Abstract
In polycystic kidney disease (PKD), persistent activation of cell proliferation and matrix production contributes to cyst growth and fibrosis, leading to progressive deterioration of renal function. Previously, we showed that periostin, a matricellular protein involved in tissue repair, is overexpressed by cystic epithelial cells of PKD kidneys. Periostin binds αVβ3-integrins and activates integrin-linked kinase (ILK), leading to Akt/mammalian target of rapamycin (mTOR)-mediated proliferation of human PKD cells. By contrast, periostin does not stimulate the proliferation of normal human kidney cells. This difference in the response to periostin is due to elevated expression of αVβ3-integrins by cystic cells. To determine whether periostin accelerates cyst growth and fibrosis, we generated mice with conditional overexpression of periostin in the collecting ducts (CDs). Ectopic CD expression of periostin was not sufficient to induce cyst formation or fibrosis in wild-type mice. However, periostin overexpression in pcy/pcy (pcy) kidneys significantly increased mTOR activity, cell proliferation, cyst growth, and interstitial fibrosis; and accelerated the decline in renal function. Moreover, CD-specific overexpression of periostin caused a decrease in the survival of pcy mice. These pathological changes were accompanied by increased renal expression of vimentin, α-smooth muscle actin, and type I collagen. We also found that periostin increased gene expression of pathways involved in repair, including integrin and growth factor signaling and ECM production, and it stimulated focal adhesion kinase, Rho GTPase, cytoskeletal reorganization, and migration of PKD cells. These results suggest that periostin stimulates signaling pathways involved in an abnormal tissue repair process that contributes to cyst growth and fibrosis in PKD.
Keywords: autosomal dominant polycystic kidney disease, extracellular matrix, matricellular protein, integrin, proliferation
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by the formation of renal cysts due to aberrant cell proliferation and accumulation of fluid within the cyst (15, 64, 73). Inexorable cyst growth leads to the loss of functional nephrons, inflammation, fibrosis, and decline in kidney function. Elevated expression of extracellular matrix (ECM) components including matricellular proteins, growth factors, surface receptors, and integrins by cystic epithelial cells (37, 42, 56, 62, 66, 72) is consistent with transcriptional reprogramming observed during tissue repair, which may contribute to disease progression (6, 31, 69–71).
Periostin is a matricellular protein that modulates biomechanical properties of tissues and binds αVβ3-integrins to activate signaling pathways involved in cell proliferation, survival, and profibrotic transforming growth factor (TGF)-β signaling (1, 24, 29, 30, 54, 55). Periostin is expressed following acute injury to tissues and activates pathways involved in tissue repair (10, 16, 19, 22, 52). We discovered that periostin is aberrantly expressed in ADPKD, autosomal recessive PKD, and rodent models of renal cystic disease, but not expressed in normal adult kidneys (66, 67). Periostin binding to αVβ3-integrins promotes the proliferation of human ADPKD cells by activating integrin-linked kinase (ILK), which stimulates Akt/mTOR signaling (46). By contrast, periostin did not stimulate the ILK/Akt/mTOR pathway or proliferation of normal human kidney (NHK) cells. This difference in the proliferative response to periostin is due to the overexpression of αVβ3-integrins by cystic epithelial cells compared with normal tubule cells (66). Genetic deletion of periostin in pcy/pcy (pcy) mice decreased renal mTOR activity, cell proliferation, and fibrosis, and delayed the decline in renal function (67).
Evidence has demonstrated a link between kidney injury and cyst initiation and PKD progression (2, 4, 17, 33, 44, 69); however, it remains unclear whether factors involved in tissue repair contribute to cyst growth and fibrosis in polycystic kidney disease (PKD) kidneys. In this study, we determined that selective overexpression of periostin in collecting ducts (CD), a predominant site for cyst formation, was not sufficient to induce cyst initiation in otherwise normal mice. However, we found that the overexpression of periostin in pcy kidneys stimulated pathways involved in tissue repair, leading to increased cyst growth and fibrosis, and accelerated the decline in renal function. These data confirm that periostin is an important factor in disease progression in renal cystic disease.
MATERIALS AND METHODS
Generation of mice with conditional overexpression of periostin.
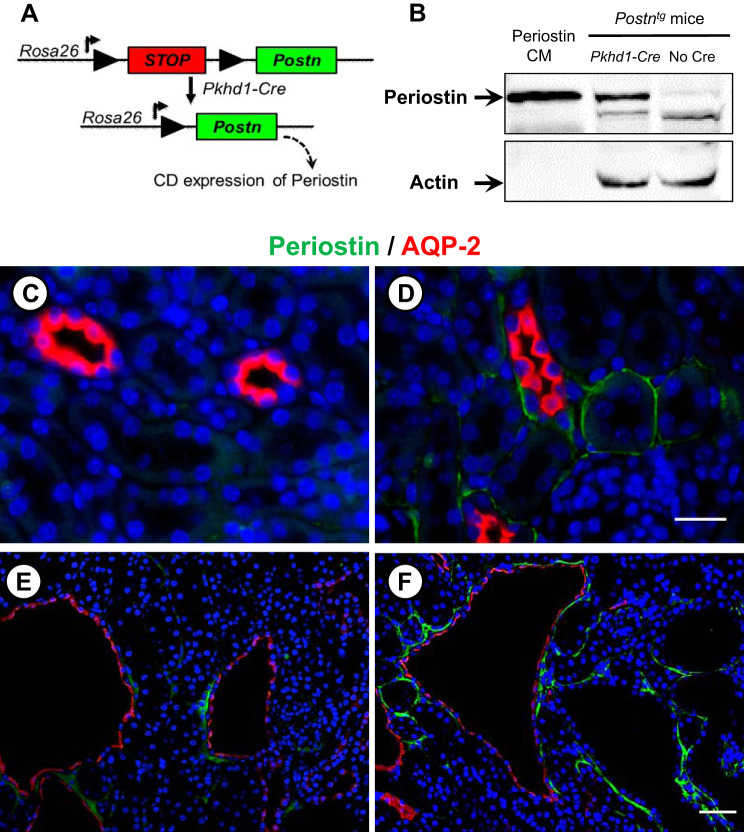
cDNA encoding mouse Postn and a 3′ bovine growth hormone polyadenylation signal were subcloned into pBT378-mCherry-LSL-MCS (J. Vivian, unpublished data). The resulting attB-flanked targeting construct was coinjected with RNA encoding the PhiC31 integrase (Applied Stem Cell, Milpitas, CA) into pronuclei of homozygous Rosa26-P3 mice (59) by the University of Kansas Medical Center (KUMC) Transgenic and Gene-Targeting Facility. Embryos were implanted in pseudopregnant females, and pups were genotyped to identify a founder with the transgene integrated into the attP sites at the Rosa26 locus via PhiC31-mediated integration. The resulting transgenic strain (Postntg) harbors the Postn coding sequences downstream of a splice acceptor and loxP-flanked-mCherry-STOP cassette under control of endogenous Rosa26 transcriptional regulatory elements (Fig. 1A). In the absence of Cre, mCherry was expressed, but periostin was not expressed. In the presence of Pkhd1-Cre, there was Cre-mediated excision of the floxed-mCherry-STOP resulting in periostin expression.
Fig. 1.
Collecting duct (CD)-specific overexpression of periostin. A: we generated a transgenic mouse (Postntg) carrying a full-length periostin cDNA construct downstream of a floxed mCherry STOP cassette that was inserted in front of the Rosa26 promoter. These mice were bred to Pkhd1-Cre mice to specifically overexpress periostin in the CD. B: to confirm Cre-mediated transgene expression, protein lysates from Postntg mice with or without Pkhd1-Cre were immunoblotted for periostin. Conditioned media (CM) from M1 cells transfected with full-length mouse periostin cDNA was used as a positive control for periostin expression. We crossed Postntg;Pkhd1-Cre (PostnCD) mice to pcy mice to generate Postntg;Pkhd1-Cre;pcy mice (PostnCD pcy). Sections were stained for periostin (green), AQP-2 (CD marker, red), and DAPI (nuclear stain, blue). Wild-type (C), PostnCD (D), pcy (E), and PostnCD pcy (F). C and D are the same magnification. A lower magnification was used of E and F to visualize multiple cysts. Scale bars: 5 µm.
Characterization of mice with CD overexpression of periostin.
Postntg mice were crossed to Pkhd1-Cre mice, which express Cre-recombinase specifically in CD cells (32) to generate Postntg;Pkhd1-Cre (PostnCD). Littermates that lacked Cre or the transgene were used as wild-type (WT) controls. pcy mice represent a slowly progressive PKD model (65, 67), orthologous to human nephronophthisis type 3. The course of disease progression in pcy mice phenocopies human ADPKD with cystic enlargement of the kidneys, interstitial fibrosis, and progressive decline in renal function by 48 wk of age (65). Postntg and Pkhd1-Cre mice were crossed to pcy mice to generate Postntg;Pkhd1-Cre;pcy/pcy (PostnCD pcy) mice. Littermates lacking Cre (Postntg;pcy/pcy mice) or the transgene (Pkhd1-Cre;pcy/pcy mice) were used as pcy mice. We observed normal Mendelian inheritance. Mice were backcrossed for five or six generations. The Institutional Animal Care and Use Committee at University of Kansas Medical Center (KUMC) approved the protocols for the use of these mice.
Genotyping.
Mice were genotyped for Postntg by PCR using a forward primer 5′-GCCATCATCAAGGAGTTCATGCGCTTC-3′ and reverse 5′-GGAGGTGATGTCCAACTTGATGTTGACG-3′. For genotyping for Cre alleles, the forward was 5′-GCGGTCTGGCAGTAAAAACTATC-3′, and the reverse primer was 5′-GTGAAACAGCATTGCTGTCACTT-3′. Cre-mediated excision of the floxed-mCherry STOP was validated using forward primer 5′-GCGCAGTAGTCCAGGGTTTCCTTGATGATG-3′ and reverse primer 5′-AGAATTTGCTGGAGGGCACAGACGTTTGGG-3′.
Immunofluorescence and immunocytochemistry.
Paraformaldehyde-fixed kidney sections were incubated with primary antibodies overnight at 4°C and secondary antibodies for 1 h at room temperature. Antibodies that were used include PCNA [AbCam (ab), Boston, MA; ab29, Ki-67 (ab15880)], periostin (Origene, Rockville, MD; TA500070), phosphorylated S6 (P-S6; Cell Signaling Technology (CS), Beverly, MA; CS4858], collagen I (ab34710), αSMA (ab5694), vimentin (ab92547), αVβ3-antibody (Millipore, Burlington, MA; MAB1976), phalloidin-Alexa Fluor-488 (ThermoFisher, Waltham, MA; A12379), and secondary antibodies (ab150084, CS4408). Sections were costained with FITC- or rhodamine-conjugated Dolichos biflorus agglutinin (DBA; FL-1031 or RL-1032, Vector Laboratories, Burlington, CA) to label CD cells and mounted with a mounting medium that contained DAPI (P36931). Slides were coded to deidentify the genotype and three nonoverlapping fields per section were imaged using an Eclipse Ti microscope (Nikon, Melville, NY).
Measurement of cystic area.
Slides with tissue sections were coded, stained with hematoxylin and eosin, and imaged using a dissecting microscope connected to a digital color camera. Cysts were defined as having a diameter ≥50 µm (46), which is approximately twice the average diameter of collecting ducts in mice (36). The number of cysts, cystic area, and total area of the kidney were measured using Image-Pro Premiere (Media Cybernetics, Silver Spring, MD) (67).
Measurement of blood urea nitrogen.
A colorimetric QuantiChrom urea assay kit (Bioassay Systems, Hayward, CA) was used to quantitate urea concentration in the serum, and blood urea nitrogen (BUN) was calculated using the formula BUN = (urea/2.14).
ADPKD and normal human kidney cells.
ADPKD kidneys (n = 23) were obtained from the surgery department at KUMC with the assistance of the KU Cancer Center’s Biospecimen Resource Core and hospitals participating in the Tissue Donation Program at the PKD Foundation (Kansas City, MO). Average age of patients at the time of nephrectomy was 52.5 yr (29–73 yr), and most patients were at or near end-stage renal disease (ESRD). Most likely, all samples were from PKD1 patients since 85% of cases are PKD1 mutations, and individuals with PKD2 mutations have a milder phenotype with later onset of ESRD (54 yr for PKD1 vs. 74 yr for PKD2 mutations) (45). NHK tissues were obtained from nephrectomy specimens by the surgery department at KUMC. Primary cultures of ADPKD and NHK epithelial cells were generated as described previously (49). Cultures were not passaged more than twice before being used in experiments. Use of deidentified surgically discarded tissues complies with federal regulations and was approved by the Institutional Review Board at KUMC.
Cell migration assay.
Confluent cell monolayers were wounded, and the scratched area was imaged using a Nikon Eclipse Ti microscope with a motorized stage. The x-y position was controlled by computer software allowing repeated measurements to be made at the same location. Wound closure was measured using Metamorph Image Analysis software and normalized to control. Treatments were carried out in triplicate, and two fields/well were examined.
Quantitative RT-PCR.
RNA was isolated using RNeasy isolation kit (Qiagen), and its integrity was verified using an Agilent Bioanalyzer (Santa Clara, CA). cDNA was synthesized from 1 µg of total RNA, using the iScript advanced cDNA synthesis kit for RT-qPCR (Bio-Rad, Irvine, CA). Real-time PCR reactions were set up by preparing a master mix of cDNA samples (1.92 µg/96-well plate) and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad; 960 µl/96-well plate). Bio-Rad PrimePCR (H96) array plates were used to screen genes involved in tissue repair. GAPDH, HPRT1, and TBP were used as reference genes, and results were analyzed with the PrimePCR Analysis Software (Bio-Rad). Results were validated by quantitative PCR in a separate study with primers purchased from SuperArray (Frederick, MD) or Genecopoeia (Rockville, MD).
Measurement of activated Rho.
Rho was immunoprecipitated from cell lysates using GST-RBD beads that have a high affinity for GTP-bound Rho and immunoblotted using a Rho antibody. Rho activity was defined as GTP-bound Rho to total Rho, normalized to control.
Immunoblot analysis.
Cell and tissue lysates were prepared as described previously (38, 46). The following antibodies were used: P-FAK (CS3283), FAK (ThermoFisher, AHO0502), GAPDH (CS2118), integrin β1 (ab529751), integrin αV (ab179475), integrin β3 (CS4702), and periostin (ab14041). Blots were incubated with an horseradish peroxidase-linked secondary antibody against rabbit or mouse (GE Healthcare, Aurora, OH; NA934, NA931). Bands were detected with ECL (RPN2232; GE Healthcare) and quantified with Bio-Rad Quantity One program.
Statistics.
Data are expressed as means ± SE. Statistical significance (P < 0.05) was determined by unpaired t-test. For multiple experimental conditions, an ANOVA followed by Student-Newman-Keuls post hoc test was used.
RESULTS
Generation of mice with CD-specific overexpression of periostin.
We generated transgenic mice that conditionally overexpress periostin selectively in renal CDs (PostnCD mice; Fig. 1A). Periostin overexpression was confirmed in PostnCD kidneys by immunoblot analysis (Fig. 1B). By contrast, periostin was not detected in kidneys of mice without the Pkhd1-Cre allele, consistent with the lack of periostin expression in WT kidneys (67). Periostin expression was visualized by immunofluorescence using antibodies to periostin (green) and AQP-2 (red), a CD marker. There was no staining for periostin in WT kidneys (Fig. 1C). By contrast, periostin localized to the matrix adjacent to AQP2-positive and AQP2-negative tubules of PostnCD kidneys (Fig. 1D). PostnCD mice had normal renal histology, kidney weight (KW), and body weight (BW), and the mice survived beyond one year (data not shown), suggesting that periostin overexpression was not sufficient to initiate cyst formation or fibrosis in kidneys that were otherwise normal.
Effect of CD-specific overexpression of periostin on renal mTOR and cyst growth in pcy mice.
We crossed Postntg and Pkhd1-Cre mice to pcy mice to obtain Postntg;Pkhd1-Cre;pcy/pcy (PostnCD pcy) mice and littermate pcy mice. Periostin was found in the ECM adjacent to cysts in pcy kidneys (Fig. 1E), as reported previously (46). Pericystic accumulation of periostin was greatly increased in PostnCD pcy kidneys (Fig. 1F). Kidney weight (KW), represented as percentage of body weight (BW) or KW (%BW), increased from 1.14 ± 0.04 in WT mice to 3.45 ± 0.25 (P < 0.005) in pcy mice at 10 wk. CD overexpression of periostin in PostnCD pcy mice had no effect on BW, but caused a 38% increase in KW (%BW) (Fig. 2, A–D). There was a 68% increase in cystic area and more cysts in PostnCD pcy mice compared with littermate pcy mice (Fig. 2, B, E, F).
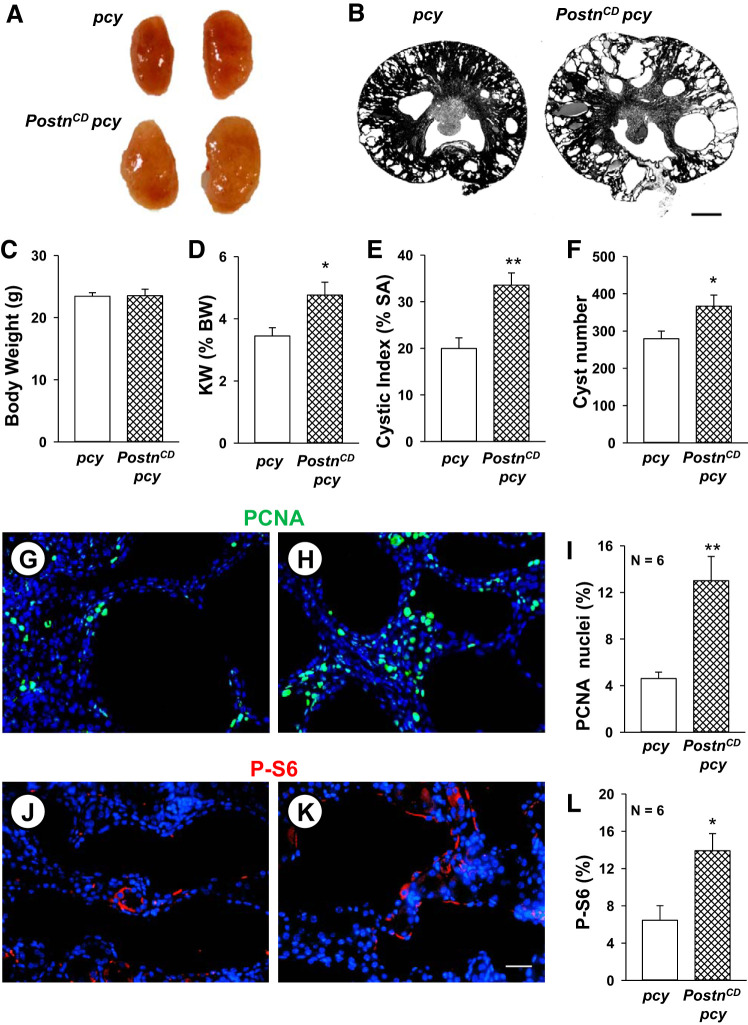
Fig. 2.
CD-specific periostin expression accelerates mammalian target of rapamycin (mTOR)-mediated cell proliferation and cyst growth in pcy kidneys. Representative whole kidneys (A) and kidney sections (B) from 10-wk-old pcy and PostnCD pcy mice. Scale bar: 1 mm. Bar graphs show means ± SE for body weight (BW) (C), kidney weight (KW) as a percentage of BW, indicated as KW (%BW) (D), percentage of cystic area per total cross-sectional surface area (% SA) (E) and cyst number per kidney section (F) of pcy (n = 6) and PostnCD pcy (n = 8) mice. pcy (G, J) and PostnCD pcy (H, K) kidney sections were stained for PCNA (green) (G, H) or phosphorylated S6 (P-S6; red) and nuclear stain DAPI (blue) (J, K). Percentage of PCNA-positive nuclei (I) and the percentage of P-S6-positive cells (L) normalized to total nuclei. *P < 0.05 and **P < 0.01, compared with pcy. Scale bars: 5 µm.
To determine whether periostin overexpression increased cell proliferation, the number of PCNA-positive cells was evaluated (Fig. 2, G and H). PostnCD pcy mice had a 2.8-fold increase in total PCNA-positive nuclei compared with pcy littermates (Fig. 2I). Increased proliferation was observed in cyst-lining cells (6.5 ± 1.0% for pcy kidneys vs. 19.0 ± 2.8% for PostnCD pcy kidneys, P < 0.01; n = 4), as well as interstitial cells (6.2 ± 1.5% for pcy kidneys vs. 14.5 ± 1.6% for PostnCD pcy, P < 0.01; n = 4). To confirm these results, tissue sections were stained with an antibody to Ki-67, another marker for cell proliferation, and imaged using immunofluorescence. We found that percentage of Ki-67-positive cells per section, relative to total nuclei, was 1.55 ± 0.18% in pcy kidneys compared with 3.27 ± 0.34% in PostnCD pcy kidneys (P < 0.005, n = 4). Previously, we showed that periostin induced cell proliferation and cyst growth by activation of the Akt/mTOR pathway (46). Compared with WT mice, pcy mice displayed increased renal levels of P-S6, a downstream target of mTOR activation (67). In the current study, the level of P-S6 staining was twofold higher in PostnCD pcy kidneys compared with pcy kidneys (Fig. 2, J–L), demonstrating that periostin overexpression caused a further increase in mTOR signaling.
Effect of CD-specific overexpression of periostin on the expression of mesenchymal markers.
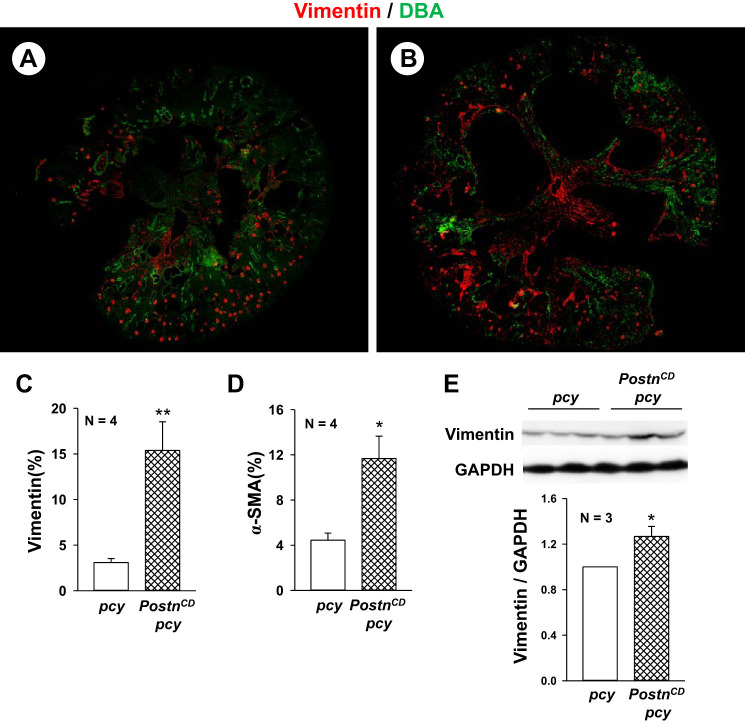
Cystic epithelial cells express mesenchymal markers, including vimentin and α-SMA (7, 60). Several studies have shown that periostin promotes epithelial mesenchymal transition (EMT) by activating integrins on epithelial cells and can stimulate myofibroblast activation, contributing to fibrosis during disease progression (10, 12, 13, 20, 23, 63, 74). To determine whether periostin overexpression in pcy kidneys increases vimentin and α-SMA, kidney sections of 10-wk old pcy and PostnCD pcy mice were stained with an antibody to vimentin (red, Fig. 3, A and B). Measurement of fluorescence intensity of entire tissue sections indicated a five-fold increase in vimentin staining in PostnCD pcy mice compared with pcy mice (Fig. 3C). Similarly, we found a 2.6-fold increase in α-SMA (Fig. 3D). Vimentin levels remained elevated in 20-wk old PostnCD pcy kidneys compared with pcy kidneys, determined by immunoblot analysis (Fig. 3E). Elevated vimentin and α-SMA staining in PostnCD pcy cyst-lining epithelial cells and renal interstitium are consistent with a pronounced mesenchymal phenotype (28). In addition, increased α-SMA expression by interstitial cells suggests that periostin secreted into the matrix activates myofibroblasts (12, 13, 60).
Fig. 3.
Collecting duct (CD)-specific expression of periostin increases the expression of vimentin and α-smooth muscle actin (α-SMA) in pcy kidneys. Representative kidney sections from pcy (A) and PostnCD pcy (B) mice were stained with antibodies to vimentin (red) and DBA (green). There was vimentin staining of mesangial cells in glomeruli in both pcy and PostnCD pcy sections. Cyst-lining cells and interstitial cells of PostnCD pcy kidneys had visually more vimentin staining compared with the kidneys of pcy littermates. Entire kidney sections were imaged using a Nikon Eclipse Ti microscope, and fluorescence for the section was measured using the threshold function in ImageJ software. Bar graphs (means ± SE) represent percentage of positively stained area in tissue section for vimentin (C) and α-SMA (D). E: immunoblots for vimentin and GAPDH levels from 20-wk old pcy and PostnCD pcy kidneys. Bar graph represents the levels of vimentin/GADPH, normalized to pcy (set to 1.0). *P < 0.05, **P < 0.01, compared with pcy mice.
Effect of CD overexpression of periostin on renal fibrosis in pcy kidneys.
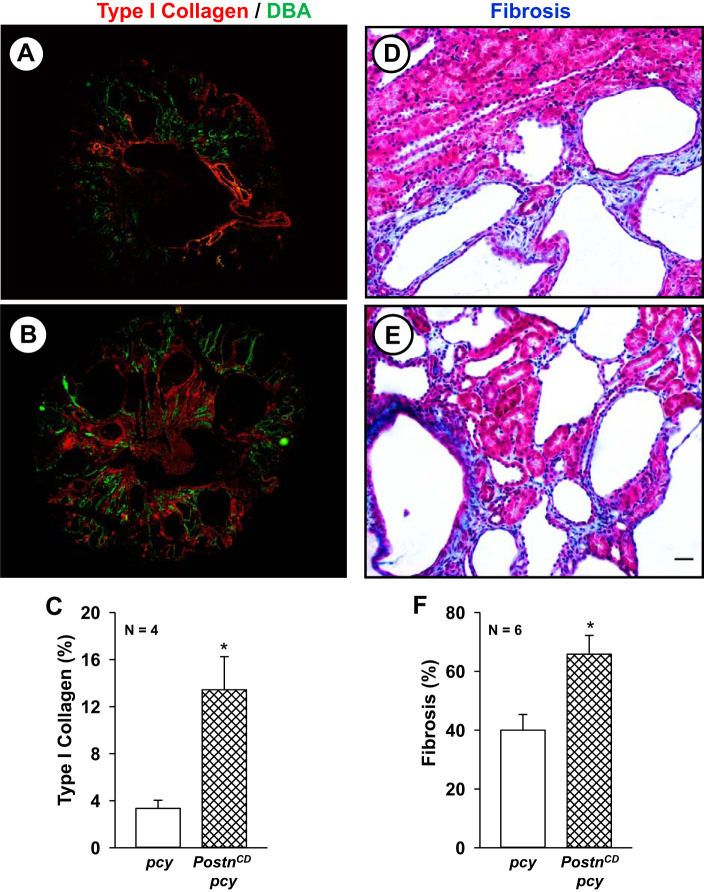
Periostin levels were highly elevated in kidneys of 20-wk old pcy mice, a time corresponding with the development of interstitial fibrosis (67). We found that periostin overexpression caused a four-fold increase in the staining for type I collagen (red, Fig. 4, A–C) in PostnCD pcy kidneys compared with pcy kidneys. There was prominent interstitial fibrosis in the kidneys of pcy mice by 10 wk of age (46) and that CD-overexpression of periostin caused a further increase in fibrosis (Fig. 4, D–E).
Fig. 4.
Collecting duct (CD)-specific expression of periostin increases renal type I collagen and interstitial fibrosis in pcy mice. Representative sections from pcy (A) and PostnCD pcy (B) mice stained with antibodies to type I collagen (red) and Dolichos biflorus agglutinin (DBA; green). C: bar graph (means ± SE) represents the percentage of entire tissue sections that stained for type I collagen (n = 4), normalized to total surface area (SA). Representative sections from pcy (D) and PostnCD pcy (E) mice were stained with Masson’s trichrome to visualize collagen fibers (blue) and prefibrotic edematous areas characterized by an increase in spacing between tubules (pale blue) (67). F: tissue sections were scored by a naïve observer assigning a percentage of fibrotic/edematous cortical area per total area of the cortex. Bar graph represents fibrosis as a percentage of total sectional area. *P < 0.05, compared with pcy. Scale bar: 5 µm.
Effect of CD overexpression of periostin on renal function and survival of pcy mice.
At 20 wk, KW (%BW) and cystic index of the pcy mice plateaued (Fig. 5, A and B), consistent with previous results (65); however, KW (%BW) continued to increase in PostnCD pcy mice. By 30 wk, KW (%BW) was significantly higher in PostnCD pcy mice compared with their pcy littermates. This difference in KW was notably greater than the change in cystic area (Fig. 5, A and B) at 30 wk. To determine whether apoptosis may account for the discrepancy, we stained tissue sections with an antibody to cleaved caspase 3. At 20 wk, we found that the percentage of apoptotic cells in pcy kidneys was higher than WT kidneys (1.2 ± 0.2 for pcy vs. 0.2 ± 0.02% for WT, P < 0.05; n = 3). CD overexpression of periostin did not affect the level of apoptosis (1.3 ± 0.4%). Similarly, there was no change in apoptosis in 30-wk-old pcy (1.8 ± 0.3%) compared with PostnCD: pcy (1.9 ± 0.6%) kidneys. We speculate that the larger increase in KW than could be accounted for by the change in cystic index was due to proliferation of interstitial cells, including myofibroblasts, and deposition of dense fibrotic material.
Fig. 5.
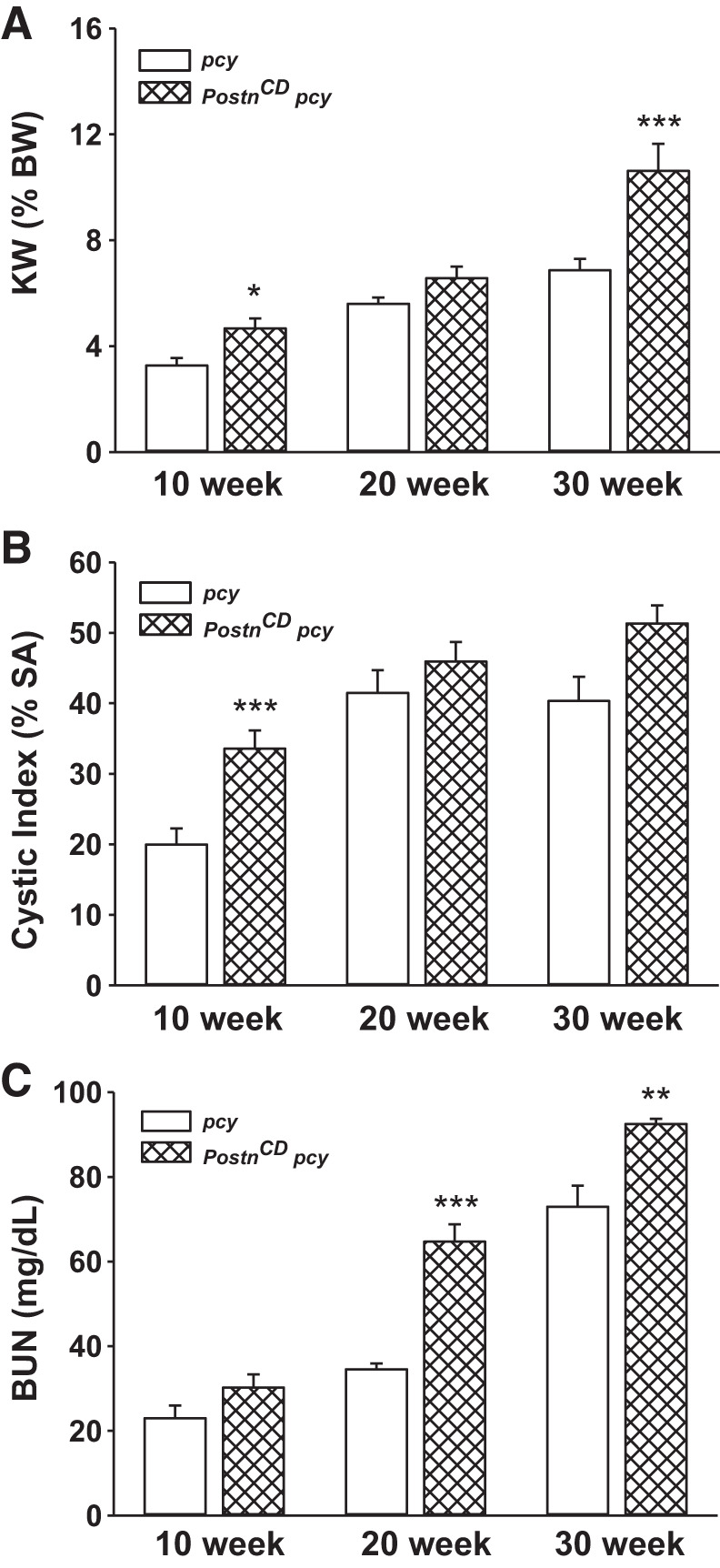
Collecting duct (CD)-specific periostin expression accelerates renal cyst growth and functional decline in pcy mice. pcy and PostnCD pcy mice were killed at 10 (n = 6–9 mice), 20 (n = 4–6 mice), and 30 (n = 3 or 4 mice) weeks of age for measurements of KW (%BW) (A), cystic index as percentage of surface area (SA) (B), and blood urea nitrogen (BUN) (C). *P < 0.05, **P < 0.01, and ***P < 0.001, compared with littermate pcy mice.
Renal function was assessed by measuring BUN levels of pcy and PostnCD pcy mice (Fig. 5C). BUN levels were 23.0 ± 3.0 and 34.5 ± 1.4 mg/dl for the pcy mice at 10 and 20 wk, respectively (normal range is 8–33 mg/dl). By 30 wk, BUN levels in pcy mice increased to 72.9 ± 5.0 mg/dl, which was more than twice the normal level. CD overexpression of periostin in PostnCD pcy mice caused a significant increase in BUN by 20 wk (65.0 ± 4.1 mg/dl), which was similar to 30-wk old pcy mice. There was a further increase in BUN in PostnCD pcy mice at 30 wk. These data demonstrate that periostin accelerates kidney enlargement and the decline in renal function in pcy mice.
The mean survival of pcy mice was 47.6 ± 0.6 wk, whereas overexpression of periostin reduced survival to 45.3 ± 0.6 wk (P < 0.05) (Fig. 6). Taken together, these data show that CD overexpression of periostin increased mTOR-mediated cell proliferation, cystic area, expression of EMT markers, and interstitial fibrosis, leading to a decline in renal function and shortened lifespan of pcy mice.
Fig. 6.
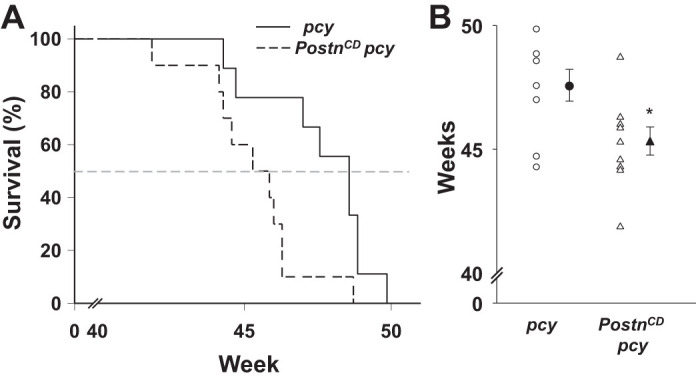
Collecting duct (CD)-specific overexpression of periostin decreases the survival of pcy mice. pcy (n = 9) and PostnCD pcy (n = 10) mice were given standard chow and water ad libitum and monitored regularly until a humane end point was reached (46, 48). A: Gehan-Breslow survival curves indicate that CD-specific overexpression of periostin significantly decreased the survival of pcy mice. B: scatterplot in which the open symbols represent the ages of pcy and PostnCD pcy mice at the time of death. Closed symbols indicate the means ± SE for each group of mice. *P < 0.05, compared with littermate age-matched pcy mice.
Effect of periostin on tissue repair pathways in human ADPKD cells.
Periostin promotes tissue repair following injury (10, 25, 29, 51). To determine whether periostin stimulates signaling pathways involved in tissue repair, we treated human ADPKD cells with periostin for 24 h and used Bio-Rad Human PrimePCR (H96) Integrin Outside-In Signaling, Collagen Diseases, Wounds and Injuries, and Transcription Factors (Tier 1) array plates to screen 220 genes, many of which are included in pathways for tissue repair. Expression levels were determined by quantitative real-time RT-PCR (qRT-PCR) analysis and compared with untreated ADPKD cells. We found that periostin significantly increased the expression of genes encoding cytoskeletal elements filamin A, β-actin, and actinins α1 and α4, as well as ECM-integrin signaling, including collagens Iα1 and IVα4, αV- and α5-integrins, ILK, Akt, and Src (Table 1). Confirmatory qRT-PCR showed elevated transcript levels for αV-integrin, collagen Iα1, β-actin, filamin A, and vinculin, and decreased levels for collagen 2α1 (Fig. 7), consistent with an EMT response.
Table 1.
Effect of periostin on gene expression in ADPKD cells
| Gene Name | Description | Fold Change |
|---|---|---|
| ECM | ||
| COL1A1 | collagen I α1 | 4.0 |
| COL4A4 | collagen IV α4 | 1.8 |
| Cytoskeleton | ||
| FLNA | filamin A | 2.1 |
| ACTB | β-actin | 2.0 |
| ACTN1 | actinin α1 | 1.7 |
| ACTN4 | actinin α4 | 1.6 |
| Integrins | ||
| ITGAV | αV-integrin | 2.0 |
| ITGA5 | α5-integrin | 1.5 |
| Focal adhesion-integrin signaling | ||
| AKT1 | AKT serine/threonine kinase | 1.6 |
| ILK | integrin-linked kinase | 1.6 |
| Growth factor signaling | ||
| SRC | Src protooncogene | 2.5 |
Human primary autosomal dominant polycystic kidney disease (ADPKD) cells (n = 3 kidney preparations) were grown on Transwells to form polarized epithelial monolayers. Cells were treated with or without periostin for 24 h. Total cellular RNA was isolated and reverse transcribed. mRNA levels of 220 genes involved integrin outside-in signaling were detected using pre-designed PrimePCR arrays. The results were analyzed using Bio-Rad CFX Manager software. Genes that showed a greater than ± 1.5-fold significant change in gene regulation with P < 0.05, compared with control, are represented here.
Fig. 7.
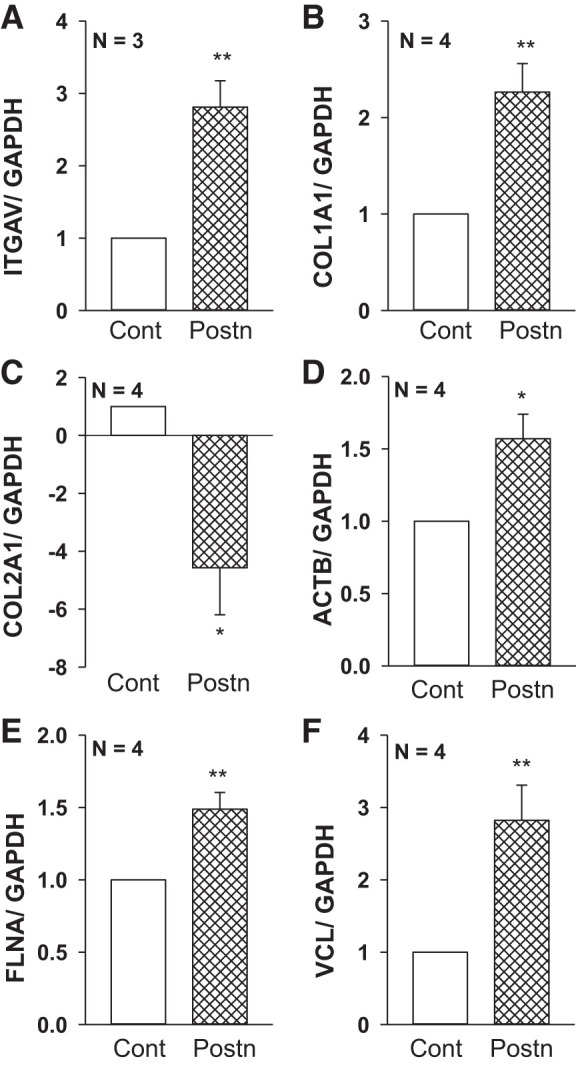
Periostin regulates genes involved in integrin/ECM signaling in autosomal dominant polycystic kidney disease (ADPKD) cells. Human ADPKD cells (n = 3 or 4 kidneys) were grown as polarized monolayers on permeable Transwells and incubated with control media (Cont) or 250 ng/ml recombinant periostin (Postn) for 24 h. Total RNA was isolated and reverse transcribed, and mRNA levels for the genes of interest were detected using quantitative RT-PCR and normalized to GAPDH. Bar graphs are expressed as means ± SE for relative changes in the expression of αV-integrin (ITGAV) (A), type I collagen (COL1A1) (B), type II collagen (COL2A1) (C), β-actin (ACTB) (D), filamin-A (FLNA) (E) and vinculin (VCL) (F). These genes were selected since they were found to be altered by periostin in a Bio-Rad PrimePCR array (Table 1). *P < 0.05 and **P < 0.01, compared with Cont.
Previously, we showed that ADPKD cells overexpress αV-integrin (66). Here, we found that treatment with periostin significantly increased levels for both αV- and β3-integrins in ADPKD cells (Fig. 8). By contrast, the expression of these integrins in NHK cells was unaffected by periostin treatment (data not shown), consistent with the lack of an effect of periostin on normal cells (66). We did not observe a difference in αV-integrin expression in PostnCD pcy kidneys compared with pcy kidneys by Western blot analysis (data not shown). Previously, renal periostin levels were found to be 20-fold higher in pcy compared with wild-type mice (67). We think that elevated endogenous periostin in pcy kidneys causes persistent expression of αV-integrin that cannot be further increased by CD overexpression of periostin.
Fig. 8.
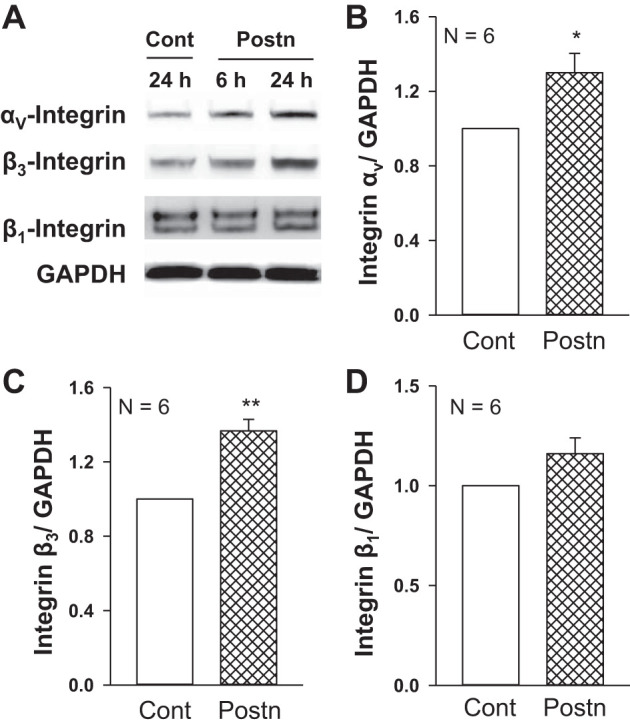
Periostin increased the expression of αV-integrins and β3-integrins in autosomal dominant polycystic kidney disease (ADPKD) cells. ADPKD cells were grown on Transwells and incubated in Cont or Postn for 6 or 24 h. A: representative immunoblots show protein levels of αV-, β3-, and β1- integrins and GAPDH. Graphs (means ± SE) display the relative expression of αV-integrin/GAPDH (B), β3-integrin/GAPDH (C), and β1-integrin/GAPDH (D). *P < 0.05, **P < 0.01, compared with Cont (set to 1.0).
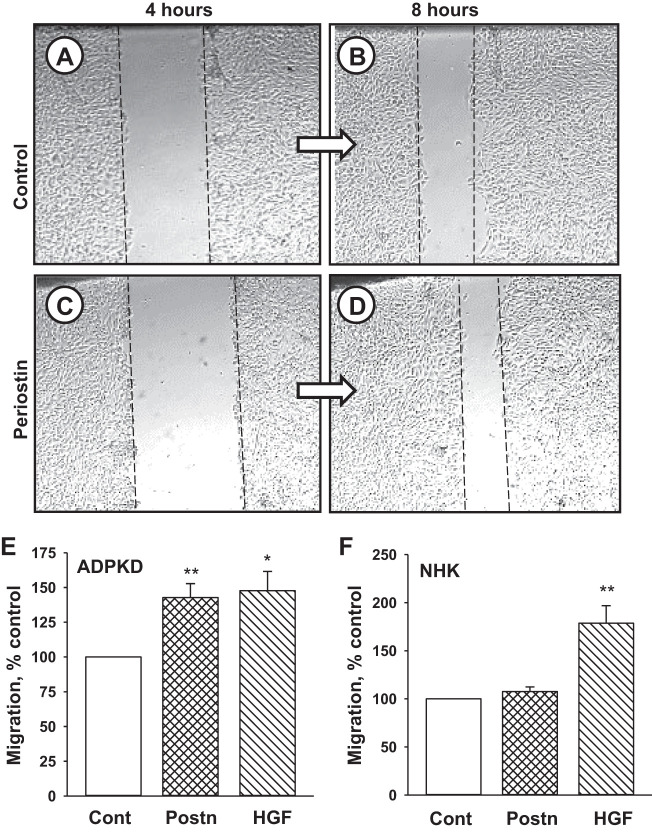
Epithelial cell migration is important feature of tissue repair process. To determine whether periostin promotes the migration of human ADPKD cells, we performed a wound-closure assay (Fig. 9). Confluent monolayers were scratched using a sterile pipette tip, and images were captured at 4 and 8 h. The monolayer in control media (Fig. 9, A and B) had a noticeable reduction in the wound. Despite a larger initial scratch, the monolayer treated with periostin (Fig. 9, C and D) had a more pronounced closure of the wound. Results from a composite of six different cell preparations showed that periostin treatment caused a 42% increase in wound repair above control (Fig. 9E). By contrast, periostin had no effect on wound closure in NHK cell monolayers (Fig. 9F). As a positive control, hepatocyte growth factor stimulated the migration of both ADPKD and NHK cells (Fig. 9, E and F).
Fig. 9.
Periostin promotes the migration of autosomal dominant polycystic kidney disease (ADPKD) cells. A: ADPKD cells were seeded into individual wells of 12-well plates containing media + 1% FBS. Serum was reduced to 0.05% for 24 h, and a scratch in each monolayer was made using a 200-µm pipette tip. Wounded monolayers were incubated in control media (A and B) or media containing 250 ng/ml periostin (C and D), and images of the monolayers were taken at 4 h (A and C) and 8 h (B and D). Bar graphs (means ± SE) represent the quantification of cell migration (wound closure) between 4 and 8 h after wounding for ADPKD (n = 6) (E) and normal human kidney (NHK; n = 3) (F) cells in Cont, periostin (Postn) or 40 ng/ml hepatocyte growth factor (HGF), a positive control for promoting cell migration (50). **P < 0.01, *P < 0.05, compared with Cont.
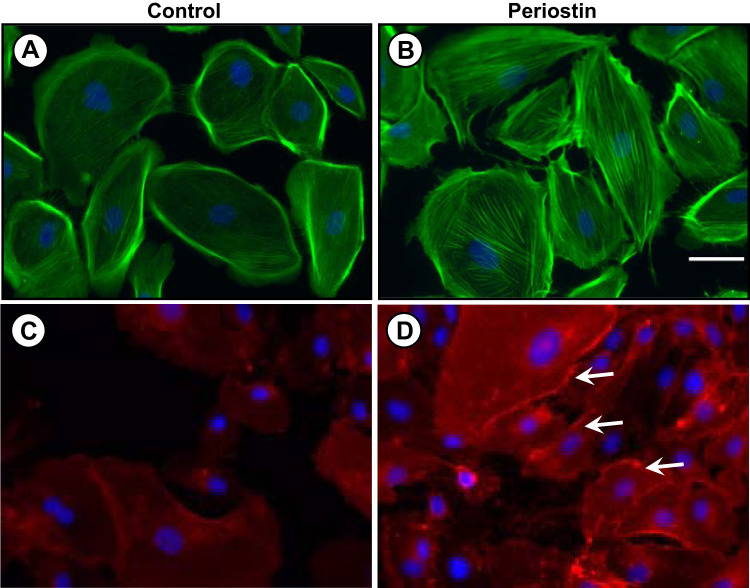
Cell migration involves the coordinated process of formation and disassembly of focal adhesions and changes in actin filaments, which are promoted by the interaction between the ECM, integrins, and the actin cytoskeleton (35, 61). Activation of focal adhesion kinase (FAK) is a rapid event that promotes changes in the actin cytoskeletal by activating Rho-family GTPases (35, 43). We found that periostin increased tyrosine phosphorylation of FAK (Fig. 10A) and caused a 65% increase in activated RhoA (Fig. 10B). To determine whether periostin induced structural changes in the actin cytoskeleton, ADPKD cells were treated with phalloidin, a stain for F-actin (Fig. 11, A and B). We found that periostin caused stress fiber formation and bundling of actin filaments into transverse and ventral stress fibers. It has been proposed that integrin activation correlates with integrin clustering, which is involved in the formation of focal adhesions (8). We found that periostin caused αVβ3-integrin clustering and accumulation at focal adhesion junctions (Fig. 11, C and D). These results are consistent with periostin activation of cytoskeletal rearrangement and migration of ADPKD cells, consistent with an injury repair response.
Fig. 10.
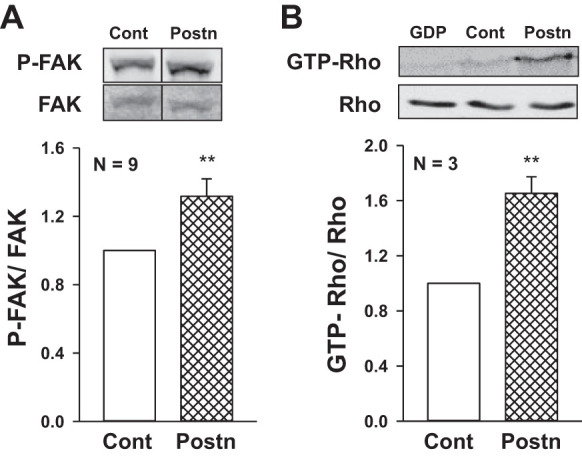
Periostin stimulates focal adhesion kinase (FAK) and Rho-mediated cytoskeletal changes in autosomal dominant polycystic kidney disease (ADPKD) cells. A: human ADPKD cells were incubated in media containing 250 ng/ml Postn for 30 to 120 min. Immunoblot analysis was used to measure levels of phosphorylated FAK (P-FAK) and total FAK. In the representative immunoblot, splice marks indicate the removal of extraneous lanes within the same blot. Bar graph (means ± SE) represents P-FAK/FAK. B: Rho was immunoprecipitated from cell lysates using GST-RBD beads with a high affinity for GTP-bound Rho. For a negative control, GDP was added to the lysate to inactivate endogenous Rho before immunoprecipitation (lane 1). GTP-Rho from the immunoprecipitate and total Rho from whole cell lysates were detected by immunoblotting using a Rho antibody. Bar graph represents GTP-bound Rho/total Rho. **P < 0.01, compared with Control (Cont; set to 1.0).
Fig. 11.
Periostin stimulates changes in the actin cytoskeleton and αVβ3-integrin clustering in autosomal dominant polycystic kidney disease (ADPKD) cells. Cells were incubated in control (A, C) media or media containing periostin (B, D) for 1 h, and then stained with phalloidin (green) to visualize F-actin fibers (A, B) or αVβ3-integrin antibody to visualize the cellular location of the integrin (C, D). Arrows denote areas of integrin clustering. Scale bar: 5 µm.
DISCUSSION
CD-specific overexpression of periostin increased renal Akt-mTOR signaling, cell proliferation, and cystic index in pcy mice. There was increased expression of markers for EMT and myofibroblast activation, greater levels of interstitial fibrosis, more rapid decline in renal function, and reduced survival of the mice. By contrast, ectopic CD expression of periostin in normal mice had no effect on renal morphology or function, indicating that periostin expression alone was not sufficient to initiate cyst formation or fibrosis in otherwise normal kidneys. Treatment of human ADPKD cells with periostin increased FAK signaling, Rho activation, integrin clustering, actin stress fiber formation, and cell migration. Increased expression of molecules involved in integrin/ECM signaling and wound healing are consistent with periostin activation of pathways involved in tissue repair.
Periostin belongs to a superfamily of TGF-β-inducible proteins. It is found in collagen-rich tissues, such as those subjected to mechanical stress conditions, including periodontal ligaments, periosteum and cardiac valves (40), and is highly expressed in several tissues following injury (10, 25, 29, 51). Periostin directly interacts with components of the ECM and promotes collagen cross-linking through activation of lysyl oxidase (26, 34). The stimulation of collagen fibrillogenesis by periostin maintains the integrity of the ECM and influences the biomechanical properties of connective tissue (30, 40, 41). However, excessive or persistent expression of periostin is associated with organ fibrosis (16, 39, 52, 53, 57, 67). Periostin contains highly conserved FAS1 domains, allowing it to serve as a ligand for specific integrins, particularly αVβ3-integrin. The binding of periostin to its integrin receptor stimulates pathways involved in tissue repair, including EMT, cell proliferation, survival, and matrix production (3, 9, 10, 22–24, 41, 68).
Periostin is expressed within the nephrogenic zone during renal development, but it is not expressed in normal adult kidney (21). We discovered that periostin was highly overexpressed in human ADPKD and autosomal recessive PKD, and in several rodent models of PKD (66, 67), indicating that its expression is a general feature of renal cystic disease regardless of the underlying genetic mutation. Periostin is secreted by cystic epithelial cells and accumulates within the ECM adjacent to cysts of ADPKD kidneys. The mechanisms regulating the expression of periostin in PKD kidneys remain unclear. It is possible that periostin expression is a response to mechanical stress due to hydrostatic pressure within the cyst. On the other hand, ADPKD cells grown in culture also overexpress periostin compared with NHK cells. Elevated periostin synthesis by ADPKD cells may be a memory effect of the cystic environment, driven by excessive TGF-β production, or the result of intrinsic differences due to the PKD cellular phenotype.
Cystic epithelial cells are characterized as being incompletely differentiated with increased c-myc expression, persistent activation of mTOR, aberrant cell proliferation, and elevated secretion of growth factors, chemokines, and matrix components (5, 11, 47, 56, 58). Analysis of PKD-related gene expression revealed that genes involved in tissue repair pathways were highly enriched in the PKD gene profile (33). Cyst cells have a loss of expression of kidney epithelium-specific genes and upregulation of developmental and mitogenic pathways (56), as well as TGF-β-Smad signaling, matrix production, Rho signaling, and cytoskeleton remodeling (7, 18). These observations support the hypothesis that PKD cells are in a perpetual state of futile repair (47, 70, 71).
Previously, we showed that recombinant periostin stimulated cell proliferation and in vitro cyst growth of ADPKD cells cultured within a collagen matrix. Also, treatment with an αV-integrin blocking antibody, ILK inhibitor CPD-22, or ILK knockdown using shRNA attenuated the mitogenic effect of periostin (46, 66). Global knockout of periostin in pcy mice caused a marked reduction in kidney weight, mTOR signaling, renal cyst growth, and fibrosis and extended the lifespan of the mice (67). Here, we showed that CD-specific overexpression of periostin increased renal mTOR activity and proliferation of cyst-lining epithelial cells, thus, accelerating cyst growth in pcy mice. There was increased staining for EMT markers αSMA and vimentin in cystic epithelial cells, myofibroblast activation, type 1 collagen expression, and tissue fibrosis, leading to a more rapid decline in renal function.
Periostin binding to αVβ3-integrin has been shown to elicit tissue remodeling (9). Stimulation of this integrin activates Rho kinase (ROCK) pathways, which are essential for stress fiber formation (3, 41). Activation of ROCK pathways, in conjunction with FAK, stimulates cell migration during tissue repair. The mechanisms by which periostin-integrin signaling induces cytoskeletal changes are not well understood. One possibility is that integrin signaling involves an interaction between ROCK and the actin-binding protein filamin-A (14, 75). Small GTPase Rho and its effector ROCK regulate actin cytoskeletal organization through interactions with filamin-A. We found that periostin activated pathways involved in wound healing and integrin/ECM signaling, and promoted integrin clustering, actin cytoskeletal changes, and migration of ADPKD cells. There was increased activity of FAK and Rho and increased gene expression of cytoskeletal elements including filamin-A, β-actin, and actinins α1 and α4. Periostin also increased the level of vinculin, an actin-binding protein involved in the linkage between integrin adhesion molecules and actin cytoskeleton. Interestingly, periostin increased αV- and β3-integrin expression, suggesting a feed-forward mechanism that would further amplify the cell’s response to periostin. Taken together, these results indicate that the secretion of periostin into the environment of the PKD kidney leads to persistent activation of pathways involved in tissue repair that accelerates cyst growth, fibrosis, and the decline in renal function.
CD-specific overexpression of periostin in wild-type mice did not induce cyst formation or fibrosis, suggesting that periostin alone was not sufficient to induce a PKD cellular phenotype. Moreover, periostin did not stimulate cell migration or expression of αV- and β3-integrin in NHK cells. One reason for the difference in the periostin response may be that the expression of αV-integrin is several-fold higher in cystic cells compared with normal renal cells (66), making cystic cells sensitive to periostin.
While ectopic expression of periostin was not cystogenic in wild-type mice, its overexpression in pcy mice increased the number of cysts within the kidneys. Increased cyst number could be due to enlargement of existing cysts that, otherwise, would be below the detection threshold, or conceivably, periostin initiates new cyst formation in tubule cells that are genetically primed to form cysts. As such, our data suggest that periostin secreted into the matrix stimulates aberrant repair responses in cystic cells and adjacent tubules, contributing to a “snowball effect” of cyst formation within the PKD kidney (27). Periostin overexpression accelerated cyst growth in PostnCD pcy mice leading to a greater increase in KW compared with pcy mice at 10 wk of age. The greater change in KW observed in PostnCD pcy mice at 30 wk may reflect an increase in interstitial cells and deposition of dense fibrotic material.
In summary, aberrant expression of periostin and αV-integrin, the receptor for periostin, promotes tissue repair pathways that accelerate cyst growth and fibrosis in PKD kidneys, and contributes to the decline in renal function. Since periostin is not typically expressed in a normal adult kidney, treatments to antagonize periostin in ADPKD are unlikely to affect normal renal processes. An understanding of the regulation and effects of periostin and other matricellular proteins on cyst growth and fibrosis may provide new opportunities for therapeutic intervention to slow PKD progression.
GRANTS
This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK-081579; to D. P. Wallace) and a fellowship through the University of Kansas Medical Center (KUMC) Biomedical Research Training Program (to A. Raman). The periostin conditional transgenic mouse strain was generated with the assistance of the KUMC Transgenic and Gene Targeting Institutional Facility supported by the KU Cancer Center, COBRE in Molecular Regulation of Cell Development and Differentiation, and the Kansas IDeA Network of Biomedical Research Excellence (P30 CA168524, P30 GM122731, and P20 GM103418). Human tissues were obtained with the assistance of the KU Cancer Center’s Biospecimen Repository (P30 CA168524) and hospitals participating in the PKD Foundation’s tissue donation program. Primary cultures of ADPKD and normal human kidney cells were generated by the PKD Biomarkers and Biomaterials Core in the Kansas PKD Research and Translational Core Center at KUMC (DK-106912; to J. Calvet and D. P. Wallace).
DISCLOSURES
D. P. Wallace is a consultant for Vertex Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.R., S.C.P., and D.P.W. conceived and designed research; A.R., S.C.P., Y.Z., G.A.R., Y.D., A.K., E.D., C.W., and J.L.V. performed experiments; A.R., Y.Z., G.A.R., Y.D., and D.P.W. analyzed data; A.R. and D.P.W. interpreted results of experiments; A.R., S.C.P., J.L.V., and D.P.W. prepared figures; A.R., S.C.P., G.A.R., and D.P.W. drafted manuscript; A.R., S.C.P., Y.Z., and D.P.W. approved final version of manuscript; D.P.W. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. James Calvet for helpful suggestions during the preparation of the manuscript and Marsha Danley for technical assistance. Pkhd1-Cre mice were received from the UTSW O’Brien Center Core (NIH P30DK079328).
Portions of this work were published previously in abstract form (J Am Soc Nephrol 22: 579A, 2011 and J Am Soc Nephrol 27: 773A, 2016).
REFERENCES
- 1.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 5: 329–339, 2004. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 2.Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011. doi: 10.1681/ASN.2010050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol 302: 256–266, 2007. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvet JP. Injury and development in polycystic kidney disease. Curr Opin Nephrol Hypertens 3: 340–348, 1994. doi: 10.1097/00041552-199405000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Calvet JP. Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int 43: 101–108, 1993. doi: 10.1038/ki.1993.17. [DOI] [PubMed] [Google Scholar]
- 6.Chang-Panesso M, Humphreys BD. Cellular plasticity in kidney injury and repair. Nat Rev Nephrol 13: 39–46, 2017. doi: 10.1038/nrneph.2016.169. [DOI] [PubMed] [Google Scholar]
- 7.Chea SW, Lee KB. TGF-β-mediated epithelial-mesenchymal transition in autosomal dominant polycystic kidney disease. Yonsei Med J 50: 105–111, 2009. doi: 10.3349/ymj.2009.50.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. The mechanisms and dynamics of αvβ3 integrin clustering in living cells. J Cell Biol 171: 383–392, 2005. doi: 10.1083/jcb.200503017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobo T, Viloria CG, Solares L, Fontanil T, González-Chamorro E, De Carlos F, Cobo J, Cal S, Obaya AJ. Role of periostin in adhesion and migration of bone remodeling cells. PLoS One 11: e0147837, 2016. doi: 10.1371/journal.pone.0147837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics 9: 548–555, 2008. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley BD Jr, Smardo FL Jr, Grantham JJ, Calvet JP. Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc Natl Acad Sci USA 84: 8394–8398, 1987. doi: 10.1073/pnas.84.23.8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford J, Nygard K, Gan BS, O’Gorman DB. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp Dermatol 24: 120–126, 2015. doi: 10.1111/exd.12601. [DOI] [PubMed] [Google Scholar]
- 13.Elliott CG, Wang J, Guo X, Xu SW, Eastwood M, Guan J, Leask A, Conway SJ, Hamilton DW. Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. J Cell Sci 125: 121–132, 2012. doi: 10.1242/jcs.087841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 6: 1034–1038, 2004. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 15.Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney Int 64: 1157–1162, 2003. doi: 10.1046/j.1523-1755.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 16.Guerrot D, Dussaule JC, Mael-Ainin M, Xu-Dubois YC, Rondeau E, Chatziantoniou C, Placier S. Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One 7: e31974, 2012. doi: 10.1371/journal.pone.0031974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Happé H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- 18.Hassane S, Leonhard WN, van der Wal A, Hawinkels LJ, Lantinga-van Leeuwen IS, ten Dijke P, Breuning MH, de Heer E, Peters DJ. Elevated TGFβ-Smad signalling in experimental Pkd1 models and human patients with polycystic kidney disease. J Pathol 222: 21–31, 2010. doi: 10.1002/path.2734. [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res 14: 1239–1249, 1999. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, Sun C, Xia G. Periostin Mediates TGF-β-iduced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem 36: 799–809, 2015. doi: 10.1159/000430139. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Suzuki A, Imai E, Horimoto N, Ohnishi T, Daikuhara Y, Hori M. Tornado extraction: a method to enrich and purify RNA from the nephrogenic zone of the neonatal rat kidney. Kidney Int 62: 763–769, 2002. doi: 10.1046/j.1523-1755.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- 22.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal 3: 125–133, 2009. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, Hamada S, Satoh A, Egawa S, Motoi F, Unno M, Shimosegawa T. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer 122: 2707–2718, 2008. doi: 10.1002/ijc.23332. [DOI] [PubMed] [Google Scholar]
- 24.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 68: 3201–3207, 2011. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 13: 962–969, 2007. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 26.Laczko R, Szauter KM, Jansen MK, Hollosi P, Muranyi M, Molnar J, Fong KS, Hinek A, Csiszar K. Active lysyl oxidase (LOX) correlates with focal adhesion kinase (FAK)/paxillin activation and migration in invasive astrocytes. Neuropathol Appl Neurobiol 33: 631–643, 2007. doi: 10.1111/j.1365-2990.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 27.Leonhard WN, Zandbergen M, Veraar K, van den Berg S, van der Weerd L, Breuning M, de Heer E, Peters DJ. Scattered deletion of PKD1 in kidneys causes a cystic snowball effect and recapitulates polycystic kidney disease. J Am Soc Nephrol 26: 1322–1333, 2015. doi: 10.1681/ASN.2013080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009. doi: 10.1681/ASN.2008090930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol 25: 77–83, 2005. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 30.Litvin J, Zhu S, Norris R, Markwald R. Periostin family of proteins: therapeutic targets for heart disease. Anat Rec A Discov Mol Cell Evol Biol 287: 1205–1212, 2005. doi: 10.1002/ar.a.20237. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7: 684–696, 2011. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013. doi: 10.1038/ng.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malas TB, Formica C, Leonhard WN, Rao P, Granchi Z, Roos M, Peters DJ, ’t Hoen PA. Meta-analysis of polycystic kidney disease expression profiles defines strong involvement of injury repair processes. Am J Physiol Renal Physiol 312: F806–F817, 2017. doi: 10.1152/ajprenal.00653.2016. [DOI] [PubMed] [Google Scholar]
- 34.Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 285: 13294–13303, 2010. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68, 2005. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 36.Morla L, Brideau G, Fila M, Crambert G, Cheval L, Houillier P, Ramakrishnan S, Imbert-Teboul M, Doucet A. Renal proteinase-activated receptor 2, a new actor in the control of blood pressure and plasma potassium level. J Biol Chem 288: 10124–10131, 2013. doi: 10.1074/jbc.M112.446393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moskowitz DW, Bonar SL, Liu W, Sirgi CF, Marcus MD, Clayman RV. Epidermal growth factor precursor is present in a variety of human renal cyst fluids. J Urol 153: 578–583, 1995. doi: 10.1016/S0022-5347(01)67652-3. [DOI] [PubMed] [Google Scholar]
- 38.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int 73: 269–277, 2008. doi: 10.1038/sj.ki.5002629. [DOI] [PubMed] [Google Scholar]
- 39.Naik PK, Bozyk PD, Bentley JK, Popova AP, Birch CM, Wilke CA, Fry CD, White ES, Sisson TH, Tayob N, Carnemolla B, Orecchia P, Flaherty KR, Hershenson MB, Murray S, Martinez FJ, Moore BB; COMET Investigators . Periostin promotes fibrosis and predicts progression in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L1046–L1056, 2012. doi: 10.1152/ajplung.00139.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101: 695–711, 2007. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 3: 275–286, 2009. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int 47: 490–499, 1995. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- 43.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303: 836–839, 2004. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 44.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pei Y. Practical genetics for autosomal dominant polycystic kidney disease. Nephron Clin Pract 118: c19–c30, 2011. doi: 10.1159/000320887. [DOI] [PubMed] [Google Scholar]
- 46.Raman A, Reif GA, Dai Y, Khanna A, Li X, Astleford L, Parnell SC, Calvet JP, Wallace DP. Integrin-linked kinase signaling promotes cyst growth and fibrosis in polycystic kidney disease. J Am Soc Nephrol 28: 2708–2719, 2017. doi: 10.1681/ASN.2016111235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rankin CA, Grantham JJ, Calvet JP. C-fos expression is hypersensitive to serum-stimulation in cultured cystic kidney cells from the C57BL/6J-cpk mouse. J Cell Physiol 152: 578–586, 1992. doi: 10.1002/jcp.1041520318. [DOI] [PubMed] [Google Scholar]
- 48.Ray MA, Johnston NA, Verhulst S, Trammell RA, Toth LA. Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci 49: 282–288, 2010. [PMC free article] [PubMed] [Google Scholar]
- 49.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1013, 2011. doi: 10.1152/ajprenal.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren Y, Cao B, Law S, Xie Y, Lee PY, Cheung L, Chen Y, Huang X, Chan HM, Zhao P, Luk J, Vande Woude G, Wong J. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res 11: 6190–6197, 2005. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 51.Satirapoj B, Tassanasorn S, Charoenpitakchai M, Supasyndh O. Periostin as a tissue and urinary biomarker of renal injury in type 2 diabetes mellitus. PLoS One 10: e0124055, 2015. doi: 10.1371/journal.pone.0124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant 27: 2702–2711, 2012. doi: 10.1093/ndt/gfr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol 179: 1756–1767, 2011. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 24: 3992–4003, 2004. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 107: 14170–14175, 2010. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18: 2328–2343, 2009. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 57.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118: 98–104, 2006. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 58.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16: 46–51, 2005. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 59.Tasic B, Hippenmeyer S, Wang C, Gamboa M, Zong H, Chen-Tsai Y, Luo L. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc Natl Acad Sci USA 108: 7902–7907, 2011. doi: 10.1073/pnas.1019507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Togawa H, Nakanishi K, Mukaiyama H, Hama T, Shima Y, Sako M, Miyajima M, Nozu K, Nishii K, Nagao S, Takahashi H, Iijima K, Yoshikawa N. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am J Physiol Renal Physiol 300: F511–F520, 2011. doi: 10.1152/ajprenal.00038.2010. [DOI] [PubMed] [Google Scholar]
- 61.Vallenius T. Actin stress fibre subtypes in mesenchymal-migrating cells. Open Biol 3: 130001, 2013. doi: 10.1098/rsob.130001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijayakumar S, Dang S, Marinkovich MP, Lazarova Z, Yoder B, Torres VE, Wallace DP. Aberrant expression of laminin-332 promotes cell proliferation and cyst growth in ARPKD. Am J Physiol Renal Physiol 306: F640–F654, 2014. doi: 10.1152/ajprenal.00104.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker JT, McLeod K, Kim S, Conway SJ, Hamilton DW. Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res 365: 453–465, 2016. doi: 10.1007/s00441-016-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallace DP, Grantham JJ, Sullivan LP. Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996. doi: 10.1038/ki.1996.445. [DOI] [PubMed] [Google Scholar]
- 65.Wallace DP, Hou YP, Huang ZL, Nivens E, Savinkova L, Yamaguchi T, Bilgen M. Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int 73: 778–781, 2008. doi: 10.1038/sj.ki.5002771. [DOI] [PubMed] [Google Scholar]
- 66.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 85: 845–854, 2014. doi: 10.1038/ki.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe T, Yasue A, Fujihara S, Tanaka E. PERIOSTIN regulates MMP-2 expression via the αvβ3 integrin/ERK pathway in human periodontal ligament cells. Arch Oral Biol 57: 52–59, 2012. doi: 10.1016/j.archoralbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 70.Weimbs T. Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair? Cell Cycle 5: 2425–2429, 2006. doi: 10.4161/cc.5.21.3408. [DOI] [PubMed] [Google Scholar]
- 71.Weimbs T, Olsan EE, Talbot JJ. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAKSTAT 2: e23650, 2013. doi: 10.4161/jkst.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson PD, Hreniuk D, Gabow PA. Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992. doi: 10.1002/jcp.1041500220. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279: 40419–40430, 2004. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 74.Zheng QM, Lu JJ, Zhao J, Wei X, Wang L, Liu PS. Periostin facilitates the epithelial-mesenchymal transition of endometrial epithelial cells through ILK-Akt signaling pathway. BioMed Res Int 2016: 9842619, 2016. doi: 10.1155/2016/9842619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou X, Borén J, Akyürek LM. Filamins in cardiovascular development. Trends Cardiovasc Med 17: 222–229, 2007. doi: 10.1016/j.tcm.2007.08.001. [DOI] [PubMed] [Google Scholar]