Abstract
Muscle dysfunction is an important cause of morbidity among patients with chronic kidney disease (CKD). Although muscle fibrosis is present in a CKD rodent model, its existence in humans and its impact on physical function are currently unknown. We examined isometric leg extension strength and measures of skeletal muscle fibrosis and inflammation in vastus lateralis muscle from CKD patients (n = 10) and healthy, sedentary controls (n = 10). Histochemistry and immunohistochemistry were used to assess muscle collagen and macrophage and fibro/adipogenic progenitor (FAP) cell populations, and RT-qPCR was used to assess muscle-specific inflammatory marker expression. Muscle collagen content was significantly greater in CKD compared with control (18.8 ± 2.1 vs. 11.7 ± 0.7% collagen area, P = 0.008), as was staining for collagen I, pro-collagen I, and a novel collagen-hybridizing peptide that binds remodeling collagen. Muscle collagen was inversely associated with leg extension strength in CKD (r = −0.74, P = 0.01). FAP abundance was increased in CKD, was highly correlated with muscle collagen (r = 0.84, P < 0.001), and was inversely associated with TNF-α expression (r = −0.65, P = 0.003). TNF-α, CD68, CCL2, and CCL5 mRNA were significantly lower in CKD than control, despite higher serum TNF-α and IL-6. Immunohistochemistry confirmed fewer CD68+ and CD11b+ macrophages in CKD muscle. In conclusion, skeletal muscle collagen content is increased in humans with CKD and is associated with functional parameters. Muscle fibrosis correlated with increased FAP abundance, which may be due to insufficient macrophage-mediated TNF-α secretion. These data provide a foundation for future research elucidating the mechanisms responsible for this newly identified human muscle pathology.
Keywords: chronic kidney disease, fibrosis, inflammation, skeletal muscle
INTRODUCTION
Impaired physical function is a major determinant of poor overall health and quality-of-life in patients with chronic kidney disease (CKD) (4, 52, 55, 65). A number of deficits in physical function have been described, including loss of muscle strength, reduced exercise capacity, and the development of mobility impairment and disability (3, 17, 28, 34, 40, 50, 53, 54, 60, 64). Altered muscle physiology contributes to these functional deficits (39, 63).
Prior research has focused on intrinsic muscle fiber deficits and has scarcely examined how kidney disease affects the interstitial muscle extracellular matrix (ECM). The paucity of such investigations is important because the ECM performs several important functions: It provides structural integrity; transfers force both longitudinally and transversely within muscle; protects muscle fibers from injury; and regulates the function of myogenic progenitor cells that reside within the ECM (2, 7, 22, 23, 41, 44). It has been reported that excess ECM accumulation and fibrosis negatively impact muscle force production (19), suggesting that alterations of the ECM can have significant functional implications.
Muscle fibrosis was recently identified in an animal model of advanced CKD, but this has not been investigated in humans; thus its relevance to clinically meaningful functional outcomes is unknown (14, 79). We sought to determine whether skeletal muscle fibrosis was present in humans with CKD, fibrotic burden was associated with a clinically relevant functional outcome, and muscle fibrosis was associated with alterations in cellular constituents that are crucial for appropriate muscle repair pathways.
MATERIALS AND METHODS
Study Population
We performed cross-sectional comparisons between ambulatory participants with non-dialysis-dependent stage 4 or 5 CKD and healthy sedentary volunteers. CKD patients were recruited for this study from a prospective cohort study of patients with CKD stages 4 and 5 [estimated glomerular filtration rate (eGFR) <30 ml·min−1·1.73 m−2] who were not receiving renal replacement therapy with dialysis. Patients were eligible for participation in the parent study if they were ≥21 yr of age, able to provide informed consent, and were ambulatory. Exclusion criteria included bilateral lower extremity amputations, use of immunosuppressive medications in the prior 3 mo, and an active cancer diagnosis or receiving treatment for cancer. Patients were ineligible for a muscle biopsy if they were taking anticoagulant medications. Controls were generally healthy with no physical limitations to activity. They were required to be sedentary, which was defined as not being engaged in strenuous work, regular brisk leisure physical activity, or a formal exercise session more than once per week for at least the previous 3 mo (30, 46). Medical history data were collected via standardized questionnaire and medical record review. The study protocol was approved by the Institutional Review Board of the Albert Einstein College of Medicine. Written informed consent was obtained from all participants before inclusion in the study.
Study Design
Following screening, an enrollment visit was conducted during which questionnaires and physical function testing were administered. Health-related quality-of-life was assessed with the 36-Item Short Form Health Survey (SF-36), and functional independence was assessed using the Katz Index of Activities of Daily Living (31).
Physical function.
Unilateral knee extensor strength was measured using isometric dynamometry with a handheld dynamometer (Manual Muscle Test System, Lafayette Instrument, Lafayette, IN). To ensure assessment of maximum strength, subjects were instructed to perform a maximal exertion contraction, and two trials were recorded. The highest result achieved in the biopsied leg was used for analysis. Results were normalized for body weight. To measure gait speed, participants walked a 4-m course at their usual pace, with the fastest time used for analysis. Endurance capacity was measured by the 2-min walk test (62): participants were asked to walk back and forth over a 50-foot course as far as possible over 2 min. The distance covered is highly correlated with 6-min walk distance (5).
Physical activity.
After the enrollment visit, all participants wore a triaxial accelerometer (Actigraph GT3X-BT, Actigraph, Pensacola, FL) around the waist for 7 consecutive days to measure physical activity level. Participants were instructed to wear the accelerometer at all times, except when showering, bathing, or swimming. Data processing was performed using 60-s epochs in ActiLife 6.13.3. Wear-time validation was performed as per Troiano et al. (70). Intensity levels of physical activity were defined as sedentary time [<100 counts/min (cpm)] and light (100–1,951 cpm), moderate (1,952–5,724 cpm), vigorous (5,725–9,498 cpm), and very vigorous (≥9,499 cpm) intensity (16, 47). Sedentary time was classified according to daily time spent in sedentary bouts of 10 or more consecutive minutes, excluding sleep time (70).
Laboratory testing.
Serum creatinine was measured by a modified kinetic Jaffé reaction in the clinical laboratory at Montefiore Medical Center. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (36). Timed 24-h urine collections were performed after the provision of detailed instructions. Urine urea nitrogen (UUN) was measured enzymatically.
Body composition and nutrition.
Body composition was assessed using whole-body dual-energy X-ray absorptiometry scans (Lunar Prodigy Advance DXA System, GE Medical Systems Lunar; Madison, WI; software v13.31). The appendicular skeletal muscle mass index (ASMI) was calculated as the total lean mass of the four extremities divided by the square of the height (10). Daily dietary protein intake was calculated as 6.25 × [UUN (g/day) + weight (kg) × 0.031] (45). Protein-energy wasting (PEW) was defined, with slight modification based on the data available, according to the International Society of Renal Nutrition and Metabolism, as satisfying at least one criterion in at least three of four categories (15): Serum chemistry: serum albumin <3.8 g/dl; body mass: BMI <23 kg/m2, ≥10 lb. unintentional weight loss over 12 mo, or total body fat percentage <10%; Muscle mass: low muscle mass as recommended by the European Working Group on Sarcopenia in Older People (ASMI <5.45 kg/m2 in women and <7.26 kg/m2 in men) (10); Dietary intake: dietary protein intake <0.6 g·kg−1·day−1.
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the Albert Einstein College of Medicine (25).
Muscle Biopsies
Patients were scheduled for muscle biopsies after completing the accelerometer wear period. On the morning of the study, participants were admitted to the Clinical Research Center study room at 8:00 AM after a 12-h overnight fast. Serum samples were collected and stored at −80°C. Tissue (100–150 mg) was obtained from the vastus lateralis via an incision site 15 cm proximal to the superior border of the patella using a 12-gauge biopsy needle (Bard Monopty, Bard Biopsy Systems, Tempe, AZ). Muscle tissue was blotted to remove extraneous blood and was immediately frozen in liquid nitrogen and then stored at −80°C. Serum TNF-α (ALPCO Diagnostics, Salem, NH) and IL-6 Chemiluminescence (R&D Systems, Minneapolis, MN) were measured in duplicate by ELISA.
Histochemistry and Immunohistochemistry
Tissue from one control participant was unavailable for histochemistry and immunohistochemistry. Frozen tissue was sectioned (7 µm) on a cryostat (HM525 NX, ThermoFisher), and slides were air-dried for 1 h. For collagen staining, slides were fixed for 1 h at 56°C in Bouin’s fixative then incubated in picro-sirius red, washed in 0.5% acetic acid, dehydrated, equilibrated with xylenes, and then mounted with cytoseal XYL (ThermoFisher, Waltham, MA).
For collagen 1 and pro-collagen 1 staining, slides were fixed in ice-cold acetone (−20°C) for 10 min, rinsed with PBS, and blocked in 1% BSA in PBS for 1 h at room temperature (RT). Slides were then incubated with primary antibody: collagen 1 [ab34710, 1:200, Abcam, Cambridge, MA) and pro-collagen (SP1.D8, mouse monoclonal, supernatant, Developmental Studies Hybridoma Bank (DSHB)] overnight at 4°C. The SP1.D8 pro-collagen 1 antibody was obtained from the DSHB and deposited by H. Furthmayr, created by the NICHD of the National Institutes of Health (NIH), and maintained at The University of Iowa, Department of Biology (Iowa City, IA). The next day, slides were incubated in goat anti-mouse IgG1 AF555 (ThermoFisher), goat anti-rabbit AF488 (ThermoFisher) for 1 h at RT. Slides were costained with 4′,6-diamidino-2-phenylindole (DAPI).
For collagen 4 and collagen-hybridizing peptide (CHP), immunohistochemical methods were modified according to the manufacturer’s instruction (3Helix, Salt Lake City, UT). In brief, sections were fixed in ice-cold acetone (−20°C) for 10 min and blocked in 2.5% normal horse serum for 1 h at RT. 3Helix-5-FAM conjugate (3Helix) was diluted to a working solution (20 μM) and placed on a heating block at 80°C for 5 min to denature 3Helix trimers, then quickly cooled on wet ice for 2 min. Immediately after cooling, anti-collagen 4 (ab6586, rabbit, 1:200, Abcam) was added to the 3Helix-PBS solution, and slides were incubated overnight at 4°C. The next day, slides were washed in PBS and incubated with goat anti-rabbit AF555 (ThermoFisher), and then costained with DAPI.
Platelet-derived growth factor receptor-α (PDGFRα) immunohistochemical methods have been published previously (18). Briefly, sections were fixed in 4% paraformaldehyde, followed by a blocking step in 2.5% normal horse serum (Vector Laboratories, Burlingame, CA), and then incubated overnight with goat anti-PDGFRα (AF-307-NA, 1:100, R&D Systems) and AF488-conjugated wheat germ agglutinin (WGA; W11261; ThermoFisher) at 4°C. The following day, slides were incubated in rabbit anti-goat AF555 (A-21431, ThermoFisher), costained with DAPI (D35471, ThermoFisher), and then mounted with fluorescent mounting media.
For CD68, CD11b, and CD206 macrophages, immunohistochemical protocols were adapted from Reidy et al. (61). Sections were fixed in −20°C acetone for 10 min followed by blocking for 1 h in 2.5% normal horse serum at RT and subsequently incubated overnight at 4°C with AF488-conjugated WGA and one of three primary antibodies against either CD68 (1:100, M0814, Dako, Santa Clara, CA), CD11b (1:100, MON1019-1, Cell Sciences, Newburyport, MA), or CD206 (1:100, AF2534, R&D Systems) diluted in 2.5% normal horse serum. On day 2, sections were incubated for 1 h with one of two secondary antibodies: rabbit anti-goat IgG AF555 (1:500, A-21431, ThermoFisher) or goat anti mouse IgG1 AF555 (1:500, A-21121, ThermoFisher). Sections were then costained with DAPI and then mounted with fluorescent mounting media.
Image Acquisition and Analysis
Images were captured at ×200 total magnification using a Zeiss AxioImager M1 microscope (Carl Zeiss, Thornwood, NY), and analysis was carried out using AxioVision Rel software (v4.9). Picro-sirius red staining was quantified to measure expansion of collagen between muscle fibers using the thresholding feature of FIJI software (https://fiji.sc/), and the area occupied by collagen was expressed relative to the total muscle area (mm2). Fibro/adipogenic progenitors (FAPs; PDGFRα+/DAPI+) were identified as previously described (18). Briefly, FAPs were identified with PDGFRα+ staining surrounding a DAPI+ nucleus, to denote the presence of PDGFRα+ cell surface expression that has been used by others to identify FAPs in human skeletal muscle (71). We only identified cells as FAPs when the PDGFRα+ staining pattern exhibited clear cell surface/membrane staining, i.e., no overlap with a DAPI+ nucleus. PDGFRα is a cell surface protein, not a transcription factor, and this distinction was our justification for the inclusion of cells that only display PDGFRα surrounding a DAPI+ nucleus. Our assessment is in line with others in the field who have quantified FAPs in human muscle biopsies (71). Cell counts were normalized to the total area of the muscle cross section (mm2). Collagen 1 and collagen 4 were quantified to measure expansion of key components of the ECM using the thresholding feature of Zeiss AxioVision software (v4.9), and the area occupied by collagen 1 and 4 were independently expressed relative to the total muscle area (mm2). CHP analysis was conducted in a similar manner to determine areas of active collagen remodeling. The binding area of the CHP was determined using the thresholding feature of AxioVision and expressed relative to the total muscle area (mm2). Muscle macrophage content was quantified as either CD68+/DAPI+, CD11b+/DAPI+, or CD206+/DAPI+, and cellular density was normalized per unit area (mm2). All immunohistochemical images were analyzed by a single assessor in a blinded manner to control or CKD subject status.
RT-qPCR
Muscle samples were homogenized with QIAzol Lysis Reagent (Qiagen), and then the aqueous phase was used for RNA isolation. Total RNA (1 mg) isolated with an RNeasy Mini Kit (Qiagen Sciences) was reverse transcribed to cDNA using the SuperScript VILO cDNA synthesis kit (Invitrogen). TaqMan (Applied Biosystems) RT-qPCR was performed for measurement of mRNA using standard curves. Gene expression was adjusted by comparison with human RPL7 expression. Primer-probe mixtures for human RPL7 were customized, and other primer-probe mixtures were from Applied Biosystems. Primers and probes used to detect human RPL7 mRNA were forward primer 5′-AAGAAGCGAATTGCTTTGACAGA-3′, reverse primer 5′-CAAATCCTCCATGCAGATGATG-3′, and probe 5′-[6-FAM] ACGCTTTGATTGCTCGATCTCTTGGTAAATACG[TAMRA-6-FAM]-3′. Tissue from one CKD patient was unavailable for RT-qPCR.
Statistics
Baseline characteristics, histochemistry, immunohistochemistry, and gene expression data were compared between CKD patients and controls using χ2 tests or Fisher’s exact test for categorical variables and two-tailed t-tests or Wilcoxon rank-sum tests for continuous variables. Correlations were tested by graphically assessing the existence of a linear fit between variables and calculating Pearson correlation coefficients. All analyses were performed with Stata 13.1 (StataCorp, College Station, TX). A P value <0.05 was considered statistically significant.
RESULTS
Participant Characteristics
Ten patients with CKD and 10 control individuals participated in this study (Table 1). Age did not differ between the groups (P = 0.89). Compared with the controls, participants in the CKD group were more likely to have hypertension. Control participants did not have other comorbidities. Among the CKD patients, 60% had diabetes, 20% had coronary artery disease, 20% had congestive heart failure, and none had a diagnosis of peripheral vascular disease. Their median eGFR was 12.9 ml·min−1·1.73 m−2 (interquartile range, 7.7–18.1), reflective of their advanced kidney disease. Based on the Physical Component Score from the SF-36, compared with controls, CKD patients had poorer self-reported physical functioning. Physical activity levels did not differ between the groups. All CKD patients and controls performed all activities of daily living independently. Lean body mass, ASMI, serum albumin, and dietary protein intake did not differ between CKD patients and controls. No participants met criteria for PEW.
Table 1.
Participant characteristics
| Control | Chronic Kidney Disease | P | |
|---|---|---|---|
| Age, yr | 61.4 ± 15.2 | 62.2 ± 7.8 | 0.89 |
| Women – n (%) | 4 (40) | 3 (30) | 1.0 |
| Race/ethnicity – n (%) | 0.09 | ||
| Non-Hispanic white | 4 (40) | 0 | |
| Black | 4 (40) | 8 (80) | |
| Hispanic/multiracial | 2 (20) | 2 (20) | |
| Hypertension – n (%) | 3 (30) | 9 (90) | 0.02 |
| Diabetes – n (%) | 0 | 6 (60) | 0.01 |
| Coronary artery disease – n (%) | 0 | 2 (20) | 0.47 |
| Congestive heart failure – n (%) | 0 | 2 (20) | 0.47 |
| Peripheral vascular disease – n (%) | 0 | 0 | .... |
| Medication – n (%) | |||
| ACE inhibitor or ARB | 1 (10) | 5 (50) | 0.14 |
| Statin | 1 (10) | 7 (70) | 0.02 |
| Vitamin D2 or D3 | 2 (20) | 6 (60) | 0.19 |
| Activated vitamin D analogs | 0 | 2 (20) | 0.47 |
| Body mass index, kg/m2 | 28.7 (27.2–30.0) | 33.4 (24.4–39.8) | 0.17 |
| eGFR, ml·min−1·1.73 m−2 | 80.0 (69.5–89.0) | 12.9 (7.7–18.1) | <0.001 |
| SF-36 Physical Component Score | 51 ± 3 | 36 ± 9 | <0.001 |
| SF-36 Mental Component Score | 49 ± 4 | 47 ± 7 | 0.50 |
| Daily physical activity, h/day* | |||
| Sedentary time† | 8.1 ± 1.7 | 8.5 ± 1.7 | 0.66 |
| Light intensity | 4.2 ± 1.8 | 4.7 ± 1.3 | 0.54 |
| Moderate intensity | 0.5 (0.3–0.6) | 0.1 (0.1–0.3) | 0.10 |
| Vigorous intensity | 0 (0–0.04) | 0 (0–0) | 0.40 |
| Quadriceps strength, kg/kg body wt | 0.21 ± 0.10 | 0.22 ± 0.04 | 0.72 |
| Gait speed, m/s | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.25 |
| 2-Min walk distance, ft | 546 ± 100 | 455 ± 96 | 0.05 |
| Lean body mass, kg | 49.6 ± 9.1 | 56.3 ± 14.6 | 0.24 |
| Appendicular skeletal muscle mass index, kg/m2 | 7.27 ± 1.18 | 8.16 ± 2.15 | 0.26 |
| Serum albumin, g/dl | 4.38 ± 0.24 | 4.32 ± 0.46 | 0.72 |
| Dietary protein intake, g/day§ | 60.7 ± 21.2 | 56.2 ± 20.2 | 0.87 |
Data are presented as means ± SD or median (interquartile range) for continuous variables.
Accelerometer data were unavailable for one control participant who did not wear the Actigraph device. No very-vigorous intensity activity was recorded for any of the participants.
Sedentary time was classified according to daily time spent in sedentary bouts of 10 or more consecutive min, excluding sleep time.
Calculated from 24-h urine urea nitrogen excretion. Urine collections were unavailable in 2 control participants.
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; SF-36, 36-Item Short Form Health Survey.
Skeletal Muscle Collagen and Muscle Strength
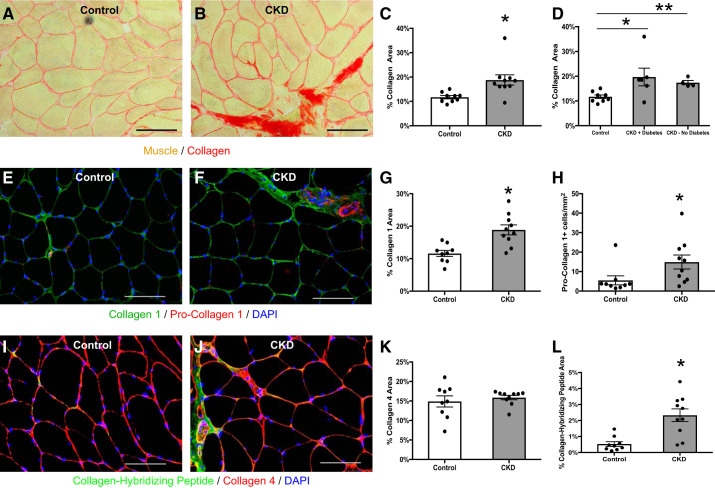
Muscle collagen content was quantified using histochemistry with picro-sirius red staining. Total muscle collagen was increased in patients with CKD compared with controls (Fig. 1, A–C, 18.8 ± 2.1 vs. 11.7 ± 0.7% collagen area, P = 0.008). Staining for type I collagen, one of the main fibrillar collagens in skeletal muscle and structural components of the ECM (57), was greater in the CKD patients (Fig. 1, E–G). The number of pro-collagen I-expressing cells was also higher in CKD (Fig. 1, E–H). There was no difference in staining for type IV collagen, which is the major basement membrane collagen (Fig. 1, I–K) (57).
Fig. 1.
Increased collagen content within the m. vastus lateralis muscle of chronic kidney disease (CKD) patients. A and B: representative histochemical image of picro-sirius red collagen stain in control (A; n = 9) and CKD (B; n = 10) muscle biopsies. Scale bar = 100 μm. C: quantification of collagen content within the muscle represented as mean percentage of total muscle area ± SE. D: quantification of collagen content stratified by diabetes status (n = 6, CKD with diabetes; n = 4, CKD without diabetes). P values were calculated for comparisons between each CKD subgroup and control. E and F: representative immunohistochemical images demonstrating staining for collagen 1 (green), pro-collagen 1 (red), and DAPI (blue) in control (E) and CKD (F) muscle biopsies. Scale bar = 100 μm. G and H: quantification of collagen 1 content (G) and pro-collagen 1+ cells (H) within the muscle represented as mean percentage of total muscle area ± SE. I and J: representative immunohistochemical images demonstrating staining for collagen hybridizing peptide (CHP; green), collagen 4 (red), and DAPI (blue) in control (I) and CKD (J) muscle biopsies. Scale bar = 100 μm. K and L: quantification of collagen 4 content (K), and CHP binding (L) represented as mean percentage of total muscle area ± SE. *P < 0.05. **P < 0.001.
We performed several analyses to address possible confounding. Adjustment for race in a linear regression model did not affect the difference in muscle collagen between CKD and controls: CKD patients had 7.2% [95% confidence interval (CI) 1.4–13.0; P = 0.02] greater muscle collagen content after adjustment, compared with a 7.1% (95% CI 2.1–12.0; P = 0.008) difference in an unadjusted model. Muscle collagen content did not differ between diabetic and nondiabetic CKD patients (19.7 ± 3.6 vs. 17.4 ± 0.9%, respectively; P = 0.63), and was significantly greater than controls irrespective of diabetes status (Fig. 1D). Greater muscle collagen was also present in CKD after excluding patients with either coronary artery disease or congestive heart failure (20.2 ± 2.7% (n = 7), P = 0.004 vs. control). To address the possibility that hypertension could explain the increase in muscle collagen, we compared muscle collagen content in controls with and without hypertension and found no difference (12.7 ± 1.3 vs. 11.2 ± 0.8, respectively; P = 0.33). In addition, muscle collagen in patients with CKD was not associated with the number of antihypertensive medications used (r = −0.11, P = 0.77). To address possible confounding by medication use, we also compared muscle collagen between users and non-users of medications that could impact skeletal muscle and found similar results regardless of medication use (Table 2). Furthermore, muscle collagen was not associated with the total number of medications used by CKD patients (r = −0.11, P = 0.76). Muscle collagen content was not associated with muscle mass (r = 0.39, P = 0.10 for LBM; r = 0.43, P = 0.07 for ASMI) and remained significantly greater in CKD after adjustment for age, sex, and either lean body mass (5.9%, 95% CI 0.6–11.3; P = 0.03) or ASMI (6.1%, 95% CI 1.0–11.1; P = 0.02).
Table 2.
Muscle collagen content based on medication use*
| Control Non-user | CKD |
P Value |
|||
|---|---|---|---|---|---|
| Non-user | User | CKD Non-user vs. Control | CKD Non-user vs. CKD User | ||
| ACE inhibitor or ARB | 11.9 ± 0.8 | 19.9 ± 4.4 | 17.6 ± 0.7 | 0.04 | 0.62 |
| Statin | 11.7 ± 0.8 | 17.7 ± 1.0 | 19.2 ± 3.1 | 0.002 | 0.75 |
| Vitamin D† | 11.8 ± 0.9 | 16.0 ± 2.3 | 20.7 ± 3.1 | 0.07 | 0.31 |
Quantification of collagen content within the muscle is presented as mean percentage of total muscle area ± SE.
User and non-user refer to use of each medication class separately.
Vitamin D2 or D3 or activated vitamin D analogs.
CKD, chronic kidney disease; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
We next examined dynamic collagen remodeling using a novel collagen hybridizing peptide (CHP). CHP binds only remodeling, unfolded collagen chains as the static collagen triple helix is inaccessible to peptide binding (26). CHP staining is also indicative of microstructural damage to collagen fibrils (80). CHP staining was significantly greater in CKD patients compared with controls (Fig. 1, I, J, and L).
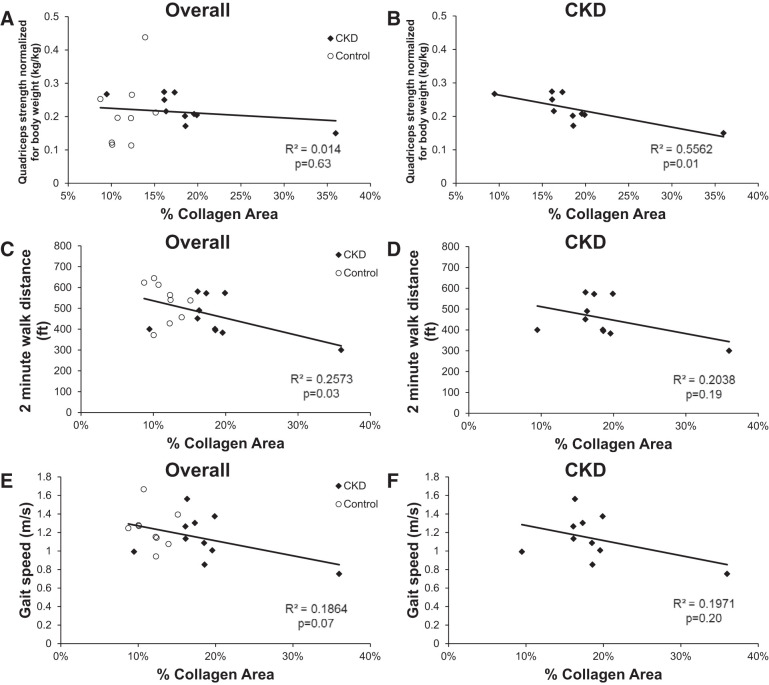
To examine whether muscle fibrosis might impact physical function, we next examined isometric knee extensor strength from the biopsied leg. Higher collagen content was associated with lower knee extensor strength in CKD patients (r = −0.74, P = 0.01; Fig. 2A). In the overall cohort including controls, muscle collagen content was not associated with knee extensor strength (r = −0.12, P = 0.63; Fig. 2B), but was inversely associated with 2-min walk distance (r = −0.51, P = 0.03; Fig. 2C), with a similar trend for gait speed (r = −0.43, P = 0.07; Fig. 2E). In the CKD group alone, although not reaching statistical significance, these associations were of similar magnitude (2-min walk distance: r = −0.45, P = 0.19; Fig. 2D; gait speed: r = −0.44, P = 0.20; Fig. 2F).
Fig. 2.
Correlation of collagen content within the m. vastus lateralis muscle with physical function in all participants (A, C, and E) and CKD patients (B, D, and F). A and B: unilateral knee extensor strength was measured in the leg to be biopsied using isometric dynamometry with a handheld dynamometer. C and D: gait speed was measured by having participants walk a 4-m course at their usual pace. E and F: endurance capacity was measured by asking participants to walk back and forth over a 50-ft course as far as possible over 2 min.
FAP Cells and Skeletal Muscle Inflammation
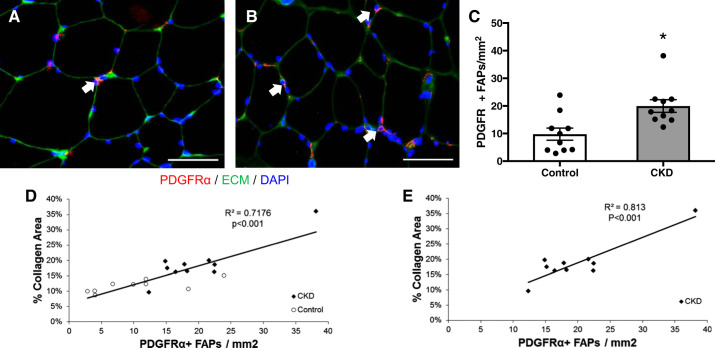
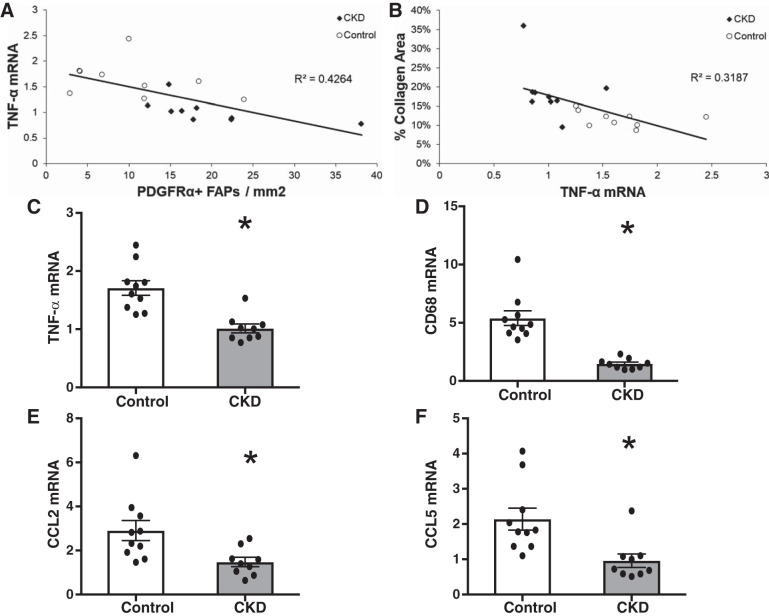
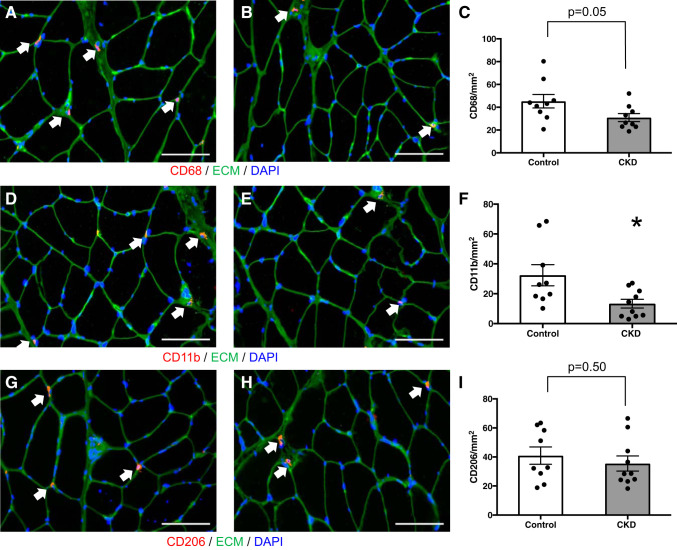
FAP cells expressing the PDGFRα surface marker have recently been implicated in the development of muscle fibrosis, including in an animal model of CKD (14, 72). Using immunohistochemical quantification, PDGFRα+ cells, located in the interstitial space between muscle fibers, were more abundant in the muscle of CKD patients (Fig. 3, A–C), and their abundance strongly correlated with muscle collagen content (r = 0.84, P < 0.001 overall; r = 0.90, P < 0.001 in CKD) (Fig. 3, D and E). FAP pools expand in response to muscle damage; subsequent reduction in FAP numbers is dependent upon signaling by proinflammatory macrophages (35). Specifically, in the absence of TNF-α secreted by proinflammatory macrophages, the expansion of FAP pools persists and causes fibrosis (35). To determine whether differences in inflammatory gene expression could explain our findings, mRNA was quantified using qRT-PCR in whole muscle lysates. TNF-α expression was inversely correlated with both FAP abundance (r = −0.65, P = 0.003) (Fig. 4A) and with muscle collagen content (r = −0.56, P = 0.01) (Fig. 4B), and TNF-α expression was significantly reduced in the muscle of CKD patients compared with controls (Fig. 4C), despite higher systemic inflammatory markers (CKD vs. control: IL-6, 3.4 (2.6–5) vs. 2.4 (1.6–3.4) pg/ml, P = 0.04; TNF-α, 10.2 (8.1–16.4) vs. 7.0 (6.2–9.6) pg/ml, P = 0.09). We hypothesized this could be due to differences in muscle macrophage content or phenotype. Muscle expression of the macrophage marker CD68 and chemokines CCL2 and CCL5 was also significantly reduced in CKD patients (Fig. 4, D–F). Immunohistochemical quantification demonstrated fewer CD68+ cells (Fig. 5, A–C; monocyte and panmacrophage marker) and fewer CD11b+ cells (Fig. 5, D–F; proinflammatory macrophage marker) in CKD patients compared with controls but no difference in CD206+ cells (Fig. 5, G–I; anti-inflammatory macrophage marker) between the two groups.
Fig. 3.
Increased fibrogenic/adipogenic progenitor (FAP) cell abundance within the m. vastus lateralis muscle of CKD patients. A and B: representative immunohistochemical image demonstrating PDGFRα+ muscle FAPs (PDGFRα+ cell surface expression surrounding DAPI+ nucleus; white arrows, red), extracellular matrix (ECM; green) and DAPI (blue) in control (A) and CKD (B) muscle biopsies. Scale bar = 50 μm. C: quantification of FAP content within the muscle represented as mean number of PDGFRα+ FAPs per total muscle area ± SE. D and E: correlation of collagen content within the m. vastus lateralis muscle with FAP content in all participants (D) and CKD patients (E). *P < 0.05.
Fig. 4.
Reduced mRNA expression of inflammatory and macrophage genes within the m. vastus lateralis muscle of CKD patients. A and B: correlation of TNF-α expression with PDGFRα+ muscle fibrogenic/adipogenic progenitor (FAP) cell abundance (A) and muscle collagen content (B). C–F: mRNA expression of TNF-α, CD68, CCL2, and CCL5 in control (n = 10) and CKD (n = 9) muscle biopsies. Gene expression was normalized to RPL7 expression. Data are means ± SE. *P < 0.05.
Fig. 5.
Macrophage marker immunofluorescence within the m. vastus lateralis muscle of CKD patients and controls. A and B: representative immunohistochemical image demonstrating CD68+ muscle macrophages (white arrows, red), extracellular matrix (ECM; green), and DAPI (blue) in control (A) and CKD (B) muscle biopsies. Scale bar = 100 μm. C: quantification of CD68+ macrophage content within the muscle represented as mean number of CD68+ macrophages per total muscle area ± SE. D and E: representative immunohistochemical image demonstrating CD11b+ muscle macrophages (white arrows, red), ECM (green), and DAPI (blue) in control (D) and CKD (E) muscle biopsies. Scale bar = 100 μm. F: quantification of CD11b+ macrophage content within the muscle represented as mean number of CD11b+ macrophages per total muscle area ± SE. G and H: representative immunohistochemical image demonstrating CD206+ muscle macrophages (white arrows, red), ECM (green), and DAPI (blue) in control (G) and CKD (H) muscle biopsies. Scale bar = 100 μm. I: quantification of CD206+ macrophage content within the muscle represented as mean number of CD206+ macrophages per total muscle area ± SE. *P < 0.05.
DISCUSSION
Preventing functional decline and loss of mobility among patients with CKD is a major unmet need: mobility impairment leads to falls, hospitalizations, and significant morbidity (6, 51, 56). Based on our results, this functional impairment may partly be due to skeletal muscle fibrosis. Muscle collagen content was increased in CKD patients; based on collagen I staining, this was explained by increased fibrillar collagen deposition. As pro-collagen I staining was also greater in CKD, the increased muscle collagen is likely, at least in part, due to increased collagen synthesis; increased CHP staining suggests there is also increased collagen proteolytic remodeling in CKD. As muscle collagen content was inversely associated with measures of physical function, it is possible that collagen deposition in CKD could be pathological and have functional implications.
An effect of excess muscle collagen on physical function could be due to impaired transfer of force generated by myofibril contractile units. Maximal transfer to tendons of the force generated by myofibril contraction requires intact ECM (20, 33, 42, 49, 57, 68, 69). Whereas single-fiber force was preserved in an animal model of muscle fibrosis, whole-muscle force per cross-sectional area was reduced (19). Compared with isolated muscle fiber bundles without ECM, the load-bearing potential of fiber bundles with intact ECM is about five times greater (38, 48, 58). Therefore, while producing minimal changes in muscle mass, pathological alteration of the ECM can cause meaningful changes in muscle function.
Impaired force generation could also explain the association of muscle collagen with endurance capacity; alternatively, since fibrosis may be accompanied by capillary rarefaction (12), muscle collagen could be a marker for impaired muscle perfusion, which would promote fatigability and limit endurance. Another possibility is that fibrosis accompanies muscle atrophy, and that loss of muscle mass explains the associations of muscle collagen with physical function. Our data, however, did not support this hypothesis: muscle mass was not lower in the CKD patients than controls; greater muscle collagen content was not associated with lower muscle mass; and after adjusting for muscle mass, muscle collagen remained higher in CKD.
No prior studies have examined muscle ECM homeostasis in humans with CKD. However, muscle fibrosis has been observed in the partial nephrectomy model of uremia (14, 79), and our results confirm the relevance of this finding for human pathology. As in that animal model, FAP content was increased in human CKD muscle, and here FAP abundance was highly correlated with muscle collagen content. Prior work has shown that FAPs are elevated in severe fibrotic muscle diseases such as Duchenne muscular dystrophy (72). More recently, elevated FAPs were seen in association with muscle fibrosis following anterior cruciate ligament injury (18). Here, we report increased FAPs in association with lesser expansion of muscle collagen than is seen in classic fibrotic muscle diseases, and not following known injury. To the best of our knowledge, this is the first report of such an occurrence.
Although the CKD patients had not experienced clinically apparent muscle injury, increased CHP staining, compared with controls, may indicate subclinical muscle damage. Subfailure damage, in which macroscale damage is not detected, causes unfolding of collagen fibrils (80), which could provide a signal for the initiation of repair mechanisms. While such damage has not been described previously in patients with advanced CKD, studies have documented disrupted sarcomeres and altered muscle architecture (1, 13, 37, 67). Previously, there were no tools to detect molecular level damage to collagen in the absence of macroscale damage (80); therefore, such damage may have been undetected. If subfailure damage to collagen is present, its etiology merits further study: one possibility is increased susceptibility to muscle contraction-induced injury in CKD patients. This could be an initiating factor of the fibrotic process; conversely, because the ECM provides structural support to muscle fibers, fibrosis itself could increase susceptibility to damage (43). Such hypotheses require additional study; regardless, the CHP results, in conjunction with pro-collagen I staining, support increased collagen remodeling in patients with CKD.
An underlying cause of muscle fibrosis in CKD may be ineffective macrophage-mediated muscle repair. The initial response to injury is characterized by influx of pro-inflammatory macrophages and concomitant expansion of the FAP pool, which is needed for repair of the ECM (29). Appropriate repair, and prevention of fibrosis, is dependent upon the macrophage-FAP interaction. TNF-α, secreted by proinflammatory macrophages, causes contraction of the FAP pool via apoptosis; if TNF-α is absent, persistently expanded FAP pools result, causing fibrosis (9, 14, 35, 72). Our findings link muscle TNF-α with muscle fibrosis in CKD. Lower TNF-α expression correlated with both greater FAP abundance and greater collagen content. Furthermore, we found lower muscle expression of TNF-α and other inflammatory and macrophage genes in CKD compared with control; this was remarkable given the greater systemic inflammation in the CKD patients. CKD is recognized as a state of increased inflammation (24); thus the discrepancy between muscle inflammation and systemic inflammation is rather striking. As proinflammatory macrophages are a likely source of TNF-α, the reduction of TNF-α in CKD muscle is consistent with the reduced macrophage content detected. It is noteworthy that the associations between muscle collagen, FAPs, and TNF-α appeared similar in CKD patients and controls. This suggests both an important role for macrophage-mediated regulation of FAPs in human skeletal muscle homeostasis and that loss of this regulation contributes to muscle fibrosis.
Given the importance of inflammation in CKD-associated morbidity, several factors may explain our finding of lower skeletal muscle inflammation in CKD patients. First, the possibility of a divergence between systemic inflammation and that within muscle has been hinted at by prior literature. Both aerobic and resistance exercise in non-dialysis-dependent CKD patients reduce systemic inflammation (8, 27, 75), yet in skeletal muscle inflammatory gene expression did not decline (76). Second, prior studies have either examined end-stage renal disease patients receiving dialysis (as opposed to non-dialysis-dependent CKD, as in our study); studied patients during the hemodialysis treatment itself, which may be proinflammatory (59); sampled non-locomotor muscles, e.g., rectus abdominus, as opposed to vastus lateralis in our study (21, 73, 74, 78); or relied on systemic markers of inflammation only (11). Each of these differences may explain the divergence with our findings. Hemodialysis is thought to be a proinflammatory stimulus; for this and other reasons, end-stage renal disease patients appear to have a greater inflammatory burden than non-dialysis-dependent CKD patients, which might translate to differences within the muscle. Our data set does not enable us to explore whether such differences exist. Inflammatory cytokine expression might differ between locomotor and non-locomotor muscles; this could be especially important for a muscle such as the rectus abdominus that lies in close proximity to abdominal fat depots, which are recognized sources of inflammation (32). Furthermore, few studies in pre-dialysis CKD patients have included comparison with a non-CKD control group, and all sampled rectus abdominus during placement of a peritoneal dialysis catheter (21, 73, 74, 78). Two other differences between those four studies and ours deserve mention: the mean eGFR of those patients was below 10 ml·min−1·1.73 m−2, and many experienced wasting and malnutrition. Although it is possible that our findings would have differed had we studied patients with lower eGFR who were initiating dialysis, given the relatively advanced CKD in our cohort, this seems a less likely explanation for the lower muscle inflammation that we observed. However, as PEW is strongly associated with inflammation, we might have observed increased inflammation in skeletal muscle had we studied patients with symptomatic uremia and severe muscle wasting. If true, this would imply that the well-described manifestations of inflammation and muscle wasting are preceded during earlier stages of CKD by a relative paucity of inflammation in skeletal muscle and by the development of muscle fibrosis.
Several limitations of our study should also be considered. There were important differences between the CKD and control groups in terms of comorbidities and medication use. We accounted for these differences using stratified analyses and adjusted regression models, which indicated that our findings were not explained by these factors. The modest sample size of our cohort precluded more detailed statistical analyses. As this was a cross-sectional study, our findings do not prove a cause-effect relationship, and we lack temporal data on the development of fibrosis and the cellular abnormalities we have characterized. We cannot rule out reverse causality: for example, the possibility that the differences in FAPs and macrophages in CKD muscle were a response to fibrosis. Nevertheless, our findings agree with animal model data demonstrating the central role of macrophages and FAPs in the repair of muscle ECM and prevention of fibrosis (35). We used validated markers of pro- and anti-inflammatory macrophages to define macrophage subsets (66). However, in vivo there is a continuum of macrophage subsets with overlapping but relatively polarized gene expression profiles (77). Future studies are needed to characterize the effects of CKD on muscle macrophages in more detail.
In summary, skeletal muscle fibrosis is a previously unrecognized muscle pathology in patients with CKD and a potentially important contributor to physical function impairment. Increased FAP abundance, possibly caused by insufficient macrophage-mediated TNF-α secretion, may contribute to this pathology. The disparity between systemic and local inflammation highlights the need to study human muscle tissue directly rather than extrapolating from systemic measurements. These data provide a framework for further investigations into the mechanisms causing this pathology in CKD.
GRANTS
This research was supported by NIH Grants DK099438, DK116023, DK069861, DK079974, and AR072061; by Einstein-Montefiore NIH CTSA Grants UL1TR001073 and UL1TR002556 from the National Center for Research Resources (NCRR); and by the Einstein-Mount Sinai Diabetes Research Center (5P30DK020541-41).
DISCLAIMERS
Its contents are solely the responsibility of the authors and do not necessarily represent the official views or policies of the NCRR or NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K.A., H.K., J.E.P., M.H., and C.S.F. conceived and designed study; M.K.A., W.P., K.Z., C.R.B., J.N.N., H.K., M.C., R.S.B., H.F., B.Z., R.P., and C.S.F. performed experiments; M.K.A. and C.S.F. analyzed data; M.K.A., J.E.P., M.H., and C.S.F. interpreted results of experiments; M.K.A. and C.S.F. prepared figures; M.K.A. drafted manuscript; M.K.A., W.P., K.Z., C.R.B., J.N.N., H.K., M.C., R.S.B., H.F., B.Z., R.P., J.E.P., M.H., and C.S.F. edited and revised manuscript; M.K.A., W.P., K.Z., C.R.B., J.N.N., H.K., M.C., R.S.B., H.F., B.Z., R.P., J.E.P., M.H., and C.S.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of M. Custodio: SUNY Downstate College of Medicine, Brooklyn, NY; H. Farooq: Louis A. Weiss Memorial Hospital, Chicago, IL; B. Zaidi: BronxCare Health System, Bronx, NY; and R. Pai: Beth Israel Deaconess Medical Center, Boston, MA.
REFERENCES
- 1.Ahonen RE. Striated muscle ultrastructure in uremic patients and in renal transplant recipients. Acta Neuropathol 50: 163–166, 1980. doi: 10.1007/BF00692869. [DOI] [PubMed] [Google Scholar]
- 2.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol 293: C661–C669, 2007. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 3.Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci 69: 315–322, 2014. doi: 10.1093/gerona/glt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med 172: 1071–1077, 2012. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohannon RW, Bubela D, Magasi S, McCreath H, Wang YC, Reuben D, Rymer WZ, Gershon R. Comparison of walking performance over the first 2 minutes and the full 6 minutes of the Six-Minute Walk Test. BMC Res Notes 7: 269, 2014. doi: 10.1186/1756-0500-7-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowling CB, Hall RK, Khakharia A, Franch HA, Plantinga LC. Serious fall injury history and adverse health outcomes after initiating hemodialysis among older U.S. adults. J Gerontol A Biol Sci Med Sci 73: 1216–1221, 2018. doi: 10.1093/gerona/glx260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3: 226–237, 2007. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 8.Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 43: 607–616, 2004. doi: 10.1053/j.ajkd.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Contreras O, Rebolledo DL, Oyarzún JE, Olguín HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res 364: 647–660, 2016. doi: 10.1007/s00441-015-2343-0. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412–423, 2010. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deger SM, Hung AM, Gamboa JL, Siew ED, Ellis CD, Booker C, Sha F, Li H, Bian A, Stewart TG, Zent R, Mitch WE, Abumrad NN, Ikizler TA. Systemic inflammation is associated with exaggerated skeletal muscle protein catabolism in maintenance hemodialysis patients. JCI Insight 2: 95185, 2017. doi: 10.1172/jci.insight.95185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68: 762–773, 2009. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 13.Diesel W, Emms M, Knight BK, Noakes TD, Swanepoel CR, van Zyl Smit R, Kaschula RO, Sinclair-Smith CC. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis 22: 677–684, 1993. doi: 10.1016/S0272-6386(12)80430-6. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int 91: 119–128, 2017. doi: 10.1016/j.kint.2016.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Treviño-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 16.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Fried LF, Lee JS, Shlipak M, Chertow GM, Green C, Ding J, Harris T, Newman AB. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc 54: 750–756, 2006. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res 35: 1876–1885, 2017. doi: 10.1002/jor.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garfin SR, Tipton CM, Mubarak SJ, Woo SL, Hargens AR, Akeson WH. Role of fascia in maintenance of muscle tension and pressure. J Appl Physiol Respir Environ Exerc Physiol 51: 317–320, 1981. doi: 10.1152/jappl.1981.51.2.317. [DOI] [PubMed] [Google Scholar]
- 21.Garibotto G, Sofia A, Procopio V, Villaggio B, Tarroni A, Di Martino M, Cappelli V, Gandolfo MT, Aloisi F, De Cian F, Sala MR, Verzola D. Peripheral tissue release of interleukin-6 in patients with chronic kidney diseases: effects of end-stage renal disease and microinflammatory state. Kidney Int 70: 384–390, 2006. doi: 10.1038/sj.ki.5001570. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, Shah VO, Balakrishnan VS, Guzman NJ, Girndt M, Periera BG, Feldman HI, Kusek JW, Joffe MM, Raj DS; CRIC Study Investigators . Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol 7: 1938–1946, 2012. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J, Huang Y, Burwell TJ, Peterson NC, Connor J, Weiss SJ, Yu SM, Li Y. In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano 11: 9825–9835, 2017. doi: 10.1021/acsnano.7b03150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikizler TA, Robinson-Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, Evans EE, Milch CM, Moody KA, Germain M, Limkunakul C, Bian A, Stewart TG, Himmelfarb J. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol 29: 250–259, 2018. doi: 10.1681/ASN.2017010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 29.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansen KL, Chertow GM, Ng AV, Mulligan K, Carey S, Schoenfeld PY, Kent-Braun JA. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 57: 2564–2570, 2000. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 31.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31: 721–727, 1983. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 32.Kishore P, Li W, Tonelli J, Lee DE, Koppaka S, Zhang K, Lin Y, Kehlenbrink S, Scherer PE, Hawkins M. Adipocyte-derived factors potentiate nutrient-induced production of plasminogen activator inhibitor-1 by macrophages. Sci Transl Med 2: 20ra15, 2010. doi: 10.1126/scitranslmed.3000292. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 34.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, Kopple JD. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol (1985) 112: 72–78, 2012. doi: 10.1152/japplphysiol.00556.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28: 464–471, 2003. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 39.Lim VS, Yarasheski KE, Flanigan MJ. The effect of uraemia, acidosis, and dialysis treatment on protein metabolism: a longitudinal leucine kinetic study. Nephrol Dial Transplant 13: 1723–1730, 1998. doi: 10.1093/ndt/13.7.1723. [DOI] [PubMed] [Google Scholar]
- 40.Liu CK, Lyass A, Massaro JM, D’Agostino RB Sr, Fox CS, Murabito JM. Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: the Framingham Offspring Study. J Gerontol A Biol Sci Med Sci 69: 301–307, 2014. doi: 10.1093/gerona/glt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lund DK, Cornelison DD. Enter the matrix: shape, signal and superhighway. FEBS J 280: 4089–4099, 2013. doi: 10.1111/febs.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas H, Baan GC, Huijing PA. Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech 34: 927–940, 2001. doi: 10.1016/S0021-9290(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 43.Mackey AL, Brandstetter S, Schjerling P, Bojsen-Moller J, Qvortrup K, Pedersen MM, Doessing S, Kjaer M, Magnusson SP, Langberg H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. FASEB J 25: 1943–1959, 2011. doi: 10.1096/fj.10-176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1: 21, 2011. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 46.Martins C, Truby H, Morgan LM. Short-term appetite control in response to a 6-week exercise programme in sedentary volunteers. Br J Nutr 98: 834–842, 2007. doi: 10.1017/S000711450774922X. [DOI] [PubMed] [Google Scholar]
- 47.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 167: 875–881, 2008. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monti RJ, Roy RR, Hodgson JA, Edgerton VR. Transmission of forces within mammalian skeletal muscles. J Biomech 32: 371–380, 1999. doi: 10.1016/S0021-9290(98)00189-4. [DOI] [PubMed] [Google Scholar]
- 50.Moore GE, Brinker KR, Stray-Gundersen J, Mitchell JH. Determinants of VO2peak in patients with end-stage renal disease: on and off dialysis. Med Sci Sports Exerc 25: 18–23, 1993. doi: 10.1249/00005768-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Nastasi AJ, McAdams-DeMarco MA, Schrack J, Ying H, Olorundare I, Warsame F, Mountford A, Haugen CE, González Fernández M, Norman SP, Segev DL. Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant 18: 189–196, 2018. doi: 10.1111/ajt.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Hare AM, Tawney K, Bacchetti P, Johansen KL. Decreased survival among sedentary patients undergoing dialysis: results from the dialysis morbidity and mortality study wave 2. Am J Kidney Dis 41: 447–454, 2003. doi: 10.1053/ajkd.2003.50055. [DOI] [PubMed] [Google Scholar]
- 53.Padilla J, Krasnoff J, Da Silva M, Hsu CY, Frassetto L, Johansen KL, Painter P. Physical functioning in patients with chronic kidney disease. J Nephrol 21: 550–559, 2008. [PubMed] [Google Scholar]
- 54.Painter P, Messer-Rehak D, Hanson P, Zimmerman SW, Glass NR. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron 42: 47–51, 1986. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- 55.Painter P, Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens 22: 615–623, 2013. doi: 10.1097/MNH.0b013e328365b43a. [DOI] [PubMed] [Google Scholar]
- 56.Plantinga LC, Lynch RJ, Patzer RE, Pastan SO, Bowling CB. Association of serious fall injuries among United States end stage kidney disease patients with access to kidney transplantation. Clin J Am Soc Nephrol 13: 628–637, 2018. doi: 10.2215/CJN.10330917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol 133: 947–966, 2002. doi: 10.1016/S1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 58.Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil 15: 299–308, 1994. doi: 10.1007/BF00123482. [DOI] [PubMed] [Google Scholar]
- 59.Raj DS, Dominic EA, Pai A, Osman F, Morgan M, Pickett G, Shah VO, Ferrando A, Moseley P. Skeletal muscle, cytokines, and oxidative stress in end-stage renal disease. Kidney Int 68: 2338–2344, 2005. doi: 10.1111/j.1523-1755.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 60.Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, Baker JF, Carlow D, Sulik MJ, Lo JC, Go AS, Ky B, Mariani L, Feldman HI, Leonard MB; CRIC Study Investigators . Physical performance and frailty in chronic kidney disease. Am J Nephrol 38: 307–315, 2013. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reidy PT, Lindsay CC, McKenzie AI, Fry CS, Supiano MA, Marcus RL, LaStayo PC, Drummond MJ. Aging-related effects of bed rest followed by eccentric exercise rehabilitation on skeletal muscle macrophages and insulin sensitivity. Exp Gerontol 107: 37–49, 2018. doi: 10.1016/j.exger.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reuben DB, Magasi S, McCreath HE, Bohannon RW, Wang YC, Bubela DJ, Rymer WZ, Beaumont J, Rine RM, Lai JS, Gershon RC. Motor assessment using the NIH Toolbox. Neurology 80, Suppl 3: S65–S75, 2013. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roshanravan B, Kestenbaum B, Gamboa J, Jubrias SA, Ayers E, Curtin L, Himmelfarb J, de Boer IH, Conley KE. CKD and muscle mitochondrial energetics. Am J Kidney Dis 68: 658–659, 2016. doi: 10.1053/j.ajkd.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roshanravan B, Patel KV, Robinson-Cohen C, de Boer IH, O’Hare AM, Ferrucci L, Himmelfarb J, Kestenbaum B. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis 65: 737-747, 2015. doi: 10.1053/j.ajkd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roshanravan B, Robinson-Cohen C, Patel KV, Ayers E, Littman AJ, de Boer IH, Ikizler TA, Himmelfarb J, Katzel LI, Kestenbaum B, Seliger S. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 24: 822–830, 2013. doi: 10.1681/ASN.2012070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31: 384–396, 2013. doi: 10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 67.Shah AJ, Sahgal V, Quintanilla AP, Subramani V, Singh H, Hughes R. Muscle in chronic uremia–a histochemical and morphometric study of human quadriceps muscle biopsies. Clin Neuropathol 2: 83–89, 1983. [PubMed] [Google Scholar]
- 68.Smith LR, Fowler-Gerace LH, Lieber RL. Muscle extracellular matrix applies a transverse stress on fibers with axial strain. J Biomech 44: 1618–1620, 2011. doi: 10.1016/j.jbiomech.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tidball JG. Force transmission across muscle cell membranes. J Biomech 24, Suppl 1: 43–52, 1991. doi: 10.1016/0021-9290(91)90376-X. [DOI] [PubMed] [Google Scholar]
- 70.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 40: 181–188, 2008. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 71.Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis 5: e1186, 2014. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124: 3654–3664, 2011. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 73.Verzola D, Bonanni A, Sofia A, Montecucco F, D’Amato E, Cademartori V, Parodi EL, Viazzi F, Venturelli C, Brunori G, Garibotto G. Toll-like receptor 4 signalling mediates inflammation in skeletal muscle of patients with chronic kidney disease. J Cachexia Sarcopenia Muscle 8: 131–144, 2017. doi: 10.1002/jcsm.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verzola D, Procopio V, Sofia A, Villaggio B, Tarroni A, Bonanni A, Mannucci I, De Cian F, Gianetta E, Saffioti S, Garibotto G. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int 79: 773–782, 2011. doi: 10.1038/ki.2010.494. [DOI] [PubMed] [Google Scholar]
- 75.Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, Smith AC. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol 25: 2121–2130, 2014. doi: 10.1681/ASN.2013070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watson EL, Viana JL, Wimbury D, Martin N, Greening NJ, Barratt J, Smith AC. The effect of resistance exercise on inflammatory and myogenic markers in patients with chronic kidney disease. Front Physiol 8: 541, 2017. doi: 10.3389/fphys.2017.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455, 2013. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Pan J, Dong Y, Tweardy DJ, Dong Y, Garibotto G, Mitch WE. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab 18: 368–379, 2013. doi: 10.1016/j.cmet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L, Wang XH, Wang H, Du J, Mitch WE. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 21: 419–427, 2010. doi: 10.1681/ASN.2009060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zitnay JL, Li Y, Qin Z, San BH, Depalle B, Reese SP, Buehler MJ, Yu SM, Weiss JA. Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun 8: 14913, 2017. doi: 10.1038/ncomms14913. [DOI] [PMC free article] [PubMed] [Google Scholar]