Abstract
Renal fibrosis is a common pathological feature in chronic kidney disease (CKD), including diabetic kidney disease (DKD) and obstructive nephropathy. Multiple microRNAs have been implicated in the pathogenesis of both DKD and obstructive nephropathy, although the overall role of microRNAs in tubular injury and renal fibrosis in CKD is unclear. Dicer (a key RNase III enzyme for microRNA biogenesis) was specifically ablated from kidney proximal tubules in mice via the Cre-lox system to deplete micoRNAs. Proximal tubular Dicer knockout (PT-Dicer KO) mice and wild-type (WT) littermates were subjected to streptozotocin (STZ) treatment to induce DKD or unilateral ureteral obstruction (UUO) to induce obstructive nephropathy. Renal hypertrophy, renal tubular apoptosis, kidney inflammation, and tubulointerstitial fibrosis were examined. Compared with WT mice, PT-Dicer KO mice showed more severe tubular injury and renal inflammation following STZ treatment. These mice also developed higher levels of tubolointerstitial fibrosis. Meanwhile, PT-Dicer KO mice had a significantly higher Smad2/3 expression in kidneys than WT mice (at 6 mo of age) in both control and STZ-treated mice. Similarly, UUO induced more severe renal injury, inflammation, and interstitial fibrosis in PT-Dicer KO mice than WT. Although we did not detect obvious Smad2/3 expression in sham-operated mice (2–3 mo old), significantly more Smad2/3 was induced in obstructed PT-Dicer KO kidneys. These results supported a protective role of Dicer-dependent microRNA synthesis in renal injury and fibrosis development in CKD, specifically in DKD and obstructive nephropathy. Depletion of Dicer and microRNAs may upregulate Smad2/3-related signaling pathway to enhance the progression of CKD.
Keywords: diabetic kidney disease, Dicer, fibrosis, microRNA, Smad2/3, unilateral urethral obstruction
INTRODUCTION
MicroRNAs (miRNAs) are small, noncoding RNAs that function as posttranscriptional regulators of gene expression. By binding to the 3′-untranslated region (3′-UTR) of target genes, miRNAs induce translational repression or mRNA degradation. The biogenesis of miRNAs involves Dicer, an RNase III enzyme that cleaves pre-microRNAs to produce functional, mature miRNAs. In kidneys, Dicer and associated microRNAs contribute to renal development, including the development of nephron and the differentiation of nephron progenitor (2–4). In addition, the ablation of Dicer in podocytes led to cytoskeletal dynamics alterations (5), glomerular pathologies, and proteinuria 4 to 5 wk after birth followed by animal death several weeks later (3). Interestingly, deletion of Dicer from renal proximal tubules protected against renal ischemia-reperfusion injury (6), but it did not have significant effects on cisplatin-induced nephrotoxicity (7), indicating that the pathological role of Dicer and related miRNAs in kidneys is disease type- or model-dependent. However, the overall role of miRNAs in chronic kidney disease (CKD) is not clear.
Diabetes and kidney obstruction are two important causes of CKD. Diabetic kidney disease (DKD) is currently the most common cause of CKD worldwide, accounting for 42.2% in 5,718 CKD patients in a recent single-center retrospective hospital-based study (8). Although DKD is traditionally considered as a glomerular disease, it has been recognized in recent years that tubulointerstitial injury is an important predictor of renal dysfunction in diabetes (9, 10), and tubulointerstitial pathologies contribute significantly to the development of DKD (11–13). In fact, the tubular hypertrophy and the severity of tubulointerstitial lesions (inflammation and fibrosis) are strongly correlated with the renal outcome in diabetes (14–16). Tubulointerstitial injuries, including cell apoptosis, tubule atrophy, inflammatory infiltration, and interstitial fibrosis, are also presumed to play a key role in obstructive nephropathy-related CKD progression (17–19).
miRNAs have been reported to play important roles in the pathogenesis of CKD (20–23). In diabetes, multiple miRNAs are induced in kidney tissues and have been shown to promote the pathological changes in kidneys, including renal fibrosis. For example, miR-192 is one of the first microRNAs identified to have an important role in renal fibrogenesis in DKD by regulation of TGF-β/Smad pathway (24). miR-192 was also shown to mediate TGF-β/Smad-driven renal fibrosis in the unilateral ureteral obstruction (UUO) (25). miR-214 was upregulated and promoted renal fibrosis during UUO (26). In contrast, miR-196a/b attenuated renal fibrosis by silencing TGF-β-Smad signaling in the UUO model (27). In addition, changes in the expression of miR-29 family members have been reported in diabetic and nondiabetic models of renal fibrosis (28–30). More recently, miR-21 was reported to target specific genes Smad7 and PTEN to induce fibrosis and other relevant pathologies in DKD (31–34). As such, manipulation of these microRNAs may effectively reduce renal fibrosis and slow down the progression of CKD (25, 26, 33, 35, 36).
We have established a proximal tubule-specific Dicer knockout (PT-Dicer KO) mouse model, which showed the depletion of microRNAs in kidney proximal tubules and was resistant to renal ischemia-reperfusion injury, demonstrating a critical role of Dicer and associated miRNAs in the pathogenesis of acute kidney injury (6). In this study, we will examine PT-Dicer KO mouse for its renal injury related to DKD and UUO to determine the role of Dicer-dependent microRNA synthesis in renal tubular injury, inflammation, and fibrosis in CKD condition. Our results show that compared with WT mice, PT-Dicer KO mice had a more severe renal injury, inflammation, and interstitial fibrosis following streptozotocin (STZ)-induced diabetes. Similarly, these mice developed more renal fibrosis in the model of UUO. Interestingly, there was a significant upregulation of Smad2/3 in PT-Dicer-KO kidney tissue, which may account for the sensitivity of these mice to renal fibrosis.
MATERIALS AND METHODS
Antibodies and reagents.
Primary antibodies were from the following sources: anticyclophilin B (ab16045), antimacrophage (ab56297), anti-α-SMA (ab5694), antiibronectin (ab2413), and anticollagen I (NBP1-30054) (Novus Biologicals). Secondary antibodies for immunoblot analysis were purchased from Jackson ImmunoResearch Laboratories.
Animals.
C57BL/6 mice were purchased from the Jackson Laboratory. A conditional knockout mouse model with Dicer specifically deleted from renal proximal tubules (PT-Dicer WT and PT-Dicer KO) was established and characterized in our laboratory Using Cre-loxP technology (6). Mice were housed in a standard animal facility in Charlie Norwood VA Medical Center under a 12:12-h light-dark pattern.
STZ treatment.
STZ dissolved in monohydrate Na citrate solution with pH 4.5 was given to male mice of 8-wk-old intraperitoneally at a dosage of 50 mg/kg 5 days consecutively. Mice injected monohydrate Na citrate solution served as a control. Body weight and blood glucose concentration were monitored before STZ injection and every 2 wk thereafter. Mice having fast blood glucose higher than 280 mg/dl were considered diabetic.
Albumin-to-creatinine ratio determination.
Urine was collected using metabolic cages and stored at −80°C until analysis. Urinary albumin and creatinine levels were determined using Albuwell M and creatinine companion kit, respectively (Exocell, Philadelphia, PA), according to the manufacturer’s instruction. Albumin-to-creatinine ratio (ACR) was calculated.
Mouse model of UUO.
UUO surgery was conducted in 8- to 10-wk-old male mice that were littermates and age-matched nonlittermates. Mice were anesthetized with pentobarbital sodium (50 mg/kg ip) and kept on a homeothermic blanket control unit (Harvard Apparatus) with a rectal probe to monitor and maintain body temperature at ~36.5°C. Mice were given buprenorphine (0.05 mg/kg) before surgery. The left ureter was isolated following left flank incision. The midureter was obstructed by two-point ligations with 4-0 silk sutures. Mice subjected to sham operation without obstruction of the left ureter were used as the control. Mice were euthanized at different time points (4 days, 1 wk, or 2 wk) following UUO for biochemical and histological analyses.
All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of Charlie Norwood Veterans Affairs Medical Center.
Immunohistochemistry analysis.
Collected kidney samples were fixed immediately in 4% paraformaldehyde. Four-micrometer-thick sections were used for immunohistochemistry analysis. After rehydration and antigen retrieval, slides were incubated in blocking buffer containing 2% BSA, 0.2% milk, 2% normal goat serum in PBS with 0.8% Triton X-100, followed by incubation with primary antibodies overnight at 4°C. Tissue sections were exposed to horseradish peroxidase-conjugated secondary antibody incubation (DAKO) secondary antibody for 1 h at room temperature. The slides were developed with VECTASTAIN Elite ABC kit and ImmPACT DAB peroxidase substrate (Vector Laboratories). Quantification analysis was performed using ImageJ.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay.
Four percent paraformaldehyde-fixed and paraffin-embedded kidney tissues were stained with in situ cell death detection kit (Roche Applied Science), according to the manufacturerʼs instruction. After mounting slides with ProLong Gold Antifade Reagent (Life Technologies), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells were counted from 10 random images for each specimen from the outer medulla and kidney cortex.
Real-time PCR.
Total RNA was isolated from frozen mouse kidney samples with mirVana miRNA isolation kit (Life Technologies) following the manufacturer’s instruction. One microgram of RNA was reverse transcribed using cDNA transcription kit (Bio-Rad), and RT-qPCR was performed using SYBR Green PCR Master Mix (Bio-Rad). β-actin was used for normalization in all experiments. The primer sequence of actin, Smad2, and Smad3 is as follows: β-actin primer 1: 5′-GAT TAC TGC TCT GGC TCC TAG-3′, β-actin primer 2: 5′-GAC TCA TCG TAC TCC TGC TTG-3′, Smad2 primer 1: 5′- GCA CTA TCA CTT AGG CAC TCA G-3′, Smad2 primer 2: 5′- CGA AAT GCC ACT GTA GAA ATG AC-3′, Smad3 primer 1: 5′- GCG GCA GTA GAT AAC GTG AG-3′, and Smad3 primer 2: 5′- GAA CAC CAA GTG CAT TAC CAT C-3′.
Statistics.
Differences between two groups were assessed using two-tailed t-tests. Differences between more than two groups were assessed by one-way ANOVA using GraphPad. Data were expressed as means ± SD. P values <0.05 were considered to be significant.
RESULTS
Dicer deficiency in proximal tubules aggravates renal tubular injury and interstitial inflammation in diabetic mice.
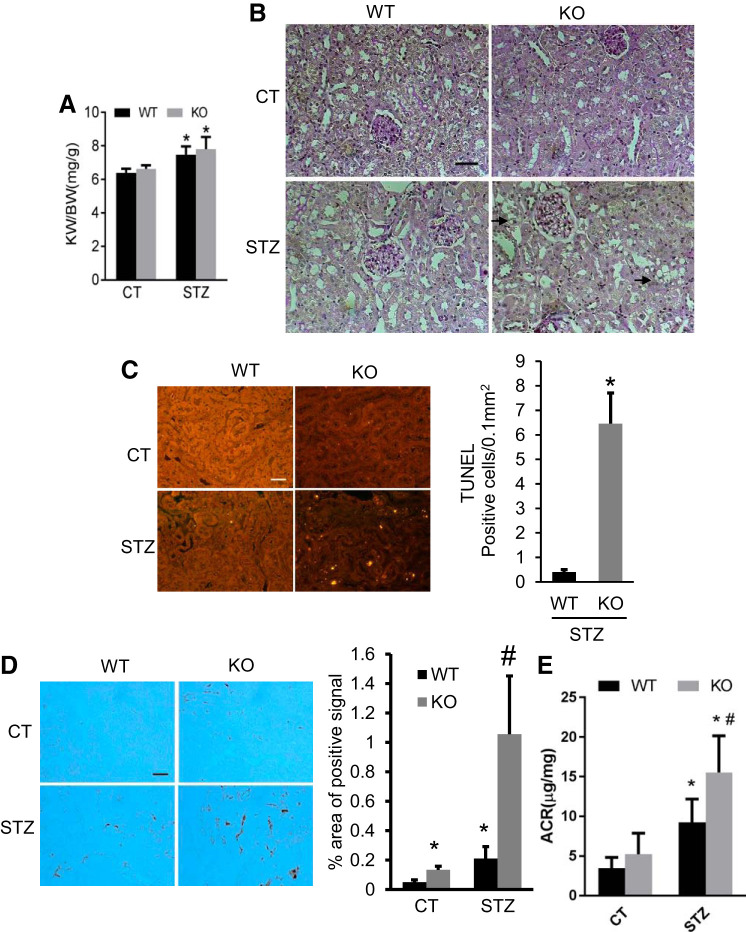
In our previous work, we established the proximal tubule-specific Dicer knockout (PT-Dicer KO) mouse model, which showed >80% microRNAs depletion in the cortex and outer medulla (6). We used this model to examine the role of Dicer and associated microRNAs in STZ-induced DKD. The successful induction of diabetes was confirmed by biweekly blood glucose measurement. Consistent with previous studies (37–39), renal hypertrophy was detected by significant increases in kidney size shown by higher kidney weight/body weight (KW/BW) ratio at 16 wk after STZ treatment (Fig. 1A). Of note, the KW/BW ratio was not significantly different between WT and PT-Dicer KO diabetic mice, indicating that ablation of Dicer from proximal tubules did not affect renal hypertrophy in STZ-induced DKD. The diabetic nephropathy was shown as obvious loss of brush border in kidney proximal tubules in both WT and PT-Dicer KO diabetic mice by periodic acid-Schiff (PAS) staining (Fig. 1B). In addition to the loss of brush border, PT-Dicer KO mice also showed vacuolization in tubular cells, indicating more severe injury in this group of mice (Fig. 1B).
Fig. 1.
Dicer deficiency in proximal tubules aggravates renal tubular injury and interstitial inflammation in diabetic mice. Eight weeks old proximal tubule (PT)-Dicer wild-type (WT) and knockout (KO) mice were treated with streptozotocin (STZ) or without STZ (Control, CT) and examined after 16 wk. A: kidney weight/body weight. Data are expressed as means ± SD. B: representative images of periodic acid-Schiff (PAS) staining showing the vacuolization (arrowheads) in tubules in PT-Dicer KO diabetic. Scale bar: 50 µm. C: representative images of TUNEL assay and TUNEL-positive signal counting showing more apoptotic tubular cells in renal cortex and outer medulla in PT-Dicer KO diabetic compared with PT-Dicer WT diabetic mice. D: representative images of immunohistochemistry staining of macrophage showing more obvious macrophage infiltration in kidney cortex from PT-Dicer KO diabetic mice. E: urine albumin-to-creatinine ratio (ACR). n ≥ 7.*P < 0.05, vs. WT-Control (WT-CT); #P < 0.05, vs. STZ-treated PT-Dicer WT mice.
As PT-Dicer KO will first affect proximal tubular cells, we further tested renal tubular apoptosis in diabetic condition by TUNEL staining (Fig. 1C). Few TUNEL-positive cells were detected in wild-type and knockout mouse kidneys under the control condition (Fig. 1C). After STZ treatment, WT kidneys showed a few apoptotic cells mainly in renal tubules, and the number of apoptotic cells was remarkably increased in PT-Dicer KO diabetic kidneys (Fig. 1C).
Inflammation is another major pathology in DKD, which is closely related to apoptosis. We analyzed macrophage infiltration by immunohistochemistry staining to indicate interstitial inflammation. As shown in Fig. 1D, macrophages were rarely seen in the kidneys of control WT mice, but some macrophages were detected in the kidneys of control PT-Dicer KO mice. At 16 wk after diabetic induction, the number of macrophages obviously increased in WT mice kidneys, and the increase was even more in PT-Dicer KO diabetic kidneys, implicating that Dicer deficiency in proximal tubules can significantly promote interstitial inflammation, especially in the diabetic condition.
Consistent with these histopathological changes, STZ-treated mice showed an increase of urine ACR as compared with nondiabetic controls, and the increase was significantly higher in PT-Dicer KO diabetic mice (Fig. 1E). Together, these results indicate that PT-Dicer KO mice experience more severe diabetic renal injury than WT mice.
Dicer deficiency in proximal tubules aggravates renal interstitial fibrosis in diabetic mice.
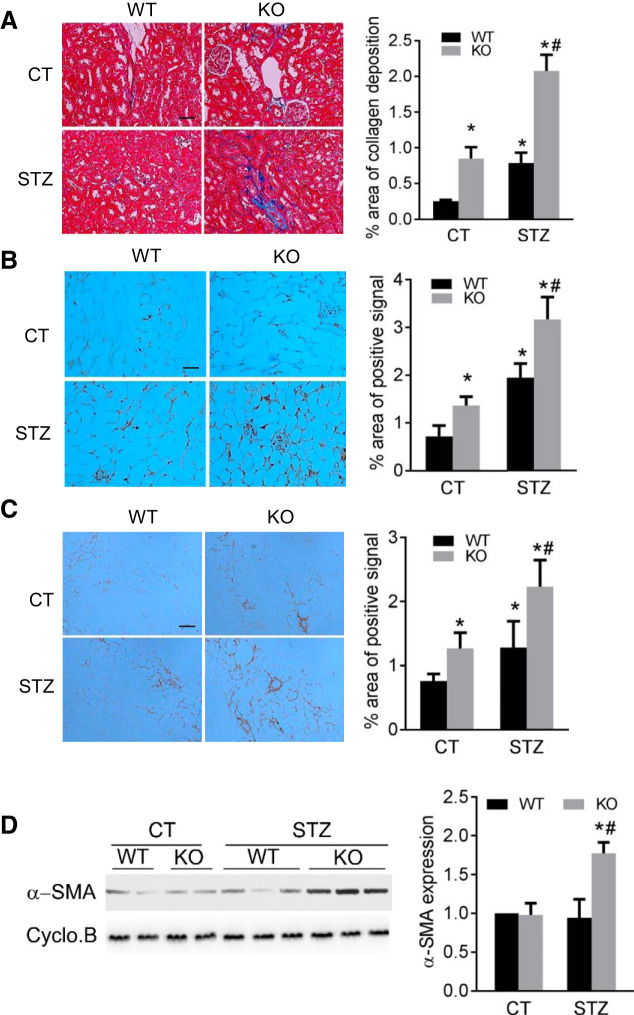
To further examine the effect of PT-Dicer KO on diabetic nephropathy, we determined renal interstitial fibrosis in these mice after STZ treatment. Masson’s trichrome staining indicated mostly negative collagen deposition in the kidneys of control WT mice, while some tubulointerstitial staining (blue staining) was detected in PT-Dicer KO kidneys, showing some collagen deposition (Fig. 2A). At 16 wk after STZ treatment, WT mouse kidneys showed induction of collagen deposition in renal interstitium, and even more collagen deposition was detected in PT-Dicer KO kidneys (Fig. 2A). Quantification by morphometric analysis showed that there was twofold more collagen deposition in STZ-treated Dicer KO mice than in STZ-treated WT mice kidneys. Consistently, immunohistochemistry staining detected significantly higher interstitial accumulation of fibronectin in kidneys of PT-Dicer KO mice than that of WT mice (Fig. 2B). Similarly, accumulation of collagen I in kidney tissues was also higher in PT-Dicer KO mice by immunohistochemistry analysis (Fig. 2C). In addition, at 16 wk after STZ-treatment, α-SMA (myofibroblast marker protein) was significantly induced in PT-Dicer KO mouse kidneys, but not in WT mouse kidneys (Fig. 2D). Together, these results indicate that ablation of Dicer from proximal tubules significantly enhances renal interstitial fibrosis in diabetic kidneys.
Fig. 2.
Dicer deficiency in proximal tubules aggravates renal interstitial fibrosis in diabetic mice. Eight-week-old proximal tubule (PT)-Dicer wild-type (WT) and PT-Dicer knockout (KO) mice were treated with streptozotocin (STZ) or without STZ (Control, CT) and examined after 16 wk. A: representative images of Masson trichrome staining and quantification of collagen deposition area ratio. Scale bar: 50 µm. B: representative images of immunohistochemistry staining of fibronectin and quantification of fibronectin staining positive area percentage. Scale bar: 50 µm. C: representative images of immunohistochemistry staining of collagen I- and quantification of collagen I-positive area percentage. Scale bar: 50 µm. D: representative immunoblots and densitometry quantification showing a significant increase of α-SMA in kidney cortex samples from PT-Dicer KO mice compared with WT diabetic mice. Cyclophilin B was used as loading control. n ≥ 7. *P < 0.05, vs. WT-CT; #P < 0.05, vs. PT-Dicer WT diabetic mice.
Dicer deficiency in proximal tubules aggravates renal tubular injury and interstitial inflammation in UUO.
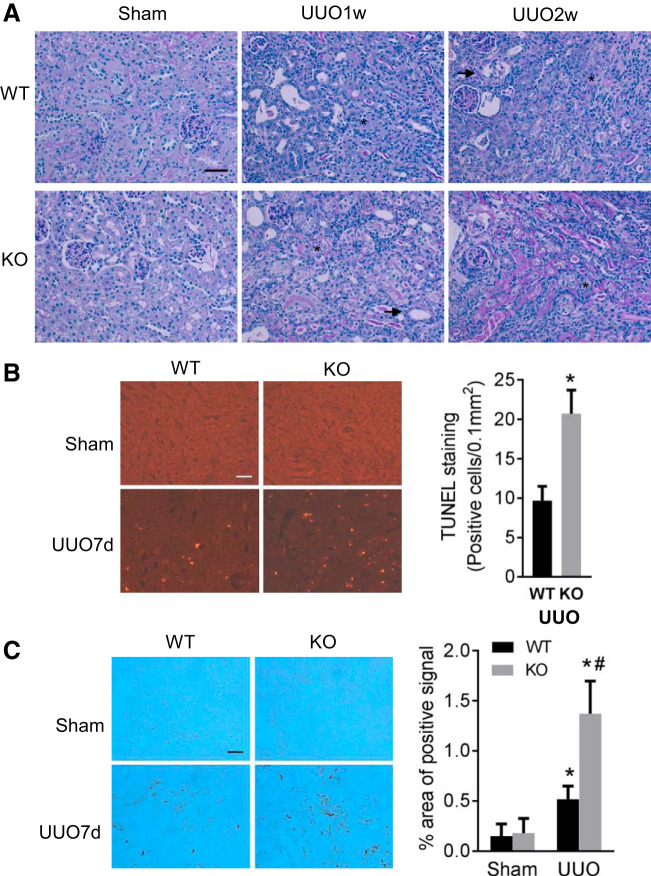
To further confirm the role of Dicer and associated microRNAs in CKD, we tested another CKD model of UUO with PT-Dicer KO mice. As shown in Fig. 3A, compared with sham controls, both WT and PT-Dicer KO mice after 1–2 wk of UUO showed significant decreases in normal healthy kidney proximal tubules with brush border shown by PAS staining. Some tubules became atresia (see arrowheads in Fig. 3A), indicating tubular degeneration, while others were dilated with thin epithelium devoid of brush borders at 1–2 wk of UUO (asterisks), indicating tubular atrophy. Meanwhile, the peritubular interstitium was expanded and filled with infiltrated cells. In general, these signs of tubular degeneration and atrophy were more severe in PT-Dicer KO mice kidneys (Fig. 3A).
Fig. 3.
Dicer deficiency in proximal tubules aggravates renal tubular injury and interstitial inflammation in unilateral ureteral obstruction (UUO). Proximal tubule (PT)-Dicer wild-type (WT) and PT-Dicer knockout (KO) mice were subjected to either sham operation (Sham) or UUO surgery. Injured mice were euthanized 1 wk (UUO1w) or 2 wk (UUO2w) after UUO, and left kidneys were collected for histological and immunoblotting analyses. A: representative images of Periodic acid-Schiff (PAS) staining showing more severe renal injury in PT-Dicer KO mice after UUO. Scale bar: 50 µm. Arrowheads and asterisks stand for atrophic and atresic tubules, respectively. B: representative images of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining and quantification of TUNEL-positive signals showing more apoptotic tubular cells in renal cortex and outer medulla in PT-Dicer KO mice compared with PT-Dicer WT after UUO injury. Scale bar: 50 µm. *Statistically significant difference compared with WT group. C: representative images of immunohistochemistry staining of macrophage showing more macrophage infiltration in kidney cortex in PT-Dicer KO mice compared with WT mice after UUO injury. Scale bar: 50 µm. n ≥ 10. *P < 0.05, vs. WT-sham; #P < 0.05, vs. PT-Dicer WT UUO.
We further examined renal apoptosis and inflammation during UUO. Few TUNEL-positive cells were detected in sham-operated kidneys. Following UUO, the number of apoptotic tubular cells was significantly increased in WT kidneys, and the increase was more in PT-Dicer KO. Quantification of TUNEL-positive cells further verified the increase in apoptosis following UUO in PT-Dicer KO mice (Fig. 3B). UUO-induced interstitial inflammation was assessed by immunohistochemistry analysis of macrophage infiltration. As shown in Fig. 3C, macrophage was rarely seen in sham operation mice regardless of genotype. However, UUO induced obvious peritubular accumulation of macrophages in both WT and PT-Dicer KO mice, with the latter showing much more intense staining. Quantitatively, the area of tubulointerstitial macrophage infiltration in PT-Dicer KO mice was more than twofold higher than in wild-type mice. Together, these studies provide evidence that Dicer deficiency in proximal tubules promotes kidney interstitial inflammation and apoptotic injury during UUO, similar as in DKD.
Dicer deficiency in proximal tubules aggravates renal interstitial fibrosis in UUO.
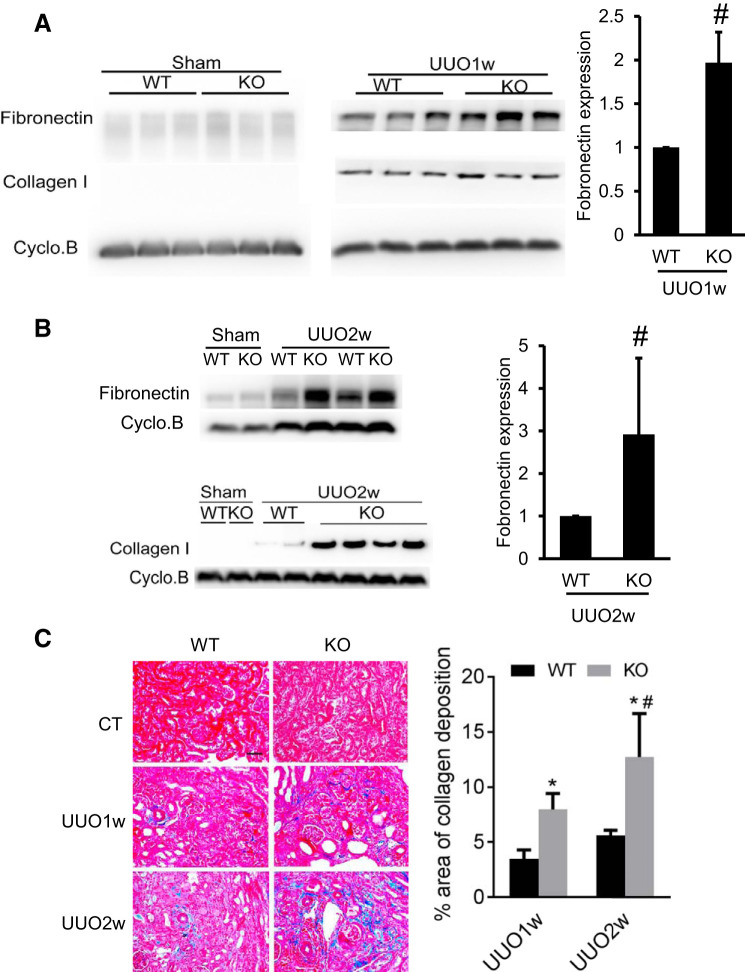
We further examined the effect of PT-Dicer KO on renal fibrosis in the UUO model. As shown in Fig. 4A, compared with sham control mice, fibronectin was induced in WT kidney tissues following 1 wk of UUO, and the induction was much more remarkable in PT-Dicer KO kidneys. UUO also induced collagen I expression in kidney tissues of both WT and PT-Dicer KO mice, but the difference between WT and PT-Dicer KO group was marginal. However, at 2 wk of UUO, the induction of fibronectin and collagen I was more prominent in PT-Dicer KO mice (Fig. 4B). We further examined kidney fibrosis by Masson’s trichrome staining (Fig. 4C). Obviously, UUO induced tubulointerstitial collagen staining in both WT and PT-Dicer KO mice kidneys, but more collagen deposition was detected in KO tissues. In the morphometric analysis, 1 and 2 wk of UUO induced 3.5% and 5.6% interstitial fibrosis in WT kidneys, which was significantly increased to 8% and 12.7% in PT-Dicer KO kidneys, respectively. Collectively, these results indicate that Dicer deficiency in proximal tubules promotes renal injury, along with the progression of renal interstitial fibrosis during UUO.
Fig. 4.
Dicer deficiency in proximal tubules aggravates renal interstitial fibrosis in unilateral ureteral obstruction (UUO). Proximal tubule (PT)-Dicer wild-type (WT) and PT-Dicer knockout (KO) mice were subjected to either sham operation (sham) or UUO surgery. Mice were euthanized at 1 wk (UUO1w) or 2 wk (UUO2w) after UUO injury, and left kidneys were collected for histological and immunoblotting analyses. A: representative Immunoblots showing more fibronectin induced in kidney cortex from PT-Dicer KO mice at 1 wk after UUO. Cyclophilin B was used as a loading control. B: representative immunoblots showing more induction of fibronectin and collagen I in PT-Dicer KO mice compared with PT-Dicer WT at 2 wk after UUO. Cyclophilin B was used as a loading control. D: representative images of Masson Trichrome staining and quantification of collagen deposition area. Scale bar = 50 µm. n ≥ 10.*P < 0.05, vs. WT-sham; #P < 0.05, vs. PT-Dicer WT UUO.
Increased Smad2/3 expression in PT-Dicer KO kidney may contribute to enhanced renal interstitial fibrosis in diabetic and UUO nephropathy.
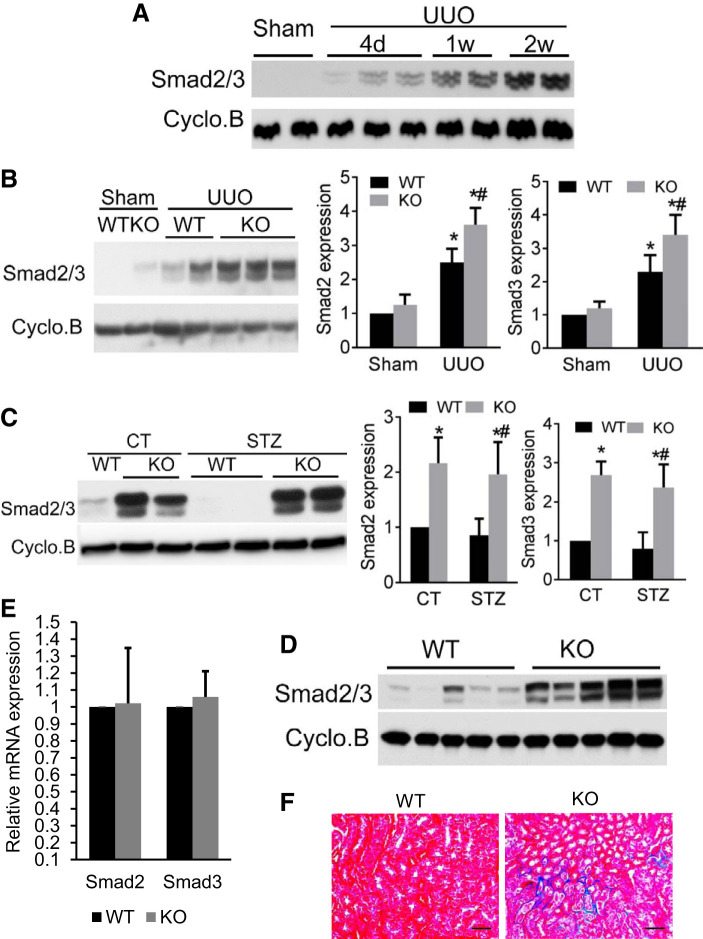
The TGF-β signaling pathway is known to play an important role in renal fibrogenesis (1, 40–42). In this pathway, Smad2/3 is the critical mediator molecules that relay TGFβ signals from the cytoplasm into the nucleus. Similar to the report that Smad2 and Smad3 were induced in UUO and human fibrotic kidneys (43, 44), we detected an increase in Smad2/3 expression during UUO in a time-dependent manner from 4 days to 2 wk (Fig. 5A).
Fig. 5.
Increased Smad2/3 expression in proximal tubule (PT)-Dicer knockout (KO) kidney may contribute to enhanced renal interstitial fibrosis in diabetic and unilateral ureteral obstruction (UUO) nephropathy. A: representative immunoblots of Smad2/3 in mouse kidney. C57BL/6 mice were subjected to either sham operation or UUO surgery. Mice were euthanized at 4 days (UUO4d), 1 wk (UUO1w), or 2 wk (UUO2w) after surgery, and left kidneys were collected for immunoblotting analyses of Smad2/3. Cyclophilin B was used as loading control. Representative immunoblots are presented. The results showed a time-dependent increase of Smad2/3 in kidney cortex following UUO. B: representative immunoblots and densitometry quantification of Smad2/3 levels. PT-Dicer wild-type (WT) and PT-Dicer KO mice were subjected to either sham operation or UUO surgery. Mice were euthanized at 1 wk after surgery, and left kidneys were collected for immunoblot analysis of Smad2/3 expression and densitometry quantification. The results showed the significantly increased Smad2/3 in kidney cortex in both PT-Dicer WT and PT-Dicer KO mice subjected to UUO compared with sham controls. The levels of Smad2/3 were significantly higher in PT-Dicer KO mice than in PT-Dicer WT mice after UUO. n ≥ 10. *P < 0.05, vs WT-sham; #P < 0.05, vs. PT-Dicer WT UUO. Cyclophilin B was used as loading control. C: representative immunoblots and densitometry quantification of Smad2/3 levels. Eight-week-old PT-Dicer WT and PT-Dicer KO mice were treated with streptozotocin (STZ) or without STZ (Control, CT) and examined after 16 wk. The results showed that Smad2/3 levels were significantly elevated in PT-Dicer KO mice compared with PT-Dicer WT mice in both control and diabetic mice. n ≥ 7. *P < 0.05, vs. WT-CT; #P < 0.05, vs. PT-Dicer WT diabetic mice. Cyclophilin B was used as loading control. D: representative immunoblot of Smad2/3. Left kidneys from 6-mo-old PT-Dicer WT and PT-Dicer KO mice were collected for immunoblot analyses. The results showed the remarkably increased Smad2/3 levels in PT-Dicer KO kidney cortex than in WT cortex. Cyclophilin B was used as loading control. E: Smad2 and Smad3 mRNA levels in the renal cortex in 6-mo-old PT-Dicer KO mice by real-time PCR; n = 5. No significant difference in Smad2/3 mRNA levels was found between WT and PT-Dicer KO mice under the control condition. F: representative images of Masson Trichrome staining of kidney samples from 7-mo-old PT-Dicer WT and PT-Dicer KO mice showing obvious deposition and accumulation of collagen in PT-Dicer KO mice compared with PT-Dicer WT mice; n = 6. Scale bar: 50 µm.
To determine whether PT-Dicer KO may affect Smad2/3 in diabetes and UUO-related renal fibrosis, we analyzed Smad2/3 expression in WT and PT-Dicer KO mice. Consistent with Fig. 5A, Smad2/3 levels were significantly increased 7 days following UUO, both in WT and in PT-Dicer KO mice. Notably, the Smad2/3 levels were significantly higher in PT-Dicer KO mice than in WT mice following UUO. However, when we examined the expression of Smad2/3 in STZ-treated mice, we also noticed that PT-Dicer KO mouse kidneys had remarkably higher levels of Smad2/3 expression than WT, even in the control condition. After STZ treatment, Smad2/3 maintained at high level in PT-Dicer KO kidney, although we did not detect obvious induction of Smad2/3 in WT kidneys. The possible reason is that Smad2/3 may have age-related enhancement, especially in PT-Dicer KO kidney, as mice we examined in DKD model were a 3–4 mo older than in the UUO model. To further confirm the effect of Dicer deficiency on Smad2/3 expression, we compared WT and PT-Dicer KO littermate mice without treatment at age of 6 mo old. As shown in Fig. 5D, compared with the WT mice, significant higher Smad2/3 expression was observed in PT-Dicer KO mice. To investigate whether the increased Smad2/3 proteins expression in PT-Dicer KO mice was the result of increased gene transcription or not, we did real-time PCR to test the mRNA levels in 6-mo-old mice under control conditions. As shown in Fig. 5E, Smad2 and Smad3 mRNA levels in 6-mo-old PT-Dicer KO mice were not significantly different than in the age-matched PT-Dicer WT mice under control conditions, suggesting that Smad2/3 upregulation in PT-Dicer KO mice is mainly due to the loss of miRNA. To further clarify the role of increased Smad2/3 expression in renal interstitial fibrosis, Masson trichrome staining was performed in 7-mo-old WT and PT-Dicer KO littermate mice. As shown in Fig. 5F, no collagen deposition was seen in kidney tissues of WT mice, but obvious peritubular collagen deposition stained in blue was observed in PT-Dicer KO mice kidneys. Thus, Dicer deficiency in proximal tubules leads to age-related upregulation of Smad2/3, sensitizing the kidneys to injury and tubulointerstitial fibrosis.
DISCUSSION
MicroRNAs are important regulators in CKD, including renal fibrosis in DKD, UUO, and others. While some microRNAs may be beneficial for the kidney and protective against renal diseases, others may be pathogenic. Despite these studies, the role of microRNAs as a whole class of regulatory molecules in CKD has not been reported. In the current study using a proximal tubule-specific Dicer knockout mouse model, we examined the role of miRNA depletion in renal cell death, inflammation, and fibrosis in CKD. Our results show that suppression of Dicer and associated microRNAs in these mice significantly aggravated renal tubular apoptosis, macrophage infiltration, and interstitial fibrosis in both models of STZ-induced DKD and UUO treatment. Mechanistically, PT-Dicer KO mice expressed significantly more Smad2/3 in kidneys in an age-dependent manner, providing an explanation for the sensitivity of these mice to injury and fibrosis.
In the present study, the suppression of Dicer and associated microRNAs in PT-Dicer diabetic mice did not show much effect on renal hypertrophy. The downregulated miRNAs included both known renal hypertrophy promotion and renal hypertrophy inhibition-related miRNAs. For example, miR-192 and miR-214 were upregulated and promoted renal hypertrophy during diabetes (35, 45, 46). miR-455-3p was downregulated in high glucose-treated human mesangial cells and human proximal tubule epithelial cells; miR-455-3p overexpression improved the pathological changes of glomerular hypertrophy (47). Thus, their effects may be neutralized by each other, leading to no obvious alterations in renal hypertrophy in PT-Dicer KO diabetic mice relative to WT diabetic mice. Moreover, miRNA depletion was not complete, and some miRNAs were even upregulated in PT-Dicer KO kidney (6), which may provide another possible explanation for this phenomenon.
Of note, lacking Dicer in proximal tubules aggravated the renal tubular injury and interstitial inflammation in diabetes, despite an inability to alter renal hypertrophy, as demonstrated by more apoptotic tubular cells and more macrophage infiltration in these mice. Meanwhile, the expression of α-SMA, ECM deposition, and renal interstitial fibrosis was increased in PT-Dicer KO diabetic mice. In agreement with our results, it has been widely reported that α-SMA expression was upregulated, and obvious fibrosis observed in diabetic kidneys compared with the controls (48–51). Consistent with the deteriorated histological changes and increased tubulointerstitial fibrosis, diabetic mice with Dicer deleted from the proximal tubules showed higher ACR level than the WT diabetic mice, suggesting Dicer deficiency in the proximal tubules accelerated the progression of diabetic nephropathy.
Dicer deletion in proximal tubules increased renal tubular injury and interstitial inflammation in the UUO model as well. In addition, PT-Dicer KO mice showed more remarkable atresia and atrophy compared with WT mice following UUO. In agreement with these pathological changes and the increase in tubular cell apoptosis, the fibronectin expression, and collagen deposition were enhanced in PT-Dicer KO mice following UUO. Altogether, Dicer deficiency in proximal tubules may aggravate the common renal pathology in CKD condition, including renal apoptosis, inflammation, and fibrosis.
TGF-β/Smad signaling pathway plays an important role in regulating renal fibrosis in CKD condition (1, 52, 53). Smad2 and Smad3, two important downstream signaling mediators, are phosphorylated and activated by the TGF-β receptor after its binding to TGF-β. Phosphorylated Smad2/3 bind to Smad4 proteins, form heterooligomeric complexes, and translocate into the nuclei, inducing the transcription of fibrotic genes. The activation of Smad2 and Smad3 is observed in obstructed mouse kidneys (54, 55), Type 1 diabetes patients, and diabetic mice (56–58), as illustrated by the increase in phosphorylated Smad2 (p-Smad2) and phosphorylated Smad3 (p-Smad3) expression.
In addition to the increases in phosphorylated Smad2 (p-Smad2) and Smad3 (p-Smad3) observed in diabetes (56–58), it was reported that Smad2/3 expression was upregulated relative to controls in diabetic kidneys in mice accompanied by obvious fibrosis (48, 50, 59). In our studies, Smad2/3 expression was remarkably greater in PT-Dicer KO diabetic mice compared with nondiabetic and PT-Dicer WT diabetic controls. Correspondingly, renal fibrosis was remarkably greater in PT-Dicer KO diabetic mice than in WT diabetic mice. Even though we did not detect obvious Smad2/3 in relatively younger mice in the UUO model, Smad2/3 expression was significantly higher in PT-Dicer WT, PT-Dicer KO, and C57BL/6 mice following UUO injury compared with sham control mice. Additionally, the induction of Smad2/3 was significantly higher in PT-Dicer KO mice than in PT-Dicer WT mice following UUO. Consistent with the changes in Smad2/3, renal fibrosis was much more prominent in PT-Dicer KO mice than in PT-Dicer WT mice. All of these results imply the strong correlation between the levels of Smad2/3 and the severity of renal fibrosis in both diabetes and UUO, showing the necessary pathogenesis role of Smad2/3 in these models. Meanwhile, the increase in Smad2/3 expression in proximal tubules is sufficient to induce renal fibrosis, even without treatment in PT-Dicer KO mice, suggesting an essential role of Dicer and miRNAs in maintaining the renal structure and the health and physiology in normal kidneys, and further confirming the pivotal fibrogenesis role of Smad2/3 in diabetic or obstructive kidneys in PT-Dicer KO mice.
This difference in Smad2/3 protein expression is attributable to the difference in age of the mice. Meanwhile, older mice showed renal injury and interstitial fibrosis, which was not detectable in young mice. These findings suggest that Dicer may play a role in aging-related kidney injury. The possibility that Dicer deficiency may be associated with the vulnerability of aging kidneys to injury, as well as influencing the course of chronic kidney disease, needs to be investigated.
Smad2/3 upregulation in PT-Dicer KO mice was not paralleled by the increases in mRNA levels (Fig. 5E), suggesting that Smad2/3 upregulation in these mice is mainly attributable to posttranscriptional mechanisms, including miRNAs. It has been shown that Smad2 and Smad3 can be regulated by microRNAs. For example, miR-542-3p, miR-26a, and miR-486-5p are reported to target Smad2 (60–62), and miR-637, miR-16-5p, and miR-145 target Smad3 (63–66). However, microRNAs that can simultaneously regulate Smad2/3 expression remain largely unclear. So far, miR-136, miR-323-3p, and miR-146a are reported to directly downregulate both Smad2 and Smad3 (67–69). The potential miRNAs that upregulated the expression of Smad2/3 in PT-Dicer KO mice need to be identified in the future.
Collectively, Dicer deficiency in proximal tubules induced Smad2/3 expression and enhanced the activity of TGF-β/Smad signaling pathway, increased tubular cell death, and enhanced inflammation, thus promoting final renal interstitial fibrosis in diabetic and obstructive nephropathy. These data present a key role for Dicer and associated miRNAs in the regulation of renal tubular injury and extracellular matrix accumulation and provide important insights into the molecular pathways implicated in the progression of diabetic and obstructive nephropathy. Targeting the potential miRNAs that can regulate Smad2/3 expression and fibrosis progression may represent a novel and effective strategy for the treatment of diabetic and obstructive nephropathy.
GRANTS
The work was supported partly by the grants from National Natural Science Foundation of China (81430017), the National Institutes of Health, and the U.S. Department of Veterans Affairs. Z. Dong was a recipient of Senior Career Scientist award from U.S. Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.M. and Z.D. conceived and designed research; Z.M. performed experiments; Z.M. and Z.D. analyzed data; Z.M., Q.W., M.Z., J.-K.C., and Z.D. interpreted results of experiments; Z.M. prepared figures; Z.M. drafted manuscript; Z.M., Q.W., M.Z., J.-K.C., and Z.D. edited and revised manuscript; Z.M., Q.W., M.Z., J.-K.C., and Z.D. approved final version of manuscript.
REFERENCES
- 1.Afroz T, Sagar R, Reddy S, Gandhe S, Rajaram KG. Clinical and histological correlation of diabetic nephropathy. Saudi J Kidney Dis Transpl 28: 836–841, 2017. [PubMed] [Google Scholar]
- 2.Bera A, Das F, Ghosh-Choudhury N, Mariappan MM, Kasinath BS, Ghosh Choudhury G. Reciprocal regulation of miR-214 and PTEN by high glucose regulates renal glomerular mesangial and proximal tubular epithelial cell hypertrophy and matrix expansion. Am J Physiol Cell Physiol 313: C430–C447, 2017. doi: 10.1152/ajpcell.00081.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt K, Kato M, Natarajan R. Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 310: F109–F118, 2016. doi: 10.1152/ajprenal.00387.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 187: 251–259, 1991. doi: 10.1016/S0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, Chen DQ, Vaziri ND, Zhao YY. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother 101: 670–681, 2018. doi: 10.1016/j.biopha.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KS, Sposito N, Stumpf PS, Wilson DI, Sanchez-Elsner T, Oreffo RO. MicroRNA-146a regulates human foetal femur derived skeletal stem cell differentiation by down-regulating SMAD2 and SMAD3. PLoS One 9: e98063, 2014. doi: 10.1371/journal.pone.0098063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016. doi: 10.1152/ajprenal.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier RL, Thornhill BA, Forbes MS, Kiley SC. Mechanisms of renal injury and progression of renal disease in congenital obstructive nephropathy. Pediatr Nephrol 25: 687–697, 2010. doi: 10.1007/s00467-009-1316-5. [DOI] [PubMed] [Google Scholar]
- 9.Chu JY, Sims-Lucas S, Bushnell DS, Bodnar AJ, Kreidberg JA, Ho J. Dicer function is required in the metanephric mesenchyme for early kidney development. Am J Physiol Renal Physiol 306: F764–F772, 2014. doi: 10.1152/ajprenal.00426.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-β/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung AC, Huang XR, Zhou L, Heuchel R, Lai KN, Lan HY. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant 24: 1443–1454, 2009. doi: 10.1093/ndt/gfn699. [DOI] [PubMed] [Google Scholar]
- 12.Denby L, Ramdas V, Lu R, Conway BR, Grant JS, Dickinson B, Aurora AB, McClure JD, Kipgen D, Delles C, van Rooij E, Baker AH. MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol 25: 65–80, 2014. doi: 10.1681/ASN.2013010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande S, Abdollahi M, Wang M, Lanting L, Kato M, Natarajan R. Reduced autophagy by a microRNA-mediated signaling cascade in diabetes-induced renal glomerular hypertrophy. Sci Rep 8: 6954, 2018. doi: 10.1038/s41598-018-25295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao J, Lou Q, Wei Q, Mei S, Li L, Wu G, Mi QS, Mei C, Dong Z. MicroRNA-375 is induced in cisplatin nephrotoxicity to repress hepatocyte nuclear factor 1-β. J Biol Chem 292: 4571–4582, 2017. doi: 10.1074/jbc.M116.754929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of Dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol 27: 901–909, 2012. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Høj Thomsen L, Fog-Tonnesen M, Nielsen Fink L, Norlin J, de Vinuesa AG, Krarup Hansen T, de Heer E, Ten Dijke P, Rosendahl A. Smad2 phosphorylation in diabetic kidney tubule epithelial cells is associated with modulation of several transforming growth factor-β family members. Nephron 135: 291–306, 2017. doi: 10.1159/000453337. [DOI] [PubMed] [Google Scholar]
- 18.Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-β1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 158: 1653–1663, 2001. doi: 10.1016/S0002-9440(10)64121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Sun P, Lei Z, Li M, Wang Y, Zhang HT, Liu J. miR-145 inhibits invasion and metastasis by directly targeting Smad3 in nasopharyngeal cancer. Tumour Biol 36: 4123–4131, 2015. doi: 10.1007/s13277-015-3046-6. [DOI] [PubMed] [Google Scholar]
- 20.Iervolino A, Trepiccione F, Petrillo F, Spagnuolo M, Scarfò M, Frezzetti D, De Vita G, De Felice M, Capasso G. Selective Dicer suppression in the kidney alters GSK3β/β-catenin pathways promoting a glomerulocystic disease. PLoS One 10: e0119142, 2015. doi: 10.1371/journal.pone.0119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inazaki K, Kanamaru Y, Kojima Y, Sueyoshi N, Okumura K, Kaneko K, Yamashiro Y, Ogawa H, Nakao A. Smad3 deficiency attenuates renal fibrosis, inflammation,and apoptosis after unilateral ureteral obstruction. Kidney Int 66: 597–604, 2004. doi: 10.1111/j.1523-1755.2004.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6: 395–423, 2011. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kölling M, Kaucsar T, Schauerte C, Hübner A, Dettling A, Park JK, Busch M, Wulff X, Meier M, Scherf K, Bukosza N, Szénási G, Godó M, Sharma A, Heuser M, Hamar P, Bang C, Haller H, Thum T, Lorenzen JM. Therapeutic miR-21 silencing ameliorates diabetic kidney disease in mice. Mol Ther 25: 165–180, 2017. doi: 10.1016/j.ymthe.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 7: 1056–1067, 2011. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Qu X, Yao J, Caruana G, Ricardo SD, Yamamoto Y, Yamamoto H, Bertram JF. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59: 2612–2624, 2010. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Chung AC, Yu X, Lan HY. MicroRNAs in diabetic kidney disease. Int J Endocrinol 2014: 593956, 2014. doi: 10.1155/2014/593956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang G, Song L, Chen Z, Qian Y, Xie J, Zhao L, Lin Q, Zhu G, Tan Y, Li X, Mohammadi M, Huang Z. Fibroblast growth factor 1 ameliorates diabetic nephropathy by an anti-inflammatory mechanism. Kidney Int 93: 95–109, 2018. doi: 10.1016/j.kint.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Sun J, Lei X, Zhu Z, Pei C, Qin L. MicroRNA-486-5p suppresses TGF-β2-induced proliferation, invasion and epithelial-mesenchymal transition of lens epithelial cells by targeting Smad2. J Biosci 42: 575–584, 2017. doi: 10.1007/s12038-017-9709-2. [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Lee K, He JC. Genetics and epigenetics of diabetic nephropathy. Kidney Dis (Basel) 1: 42–51, 2015. doi: 10.1159/000381796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm 2016: 8319283, 2016. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loeffler I, Liebisch M, Allert S, Kunisch E, Kinne RW, Wolf G. FSP1-specific SMAD2 knockout in renal tubular, endothelial, and interstitial cells reduces fibrosis and epithelial-to-mesenchymal transition in murine STZ-induced diabetic nephropathy. Cell Tissue Res 372: 115–133, 2018. doi: 10.1007/s00441-017-2754-1. [DOI] [PubMed] [Google Scholar]
- 33.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837–11848, 2011. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucarelli G, Mancini V, Galleggiante V, Rutigliano M, Vavallo A, Battaglia M, Ditonno P. Emerging urinary markers of renal injury in obstructive nephropathy. BioMed Res Int 2014: 303298, 2014. doi: 10.1155/2014/303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma ZJ, Zhang XN, Li L, Yang W, Wang SS, Guo X, Sun P, Chen LM. Tripterygium glycosides tablet ameliorates renal tubulointerstitial fibrosis via the Toll-like receptor 4/nuclear factor κB signaling pathway in high-fat diet fed and streptozotocin-induced diabetic rats. J Diabetes Res 2015: 390428, 2015. doi: 10.1155/2015/390428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129: 1237–1249, 2015. doi: 10.1042/CS20150427. [DOI] [PubMed] [Google Scholar]
- 37.Meng J, Li L, Zhao Y, Zhou Z, Zhang M, Li D, Zhang CY, Zen K, Liu Z. MicroRNA-196a/b mitigate renal fibrosis by targeting TGF-β receptor 2. J Am Soc Nephrol 27: 3006–3021, 2016. doi: 10.1681/ASN.2015040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng XM, Huang XR, Xiao J, Chen HY, Zhong X, Chung AC, Lan HY. Diverse roles of TGF-β receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol 227: 175–188, 2012. doi: 10.1002/path.3976. [DOI] [PubMed] [Google Scholar]
- 39.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12: 325–338, 2016. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 40.Mise K, Hoshino J, Ueno T, Hazue R, Hasegawa J, Sekine A, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Sawa N, Fujii T, Hara S, Ohashi K, Takaichi K, Ubara Y. Prognostic value of tubulointerstitial lesions, urinary N-Acetyl-β-d-glucosaminidase, and urinary β2-microglobulin in patients with Type 2 diabetes and biopsy-proven diabetic nephropathy. Clin J Am Soc Nephrol 11: 593–601, 2016. doi: 10.2215/CJN.04980515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagalakshmi VK, Ren Q, Pugh MM, Valerius MT, McMahon AP, Yu J. Dicer regulates the development of nephrogenic and ureteric compartments in the mammalian kidney. Kidney Int 79: 317–330, 2011. doi: 10.1038/ki.2010.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada T, Nagao T, Matsumoto H, Nagaoka Y, Wada T, Nakao T. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 17: 68–75, 2012. doi: 10.1111/j.1440-1797.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 43.Petersen M, Thorikay M, Deckers M, van Dinther M, Grygielko ET, Gellibert F, de Gouville AC, Huet S, ten Dijke P, Laping NJ. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int 73: 705–715, 2008. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 44.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23: 458–469, 2012. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi J, Liu Y, Hu K, Zhang Y, Wu Y, Zhang X. MicroRNA-26a inhibits hyperplastic scar formation by targeting Smad2. Exp Ther Med 15: 4332–4338, 2018. doi: 10.3892/etm.2018.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474, 2011. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramdas V, McBride M, Denby L, Baker AH. Canonical transforming growth factor-β signaling regulates disintegrin metalloprotease expression in experimental renal fibrosis via miR-29. Am J Pathol 183: 1885–1896, 2013. doi: 10.1016/j.ajpath.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satirapoj B, Aramsaowapak K, Tangwonglert T, Supasyndh O. Novel tubular biomarkers predict renal progression in type 2 diabetes mellitus: a prospective cohort study. J Diabetes Res 2016: 3102962, 2016. doi: 10.1155/2016/3102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma M, Doley P, Das HJ. Etiological profile of chronic kidney disease: A single-center retrospective hospital-based study. Saudi J Kidney Dis Transpl 29: 409–413, 2018. doi: 10.4103/1319-2442.229297. [DOI] [PubMed] [Google Scholar]
- 50.Tang F, Hao Y, Zhang X, Qin J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther 11: 2813–2826, 2017. doi: 10.2147/DDDT.S143805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res 2015: 697010, 2015. doi: 10.1155/2015/697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trionfini P, Benigni A. MicroRNAs as master regulators of glomerular function in health and disease. J Am Soc Nephrol 28: 1686–1696, 2017. doi: 10.1681/ASN.2016101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Liu P, Wu H, Cui P, Li Y, Liu Y, Liu Z, Gou S. MicroRNA-323-3p inhibits cell invasion and metastasis in pancreatic ductal adenocarcinoma via direct suppression of SMAD2 and SMAD3. Oncotarget 7: 14912–14924, 2016. doi: 10.18632/oncotarget.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. Cell Biochem Biophys 67: 537–546, 2013. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 55.Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, Yang JK. miR-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol 392: 163–172, 2014. doi: 10.1016/j.mce.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Lin C, Ren Q, Liu Y, Yang X. Astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissues of diabetic KKAy mice. Int J Clin Exp Pathol 8: 6828–6834, 2015. [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 21: 756–761, 2010. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56: 393–405, 1999. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu H, Kong L, Tan Y, Epstein PN, Zeng J, Gu J, Liang G, Kong M, Chen X, Miao L, Cai L. C66 ameliorates diabetic nephropathy in mice by both upregulating NRF2 function via increase in miR-200a and inhibiting miR-21. Diabetologia 59: 1558–1568, 2016. doi: 10.1007/s00125-016-3958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J, Liu J, Ding Y, Zhu M, Lu K, Zhou J, Xie X, Xu Y, Shen X, Chen Y, Shao X, Zhu C. MiR-455-3p suppresses renal fibrosis through repression of ROCK2 expression in diabetic nephropathy. Biochem Biophys Res Commun 503: 977–983, 2018. doi: 10.1016/j.bbrc.2018.06.105. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, You J, Li F, Wang F, Wang Y. MicroRNA-542-3p suppresses tumor cell proliferation via targeting Smad2 inhuman osteosarcoma. Oncol Lett 15: 6895–6902, 2018. doi: 10.3892/ol.2018.8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X, Yuan J, Chen S, Li M. Targeting Smad2 and Smad3 by miR-136 suppresses metastasis-associated traits of lung adenocarcinoma cells. Oncol Res 21: 345–352, 2013. doi: 10.3727/096504014X14024160459285. [DOI] [PubMed] [Google Scholar]
- 63.Yao Z, Yang S, He W, Li L, Xu R, Zhang X, Li H, Zhan R, Sun W, Tan J, Zhou J, Luo G, Wu J. P311 promotes renal fibrosis via TGFβ1/Smad signaling. Sci Rep 5: 17032, 2015. doi: 10.1038/srep17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin W, Xu S, Wang Z, Liu H, Peng L, Fang Q, Deng T, Zhang W, Lou J. Recombinant human GLP-1(rhGLP-1) alleviating renal tubulointestitial injury in diabetic STZ-induced rats. Biochem Biophys Res Commun 495: 793–800, 2018. doi: 10.1016/j.bbrc.2017.11.076. [DOI] [PubMed] [Google Scholar]
- 65.Yu FY, Xie CQ, Sun JT, Peng W, Huang XW. Overexpressed miR-145 inhibits osteoclastogenesis in RANKL-induced bone marrow-derived macrophages and ovariectomized mice by regulation of Smad3. Life Sci 202: 11–20, 2018. doi: 10.1016/j.lfs.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 66.Yu SM, Bonventre JV. Acute kidney injury and progression of diabetic kidney disease. Adv Chronic Kidney Dis 25: 166–180, 2018. doi: 10.1053/j.ackd.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Yang K, Ren T, Huang Y, Tang X, Guo W. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis 9: 680, 2018. doi: 10.1038/s41419-018-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Guo B, Hui Q, Li W, Chang P, Tao K. Downregulation of miR-637 promotes proliferation and metastasis by targeting Smad3 in keloids. Mol Med Rep 18: 1628–1636, 2018. doi: 10.3892/mmr.2018.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao K, He J, Zhang Y, Xu Z, Xiong H, Gong R, Li S, Chen S, He F. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci Rep 6: 37234, 2016. doi: 10.1038/srep37234. [DOI] [PMC free article] [PubMed] [Google Scholar]