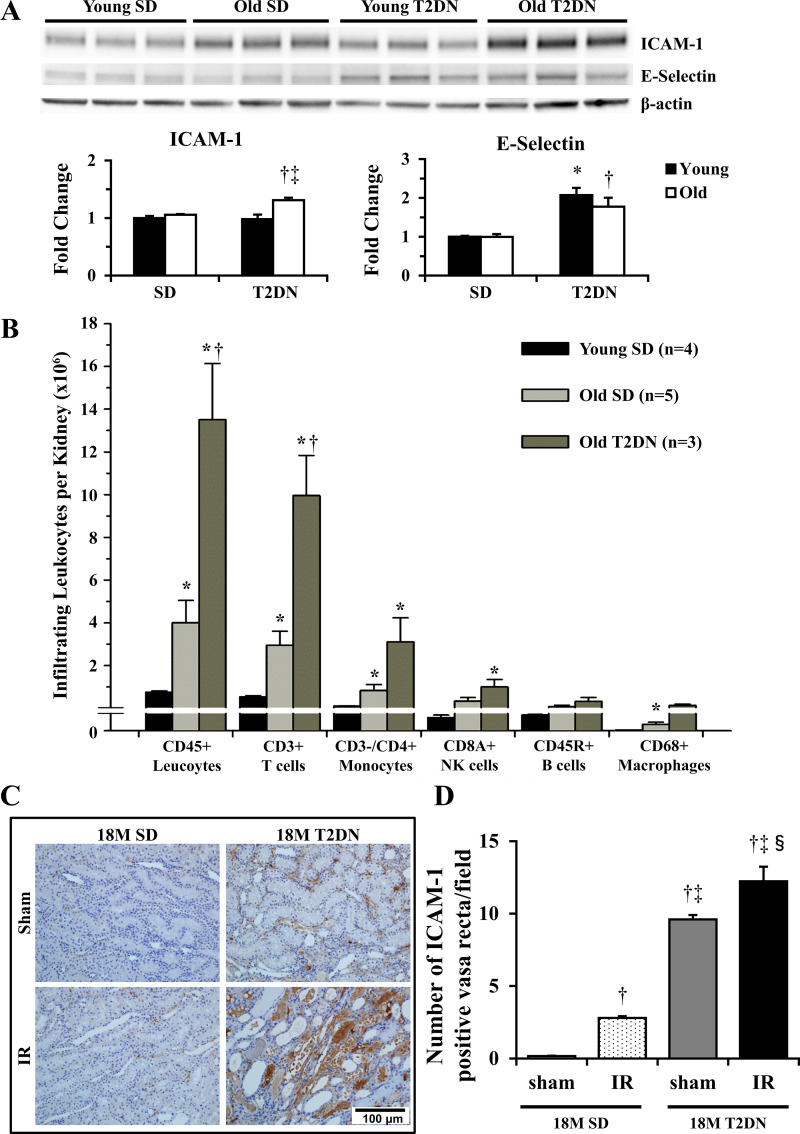
Comparison of renal inflammation in SD and T2DN rats.
To investigate the mechanism by which IR caused more severe renal injury in 18M T2DN rats, we examined the expression of cell adhesion molecules in the kidney of young and old SD and T2DN rats. Western blots showed that the basal expression of the adhesion molecules ICAM-1 and E-selectin was significantly increased in the kidney of elderly T2DN relative to age-matched SD and young SD and middle-aged T2DN rats before the induction of IR (Fig. 8A). This was associated with increased number of infiltrating leukocytes in the kidney of elderly T2DN rats (Fig. 8B). We further examined the number of ICAM-1 positive vasa recta before and after renal IR. The baseline expression of ICAM-1 was elevated in vasa recta capillaries (Fig. 8, C and D) in elderly T2DN rats, and it increased further following renal IR (Fig. 8, C and D).
Fig. 8.
Comparison of renal inflammation in SD and T2DN rats. A: expression of ICAM-1 and E-selectin in the kidney of elderly T2DN rats was significantly increased compared with that of age-matched SD rats. Mean values ± 1 SE from 3–7 rats per group are presented. *P < 0.05 compared with the corresponding value in young SD rats. †P < 0.05 compared with the corresponding value in age matched elderly SD rats. ‡P < 0.05 compared with the corresponding value in young T2DN rats. B: number of infiltrating leukocytes in the kidneys of SD and T2DN rats measured by FACS. Mean values ± 1 SE from 3–5 rats per group are presented. *P < 0.05 compared with young SD rats. †P < 0.05 compared with 18M elderly SD rats. C and D: number of ICAM-1 positive vasa recta in outer medulla (OM) was significantly elevated in 18M elderly T2DN rats as compared with the corresponding value seen in age-matched SD rats. Scale bar = 100 μm. Quantitative analysis was performed on 10 random nonoverlapping fields per rat in the renal OM. Mean values ± 1 SE from 3 rats per group are presented. †P < 0.05 compared with the corresponding value in sham 18M SD rats. ‡P < 0.05 compared with the corresponding value in 18M SD rats following IR. §P < 0.05 compared with the corresponding value in sham 18M T2DN rats. 12 M, 12 mo old; 18M, 18 mo old; FACS, fluorescence-activated cell sorting; IR, ischemia-reperfusion; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
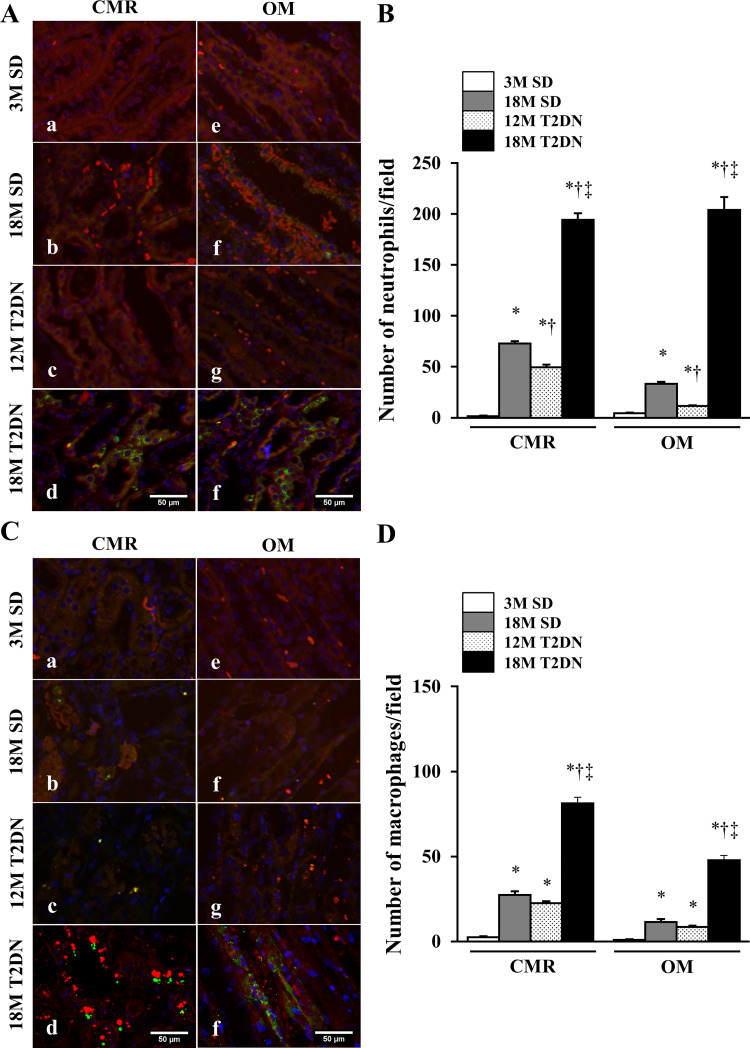
We also examined neutrophil and macrophage infiltration 3 and 24 h after renal IR. The number of NIMP-R14 positive neutrophils in the corticomedullary region and outer medulla was significantly higher in 18M T2DN rats than in 3M and 12M SD rats and 12M T2DN rats (Fig. 9, A and B). The number of ED1 positive macrophages in the corticomedullary region and outer medulla of 18M T2DN rats was also significantly higher than in all the other groups (Fig. 9, C and D).
Fig. 9.
Comparison of the number of infiltrating neutrophils (CD45+ cells) and macrophages (CD68+ cells) in SD and T2DN rats 3 h following 30 min of ischemia and reperfusion. A and B: number of neutrophils in the corticomedullary region (CMR) and outer medulla (OM) was counted in 10 random nonoverlapping fields per rat. C and D: number of macrophages in the CMR and OM was counted in 10 random nonoverlapping fields per rat. Mean values ± 1 SE from 5 rats per group are presented. *P < 0.05 compared with the corresponding value in 3M SD rats. †P < 0.05 compared with the corresponding value in 18M SD rats. ‡P < 0.05 compared with the corresponding value in 12M T2DN rats. 3M, 3 mo old; 12 M, 12 mo old; 18M, 18 mo old; SD, Sprague-Dawley; T2DN, type 2 diabetic nephropathy rat.
DISCUSSION
Aging and CKD are the risk factors for AKI (24, 40, 46). The incidence and severity of AKI in human patients increases with age (3, 46–48). Previous studies have also indicated that DM is associated with higher risks for AKI (8, 17, 21, 24, 30). In addition, Otsuka Long-Evans Tokushima Fatty rats, a model of type 2 DM, suffer more severe renal IR injury (26). However, the mechanisms involved remain to be determined. Therefore the present study compared the degree of renal injury in middle-aged (12M) and elderly (18M) T2DN rats with mild versus severe diabetic nephropathy and in young (3M) and age-matched elderly, non-diabetic SD rats following 30 min of bilateral renal IR.
The results indicate that that elderly 18M SD and middle-aged 12M T2DN rats exhibited a similar mild degree of baseline renal injury, as reflected by an elevation in proteinuria, glomerular matrix expansion, and renal fibrosis as compared with young SD rats. However, plasma creatinine concentration and GFR were not significantly altered in young versus elderly SD and middle-aged T2DN rats. In contrast, the elderly 18M T2DN rats exhibited more severe diabetic nephropathy as evidenced by markedly elevated protein excretion, a 50% decline in baseline GFR, elevated plasma creatinine level, severe glomerular injury, renal fibrosis, tubular damage, and inflammation. Baseline cortical perfusion was similar in 3M and 18M SD rats. However, cortical perfusion was elevated in 12M T2DN rats and reduced in old T2DN rats with diabetic nephropathy. The elevated CBF in the 12M T2DN rats is consistent with renal hypertrophy and hyperfiltration typically seen in the early stage of diabetes, whereas the reduced CBF in 18M T2DN rats is consistent with the progressive fall in GFR in patients and animal models with advanced diabetic nephropathy (2, 34).
The degree of renal injury following IR correlated well with baseline GFRs and plasma creatinine concentrations in the young and elderly SD and middle-aged and elderly T2DN rats. The rise in plasma creatinine concentration following IR was similar in young and elderly SD and middle-aged T2DN rats despite the fact that baseline proteinuria was higher in elderly SD and middle-aged T2DN rats. The only differences among the groups were that the degree of tubular necrosis and apoptotic cells were significantly higher in the elderly SD and middle-aged T2DN rats following IR than in the young SD rats. In contrast, the increase in plasma creatinine concentration, fall in GFR, and degree of tubular necrosis were significantly elevated in the elderly T2DN rats with pre-existing diabetic nephropathy than the other three groups. These findings suggest that the degree of pre-existing CKD, but not aging or DM, is the most important predicator of the severity of IR-induced injury. Our results are also consistent with previous clinical studies suggesting that the severity of pre-existing CKD as defined by a reduction in estimated GFR and elevated protein excretion is associated with increased risk of AKI in elderly patients both with and without diabetes (4, 11, 12).
A recent study by Shi et al. (35) reported that the degree of IR injury in type 2 db/db mice is associated with delayed recovery of cortical and medullary blood flow for ~10 min following reperfusion. In our study, CBF rapidly and fully recovered in both the non-diabetic and diabetic animals. Similarly, MBF rapidly returned to values ~65% of control in all groups of rats. The only difference between the groups was that there were vasocongestion and a progressive secondary fall in MBF that developed 2 h following renal IR in the 18M T2DN rats. This was associated with prolonged renal medullary ischemia that can exacerbate tubular epithelial cell injury and may have enhanced the severity of IR injury. The secondary fall of MBF was not due to renal vasoconstriction, since CBF fully recovered immediately after IR. It was more likely caused by reduction in MBF secondary to endothelial dysfunction (10), inflammation (20, 33), and elevated oxidative stress (28, 41), all of which have been reported in DM.
Another important finding is that GFR failed to recover in 18M T2DN rats to the same extent as that seen 18M SD and 12M T2DN rats. This was not due to changes in renal hemodynamics, since CBF fully recovered after IR in all groups. Nor does it appear to be related to a decrease in glomerular capillary pressure secondary to dilation of efferent arteriole, since in this case CBF should have then been elevated. The most likely possibility is tubular obstruction and elevated tubular pressure due to pre-existing CKD in old T2DN rats that enhanced IR-induced proximal tubular necrosis.
Endothelial injury and dysfunction have been reported to play an important role in the extension phase of AKI (25). Increased expression of adhesion molecules on endothelial cells has been observed after ischemic injury, whereas inhibition of these molecules decreases binding of leukocytes and renal injury (15, 38). Our results indicate that the basal expression of cell adhesion molecules ICAM-1 and E-selectin were significantly elevated in the kidney of elderly T2DN rats compared with the age-matched SD rats. This was associated with an increased number of infiltrating leukocytes. These results are consistent with the view that the expression of adhesion molecules and the number of infiltrating leukocytes are elevated in the kidney of elderly T2DN rats with pre-existing renal injury. Thus, the kidney is primed for greater vasocongestion, more prolonged ischemia, and enhanced injury following IR.
The heightened inflammatory response increases the susceptibility of IR injury in diabetic mice (7), which is consistent with our results of the current finding that the numbers of infiltrating neutrophils and macrophages were significantly higher in 18M T2DN rats than in the other groups. Increased adherence of leukocytes to endothelial cells following IR injury may cause plugging of vasa recta capillaries (vasocongestion). Moreover, hypoxia in the medulla might be more pronounced in diabetic animals because of the increased workload of the Na+, K+-ATPase secondary to hyperfiltration and increased delivery of glucose and sodium to the pars recta of the proximal tubule (44, 45). This could contribute to the enhanced susceptibility to hypoxic injury following renal IR in DM rats.
Overall, the present results indicate that pre-existing diabetic nephropathy and CKD, but not hyperglycemia, elevated blood pressure, or aging alone, contribute to the increased susceptibility to renal IR in elderly T2DN rats. The increased susceptibility to ischemic AKI is associated with a secondary fall in MBF, prolonged tissue hypoxia, and increased renal inflammation. In addition, in the setting of DM and aging, there is a significant interaction between the baseline degree of CKD indicated by GFR and plasma creatinine concentration and the susceptibility to ischemic AKI. This finding is consistent with clinical findings linking aging and the degree of pre-existing CKD to the incidence and severity of AKI (36, 46). Overall, the present results suggest that therapies that improve renal MBF, like calcium channel blockers or agents that reduce the inflammatory response, may reduce the severity of AKI in patients with diabetes with moderate CKD.
GRANTS
This work was supported by NIH Grants DK-104184, HL-36279, and HL-138685 (to R. J. Roman); AG-050049 (to F. Fan); and GM-104357 (to R. J. Roman and F. Fan); American Heart Association Grant 16GRNT31200036 (to F. Fan); and Ministry of Education, Culture, Sports, Science, and Technology Grant 17K01462 (to Y. Muroya).
DISCLAIMERS
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M., F.F., and R.J.R. conceived and designed research; Y.M., X.H., L.F., and R.X. performed experiments; Y.M., X.H., L.F., and R.J.R. analyzed data; Y.M., X.H., L.F., F.F., and R.J.R. interpreted results of experiments; Y.M., X.H., and L.F. prepared figures; Y.M. and R.J.R. drafted manuscript; Y.M., X.H., L.F., S.W., R.X., F.F., and R.J.R. edited and revised manuscript; Y.M., X.H., L.F., S.W., R.X., F.F., and R.J.R. approved final version of manuscript.
REFERENCES
- 1.Aydin Z, van Zonneveld AJ, de Fijter JW, Rabelink TJ. New horizons in prevention and treatment of ischaemic injury to kidney transplants. Nephrol Dial Transplant 22: 342–346, 2007. doi: 10.1093/ndt/gfl690. [DOI] [PubMed] [Google Scholar]
- 2.Barai S, Gambhir S, Prasad N, Sharma RK, Ora M. Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton) 15: 350–353, 2010. doi: 10.1111/j.1440-1797.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi A, Ballestri M, Rapanà R, Lucchi L, Borella P, Leonelli M, Furci L, Lusvarghi E. Acute renal failure of medical type in an elderly population. Nephrol Dial Transplant 13, Suppl 7: 25–29, 1998. doi: 10.1093/ndt/13.suppl_7.25. [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, Cho KC, Hsu CY. Acute kidney injury in the elderly: predisposition to chronic kidney disease and vice versa. Nephron Clin Pract 119, Suppl 1: c19–c24, 2011. doi: 10.1159/000328023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St. Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2008 Annual Data Report. Am J Kidney Dis 53, Suppl: A6–A7, 2009. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Efrati S, Berman S, Hamad RA, Siman-Tov Y, Ilgiyaev E, Maslyakov I, Weissgarten J. Effect of captopril treatment on recuperation from ischemia/reperfusion-induced acute renal injury. Nephrol Dial Transplant 27: 136–145, 2012. doi: 10.1093/ndt/gfr256. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Zhang B, Ramesh G, Betterly D, Tadagavadi RK, Wang W, Reeves WB. TNF-α mediates increased susceptibility to ischemic AKI in diabetes. Am J Physiol Renal Physiol 304: F515–F521, 2013. doi: 10.1152/ajprenal.00533.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girman CJ, Kou TD, Brodovicz K, Alexander CM, O’Neill EA, Engel S, Williams-Herman DE, Katz L. Risk of acute renal failure in patients with type 2 diabetes mellitus. Diabet Med 29: 614–621, 2012. doi: 10.1111/j.1464-5491.2011.03498.x. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Goligorsky MS. Whispers and shouts in the pathogenesis of acute renal ischaemia. Nephrol Dial Transplant 20: 261–266, 2005. doi: 10.1093/ndt/gfh182. [DOI] [PubMed] [Google Scholar]
- 11.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol 21: 1757–1764, 2010. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson F, Phillips D, Talabani B, Wonnacott A, Meran S, Phillips AO. The impact of acute kidney injury in diabetes mellitus. Nephrology (Carlton) 21: 506–511, 2016. doi: 10.1111/nep.12649. [DOI] [PubMed] [Google Scholar]
- 15.Kelly KJ, Williams WW Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 97: 1056–1063, 1996. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SS, Kazmi WH, Abichandani R, Tighiouart H, Pereira BJ, Kausz AT. Health care utilization among patients with chronic kidney disease. Kidney Int 62: 229–236, 2002. doi: 10.1046/j.1523-1755.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 17.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA Jr. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 110: 505–515, 2009. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 18.Kojima N, Slaughter TN, Paige A, Kato S, Roman RJ, Williams JM. Comparison of the development diabetic induced renal disease in strains of Goto-Kakizaki rats. J Diabetes Metab 2013, Suppl 9: S9-005, 2013. doi: 10.4172/2155-6156.S9-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345: 464–472, 2013. doi: 10.1124/jpet.113.203869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lameire NH, Vanholder R. Pathophysiology of ischaemic acute renal failure. Best Pract Res Clin Anaesthesiol 18: 21–36, 2004. doi: 10.1016/j.bpa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RH, Grab JD, O’Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED; Society of Thoracic Surgeons National Cardiac Surgery Database Investigators . Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 114: 2208–2216, 2006. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 22.Melin J, Hellberg O, Akyürek LM, Källskog O, Larsson E, Fellström BC. Ischemia causes rapidly progressive nephropathy in the diabetic rat. Kidney Int 52: 985–991, 1997. doi: 10.1038/ki.1997.420. [DOI] [PubMed] [Google Scholar]
- 23.Melin J, Hellberg O, Larsson E, Zezina L, Fellström BC. Protective effect of insulin on ischemic renal injury in diabetes mellitus. Kidney Int 61: 1383–1392, 2002. doi: 10.1046/j.1523-1755.2002.00284.x. [DOI] [PubMed] [Google Scholar]
- 24.Mittalhenkle A, Stehman-Breen CO, Shlipak MG, Fried LF, Katz R, Young BA, Seliger S, Gillen D, Newman AB, Psaty BM, Siscovick D. Cardiovascular risk factors and incident acute renal failure in older adults: the cardiovascular health study. Clin J Am Soc Nephrol 3: 450–456, 2008. doi: 10.2215/CJN.02610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int 66: 496–499, 2004. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 26.Muratsubaki S, Kuno A, Tanno M, Miki T, Yano T, Sugawara H, Shibata S, Abe K, Ishikawa S, Ohno K, Kimura Y, Tatekoshi Y, Nakata K, Ohwada W, Mizuno M, Miura T. Suppressed autophagic response underlies augmentation of renal ischemia/reperfusion injury by type 2 diabetes. Sci Rep 7: 5311, 2017. doi: 10.1038/s41598-017-05667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muroya Y, Fan F, Regner KR, Falck JR, Garrett MR, Juncos LA, Roman RJ. Deficiency in the formation of 20-hydroxyeicosatetraenoic acid enhances renal ischemia-reperfusion injury. J Am Soc Nephrol 26: 2460–2469, 2015. doi: 10.1681/ASN.2014090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilakantan V, Hilton G, Maenpaa C, Van Why SK, Pieper GM, Johnson CP, Shames BD. Favorable balance of anti-oxidant/pro-oxidant systems and ablated oxidative stress in Brown Norway rats in renal ischemia-reperfusion injury. Mol Cell Biochem 304: 1–11, 2007. doi: 10.1007/s11010-007-9480-z. [DOI] [PubMed] [Google Scholar]
- 29.Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes 53: 735–742, 2004. doi: 10.2337/diabetes.53.3.735. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira JF, Silva CA, Barbieri CD, Oliveira GM, Zanetta DM, Burdmann EA. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob Agents Chemother 53: 2887–2891, 2009. doi: 10.1128/AAC.01430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr Opin Nephrol Hypertens 21: 33–38, 2012. doi: 10.1097/MNH.0b013e32834d085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int 75: 511–517, 2009. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlichting CL, Schareck WD, Weis M. Renal ischemia-reperfusion injury: new implications of dendritic cell-endothelial cell interactions. Transplant Proc 38: 670–673, 2006. doi: 10.1016/j.transproceed.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 34.Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127: 94–100, 2014. doi: 10.1159/000363721. [DOI] [PubMed] [Google Scholar]
- 35.Shi H, Patschan D, Epstein T, Goligorsky MS, Winaver J. Delayed recovery of renal regional blood flow in diabetic mice subjected to acute ischemic kidney injury. Am J Physiol Renal Physiol 293: F1512–F1517, 2007. doi: 10.1152/ajprenal.00215.2007. [DOI] [PubMed] [Google Scholar]
- 36.Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol 5: 1690–1695, 2010. doi: 10.2215/CJN.00830110. [DOI] [PubMed] [Google Scholar]
- 37.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007. doi: 10.1152/ajprenal.00279.2006. [DOI] [PubMed] [Google Scholar]
- 38.Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 99: 2682–2690, 1997. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 334: 1448–1460, 1996. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 40.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators . Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 41.Villanueva S, Céspedes C, González AA, Vio CP, Velarde V. Effect of ischemic acute renal damage on the expression of COX-2 and oxidative stress-related elements in rat kidney. Am J Physiol Renal Physiol 292: F1364–F1371, 2007. doi: 10.1152/ajprenal.00344.2006. [DOI] [PubMed] [Google Scholar]
- 42.von Overbeck J, Saraga P, Gardiol D. An autofluorescence method for the diagnosis of early ischaemic myocardial lesions. A systematic study on 732 autopsies, including 182 cases of sudden death. Virchows Arch A Pathol Anat Histopathol 409: 535–542, 1986. doi: 10.1007/BF00705423. [DOI] [PubMed] [Google Scholar]
- 43.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 44.Wald H, Markowitz H, Zevin S, Popovtzer MM. Opposite effects of diabetes on nephrotoxic and ischemic acute tubular necrosis. Proc Soc Exp Biol Med 195: 51–56, 1990. doi: 10.3181/00379727-195-43117. [DOI] [PubMed] [Google Scholar]
- 45.Wald H, Scherzer P, Popovtzer MM. Enhanced renal tubular ouabain-sensitive ATPase in streptozotocin diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 251: F164–F170, 1986. doi: 10.1152/ajprenal.1986.251.1.F164. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci 15: 15358–15376, 2014. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol 21: 1436–1439, 2010. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Z, Zhu B, Anderson J, Fu H, LeNarz L. Resource utilization and healthcare costs for acute coronary syndrome patients with and without diabetes mellitus. J Med Econ 13: 748–759, 2010. doi: 10.3111/13696998.2010.535661. [DOI] [PubMed] [Google Scholar]
- 50.Zhong Z, Arteel GE, Connor HD, Yin M, Frankenberg MV, Stachlewitz RF, Raleigh JA, Mason RP, Thurman RG. Cyclosporin A increases hypoxia and free radical production in rat kidneys: prevention by dietary glycine. Am J Physiol Renal Physiol 275: F595–F604, 1998. doi: 10.1152/ajprenal.1998.275.4.F595. [DOI] [PubMed] [Google Scholar]
- 51.Zou AP, Muirhead EE, Cowley AW, Mattson DL, Falck JR, Jiang J, Roman RJ. Role of changes in renal hemodynamics and P-450 metabolites of arachidonic acid in the reversal of one-kidney, one clip hypertension. J Hypertens 13: 557–566, 1995. doi: 10.1097/00004872-199505000-00012. [DOI] [PubMed] [Google Scholar]