Abstract
Proteinuria is not only a common feature of chronic kidney diseases (CKD) but also an independent risk factor promoting CKD progression to end-stage renal failure. However, the underlying molecular mechanisms for protein overload-induced renal injury remain elusive. The present study examined the role of (pro)renin receptor (PRR) in pathogenesis of albumin overload (AO)-induced nephropathy and activation of the intrarenal renin-angiotensin system (RAS) in rats. Wistar rats underwent unilateral nephrectomy and were treated for 7 wk with vehicle, bovine serum albumin (5 g·kg−1·day−1 via a single ip injection), alone or in conjunction with the PRR decoy inhibitor PRO20 (500 μg·kg−1·day−1 via 3 sc injections). The AO rat model exhibited severe proteinuria, tubular necrosis, and interstitial fibrosis, oxidative stress, and inflammation, accompanied by elevated urinary N-acetyl-β-d-glucosaminidase activity and urinary β2-microglobulin secretion, all of which were significantly attenuated by PRO20. Urinary and renal levels of renin, angiotensinogen, and ANG II were elevated by AO and suppressed by PRO20, contrasting to largely unaltered plasma levels of the RAS parameters. The AO model also showed increased renal expression of full-length PRR and soluble PRR (sPRR) and urinary excretion of sPRR. Taken together, we conclude that PRR antagonism with PRO20 alleviates AO-induced nephropathy via inhibition of intrarenal RAS.
Keywords: albumin overload, chronic kidney disease, proteinuria, PRO20, (pro)renin receptor, renin-angiotensin system
INTRODUCTION
Insight into the mechanisms underlying the progression of chronic proteinuric nephropathies has attracted the interest of the renal community in the last two decades. Proteinuria induces a multitude of inflammatory and fibrogenic mediators, all of which contribute to the progression of renal damage (21, 41, 51). Thus, persistent proteinuria is considered as a deleterious prognostic factor in most progressive renal diseases (2, 35). Although the mechanisms by which proteinuria induces renal damage remain undetermined, proteinuria has been shown to elicit the local activation of the renin-angiotensin system (RAS) in the kidney (4). Takase et al. found that increase in intrarenal ANG II induced by proteinuria depends on nuclear factor-κB activation (39). Cao et al. found that AO activates the intrarenal RAS through the protein kinase C-nicotinamide adenine dinucleotide phosphate oxidase-dependent pathway (4). The RAS inhibitors offer greater renoprotection than other antihypertensive drugs in chronic kidney disease (CKD) patients that usually exhibit unaltered circulating levels of RAS components, suggesting involvement of intrarenal RAS (18). However, the mechanism of how intrarenal RAS is activated in CKD remains unclear. Furthermore, the existing therapies for CKD are suboptimal, and patients with end-stage renal disease largely rely on dialysis and renal transplantation. Therefore, it is imperative to identify novel therapeutic targets for CKD.
In 2002, Nguyen and coworkers cloned (pro)renin receptor (PRR), which specifically binds both renin and prorenin (1, 5). PRR is a 39-kDa protein that belongs to the type I transmembrane receptor family and interestingly contains a protease cleavage site (7, 31). Within the kidney, PRR mRNA and protein are predominantly expressed in collecting duct where it is most abundant in microvilli at the apical surface of A-type intercalated cells (1). It induces a fivefold increase in the activity of bound renin by increasing the affinity of renin for the oxidized form of its substrate, angiotensinogen (AGT) (50).
PRR has attracted much attention in light of its potential as a new therapeutic target for cardiovascular and renal diseases (30). The handle region peptide (HRP) is the first PRR-blocking peptide, but its specificity in inhibiting PRR signaling is highly debated (11, 15, 19). PRO20, a newly developed PRR decoy inhibitor (the first 20 amino acid residues of the prorenin prosegment, L1PTDTASFGRILLKKMPSVR20) (22), similarly targets the prosegment of prorenin and renin but covers a longer region than the 10-amino-acid HRP. PRO20 has been validated as a specific and effective PRR antagonist by multiple recent studies (25, 34, 38, 43, 45, 47–49). The main purpose of the present study was to examine the effect of PRO20 on albumin-induced kidney injury and the underlying mechanism involving activation of intrarenal RAS in rats.
MATERIALS AND METHODS
Preparation of albumin solution.
For animal treatment, bovine serum albumin (BSA, 10735108001; Roche, Indianapolis, IN) was dissolved in normal saline to a final concentration of 330 mg/ml. The prepared BSA solution was passed through a DetoxiGel column (Pierce, Rockford, IL) to remove endotoxin.
Animals.
Male Wistar rats (160–180 g) were purchased from Beijing Vital River Laboratories (Beijing, China). All animals were cage housed and maintained in a temperature-controlled room with a 12:12-h light-dark cycle, with free access to tap water and standard rat chow. The animal protocols were approved by the Animal Care and Use Committee at Sun Yat-sen University, China.
Albumin overload model and PRO20 treatment.
After 1 wk of adaptive feeding, the rats were subjected to unilateral nephrectomy. After the operation (1 wk), the rats were randomized to receive the following treatments for 7 wk: daily intraperitoneal injection of BSA dissolved in saline (pH 7.4) at 5.0 g·kg−1·day−1 [albumin overload (AO) group], vehicle (saline, pH 7.4) (CTR group), or intraperitoneal injection of BSA at 5.0 g·kg−1·day−1 in conjunction with a subcutaneous injection of PRO20 (500 μg·kg−1·day−1 via 3 sc injections) (AO + PRO20 group). At week 0, 2, 5, and 7, each rat was placed in a metabolic cage to collect 24-h urine, and the urine samples were snap-frozen in liquid nitrogen and then stored at −80°C. Proteinuria was measured by using the Coomassie blue method (24), and N-acetyl-β-d-glucosaminidase (NAG) activity was determined by using an ELISA kit (A031; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). At the end of the experiment, the animals were anesthetized with isoflurane, blood was collected, and kidneys were harvested for analysis of protein and mRNA expression as well as histological analysis. Plasma creatinine and urea nitrogen were measured by using commercial kits (BioAssay Systems) according to the manufacturer’s instructions. β2-Microglobulin (β2-MG) levels in urine were measured by using a commercial ELISA kit (AE90428Ra; Shanghai Lianshuo Biological Technology, Shanghai, China).
Measurement of blood pressure.
Systolic blood pressure (SBP) was measured by tail-cuff plethysmography using a Visitech Systems BP-2000 Blood Pressure Analysis System (Apex, NC), as previously described (16). All animals were habituated to the blood pressure measurement device for 7 days. They all underwent two cycles of 20 measurements reordered per day for a minimum of 3 days.
Histopathological assessment.
One-fourth of the kidneys was immersion fixed in 10% buffered formalin, embedded in paraffin, and sectioned to 5 μm thickness. Periodic acid-Schiff staining was used to examine kidney histology. Renal tubulointerstitial indexes were assessed using a semiquantitative scoring system, as previously described (12, 42). In brief, tubulointerstitial injury was scored in a blinded manner according to the percentage of damage, including tubular atrophy and dilation, protein casts, and inflammatory cell infiltration (the higher the number, the more severe the injury): 0, normal; 1, lesions involving <25% of the cortical area; 2, 25–50%; and 3, >50%. The score index in each rat was expressed as a mean value of all scores obtained. The images were captured by a Leica fluorescence microscope. The random pictures per kidney section were quantified. Picrosirius red staining was performed for analysis of renal fibrosis. The amount of cortical collagen was determined by quantify the percentage of positive staining areas with picrosirus red. The kidney index was calculated as kidney weight/body weight = kidney weight (g)/animal weight (g) (‰).
Immunoblotting.
Western blot analysis was carried out as previously described (44). Briefly, 30 μg of protein for each sample were denatured in a boiling water bath for 10 min, separated by SDS-PAGE, and transferred to polyvinylidene fluoride membranes (Immobilion-P; Millipore, Bedford, MA). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) for 1 h at room temperature followed by incubation with primary antibodies [PRR, 1:1,000 dilution (HPA003156; Sigma-Aldrich); AGT, 1:1,000 dilution (SAB2100072; Sigma-Aldrich); tumor necrosis factor-α (TNF-α), 1:1,000 dilution (sc-52746, Santa Cruz); interleukin-8 (IL-8), 1:1,000 dilution (sc-8427, Santa Cruz); transforming growth factor-β1 (TGF-β1), 1:1,000 dilution (ab31013, Abcam); collagen 1 (COL-1), 1:1,000 dilution (sc-59772, Santa Cruz); fibronectin (FN), 1:1,000 dilution (F3648, Sigma-Aldrich); and β-actin, 1:10,000 dilution (A-2066; Sigma-Aldrich)] overnight at 4°C. After being washed with TBST, membranes were incubated with secondary antibodies (goat anti-rabbit/mouse horseradish peroxidase-conjugated secondary antibody; Thermo Scientific) for 1 h at room temperature and visualized with enhanced chemiluminescence (Thermo Scientific). Signals on immunoblots were detected using a Tanon 5200 Luminescent Imaging Workstation (Tanon, Shanghai, China) and quantitated using Image-Pro Plus version 6.0 software. β-Actin was used as the internal control.
Biochemical analysis of renin, soluble PRR, AGT, and ANG II.
Renin activity assay was performed as previously described (3). Briefly, plasma and urine samples were centrifuged at 4,000 revolutions/min at 4°C for 20 min, and the supernatant was collected. Renin activity was determined by the delta value of the ANG I generation using an ELISA kit after a 1-h incubation at 37°C vs. 4°C. ANG I generation was assayed by using an ANG I EIA kit (S-1188; Peninsula Laboratories) according to the manufacturer’s instructions. Active renin content was measured with excessive AGT. Urine samples were spiked with 1 μM synthetic renin substrate tetradecapeptide (R8129; Sigma-Aldrich). The values were expressed as nanogram per milliliter per hour of generated ANG I. Soluble PRR (sPRR) concentrations in urine and plasma were measured by using a commercial ELISA kit (27781; Immuno-Biological Laboratories, Takasaki, Japan). Total rat prorenin/renin concentrations in urine were measured by using a commercial ELISA kit (RPRENKT-TOT; Molecular Innovations). AGT and ANG II concentrations in urine and plasma were measured by using a commercial ELISA kit (SEA797Ra; CEA005Ra; Cloud-Clone).
Measurement of thiobarbituric acid reactive substances.
The measurement of thiobarbituric acid reactive substances (TBARS) was based on the formation of malondialdehyde by using a commercially available TBARS Assay kit (10009055; Cayman Chemical) according to the manufacturer’s instructions (18, 46).
Quantitative reverse transcription-polymerase chain reaction.
Snap-frozen renal samples were homogenized in TRIzol reagent (15596018; Life Technologies). Total RNA isolation and reverse transcription were performed as previously described (32). Total RNA concentrations were determined using a NANODROP 2000 spectrophotometer (Thermo Scientific) according to the manufacturer’s instructions. We used 1 μg of total RNA as a template for RT by using the Transcriptor First Strand cDNA Synthesis Kit (04379012001; Roche, Berlin, Germany) according to the manufacturer’s instructions. Real-time quantitative PCR was performed using the ABI Prism StepOnePlus System (Applied Biosystems, Life Technologies) and the FastStart Universal SYBR Green Master (ROX) (04913914001; Roche) according to the manufacturer’s instructions. Oligonucleotides were designed using Primer3 software (available at http://bioinfo.ut.ee/primer3-0.4.0/), and their sequences were shown in Table 1. All reactions were run in duplicate. Relative mRNA expression levels were calculated from threshold cycle numbers (CT), i.e., 2−ΔΔCT, according to the manufacturer's suggestion. The data were shown as a relative value normalized by β-actin.
Table 1.
Primer sequences for qRT-PCR
| Target Gene | Primer Sequence |
|---|---|
| Rat β-actin | |
| Forward | CACCCGCGAGTACAACCTTC |
| Reverse | CCCATACCCACCATCACACC |
| Rat IL-6 | |
| Forward | AGAGACTTCCAGCCAGTTGC |
| Reverse | AGTCTCCTCTCCGGACTTGT |
| Rat IL-8 | |
| Forward | CCCCCATGGTTCAGAAGATTG |
| Reverse | TTGTCAGAAGCCAGCGTTCAC |
| Rat MCP-1 | |
| Forward | TAGCATCCACGTGCTGTCTC |
| Reverse | CAGCCGACTCATTGGGATCA |
| Rat TNF-α | |
| Forward | CGTCAGCCGATTTGCCATTT |
| Reverse | TCCCTCAGGGGTGTCCTTAG |
| Rat TGF-β1 | |
| Forward | CTCAACACCTGCACAGCTCC |
| Reverse | AGTTGGCATGGTAGCCCTTG |
| Rat PRR | |
| Forward | ATCCTTGAGACGAAACAAGA |
| Reverse | AGCCAGTCATAATCCACAGT |
| Rat REN | |
| Forward | GATCACCATGAAGGGGGTCTCTGT |
| Reverse | GTTCCTGAAGGGATTCTTTTGCAC |
| Rat AGT | |
| Forward | AGCATCCTCCTTGAACTCCA |
| Reverse | TGATTTTTGCCCAGGATAGC |
| Rat ACE | |
| Forward | GAGCCATCCTTCCCTTTTTC |
| Reverse | CCACATGTTCCCTAGCAGGT |
qRT-PCR, quantitative reverse transcription-polymerase chain reaction; IL, interleukin; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1; PRR, (pro)renin receptor; REN, renin; AGT, angiotensinogen; ACE, angiotensin-converting enzyme.
Statistical analysis.
Data are summarized as means ± SE. All data points were included in the statistical analyses. Statistical analysis was performed by using analysis of variance with the Bonferroni test for multiple comparisons, or by unpaired Student’s t-test for two comparisons. A P value <0.05 was considered statistically significant.
RESULTS
PRO20 attenuated proteinuria and improved renal damage induced by AO.
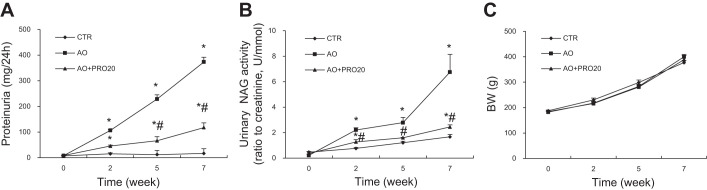
Following intraperitoneal injections of BSA, proteinuria was detectable at week 2, progressively increased with time, and reached the peak at week 7 as shown in the AO group (Fig. 1A). In contrast, the progression of proteinuria was remarkably attenuated in the AO + PRO20 group compared with the AO group (P < 0.05, Fig. 1A). Urinary NAG was monitored to reflect the degree of renal injury. The NAG level in the AO rats exhibited time-dependent increases in parallel with proteinuria. Similarly, the rise in urinary NAG excretion was attenuated by PRO20 (Fig. 1B). The growth curves of body weight were not different among any groups (Fig. 1C). At the end of the experiment, the urine samples were assayed for albumin, β2-MG, and plasma samples for blood creatinine and blood urea nitrogen, and the kidney weight was determined (Table 2). Severe renal hypertrophy developed as evidenced by 100% increase in kidney weight in the AO group, which was much less in the AO + PRO20 group. PRO20 induced a 70% decrease in urinary albumin and a 62% decrease in β2-MG. However, blood creatinine and blood urea nitrogen remained quite constant among the three groups. Table 2 shows the SBP at the end of the experiment, which tended to be higher in the AO group compared with the control, but this difference did not reach a statistical significance, and PRO20 was without an effect.
Fig. 1.
Analysis of proteinuria, N-acetyl-β-d-glucosaminidase (NAG), and body weight. Wistar rats were treated for 7 wk with vehicle, albumin overload (AO), or AO + (pro)renin receptor decoy inhibitor (the first 20 amino acid residues of the prorenin prosegment, L1PTDTASFGRILLKKMPSVR20) (PRO20). Proteinuria, urinary NAG activity, and body weight were monitored at the indicated time points. A: time courses of proteinuria. B: time courses of urinary NAG activity. C: body weight (BW). Data are means ± SE; n = 6/group. *P < 0.05 vs. control (CTR); #P < 0.05 vs. AO.
Table 2.
Analysis of systolic blood pressure, renal hypertrophy, and renal injury biomarkers
| Group | Systolic BP, mmHg | KW, g | KW/BW, ‰ | Alb, mg/24 h | β2-MG, μg/24 h | Bcr, μmol/l | BUN, mmol/l |
|---|---|---|---|---|---|---|---|
| CTR | 124 ± 1.21 | 1.21 ± 0.03 | 4.25 ± 0.07 | 11.17 ± 2.84 | 0.07 ± 0.01 | 47.36 ± 1.61 | 3.98 ± 0.95 |
| AO | 130 ± 2.60 | 2.67 ± 0.25* | 8.49 ± 0.98* | 109.74 ± 23.95* | 0.13 ± 0.01* | 46.84 ± 1.27 | 4.64 ± 0.48 |
| AO + PRO20 | 132 ± 4.23 | 1.89 ± 0.23*# | 5.99 ± 0.52*# | 33.45 ± 5.40*# | 0.05 ± 0.01# | 45.90 ± 1.07 | 4.24 ± 0.53 |
Data are means ± SE; n = 5~6.
P < 0.05 vs. CTR;
P < 0.05 vs. AO.
BP, blood pressure; KW, kidney weight; KW/BW, ratio of kidney weight to body weight; Alb, urinary albumin excretion; β2-MG, urinary β2-microglobulin; Bcr, blood creatinine; BUN, blood urea nitrogen; CTR, control; AO, albumin overload; PRO20, (pro)renin receptor decoy inhibitor (the first 20 amino acid residues of the prorenin prosegment, L1PTDTASFGRILLKKMPSVR20).
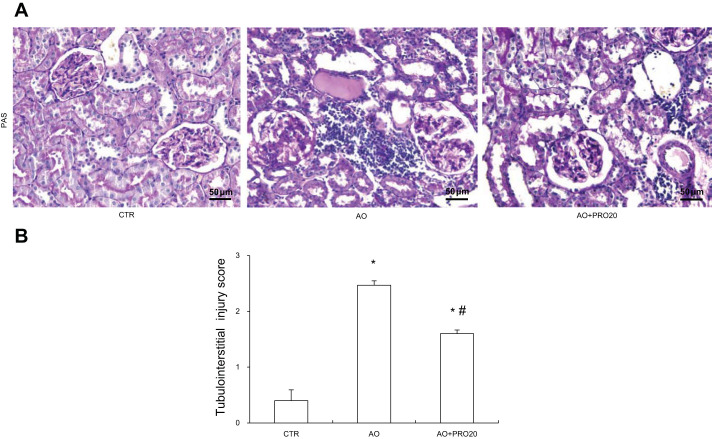
By Periodic acid-Schiff staining analysis, the AO group exhibited tubular atrophy and dilation, protein casts, and inflammatory cell infiltration (Fig. 2A). PRO20 has improved the histological damage induced by AO (Fig. 2A). These results were semiquantitatively analyzed as tubulointerstitial injury score (Fig. 2B).
Fig. 2.
Histological analysis of renal injury. A: Periodic acid-Schiff staining of renal cortex. Original magnification, ×200. B: renal tubulointerstitial injury score from semiquantitative analysis of renal pathologies (average of 10 fields of area/rat, n = 5/group). Data are means ± SE. *P < 0.05 vs. control (CTR); #P < 0.05 vs. albumin overload (AO).
PRO20 attenuated renal oxidative stress, inflammation, and fibrosis induced by AO.
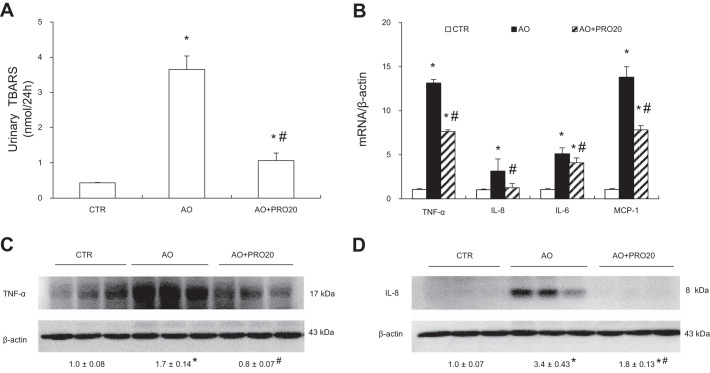
It has been shown that AO is associated with increased renal oxidative stress (28, 40). We therefore examined urinary TBARS level, a marker for oxidative stress. Urinary TBARS exhibited a threefold increase in the AO group compared with the control group, and this elevation was less in the AO + PRO20 group (P < 0.05, Fig. 3A).
Fig. 3.
Analysis of renal oxidative stress and proinflammatory cytokine expression. A: urinary thiobarbituric acid reactive substances (TBARS). B: quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of mRNA expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-8, IL-6, and monocyte chemoattractant protein 1 (MCP-1) in the renal cortex. The values were normalized by β-actin. C: immunoblotting analysis of TNF-α. The protein abundance of TNF-α was analyzed by densitometry, and the values were normalized by β-actin and shown underneath the blot. D: immunoblotting analysis of IL-8. The densitometry of IL-8 was performed as for TNF-α. Data are means ± SE; n = 6/group. *P < 0.05 vs. control (CTR); #P < 0.05 vs. albumin overload (AO).
Inflammation is an important feature of AO-induced nephropathy (1). To test whether PRO20 influenced inflammation, kidneys were collected and assayed for proinflammatory factors TNF-α and IL-8 using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blot analysis. As shown in Fig. 3B, mRNA levels of TNF-α and IL-8 were significantly increased in AO rats, and these increases were less in the AO + PRO20 rats (P < 0.05, Fig. 3B). Similar results were obtained by qRT-PCR analysis of renal interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) (Fig. 3B). Subsequently, we validated the protein expression of selected cytokines such as TNF-α and IL-8 by Western blot analysis. Renal protein expression of TNF-α and IL-8 was increased in the AO rats compared with the control group (P < 0.05), and these increases were both blocked by PRO20 treatment (P < 0.05, Fig. 3, C and D).
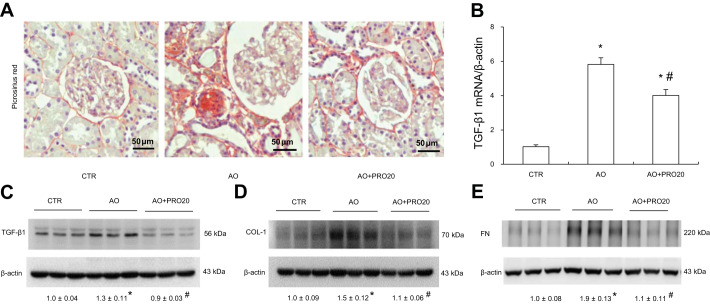
Fibrosis results from the excessive deposition of extracellular matrix components, which ultimately leads to organ failure. Renal fibrosis is a common pathological change shared by various types of renal disease. We therefore evaluated various renal fibrosis markers. To ascertain the renoprotective effect of PRO20 in progression of AO-induced nephropathy, we examined renal fibrosis by Picrosirius red staining and immunoblotting of COL-1, TGF-β1, and FN. As shown in Fig. 4A, PRO20 significantly attenuated the percentage of Picrosirius red staining in the cortex area (8.17 ± 0.81 in AO vs. 3.08 ± 0.43 in AO + PRO20, P < 0.05). Consistently, PRO20 suppressed mRNA levels of TGF-β1 (P < 0.05, Fig. 4B) as well as TGF-β1, COL-1, and FN (P < 0.05, Fig. 4, C, D, and E). Taken together, these results strongly suggest renoprotective action of PRO20 in the AO-induced nephropathy.
Fig. 4.
Analysis of renal fibrosis. A: Picrosirius red staining of the kidney. Shown are representative images from 6 rats/group. B: quantitative reverse transcription-polymerase chain reaction (q-RT-PCR) analysis of renal mRNA expression of transforming growth factor-β1 (TGF-β1) with normalization by β-actin. C–E: immunoblotting analysis of TGF-β1, collagen 1 (COL-1), and fibronectin (FN) in the cortex. Shown are representative blots with densitometry data. β-Actin was used as an internal control. Data are means ± SE; n = 6/group. *P < 0.05 vs. control (CTR); #P < 0.05 vs. albumin overload (AO).
PRO20 inhibited renal local RAS activation induced by AO.
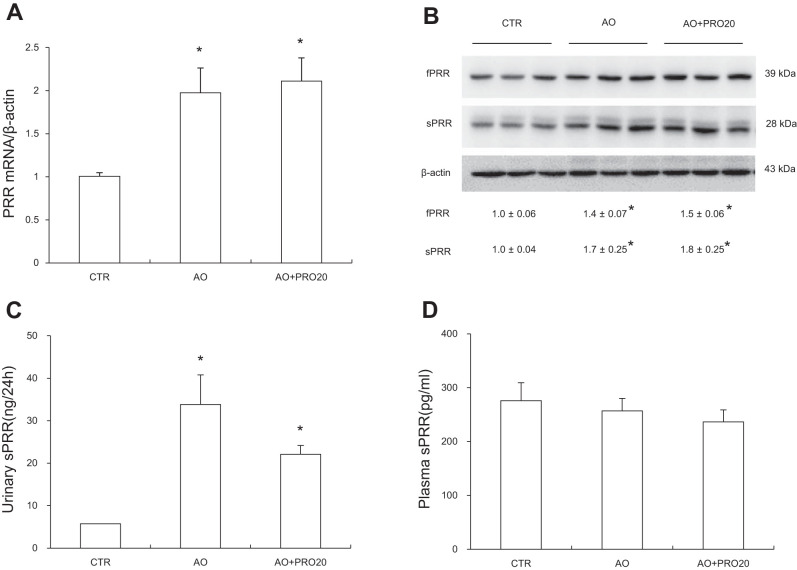
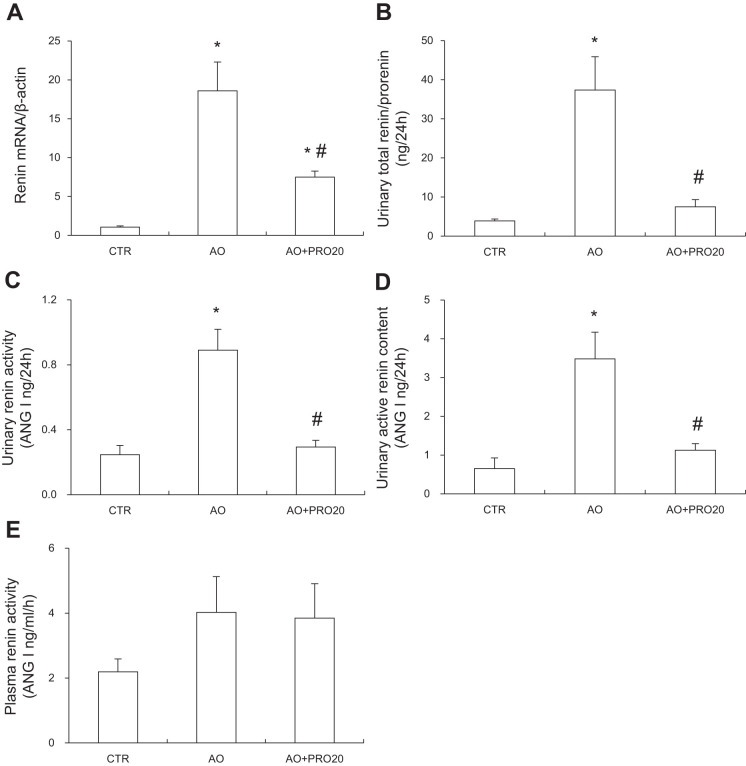
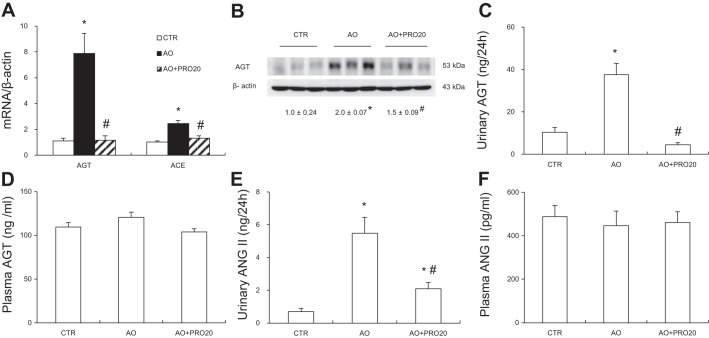
Activation of intrarenal RAS contributes to pathogenesis of AO-induced nephropathy and other types of renal disease. Furthermore, PRR has recently shown to be a key regulator of intrarenal RAS during ANG II-induced hypertension. We therefore examined the possibility that PRR may control the activity of intrarenal RAS during AO-induced nephropathy. Renal mRNA and protein expressions of PRR along with renal sPRR expression and urinary sPRR excretion were all elevated in the AO group compared with the control group (P < 0.05, Fig. 5, A, B, and C), contrasting to unchanged circulating sPRR concentration (Fig. 5D). These results represent evidence of activation of intrarenal PRR/sPRR. Similarly, renal renin mRNA expression and urinary total renin level were significantly increased in the AO group (P < 0.05, Fig. 6, A and B). However, circulating renin activity remained constant (Fig. 6E). PRO20 significantly suppressed AO-induced urinary total prorenin/renin concentrations [33.01 ± 8.49 in AO vs. 7.52 ± 1.83 urinary total prorenin/renin (ng/ ml) in AO + PRO20, P < 0.05, Fig. 6B], urinary renin activity [0.89 ± 0.13 in AO vs. 0.29 ± 0.04 ANG I (ng/24 h) in AO + PRO20, P < 0.05, Fig. 6C], and urinary active renin content [3.48 ± 0.27 in AO vs. 1.12 ± 0.17 ANG I (ng/24 h) in AO + PRO20, P < 0.05, Fig. 6D]. In addition, PRO20 significantly reduced renal AGT and angiotensin-converting enzyme (ACE) mRNA levels and AGT protein expression (P < 0.05, Fig. 7A and B). Furthermore, urinary AGT and ANG II levels were significantly increased compared with the control group (P < 0.05), which were both inhibited by PRO20 treatment (P < 0.05, Fig. 7, C and E). In contrast, AGT and ANG II levels in plasma were largely unchanged among the three groups (Fig. 7, D and F). These data suggest PRR-dependent activation of intrarenal RAS during AO-induced nephropathy.
Fig. 5.
Analysis of (pro)renin receptor (PRR)/soluble (pro)renin receptor (sPRR) expression. A: quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of PRR in the renal cortex. The expression was normalized by β-actin. B: immunoblotting analysis of full-length PRR and sPRR in the renal cortex. Shown are representative blots with densitometry data. β-Actin was used as an internal control. C: ELISA analysis of urinary sPRR excretion. D: ELISA analysis of plasma sPRR. Data are means ± SE; n = 6/group. *P < 0.05 vs. control (CTR).
Fig. 6.
Analysis of intrarenal renin levels compared with plasma renin activity. A: quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of renin mRNA expression in the renal cortex. The expression was normalized by β-actin. B: urinary total prorenin/renin. C: urinary renin activity. D: urinary active renin content. E: plasma renin activity. Data are means ± SE; n = 6/group. *P < 0.05 vs. control (CTR); #P < 0.05 vs. albumin overload (AO).
Fig. 7.
Analysis of other intrarenal renin-angiotensin system (RAS) components. A: quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of mRNA expression of angiotensinogen (AGT) and angiotensin-converting enzyme (ACE) in the renal cortex. β-Actin was used as an internal control. B: immunoblotting analysis of AGT in the renal cortex. Shown are representative blots with densitometry data. β-Actin was used as an internal control. C: ELISA measurement of urinary AGT excretion. D: ELISA measurement of plasma AGT concentration. E: ELISA measurement of urinary ANG II excretion. F: ELISA measurement of plasma ANG II concentration. Data are means ± SE; n = 6. *P < 0.05 vs. control (CTR); #P < 0.05 vs. albumin overload (AO).
DISCUSSION
Proteinuria is well known to play a pathogenic role in promoting renal injury. Inappropriate activation of the intrarenal RAS has been implicated in the pathogenesis of AO nephropathy. In the present study, we tested the hypothesis that PRR mediates AO-induced renal injury via upregulation of intrarenal RAS. We demonstrated that AO treatment in rats induced renal PRR expression and urinary sPRR excretion associated with overactivation of intrarenal RAS as well as oxidative and proinflammatory responses. PRO20 treatment protested against AO-induced proteinuria and renal pathologies and suppressed intrarenal RAS. The present study examined the potential pathogenic role of PRR in a rat model of AO-induced nephropathy and further elucidates the underlying mechanism. AO treatment for 7 wk induced PRR protein expression and urinary sPRR excretion, in parallel with increased urinary excretion of renin activity, AGT, and ANG II levels. PRR antagonism with the novel PRR competitive peptide inhibitor PRO20 attenuated the indexes of intrarenal RAS and improved albuminuria, renal hypertrophy, TGF-β1, COL-1, and FN protein expression and oxidative and inflammatory responses. This study for the first time elucidates the pathogenic role of PRR in AO-induced nephropathy and demonstrated the therapeutic potential of PRO20 for this devastating disease.
Ichihara et al. synthesize a site-specific binding peptide that interacts with the “handle” region of the prorenin prosegment (HRP, R10IPLKKMPSV19) as the first version of the competitive PRR peptide inhibitor (15). Whereas HRP was initially shown to alleviate nephropathy and retinopathy induced by diabetes and hypertension, subsequent studies yield negative results (11, 13, 37). In particular, HRP was unable to block PRR-mediated activation of phosphorylated extracellular signal-regulated kinase 1/2 in cultured vascular smooth muscle cells, raising a serious doubt about HRP as a specific inhibitor of PRR (11). Recently, Li et al. designed and developed a novel PRR inhibitory peptide, PRO20 (the first 20 amino acid residues of the prorenin prosegment, L1PTDTASFGRILLKKMPSVR20) (22), which covers a longer region than the 10-amino-acid HRP; it directly inhibits the binding of prorenin and PRR, effectively reduces prorenin-induced ANG II-dependent calcium influx in human neuronal cells, and suppresses DOCA-salt-induced hypertension, whereas HRP has no such effect. In agreement with these observations, multiple studies from our group have validated PRO20 in a number of rodent models with hypertension and dysregulation of fluid and electrolyte homeostasis. In particular, we employed this agent to generate the first in vivo evidence of PRR regulation of intrarenal renin activity during ANG II-induced hypertension (43). This agent was also successfully used to demonstrate PRR regulation of intrarenal RAS under conditions of water deprivation, high K+ intake, and fructose/salt-induced hypertension (45, 48, 49). The present study is the first to report a beneficial effect of PRO20 on AO-induced nephropathy.
An inappropriate activation of intrarenal RAS has been implicated in the pathogenesis of CKD induced by protein overload, but there is a knowledge gap in understanding the underlying mechanism. Most reports rely on in vitro investigation of the effects of albumin on the components of RAS or the response of albumin-induced fibrogenesis to angiotensin type 1 receptor blockers (9, 23, 27). Largo et al. show that AO in uninephrectomized Wistar-Kyoto rats induced renal mRNA and protein expression of AGT and ACE but suppressed renal mRNA expression of renin (20). In this study, the mRNA expression was assessed by conventional reverse transcription-PCR (RT-PCR). In the present study, we performed extensive analysis of various parameters of intrarenal RAS in AO rats with or without PRO20 treatment. Consistent with the previous report (18), we found that renal AGT protein expression was elevated in AO. However, inconsistent with this report (18), we observed increased renal renin mRNA expression as well. The reason for the discrepancy is unknown but may be related to differences in mRNA detection techniques (qRT-PCR vs. RT-PCR) or the duration of BSA treatment (7 wk vs. 8 days). Previous studies from our group and others have shown that urinary excretion of some components of RAS reflects the activity of intrarenal RAS. For example, renin levels are elevated in the urine but suppressed in the plasma during ANG II infusion, highlighting the activation of intrarenal RAS (43). Therefore, a direct comparison of urinary vs. plasma levels of the RAS components will offer a unique assessment to intrarenal RAS, representing a major strength of the current study. We observed parallel increases in urinary renin, AGT, and ANG II in response to AO, but plasma levels of these RAS components all remained constant. These results represent strong evidence for overactivation of intrarenal vs. systemic RAS during AO-induced nephropathy. It is interesting to note that, as a competitive PRR inhibitor, PRO20 presumably acted at the level of renin activity but in fact suppressed mRNA and protein expression of various RAS components, including renin and AGT. The later effect of PRO20 is considered to be secondary to the change in renin activity.
PRR was originally discovered as a potential regulator of local renin activity given its wide distribution beyond the juxtaglomerular cells. However, subsequent studies yield conflicting reports regarding the relationship between PRR and the RAS.
The doubt about such relationship derives from a series of evidence. For example, PRR is known to act as a subunit of V-ATPase complex involved in a number of essential cellular functions such as acidification of cellular organelles and Wnt/β-catenin signaling (8). In addition, overexpression of human PRR fails to affect tissue ANG II concentrations (17). However, increasing evidence from renal PRR studies supports a potential role of PRR in regulation of intrarenal RAS. PRO20 (22) administered via the intramedullary infusion technique remarkably suppressed the increases in urinary and renal medullary renin activity during ANG II-induced hypertension (43). In vitro evidence further demonstrated that the action of PRO20 in inhibiting renin activity was direct (43). Positive results were also observed in mice with collecting duct-specific deletion of PRR that showed suppressed urinary renin activity at basal condition and during ANG II-induced hypertension (34). In the present study, PRO20 was highly effective in attenuating the enhancement of various intrarenal RAS components during AO nephropathy, providing additional support of the association of PRR and intrarenal RAS. This notion is also supported by our in vitro study (10) that recapitulates most of the in vivo findings shown by the present study. Of note, in cultured renal epithelial cells, albumin induced inflammatory and fibrogenic response and renin activity, all of which were blunted by PRO20. Therefore, the compelling in vivo and in vitro evidence demonstrates that PRR plays a pathogenic role in AO-induced nephropathy via triggering intrarenal RAS.
Activation of oxidative, inflammatory, and fibrogenic responses represents a universal downstream pathway responsible for pathogenesis of various types of renal diseases. These responses are likely affected by PRO20 in the AO model. We found that PRO20 treatment blocked the increase of urinary TBARS in the AO rats, consistent with our previous report that enhanced intrarenal PRR was associated with oxidative stress (25). Here we also found that PRO20 inhibited overexpression of renal TNF-α, IL-6, IL-8, and MCP-1 in the AO rats. Moreover, PRO20 significantly reduced expression of fibrotic factors, such as TGF-β1, COL-1, and FN. However, it remains unclear whether these beneficial effects of PRO20 are a direct consequence of the suppressed intrarenal RAS. Indeed, PRR can act in a RAS-dependent and -independent manner. Apart from the regulation of the activity of prorenin and renin, activation of PRR induces multiple signaling pathways, including mitogen-activated protein kinase (36), oxidative stress (33), Wnt/β-catenin signaling (8), fibrogenic response (5, 14), etc. Therefore, the therapeutic value of PRO20 may go beyond the RAS inhibition. Furthermore, it becomes increasingly apparent that PRR also can serve as an integral component of the V-ATPase complex.
sPRR, the 28-kDa product of protease-mediated cleavage of PRR, is elevated in patients with CKD and correlates with the fall of estimated glomerular filtration rate and disease progress. It is intriguing that sPRR may not merely serve as a disease biomarker but play an active role in pathogenesis of CKD. This possibility is supported by emerging evidence from our recent studies. We for the first time report that sPRR exerts a biological function in stimulating renal AQP2 expression and thus enhancing urine concentrating capability (26). A more recent study from our group shows that sPRR is involved in albumin-induced signaling in cultured renal epithelial cells, which provides a rationale for conducting the current in vivo investigation (10). Exposure of these cells to albumin induced a >10-fold increase in sPRR release, consistent with the increases of renal sPRR abundance and urinary sPRR excretion in AO rats. Furthermore, we discovered site-1 protease (S1P) but not furin or ADAM19 as the predominant source of albumin-induced sPRR production (10). This finding aligns well with the study of Nakagawa et al. who similarly report S1P as a major PRR cleavage protease (29). Future studies are needed to test the possible effect of albumin on S1P activity/expression and its in vivo relevance to pathogenesis of albumin-induced nephropathy.
In vitro evidence originally demonstrated that PRR binding to prorenin or renin leads to enhanced ANG I generation, suggesting that PRR-dependent renin regulation may primarily occur at the activity level. In support of this notion, we have previously shown that PRR antagonism with PRO20 only reduces renin activity in the kidney and urine but not renin mRNA during ANG II-induced hypertension (43). To our surprise, the present study shows that PRO20 treatment suppressed multiple RAS components, including renin and AGT, at the levels of both activity and expression, indicating a distinct mechanism of PRR regulation of intrarenal RAS during AO nephropathy. It remains elusive whether the regulation of the RAS component expression is secondary to the changes in renin activity or reflects another mode of action of PRR. It seems conceivable that PRR may affect a multitude of cellular functions via generation of sPRR that induces multiple signaling pathways.
In conclusion, the present study tested the role of renal PRR in pathogenesis of AO-induced nephropathy. We present strong in vivo evidence for activation of renal PRR and intrarenal RAS during AO in rats. Administration of PRR decoy inhibitor PRO20 exerts renoprotective action against AO-induced nephropathy associated with suppressed intrarenal RAS. Overall, these results support therapeutic potential of PRO20 for management of proteinuric CKD.
GRANTS
This work was supported by National Natural Science Foundation of China Grant Nos. 31330037, 81570377, 91439205, and 81630013; Beijing Novo Nordisk Pharmaceuticals Science & Technology; National Institutes of Health Grants DK-104072, HL-139689, and HL-135851; and a VA Merit Review from the Department of Veterans Affairs. T. Yang is Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.F., M.D., L. Zhang, A.L., J.S., C.X., L. Zhou, and L.W. performed experiments; H.F., M.D., L. Zhang, A.L., J.S., C.X., L. Zhou, L.W., and T.Y. analyzed data; H.F., J.-S.O., W.W., and T.Y. interpreted results of experiments; H.F. prepared figures; H.F. and T.Y. drafted manuscript; J.-S.O., W.W., and T.Y. edited and revised manuscript; T.Y. conceived and designed research; T.Y. approved final version of manuscript.
REFERENCES
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis 27: 765–775, 1996. doi: 10.1016/S0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Nussberger J, Stowasser M, Danser AH, Morganti A, Frandsen E, Ménard J. Activity assays and immunoassays for plasma Renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem 55: 867–877, 2009. doi: 10.1373/clinchem.2008.118000. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, Hou FF. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens 29: 1411–1421, 2011. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]
- 5.Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol Renal Physiol 300: F1310–F1318, 2011. doi: 10.1152/ajprenal.00119.2010. [DOI] [PubMed] [Google Scholar]
- 7.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 8.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463, 2010. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 9.Ding LH, Liu D, Xu M, Liu H, Wu M, Tang RN, Lv LL, Ma KL, Liu BC. Enalapril inhibits tubulointerstitial inflammation and NLRP3 inflammasome expression in BSA-overload nephropathy of rats. Acta Pharmacol Sin 35: 1293–1301, 2014. doi: 10.1038/aps.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang H, Xu C, Lu A, Zou CJ, Xie S, Chen Y, Zhou L, Liu M, Wang L, Wang W, Yang T. (Pro) renin receptor mediates albumin induced cellular responses: role of site-1 protease-derived soluble (pro) renin receptor in renal epithelial cells. Am J Physiol Cell Physiol 313: C632–C643, 2017. doi: 10.1152/ajpcell.00006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol 19: 743–748, 2008. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadola L, Noboa O, Márquez MN, Rodriguez MJ, Nin N, Boggia J, Ferreiro A, García S, Ortega V, Musto ML, Ponte P, Sesser P, Pizarrosa C, Ravaglio S, Vallega A. Calcium citrate ameliorates the progression of chronic renal injury. Kidney Int 65: 1224–1230, 2004. doi: 10.1111/j.1523-1755.2004.00496.x. [DOI] [PubMed] [Google Scholar]
- 13.Giese MJ, Speth RC. The ocular renin-angiotensin system: a therapeutic target for the treatment of ocular disease. Pharmacol Ther 142: 11–32, 2014. doi: 10.1016/j.pharmthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- 15.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Z, Wang H, Yang T. Microsomal prostaglandin E synthase 1 deletion retards renal disease progression but exacerbates anemia in mice with renal mass reduction. Hypertension 59: 122–128, 2012. doi: 10.1161/HYPERTENSIONAHA.111.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 18.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 19.Krop M, Lu X, Danser AH, Meima ME. The (pro)renin receptor. A decade of research: what have we learned? Pflugers Arch 465: 87–97, 2013. doi: 10.1007/s00424-012-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Largo R, Gómez-Garre D, Soto K, Marrón B, Blanco J, Gazapo RM, Plaza JJ, Egido J. Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension 33: 732–739, 1999. doi: 10.1161/01.HYP.33.2.732. [DOI] [PubMed] [Google Scholar]
- 21.Lee EK, Jeong JU, Chang JW, Yang WS, Kim SB, Park SK, Park JS, Lee SK. Activation of AMP-activated protein kinase inhibits albumin-induced endoplasmic reticulum stress and apoptosis through inhibition of reactive oxygen species. Nephron, Exp Nephrol 121: e38–e48, 2012. doi: 10.1159/000342802. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu BC, Gao J, Li Q, Xu LM. Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin Cim Acta 403: 23–30, 2009. doi: 10.1016/j.cca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Lott JA, Stephan VA, Pritchard KA Jr. Evaluation of the Coomassie Brilliant Blue G-250 method for urinary protein. Clin Chem 29: 1946–1950, 1983. [PubMed] [Google Scholar]
- 25.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui T, Yamagishi S, Ueda S, Fukami K, Okuda S. Irbesartan inhibits albumin-elicited proximal tubular cell apoptosis and injury in vitro. Protein Pept Lett 17: 74–77, 2010. doi: 10.2174/092986610789909430. [DOI] [PubMed] [Google Scholar]
- 28.Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol 13: 1179–1189, 2002. [PubMed] [Google Scholar]
- 29.Nakagawa T, Suzuki-Nakagawa C, Watanabe A, Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN, Suzuki F. Site-1 protease is required for the generation of soluble (pro)renin receptor. J Biochem 161: 369–379, 2017. doi: 10.1093/jb/mvw080. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen G. The (pro)renin receptor in health and disease. Ann Med 42: 13–18, 2010. doi: 10.3109/07853890903321567. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen G. Renin, (pro)renin and receptor: an update. Clin Sci (Lond) 120: 169–178, 2011. doi: 10.1042/CS20100432. [DOI] [PubMed] [Google Scholar]
- 32.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E2. Am J Physiol Renal Physiol 287: F152–F159, 2004. doi: 10.1152/ajprenal.00287.2003. [DOI] [PubMed] [Google Scholar]
- 33.Peng H, Li W, Seth DM, Nair AR, Francis J, Feng Y. (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS One 8: e58339, 2013. doi: 10.1371/journal.pone.0058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol 312: F245–F253, 2017. doi: 10.1152/ajprenal.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 51: 2–15, 1997. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 36.Saris JJ, ’t Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 37.Seki Y, Ichihara A, Mizuguchi Y, Sakoda M, Kurauchi-Mito A, Narita T, Kinouchi K, Bokuda K, Itoh H. Add-on blockade of (pro)renin receptor in imidapril-treated diabetic SHRsp. Front Biosci (Elite Ed) 2: 972–979, 2010. doi: 10.2741/e156. [DOI] [PubMed] [Google Scholar]
- 38.Su J, Liu X, Xu C, Lu X, Wang F, Fang H, Lu A, Qiu Q, Li C, Yang T.. NF-κB-dependent upregulation of (pro)renin receptor mediates high NaCl-induced apoptosis in mouse inner medullary collecting duct cells. Am J Physiol Cell Physiol 313: C612–C620, 2017. doi: 10.1152/ajpcell.00068.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takase O, Marumo T, Imai N, Hirahashi J, Takayanagi A, Hishikawa K, Hayashi M, Shimizu N, Fujita T, Saruta T. NF-kappaB-dependent increase in intrarenal angiotensin II induced by proteinuria. Kidney Int 68: 464–473, 2005. doi: 10.1111/j.1523-1755.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 40.Tang S, Leung JC, Abe K, Chan KW, Chan LY, Chan TM, Lai KN. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 111: 515–527, 2003. doi: 10.1172/JCI16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol 283: F640–F647, 2002. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- 42.Véniant M, Heudes D, Clozel JP, Bruneval P, Ménard J. Calcium blockade versus ACE inhibition in clipped and unclipped kidneys of 2K-1C rats. Kidney Int 46: 421–429, 1994. doi: 10.1038/ki.1994.290. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE2 Receptor EP4. J Am Soc Nephrol 27: 3022–3034, 2016. doi: 10.1681/ASN.2015050592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Liu H, Jia Z, Guan G, Yang T. Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese Zucker rats. PPAR Res 2010: 601562, 2010. doi: 10.1155/2010/601562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C, Fang H, Zhou L, Lu A, Yang T.. High potassium promotes mutual interaction between (pro)renin receptor and the local renin-angiotensin-aldosterone system in rat IMCD cells. Am J Physiol Cell Physiol 311: C686–C695 2016. doi: 10.1152/ajpcell.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu C, Lu A, Lu X, Zhang L, Fang H, Zhou L, Yang T. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension 69: 339–348, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu C, Lu A, Wang H, Fang H, Zhou L, Sun P, Yang T. (Pro)Renin receptor regulates potassium homeostasis through a local mechanism. Am J Physiol Renal Physiol 313: F641–F656, 2017. doi: 10.1152/ajprenal.00043.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature 468: 108–111, 2010. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zoja C, Benigni A, Remuzzi G. Cellular responses to protein overload: key event in renal disease progression. Curr Opin Nephrol Hypertens 13: 31–37, 2004. doi: 10.1097/00041552-200401000-00005. [DOI] [PubMed] [Google Scholar]