Abstract
Acute kidney injury (AKI) is a major complication in hospitalized patients and is associated with elevated mortality rates. Numerous recent studies indicate that AKI also significantly increases the risk of chronic kidney disease (CKD), end-stage renal disease (ESRD), hypertension, cardiovascular disease, and mortality in those patients who survive AKI. Moreover, the risk of ESRD and mortality after AKI is substantially higher in patients with preexisting CKD. However, the underlying mechanisms by which AKI and CKD interact to promote ESRD remain poorly understood. The recently developed models that superimpose AKI on rodents with preexisting CKD have provided new insights into the pathogenic mechanisms mediating the deleterious interactions between AKI and CKD. These studies show that preexisting CKD impairs recovery from AKI and promotes the development of mechanisms of CKD progression. Specifically, preexisting CKD exacerbates microvascular rarefaction, failed tubular redifferentiation, disruption of cell cycle regulation, hypertension, and proteinuria after AKI. The purpose of this review is to discuss the potential mechanisms by which microvascular rarefaction and hypertension contribute to impaired recovery from AKI and the subsequent progression of renal disease in preexisting CKD states.
Keywords: acute kidney injury, chronic kidney disease, hypertension, microvascular rarefaction
INTRODUCTION
End-stage renal disease (ESRD), or the permanent loss of kidney function, is a major health burden in the United States, which is associated with increased morbidity, mortality, and health care costs (51a). An emerging body of clinical data indicates that acute kidney injury (AKI) significantly increases the risk of ESRD (18, 20, 38). AKI, defined as an abrupt, but potentially reversible, loss of kidney function over a few days, is commonly observed in hospitalized patients and is associated with high mortality rates (51a). Common causes of AKI include prolonged renal ischemia, exposure to nephrotoxic agents, and sepsis (51a). Historically, the long-term renal outcome in patients surviving an episode of AKI was presumed to be good (18). However, several recent epidemiological studies indicate that AKI has major long-term consequences on kidney structure and function, morbidity, and mortality. For example, AKI substantially increases the risk of chronic kidney disease (CKD), or the permanent loss of individual nephrons, ESRD, cardiovascular disease, hypertension, and long-term mortality (16–18, 35–38, 47, 53). If AKI occurs in patients with CKD, the prognosis is significantly worse (38, 53, 66, 67). Clinical studies show that AKI accelerates the progression of established CKD and increases the risk of ESRD and mortality, above and beyond that observed in patients with either AKI or CKD (38). In light of these observations, AKI and CKD are now viewed as an interconnected clinical syndrome (18). The purpose of this review is to summarize the potential role of microvascular rarefaction and hypertension in contributing to impaired recovery from AKI in preexisting CKD states and the subsequent rapid progression to ESRD.
IMPAIRED RECOVERY AND ACCELERATED PROGRESSION OF KIDNEY DISEASE AFTER AKI IN PREEXISTING CKD STATES
Although there is strong evidence that AKI and CKD interact to promote ESRD in clinical populations, there is a paucity of animal models that recapitulate the AKI-CKD syndrome. For this reason, the development of AKI-CKD animal models that mimic clinical AKI-CKD populations has been recommended (23, 48, 74). It should be emphasized that there are numerous models being used to investigate the pathogenesis by which AKI directly leads to the permanent loss of nephrons (i.e., AKI-CKD transition) in animals without preexisting CKD (28). Moreover, several models exist in which animals are subjected to multiple AKI episodes over time to examine the subsequent mechanisms of CKD progression (28). However, only a couple of studies have examined renal function and structure several weeks after a single episode of AKI in animals with preexisting CKD associated with greater than 50% renal mass reduction (RMR) (45, 58), which is the focus of this review. We recently developed an AKI-CKD model using renal ischemia-reperfusion (IR) in rats with varying degrees of preexisting RMR to investigate whether decreased renal mass, per se, alters the severity of and recovery from IR-induced AKI (58). The surgical excision model of CKD (31) was used to create rats with different levels of normotensive RMR (75%, 50% and 0% RMR), and renal IR was performed two weeks after RMR surgery. While the severity of AKI was not different between groups, rats with 75% RMR exhibited impaired recovery four weeks after AKI, as evidenced by sustained elevations in plasma creatinine, a greater percentage of tubular epithelial cells (TEC) that failed to redifferentiate, and greater microvascular rarefaction and tubulointerstitial fibrosis (TIF) (Fig. 1). Of note, microvascular rarefaction and TIF are hallmarks of kidney disease, and the progression of CKD correlates best with the level of TIF (6, 51, 66, 67). Because the degree of injured, dedifferentiated tubules was similar among rats with different levels of RMR at 1 wk post-AKI, these data indicate that impaired recovery of injured TEC in rats with 75% RMR is manifest between weeks 1 and 4 following AKI. Evidence of impaired recovery following a single episode of AKI has also recently been reported in mice with preexisting CKD (45). It should also be noted that normotensive rats with 75% RMR spontaneously developed hypertension and proteinuria after AKI, major risk factors for CKD progression and ESRD, while those with 50% or 0% RMR did not.
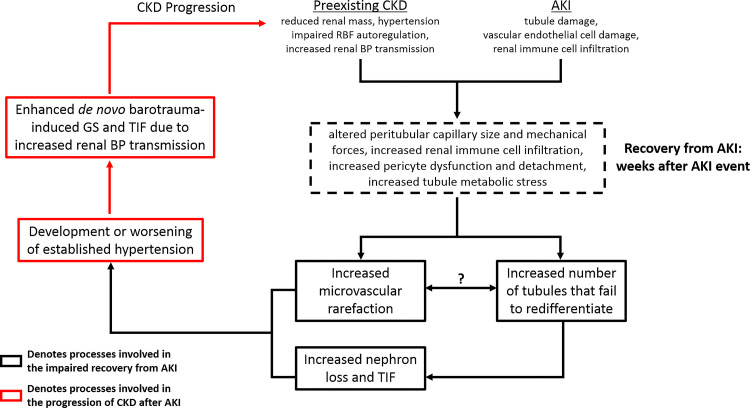
Fig. 1.
Proposed mechanisms by which acute kidney injury (AKI) and chronic kidney disease (CKD) interact to impair recovery from AKI and accelerate the progression to end-stage renal disease (ESRD). Impaired recovery from AKI in preexisting CKD states is manifested by a greater percentage of tubules that fail to redifferentiate, which enhances nephron loss and tubulointerstitial fibrosis (TIF), as well as augmented microvascular rarefaction (indicated by black boxes). The potential mechanisms by which CKD–AKI interactions hinder tubule redifferentiation and promote capillary rarefaction are indicated by the dashed box. The presence of hypertension and impaired renal blood flow (RBF) autoregulation in CKD states alters peritubular capillary size and intracapillary forces that may synergize with AKI-induced endothelial injury to promote pericyte dysfunction and detachment, increase renal immune cell infiltration, and promote loss of capillaries. Moreover, tubule metabolic stress after AKI in preexisting CKD states may result in hypoxia, which could hinder normal cellular repair mechanisms. An important unanswered question is whether microvascular rarefaction drives failed tubule redifferentiation or vice versa. The progression of renal injury following AKI in preexisting CKD states is due to mechanisms that are independent of AKI. We propose that the development of hypertension following AKI in CKD states has a major role in the subsequent progression to ESRD. Augmented renal microvascular rarefaction and nephron loss both significantly increase the risk of hypertension. Even modest increases in blood pressure (BP) in the presence of CKD-associated impaired RBF autoregulation greatly increase the risk of barotrauma-mediated glomerulosclerosis (GS) and TIF, which could explain the rapid progression to ESRD in clinical AKI-CKD populations. Black boxes indicate processes involved in the impaired recovery from AKI, while red boxes indicate processes involved in the progression of CKD.
AUGMENTED MICROVASCULAR RAREFACTION AFTER AKI IN PREEXISTING CKD STATES
Renal microvascular damage and rarefaction are common after AKI and are thought to contribute to the AKI-CKD transition (6, 8, 9, 11, 21, 27, 30, 65, 67). We found that microvascular rarefaction was more severe in rats with preexisting CKD and was observed in close proximity to atrophic, vimentin-positive tubules 4 wk post-AKI (58). This raises the possibility that microvascular rarefaction directly impairs the ability of injured TEC to recover normal structure and function. A recent study by Kramann et al. (42), demonstrating that loss of peritubular capillaries promotes tubular injury, supports this concept. Loss of capillary density could predispose to hypoxia leading to growth arrest, oxidative stress, and aberrant cellular metabolism, which could hinder normal repair mechanisms (7, 27, 65, 67). Consistent with this concept, increased cell cycle arrest has been observed in mice with preexisting CKD during recovery from a single episode of IR-induced AKI (45).
An important, but unanswered question, is why does preexisting CKD promote capillary dropout after AKI? One possibility is altered mechanical forces within peritubular capillaries. CKD is associated with hypertension, impaired renal blood flow (RBF) autoregulation (12), endothelial dysfunction (72), inflammation (1), and increased peritubular capillary size and pressure (3, 15, 25, 34, 52). In conjunction with increased endothelial permeability and damage after AKI (5, 11, 63, 64), altered mechanical forces within peritubular capillaries could negatively impact cells that regulate capillary stability and TIF (Fig. 1). This is relevant given that pericytes, contractile cells adjoined to the outside of endothelial cells, play a major role in stabilizing capillaries, and regulating tissue fibrosis and signaling between TEC and endothelial cells (40, 41, 44, 50, 61, 67, 73). Altered mechanical forces within peritubular capillaries and impaired endothelial integrity can alter pericyte shape and function (24, 33, 60) and promote extravasation of immune cells to the interstitium and nearby TEC (33, 59, 69). Such inflammatory processes are thought to initiate or alter cellular pathways within pericytes, promote pericyte detachment, and ultimately lead to TIF (19, 26, 33, 41, 44, 46, 62, 67, 70).
Conversely, the exaggerated microvascular rarefaction after AKI in preexisting CKD states could be a result of the greater number of TEC that remain in a dedifferentiated state (Fig. 1). Such TEC are atrophic and growth-arrested but continue to secrete profibrotic and proinflammatory factors that are able to influence pericyte function and detachment (29, 66, 67). Supportive of this concept is evidence that specific injury to TEC promotes inflammation, fibrosis, and the loss of nearby capillaries (13, 67, 71). Of note, injured and dedifferentiated TEC are associated with decreased VEGF expression, which can also promote pericyte detachment and microvascular rarefaction (10, 46, 67, 71).
If the failure of more TEC to redifferentiate after AKI in preexisting CKD states is causing greater loss of capillaries, then what is driving failed TEC redifferentiation in such settings? As we have previously speculated (58, 66, 67), it is possible that the metabolic stress associated with CKD may impair the ability of injured TEC to regain normal structure and function. Alternatively, the presence of hypertension in AKI-CKD settings may also impair recovery of TEC. In the setting of hypertension and impaired RBF autoregulation in CKD states, increased renal blood pressure (BP) transmission can lead to additional, de novo, injury, which may impair the ability of previously injured TEC to recover from AKI. This concept is supported by a study in stroke-prone spontaneously hypertensive rats, in which repair of previously injured vasculature was greatly compromised by the presence of hypertension and new injury (32). De novo barotrauma-mediated injury to glomerular capillaries or peritubular capillaries could contribute to focal areas of inflammation and extracellular matrix production that may alter recovery of nearby previously injured TEC. Nevertheless, a fundamental question that remains to be answered is, does microvascular rarefaction predispose to impaired recovery of injured TEC or does the presence of more dedifferentiated TEC predispose to microvascular rarefaction?
AKI IN PREEXISTING CKD STATES PROMOTES HYPERTENSION AND CKD PROGRESSION
The TIF and loss of nephrons that result from AKI (i.e., the AKI-CKD transition) are separate processes from the subsequent progression of renal injury (43, 57, 67, 68). TIF resulting from AKI is a self-limiting process that circumscribes areas of dead tissue and results in a scar (39, 43, 67). Conversely, new pathological processes that injure cells that were either uninjured or had fully recovered from AKI are required for CKD progression (57, 67). In this respect, evidence from clinical (35) and experimental (30, 57, 58) studies showing that AKI results in hypertension is relevant, especially in preexisting CKD states.
Renal microvascular rarefaction after AKI increases the risk of hypertension by impairing the pressure-natriuresis mechanism (49, 56). Moreover, loss of additional nephrons following AKI in preexisting CKD states can diminish renal reserve and also promote hypertension (14). The development or worsening of hypertension after AKI in settings of CKD likely has major consequences (Fig. 1). Hypertension in settings of impaired RBF autoregulation increases renal BP transmission and the risk of barotrauma-mediated glomerulosclerosis (GS) (12). Of note, we found that rats with preexisting normotensive 75% RMR developed hypertension within 4 wk after AKI, and the rats with the most severe levels of hypertension within this group exhibited GS (58). In a more recent study, we found that significant levels of TIF 16 wk after IR-induced AKI in uninephrectomized rats was only observed in rats that developed hypertension, albeit modest, and GS (57). GS and TIF were minimal in rats that did not exhibit elevations in BP 16 wk after AKI. This is consistent with studies that have documented extensive levels of GS several months after AKI in uninephrectomized rats that also exhibited extensive levels of TIF (9, 22, 54, 55). Moreover, significant GS has also been observed after severe AKI in rats with intact kidneys (4), in which hypertension is likely present, although this has never been documented with BP radiotelemetry techniques. Despite the evidence to support this pathway of CKD progression after AKI, there are no studies that have addressed the role that even modest elevations in BP have in contributing to the progression of renal injury after AKI. Such studies would require careful monitoring of BP (24 h/day) via radiotelemetry for several months after AKI in rats treated with antihypertensive therapy vs. untreated rats. It is possible that modest increases in BP, even considered normotensive by current guidelines, may be the dominant factor in the progression to ESRD after AKI in preexisting CKD states. The recent evidence that AKI increases the risk of hypertension in clinical populations further supports the need for such studies (35). A better understanding of this important aspect of the AKI-CKD nexus could lead to new treatment strategies to reduce the risk of ESRD in this population.
CONCLUSIONS
Deleterious interactions between AKI and CKD are a major contributor to ESRD. The recent development of AKI-CKD models that mimic the clinical AKI-CKD syndrome will provide important insights into the underlying pathogenic mechanisms. These models show that preexisting CKD exacerbates renal microvascular rarefaction, impairs recovery of injured TEC, and alters cell cycle regulation after AKI (45, 58). Targeting these pathways may improve recovery and mitigate the subsequent rapid progression to ESRD after AKI in CKD patients. Moreover, recent studies suggest that even modest levels of hypertension after AKI in CKD states could play a major role in the subsequent progression of kidney disease. The development of additional AKI-CKD models that include preexisting hypertension, diabetes, and renal injury will provide even further insights into the clinical AKI-CKD nexus.
GRANTS
A. J. Polichnowski is currently supported by a Carl Gottschalk Research Scholar Grant from the American Society of Nephrology Foundation for Kidney Research, the American Heart Association (17AIREA33660433) and the NIH (C06RR0306551). The work presented in this review from A. J. Polichnowski has also been supported by grants from the Veterans Administration (IK2BX001285) and National Kidney Foundation of IL.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
A.J.P. prepared figure; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
ACKNOWLEDGMENTS
The author acknowledges Dr. Manjeri Venkatachalam for his insights and helpful discussions regarding the interactions between acute kidney injury and chronic kidney disease.
REFERENCES
- 1.Amdur RL, Feldman HI, Gupta J, Yang W, Kanetsky P, Shlipak M, Rahman M, Lash JP, Townsend RR, Ojo A, Roy-Chaudhury A, Go AS, Joffe M, He J, Balakrishnan VS, Kimmel PL, Kusek JW, Raj DS; CRIC Study Investigators . Inflammation and progression of CKD: The CRIC Study. Clin J Am Soc Nephrol 11: 1546–1556, 2016. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azar S, Tobian L, Johnson MA. Glomerular, efferent arteriolar, peritubular capillary, and tubular pressures in hypertension. Am J Physiol 227: 1045–1050, 1974. doi: 10.1152/ajplegacy.1974.227.5.1045. [DOI] [PubMed] [Google Scholar]
- 4.Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, Reyna J, Uribe N, Gamba G, Bobadilla NA. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int 83: 93–103, 2013. doi: 10.1038/ki.2012.352. [DOI] [PubMed] [Google Scholar]
- 5.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 72: 151–156, 2007. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001. doi: 10.1152/ajprenal.00050.2001. [DOI] [PubMed] [Google Scholar]
- 9.Basile DP, Donohoe DL, Roethe K, Mattson DL. Chronic renal hypoxia after acute ischemic injury: effects of l-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003. doi: 10.1152/ajprenal.00169.2002. [DOI] [PubMed] [Google Scholar]
- 10.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 11.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2013. doi: 10.1097/MNH.0b013e32835b36c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonventre JV. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int Suppl (2011) 4: 39–44, 2014. doi: 10.1038/kisup.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 15.Brown SA, Finco DR, Crowell WA, Choat DC, Navar LG. Single-nephron adaptations to partial renal ablation in the dog. Am J Physiol Renal Fluid Electrolyte Physiol 258: F495–F503, 1990. doi: 10.1152/ajprenal.1990.258.3.F495. [DOI] [PubMed] [Google Scholar]
- 16.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE II, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012. doi: 10.1038/ki.2011.405. [DOI] [PubMed] [Google Scholar]
- 17.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 20.Chew ST, Ng RR, Liu W, Chow KY, Ti LK. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol 18: 60, 2017. doi: 10.1186/s12882-017-0476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8: e70464, 2013. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruzado JM, Torras J, Riera M, Herrero I, Hueso M, Espinosa L, Condom E, Lloberas N, Bover J, Alsina J, Grinyó JM. Influence of nephron mass in development of chronic renal failure after prolonged warm renal ischemia. Am J Physiol Renal Physiol 279: F259–F269, 2000. doi: 10.1152/ajprenal.2000.279.2.F259. [DOI] [PubMed] [Google Scholar]
- 23.de Caestecker M, Humphreys BD, Liu KD, Fissell WH, Cerda J, Nolin TD, Askenazi D, Mour G, Harrell FE Jr, Pullen N, Okusa MD, Faubel S; ASN AKI Advisory Group . Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol 26: 2905–2916, 2015. doi: 10.1681/ASN.2015070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demolli S, Doddaballapur A, Devraj K, Stark K, Manavski Y, Eckart A, Zehendner CM, Lucas T, Korff T, Hecker M, Massberg S, Liebner S, Kaluza D, Boon RA, Dimmeler S. Shear stress-regulated miR-27b controls pericyte recruitment by repressing SEMA6A and SEMA6D. Cardiovasc Res 113: 681–691, 2017. doi: 10.1093/cvr/cvx032. [DOI] [PubMed] [Google Scholar]
- 25.Diezi J, Michoud P, Grandchamp A, Giebisch G. Effects of nephrectomy on renal salt and water transport in the remaining kidney. Kidney Int 10: 450–462, 1976. doi: 10.1038/ki.1976.132. [DOI] [PubMed] [Google Scholar]
- 26.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018. doi: 10.1152/ajprenal.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, Barnes JL, Saikumar P, Weinberg JM, Venkatachalam MA. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 174: 1291–1308, 2009. doi: 10.2353/ajpath.2009.080295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greite R, Thorenz A, Chen R, Jang MS, Rong S, Brownstein MJ, Tewes S, Wang L, Baniassad B, Kirsch T, Bräsen JH, Lichtinghagen R, Meier M, Haller H, Hueper K, Gueler F. Renal ischemia-reperfusion injury causes hypertension and renal perfusion impairment in the CD1 mice which promotes progressive renal fibrosis. Am J Physiol Renal Physiol 314: F881–F892, 2018. doi: 10.1152/ajprenal.00519.2016. [DOI] [PubMed] [Google Scholar]
- 31.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol 4: 2023–2031, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Griffin KA, Polichnowski A, Litbarg N, Picken M, Venkatachalam MA, Bidani AK. Critical blood pressure threshold dependence of hypertensive injury and repair in a malignant nephrosclerosis model. Hypertension 64: 801–807, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Volarevic V. Molecular mechanisms underlying therapeutic potential of pericytes. J Biomed Sci 25: 21, 2018. doi: 10.1186/s12929-018-0423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayslett JP. Functional adaptation to reduction in renal mass. Physiol Rev 59: 137–164, 1979. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS. Elevated BP after AKI. J Am Soc Nephrol 27: 914–923, 2016. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu CY, Liu KD. Cardiovascular events after AKI: a new dimension. J Am Soc Nephrol 25: 425–427, 2014. doi: 10.1681/ASN.2013121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, Slinin Y, Ensrud KE. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171: 226–233, 2011. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 38.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta 1832: 931–939, 2013. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami T, Mimura I, Shoji K, Tanaka T, Nangaku M. Hypoxia and fibrosis in chronic kidney disease: crossing at pericytes. Kidney Int Suppl (2011) 4: 107–112, 2014. doi: 10.1038/kisup.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34: 374–383, 2014. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int 93: 27–40, 2018. doi: 10.1016/j.kint.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Lim SY, Ko YS, Lee HY, Yang JH, Kim MG, Jo SK, Cho WY. The impact of preexisting chronic kidney disease on the severity and recovery of acute kidney injury. Nephron 139: 254–268, 2018. doi: 10.1159/000487492. [DOI] [PubMed] [Google Scholar]
- 46.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C; ADQI XIII Work Group . Renal hemodynamics in AKI: in search of new treatment targets. J Am Soc Nephrol 27: 49–58, 2016. doi: 10.1681/ASN.2015030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 284: R13–R27, 2003. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 50.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992. doi: 10.1016/S0272-6386(12)80312-X. [DOI] [PubMed] [Google Scholar]
- 51a.National Institutes of Health United States Renal Data System. 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2017. [Google Scholar]
- 52.Nishi S, Imai N, Alchi B, Iguchi S, Ueno M, Fukase S, Mori H, Arakawa M, Saito K, Takahashi K, Gejyo F. The morphological compensatory change of peritubular capillary network in chronic allograft rejection. Clin Transplant 19, Suppl 14: 7–11, 2005. doi: 10.1111/j.1399-0012.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 53.Okusa MD, Chertow GM, Portilla D; Acute Kidney Injury Advisory Group of the American Society of Nephrology . The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009. doi: 10.2215/CJN.06711208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pagtalunan ME, Olson JL, Meyer TW. Contribution of angiotensin II to late renal injury after acute ischemia. J Am Soc Nephrol 11: 1278–1286, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Pagtalunan ME, Olson JL, Tilney NL, Meyer TW. Late consequences of acute ischemic injury to a solitary kidney. J Am Soc Nephrol 10: 366–373, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 297: R1358–R1363, 2009. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picken M, Long J, Williamson GA, Polichnowski AJ. Progression of chronic kidney disease after acute kidney injury: role of self-perpetuating versus hemodynamic-induced fibrosis. Hypertension 68: 921–928, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polichnowski AJ, Lan R, Geng H, Griffin KA, Venkatachalam MA, Bidani AK. Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J Am Soc Nephrol 25: 1496–1507, 2014. doi: 10.1681/ASN.2013040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209: 1219–1234, 2012. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrimpf C, Koppen T, Duffield JS, Böer U, David S, Ziegler W, Haverich A, Teebken OE, Wilhelmi M. TIMP3 is regulated by pericytes upon shear stress detection leading to a modified endothelial cell response. Eur J Vasc Endovasc Surg 54: 524–533, 2017. doi: 10.1016/j.ejvs.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res 51: 247–258, 2014. doi: 10.1159/000365149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res 77: 4–7, 2009. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307: F1187–F1195, 2014. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 66.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venner JM, Famulski KS, Reeve J, Chang J, Halloran PF. Relationships among injury, fibrosis, and time in human kidney transplants. JCI Insight 1: e85323, 2016. doi: 10.1172/jci.insight.85323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One 7: e45499, 2012. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, Wu TH, Linn GR, Ling H, Wu KD, Tsai TJ, Chen YM, Duffield JS, Lin SL. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182: 118–131, 2013. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol 163: 2289–2301, 2003. doi: 10.1016/S0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. Am J Kidney Dis 66: 1083–1094, 2015. doi: 10.1053/j.ajkd.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 73.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med 67: 293–307, 2016. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zuk A, Palevsky PM, Fried L, Harrell FE Jr, Khan S, McKay DB, Devey L, Chawla L, de Caestecker M, Kaufman JS, Thompson BT, Agarwal A, Greene T, Okusa MD, Bonventre JV, Dember LM, Liu KD, Humphreys BD, Gossett D, Xie Y, Norton JM, Kimmel PL, Star RA. Overcoming translational barriers in acute kidney injury: a report from an NIDDK workshop. Clin J Am Soc Nephrol 13: 1113–1123, 2018. doi: 10.2215/CJN.06820617. [DOI] [PMC free article] [PubMed] [Google Scholar]