Abstract
Acupuncture with low-frequency electrical stimulation (Acu/LFES) can prevent muscle atrophy by increasing muscle protein anabolism in mouse models of chronic kidney disease. During the treatment of muscle wasting, we found that Acu/LFES on the gastrocnemius muscle of the leg enhances renal blood flow. We also found that Acu/LFES increases exosome abundance and alters exosome-associated microRNA expression in the circulation. When exosome secretion was blocked using GW4869, the Acu/LFES-induced increase in renal blood flow was limited. This provided evidence that the increased renal blood flow is exosome mediated. To identify how exosomes regulate renal blood flow, we performed microRNA deep sequencing in exosomes isolated from treated and untreated mouse serum and found that the 34 microRNAs are altered by Acu/LFES. In particular, miR-181d-5p is increased in the serum exosome of Acu/LFES-treated mice. In silico searching suggested that miR-181d-5p could target angiotensinogen. Using a luciferase reporter assay, we demonstrated that miR-181 directly inhibits angiotensinogen. When Acu/LFES-treated muscle was excised and incubated in culture medium, we found that the amount of exosomes and miR-181d-5p was increased in the medium providing evidence that Acu/LFES can increase miR-181 secretion. We conclude that Acu/LFES on leg hindlimb increases miR-181 in serum exosome leading to increased renal blood flow. This study provides important new insights about the mechanism(s) by which acupuncture may regulation of muscle-organ cross talk through exosome-derived microRNA.
Keywords: Acu/LFES, angiotensinogen, exosome, microRNA, renal blood flow
INTRODUCTION
Acupuncture with low-frequency electrical stimulation (Acu/LFES) is widely used as a therapeutic intervention in the United States and around the world (24a).
In the course of our studies, we found that Acu/LFES increases muscle mass, myogenesis, and insulin-like growth factor-1 and decreases myostatin in the muscle of chronic kidney disease, diabetic, and denervated mice (13, 28, 29). Previous research, including ours, showed that treating the leg muscle has whole body consequences. Acu/LFES can ameliorate symptoms, such as fatigue, stress, hypertension, and pruritus in patients with end-stage renal disease (3, 16, 27). However, the mechanisms by which acupuncture in the limb improves distant organ function is not clear. Muscle wasting is associated with decline of renal function (1, 31). Little is known about potential beneficial consequences of Acu/LFES treatment of muscle on renal hemodynamics. Our results suggest that new strategies to ameliorate muscle wasting may provide new effective treatments for renal dysfunction.
The use of Acu/LFES is a simple and safe technique. Acu/LFES is an acupuncture technique that replicates the benefits of resistance exercise through stimulation of muscle contraction. The British Acu/LFES Council concluded that Acu/LFES is a relatively safe form of treatment after their prospective survey of 34,407 Acu/LFES treatments (22). The equipment needed to perform this technique is simple and inexpensive. In the case of manual acupuncture alone, simple acupuncture needles are inserted into key points in the body; in Acu/LFES, an adjustable power source, battery operated and very portable device, similar to a transcutaneous electrical nerve stimulation unit, is connected to the needles to provide electrical stimulation at those key points (8a). This minimally invasive treatment can potentially overcome a huge problem. This study presents evidence that exosomes are influenced by Acu/LFES. There is currently no published experimental evidence that acupuncture can regulate exosomes.
Exosomes are a subtype of extracellular vesicle (EV). EVs are spherical bilayered proteolipids with an average diameter of 20–1,000 nm that contain proteins, mRNAs, microRNAs (miRs), lipids, and metabolites that reflect their originating cell types and conditions (26). The release of exosomes is a common cellular function in most living biological systems (15). EVs are found in various biological fluids including serum and urine. Exosomes (30–130 nm) and microparticles (100–1,000 nm) are the most studied of the EVs and have various functions in intercellular and interorgan communication (30). Exosomes can provide biomarkers for noninvasive diagnosis and prognosis of diseases (11) and are being investigated as a means for delivery of vaccines, chemotherapeutic drugs, and siRNA (14). Both pre-miRNAs and mature miRNA have been detected in exosomes, and packaged miRs in exosomes are quite stable (19).
Our hypothesis is that during acupuncture there is cross talk between skeletal muscle and other organs through serum-derived exosomes containing miR. As proof of this hypothesis we 1) measured renal blood flow (RBF) after Acu/LFES; 2) measured exosome secretion after Acu/LFES; 3) performed miR deep sequencing in the RNA isolated from serum exosomes and found that miR-181 was significantly increased by Acu/LFES; and 4) tested impact of miR-181 on angiotensinogen.
METHODS
Animals.
These experiments were approved by the Emory University Institutional Animal Care and Use Committee (Protocol No. 4000152). The mice (C57BL/6J) were from Jackson Laboratories (Bar Harbor, ME) and were housed with a 12:12-h light-dark cycle.
Acu-LFES treatment.
The mice were kept in specially designed restraints so that they would remain in a recumbent position during Acu-LFES treatment. Mice were awake without any anesthesia and appeared to be comfortable throughout the treatments. Acupuncture points selected were according to the World Health Organization Standard Acupuncture guidelines (20). The positive point (anode: Yang Ling Quan, GB34) is in the hollow of the exterior-inferior of the caput fibulae ~6-mm deep. This position is close to the superficial fibular nerve and deep fibular nerve. The negative point (cathode: Zu San Li, ST36) is 5 mm beneath the capitulum fibulae and located laterally and posterior to the knee-joint ~7-mm deep and close to fibular nerve. The impulses were delivered between the two acupuncture needles. Disposable sterile needles with a diameter of 0.25 mm (Shen Li Medical & Health Material, Wujiang, China) were used. The needles were connected into SDZ-II electronic acupuncture instrument using consistent pulse with an electric frequency of 20 Hz and electric current of 2 mA (13).
Renal plasma flow for mice.
The renal plasma flow was measure by p-aminohippuric acid (PAH; A3759; Sigma-Aldrich, St. Louis, MO) infusion. Experiments were performed in the sham (acupuncture needle in, proximal to the treatment point, without LFES) and Acu/LFES (point GB34 and S36) mice. Mice were anesthetized by 10 mg/kg xylazine and 90 mg/kg ketamine ip. The right carotid artery was cannulated with PE-10 tubing for blood collection. The right jugular vein was catheterized with PE-10 tubing for fluid infusion. The bladder was catheterized with PE-50 tubing via a suprapubic incision for urine collections. Then, 1.5% PAH (A3759; Sigma) was injected by peristaltic pump into jugular vein (use Standard Infusion PHD 22/2000 Syringe Pumps; Harvard Apparatus, Holliston, MA) at 5 ul/min. After a 60-min equilibration period, sham and Acu/LFES were performed for 15 min, and then mice were rested for an additional 40 min after which 400 µl blood and urine were collected for measuring PAH using a PAH colorimetric assay kit (K860; BioVision, Milpitas, CA). The mice were euthanatized using American Veternary Medical Association guidelines. The PAH clearance were calculated by urine PAH multiplied by urine volume (2 h) divided by plasma PHA; all values were normalized per gram of kidney weight (2).
Isolation of exosomes from serum and conditioned medium.
Exosomes were isolated using series centrifugation. Serum was diluted 1:4 with 0.9% sodium chloride and centrifuged at 1,000 g for 10 min, 4°C. The supernatant fraction was further centrifuged at 16,000 g for 30 min. The second supernatant was sterile filtered through a 0.22-μm filter. Exosomes were pelleted at 120,000 g for 90 min at 4°C (L8–70M ultracentrifuge; Beckman-Coulter, Indianapolis IN). Finally, the exosome pellet was resuspended in 100–400 μl PBS. For exosomes isolated from incubated muscle following acupuncture: tibialis anterior muscles were isolated immediately after Acu/LFES and then incubated in Ham's F-10 nutrient mixture medium with 2% exosome-free serum in an incubator with 5% CO2 to allow muscles to release exosomes. After 16 h, the conditioned medium was centrifuged at 800 g for 5 min, 4°C. The exosomes were isolated from the conditioned medium using a series of centrifugation as described above. Exosome size, concentration, and distribution of particles were identified using a NanoSight instrument (Malvern, Westborough, MA). The exosomal marker TSG101 was assessed by Western blot. GW4869, an inhibitor of exosome release, was purchased from Sigma-Aldrich.
Reverse transcription and quantitative PCR for RNA.
Total RNA was extracted using Tri-Reagent (Molecular Research, Cincinnati, OH). For miR, the miRCURY LNA Universal cDNA Synthesis kit (Exiqon, Woburn, MA) was used for reverse transcription of total RNA. The primers were purchased from Exiqon. The miRCURY LNA miR PCR SYBR Green master mix (Exiqon INC) was used for quantitative PCR (qPCR). For mRNA expression, total RNA (1–2 μg) was reverse transcribed using a Thermoscript RT-PCR kit (Invitrogen, Carlsbad, CA). Real-time qPCR was performed with the SYBR Green PCR reagent (Bio-Rad). Expression of individual miR was standardized to the mouse U6 gene (miR tissue), miR103 (miR serum), or 18S (mRNA) (13, 29). The quantification cycle (Cq) values was defined as the number of cycles required for the fluorescence signal to exceed the detection threshold. Individual mRNA expression was calculated as the difference between the threshold values of the two genes (2−Δcq). Melting curve analysis was routinely performed to verify the specificity of the reaction. The mRNA expression were used following primers: CD4 (NM_013488.3), forward 5′-CCA GCT GTC TGC TTG GAT CA-3′ and reverse 5′-AGC CCT CTC GTA AAC TGT GC-3′ (amplicon 96 nt); and CD8 (NM_001081110.2), forward 5′-CAG AGA CCA GAA GAT TGT CG-3′ and reverse 5′-TGA TCA AGG ACA GCA GAA GG-3′ (amplicon 124 nt).
miRNA-Seq library preparation and sequencing.
Qualitative and quantitative analysis of the total RNA was performed using the Thermo Nanodrop 2000 and Agilent 2100 Bioanalyzer, respectively. Small RNA libraries were prepared using the SeqMatic tailormix miRNA sample preparation kit (SeqMatic, Union City, CA) as per manufacturer’s instructions. Briefly, 100 ng of total RNA were used for library preparation. Small RNAs were ligated with Illumina compatible adapters, and each sample was tagged with a unique barcode to allow multiplexing. The adapter-ligated libraries were then enriched using PCR amplification followed by gel enrichment for mature miRNA library. The amplified library was validated using a High Sensitivity DNA chip on the Agilent Bioanalyzer. The libraries were further quantified on Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY) using the High Sensitivity dsDNA assay. Libraries from all the samples were multiplexed and run in a single lane of Illumina 3K flowcell. PhiX was used as an internal control on each lane to monitor the error statistics. Cluster generation was performed on the v3 flowcell on the Illumina cBot. The clustered flowcell was sequenced on the Illumina HiSeq3000 system as a 100-cycle single read multiplexed run.
Immunoblotting and antibodies.
To identify protein in skeletal muscle, hindlimb muscles were homogenized in RIPA buffer and proteins in the soluble fraction of tissue homogenates were assessed by Western blot, performed as previously described. Protein bands were probed with angiotensinogen antibody (ab213705; Abcam, Cambridge, MA; 1:1,000 dilution). The angiotensinogen densities were normalized to the GAPDH of each sample. Anti-GAPDH was purchased from Millipore (Burlington, MA).
Luciferase reporter assay and transfection.
Effectene transfection reagent was used for transfection (Qiagen, Valencia, CA). Firefly and Renilla luciferase activities were measured by dual-luciferase assays (Promega) using a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) (9). The luciferase report vectors (pMIR-REPORT Luciferase) were purchase from Applied BIOSYSTEMS (Waltham, MA), and constructs were made by Emory Integrated Genomics Core.
Creatine kinase activity assay.
Plasma and gastrocnemius muscles were isolated from sham and Acu/LFES-treated mice. Muscles were lysed in Tris-MES buffer (50 mM Tris-MES and 1% Triton X-100 pH 7.7). The creatine kinase activity assay kit was purchased from Catachem (Oxford, CT). The activity was assessed using a chromogenic substrate and monitoring absorbance at 420 nm according to manufacturer’s instructions. Enzymatic activity for creatine kinase was normalized to grams of muscle weight.
Statistical analysis.
Data were presented as means ± SE. To identify significant differences between two groups, comparisons were made by using the Student’s t-test. When multiple treatments were compared, ANOVA was performed with a post hoc analysis by the Student-Newman-Keuls test. The relationship between muscle and kidney fluorescence intensity was calculated by linear regression modeling. Differences with P < 0.05 were considered significant.
RESULTS
Acupuncture increased RBF.
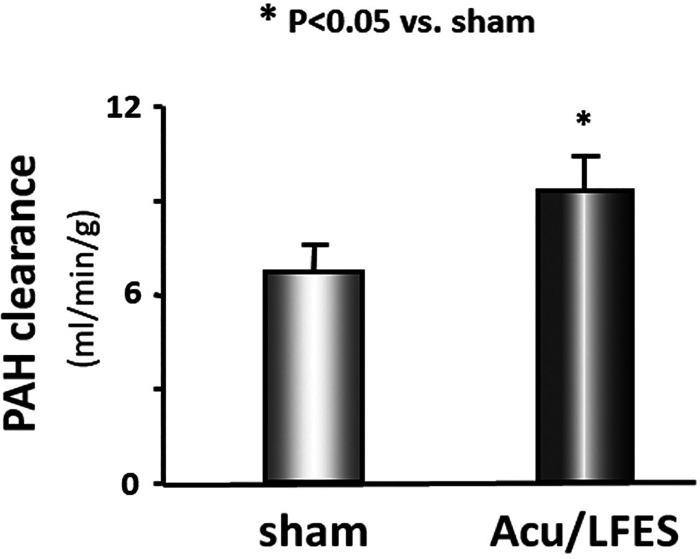
Previous studies have found that Acu/LFES decreases urinary albumin secretion and improves RBF and glomerular filtration rate in nephropathy patients and animals (4, 8, 10, 34). To investigate how Acu/LFES increases RBF, we first verified the increased blood flow in normal mice. Acu/LFES mice received needles in point GB34 and S36 using a consistent pulse, an electrical frequency of 20 Hz, and electrical current of 2 mA for 30 min of electrical stimulation. Sham mice had acupuncture needles inserted in close proximity to the LFES insertion position, and needles were connected to the LFES device, but electrical stimulation was not applied. RBF was measured by PAH (A3759; Sigma-Aldrich) infusion before and during Acu/LFES. We found that PAH clearance is increased by 40% in the mice after 15 min of Acu/LFES vs. mice with sham Acu/LFES (Fig. 1).
Fig. 1.
Acupuncture with low-frequency electrical stimulation (Acu/LFES) increases renal blood flow. Experiments were performed in the sham-treated (acupuncture needle inserted near, but not at, treatment point, without LFES) and Acu/LFES-treated mice. Renal flood flow was measured using p-aminohippuric acid (PAH) infusion during the Acu/LFES. PAH concentration was tested using a PAH colorimetric assay kit. The bar graph shows mean PAH clearance from sham and Acu/LFES groups of mice. Results are normalized to grams of body weight (bars: means ± SE; n = 12/group; *P < 0.05 vs. sham).
Acupuncture increased exosome secretion.
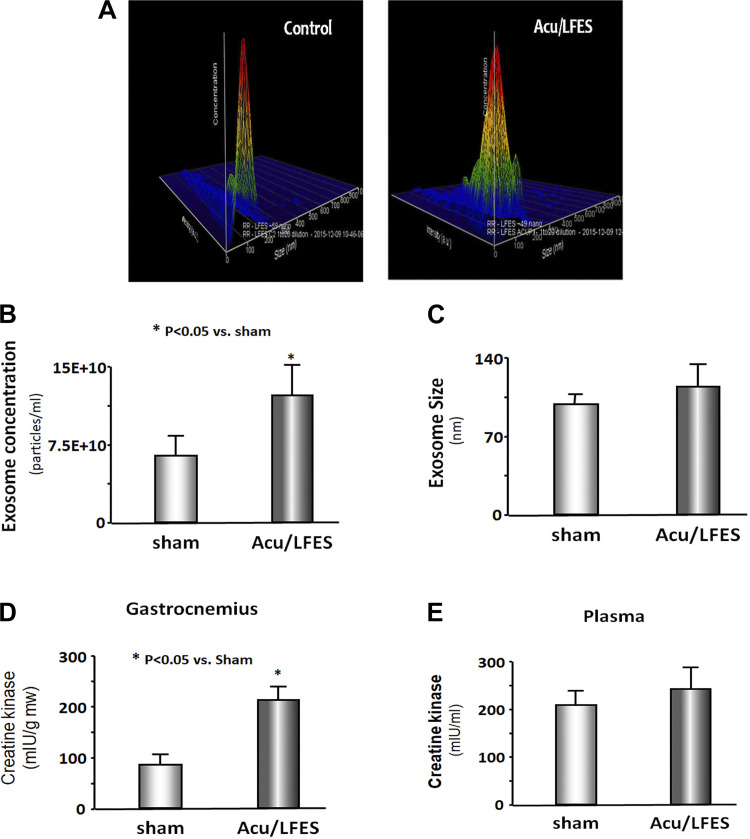
A possible explanation for Acu/LFES increase of RBF is altered circulating exosomes. To elucidate the role of exosomes in Acu/LFES, we isolated exosomes from both sham and Acu/LFES-treated mice. Using a Nanosight instrument, we found that Acu/LFES changed exosome distribution in the serum (Fig. 2A). The concentration of exosomes was increased twofold by Acu/LFES (Fig. 2B). The exosome size had a tendency to be increased by Acu/LFES, but this did not reach statistical significance (Fig. 2C). Creatine kinase is an important enzyme in muscle and is elevated in both healthy (e.g., exercised) or damaged (after myocardial infarction) muscle. We measured the plasma creatine kinase level and found it is significantly increased in skeletal muscle following Acu/LFES. We also observed a tendency to increase in plasma from the treated animals, but this did not reach a statistical difference (Fig. 2, D and E).
Fig. 2.
Acupuncture increases exosome secretion. A–C: exosomes were isolated from sham and acupuncture with low-frequency electrical stimulation (Acu/LFES)-treated mice. The distribution (A), concentration (B), and size (C) of exosomes were measured using a NanoSight instrument. Typical color 3-dimensioanl graphs from 1 sham-treated mouse (left) and 1 Acu/LFES-treated mouse (right) are provided in A. The readout shows the size (x-axis, nanometer diameter) and concentration (y-axis, -articles/ml) of exosomes from mice serum. The bar graph in B shows mean exosome concentration and in C shows exosome size from sham and Acu/LFES groups of mice (bars: means ± SE; n = 5/group; *P < 0.05 vs. sham). D and E: creatine kinase activity was assessed using a chromogenic substrate in gastrocnemius muscle lysate (D) and plasma (E) (bars: means ± SE; n = 6; *P < 0.05 vs. sham).
Blocking exosome secretion limited the acupuncture-induced increase in RBF.
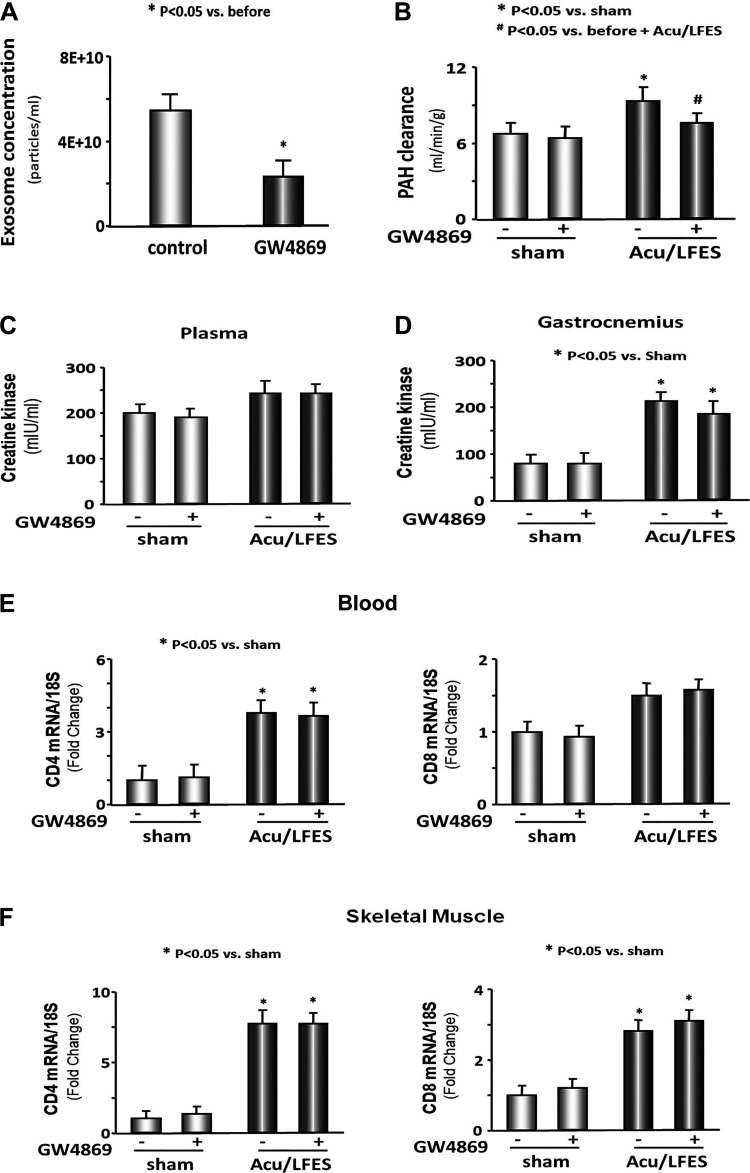
To prove the increase in RBF is due to exosome-mediated changes, we used GW4869 to inhibit exosome secretion. GW4689 is the most widely used pharmacological agent for blocking exosome generation. It inhibits the ceramide-mediated inward budding of multivesicular bodies and release of mature exosomes from multivesicular bodies (17). Multiple trials of time and concentration were performed to find optimal conditions for blocking exosome secretion using GW4869. Mice were injected with 1 μg/g body wt of GW4869 16 h before treatment with Acu/LFES. We found that GW4869 decreased serum exosome secretion by 49.4% in control mice that did not have Acu/LFES treatment (Fig. 3A). A reduced PAH clearance was observed in GW4869-treated Acu/LFES mice compared with Acu/LFES mice that did not receive GW4869 (Fig. 3B). There was no difference in PAH clearance in sham mice with or without GW4869 treatment. These results suggest that exosome secretion resulting from Acu/LFES treatment of muscle is associated with enhanced RBF. Next, we asked whether the increase in RBF might be associated with immune response or general changes in muscle metabolism. We measured the creatine kinase level in plasma and gastrocnemius muscle lysate under conditions where exosomes secretion was inhibited using GW4869. The creatine kinase amount unchanged by GW4869 in Acu/LFES-treated mice (Fig. 3, C and D). This suggests that the decrease in RBF that accompanied GW4869 treatment of Acu/LFES mice was not the result of the increased muscle metabolism. Our previous study found that Acu/LFES increase inflammation cytokines in the plasma and skeletal muscle of Acu/LFES-treated mice (28). In the current study, we measured the expression of CD4 and CD8 in blood and skeletal muscle. Acu/LFES increased CD4 expression in plasma and skeletal muscle, Inhibition of exosome secretion did not alter the CD4 or CD8 responses, again, suggesting that the GW4869-induced decrease in RBF was not the result of a decrease in immune response (Fig. 3, E and F).
Fig. 3.
Blocking exosome secretion limits acupuncture-induced increase in renal blood flow (RBF). A: exosomes were isolated from normal mice with or without GW4869 treatment for 16 h. The concentration of exosomes was measured using a NanoSight instrument. The bar graph shows mean concentration (particles/ml) from control and GW4869-treated mice (bars: means ± SE; n = 5/group; *P < 0.05 vs. control). B: mice were treated with GW4869 to reduce exosomes secretion and then received either sham needle insertion or acupuncture with low-frequency electrical stimulation (Acu/LFES) treatment. RBF was measured by p-aminohippuric acid (PAH) infusion during the Acu/LFES or sham treatments. PAH concentration was tested using a PAH colorimetric assay. The bar graph shows mean PAH clearance from sham and Acu/LFES groups of mice treated with or without GW4869 for 16 h. The results are normalized to g body weight (bars: means ± SE; n = 12/group; *P < 0.05 vs. sham; #P < 0.05 vs. Acu/LFES without GW4869). C: plasma was isolated from sham and Acu/LFES mice with or without GW4869 treatment. Creatine kinase activity was assessed using a chromogenic substrate (bars: means ± SE; n = 6). D: gastrocnemius muscle lysate from sham and Acu/LFES mice with or without GW4869 treatment were assayed for creatine kinase activity (bars: means ± SE; n = 6; *P < 0.05 vs. sham). E: total RNA was extracted from blood of sham and Acu/LFES mice. The expression of CD4 and CD8 were assayed by real-time quantitative (q)PCR. The bar graph shows mRNA from the blood of Acu/LFES mice compared with levels in shams (defined as 1-fold). Results are normalized to 18S (bars: means ± SE; n = 6/group; *P < 0.05 vs. sham). F: total RNA was extracted from gastrocnemius muscle of sham and Acu/LFES mice. The expression of CD4 and CD8 were assayed by real-time qPCR. The bar graph shows mRNA from the muscle of Acu/LFES mice compared with levels in shams (defined as 1-fold). Results are normalized to 18S (bars: means ± SE; n = 6/group; *P < 0.05 vs. sham).
Acu/LFES increased miR-181 in circulation exosomes and skeletal muscle.
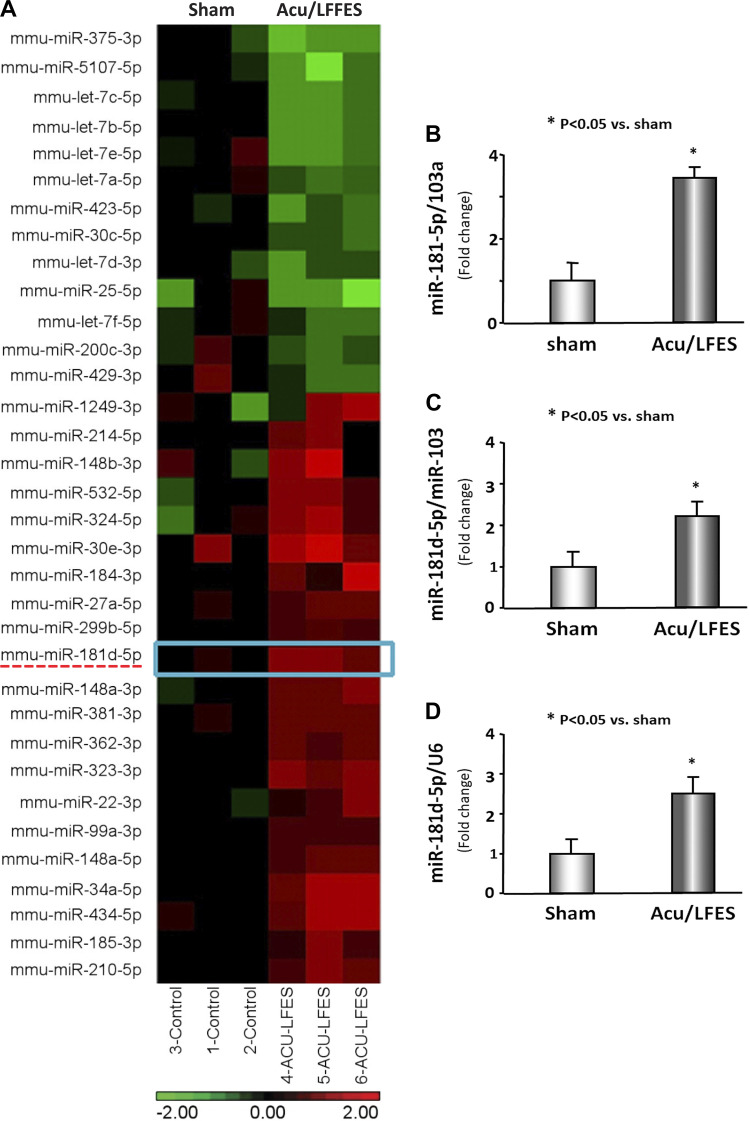
To identify possible mechanisms of Acu/LFES-induced exosome-mediated increases in RBF we performed miR deep sequencing analysis on RNA isolated from serum exosomes of mice with and without Acu/LFES. We found 34 miRs that were altered by Acu/LFES. These included miR-181d-5p, which was significantly increased by Acu/LFES (Fig. 4A). To verify the deep-sequence data, we isolated serum exosomes from sham and Acu/LFES mice and measured miR-181d-5p by real-time qPCR. The expression of miR-181d-5p was increased 3.6-fold in the serum exosomes from Acu/LFES mice vs. sham mice (Fig. 4B). To examine whether the miR-181 that was increased in serum exosomes could have originated in muscle, we isolated hindlimb muscle from Acu/LFES-treated mice and then incubated these muscles in Ham's F-10 nutrient mixture medium with 2% exosome-free serum for 16 h to allow muscles to release exosomes. The exosomes were isolated from the conditioned medium. The expression of miR-181d-5p was increased by 2.2-fold in the Acu/LFES-treated muscle over levels in sham mouse muscle (Fig. 4C). To confirm these results, we also measured the expression of miR-181d-5p in gastrocnemius muscle and found that miR-181d was increased by 2.5-fold in the muscle with Acu/LFES treatment (Fig. 4D).
Fig. 4.
Acupuncture with low-frequency electrical stimulation (Acu/LFES) increases exosome-carried miR-181. A: total RNA was isolated from serum exosomes of sham and Acu/LFES-treated mice. microRNA deep sequencing was performed and the heat map showed that there are 34 microRNA of serum exosome that are altered by Acu/LFES. Left 3 lanes: sham levels; right 3 lanes: Acu/LFES mouse levels. Green indicates decrease; red indicates increase. B: total RNA was extracted from serum exosomes of sham and Acu/LFES mice. The expression of miR-181d-5p was assayed by real-time quantitative (q)PCR. The bar graph shows microRNA from the exosomes of Acu/LFES mice compared with levels in shams (defined as 1-fold). Results are normalized to miR-103a (bars: means ± SE; n = 9/group; *P < 0.05 vs. sham). C: hindlimb muscles were isolated from Acu/LFES or sham-treated mice immediately following treatment and incubated in Ham's F-10 nutrient mixture medium with 2% exosome-free serum for 16 h. Exosomes were isolated from the conditioned medium. RNA was extracted from the exosomes and the expression of miR-181d-5p was assayed by real-time qPCR. The bar graph shows miR-181d-5p from the exosomes of Acu/LFES muscle compared with levels in shams (represented by 1-fold). Results are normalized to miR-103a (bars: means ± SE; n = 9/group; *P < 0.05 vs. sham). D: total RNA was extracted from gastrocnemius muscles of sham and Acu/LFES mice. The expression of miR-181d-5p was assayed by real-time qPCR. The bar graph shows microRNA from the exosomes of Acu/LFES mice compared with levels in shams (defined as 1-fold). Results are normalized to U6 (bars: means ± SE; n = 9/group; *P < 0.05 vs. sham).
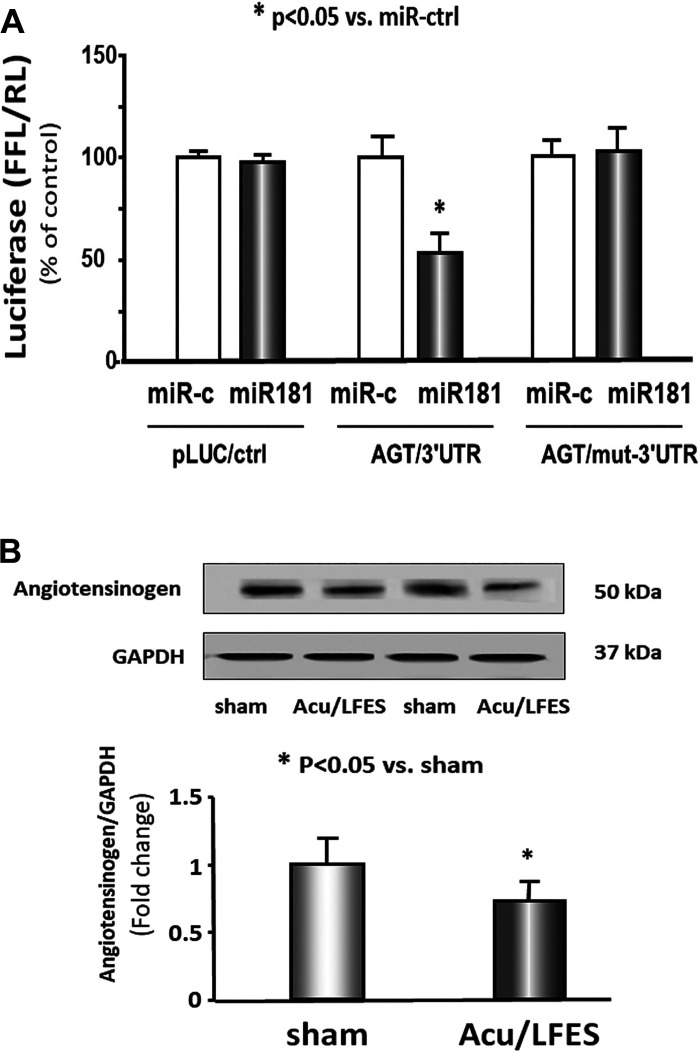
miR-181 directly targets 3′-untranslated region of angiotensinogen.
miR-181 has been implicated in modification of the renin-angiotensin system and therefore could impact RBF (23, 24). An in silico analysis of the 3′-untranslated region of angiotensinogen identified a conserved binding site for miR-181. To experimentally confirm that miR-181 interacts with the angiotensinogen mRNA, the angiotensinogen target site of miR-181 (433 to 440 nt on angiotensinogen 3′-untranslated region) was cloned into a luciferase reporter construct (pLUC-AGT). When cells were transfected with pLUC-AGT along with miR-181, luciferase activity was decreased. When cells were transduced with an angiotensinogen-luciferase construct in which the binding site had been mutated (pLUC-AGT-mut), there was no decrease (Fig. 5A). Using Western blot, we confirmed that AGT protein abundance was decreased in the kidney of Acu/LFES-treated mice (Fig. 5B). These results confirmed that miR-181 directly targets angiotensinogen and blocks its translation.
Fig. 5.
miR-181 directly targets 3′-untranlasted region (UTR) of angiotensinogen (AGT) and inhibits translation. A: 293HEK cells were transfected with luciferase pLucctrl vector (left) or the vector containing the 3′-UTR of AGT (pMIR-AGT/433-440) (middle) or a vector containing a mutated 3′-UTR of AGT (pMIR-mut-AGT) (right). Cells were cotransfected with control mimic (white bars) or miR-181 mimic (black bars). Luciferase activity in cells that received the pLucctrl with miR-ctrl was designated as the 100% (far left white bar). The other bars show the response to miR181 expressed as a percentage of this control. Triplicate determinations were made in each condition and each experiment was repeated a total of three times; the firefly luciferase (FFL) results were normalized by renilla luciferase (RL) activity (data are means ± SE; n = 9; *P < 0.05 vs. AGT/3′-UTR + miR-ctrl). B: protein was isolated from the kidney of sham and Acu/LFES-treated mice. Angiotensinogen in tissue lysates was measured by Western blots. The bar graph shows the fold change of the Angiotensinogen protein band compared with levels in sham-treated group represented by 1-fold. All protein band densities have been normalized to their corresponding GAPDH loading control. (bars: means ± SE; n = 9/group; *P < 0.05 vs. sham).
DISCUSSION
The treatment of muscle wasting is well known to be accompanied by benefits in other organs (12). However, how the muscle communicates with these organs in response to acupuncture is less understood. Studies have shown that prevention or treatment of muscle atrophy can improve renal function (12). In this study, we provide evidence that Acu/LFES administered to mice hindlimb muscles results in enhanced RBF. In addition, we show that this treatment raises miR-181d-5p that is transported by exosomes to distant organs where it inhibits angiotensinogen production. The consequence of this inhibition would be decreased angiotensin production and increased RBF.
We found that Acu/LFES increased RBF, which could potentially reduce renal injury. The other published accounts support our findings (10, 32, 33) that acupuncture decreases urinary albumin secretion and improves RBF and glomerular filtration rate in nephropathy patients and animals (4, 8, 10, 34). In addition, studies showed that acupuncture treatment can ameliorate symptoms, such as fatigue, stress, hypertension, and pruritus in patients with end-stage renal disease that are on dialysis (3, 16, 27). The Acu/LFES is a safe technique and routinely used in all over the world (22). The equipment needed to perform this technique is simple and inexpensive. In the case of manual acupuncture alone, simple acupuncture needles are inserted into key points in the body; in electrically stimulated acupuncture, an adjustable power source is connected to the needles to provide electrical stimulation at those key points (18). This minimally invasive treatment can potentially minimize some of the renal damage from kidney diseases.
The Acu/LFES-mediated increase in RBF correlated with increases in circulating EVs known as exosomes. Many investigations have demonstrated that EVs, including exosomes, can carry mediators of interorgan cross talk (20, 21). In this study, we found that the number of circulating exosomes was almost doubled by Acu/LFES treatment and 34 exosome-carried miRs were altered by this type of acupuncture. These circulating exosomes are the result of increased secretion and not leakage into the blood. Exosomes form in multivesicular bodies that fuse with muscle fiber membranes and extrude exosome out. The increase in exosomes in response to acupuncture is associated with an increase of muscle creatine kinase, which suggests that increased exosomes containing miR-181-5p could result from increased muscle activity. Although muscle creatine kinase was increased, serum creatine kinase was not changed. This suggests that the exosomes entered the circulation through secretion rather than some leakage from muscle. It seems clear that acupuncture induces exosome formation in muscle, but how it increases exosomes’ secretion will require further study.
miRs participate in the regulation of many physiologic functions and are altered in pathologic conditions, sometimes contributing to disease induction and sometimes limiting pathologic changes. Data from our serum-derived exosome miR deep sequencing analysis showed an increase in miR-181d-5p could play an important role in the regulation of RBF observed with Acu/LFES treatment of muscle. Using a luciferase reporter assay, we found miR-181d-5p directly inhibits angiotensinogen (Fig. 5). The resulting reduction in angiotensinogen translation could potentially improve renal function. Other investigators found that miR-181 inhibits renin (23, 24). Renin cleaves the angiotensin I decapeptide from angiotensinogen. Angiotensin I is then converted to the octapeptide angiotensin II by angiotensin-converting enzyme. With the use of a combination of these responses, increases in miR-181 could depress inhibit renin and angiotensinogen levels, resulting in a decreased renin-angiotensin system. It is well known that upregulation of the renin-angiotensin system causes renal injury (7). Angiotensin II decreases RBF which causes ischemia induced renal injury secondary to intrarenal vasoconstriction (21) and induces proteinuria (25). Angiotensin II also activates renal fibroblasts to become myofibroblasts and stimulates the production of the profibrotic cytokine transforming growth factor-β (5). Taken together, there are many reasons for wanting to block the upregulation of the renin-angiotensin system in subjects of renal disease. Our study provides evidence for Acu/LFES may be able to improve RBF through exosome-mediated miR transfer.
Thirty-four exosome-carried miRs were altered by Acu/LFES, which indicates the potential impact that Acu/LFES may have on regulating physiologic conditions. Our previous study found that plasma cytokines were altered by Acu/LFES, suggesting that Acu/LFES influence could extend to multiple organs (28). The focus of our current study was RBF. We recognize that the increase RBF by Acu/LFES may not be solely regulated by miR-181-5p. There were 34 miRs identified to have changed with Acu/LFES treatment, only one of which was miR-181-5p. Of the remaining identified miRs, there were no clearly identifiable targets that could regulate RBF. It is possible, however, that they have the potential to regulate RBF by indirect mechanisms. In addition, the elevation of circulating exosomes may be have been secreted from blood immune cells or sympathetic or parasympathetic nerves. Future studies would be needed to prove these additional exosome origins.
Conclusion.
Acu/LFES in the limb releases exosomes containing specific cargo (miR-181) into the circulation where it can move to and influence distance organs and increase RBF. The increase in RBF in response to Acu/LFES may be a consequence of the increase in exosome-carried miR-181.
GRANTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01-AR-060268 and American Heart Association Discovery Grant supported by Bayer Group (no. 17IBDG33780000 to X. H. Wang); National Natural Science Foundation of China Grant 81671403 and Zhejiang Provincial Natural Science Foundation Grant LY15H 270017 (to Z. Su); and National Natural Science Foundation of China Grant 81670628 (to Y. Yuan). This research project was also supported in part (microRNA deep sequencing) by the Genomics Core of Yerkes National Primate Research Center under NIH Grant ORIP/OD P51-OD-011132.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH, Department of Veterans Affairs, or US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.S., Y.Y., M.Y., and Y.L. performed experiments; Z.S., Y.Y., and X.H.W. analyzed data; X.H.W. interpreted results of experiments; X.H.W. prepared figures; Z.S., Y.Y., and X.H.W. drafted manuscript; J.D.K. and X.H.W. edited and revised manuscript; J.D.K. and X.H.W. approved final version of manuscript.
REFERENCES
- 1.Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 90: 53–66, 2016. doi: 10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Cervenka L, Mitchell KD, Navar LG. Renal function in mice: effects of volume expansion and angiotensin II. J Am Soc Nephrol 10: 2631–2636, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Che-Yi C, Wen CY, Min-Tsung K, Chiu-Ching H. Acupuncture in haemodialysis patients at the Quchi (LI11) acupoint for refractory uraemic pruritus. Nephrol Dial Transplant 20: 1912–1915, 2005. doi: 10.1093/ndt/gfh955. [DOI] [PubMed] [Google Scholar]
- 4.Chen HC, Brattberg G. Effects of moxibusting point Kuan-Yuan on cardiovascular and renal responses to histamine-induced shock. Am J Chin Med 15: 77–82, 1987. doi: 10.1142/S0192415X87000102. [DOI] [PubMed] [Google Scholar]
- 5.Chen LJ, Xu YL, Song B, Yu HM, Oudit GY, Xu R, Zhang ZZ, Jin HY, Chang Q, Zhu DL, Zhong JC. Angiotensin-converting enzyme 2 ameliorates renal fibrosis by blocking the activation of mTOR/ERK signaling in apolipoprotein E-deficient mice. Peptides 79: 49–57, 2016. doi: 10.1016/j.peptides.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Rudemiller NP. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol 28: 1350–1361, 2017. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darenkov AF, Balchiĭ-ool AA, Shemetov VD, Troitskiĭ OA, Kuznetsov VM. [Acupuncture in the combined treatment of pyelonephritis]. Urol Nefrol (Mosk) 2: 10–12, 1993. [PubMed] [Google Scholar]
- 8a.Desmeules F, Boudreault J, Roy JS, Dionne CE, Fremont P, MacDermid JC. Efficacy of transcutaneous electrical nerve stimulation for rotator cuff tendinopathy: a systematic review. Physiotherapy 102: 41–49, 2016. doi: 10.1016/j.physio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Klein JD, Hassounah F, Zhang J, Zhang C, Wang XH. Aging increases CCN1 expression leading to muscle senescence. Am J Physiol Cell Physiol 306: C28–C36, 2014. doi: 10.1152/ajpcell.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang JQ, Zhang LL, Shao XM. [Effects of the renal blood flow at different levels by transcutaneous electrical acupoint stimulation combined general anesthesia induced controlled hypotension]. Zhongguo Zhong Xi Yi Jie He Za Zhi 32: 1512–1515, 2012. [PubMed] [Google Scholar]
- 11.Franzen CA, Blackwell RH, Foreman KE, Kuo PC, Gupta GN. Urinary exosomes: the potential for biomarker utility, intercellular signaling, and therapeutics in urologic malignancy. J Urol 195: 1331–1339, 2016. doi: 10.1016/j.juro.2015.08.115. [DOI] [PubMed] [Google Scholar]
- 12.Hanatani S, Izumiya Y, Araki S, Rokutanda T, Kimura Y, Walsh K, Ogawa H. Akt1-mediated fast/glycolytic skeletal muscle growth attenuates renal damage in experimental kidney disease. J Am Soc Nephrol 25: 2800–2811, 2014. doi: 10.1681/ASN.2013091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L, Klein JD, Hassounah F, Cai H, Zhang C, Xu P, Wang XH. Low-frequency electrical stimulation attenuates muscle atrophy in CKD–a potential treatment strategy. J Am Soc Nephrol 26: 626–635, 2015. doi: 10.1681/ASN.2014020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, Go G, Nhung D, Hong K, Jang SC, Kim SH, Park KS, Kim OY, Park HT, Seo JH, Aikawa E, Baj-Krzyworzeka M, van Balkom BW, Belting M, Blanc L, Bond V, Bongiovanni A, Borràs FE, Buée L, Buzás EI, Cheng L, Clayton A, Cocucci E, Dela Cruz CS, Desiderio DM, Di Vizio D, Ekström K, Falcon-Perez JM, Gardiner C, Giebel B, Greening DW, Gross JC, Gupta D, Hendrix A, Hill AF, Hill MM, Nolte-’t Hoen E, Hwang DW, Inal J, Jagannadham MV, Jayachandran M, Jee YK, Jørgensen M, Kim KP, Kim YK, Kislinger T, Lässer C, Lee DS, Lee H, van Leeuwen J, Lener T, Liu ML, Lötvall J, Marcilla A, Mathivanan S, Möller A, Morhayim J, Mullier F, Nazarenko I, Nieuwland R, Nunes DN, Pang K, Park J, Patel T, Pocsfalvi G, Del Portillo H, Putz U, Ramirez MI, Rodrigues ML, Roh TY, Royo F, Sahoo S, Schiffelers R, Sharma S, Siljander P, Simpson RJ, Soekmadji C, Stahl P, Stensballe A, Stępień E, Tahara H, Trummer A, Valadi H, Vella LJ, Wai SN, Witwer K, Yáñez-Mó M, Youn H, Zeidler R, Gho YS. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 31: 933–939, 2015. doi: 10.1093/bioinformatics/btu741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DK, Lee J, Simpson RJ, Lötvall J, Gho YS. EVpedia: community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin Cell Dev Biol 40: 4–7, 2015. doi: 10.1016/j.semcdb.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Kim KH, Kim TH, Kang JW, Sul JU, Lee MS, Kim JI, Shin MS, Jung SY, Kim AR, Kang KW, Choi SM. Acupuncture for symptom management in hemodialysis patients: a prospective, observational pilot study. J Altern Complement Med 17: 741–748, 2011. doi: 10.1089/acm.2010.0206. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285: 17442–17452, 2010. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Shen Z, Yu XY. Transport of microRNAs via exosomes. Nat Rev Cardiol 12: 198, 2015. doi: 10.1038/nrcardio.2014.207-c1. [DOI] [PubMed] [Google Scholar]
- 20.Lim S. WHO standard acupuncture point locations. Evid Based Complement Alternat Med 7: 167–168, 2010. doi: 10.1093/ecam/nep006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long DA, Price KL, Herrera-Acosta J, Johnson RJ. How does angiotensin II cause renal injury? Hypertension 43: 722–723, 2004. doi: 10.1161/01.HYP.0000120964.22281.3e. [DOI] [PubMed] [Google Scholar]
- 22.MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34 000 treatments by traditional acupuncturists. BMJ 323: 486–487, 2001. doi: 10.1136/bmj.323.7311.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ. Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension 58: 1093–1098, 2011. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 24.Marques FZ, Romaine SP, Denniff M, Eales J, Dormer J, Garrelds IM, Wojnar L, Musialik K, Duda-Raszewska B, Kiszka B, Duda M, Morris BJ, Samani NJ, Danser AJ, Bogdanski P, Zukowska-Szczechowska E, Charchar FJ, Tomaszewski M. Signatures of miR-181a on renal transcriptome and blood pressure. Mol Med 21: 739–748, 2015. doi: 10.2119/molmed.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.National Institutes of Health NIH Consensus Conference. Acupuncture. JAMA 280: 1518–1524, 1998. doi: 10.1001/jama.280.17.1518. [DOI] [PubMed] [Google Scholar]
- 25.Patel SN, Ali Q, Hussain T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese zucker rats. Hypertension 67: 906–915, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14: 195–208, 2014. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su LH, Wu KD, Lee LS, Wang H, Liu CF. Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. Am J Chin Med 37: 215–226, 2009. doi: 10.1142/S0192415X09006783. [DOI] [PubMed] [Google Scholar]
- 28.Su Z, Hu L, Cheng J, Klein JD, Hassounah F, Cai H, Li M, Wang H, Wang XH. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates denervation-induced muscle atrophy. J Appl Physiol (1985) 120: 426–436, 2016. doi: 10.1152/japplphysiol.00175.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Z, Klein JD, Du J, Franch HA, Zhang L, Hassounah F, Hudson MB, Wang XH. Chronic kidney disease induces autophagy leading to dysfunction of mitochondria in skeletal muscle. Am J Physiol Renal Physiol 312: F1128–F1140, 2017. doi: 10.1152/ajprenal.00600.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659, 2007. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 31.Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10: 504–516, 2014. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu N. [Effect of electroacupuncture at “taixi” point on plasma thromboxane A2 and prostacyclin in the rabbit with renal ischemia]. Zhen Ci Yan Jiu 18: 240–242, 1993. [PubMed] [Google Scholar]
- 33.Xu N, Xu G, Zhu C. [Effect of electroacupuncture at “shenshu” point on renal blood flow in rabbits]. Zhen Ci Yan Jiu 20: 48–50, 1995. [PubMed] [Google Scholar]
- 34.Zhang ZL, Ji XQ, Zhang P, Zhang XH, Meng ZJ, Yang XJ. [Randomized and controlled study on needling method of harmonizing spleen-stomach for early intervention of diabetic nephropathies and the mechanism of protecting kidney]. Zhongguo Zhen Jiu 27: 875–880, 2007. [PubMed] [Google Scholar]