Abstract
Social stress causes profound urinary bladder dysfunction in children that often continues into adulthood. We previously discovered that the intensity and duration of social stress influences whether bladder dysfunction presents as overactivity or underactivity. The transient receptor potential vanilloid type 1 (TRPV1) channel is integral in causing stress-induced bladder overactivity by increasing bladder sensory outflow, but little is known about the development of stress-induced bladder underactivity. We sought to determine if TRPV1 channels are involved in bladder underactivity caused by stress. Voiding function, sensory nerve activity, and bladder wall remodeling were assessed in C57BL/6 and TRPV1 knockout mice exposed to intensified social stress using conscious cystometry, ex vivo afferent nerve recordings, and histology. Intensified social stress increased void volume, intermicturition interval, bladder volume, and bladder wall collagen content in C57BL/6 mice, indicative of bladder wall remodeling and underactive bladder. However, afferent nerve activity was unchanged and unaffected by the TRPV1 antagonist capsazepine. Interestingly, all indices of bladder function were unchanged in TRPV1 knockout mice in response to social stress, even though corticotrophin-releasing hormone expression in Barrington’s Nucleus still increased. These results suggest that TRPV1 channels in the periphery are a linchpin in the development of stress-induced bladder dysfunction, both with regard to increased sensory outflow that leads to overactive bladder and bladder wall decompensation that leads to underactive bladder. TRPV1 channels represent an intriguing target to prevent the development of stress-induced bladder dysfunction in children.
Keywords: social stress, TRPV1 channels, urinary bladder, voiding dysfunction
INTRODUCTION
The urinary bladder performs two primary functions: the storage of urine and the expulsion of urine at an appropriate time. Lower urinary tract dysfunction can occur as a direct result of multiple pathologies, including diabetes, overactive bladder, interstitial cystitis/bladder pain syndrome, bladder outlet obstruction, and spinal cord injury (20). While stress can exacerbate the symptoms of urinary bladder dysfunction in all of these conditions (18), social stress is particularly implicated in the development of bladder dysfunction in children (21). Previously, we demonstrated that social stress leads to symptoms of bladder overactivity in mice, characterized by a decrease in intermicturition interval and voided volume and an increase in sensory nerve outflow from the bladder (15). Increasing the intensity of social stress by prolonging exposure to the stressor instead caused an increase in both intermicturition interval and voided volume, symptoms that are indicative of bladder insufficiency or underactive bladder (16). Thus, it is unclear if stress-induced bladder underactivity represents a separate pathology from stress-induced overactivity or if prolonged overactivity leads to compensatory bladder remodeling, which results in decompensation and an underactive bladder.
Normal urinary tract function relies on the ability of the brain to interpret sensory information from the bladder and determine the appropriate time to void. Bladder sensory nerves not only contain many Aδ fibers but also C fibers that express transient receptor potential vanilloid family type 1 (TRPV1) channels (11). TRPV1 channels are part of a family of nonselective cation channels that activate in response to external stimuli, such as high temperature and low pH (8). The expression of TRPV1 channels is increased in suburothelial nerves during detrusor overactivity caused by spinal cord injury (14). TRPV1 channels have also been implicated in modulating pathophysiological responses to maternal separation stress, where TRPV1 inhibition prevented poststress colonic hypersensitivity (22). Previously, we discovered that TRPV1 channels are also critical to the development of social stress-induced overactive bladder, wherein intravesical instillation of the TRPV1 antagonist capsazepine reversed the symptoms of overactive bladder caused by social stress in mice (15). Whether or not TRPV1 channels play a role in the development of social stress-induced bladder underactivity, however, has not been explored.
In this study, we sought to determine the role that TRPV1 channels play in the development of bladder decompensation and underactivity in an intensified model of social stress. Mice exposed to intensified social stress had an increase in the intermicturition interval and voided volume, suggestive of bladder underactivity, as well as significant bladder wall remodeling. Interestingly, TRPV1 knockout (TRPV1-KO) mice showed no signs of social stress-induced bladder dysfunction; cystometric parameters were unchanged, and no appreciable bladder wall remodeling was present in TRPV1-KO mice exposed to social stress. However, corticotropin-releasing hormone (CRH) expression in Barrington’s Nucleus (BN) was still significantly increased in TRPV1-KO mice after social stress, suggesting that centrally mediated stress responses were intact. Together, these results suggest that TRPV1 channels in the periphery are a keystone in the development of social stress-induced bladder dysfunction with regard to increased afferent nerve activity that leads to overactive bladder and the bladder wall decompensation that ultimately leads to underactive bladder.
METHODS
Animal care and use.
Juvenile male C57BL/6 mice (4 to 6 wk old; 20–30 g; Charles River Canada, St. Constant, Canada) were used in these studies. Mice were housed in American Association for Accreditation of Laboratory Animal Care-accredited animal facilities and provided free access to food and water. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Vermont and were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed., National Institutes of Health, Bethesda, MD).
Social stress paradigm.
The “mild” social stress paradigm was followed as described previously (15, 16). Briefly, submissive 4-wk-old TRPV1-KO mice were placed in direct contact with C57BL/6 retired breeder mice (aggressor mice) for 5 min or until aggressor mice initiated traumatic aggressive behavior. Afterward, a clear plastic barrier with holes allowing for olfactory stimulation separated the mice for 1 h, after which the aggressor mouse was returned to his home cage. The process was repeated daily with a different aggressor mouse for 2 wk.
The “intensified” social stress paradigm was followed as described previously (15, 16). Briefly, submissive 4-wk-old C57BL/6 and TRPV1-KO mice were placed in direct contact with C57BL/6 retired breeder mice (aggressor mice) for 5 min or until aggressor mice initiated traumatic aggressive behavior. Afterward, a clear plastic barrier with holes allowing for olfactory stimulation separated the mice for 23 h. The process was repeated daily with a different aggressor mouse for 2 wk.
Conscious cystometry.
Cystometry was performed as described previously (15, 16). The urinary bladder was exposed through a lower midline abdominal incision under general anesthesia (2%–3% isoflurane). A saline-filled polyethylene-10 cannula with flared end was inserted into the bladder dome and secured with a 6–0 nylon suture. The distal end of the cannula was sealed. Muscle and skin layers were then closed. The distal part of the cannula was placed in the subcutaneous space, and the mice were returned to their home cages for 72 h to ensure complete recovery. Prior to cystometry, the mice were anesthetized, and the cannula was exteriorized. After regaining consciousness, mice were placed unrestrained in recording cages for the measurement of intravesical pressure changes during filling using a Small Animal Cystometry System (Med Associates, St. Albans, VT). Saline solution alone or saline with the TRPV1 antagonist capsazepine (10 μM) was infused at a rate of 25 μl/min. At least 4 reproducible micturition cycles were recorded after an initial stabilization period of 25 to 30 min. Voided saline was collected to determine void volume. Intermicturition interval and the frequency of nonvoiding contractions (NVCs) were also measured. NVCs were defined as rhythmic changes in intravesical pressure greater than 3 mmHg above baseline pressure and a minimum peak width of 1 s. Results are presented as NVCs per minute of filling cycle.
Ex vivo bladder preparation.
Ex vivo bladder pressure and afferent nerve activity were recorded as described previously (12, 16). Briefly, the urinary bladder and urethra with attached postganglionic nerves, major pelvic ganglion, and pelvic nerves were dissected and placed in a recording chamber recirculated with physiological saline solution [containing (in mM): 118.5 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgCl2, 2.0 CaCl2, 24 NaHCO3, 7 glucose, pH 7.4]. Physiological saline solution was bubbled with biological atmosphere gas (20% O2-5% CO2-75% N2) and maintained at 37°C. The bladder was cannulated via the urethra and infused with saline at a constant rate (1.8 ml/h) until intravesical pressure reached 25 mmHg, at which point the bladder was emptied and allowed to rest for 10 min before subsequent fills. Bladder afferent nerve activity was recorded by a suction electrode attached to one of the pelvic nerves. The suction electrode was connected to a NeuroLog headstage (NL100AKS Digitimer), where the extracellular action potentials were amplified with an AC amplifier (NL104, Digitimer), filtered (band pass 200–4,000 Hz, Digitimer NL125/NL126), and digitized with a Power1401 analog-to-digital interface (Cambridge Electronic Design, Cambridge, UK). Afferent nerve activity was acquired at a rate of 25,000 Hz and bladder pressure at 100 Hz. Data analysis was performed offline using Spike 2 software (Cambridge Electronic Design). The threshold for action potential detection was set at twice the root mean square of the recorded signal in the absence of action potentials. The TRPV1 antagonist capsazepine (10 μM) was applied to the bladder by addition to the superfusate. Ex vivo bladder capacity was defined as the bladder volume after filling to a maximum intravesical pressure of 25 mmHg.
Histology.
Imaging work was performed at the Microscopy Imaging Center at the University of Vermont. Tissues were embedded in paraffin blocks, sectioned (5-µm thickness), and mounted on glass slides. Sections were then deparaffinized and stained with hematoxylin, scarlet-acid fuchsin, phosphomolybdic/phosphotungstic acid, and aniline blue (Masson trichrome staining). Sections were imaged with a ×63 Leica HC PL-FL (NA 0.9) objective lens using a Leica Aperio VERSA Whole Slide Imager (Leica Biosystems, Buffalo Grove, IL). Following acquisition, images of each section were extracted for collagen content analysis using Aperio ImageScope software (Leica Biosystems). Full-width sections of intact bladder wall (250–500 µm2) were selected for analysis. Positive collagen staining (blue) was measured as a percent of total pixels in a selected area using the Positive Pixel Count algorithm in the ImageScope software.
Fluorescence in situ hybridization.
Serial coronal brain and brainstem frozen sections (10-µm thickness) through BN were isolated. Fluorescence in situ hybridization using DNA oligonucleotide probes coupled to an amplification system (RNAscope; Advanced Cell Diagnostics, Hayward, CA) were used to visualize CRH mRNA expression. The location of BN was determined based on brain anatomical landmarks, including the fourth ventricle, in accordance with the Allen Brain Map (13). Total nuclear counts were defined by staining with 4′,6-diamidino-2-phenylindole, and total CRH-positive cell counts were compared.
Statistical analysis.
For comparisons of two samples of equal variance, statistical significance between groups was established using two-tailed unpaired Student's t-tests (α = 0.05). For samples of unequal variance, the Mann-Whitney U-test was used (α = 0.05). For multiple sample comparisons, two-way ANOVA was used, followed by Bonferroni's post hoc analysis to compare individual means. Calculations were performed using Microsoft Excel or GraphPad Prism (GraphPad Software, San Diego, CA). Values are expressed as means ± SE. Differences with P values < 0.05 were considered statistically significant.
RESULTS
Intensified social stress causes bladder underactivity without altering afferent nerve activity.
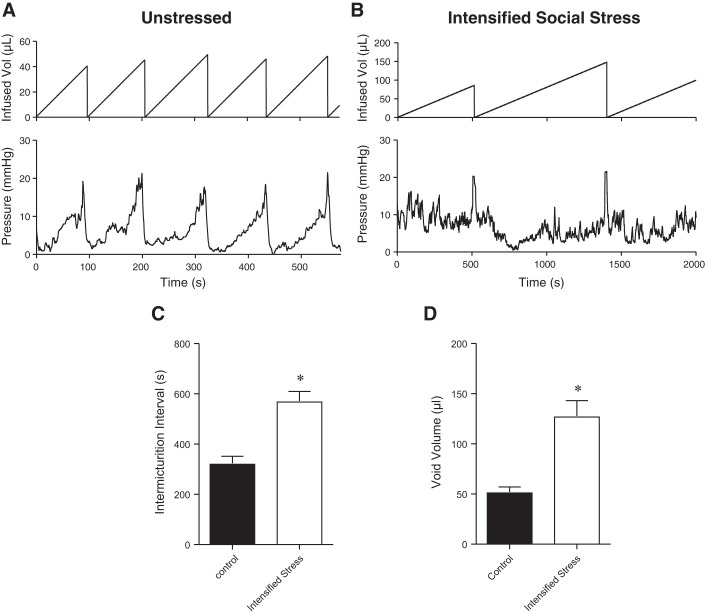
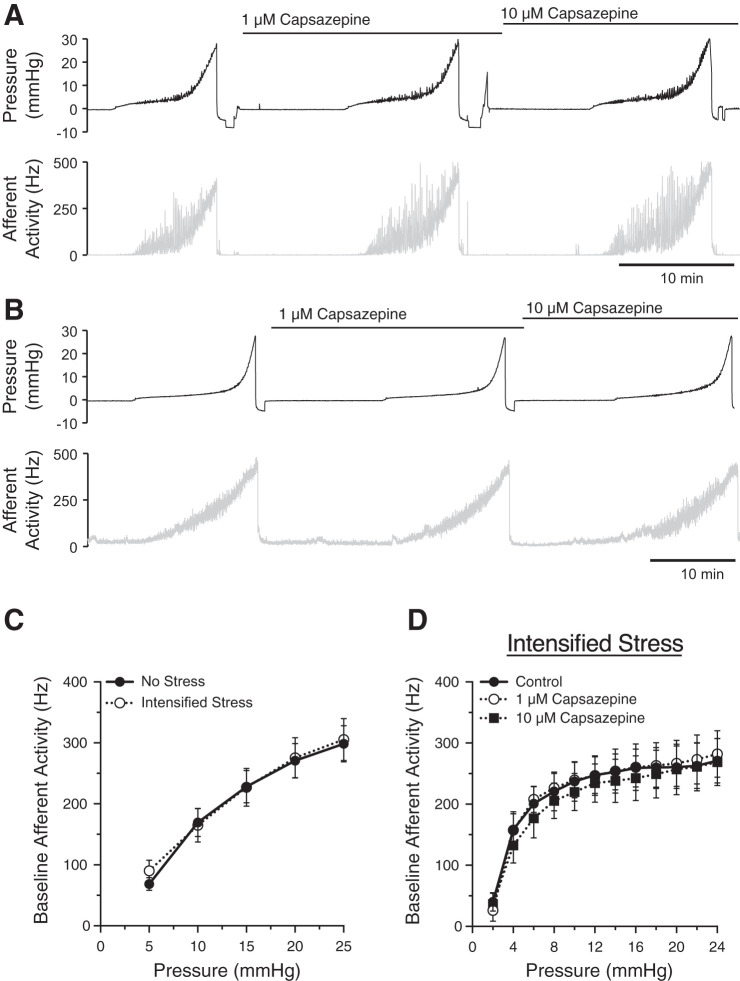
We used conscious cystometry to confirm that intensified social stress caused bladder dysfunction in C57BL/6 mice, as shown previously (16) (Fig. 1, A and B). Intensified social stress significantly increased the intermicturition interval (Fig. 1C) and voided volume (Fig. 1D) as compared with unstressed mice, indicative of bladder underactivity. However, the occurrence of NVCs was not altered significantly with intensified social stress (0.54 ± 0.07 min−1 vs. 0.86 ± 0.13 min−1). We next studied the effects of intensified social stress on bladder afferent nerve activity using an ex vivo bladder preparation (Fig. 2, A and B). Afferent nerve activity was unchanged between mice exposed to intensified stress and unstressed mice (Fig. 2C). Afferent nerve activity was also unaffected by exposure to the TRPV1 antagonist capsazepine (1–10 µM; Fig. 2D), suggesting that a TRPV1-dependent component to afferent nerve activity was absent with intensified social stress. Together, these data suggest that intensified stress-induced bladder underactivity is not due to changes in TRPV1-dependent afferent nerve activity or a decrease in sensory outflow.
Fig. 1.
Intensified social stress causes bladder underactivity in C57BL/6 mice. A and B: cystometric recordings from unstressed or stressed C57BL/6 mice, respectively. C and D: summary bar graphs showing that intensified social stress significantly increased intermicturition interval and void volume, respectively. *P ≤ 0.05; n = 5–6 mice.
Fig. 2.
Afferent nerve activity is unchanged by intensified social stress. Representative traces of intravesical pressure (top) and afferent nerve activity (bottom) from unstressed (A) or stressed mice (B). Summary graph (C) showing that intensified social stress had no effect on bladder afferent activity. Transient receptor potential vanilloid type 1 channel antagonist capsazepine (1–10 µM) (D) also had no effect on bladder afferent nerve activity in intensified stress mice. n = 6–10 mice.
Intensified social stress causes bladder wall remodeling and increased bladder volume.
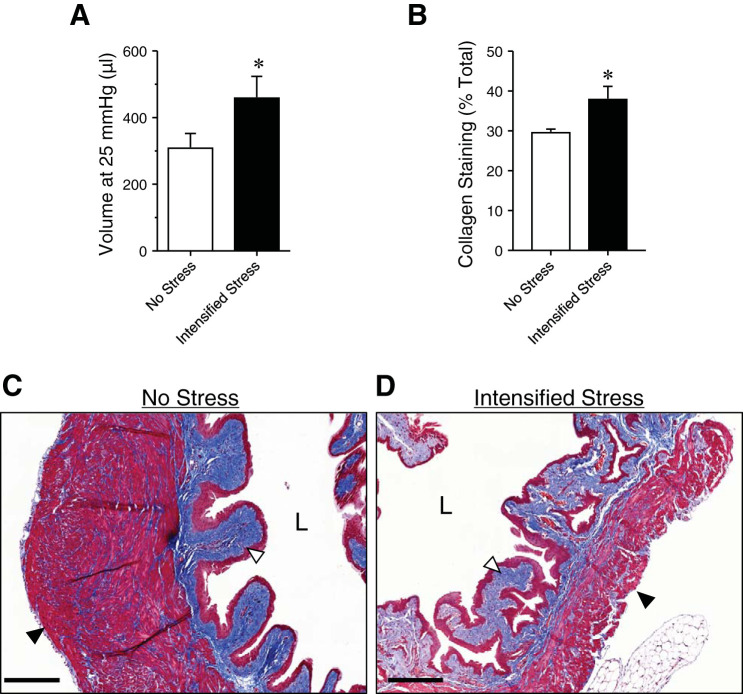
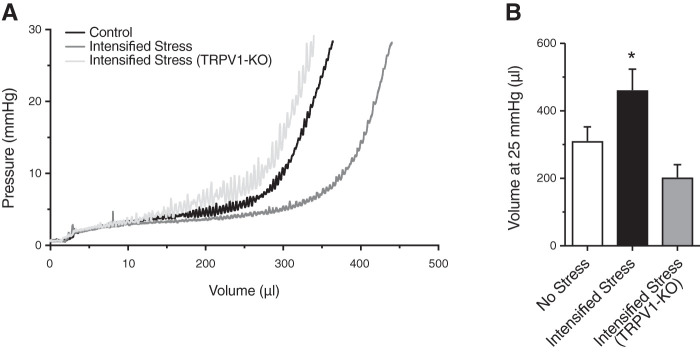
Stress-induced bladder dysfunction is thought to be caused, in part, by changes to bladder size and bladder wall structure (7). Maximum ex vivo bladder volume (at 25 mmHg) was significantly increased with intensified social stress, suggesting a general increase in bladder size (Fig. 3A). Masson’s trichrome staining revealed increased collagen deposition in bladders of mice exposed to intensified social stress, as compared with nonstressed mice (Fig. 3, B and C). Taken together, these data suggest that, while the size and structure of the bladder wall are altered by intensified social stress, bladder sensory outflow remains unchanged.
Fig. 3.
Ex vivo bladder capacity and collagen deposition are increased in intensified stress mice. Bar graph (A) showing ex vivo bladder capacity (at 25 mmHg) in unstressed and intensified stress mice. Summary graph (B) and representative images of Masson trichrome staining of bladder sections from unstressed (C) and intensified stress (D) mice, showing increased collagen deposition (blue; white arrow) and a decrease in smooth muscle (red; black arrow). Representative of samples from 6 mice. Scale bar = 200 µm. *P ≤ 0.05; n = 5 mice. L, bladder lumen.
Social stress does not cause bladder dysfunction or remodeling in TRPV1-KO mice.
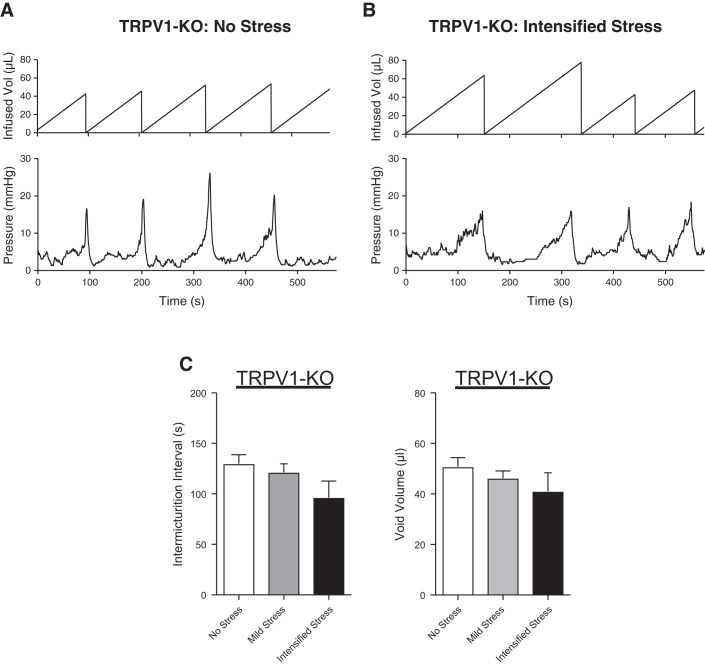
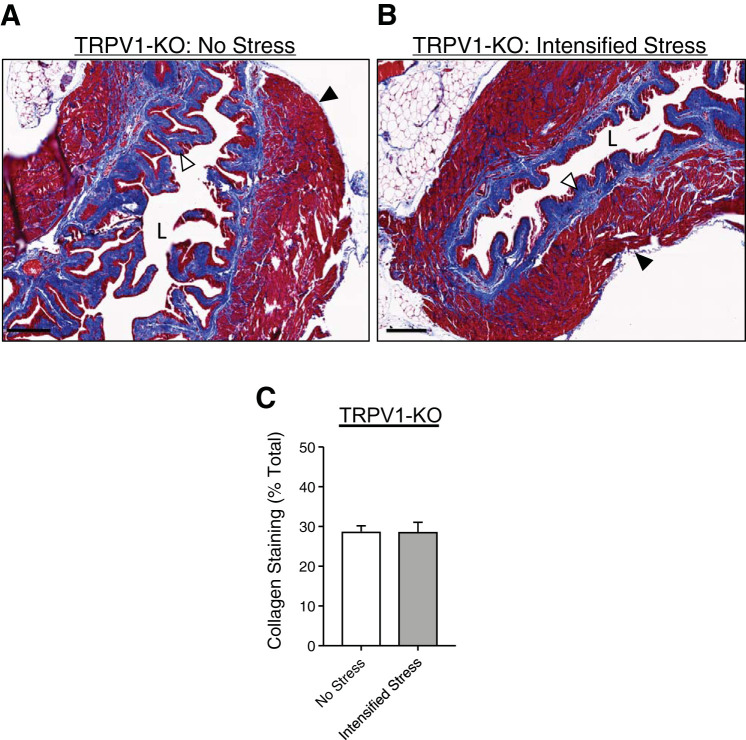
To determine if the presence of TRPV1 channels is necessary for social stress-induced bladder dysfunction to develop, we performed conscious cystometry in TRPV1-KO mice exposed to either the mild social stress paradigm or the intensified social stress paradigm (see Methods). Neither mild social stress nor intensified social stress changed void volume or intermicturition interval, as compared with nonstressed TRPV1-KO mice (Fig. 4). This suggests that TRPV1-KO mice do not develop any bladder dysfunction in response to social stress. Ex vivo bladder volume (Fig. 5) and collagen deposition (Fig. 6) were also unchanged by intensified social stress in TRPV1-KO mice, suggesting a lack of social stress-dependent bladder wall remodeling. Together, these data suggest that the presence of TRPV1 channels is required for social stress-induced bladder dysfunction to develop and for social stress-mediated bladder wall remodeling to occur.
Fig. 4.
Intensified social stress does not affect bladder function in transient receptor potential vanilloid type 1 (TRPV1) knockout (KO) mice. Cystometric recordings from unstressed (A) or stressed (B) TRPV1-KO mice. Summary bar graphs (C) showing that neither mild nor intensified social stress affects intermicturition interval and void volume in TRPV1-KO mice. * P ≤ 0.05; n = 4–6 mice.
Fig. 5.
Ex vivo bladder capacity is unaltered in transient receptor potential vanilloid type 1 (TRPV1) knockout (KO) mice after intensified stress mice. Representative pressure-volume traces (A) and summary bar graph (B) showing ex vivo bladder capacity (at 25 mmHg) in unstressed C57BL/6 mice, intensified stress C57BL/6 mice, and intensified stress TRPV1-KO. *P ≤ 0.05; n = 5.
Fig. 6.
Collagen deposition are unaltered in transient receptor potential vanilloid type 1 (TRPV1) knockout (KO) mice after intensified stress mice. Masson trichrome staining of bladder sections from unstressed TRPV1-KO (A) and intensified stress TRPV1-KO (B) mice showing collagen deposition (blue; white arrow) and smooth muscle (red, black arrow). Intensified social stress (C) did not alter urinary bladder collagen expression in TRPV1-KO mice. Representative of samples from 5 mice. Scale bar = 200 µm. *P ≤ 0.05; n = 5. L, bladder lumen.
Intensified social stress increases CRH mRNA in BN.
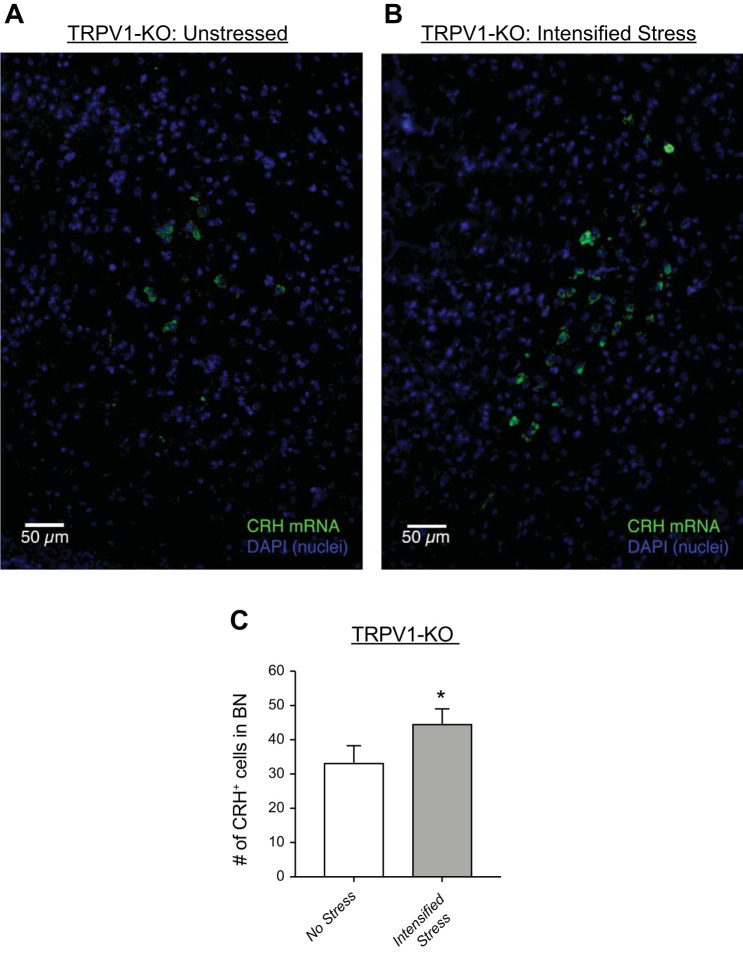
Social stress-induced bladder dysfunction has also been attributed to persistent increases in CRH in BN, the area of the brain responsible for control of micturition (5). To determine if this central effect of social stress is present in the TRPV1-KO mouse, sections of the mouse brain from unstressed and stressed mice were stained for CRH mRNA using fluorescent in situ hybridization. CRH mRNA expression was significantly increased in BN in stressed versus unstressed TRPV1-KO mice (Fig. 7). These findings are similar to other reports using C57BL/6 mice with bladder underactivity caused by intensified social stress (5). Thus, the central effect of social stress on CRH expression in BN is not mitigated in the absence of TRPV1 channels, and as such, the TRPV1-mediated changes in bladder function likely happen in the periphery because of the limited expression of TRPV1 channels in the brain (6).
Fig. 7.
Intensified stress increases corticotropin-releasing hormone (CRH) mRNA expression in Barrington’s Nucleus (BN) from transient receptor potential vanilloid type 1 (TRPV1) knockout (KO) mice. Fluorescent in situ hybridization showing CRH-positive cells (green) in brain sections of Barrington’s Nucleus from unstressed (A) and intensified stress (B) TRPV1-KO mice. Summary bar graph (C) showing a significant increase in CRH-expressing cells in response to intensified social stress. *P ≤ 0.05; n = 4.
DISCUSSION
In this study, we expand upon our current knowledge with regard to the role of TRPV1 in social stress-induced bladder dysfunction. Consistent with our previous work, increasing social stress leads to bladder underactivity, increased intermicturition interval, and increased voided volume during conscious cystometry. In contrast to bladder overactivity caused by “mild” social stress, bladder underactivity caused by intensified social stress did not result through a change in afferent nerve activity. Instead, we discovered an increase in bladder size and bladder collagen deposition in response to intensified social stress. This supports the concept that intensified social stress leads to bladder wall remodeling with probable concomitant changes in detrusor muscle reactivity and wall resistivity. Our most intriguing finding is that underactivity caused by intensified social stress is completely absent in mice lacking TRPV1 channels, even though the central effects of social stress are unchanged. Thus, TRPV1 channels are independently required for bladder underactivity to develop, with or without perturbation of central regulation of the bladder. These data suggest that TRPV1 channels play a direct role in the increased afferent activity present in social stress-induced overactivity and also have a pivotal role in the bladder wall remodeling that leads to underactive bladder with intensified social stress.
The role of TRPV1 channels in normal bladder activity is controversial because of questions as to where TRPV1 channels are expressed and if TRPV1 channels are involved in normal bladder physiology. In addition to afferent C fibers, TRPV1 channels are expressed in bladder arteriolar smooth muscle (17); however, their role in regulating vascular tone is still being investigated. TRPV1 channel expression has also been reported in the bladder urothelium (4); however, studies using reporter mice or direct current measurements from isolated urothelial cells do not support this (6, 23). Most previous studies, including ours, suggest that TRPV1 channels are expressed in afferent nerves but do not contribute to urinary bladder sensation over physiological filling pressures and filling rates in normal mice (1, 2, 10, 15, 19, 24). One additional study reported a decrease in afferent firing rate per fiber in TRPV1-KO mice during filling (9). However, this study also showed no difference in afferent nerve firing frequency between TRPV1+/+ and TRPV1−/− mice at 40 mmHg and a filling rate of 50 µl/min. Differences between TRPV1+/+ and TRPV1−/− mice were only apparent at pressures equal to or above the physiological threshold pressure for micturition (~15 mmHg). Thus, we interpret these findings to support our conclusions that TRPV1-sensitive nerve fibers are only engaged in normal mice at supraphysiological bladder pressures and/or bladder filling rates. Our current findings suggest that TRPV1 channels also have a role in regulating bladder wall remodeling, particularly in response to social stress. Social stress has been implicated in bladder wall hypertrophy previously (7), but changes in afferent activity were not investigated. Whether this is a compensatory response to social stress-induced increases in TRPV1-mediated afferent outflow, or whether this represents a separate response mediated by TRPV1 channels outside of sensory nerves, remains to be determined.
Given the profound wall remodeling, the large increase in bladder capacity, and the development of bladder underactivity uncovered in our experiments, we expected that a significant decrease in afferent nerve activity would also occur. To our surprise, no changes in baseline afferent activity were noted with intensified social stress, nor was any component of the afferent activity TRPV1 dependent. This suggests that decreases in bladder afferent outflow are not responsible for social stress-induced bladder underactivity and presents a conundrum as to why TRPV1-KO mice do not develop any bladder dysfunction with mild or intensified social stress: how does the absence of TRPV1 channels prevent social stress-induced bladder dysfunction, if not through direct changes in afferent nerve activity? Central nervous system expression of TRPV1 channels is very restricted (6), thus arguing against a central nervous system effect. It is also possible that TRPV1 channels in the bladder arteriolar smooth muscle respond to social stress to remodel the bladder through changes in perfusion since functional TRPV1 channels have been identified in the bladder vasculature. However, TRPV1 expression in bladder arterioles was predominantly seen in female, not male, mice (17). Thus, none of these explanations fully account for our findings that the development of social stress-induced bladder overactivity and underactivity are both TRPV1-dependent.
Our findings suggest a new model of social stress-induced bladder dysfunction, wherein bladder dysfunction progresses from overactivity to underactivity as the intensity of social stress increases. Initially, social stress increases TRPV1-dependent afferent nerve activity, leading to bladder overactivity without an overt change in bladder volume (15). Over time, chronic overactivity increases stretch and strain on the bladder wall, causing extracellular matrix remodeling and increased collagen deposition independently of the changes in sensory nerve outflow (3). While this remodeling increases bladder volume and returns afferent outflow to normal (Figs. 2 and 3), it does so at the expense of bladder elasticity. Increased collagen deposition reduces the ability of muscle to generate sufficient force to quickly and fully empty the bladder, which, in turn, leads to infrequent voiding and ultimately the inability to completely void when necessary. Thus, the increase in TRPV1-mediated afferent activity, which initially results in bladder overactivity, sets in motion a cascade of events that causes bladder remodeling, decompensation, and bladder underactivity that is independent of the initial change in sensory feedback.
In summary, our current findings uncover the important role that TRPV1 plays in the development of bladder underactivity in the setting of social stress. In particular, we demonstrate that the underactivity seen with intensified social stress is likely due to bladder wall remodeling and decompensation. Importantly, all social stress-induced bladder dysfunction requires TRPV1 channels. Thus, underactive bladder is likely an end-point on a spectrum of social stress-induced bladder dysfunction that progresses from overactivity to underactivity and that TRPV1 channels are necessary for this to occur. Potentially, the progression of social stress-induced bladder dysfunction could be slowed or prevented in humans as well, by preventing TRPV1 channel sensitization or activation.
GRANTS
This work was supported by grants to N. R. Tykocki from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K01-DK-103840); to M. T. Nelson from NIDDK (R37-DK-053832), the National Heart, Lung, and Blood Institute (R01-HL-121706, R01-HL-131181), the Totman Medical Research Trust, the Fondation Leducq, and European Union Horizon 2020 Research and Innovation Programme (Grant Agreement 666881); to M. A. Vizzard from NIDDK (R01-DK-051369, R01-DK-060481), COBRE Program of the National Center for Research Resources (P30-RR-032135), and the National Institute of General Medical Sciences (P30-GM-103498); and to G. C. Mingin from NIDDK (K08–082759).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R.T. and G.C.M. conceived and designed research; T.J.H., C.S.E., and J.v.B. performed experiments; N.R.T., T.J.H., C.S.E., J.v.B., and G.C.M. analyzed data; N.R.T., M.A.V., M.T.N., and G.C.M. interpreted results of experiments; N.R.T. and J.v.B. prepared figures; N.R.T. and G.C.M. drafted manuscript; N.R.T., M.A.V., M.T.N., and G.C.M. edited and revised manuscript; N.R.T., T.J.H., C.S.E., J.v.B., M.A.V., M.T.N., and G.C.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Stephen Zderic, M.D. for assistance with fluorescence in situ hybridization assays.
REFERENCES
- 1.Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 174: 977–982, 2005. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 2.Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn Schmiedebergs Arch Pharmacol 373: 287–299, 2006. doi: 10.1007/s00210-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 3.Baskin L, Howard PS, Macarak E. Effect of physical forces on bladder smooth muscle and urothelium. J Urol 150: 601–607, 1993. doi: 10.1016/S0022-5347(17)35560-X. [DOI] [PubMed] [Google Scholar]
- 4.Birder LA, Wolf-Johnston AS, Sun Y, Chai TC. Alteration in TRPV1 and muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol (Oxf) 207: 123–129, 2013. doi: 10.1111/j.1748-1716.2012.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler S, Luz S, McFadden K, Fesi J, Long C, Spruce L, Seeholzer S, Canning D, Valentino R, Zderic S. Murine social stress results in long lasting voiding dysfunction. Physiol Behav 183: 10–17, 2018. doi: 10.1016/j.physbeh.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol 297: F1101–F1108, 2009. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450, 2005. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 9.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol 583: 663–674, 2007. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everaerts W, Sepúlveda MR, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn Schmiedebergs Arch Pharmacol 379: 421–425, 2009. doi: 10.1007/s00210-008-0391-7. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie JI, van Koeveringe GA, de Wachter SG, de Vente J. On the origins of the sensory output from the bladder: the concept of afferent noise. BJU Int 103: 1324–1333, 2009. doi: 10.1111/j.1464-410X.2009.08377.x. [DOI] [PubMed] [Google Scholar]
- 12.Heppner TJ, Tykocki NR, Hill-Eubanks D, Nelson MT. Transient contractions of urinary bladder smooth muscle are drivers of afferent nerve activity during filling. J Gen Physiol 147: 323–335, 2016. doi: 10.1085/jgp.201511550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 14.Lepiarczyk E, Bossowska A, Kaleczyc J, Skowrońska A, Majewska M, Majewski M, Majewski M. the influence of resiniferatoxin (RTX) and tetrodotoxin (TTX) on the distribution, relative frequency, and chemical coding of noradrenergic and cholinergic nerve fibers supplying the porcine urinary bladder wall. Toxins (Basel) 9: 9, 2017. doi: 10.3390/toxins9100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingin GC, Heppner TJ, Tykocki NR, Erickson CS, Vizzard MA, Nelson MT. Social stress in mice induces urinary bladder overactivity and increases TRPV1 channel-dependent afferent nerve activity. Am J Physiol Regul Integr Comp Physiol 309: R629–R638, 2015. doi: 10.1152/ajpregu.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingin GC, Peterson A, Erickson CS, Nelson MT, Vizzard MA. Social stress induces changes in urinary bladder function, bladder NGF content, and generalized bladder inflammation in mice. Am J Physiol Regul Integr Comp Physiol 307: R893–R900, 2014. doi: 10.1152/ajpregu.00500.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan TX, Ton HT, Chen Y, Basha ME, Ahern GP. Sex-dependent expression of TRPV1 in bladder arterioles. Am J Physiol Renal Physiol 311: F1063–F1073, 2016. doi: 10.1152/ajprenal.00234.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 57: 422–427, 2001. doi: 10.1016/S0090-4295(00)00988-2. [DOI] [PubMed] [Google Scholar]
- 19.Sharopov BR, Gulak KL, Philyppov IB, Sotkis AV, Shuba YM. TRPV1 alterations in urinary bladder dysfunction in a rat model of STZ-induced diabetes. Life Sci 193: 207–213, 2017. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 20.Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol 4, Suppl 4: S7–S18, 2002. [PMC free article] [PubMed] [Google Scholar]
- 21.Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder-brain connection: putative role of corticotropin-releasing factor. Nat Rev Urol 8: 19–28, 2011. doi: 10.1038/nrurol.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der Coelen D, Bulmer DC, Peeters PJ, Aerssens J, de Hoogt R, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil 21: 1107–e94, 2009. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Gordon E, Lin Z, Lozinskaya IM, Chen Y, Thorneloe KS. Functional TRPV4 channels and an absence of capsaicin-evoked currents in freshly-isolated, guinea-pig urothelial cells. Channels (Austin) 3: 156–160, 2009. doi: 10.4161/chan.3.3.8555. [DOI] [PubMed] [Google Scholar]
- 24.Yoshiyama M, Araki I, Kobayashi H, Zakoji H, Takeda M. Functional roles of TRPV1 channels in lower urinary tract irritated by acetic acid: in vivo evaluations of the sex difference in decerebrate unanesthetized mice. Am J Physiol Renal Physiol 298: F1351–F1359, 2010. doi: 10.1152/ajprenal.00695.2009. [DOI] [PubMed] [Google Scholar]