Abstract
The pudendal nerve can be injured during vaginal delivery of children, and slowed pudendal nerve regeneration has been correlated with development of stress urinary incontinence (SUI). Simultaneous injury to the pudendal nerve and its target muscle, the external urethral sphincter (EUS), during delivery likely leads to slowed neuroregeneration. The goal of this study was to determine if repeat electrical stimulation of the pudendal nerve improves SUI recovery and promotes neuroregeneration in a dual muscle and nerve injury rat model of SUI. Rats received electrical stimulation or sham stimulation of the pudendal nerve twice weekly for up to 2 wk after injury. A separate cohort of rats received sham injury and sham stimulation. Expression of brain-derived neurotrophic factor (BDNF) and βII-tubulin expression in Onuf’s nucleus were measured 2, 7, and 14 days after injury. Urodynamics, leak point pressure (LPP), and EUS electromyography (EMG) were recorded 14 days after injury. Electrical stimulation significantly increased expression of BDNF at all time points and βII-tubulin 1 and 2 wk after injury. Two weeks after injury, LPP and EUS EMG during voiding and LPP testing were significantly decreased compared with sham-injured animals. Electrical stimulation significantly increased EUS activity during voiding, although LPP did not fully recover. Repeat pudendal nerve stimulation promotes neuromuscular continence mechanism recovery possibly via a neuroregenerative response through BDNF upregulation in the pudendal motoneurons in this model of SUI. Electrical stimulation of the pudendal nerve may therefore improve recovery after childbirth and ameliorate symptoms of SUI by promoting neuroregeneration after injury.
Keywords: birth injury, leak point pressure, neurotrophin, pudendal nerve, urinary incontinence, urodynamics
INTRODUCTION
Urinary incontinence, the undesired leakage of urine, is a common condition afflicting up to half of all adult women (51). Stress urinary incontinence (SUI) is urinary incontinence from increased abdominal pressure, as occurs during coughing, sneezing, laughing, or other activity (1). SUI is strongly associated with maternal injuries from childbirth, which are often complex and involve the pelvic fascia, ligaments, muscles, and nerves, including the external urethral sphincter (EUS) and pudendal nerve, which comprise the important neuromuscular urinary continence mechanism (16, 28, 42). Both the pudendal nerve and EUS are simultaneously injured during vaginal delivery, representing a unique, compound neuromuscular insult (41). In rats, both sensory and motor branches of the pudendal nerve are significantly stretched during simulated childbirth (34). Recent studies suggest pudendal nerve recovery is delayed as a result of insufficient production of neurotrophins, such as brain-derived neurotrophic factor (BDNF), by the damaged target muscle, the EUS (35). EUS damage in the setting of pudendal nerve injury impairs upregulation of BDNF and impedes functional recovery of the neuromuscular continence mechanism (24, 25, 35). This mechanistic theory is supported by clinical electrophysiologic data demonstrating delayed pudendal nerve functional recovery up to 5 yr after delivery associated with SUI (10, 33). We previously found that exogenous BDNF treatment facilitates recovery of urinary continence after simulated birth injury involving both pudendal nerve and EUS damage (16).
Acute electrical stimulation promotes neuroregeneration after injury and increases intrinsic BDNF expression within injured motor and sensory neurons and Schwann cells (2, 3, 15, 24). We previously demonstrated that acute electrical stimulation of the motor branch of the pudendal nerve after injury increases neuronal expression of BDNF and βII-tubulin, a cytoskeletal protein involved in neuroregeneration (24). However, in this acute testing, we observed minimal evidence of changes to urodynamic parameters. In the current study, we extend stimulation testing from the 1 h after injury done previously to multiple stimulation sessions up to 2 wk after injury. In addition to retesting changes in urodynamics following this prolonged period of stimulation, we also added measurements of EUS activity, including bursting discharge during voiding because this pattern is normal in rats and leak point pressure (LPP), both of which are validated outcomes indicative of SUI (25, 26). The observation of increase in BDNF in our previous study suggested that electrical stimulation could provide a means of accelerating nerve recovery after birth injury by promoting neurotrophin upregulation, which would facilitate neuroregeneration of the pudendal nerve and reinnervation of the simultaneously damaged EUS, despite its impaired BDNF production. In this study we also advanced the stimulation protocol to stimulate the sensory and motor branches of the pudendal nerve as this is both more clinically feasible and more feasible for repeated chronic stimulation. This study tests the hypothesis that electrical stimulation of the pudendal nerve in a rat model of SUI increases BDNF expression, facilitates neuroregeneration, and improves functional recovery of urinary continence after a simulated childbirth injury by bilateral pudendal nerve crush (PNC) followed by vaginal distension (VD).
MATERIALS AND METHODS
VD + PNC-simulated birth injury model of SUI.
This research was approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic. Sixty adult female Sprague-Dawley rats (250–300 g) underwent either VD + PNC simulated birth injury or sham injury and subsequently received unilateral or bilateral pudendal nerve electrical stimulation or sham stimulation twice weekly for up to 2 wk after injury (Fig. 1). Unilateral stimulation was used in one cohort of rats to assess expression of BDNF and βII-tubulin in Onuf’s nucleus via reverse-transcription polymerase chain reaction (RT-PCR) 2 days, 1 wk, or 2 wk after injury or sham injury. Ipsilateral stimulation delivered twice weekly was compared with contralateral sham stimulation after either simulated birth injury or sham injury. Another cohort underwent bilateral electrical stimulation or bilateral sham stimulation twice weekly for 2 wk after simulated birth injury or sham injury to assess bladder and urethral function 14 days after the injury. Bilateral stimulation is needed to affect functional recovery because the bladder and urethra are innervated bilaterally.
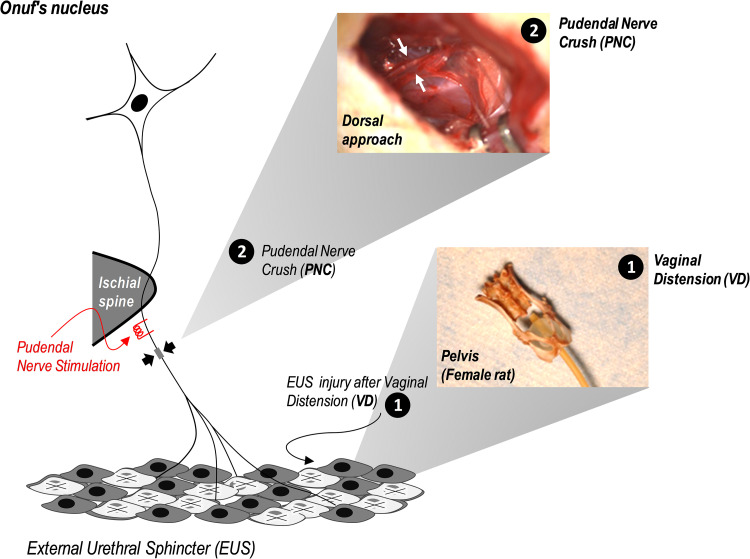
Fig. 1.
Schematic of pudendal nerve electrical stimulation after simulated birth injury, including PNC (a dorsal approach to the pudendal nerve after opening the ischiorectal fossa from the lower back) and VD (illustrated by the distended balloon in an adult female rat pelvis). EUS, external urethral sphincter; PNC, pudendal nerve crush; VD, vaginal distension.
Our model of SUI from simulated birth injury consists of PNC followed by VD, as previously described (25). In brief, under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia, sensory and motor branches of the pudendal nerve were identified bilaterally with a dorsal approach in the ischiorectal fossa and crushed twice for 30 s each with a Castroviejo needle holder (Fig. 1). Following PNC, the vagina underwent 4 h of distension using a modified 10-French Foley catheter with 3 ml water in the balloon. Sham injury was the same procedure but without filling the balloon and without crushing the nerves. All animals received postoperative analgesia (buprenorphine, 0.1 mg/kg sc) twice daily for 48 h.
Pudendal nerve electrical stimulation.
Immediately after simulated birth injury (n = 34) or sham injury (n = 26), and while maintaining anesthesia, the pudendal nerve proximal to the injury site was isolated and suspended by a bent-end bipolar parallel platinum electrode (no. PB AD08100; FHC, Bowdoin, ME) for 1 h of electrical stimulation (20 Hz, 0.3 mA, 0.1-ms pulse) unilaterally (left side, n = 18) or bilaterally (n = 8). We have shown previously that these stimulation parameters increase BDNF expression in motor neurons (24). In our previous publication this stimulation amplitude was described as subthreshold because it was selected to be just below the stimulation causing a detectable anal sphincter contraction, but it was high enough to generate an action potential (24). These stimulation parameters are similar to other published electrical stimulation studies (22).
Sham electrical stimulation consisted of placement of the electrodes without stimulation unilaterally (right side, n = 18) or bilaterally (n = 8) (Fig. 2A). Stimulation or sham stimulation was repeated twice weekly under anesthesia for 2 wk. Rats were anesthetized with inhaled isoflurane for each stimulation or sham stimulation session. Pudendal nerve stimulation was performed at the level of the ischial spine, which was accessed blindly through positioning of 2 parallel monopolar needle electrodes (model no. F-E2M, 30-gauge platinum; Grass Technologies, Warwick, RI) perpendicularly at the ischial tuberosity, which is done clinically (31) (Fig. 1). Electrodes were introduced at the level of the ischial spine and inserted to a depth of 1.5 cm to reach the sensory and motor branches before they divided (8, 36). Before conducting this experiment, we did many pudendal nerve dissections and test stimulations confirming the electrodes were in the right location using this approach.
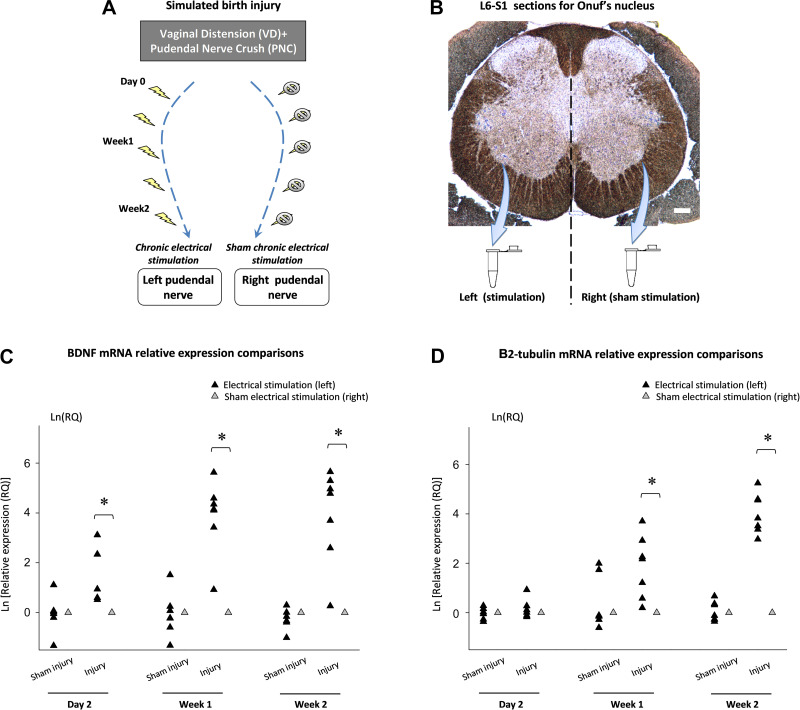
Fig. 2.
Relative expression of BDNF and βII-tubulin mRNA in Onuf’s nucleus after simulated birth injury and unilateral pudendal nerve electrical stimulation. Timeline of unilateral pudendal nerve electrical stimulation in the simulated birth injury animal model (A). Onuf’s nucleus shown by thoinin staining of neuronal cell bodies harvested bilaterally in sections from the L6-S1 spinal cord using laser microdissection, which were subsequently used for quantitative RT-PCR. Bar = 100 µm (B). Relative expression of BDNF (C) and βII-tubulin (D) mRNA in Onuf’s nucleus after simulated birth injury (n = 18) or sham injury (n = 18) plus unilateral pudendal nerve electrical stimulation. The mean relative expression (RQ) in Onuf’s nucleus of the pudendal nerve receiving sham stimulation (right side) were normalized to 1. *Statistically significant difference compared with sham stimulation (P < 0.05) in the same animals (t-test). BDNF, brain-derived neurotrophic factor. PNC, pudendal nerve crush; VD, vaginal distension.
Quantification of pudendal nerve BDNF and βII-tubulin expression in Onuf’s nucleus.
Pudendal nerve motoneuron cell body isolation and RT-PCR were completed as described previously (16, 17, 24). Two days, 1 wk, or 2 wk after injury, the lumbosacral spinal cord was flash-frozen and harvested following ketamine/xylazine anesthesia and intracardiac perfusion of heparinized saline. The L6-S1 spinal cord was sectioned transversely (12-µm thickness; Leica Microsystems, Wetzlar, Germany), and 18 sections at the level of Onuf’s nucleus were placed on 3 slides (PET membrane slides, Leica). Pudendal nerve motoneurons in the dorsolateral motor nucleus (Onuf’s nucleus) that innervate the EUS were identified morphologically after thionin staining, as done previously (Fig. 2B) (24). Spinal cord laterality was maintained, and the dorsolateral nuclei were isolated with left and right sides collected separately via laser microdissection for storage at −80°C before RT-PCR.
As done previously (16), total RNA was isolated separately from the right and left Onuf’s nucleus in each rat (TaqMan MicroRNA Assays; Applied Biosystems, Foster City, CA) and reverse transcribed (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems). RNA and cDNA quality was confirmed by Nano-Drop ND-1000 spectrophotometry (Thermo Fisher Scientific, Wilmington, DE) testing. BDNF and βII-tubulin expression were quantified by RT- PCR (StepOne Real-Time PCR, Applied Biosystems) after preamplification (TaqMan PreAmp Master Mix, Applied Biosystems). BDNF (TaqMan Gene Expression Assay, Rn02531967_s1, Applied Biosystems) and βII-tubulin (TaqMan Gene Expression Assay, Rn01435557_g1, Applied Biosystems) primers and probes consisted of a 20× mix of unlabeled PCR primers and Taqman minor groove binder probe (FAM dye-labeled reporter). The fold-change in gene expression was determined by the ΔΔCT method (30). The reference gene S18 (TaqMan Gene Expression Assay, Rn03928990_g1, Applied Biosystems) was used as an internal control (VIC dye-labeled reporter).
Cystometry and LPP.
Rats (n = 24) undergoing functional outcome testing received VD + PNC and electrical stimulation bilaterally (n = 8), bilateral injury and sham stimulation (n = 8), or bilateral sham injury with sham stimulation (n = 8). Two weeks later all animals underwent filling cystometry and LPP testing with simultaneous EUS electromyography (EMG) under urethane anesthesia (1.0 g/kg ip). A polyethylene urethral catheter (PE-50) was inserted into the bladder transurethrally and connected to both a pressure transducer (model no. P-122; Astro-Med, West Warwick, RI) and a syringe pump (model no. 200; KD Scientific, New Hope, PA). To measure EUS EMG, 2 monopolar electrodes, comprised of 50-μm teflon-insulated platinum iridium wires (A-M Systems, Carlsborg, WA) were inserted into the lateral-most aspects of the EUS (3 and 9 o’clock) via the anterior vaginal wall using a 30-gauge needle. The electrodes were connected to an analog amplifier (model no. P511 AC Amplifier, Astro-Med; band-pass frequencies: 3 Hz-3 kHz) and digital-to-analog converter with data acquisition system (DASH 8X, Astro-Med; 10-kHz sampling rate) that recorded EUS EMG simultaneously with bladder pressure during LPP testing.
The bladder was filled continuously with saline for 30 min (5 ml/h) to evaluate filling and voiding over several urination cycles while bladder pressure and EUS EMG were recorded. Bladders were emptied after the first void and before starting a 30-min continuous fill cycle, resetting bladder pressure to 0 at the start of the filling. LPP testing was performed thereafter by filling the bladder to ~0.3 ml and slowly applying a gentle external pressure to the lower abdominal wall until leakage from the urethral meatus was visualized, at which time pressure was rapidly removed. LPP testing with simultaneous EUS EMG recording was performed 3–4 times in each rat with the bladder emptied between trials. If a bladder contraction was induced during LPP testing, the results of that test were not analyzed, the bladder was drained, and the test was repeated.
Data analysis and statistics.
For RT-PCR data, target gene mean relative expressions from the stimulated side in each animal were normalized to the sham injury/sham stimulation side. Changes in gene expression were assessed via t-test. Cystometry was analyzed by determining the frequency (number/min), amplitude (cmH2O), duration (s), and interval (s) of voiding and nonvoiding bladder contractions. The latter was defined as an increase in pressure no less than 5 cmH2O above the baseline in the absence of urine leakage or voiding. LPP was calculated as baseline pressure subtracted from peak pressure (Fig. 3A). Mean amplitudes and firing rates of EUS EMG before and during LPP were calculated as done previously (26). In brief, 1-s segments selected at both baseline and peak bladder pressure were processed by LabChart 7 (ADInstrument, Dunedin, New Zealand) with 60-Hz and 120-Hz band-pass filters and a 15-μV threshold. Similar methods were used for the calculation of EUS EMG during voiding. EUS EMG bursting activity during voiding has been associated with efficiency of bladder emptying and is also a critical sign of EUS recovery (25). Therefore, in our study a bursting episode was archived as a 1-s recording sample. A 1-s recording sample at the middle of voiding was also archived even if no obvious bursting activity was identified. In each subject, three 1-s samples were analyzed. One-second signal samples were segmented during bursting episodes followed by applying both digital notch filters at 60 Hz and 120 Hz and a 0.2-μV threshold (27). Burst duration was defined as the time period between the beginning and the end of a single burst in the 1-s sample. Interburst duration was calculated as the time internal between the end of a burst and the beginning of the next burst. A mean value of each outcome variable from each animal was used to calculate a mean and standard error for each experimental group. Differences were assessed using a two-way ANOVA followed by Student-Newman-Keuls pairwise comparisons. P < 0.05 indicated a statistically significant difference between groups for all statistical tests.
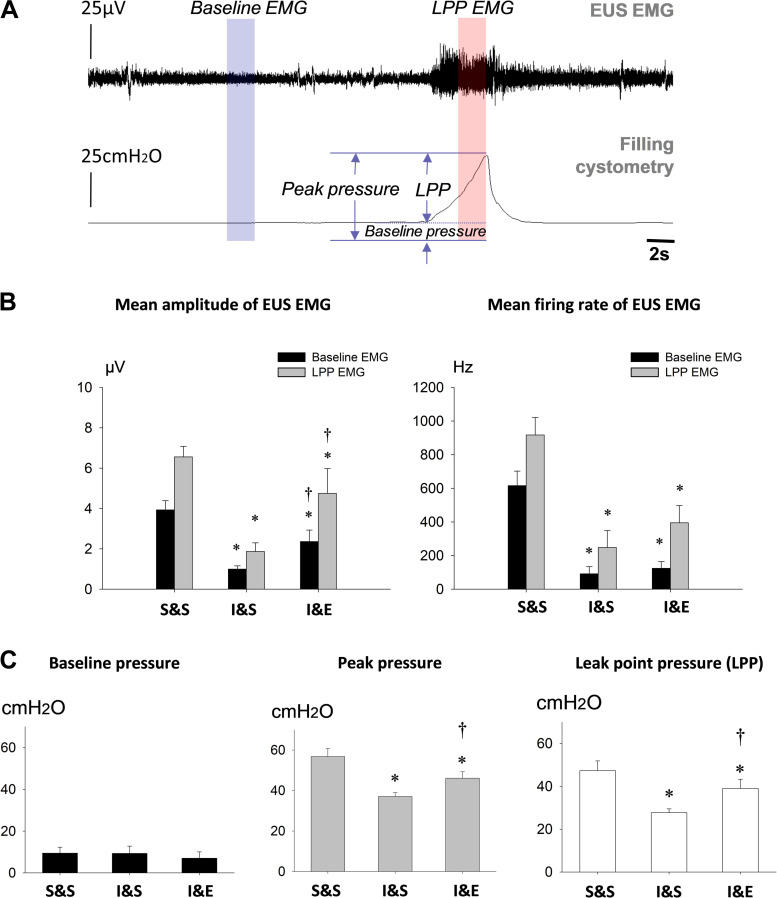
Fig. 3.
Urethral function during leak point pressure (LPP) testing after pudendal nerve stimulation. A: simultaneous external urethral sphincter (EUS) electromyogram (EMG) and bladder pressure during LPP testing in a sham-injured animal. The bars indicate the 1-s segments used to analyze EUS EMG activity at baseline and peak bladder pressure during LPP testing. B: amplitude and firing rate of EUS EMG at baseline and peak bladder pressure during LPP testing 2 wk after simulated birth injury with bilateral electrical stimulation (I&E, n = 8), injury with sham stimulation (I&S, n = 8), and sham injury with sham stimulation (S&S, n = 8). C: baseline pressure, peak pressure, and LPP during LPP testing for the same 3 groups 2 wk after injury or sham injury with stimulation or sham stimulation. *Statistically significant difference compared with the corresponding S&S group (P < 0.05). †Statistically significant difference compared with the corresponding I&S group (P < 0.05). Data are presented as mean ± standard error of data from 8 animals.
RESULTS
Increased expression of BDNF and βII-tubulin in Onuf’s nucleus with electrical stimulation of the pudendal nerve.
Expression of BDNF in Onuf’s nucleus increased significantly 2 days, 1 wk, and 2 wk after injury with ipsilateral pudendal nerve electrical stimulation compared with ipsilateral sham stimulation (Fig. 2C). Expression of βII-tubulin was significantly increased in Onuf’s nucleus 1 and 2 wk after injury with ipsilateral electrical stimulation compared with ipsilateral sham stimulation but not 2 days after injury with electrical stimulation. Neither BDNF nor βII-tubulin expression differed significantly at any time point after sham injury and electrical stimulation compared with sham injury with sham stimulation.
Electrical stimulation increased recovery of neuromuscular continence.
Typical of rats with an intact pudendal nerve (26), EUS EMG increased in amplitude and firing rate as pressure on the bladder increased with LPP testing 2 wk after sham injury (Fig. 3B). Two weeks after VD + PNC, EUS EMG amplitude and firing rate at baseline and during LPP were significantly reduced with (I&E) or without electrical stimulation (I&S), compared with sham injury and sham stimulation (S&S) (Fig. 3C). However, mean EUS EMG amplitude was significantly greater with (I&E) than without (I&S) electrical stimulation 2 wk after injury at baseline and during LPP. Baseline bladder pressures did not significantly differ between injury with stimulation (I&E) and sham injury (S&S) 2 wk after injury and commencement of stimulation. Peak bladder pressure and LPP both significantly decreased compared with sham injury, regardless of whether or not electrical stimulation was given. Nonetheless, both peak bladder pressure and LPP were significantly increased with injury and electrical stimulation (I&E) compared with sham stimulation (I&S).
Changes in bladder function after injury and bilateral stimulation.
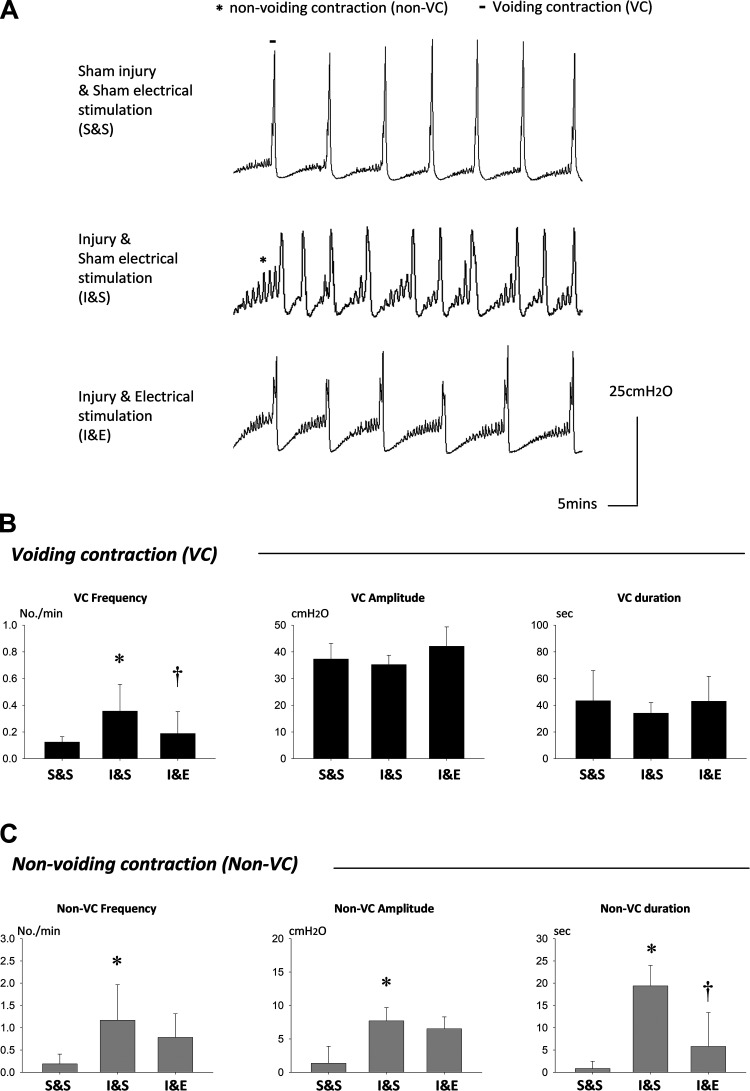
Voiding frequency was significantly increased in injured rats with sham stimulation (I&S) compared with those with sham injury (S&S) but not in rats with both injury and stimulation (I&E; Fig. 4, A and B). Rats with injury and stimulation (I&E) also had significantly decreased voiding frequency compared with rats with injury and sham stimulation (I&S). However, voiding contraction amplitude and duration did not show any significant differences between the groups.
Fig. 4.
Bladder pressure during filling cystometry 2 wk after simulated birth injury or sham injury and pudendal nerve stimulation or sham stimulation. A: bladder pressure during filling cystometry from all 3 groups showing increased frequency of voiding and nonvoiding contractions (VC and non-VC). Bladders were emptied after the first void and before starting a 30 min continuous fill cycle, not shown in Fig. 4A, resetting bladder pressure to 0 at the start of filling. B: frequency, amplitude, and duration of VC 2 wk after simulated birth injury with bilateral pudendal nerve stimulation (I&E, n = 8), simulated birth injury with sham stimulation (I&S, n = 8), and sham injury with sham stimulation (S&S, n = 8). C: frequency, amplitude, and duration of non-VC between the 3 groups. *Statistically significant difference compared with the corresponding S&S group (P < 0.05). †Statistically significant difference compared with the corresponding I&S group (P < 0.05). Data are presented as mean ± standard error of data from 8 animals.
Injured rats with sham stimulation (I&S) demonstrated increased nonvoiding contractions during the storage phase relative to sham-injured controls (S&S). Injured rats with electrical stimulation of the pudendal nerve (I&E) demonstrated fewer nonvoiding contractions than sham stimulation controls (I&S; Fig. 4, A and C). Nonvoiding contraction amplitude, frequency, and duration significantly increased in rats that underwent injury and sham stimulation (I&S) compared with those with sham injury (S&S) but not in rats that received both injury and stimulation (I&E). The latter group had significantly decreased nonvoiding contraction duration compared with rats with injury and sham stimulation (I&S).
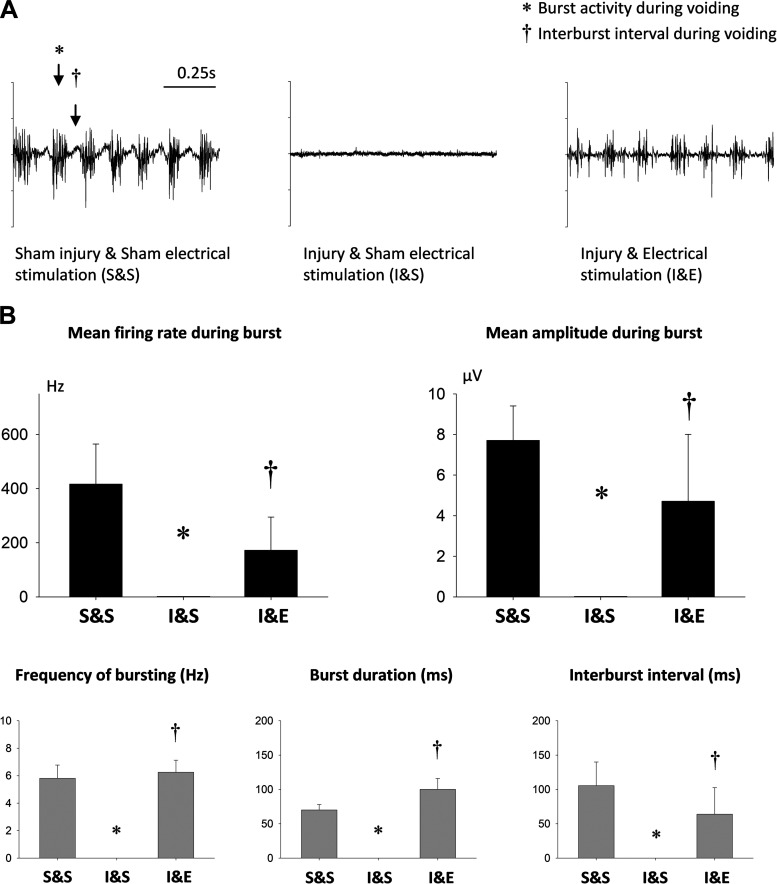
Recovery of EUS EMG bursting activity during voiding after injury and bilateral stimulation.
During voiding of sham injured animals, the EUS presented with bursting activity, an indicator of normal bladder emptying in rats. In injured rats with sham stimulation (I&S), EUS EMG burst frequency during voiding, burst duration, and interburst interval were reduced to zero 2 wk after injury (S&S; Fig. 5B). These same outcomes were all significantly recovered 2 wk after injury with electrical stimulation (I&E). Sham stimulation (I&S) had no identifiable bursting activity indicating a significant decrease in firing rate and amplitude after injury (Fig. 5B). Bursts returned and both measures were significantly recovered in injured rats receiving electrical stimulation (I&E).
Fig. 5.
External urethral sphincter (EUS) electromyogram (EMG) bursting activity during voiding 2 wk after simulated birth injury with pudendal nerve stimulation. A: example EUS EMG recordings during voiding from 3 groups. *Burst interval and †interburst interval. B: firing rate and amplitude during bursting, as well as frequency of bursting, burst duration, and interburst interval 2 wk after simulated birth injury with bilateral pudendal nerve stimulation (I&E, n = 8), simulated birth injury with sham stimulation (I&S, n = 8), and sham injury with sham stimulation (S&S, n = 8). *Statistically significant difference compared with the corresponding S&S group (P < 0.05). †Statistically significant difference compared with the corresponding I&S group (P < 0.05). Data are presented as mean ± standard error of data from 8 animals.
DISCUSSION
Birth injury from vaginal delivery is a significant risk factor for SUI both postpartum and later in life, although most women don’t develop symptoms until decades after delivery (33). In a longitudinal follow-up of women who had SUI during pregnancy or postpartum without alleviation 3 mo after delivery, 92% had symptom recurrence 5 yr later, indicating latent pathophysiological changes and delayed recovery of continence mechanisms, which could be linked to the development of SUI decades later (48, 49). Therefore, postpartum treatment targeting the pathophysiology of SUI has the potential to both treat postpartum incontinence and possibly prevent the problematic reoccurrence of SUI decades after childbirth.
As demonstrated previously, simultaneous injury of the EUS and pudendal nerve results in delayed functional recovery of the neuromuscular system compared with either injury alone and is thought to result from impaired neurotrophin upregulation in the EUS (25, 26). When an innervated target muscle is injured simultaneously, such as with the pudendal nerve and EUS system investigated in this study, neurotrophin expression within the innervated target muscle decreases and impairs neuroregeneration and functional recovery (16, 24, 25). Multiple studies have demonstrated that electrical stimulation enhances intrinsic neuronal BDNF expression and benefits both peripheral and central nervous system pathologies (19, 44, 50, 52). Al-Majed et al. (2) demonstrated that electrical stimulation led to rapid upregulation of BDNF in motoneurons along with axonal regeneration in a femoral nerve injury rat model. Subsequently, the same group developed low-frequency electrical stimulation for patients with median nerve compression and demonstrated accelerated postsurgical target organ reinnervation (20). Moreover, following deep brain stimulation for Parkinson’s disease, BDNF expression was significantly increased in the hippocampal area along with evidence of synaptic plasticity, which could play a role in the therapeutic effects of this treatment (19, 44).
Electrical stimulation may therefore overcome the potential downsides of administering BDNF after concurrent pudendal nerve and EUS injury by inducing neurotrophin upregulation and neuroregeneration in pudendal nerve cell bodies in Onuf’s nucleus after childbirth. Increasing BDNF within the EUS-pudendal nerve system was proven feasible via pudendal nerve electrical stimulation acutely after injury, which increased pudendal nerve BDNF expression in Onuf’s nucleus containing the motoneuron cell bodies of the pudendal nerve (24).Therefore, electrical stimulation may provide a postpartum therapy for the simultaneous muscle and nerve injuries sustained during childbirth. SUI treatment with electroacupuncture involving the lumbosacral region resulted in significant improvement of urinary continence after 6 wk in clinical testing (29), suggesting that electrical stimulation could have application to this condition. This treatment could not only target SUI postpartum but also potentially reduce the risk of redeveloping the condition later in life. In this study we demonstrated that electrical stimulation of the pudendal nerve increases BDNF expression, facilitates neuroregeneration, and improves functional recovery after a dual muscle and nerve simulated childbirth injury in a rat model of SUI.
The results of this study demonstrate that repeated electrical stimulation of the pudendal nerve produced a sustained increase in BDNF expression in Onuf’s nucleus compared with sham stimulation. Although this study did not directly compare BDNF RNA expression between acute stimulation and repeat stimulation at 1 wk, BDNF RNA levels at 1 wk are higher in this study compared with the acute stimulation study previously reported (24). Upregulation of βII-tubulin indicates an increased neuroregenerative response, because βII-tubulin constitutes microtubules within neurofilaments that facilitate injured axon regrowth (4, 40). The increase in BDNF expression occurred before increased βII-tubulin expression suggesting, but not proving, a causal link. Research on other models of nerve injury and electrical stimulation with deficient BDNF, neurotrophin 4, or their receptor tropomyosin receptor kinase B have demonstrated a causal link with neuroregeneration (11, 13). Sensory neurons upregulate BDNF with acute stimulation but might not regenerate with chronic stimulation because they downregulate tropomyosin receptor kinase B, the receptor for BDNF (5, 15, 21). Because both sensory and motor branches of the pudendal nerve are damaged during childbirth, it is important to determine if chronic electrical stimulation results in neuroregeneration and functional improvement after this injury. However, in the present study both branches of the pudendal nerve were electrically stimulated proximal to the nerve injury. Thus, the neurogenerative effect (increased BDNF and βII-tubulin expression in Onuf’s nucleus) of this treatment could be through either or both of two neural pathways: antidromic effect of electrical stimulation of the motor branch neuron soma or through reflex activation by a pudendal-pudendal reflex (15, 32).
To maintain urinary continence, a guarding-like reflex exists in which the EUS contracts to prevent urine leakage and counteract increased abdominal pressure upon the bladder, such as during LPP testing (27, 46). Increased contractile force generated by striated muscle, such as that comprising the EUS, involves the recruitment of additional motor units, which translates into both increased firing rate and increased amplitude of EUS EMG (39, 45). This increase in firing rate and amplitude of EUS EMG has been noted during LPP testing in uninjured rats but was impaired 2 wk after simulated birth injury by VD + PNC (25, 26) and has been confirmed in this study regardless of electrical stimulation or sham stimulation.
EUS presents tonic and bursting activity, the latter observed during voiding and considered as expression of a central pattern generator. These two types of activity in the EUS seem to involve different circuitry in spinal segments: tonic activity at L6-S1 and bursting activity between T8–9 and L3–4 (6). Axons of bursting motoneurons travel mainly through the lumbosacral trunk, before arriving to the motor branch of the pudendal nerve (8). The decrease in EUS tonic discharge during LPP and bursting activity during voiding indicate that both kinds of motor axons were damaged and electrical stimulation induced their reconnection, although tonic activity during LPP was not fully recovered 2 wk after injury.
Electrical stimulation appeared to accelerate pudendal nerve and EUS recovery compared with sham stimulation, because LPP and EUS EMG amplitude were significantly greater with stimulation compared with sham stimulation 2 wk after injury. In contrast, no difference in EUS EMG firing rate was observed with stimulation compared with sham stimulation after injury. Individual variation in EMG recordings (in some cases the electrodes may be nearer to the fibers) may influence the result but considering that each group had the same experimental variability, our finding suggests that the stimulation had a beneficial effect on recovery of EUS motor units. A future study will be needed to investigate the ability of EUS motoneurons to generate bursting activity (12). Therefore, our findings suggest that a similar number of motor units are functioning but those given electrical stimulation may be more recovered at this 2-wk time point. Investigation of later time points after injury will likely demonstrate greater recovery of EUS EMG with electrical stimulation.
The injury model used in this study is well established for the investigation of SUI pathophysiology and treatment (18, 23). However, bladder function and voiding have not been investigated in depth in this model. Previously, we found that this VD + PNC injury alters both bladder and anal function acutely, characterized by increased nonvoiding contractions during filling and diminished spontaneous anal contraction amplitude and frequency (24), which mirrors the dysfunction observed clinically immediately postpartum (14). In the present study, the frequency of nonvoiding contractions, as well as amplitude and duration of bladder contractions, significantly increased 2 wk after injury compared with sham injury; however, no increase was observed in animals given electrical stimulation. Similar effects were observed for voiding contractions, with significant increases in voiding frequency after injury if sham stimulation was given and significantly fewer contractions than sham stimulation if electrical stimulation was applied. These findings resemble the increased incidence of overactive bladder during pregnancy and immediately postpartum (47).
Interestingly, pudendal neuromodulation has been utilized clinically to treat overactive bladder (38). Therefore, it appears that electrical stimulation of the pudendal nerve improves pathologic changes in bladder activity and could potentially serve as a clinical therapy for similar postpartum conditions. The mechanism of action for this remains unknown, but it is known that EUS muscle tone during the filling phase can suppress bladder activity (known as the sphincter-to-bladder reflex); therefore, the loss of pudendal nerve control can induce the relaxation of EUS which, subsequently, leads to provocative bladder contractions (9). It is possible that restoration of the pudendal nerve innervation caused by electrical stimulation may help to reestablish this reflex. It also appears that neuroplasticity may be involved, as the reduction in overactivity persisted without ongoing stimulation (46).
In addition to bladder contractions, EUS function during voiding was also altered by electrical stimulation. In normal rats, EUS EMG bursting during voiding stems from rhythmic contraction and relaxation of the striated muscle (26). This bursting activity is central to normal voiding for bladder emptying efficiency in rats and is likely mediated in part by the efferent arm of the reflex traveling through the motor branch of the pudendal nerve. The detailed bursting reflex pathway remains unknown but may involve the pelvic and pudendal nerves, because interruption of either fiber may significantly change bursting. Although the bursting activity also depends on the pelvic nerve, which may be injured during the VD procedure in our study as the bladder and its nerves are damaged during VD, no additional injury occurred to the pelvic nerve during PNC procedure (34). Moreover, it is clear that the bursting efferent signal is carried by the pudendal nerve, and the effective organ is the EUS (37). Normal bursting activity was observed in sham-injured rats in this study. However, 2 wk after injury, bursting patterns, for the most part, were present with electrical stimulation but not with sham stimulation to the pudendal nerve, indicating that bursting activity has not recovered 2 wk after injury but recovery can be accelerated with electrical stimulation.
A limitation of the present study is the use of acute traumatic surgeries on nonpregnant quadruped animals to create a model of SUI. Nonetheless, it provides an excellent model of a combinatorial nerve-target muscle injury with reduced BDNF expression for use in investigations of the mechanism of neuromuscular regeneration and potential therapeutics. Rodents usually experience a much less traumatic delivery process, partly because of a smaller fetal head to birth canal ratio than humans and as such, postpartum pelvic floor dysfunction is uncommon in such animals (7). Despite the need for surgery to make up for a lack of delivery injury in rodents, prior work has demonstrated that this injury paradigm produces highly reproducible changes consistent with SUI (43), which is invaluable for the investigation of potential therapeutics.
Another limitation is that the repeated electrical stimulation was done blindly, with the possibility of stimulating pelvic floor musculature in addition to the pudendal nerve, although we did not observe any obvious muscle contractions. Anal sphincter contractions would be the most evident indicator of pudendal motor activation, but we did not measure anal pressure or external anal sphincter EMG in this study. Nonetheless, further studies are necessary to separate the effect of electrical stimulation of striated fibers (which could mimic Kegel exercises) and direct nerve stimulation. In addition, we did not determine if BDNF is upregulated in the sensory neuron somas in the dorsal root ganglia. Future work could investigate the pathways by which BDNF is upregulated and results in neuroregeneration.
A recent randomized clinical trial demonstrated that women with SUI treated by electroacupuncture in the lumbosacral region resulted in less urine leakage after 6 wk (29), suggesting that electrical stimulation could be a promising treatment option for SUI, although further research is needed to determine its long-term efficacy and the mechanism of action. In our study, electrical stimulation of the pudendal nerve in an injury model of nerve and target muscle injury that causes symptoms of SUI significantly improved neuroregeneration and recovery of continence, as evidenced by molecular markers of neuroregeneration, as well as functional and electrophysiological measures of EUS activity. Electrical stimulation also produced sustained increases in pudendal nerve neurotrophin levels, suggesting a mechanistic explanation for the observed functional recovery. The use of pudendal nerve electrical stimulation to treat postpartum SUI appears to be promising, and the treatment may also serve to prevent or ameliorate later development of SUI.
GRANTS
This work was supported in part by a grant from the National Institutes of Health (Grant No. R01-HD-38679 to M. S. Damaser), the Rehabilitation Research and Development Service of the Department of Veterans Affairs (No. I01 RX001262A1 to M. S. Damaser), National Natural Science Foundation of China (No. 81670695 to H.-H. Jiang), Wenzhou Science and Technology Bureau (No. Y20170101), Zhejiang Province Medical and Health for Science and Technology project (No. 2018PY031), the American Urological Association Foundation Research Scholars Program (H.-H. Jiang), and the Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction (H.-H. Jiang).
DISCLOSURES
M. S. Damaser has research agreements with Novartis and Medtronic. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
H.-H.J., Q.-X.S., B.C.G., B.M.B., R.J., and Y.C. performed experiments; H.-H.J., Q.-X.S., and R.J. analyzed data; H.-H.J., Q.-X.S., Y.C., and M.S.D. interpreted results of experiments; H.-H.J. and Q.-X.S. prepared figures; H.-H.J., Q.-X.S., B.C.G., and M.S.D. drafted manuscript; H.-H.J., Q.-X.S., B.M.B., and M.S.D. edited and revised manuscript; H.-H.J., Q.-X.S., B.C.G., B.M.B., R.J., Y.C., and M.S.D. approved final version of manuscript.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A; Standardisation Sub-committee of the International Continence Society . The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178, 2002. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 12: 4381–4390, 2000. [PubMed] [Google Scholar]
- 3.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol 24: 379–402, 2004. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PubMed] [Google Scholar]
- 4.Bré MH, Redeker V, Vinh J, Rossier J, Levilliers N. Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in paramecium. Mol Biol Cell 9: 2655–2665, 1998. doi: 10.1091/mbc.9.9.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brushart TM, Jari R, Verge V, Rohde C, Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol 194: 221–229, 2005. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292: F1044–F1053, 2007. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couri BM, Lenis AT, Borazjani A, Paraiso MF, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol 7: 249–260, 2012. doi: 10.1586/eog.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz Y, Pastelín C, Balog BM, Zaszczurynski PJ, Damaser MS. Somatomotor and sensory urethral control of micturition in female rats. Am J Physiol Renal Physiol 307: F1207–F1214, 2014. doi: 10.1152/ajprenal.00255.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 194: 91–138, 2009. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolan LM, Hosker GL, Mallett VT, Allen RE, Smith ARB. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG 110: 1107–1114, 2003. doi: 10.1111/j.1471-0528.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- 11.Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol 198: 500–510, 2006. doi: 10.1016/j.expneurol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 12.English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ. Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci 33: 2265–2271, 2011. doi: 10.1111/j.1460-9568.2011.07724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology (Bethesda) 29: 437–445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FitzGerald MP, Graziano S. Anatomic and functional changes of the lower urinary tract during pregnancy. Urol Clin North Am 34: 7–12, 2007. doi: 10.1016/j.ucl.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol 205: 347–359, 2007. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Gill BC, Balog BM, Dissaranan C, Jiang HH, Steward JB, Lin DL, Damaser MS. Neurotrophin therapy improves recovery of the neuromuscular continence mechanism following simulated birth injury in rats. Neurourol Urodyn 32: 82–87, 2013. doi: 10.1002/nau.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill BC, Lin DL, Balog BM, Dissaranan C, Jiang HH, Damaser MS. Molecular assessment of neuroregenerative response in the pudendal nerve: a useful tool in regenerative urology. SDRP J Biomed Eng 1:1, 2016. [PMC free article] [PubMed] [Google Scholar]
- 18.Gill BC, Moore C, Damaser MS. Postpartum stress urinary incontinence: lessons from animal models. Expert Rev Obstet Gynecol 5: 567–580, 2010. doi: 10.1586/eog.10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gondard E, Chau HN, Mann A, Tierney TS, Hamani C, Kalia SK, Lozano AM. Rapid modulation of protein expression in the rat hippocampus following deep brain stimulation of the fornix. Brain Stimulat 8: 1058–1064, 2015. doi: 10.1016/j.brs.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol 223: 192–202, 2010. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 43: 336–350, 2016. doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haastert-Talini K, Schmitte R, Korte N, Klode D, Ratzka A, Grothe C. Electrical stimulation accelerates axonal and functional peripheral nerve regeneration across long gaps. J Neurotrauma 28: 661–674, 2011. doi: 10.1089/neu.2010.1637. [DOI] [PubMed] [Google Scholar]
- 23.Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol 202: 45–67, 2011. doi: 10.1007/978-3-642-16499-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang HH, Gill BC, Dissaranan C, Zutshi M, Balog BM, Lin D, Damaser MS. Effects of acute selective pudendal nerve electrical stimulation after simulated childbirth injury. Am J Physiol Renal Physiol 304: F239–F247, 2013. doi: 10.1152/ajprenal.00235.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang HH, Gustilo-Ashby AM, Salcedo LB, Pan HQ, Sypert DF, Butler RS, Damaser MS. Electrophysiological function during voiding after simulated childbirth injuries. Exp Neurol 215: 342–348, 2009. doi: 10.1016/j.expneurol.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser M. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn 28: 229–235, 2009. doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang HH, Salcedo LB, Damaser MS. Quantification of neurological and other contributors to continence in female rats. Brain Res 1382: 198–205, 2011. doi: 10.1016/j.brainres.2011.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lien KC, Morgan DM, Delancey JO, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am J Obstet Gynecol 192: 1669–1676, 2005. doi: 10.1016/j.ajog.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Liu Y, Xu H, He L, Chen Y, Fu L, Li N, Lu Y, Su T, Sun J, Wang J, Yue Z, Zhang W, Zhao J, Zhou Z, Wu J, Zhou K, Ai Y, Zhou J, Pang R, Wang Y, Qin Z, Yan S, Li H, Luo L, Liu B. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trial. JAMA 317: 2493–2501, 2017. doi: 10.1001/jama.2017.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Martens FM, Heesakkers JP, Rijkhoff NJ. Surgical access for electrical stimulation of the pudendal and dorsal genital nerves in the overactive bladder: a review. J Urol 186: 798–804, 2011. doi: 10.1016/j.juro.2011.02.2696. [DOI] [PubMed] [Google Scholar]
- 32.McKenna KE, Nadelhaft I. The pudendo-pudendal reflex in male and female rats. J Auton Nerv Syst 27: 67–77, 1989. doi: 10.1016/0165-1838(89)90130-6. [DOI] [PubMed] [Google Scholar]
- 33.Meyer S, de Grandi P, Kuntzer T, Hürlimann P, Schmidt N. Birth trauma: its effect on the urine continence mechanisms. Gynakol Geburtshilfliche Rundsch 33: 236–242, 1993. doi: 10.1159/000272115. [DOI] [PubMed] [Google Scholar]
- 34.Palacios JL, Juárez M, Morán C, Xelhuantzi N, Damaser MS, Cruz Y. Neuroanatomic and behavioral correlates of urinary dysfunction induced by vaginal distension in rats. Am J Physiol Renal Physiol 310: F1065–F1073, 2016. doi: 10.1152/ajprenal.00417.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan HQ, Kerns JM, Lin DL, Sypert D, Steward J, Hoover CRV, Zaszczurynski P, Butler RS, Damaser MS. Dual simulated childbirth injury delays anatomic recovery. Am J Physiol Renal Physiol 296: F277–F283, 2009. doi: 10.1152/ajprenal.90602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastelín CF, Juárez R, Damaser MS, Cruz Y. Neural pathways of somatic and visceral reflexes of the external urethral sphincter in female rats. J Comp Neurol 520: 3120–3134, 2012. doi: 10.1002/cne.23079. [DOI] [PubMed] [Google Scholar]
- 37.Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol 294: R660–R672, 2008. doi: 10.1152/ajpregu.00270.2007. [DOI] [PubMed] [Google Scholar]
- 38.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 29: 1267–1271, 2010. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 39.Podnar S, Resman-Gaspersic A. Quantitative motor unit potential analysis in the diaphragm: a normative study. Muscle Nerve 37: 518–521, 2008. doi: 10.1002/mus.20939. [DOI] [PubMed] [Google Scholar]
- 40.Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bré MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266: 1688–1691, 1994. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- 41.Snooks SJ, Setchell M, Swash M, Henry MM. Injury to innervation of pelvic floor sphincter musculature in childbirth. Lancet 2: 546–550, 1984. doi: 10.1016/S0140-6736(84)90766-9. [DOI] [PubMed] [Google Scholar]
- 42.Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br J Surg 77: 1358–1360, 1990. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 43.Song Q-X, Balog BM, Kerns J, Lin DL, Sun Y, Damaser MS, Jiang HH. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourol Urodyn 34: 381–386, 2015. doi: 10.1002/nau.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spieles-Engemann AL, Steece-Collier K, Behbehani MM, Collier TJ, Wohlgenant SL, Kemp CJ, Cole-Strauss A, Levine ND, Gombash SE, Thompson VB, Lipton JW, Sortwell CE. Subthalamic nucleus stimulation increases brain derived neurotrophic factor in the nigrostriatal system and primary motor cortex. J Parkinsons Dis 1: 123–136, 2011. [PMC free article] [PubMed] [Google Scholar]
- 45.Stålberg E, Erdem H. Quantitative motor unit potential analysis in routine. Electromyogr Clin Neurophysiol 42: 433–442, 2002. [PubMed] [Google Scholar]
- 46.Su X, Nickles A, Nelson DE. Comparison of neural targets for neuromodulation of bladder micturition reflex in the rat. Am J Physiol Renal Physiol 303: F1196–F1206, 2012. doi: 10.1152/ajprenal.00343.2012. [DOI] [PubMed] [Google Scholar]
- 47.Thomason AD, Miller JM, Delancey JO. Urinary incontinence symptoms during and after pregnancy in continent and incontinent primiparas. Int Urogynecol J Pelvic Floor Dysfunct 18: 147–151, 2007. doi: 10.1007/s00192-006-0124-8. [DOI] [PubMed] [Google Scholar]
- 48.Viktrup L, Lose G. The risk of stress incontinence 5 years after first delivery. Am J Obstet Gynecol 185: 82–87, 2001. doi: 10.1067/mob.2001.114501. [DOI] [PubMed] [Google Scholar]
- 49.Viktrup L, Lose G, Rolff M, Barfoed K. The symptom of stress incontinence caused by pregnancy or delivery in primiparas. Obstet Gynecol 79: 945–949, 1992. [PubMed] [Google Scholar]
- 50.Willand MP, Nguyen MA, Borschel GH, Gordon T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair 30: 490–496, 2016. doi: 10.1177/1545968315604399. [DOI] [PubMed] [Google Scholar]
- 51.Wood LN, Anger JT. Urinary incontinence in women. BMJ 349, sep15 4: g4531, 2014. doi: 10.1136/bmj.g4531. [DOI] [PubMed] [Google Scholar]
- 52.Ying Z, Covalin A, Judy J, Gomez-Pinilla F. Hypothalamic stimulation enhances hippocampal BDNF plasticity in proportion to metabolic rate. Brain Stimulat 5: 642–646, 2012. doi: 10.1016/j.brs.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]