Abstract
Myogenic contraction of renal arterioles is an important regulatory mechanism for renal blood flow autoregulation. We have previously demonstrated that integrin-mediated mechanical force increases the occurrence of Ca2+ sparks in freshly isolated renal vascular smooth muscle cells (VSMCs). To further test whether the generation of Ca2+ sparks is a downstream signal of mechanotransduction in pressure-induced myogenic constriction, the relationship between Ca2+ sparks and transmural perfusion pressure was investigated in intact VSMCs of pressurized rat afferent arterioles. Spontaneous Ca2+ sparks were found in VSMCs when afferent arterioles were perfused at 80 mmHg. The spark frequency was significantly increased when perfusion pressure was increased to 120 mmHg. A similar increase of spark frequency was also observed in arterioles stimulated with β1-integrin-activating antibody. Moreover, spark frequency was significantly higher in arterioles of spontaneous hypertensive rats at 80 and 120 mmHg. Spontaneous membrane current recorded using whole cell perforated patch in renal VSMCs showed predominant activity of spontaneous transient inward currents instead of spontaneous transient outward currents when holding potential was set close to physiological resting membrane potential. Real-time PCR and immunohistochemistry confirmed the expression of Ca2+-activated Cl− channel (ClCa) TMEM16A in renal VSMCs. Inhibition of TMEM16A with T16Ainh-A01 impaired the pressure-induced myogenic contraction in perfused afferent arterioles. Our study, for the first time to our knowledge, detected Ca2+ sparks in VSMCs of intact afferent arterioles, and their frequencies were positively modulated by the perfusion pressure. Our results suggest that Ca2+ sparks may couple to ClCa channels and trigger pressure-induced myogenic constriction via membrane depolarization.
Keywords: afferent arteriole, confocal fluorescence microscopy, integrin, myogenic response, TMEM16A
INTRODUCTION
Myogenic response and tubuloglomerular feedback are the two primary mechanisms for renal blood flow autoregulation by regulating the afferent arteriolar vascular resistance (22). The macula densa in the early distal tubule in each nephron detects the flow-dependent change of luminal NaCl concentration and accordingly adjusts the afferent arteriolar resistance via the tubuloglomerular feedback mechanism (4). However, the mechanisms of mechanotransduction in pressure-induced myogenic response are not well defined in afferent arterioles (10). Many studies of myogenic response mechanotransduction have focused on the mechanosensitive gating of stretch-sensitive channels in triggering membrane depolarization and Ca2+ influx. However, the mechanical stretch on the mechanosensitive ion channels cannot provide a sustained error signal to maintain the myogenic constriction when the stretch vanishes as the vessel contracts (11, 12, 46).
Integrins are heterodimeric transmembrane proteins composed of α and β subunits, which provide the structural link between the extracellular matrix and internal cytoskeleton. Integrins are signaling receptors for both outside-in and inside-out signal transduction. In renal arterioles and renal arterial smooth muscle cells (SMCs), integrins play an important role in mechanotransduction for the myogenic response. We have previously demonstrated that the integrin binding peptide Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) can cause acute arteriolar vasoconstriction, and prolonged exposure of the peptide blocked the myogenic constrictor response in renal arterioles (1, 2, 53). Incubation of β1 or β3-integrin antibodies effectively inhibited the GRGDSP-induced Ca2+ response in renal vascular SMCs (VSMCs) (7). We have also shown that integrin-mediated mechanical force and the activation of the α5β1 integrin triggers local Ca2+ release from ryanodine receptors in the form of Ca2+ sparks in freshly isolated renal VSMCs (1). Ca2+ sparks are local Ca2+ signals originating from the coordinated opening of a group or cluster of ryanodine receptors in the sarcoplasmic reticulum (SR) (8). Ca2+ sparks in the sarcoplasmic-SR junctions of VSMCs have been shown to induce membrane depolarization and hyperpolarization by coupling to the Ca2+-activated Cl− channel (ClCa) and Ca2+-activated K channel (KCa), respectively (23, 42, 47). The manifestation of Ca2+ spark activity in membrane potential as depolarization or hyperpolarization is vascular bed-specific. For example, Ca2+ spark induces membrane hyperpolarization in cerebral artery VSMCs (23) but triggers membrane depolarization in pulmonary VSMCs (40, 42, 56). However, the effect of Ca2+ sparks on membrane potential of renal VSMCs is unclear. Membrane depolarization is the hallmark of pressure-induced myogenic constriction (36). It is intriguing that integrins on the plasma membrane of renal VSMCs may serve as mechanotransducers in pressure-induced myogenic constriction by generating Ca2+ sparks to initiate the membrane depolarization required for myogenic vasoconstriction.
To investigate whether Ca2+ sparks are involved in pressure-induced myogenic constriction in afferent arterioles, we sought to determine whether there are spontaneous Ca2+ sparks in intact VSMCs of perfused afferent arterioles and whether their occurrence is dependent on the intravascular perfusion pressure. Our results demonstrated for the first time, to our knowledge, that there are spontaneous Ca2+ sparks in VSMCs of intact afferent arteriole and that the frequency of occurrence is pressure-dependent. Moreover, the presence of the downstream effector TMEM16A for Ca2+ spark to trigger membrane depolarization was substantiated by real-time RT-PCR detection and immunolocalization of TMEM16A expression in renal afferent arterioles, electrophysiological recording of ClCa current in renal VSMCs, and pharmacological inhibition of TMEM16A in isolated pressurized afferent arterioles.
MATERIALS AND METHODS
Perfusion of afferent arterioles and Ca2+ spark detection.
All experiments were performed under protocols approved by the University of South Florida's Animal Care and Use Committee. Male Sprague-Dawley rats (120–200 g) and male spontaneous hypertensive rats (SHRs) (age 12 wk) from Harlan Laboratories were euthanized by anesthetic overdose (5% isoflurane in a chamber through a vaporizer). The kidneys were quickly removed and placed in ice-cold dissection buffer. A segment of afferent arteriole (300–400 μm) of a juxtamedullary nephron just proximal to a glomerulus was then dissected, cannulated, and perfused in a temperature-controlled perfusion chamber (Vestavia, AL) at 37°C (53, 54). The perfusion chamber was mounted on a Leica inverted microscope, which was coupled to a Leica TCS SP5 confocal imaging system. The afferent arteriole was then loaded with the calcium-indicator dye fluo-4/AM (10 μM, Molecular Probes) for 30 min, followed by 20 min of washing for de-esterification. The intraluminal pressure of the vessel was initially set at 80 mmHg. Vessels were discarded if there was fluid leakage. After vascular tone was established in the perfused vessel, confocal line scan images were collected (2 ms/scan line) immediately under the plasma membrane of VSMCs with a 63× water immersion objective (NA 1.2) for up to 16 s. Perfusion pressure was then increased from 80 to 120 mmHg in a single step. Collection of the line scan images was repeated after focal plane adjustment was made to compensate focal plane shift due to change in perfusion pressure. Ca2+ sparks in the line scan images were first identified with an algorithm developed by Bankhead et al. (3) for detecting Ca2+ sparks on varying baseline (28). The spatial and temporal characteristics of identified Ca2+ sparks were extracted with an ImageJ plugin SparkMaster (39). The composition of the dissecting solution consisted of (in mM): 115 NaCl, 25 NaHCO3, 2.5 K2HPO4, 1.2 MgSO4, 1.8 CaCl2, 5.5 glucose, 2.0 pyruvic acid, and 1 g/dl dialyzed bovine serum albumin (fraction V, Calbiochem). The luminal perfusate and bathing solutions were identical to the dissecting solution, except that no bovine serum albumin was added to the bathing solution. All solutions were gassed with 5% CO2 and 95% O2 before use, and pH was adjusted to 7.4. To monitor the pressure-induced constriction in perfused afferent arteriole, no fluo-4/AM was loaded into the vessel. The change of luminal diameter was measured from the transmitted light images using an edge-detecting algorithm based on covariance, as previously reported (53).
Electrophysiological studies in isolated renal VSMCs.
Renal VSMCs were isolated enzymatically from dissected interlobular arteries, which are capable of responding to changes in perfusion pressure (6), and the yield of isolated cells from these arterioles was much higher than the afferent arterioles (16). The arteries were first digested with 0.15% papain (Sigma-Aldrich) for 6 min and then digested with 0.2% collagenase type 4 (Worthington), 0.1% trypsin inhibitor (Sigma-Aldrich), and 0.05% elastase (Sigma-Aldrich) for 4 to 6 min. All digestions were performed in low calcium dissociation solution containing (in mM): 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.18 KH2PO4, 24 NaHCO3, 5.5 glucose, 10 HEPES, and 50 µM CaCl2, pH 7.4 at 37°C. The vessels were then triturated, and the freshly isolated VSMCs were collected and plated on Petri dishes coated with Matrigel (BD Bioscience). Electrophysiological experiments were conducted as previously reported (40). In brief, renal VSMCs were bathed in modified Tyrode’s solution containing (in mM): 137 NaCl, 5.4 KCl, 1 MgCl2, 2 Ca2+, 10 HEPES, and 10 glucose, pH 7.4 (adjusted with NaOH). They were voltage-clamped using the perforated patch technique to avoid disturbance of Ca2+ dynamics resulting from intracellular dialysis. Gigaohm seals were established with patch pipettes tip-filled with a solution containing (in mM): 35 KCl, 90 potassium gluconate, 10 NaCl, and 10 HEPES, pH 7.2 (adjusted with KOH) and were backfilled with the same solution containing 300 mg/ml amphotericin B. After an access resistance of <10 MΩ was achieved, the cell was voltage-clamped at a holding potential (Vh) of −50 mV with an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Spontaneous transient currents were recorded at a Vh of −30 mV, −60 mV, or −90 mV. Signals were filtered at 5 kHz, digitized with a Digidata 1200 analog-to-digital converter, and analyzed with pCLAMP software.
Isolation of preglomerular arteries for RT-PCR.
The abdominal aorta distal to the renal arteries was cannulated, and paramagnetic beads (2 µm diameter, Polyscience) in calcium-free HBSS were perfused into the kidney. The kidneys were then removed, and the cortex was harvested. The tissue was minced and transferred to HBSS. Paramagnetic bead-containing tissue was isolated from the suspension with a side-pull magnet and resuspended in HBSS. The suspension was passed through needles of decreasing size (18 gauge, 20 gauge, and 23 gauge) and filtered on a 200-mesh sieve. The tissue that remained on the sieve was then used for RT-PCR. Total RNA was then extracted from renal microvessels, and first-strand cDNA was synthesized as described (49). Gene-specific primers were used to amplify TMEM16A (5′-TGACGAGGATACCAAAATCCA-3′ and 5′-CGGGTCTCACTGATGTGGT-3′) and 18S rRNA (5′-CGGCTACCACATCCAAGGAA-3′ and 5′-AGCTGG AATTACCGCGGC-3′). Real-time PCR reactions were performed with SYBR Green PCR Master Mix on an iQ5 Real-Time PCR Detection System. 18S rRNA was used for internal control. The TMEM16A mRNA level was expressed as their respective copy number relative to that of 18S rRNA for each sample obtained in the same run (42).
Data analysis.
The duration and width of Ca2+ sparks were quantified as the full duration half maximum (FDHM) and full width half maximum (FWHM), respectively (40). FDHM is the duration (ms) of a Ca2+ spark where the fluorescence intensity is greater than half of its peak fluorescence intensity. FWHM is the spatial spread (µm) of a Ca2+ spark where the fluorescence intensity is greater than half of its peak fluorescence intensity. The amplitude of Ca2+ spark is defined as ΔF/F0, where F0 is the fluorescence intensity in the baseline and ΔF is the difference between the peak fluorescence intensity of the spark and F0. In intact VSMCs of small arterioles, the Ca2+ sparks can occur from the baseline and on top of prolonged elevation in [Ca2+]i (e.g., Ca2+ oscillation). The Bankhead’s algorithm was implemented with MATLAB, as described previously, to identify Ca2+ sparks with varying baseline (28). The three key parameters in Bankhead’s algorithm, the FWHM of the Gaussian spatial filter, the number of smoothing iterations, and the number of smoothing iterations to apply for estimating the baseline, were set at 10, 3, and 6, respectively. The ImageJ plugin SparkMaseter was used to extract the biophysical parameters of individual Ca2+ sparks (39). The threshold in ImageJ plugin SparkMaster was set at 3.8 × standard deviation. Ca2+ sparks were counted and included in analysis only if they were identified by both algorithms. The spark frequency of each cell was defined as the number of sparks detected per second in a scan line of 100 μm. Statistical significance (P < 0.05) was assessed by paired or unpaired Student's t-test or nonparametric Mann-Whitney U-tests, whenever applicable. Analysis of variance of regression coefficients was used to compare regression line (BMDP 1R).
RESULTS
Spontaneous Ca2+ sparks in intact VSMCs.
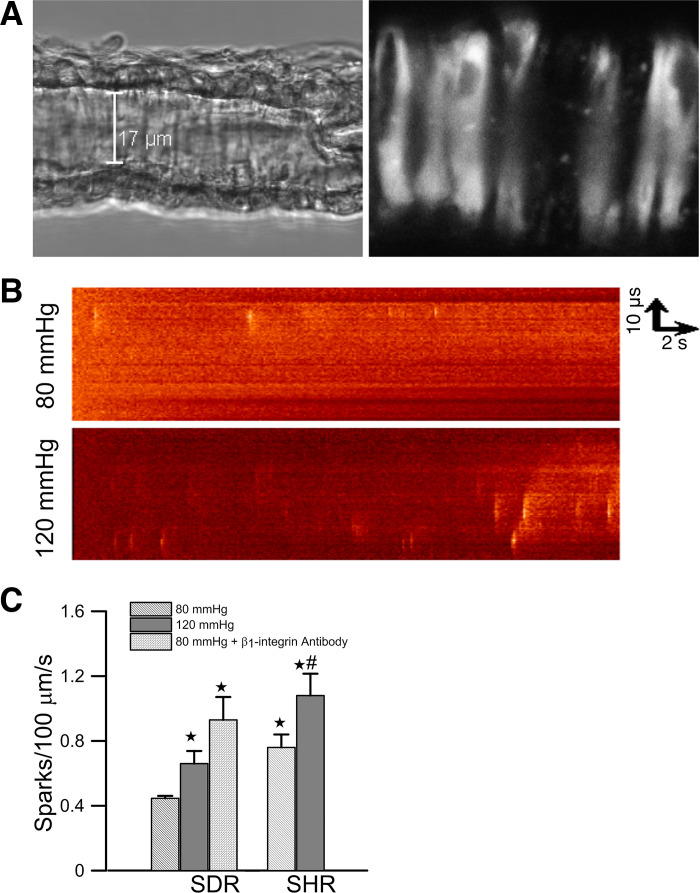
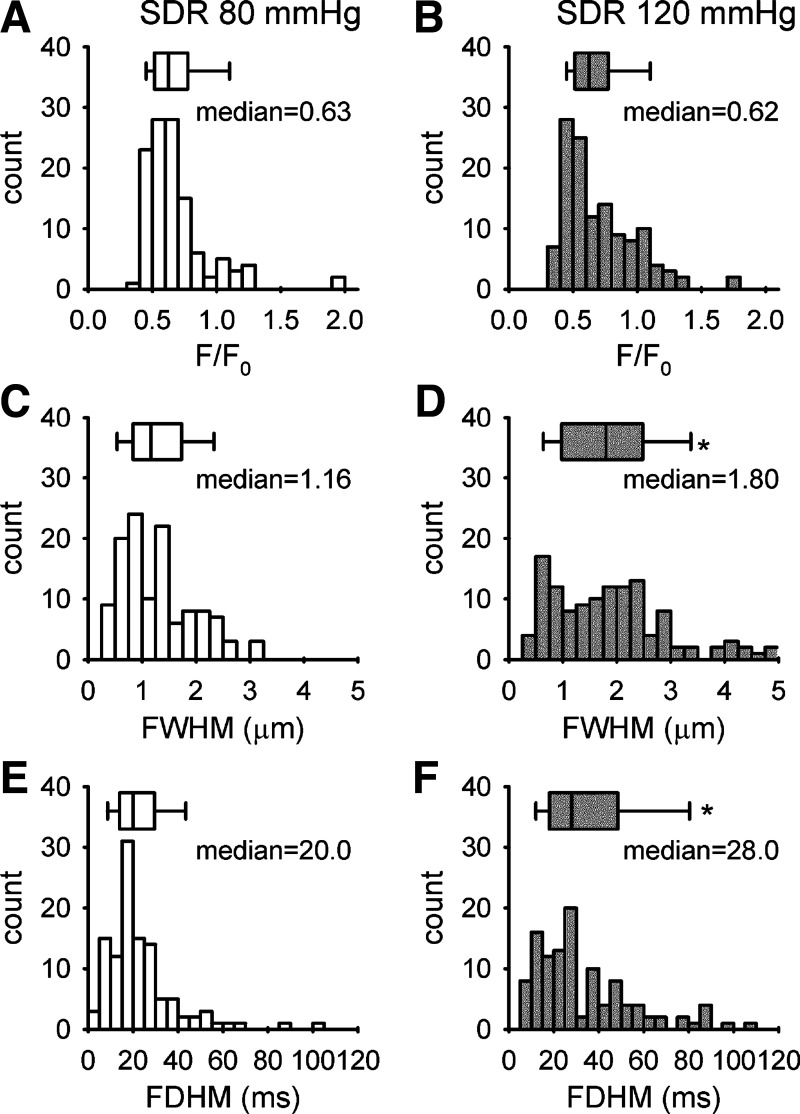
Spontaneous subcellular Ca2+ transients were monitored in intact VSMCs of pressurized afferent arteriole isolated from a Sprague-Dawley rat. The arteriole was cannulated from one end with two concentric pipettes. The other end was occluded and sealed so that there was no luminal flow. A transmitted light image and a confocal fluorescence image from the lower surface of an afferent arteriole loaded with fluo-4/AM were shown in Fig. 1A. A line scan image was collected from an individual VSMC along its longitudinal axis. Two line scan images collected from the same arteriole at two different levels of intraluminal pressure were shown in Fig. 1B. Elevation of the pressure increased the occurrence of Ca2+ sparks (bright spots). The mean Ca2+ spark frequency was increased from 0.45 ± 0.06 sparks/100 µm per second (45 cells/10 vessels) to 0.67 ± 0.12 sparks/100 µm per second (50 cells/14 vessels) when perfusion pressure was elevated from 80 mmHg to 120 mmHg (Fig. 1C). The distribution of the peak amplitude, FWHM, and FDHM of the individual Ca2+ spark was shown in Fig. 2. Ca2+ sparks (n = 120) collected at 80 mmHg and 128 Ca2+ sparks collected at 120 mmHg were analyzed. The amplitude of Ca2+ sparks (ΔF/F0) recorded at 80 mmHg (median = 0.63, 90% range = 0.42–1.25) and 120 mmHg (median = 0.62, 90% range = 0.40–1.27) were similar. In contrast, the medians of FWHM and FDHM were increased from 1.16 µm (90% range = 0.45–2.51 µm) to 1.80 µm (90% range = 0.53–4.17 µm, P < 0.001) and from 20 ms (90% range = 8–53 ms) to 28 ms (90% range = 10–94 ms, P < 0.001), respectively, when intraluminal pressure was increased to 120 mmHg.
Fig. 1.
A: differential interference contrast image and confocal fluorescence image of an afferent arteriole isolated from a Sprague-Dawley rat (SDR). The arteriole was loaded with fluo-4/AM and perfused at 80 mmHg. Individual vascular smooth muscle cells (VSMCs) are discerned in the fluorescence image. The orientation of the scan line to collect Ca2+ sparks image is shown as a dotted line. Scale bar is 17 µm. B: line scan images of spontaneous Ca2+ spark from VSMCs of afferent arterioles. Images were collected from a single VSMC of an afferent arteriole perfused at 80 mmHg and 120 mmHg, respectively. The images were collected for 16 s from 2 different cells in the same afferent arteriole isolated from a SDR. C: effects of perfusion pressure and β1-integrin activation on the mean Ca2+ spark frequency of afferent arterioles isolated from SDRs (45 cells/10 vessels at 80 mmHg, 50 cells/14 vessels at 120 mmHg, and 13 cells/3 vessels at 80 mmHg in the presence of β1-integrin antibody) and spontaneous hypertensive rats (SHRs) (29 cells/8 vessels at 80 mmHg and 28 cells/6 vessels at 120 mmHg). *Significant difference (P < 0.05) compared with 80 mmHg in arterioles of SDRs. #Significant difference (P < 0.05) compared with 120 mmHg in arterioles of SDRs.
Fig. 2.
The distribution of Ca2+ spark amplitude (A and B), full width half maximum (FWHM) (C and D), and full duration half maximum (FDHM) (E and F) collected from afferent arterioles of a Sprague-Dawley rat (SDR) perfused at 80 mmHg (45 cells/10 vessels), and 120 mmHg (50 cells/14 vessels). Box plots show the median and range of the parameters. The median of amplitude, FWHM, and FDHM are 0.63, 1.16 µm, and 20 ms at 80 mmHg (n = 120 sparks) and 0.62, 1.73 µm, and 26 ms at 120 mmHg (n = 128 sparks), respectively. *Significant difference (P < 0.001) between 80 mmHg and 120 mmHg.
Previous studies showed that the blockade of α5β1 and αVβ5 integrin inhibits myogenic response in rat cremaster arteriole (35), but a separate study showed that integrin-binding peptides containing Arg-Gly-Asp (RGD) trigger vasodilation via αVβ5 in the same preparation (37). We have also shown that the direct activation of α5β1 integrin in isolated renal arterial SMCs by mechanical pulling with paramagnetic beads coated with fibronectin or by application of α5β1 integrin antibodies triggers Ca2+ spark (1). Furthermore, α5β1 has been implicated in the myogenic response in cerebral resistance arteries (9), suggesting it is an important integrin subtype for the generation of myogenic response in different vascular beds. To test whether the activation of α5β1 integrin can trigger Ca2+ spark in VSMCs of intact afferent arterioles, an activating antibody of β1 integrin (clone Ha2/5, PharMingen) was applied to the perfusion bath (50 µg/ml) when the intraluminal pressure was set at 80 mmHg. In the presence of β1-integrin antibody, the spontaneous Ca2+ spark frequency is 0.92 ± 0.23 sparks/100 µm per second (13 cells/3 vessels), which was significantly higher (P < 0.05) than that of the control (Fig. 1C). The median values of the peak amplitude (median = 0.65, 90% range = 0.44–1.20, n = 75), FWHM (median = 1.05, 90% range = 0.39–2.38 µm), and FDHM (median = 24, 90% range = 3–50 ms) were similar to those found at a perfusion pressure of 80 mmHg. These observations suggested that the increased occurrence of Ca2+ spark in VSMCs is a robust response to an increase of intraluminal pressure or the activation of α5β1 integrin. This is consistent with the hypothesis that Ca2+ spark is an integral part of the mechanical signal transduction process in myogenic response.
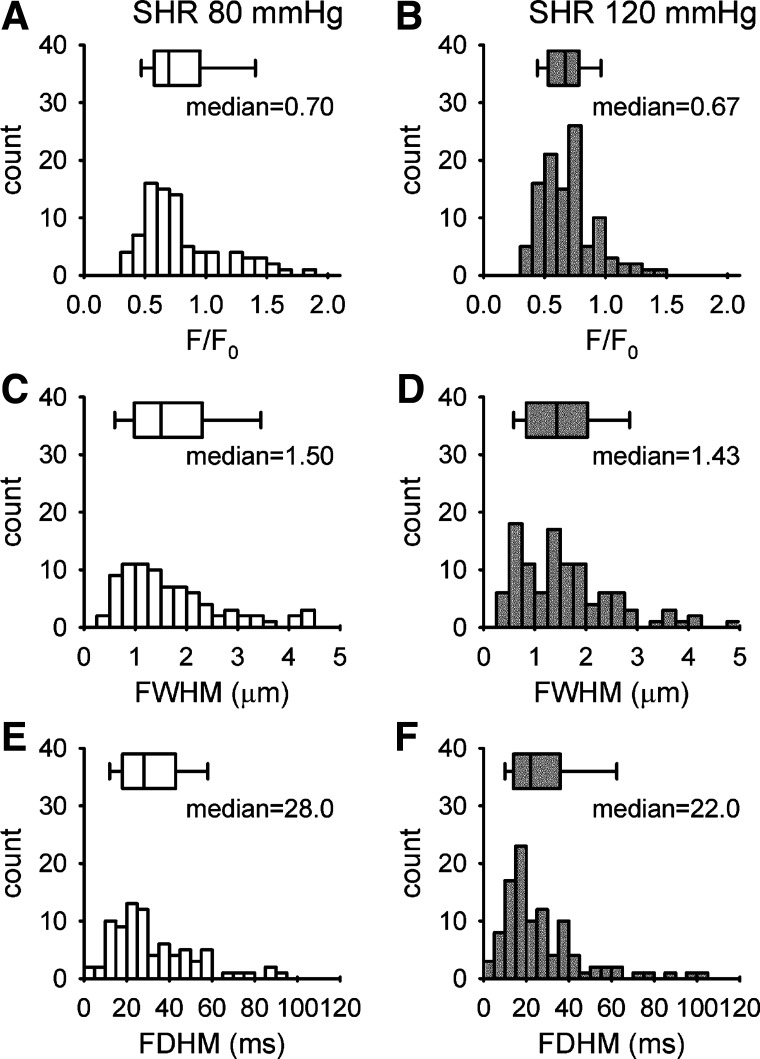
Myogenic response is known to be enhanced in SHRs (19). To further study the relationship between myogenic response and Ca2+ spark, we monitored the Ca2+ sparks in response to the elevation of pressure in the afferent arterioles isolated from SHRs. Spark frequency was significantly higher in afferent arterioles of SHRs compared with Sprague-Dawley rats when measured at the same pressure (Fig. 1C). It was 0.76 ± 0.13 sparks/100 µm per second at 80 mmHg (29 cells/8 vessels) and 1.08 ± 0.22 sparks/100 µm per second at 120 mmHg (29 cells/6 vessels), respectively. The distribution of the amplitude, FWHM, and FDHM of Ca2+ sparks detected in SHRs was shown in Fig. 3. At 80 mmHg, the amplitude of Ca2+ spark was similar (median = 0.7, 90% range = 0.43–1.52), whereas the FWHM (median = 1.50, 90% range = 0.53–4.40 µm, P < 0.001) and FDHM (median = 28, 90% range = 11–75 ms, P < 0.001) were significantly larger than those found in Sprague-Dawley rats. However, these spatiotemporal parameters of Ca2+ sparks were not significantly altered at the elevated pressure. These observations are consistent with the notion that the enhanced myogenic response in the afferent arterioles of SHRs is associated with increased frequency of Ca2+ spark.
Fig. 3.
The distribution of Ca2+ spark amplitude (A and B), full width half maximum (FWHM) (C and D), and full duration half maximum (FDHM) (E and F) collected from afferent arterioles of a spontaneous hypertensive rat (SHR) perfused at 80 mmHg (29 cells/8 vessels) and 120 mmHg (28 cells/6 vessels). The median of amplitude, FWHM, and FDHM are 0.7, 1.5 µm, and 28 ms at 80 mmHg (n = 84 sparks) and 0.67, 1.43 µm, and 22 ms at 120 mmHg (n = 107 sparks). Box plots show the median and range of the parameters. There is no significant difference in the amplitude, FWHM, and FDHM recorded at 80 mmHg and 120 mmHg.
Spontaneous transient inward currents and spontaneous transient outward currents in isolated renal VSMCs.
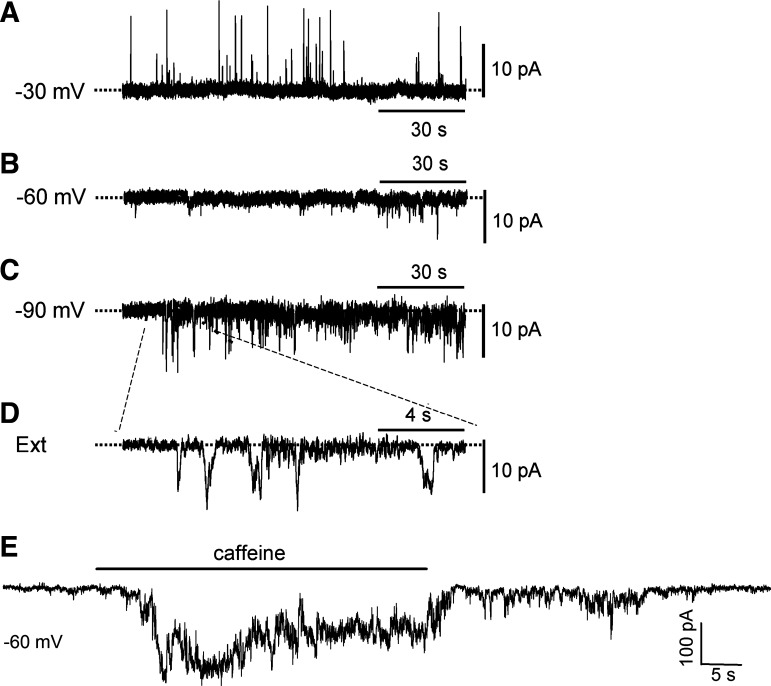
Ca2+ sparks in VSMCs can trigger spontaneous transient inward currents (STICs) and membrane depolarization when they are coupled to ClCa channels on the plasma membrane or trigger spontaneous transient outward currents (STOCs) and membrane hyperpolarization if they activate the KCa channels (47). Spontaneous membrane currents were recorded in renal arterial SMCs using whole cell perforated patch technique at a different Vh (Fig. 4). STOCs were detected at a Vh of −30 mV (~equilibrium potential for Cl−) (Fig. 4A), whereas STICs were recorded at a Vh of −90 mV (~equilibrium potential for K+) (Fig. 4, C and D). However, only STICs, but not STOCs, were found at a Vh of −60 mV (Fig. 4B), which is close to the resting membrane potential measured in renal arteries and renal VSMCs (15, 21). Furthermore, application of 0.5 mM caffeine to activate Ca2+ sparks at −60 mM elicited a prominent inward current and dramatically increased STIC activity (Fig. 4E). These observations suggested that the STICs are predominant over STOCs at the resting membrane potential and that Ca2+ sparks are most likely to trigger STICs and membrane depolarization in renal arterial SMCs.
Fig. 4.
Detection of spontaneous transient inward currents (STICs) and spontaneous transient outward currents (STOCs) in renal arterial smooth muscle cells (SMCs). The spontaneous membrane currents were recorded with perforated patch at different holding potential (Vh) in renal arterial SMCs with a pipette solution containing 120 mM K+ and 40 mM Cl− in bath-contained modified Tyrode’s solution. STOCs were detected at a Vh of −30 mV (~equilibrium potential for Cl−) (A), and STICs were detected at a Vh of −90 mV (~equilibrium potential for K+) (C and D). In contrast, only STICs, but not STOCs, were found at a Vh of −60 mV (~physiological resting membrane potential) (B). Increase in Ca2+ spark activity by applying a submaximal concentration of caffeine (0.5 mM) to cells holding at −60 mV elicited a prominent inward current and a dramatic increase in STICs without an apparent activation of STOC (E).
Expression and function of TMEM16A mRNA in renal arterioles.
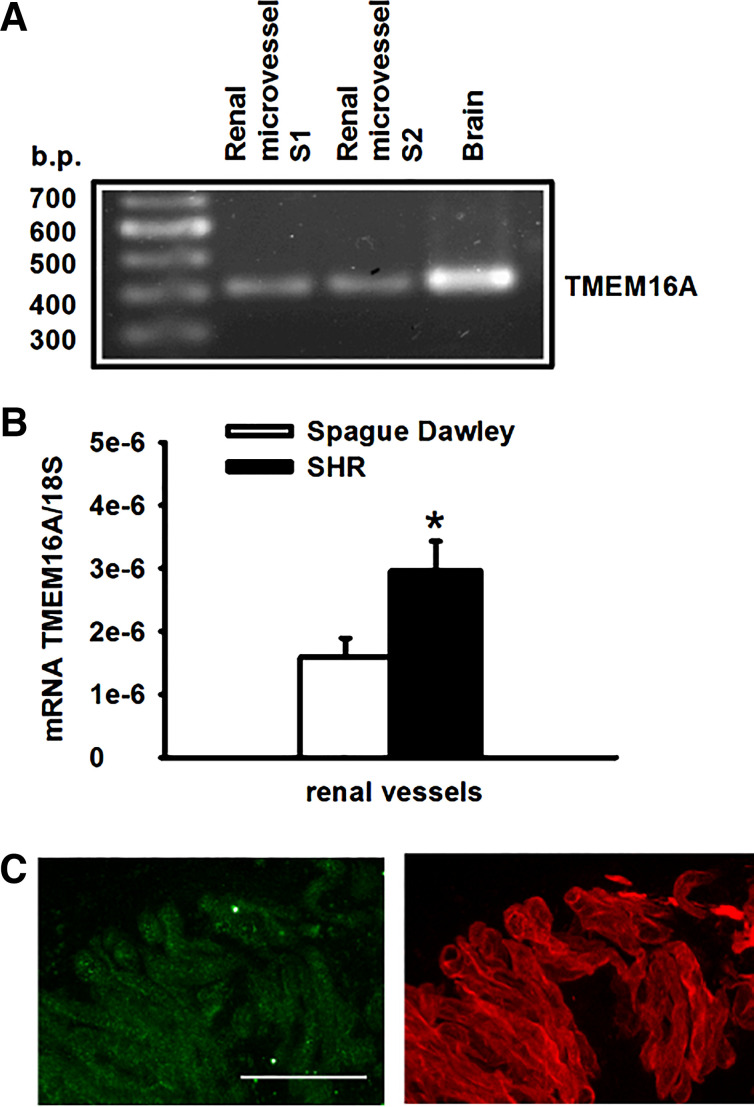
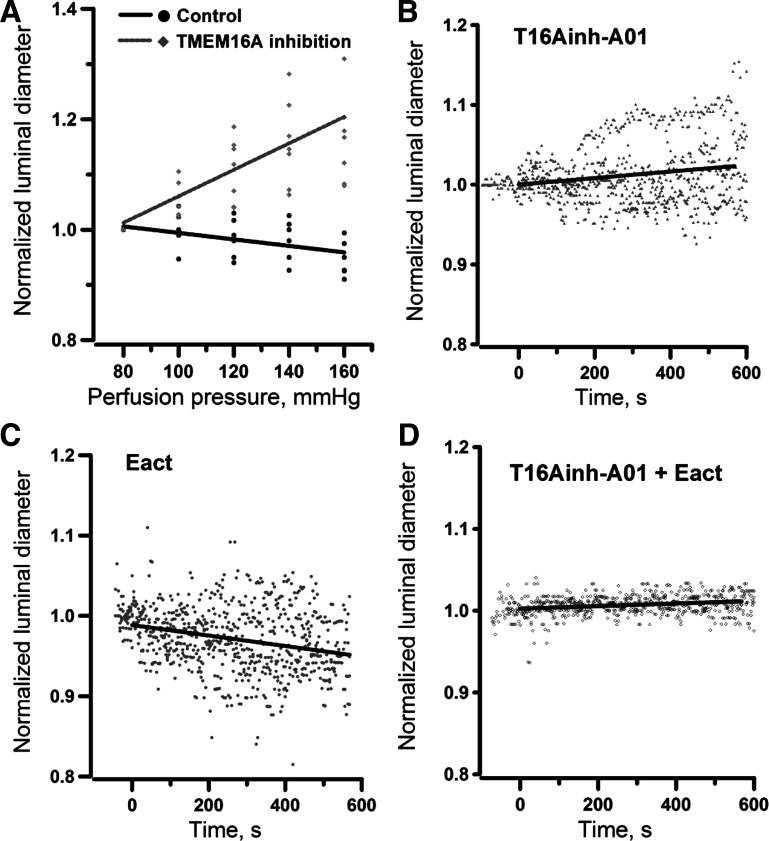
The molecular identity of the ClCa channel was recently confirmed as the transmembrane protein TMEM16A, also known as ANO1 (5, 50). TMEM16A transcripts were detected in preglomerular vessels (Fig. 5A). Using real-time RT-PCR to quantify the abundance of TMEM16A mRNA, TMEM16A expression was found significantly higher (P < 0.05) in microvessels isolated from SHRs than those from Sprague-Dawley rats (Fig. 5B). Immunofluorescence staining using a specific anti-TMEM16A antibody (Abcam, ab53212) in partially digested renal arterioles also confirmed the expression of TMEM16A protein in renal VSMCs (Fig. 5C). To further examine the functional role of TMEM16A in pressure-induced myogenic vasoconstriction, the changes in luminal diameter of afferent arterioles in response to increasing intraluminal pressure was monitored in the absence or presence of a small molecule TMEM16A inhibitor (T16Ainh-A01, 10 µM, Sigma-Aldrich). The elevation of intraluminal pressure was associated with a decrease in luminal diameter in the absence of the inhibitor, consistent with activation of myogenic response (Fig. 6A). However, the pressure-induced vasoconstriction was reversed to dilatation in the presence of T16inh-A01, indicating that the myogenic response was impaired. To test whether the activity of TMEM16A is relevant to the regulation of vascular resistance of afferent arterioles under basal condition, the effects of TMEM16A inhibition and activation were examined in arterioles perfused at 80 mmHg. Inhibition of TMEM16A with T16inh-01 triggered a subtle but significant vasodilatation (Fig. 6B). Conversely, activation of TMEM16A with Eact (10 µM, Sigma-Aldrich) induced a small but significant constriction in afferent arterioles perfused at 80 mmHg (Fig. 6C), which was abrogated when the arterioles were preincubated with T16inh-A01 (Fig. 6D). Analysis of variance of the regression coefficients was performed and shown in Table 1. The variance analysis indicated that the regression line of changes in luminal diameter induced by Eact was significantly different in the absence and presence of T16Ainh-A01.
Fig. 5.
Identification of TMEM16A mRNA in renal microvessels by real-time PCR (A), and the relative abundance of TMEM16A between a Sprague-Dawley rat and a spontaneous hypertensive rat (SHR) (B). Immunofluorescence of TMEM16A (green) and α-actin (red) in renal vascular smooth muscle cells on a partially digested renal arteriole (C). Scale bar is 30 µm. *Significant difference (n = 8, P < 0.05).
Fig. 6.
Inhibition of pressure-induced myogenic constriction in rat afferent arterioles by TMEM16A antagonist (7 vessels) (A). Time courses of change in normalized luminal diameter when afferent arterioles were exposed to a TMEM16A antagonist (T16Ainh-A01, 10 µM, 5 vessels) (B), TMEM16A agonist (Eact, 10 µM, 5 vessels) (C), and Eact after preincubation of T16Ainh-A01 (5 vessels) (D). All experiments in B, C, and D were performed at the perfusion pressure of 80 mmHg. Agonist and antagonist were administered to the perfusion bath at time = 0. The straight lines are regression lines. Analysis of variance of regression coefficients indicate that each group has different regression equations as shown in Table 1.
Table 1.
Analysis of variance of regression coefficients of Fig. 6
| Number of Vessels | Intercept | Regression Coefficient, s−1 | |
|---|---|---|---|
| Pressure step, control | 7 | 1.05 | −0.00058 ± 0.00014 |
| Pressure step, T16inh-A01 | 6 | 0.82 | 0.00240 ± 0.00032* |
| Time course, T16inh-A01 | 5 | 0.99 | 0.000046 ± 0.000008a |
| Time course, Eact | 5 | 0.98 | −0.000063 ± 0.000010b |
| Time course, T16inh-A01 + Eact | 5 | 1.01 | −0.000014 ± 0.000004c |
Regression line was significantly different (P < 0.001, degree of freedom = 91) when compared with the regression line of the control.
Regression line of each time course has a different regression coefficient when compared with the other two (P < 0.001, degree of freedom = 1,021).
DISCUSSION
The present study characterized the spatial and temporal properties of spontaneous Ca2+ sparks in VSMCs of perfused afferent arterioles and showed that the spark frequency is modulated by perfusion pressure. Activation of α5β1 integrin with antibodies simulated the effect of pressure-induced activation of Ca2+ spark. This is consistent with our previous observation in freshly isolated renal VSMCs that Ca2+ sparks were triggered by either pulling the cells with fibronectin-coated paramagnetic beads or direct activation of α5β1 integrin with antibodies (1). These observations suggest that an increase of transmural pressure can mechanically activate integrin in VSMCs of a perfused renal arteriole to initiate a sequence of signaling events leading to the increase of Ca2+ spark in VSMCs.
The mechanism by which the mechanical activation of α5β1 integrin triggers Ca2+ sparks in renal afferent arteriole is still unclear, but it could be related to the activation of the multifunctional enzyme CD38, which catalyzes the formation of the endogenous Ca2+-mobilizing messengers cyclic ADP-ribose (cADPR) and nicotinic acid adenosine dinucleotide phosphate (NAADP), which activate ryanodine receptors of SR and NAADP-sensitive Ca2+ release channels in endolysosomes, respectively (32). cADPR and NAADP have been shown to activate Ca2+ sparks and other local Ca2+ events in various types of VSMCs (27, 43, 55). Our previous study showed that an integrin ligand RGD peptide (GRGDSP) activates CD38 in pulmonary arterial SMCs, causing a significant increase in cADPR production and lysosomal alkalization, which was associated with Ca2+ release from the ryanodine receptor-gated Ca2+ stores and the lysosomal stores (43). It is likely that a similar mechanism may contribute to the activation of Ca2+ sparks in the myogenic response.
A hallmark of myogenic response is membrane depolarization (36), which drives the Ca2+ entry through the voltage-gate calcium channel to embark VSMC contraction. One possible mechanism of Ca2+ spark to trigger membrane depolarization is by coupling to ClCa on the plasma membrane of VSMCs. Both Ca2+-activated K+ outward current and Ca2+-activated Cl− inward current had been reported in renal VSMCs under appropriate conditions to optimize the respective currents (14, 16). We confirmed that STICs and STOCs were exclusively present in renal arterial SMCs when the membrane potential was held close to the equilibrium potential for K+ and Cl−, respectively. However, only STICs, but not STOCs, were found when membrane potential was set at −60 mV, which is close to the resting membrane potential measured in renal arteries and renal VSMCs (15, 21). These observations suggest that Ca2+ sparks are more effectively coupled to ClCa and contribute to membrane depolarization required by myogenic response. This is consistent with previous studies that showed mobilization of intracellular Ca2+ using endothelin-1 triggered Ca2+-activated Cl− inward current and membrane depolarization in renal VSMCs (16); and inhibition of Cl− channels with DIDS inhibited the contraction of afferent arterioles induced by agonist and potassium (24, 25). Collectively, these observations suggest that the activity of the ClCa, but not KCa, channel is a major determinant of the contractile state of renal VSMCs. Therefore, Ca2+ sparks might contribute to myogenic response by inducing membrane depolarization through ClCa and the subsequent Ca2+ influx via voltage-gated Ca2+ channels (18).
It has to be mentioned that our electrophysiological results from isolated renal arterial SMCs may not fully represent what is happening in VSMCs of pressured arterioles due to possible heterogeneity of ion channel distribution in the cells of interlobular arteries and arterioles, and due to differences in membrane potential of isolated arterial SMCs and VSMCs in pressurized arterioles. We have performed preliminary experiments to compare TMEM16A expression in rat renal interlobular arteries and microvessels isolated using the paramagnetic bead technique. TMEM16A/18S value was significantly higher in the renal microvessels than in the interlobular arteries (data not shown). Hence, the ClCa channel may play an even more important role in the arterioles. Moreover, ClCa and KCa channel activities are not only Ca2+-dependent but also voltage-dependent (30, 34, 38, 45). Membrane depolarization of VSMCs in the pressurized arterioles might have a dual effect for the activation of ClCa and KCa channels. Nevertheless, our results of an important role of ClCa channels in isolated renal arterial SMCs are supported by the observations in pressurized arterioles that the inhibition of TMEM16A with T16inh-A01 abrogated the pressure-induced myogenic constriction. Moreover, we have previously demonstrated that ryanodine impairs pressure-induced myogenic constriction in perfused afferent arterioles and attenuates Ca2+ spark occurrence in freshly isolated renal VSMCs (1). Hence, the inhibitory effect of T16inh-A01 strongly suggests that the ClCa channel is the downstream effector of Ca2+ sparks and is responsible for the myogenic vasoconstriction in afferent arterioles.
The latest mathematical model of myogenic response based on the cerebral artery also proposed that ClCa is present in the microdomain of mechanotransduction (29), which is consistent with our observations. However, Ca2+ sparks are known to couple to the KCa channel to induce membrane hyperpolarization in the cerebral artery as an integral part of cerebral blood flow autoregulation. We have not determined the functional role of the KCa channel in renal arterioles in this study. Previous studies showed that blocking KCa channels with tetraethylammonium did not exaggerate agonist-induced constriction in afferent arterioles (13), and the inhibition of KCa channels with iberiotoxin did not alter the baseline of renal blood flow (31), suggesting a relatively minor role of the KCa channel in renal circulation. Autoregulation of single nephron blood flow is very unique, and it could be quite different from that in the cerebral circulation. For example, using a noninvasive laser Doppler instrument to measure single nephron blood flow in the surface of rat kidney (41), we have shown that the dynamics of single nephron blood flow are determined by the nonlinear interactions between myogenic response and tubuloglomerular feedback (33, 52). The development of hypertension modifies these interactions and leads to bifurcation in the dynamics of single nephron renal blood flow (33, 51). In view of the complex mechanisms of autoregulation in renal circulation, it is conceivable that Ca2+ sparks in renal arterioles may employ a downstream mechanism that is different from the KCa channel in the cerebral circulation.
SHR is a genetic model of hypertension. The myogenic response in afferent arterioles is known to be enhanced compared with its inbreed control Wistar-Kyoto rats (19). The mean Ca2+ spark frequency was significantly higher in arterioles isolated from SHRs compared with afferent arterioles isolated from Sprague-Dawley rats with the same perfusion pressure. By employing the mRNA primer specific to rat TMEM16A, our RT-PCR data confirmed not only the presence of TMEM16A in renal preglomerular resistant vessels but also there are more copies of TMEM16A mRNA in renal arterioles of SHRs than in those of Sprague-Dawley rats. This is consistent with the previous finding that TMEM16A expression was increased in somatic and visceral arteries of SHRs when compared with Wistar-Kyoto rats (44). Thus, an enhanced myogenic response in SHRs may be associated with an increase in Ca2+ spark frequency and the increased level of TMEM16A expression. These observations are consistent with our hypothesis that Ca2+ spark activity is an integral part of signal transduction of myogenic response, and its activity is coupled to the ClCa channel. Furthermore, knockdown of TMEM16A by siRNA prevented hypertension development in SHRs, and attenuation of TMEM16A activity with T16inh-A01 decreased mean blood pressure in SHRs (44). Target disruption of TMEM16A expression in VSMCs of mice resulted in hypotension and reduced contractility in peripheral blood vessels (20). All of these observations indicate that TMEM16A plays a significant role in the regulation of basal vascular tone.
It is noteworthy that the myogenic response in afferent arterioles could be mediated by multiple mechanisms, in addition to the integrin-Ca2+spark-ClCa pathway (26). By using traction force microscopy, we have previously demonstrated that the cell traction force exerted by an individual renal arterial SMC was increased not only during but also after the cell was pulled with fibronectin-coated or β1-integrin antibody-coated paramagnetic beads (2). Remanent cell traction was unique to the integrin ligand-coated beads, and was not found in cells pulled with LDL-coated beads or noncoated beads. The remanent cell traction induced by integrin-mediated mechanotransduction is analogous to the changes of vascular resistance in pressure-induced myogenic response, in which vascular resistance remains elevated after myogenic constriction. Moreover, it has also been shown that the voltage-gated L-type Ca2+ current is acutely potentiated following α5β1 integrin activation in VSMCs (17, 48). Potentiation of the Ca2+ current will amplify the effects of membrane depolarization in myogenic constriction brought about by integrin-mediated Ca2+sparks or other mechanosensitive ion channels. The observations of the present study and previous findings clearly suggest that integrin-mediated increases in Ca2+ spark occurrence, remanent traction force, and L-type Ca2+ current in renal VSMCs may operate synergistically for signal transduction in the myogenic response of renal afferent arterioles.
GRANTS
This work was supported by an American Heart Association Grant-In-Aid (to K.-P. Yip) and NIH Grants HL-071834 and HL-011835 (to J. S. K. Sham).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.-P.Y., L.B., L.W., and J.S.K.S. performed experiments; K.-P.Y., L.B., C.K., L.R.-S., and J.S.K.S. analyzed data; K.-P.Y., R.L., and J.S.K.S. interpreted results of experiments; K.-P.Y., L.R.-S., and J.S.K.S. prepared figures; K.-P.Y. and J.S.K.S. drafted manuscript; K.-P.Y., R.L., and J.S.K.S. edited and revised manuscript; K.-P.Y., L.B., C.K., L.W., R.L., L.R.-S., and J.S.K.S. approved final version of manuscript.
REFERENCES
- 1.Balasubramanian L, Ahmed A, Lo CM, Sham JS, Yip KP. Integrin-mediated mechanotransduction in renal vascular smooth muscle cells: activation of calcium sparks. Am J Physiol Regul Integr Comp Physiol 293: R1586–R1594, 2007. doi: 10.1152/ajpregu.00025.2007. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanian L, Lo CM, Sham JS, Yip KP. Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction. Am J Physiol Cell Physiol 304: C382–C391, 2013. doi: 10.1152/ajpcell.00234.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bankhead P, Scholfield CN, Curtis TM, McGeown JG. Detecting Ca2+ sparks on stationary and varying baselines. Am J Physiol Cell Physiol 301: C717–C728, 2011. doi: 10.1152/ajpcell.00032.2011. [DOI] [PubMed] [Google Scholar]
- 4.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49: 251–273, 1987. doi: 10.1146/annurev.ph.49.030187.001343. [DOI] [PubMed] [Google Scholar]
- 5.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 6.Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F94–F102, 1990. doi: 10.1152/ajprenal.1990.258.1.F94. [DOI] [PubMed] [Google Scholar]
- 7.Chan WL, Holstein-Rathlou NH, Yip KP. Integrin mobilizes intracellular Ca2+ in renal vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C593–C603, 2001. doi: 10.1152/ajpcell.2001.280.3.C593. [DOI] [PubMed] [Google Scholar]
- 8.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 9.Colinas O, Moreno-Domínguez A, Zhu HL, Walsh EJ, Pérez-García MT, Walsh MP, Cole WC. α5-Integrin-mediated cellular signaling contributes to the myogenic response of cerebral resistance arteries. Biochem Pharmacol 97: 281–291, 2015. doi: 10.1016/j.bcp.2015.08.088. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 11.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008. doi: 10.1152/physiol.00034.2007. [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 13.Fallet RW, Bast JP, Fujiwara K, Ishii N, Sansom SC, Carmines PK. Influence of Ca2+-activated K+ channels on rat renal arteriolar responses to depolarizing agonists. Am J Physiol Renal Physiol 280: F583–F591, 2001. doi: 10.1152/ajprenal.2001.280.4.F583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebremedhin D, Kaldunski M, Jacobs ER, Harder DR, Roman RJ. Coexistence of two types of Ca2+-activated K+ channels in rat renal arterioles. Am J Physiol Renal Physiol 270: F69–F81, 1996. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- 15.Gelband CH, Hume JR. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res 71: 745–758, 1992. doi: 10.1161/01.RES.71.4.745. [DOI] [PubMed] [Google Scholar]
- 16.Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol Renal Fluid Electrolyte Physiol 266: F325–F341, 1994. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- 17.Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem 281: 14015–14025, 2006. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- 18.Harder DR, Gilbert R, Lombard JH. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am J Physiol Renal Fluid Electrolyte Physiol 253: F778–F781, 1987. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertension 19: 153–160, 1992. doi: 10.1161/01.HYP.19.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Heinze C, Seniuk A, Sokolov MV, Huebner AK, Klementowicz AE, Szijártó IA, Schleifenbaum J, Vitzthum H, Gollasch M, Ehmke H, Schroeder BC, Hübner CA. Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J Clin Invest 124: 675–686, 2014. doi: 10.1172/JCI70025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heumann P, Koenen A, Zavaritskaya O, Schütze K, Ramm A, Schlüter T, Steinbach A, Rettig R, Schubert R, Grisk O. Sympathetic denervation facilitates L-type Ca2+ channel activation in renal but not in mesenteric resistance arteries. J Hypertens 34: 692–703, 2016. doi: 10.1097/HJH.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 22.Holstein-Rathlou NH, Marsh DJ. Renal blood flow regulation and arterial pressure fluctuations: a case study in nonlinear dynamics. Physiol Rev 74: 637–681, 1994. doi: 10.1152/physrev.1994.74.3.637. [DOI] [PubMed] [Google Scholar]
- 23.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281: C439–C448, 2001. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 24.Jensen BL, Ellekvist P, Skøtt O. Chloride is essential for contraction of afferent arterioles after agonists and potassium. Am J Physiol Renal Physiol 272: F389–F396, 1997. doi: 10.1152/ajprenal.1997.272.3.F389. [DOI] [PubMed] [Google Scholar]
- 25.Jensen BL, Skøtt O. Blockade of chloride channels by DIDS stimulates renin release and inhibits contraction of afferent arterioles. Am J Physiol Renal Physiol 270: F718–F727, 1996. doi: 10.1152/ajprenal.1996.270.5.F718. [DOI] [PubMed] [Google Scholar]
- 26.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous beta and gammaENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 27.Jiang YL, Lin AH, Xia Y, Lee S, Paudel O, Sun H, Yang XR, Ran P, Sham JS. Nicotinic acid adenine dinucleotide phosphate (NAADP) activates global and heterogeneous local Ca2+ signals from NAADP- and ryanodine receptor-gated Ca2+ stores in pulmonary arterial myocytes. J Biol Chem 288: 10381–10394, 2013. doi: 10.1074/jbc.M112.423053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kan C, Yip KP, Yang H. Two-phase greedy pursuit algorithm for automatic detection and characterization of transient calcium signaling. IEEE J Biomed Health Inform 19: 687–697, 2015. doi: 10.1109/JBHI.2014.2312293. [DOI] [PubMed] [Google Scholar]
- 29.Karlin A. Membrane potential and Ca2+ concentration dependence on pressure and vasoactive agents in arterial smooth muscle: A model. J Gen Physiol 146: 79–96, 2015. doi: 10.1085/jgp.201511380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leblanc N, Ledoux J, Saleh S, Sanguinetti A, Angermann J, O’Driscoll K, Britton F, Perrino BA, Greenwood IA. Regulation of calcium-activated chloride channels in smooth muscle cells: a complex picture is emerging. Can J Physiol Pharmacol 83: 541–556, 2005. doi: 10.1139/y05-040. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson L, Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Renovascular BK(Ca) channels are not activated in vivo under resting conditions and during agonist stimulation. Am J Physiol Regul Integr Comp Physiol 292: R345–R353, 2007. doi: 10.1152/ajpregu.00337.2006. [DOI] [PubMed] [Google Scholar]
- 32.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88: 841–886, 2008. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 33.Marsh DJ, Sosnovtseva OV, Pavlov AN, Yip KP, Holstein-Rathlou NH. Frequency encoding in renal blood flow regulation. Am J Physiol Regul Integr Comp Physiol 288: R1160–R1167, 2005. doi: 10.1152/ajpregu.00540.2004. [DOI] [PubMed] [Google Scholar]
- 34.Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res 79: 295–301, 1996. doi: 10.1161/01.RES.79.2.295. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol 289: H322–H329, 2005. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 36.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991. doi: 10.1152/ajpheart.1991.261.3.H950. [DOI] [PubMed] [Google Scholar]
- 37.Mogford JE, Davis GE, Platts SH, Meininger GA. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res 79: 821–826, 1996. doi: 10.1161/01.RES.79.4.821. [DOI] [PubMed] [Google Scholar]
- 38.Pacaud P, Loirand G, Grégoire G, Mironneau C, Mironneau J. Calcium-dependence of the calcium-activated chloride current in smooth muscle cells of rat portal vein. Pflugers Arch 421: 125–130, 1992. doi: 10.1007/BF00374818. [DOI] [PubMed] [Google Scholar]
- 39.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 40.Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L433–L444, 2002. doi: 10.1152/ajplung.00468.2001. [DOI] [PubMed] [Google Scholar]
- 41.Smedley G, Yip KP, Wagner A, Dubovitsky S, Marsh DJ. A laser Doppler instrument for in vivo measurements of blood flow in single renal arterioles. IEEE Trans Biomed Eng 40: 290–297, 1993. doi: 10.1109/10.216413. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol 590: 3507–3521, 2012. doi: 10.1113/jphysiol.2012.232520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Umesh A, Thompson MA, Chini EN, Yip KP, Sham JS. Integrin ligands mobilize Ca2+ from ryanodine receptor-gated stores and lysosome-related acidic organelles in pulmonary arterial smooth muscle cells. J Biol Chem 281: 34312–34323, 2006. doi: 10.1074/jbc.M606765200. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Li C, Huai R, Qu Z. Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl− channel, contributes to spontaneous hypertension. J Mol Cell Cardiol 82: 22–32, 2015. doi: 10.1016/j.yjmcc.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Wang YX, Kotlikoff MI. Inactivation of calcium-activated chloride channels in smooth muscle by calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 94: 14918–14923, 1997. doi: 10.1073/pnas.94.26.14918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 47.Williams BA, Sims SM. Calcium sparks activate calcium-dependent Cl− current in rat corpus cavernosum smooth muscle cells. Am J Physiol Cell Physiol 293: C1239–C1251, 2007. doi: 10.1152/ajpcell.00553.2006. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5 beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem 276: 30285–30292, 2001. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- 49.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 50.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 51.Yip KP, Holstein-Rathlou NH, Marsh DJ. Chaos in blood flow control in genetic and renovascular hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F400–F408, 1991. doi: 10.1152/ajprenal.1991.261.3.F400. [DOI] [PubMed] [Google Scholar]
- 52.Yip KP, Holstein-Rathlou NH, Marsh DJ. Mechanisms of temporal variation in single-nephron blood flow in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F427–F434, 1993. doi: 10.1152/ajprenal.1993.264.3.F427. [DOI] [PubMed] [Google Scholar]
- 53.Yip KP, Marsh DJ. An Arg-Gly-Asp peptide stimulates constriction in rat afferent arteriole. Am J Physiol Renal Physiol 273: F768–F776, 1997. doi: 10.1152/ajprenal.1997.273.5.F768. [DOI] [PubMed] [Google Scholar]
- 54.Yip KP, Marsh DJ. [Ca2+]i in rat afferent arteriole during constriction measured with confocal fluorescence microscopy. Am J Physiol Renal Physiol 271: F1004–F1011, 1996. doi: 10.1152/ajprenal.1996.271.5.F1004. [DOI] [PubMed] [Google Scholar]
- 55.Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger - cyclic ADP-ribose. J Cell Mol Med 10: 407–422, 2006. doi: 10.1111/j.1582-4934.2006.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]