Abstract
There is currently no technique to unambiguously diagnose antemortem kidney injury on postmortem examination since postmortem tissue damage and autolysis are common. We assessed the ability to detect kidney injury molecule-1 (KIM-1) expression in adult and fetal kidneys examined at autopsy. In adult kidneys (n = 52 subjects), we found that the intensity of KIM-1 staining significantly correlated with the antemortem level of serum creatinine, and this was independent of the extent of tissue autolysis. In addition, kidneys from a total of 52 fetal/neonatal subjects, 30 stillborns and 22 liveborns, were assessed for KIM-1 staining. Given that serum creatinine is unreliable and often unavailable in fetuses and newborns, we assessed preterminal hypoxia in fetuses by the presence of squames in pulmonary alveoli and by required intubation. KIM-1 expression correlated with these clinical indexes of hypoxia. The expression of KIM-1 was seen in a majority of the fetal and neonatal autopsy kidneys (77%, 40/52) as early as 16 wk of gestation, even in the presence of autolysis. Thus KIM-1 is a specific and stable marker of antemortem tubular injury in kidneys of adults and fetuses despite postmortem autolysis.
Keywords: acute kidney injury, acute renal failure, autopsy kidney, biomarker, kidney hypoxia, TIM-1, KIM-1
INTRODUCTION
Assessment of antemortem acute tubule injury on postmortem exam has been a persistent problem for pathologists due to the autolysis in the kidney that results in tubule degenerative changes. This makes histological evaluation of antemortem tubular injury difficult, especially when that injury is acute (8, 12). No reliable histochemical test is currently available to establish the diagnosis of premortem tubular injury in postmortem kidneys. Kidney injury molecule-1 (KIM-1), a type I transmembrane protein, is undetectable in noninjured adult and fetal kidneys, or in inflammatory cells found in kidney, thymus, or tonsils (34), while its expression is markedly induced on the apical surface of the proximal epithelial cells in rodents and humans after injury (10, 17, 18). In human allograft biopsies and native human biopsies with acute tubular injury, KIM-1 is upregulated only on the injured proximal tubules but not on distal tubules, vasculature, or interstitium (10, 27, 34).
KIM-1 is also a sensitive and stable urine and blood biomarker, in adult and pediatric patients with acute kidney injury (3, 4, 7, 10, 27). We hypothesized that KIM-1 could be very useful for detecting antemortem proximal tubular injury in autolyzed kidneys upon postmortem examination. We tested whether the intensity of KIM-1 expression correlated with patient antemorterm levels of serum creatinine. We also evaluated KIM-1 expression in fetal autopsy kidneys and correlated this expression with evidence for prenatal hypoxia.
MATERIALS AND METHODS
Adult patients.
Retrospective adult and pediatric studies were both approved by the human investigation committees from Geisinger Health System (Danville, PA) and William Beaumont Health System (Royal Oak, MI). Fifty-two adult autopsy cases were included in the study. Autopsies were performed within 24 h of death. The time interval between death and kidney tissue fixation was similar in the KIM-1-positive and KIM-1-negative groups. We processed the tissues from both groups using the same protocol.
We excluded advanced/severe chronic kidney diseases, based on both medical history and kidney histology features of diffusely global glomerulosclerosis and diffuse interstitial fibrosis. Autolysis did not compromise evaluation of fibrosis and glomerulosclerosis on routine hematoxylin-eosin-stained sections, since the glomeruli did not show the extent of autolysis seen in the renal tubules and the extent of fibrosis could be determined by expanded fibrotic spaces among the tubular structures. The causes of death in adult cases included acute myocardial infarction (8), liver cirrhosis (15), pulmonary embolism (6), and other causes (including abdominal aortic dissection, cerebral vascular rupture/embolism, congestive heart failure with/without bypass surgery, metastatic malignant neoplasms, and sepsis-associated multiple organ failure). Cases (n = 52) were divided into two groups based on negative (group 1, n = 19) or positive (group, n = 33) staining of KIM-1 in the kidney sections, regardless of the cause of death. For each deceased adult patient, the last antemortem serum creatinine and blood urea nitrogen values were obtained from the medical record.
Stillborn and liveborn infants.
Randomly selected 52 autopsy cases of either stillborn ≥ 20 wk of gestation (n = 30) or liveborn (n = 22) infants were studied. Serum creatinine levels were not available in this age group. The presence of aspirated amniotic fluid contents (identification of epithelial squamous cells or squames in pulmonary alveoli) was taken as an index for hypoxia in stillborns and liveborns (23). In liveborn infants, the need for intubation was taken as an additional indicator of antemortem hypoxia. In addition, the final autopsy kidney diagnosis was identified for each infant.
Kidney histologic staining and immunohistochemical assessment of biomarker expression.
During autopsies, the kidneys of the decreased individuals were removed for examination and two large cross sections of kidney parenchyma (cortex and medulla) were taken, fixed in formalin, embedded in paraffin, sectioned, and hematoxylin-eosin-stained for light microscopy. Formalin-fixed, paraffin-embedded 4-μM sections were rehydrated and antigens retrieved using heated Tris-EDTA buffer. Immunohistochemical staining methods for KIM-1 and cytokeratin-7 have been previously described (34). KIM-1 was detected with mouse monoclonal antibody (AKG7 at 1:8 dilution) directed against the ectodomain of human KIM-1. Cytokeratin-7 (rabbit polyclonal; 1:200; Biocare Medical) expression marked distal tubules. CD133 is expressed in developing renal tubules of fetal kidneys (2, 19). Loss of CD133 staining reflected cellular autolysis in fetal kidney. The Immunohistochemical staining methodology used for CD133 (monoclonal antibody AC133; 1:50; Miltenyi Biotec) was similar to that used for KIM-1 (32).
Quantitation of immunohistochemical staining and identification of renal function.
The staining intensity for KIM-1 along the luminal surface of nonatrophic proximal tubular epithelial cells was graded from 0 to 3+ (20, 34) (0, no staining; +/−, focal weak fine granular staining; 1+, weak fine granular staining along the complete luminal surface; 2+, moderate granular staining; and 3+, strong large granular staining) by P. L. Zhang, who was unaware of the renal function or hypoxia status of the patient before death. CD133 staining along the apical membrane of tubular epithelium was also scored in a way similar to KIM-1. This semiquantitative approach to determining KIM-1 intensity expression was chosen since, as we have previously shown (34), this approach can identify the injury in nonatrophic proximal tubules in the setting of chronic injury which is characterized by shrunken atrophic tubules and surrounding fibrosis. In chronic kidney disease, KIM-1 staining is also present on either atrophic debris in the lumen of the chronic atrophic tubule or at low intensity on the apical membrane of the atrophic epithelium. Antemortem serum creatinine and blood urea nitrogen level in adult cases were measured in the clinical laboratory at Geisinger Medical Center.
Conventional evaluation of autolysis.
Autolysis was scored from 0 to 3+ in proximal tubular epithelium as follows: 0, intact cytoplasm and clear hematoxylin nuclear staining; 1+, minimal autolysis with relatively intact cytoplasm and weakened hematoxylin nuclear staining; 2+, moderate autolysis with some degenerative cytoplasmic changes and some unstained nuclei by hematoxylin; and 3+, severe autolysis with diffuse tubular cytoplasmic changes and diffuse unstained nuclei with hematoxylin.
Statistics.
Results are expressed as the means ± SE. The two groups were compared using unpaired Student’s t-test. Pearson Correlation between KIM-1 staining scores and either renal function indexes or autolysis was assessed (StatView program). P < 0.05 was considered statistically significantly different.
RESULTS
KIM-1 in adult kidneys.
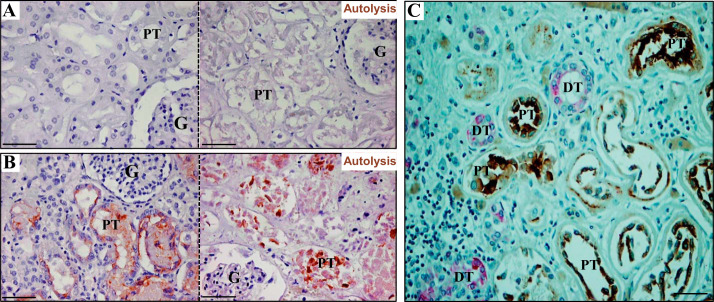
In adult cadaveric kidneys that stained negatively (group 1) or positively (group 2) for KIM-1 expression, moderate to severe autolysis was present in approximately half of cases (27/52, 52%). In group 1, KIM-1 staining was entirely negative in proximal tubules, either without autolysis (Fig. 1A, left) or with autolysis (Fig. 1A, right). In group 2, KIM-1 expression was clearly detectable on the luminal surface and in the cytoplasm of proximal tubules of autopsy kidneys, regardless of the state of autolysis [Fig. 1B, left (without autolysis) and right (with autolysis)]. KIM-1 expression was present predominantly on the apical luminal aspect of proximal tubule cells and not in cytokeratin-7-expressing distal tubule cells (Fig. 1C). Both groups had similar intervals between death and autopsy (within 24 h). Mean patient ages and autolysis scores were similar between the two groups (Table 1). Antemortem serum levels of creatinine and blood urea nitrogen were significantly higher in the KIM-1 positive (group 2) when compared with KIM-1 negative (group 1) kidneys (Table 1).
Fig. 1.
Kidney injury molecule-1 (KIM-1) expression in adult cadaver kidneys. A: the expression of KIM-1 was undetectable in cadaver kidneys when there was no clinical evidence for kidney injury in the absence (left) or in the presence of prominent autolysis (right). B: by contrast, the expression of KIM-1 was shown on the apical surface of proximal tubular cells in kidneys from patients with increased premortem serum creatinine, regardless of the absence (left) or presence (right) of marked autolysis. C: KIM-1 was specifically expressed on the injured proximal tubules (brown) but not on cytokeratin-7 positive (pink) distal tubules. G, glomerulus; PT, proximal tubule; DT, distal tubule. Scale bar = 50 µm (magnifications: ×200 in A–C).
Table 1.
The expression of KIM-1 and other indexes in adult autopsy kidneys
| Age, yr | Autolysis Scores, arbitrary units | KIM-1 Scores, arbitrary units | Antemortem Serum Creatinine, mg/dl | Antemortem BUN, mg/dl | |
|---|---|---|---|---|---|
| KIM-1 negative (n = 19) | 65 ± 4 | 1.63 ± 0.17 | 0.00 ± 0.00 | 1.23 ± 0.14 | 26.5 ± 4.3 |
| KIM-1 positive (n = 33) | 69 ± 2 | 1.97 ± 0.14 | 1.66 ± 0.15 | 2.61 ± 0.35 | 66.47 ± 8.2 |
| P value | 0.36 | 0.15 | <0.0001 | <0.01 | <0.01 |
KIM-1, kidney injury molecule-1; BUN, blood urea nitrogen.
P value: comparing KIM-1 positive and negative cases.
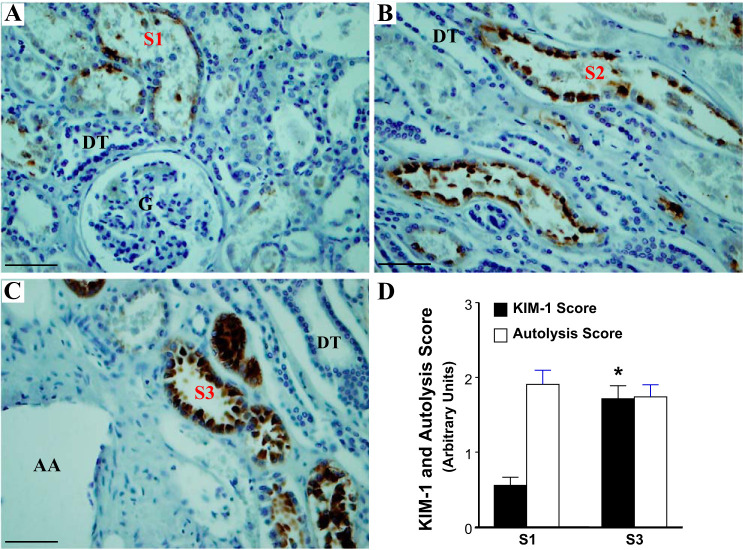
To evaluate whether there was differential vulnerability to injury of different segments of proximal tubules (22), the expression patterns of KIM-1 in S1 and S3 segments of proximal tubules from an adult autopsy kidney with antemortem acute tubular injury are shown in Fig. 2. The expression of KIM-1 was greater in S3 segments localized in the outer stripe of the outer medulla when compared with the S1 segments identified by their location around the glomerulus. By location we can be more confident about the identity of S1 segments around the glomeruli and S3 segments of the proximal tubule near the arcuate vessels in the inner cortex and outer medulla, but we cannot definitively distinguish the extent of the S2 segment in the cortex away from glomeruli (Fig. 2, A–C). We show KIM-1 staining in a medullary ray in Fig. 2B, but we only present quantitation of the S1 and S3 segments (Fig. 2D). The extent of autolysis did not differ appreciably along the various segments of proximal tubules (Fig. 2D).
Fig. 2.
The expression pattern of kidney injury molecule-1 (KIM-1) in S1, S2, and S3 segments of proximal tubules from an adult autopsy kidney with antemortem acute tubular injury. A: a weak expression of KIM-1 (brown) is demonstrated in S1 segments of proximal tubules close to a glomerulus. B: a moderate level of expression of KIM-1 is observed in S2 segments of proximal tubules along the medullary rays; C: strong expression of KIM-1 is observed in S3 segments of proximal tubules near an arcuate artery, located in the outer stripe of the outer medulla. D: quantification of KIM-1 expression in autopsy kidneys with antemortem tubular injury in the S1 and S3 segments of the proximal tubule. The KIM-1 score is significantly higher in injured S3 segments compared with S1 segments of proximal tubule. AA, arcuate artery; PT, proximal tubule; DT, distal tubule. Data are expressed as means ± SE. *P < 0.05. Scale bar = 50 µm (magnifications: ×200 in A–C).
KIM-1 expression was not significantly associated with the extent of autolysis.
When all cases were analyzed as a single group, there was a significant correlation between KIM-1 expression and renal dysfunction (Table 2). When only KIM-1-positive cases were considered, there was also a significant correlation between KIM-1 expression and both serum creatinine (P < 0.001) and blood urea nitrogen levels (P < 0.01), consistent with proximal tubular injury and decreased renal function before death. In the adult biopsy cohort, the sensitivity and specificity of a sCr value ≥1.1 mg/dl to identify acute tubular injury (KIM-1 expression) were 79 % and 53% respectively. There were 9 cases with sCr ≥1.1 but negative KIM-1 staining and 7 cases with positive KIM-1 expression but serum creatinine levels <1.1 mg/dl. Of 21 cases with serum creatinine levels ≥1.8 only two (sCr 2.2 and 2.9) had negative KIM-1 staining. One had a final autopsy diagnosis of cirrhosis and the other had multiple organ failure as the cause of death. In cases of negative KIM-1 staining and elevated antemortem serum creatinine, it is likely that prerenal factors might have contributed to higher levels of antemortem serum creatinine without significant proximal tubule injury.
Table 2.
Linear regression analysis between sCr or BUN or autolysis score versus the expression of KIM-1 in adult patients
| sCr vs KIM-1 | BUN vs KIM-1 | Autolysis Scores vs KIM-1 | |
|---|---|---|---|
| n | 52 | 52 | 52 |
| R | 0.66 | 0.59 | 0.11 |
| R2 | 0.44 | 0.34 | 0.01 |
| Intercept and slope | 0.98 and 1.11 | 26.08 and 24.58 | 1.73 and 0.08 |
| Beta coefficient | 0.17 | 4.81 | 0.11 |
| P value | <0.0001 | <0.0001 | 0.43 |
sCr, serum creatinine; BUN, blood urea nitrogen; KIM-1, kidney injury molecule-1; n, number of samples; R, the correlation coefficient; R2, the coefficient of determination.
KIM-1 in stillborn and deceased liveborn kidneys.
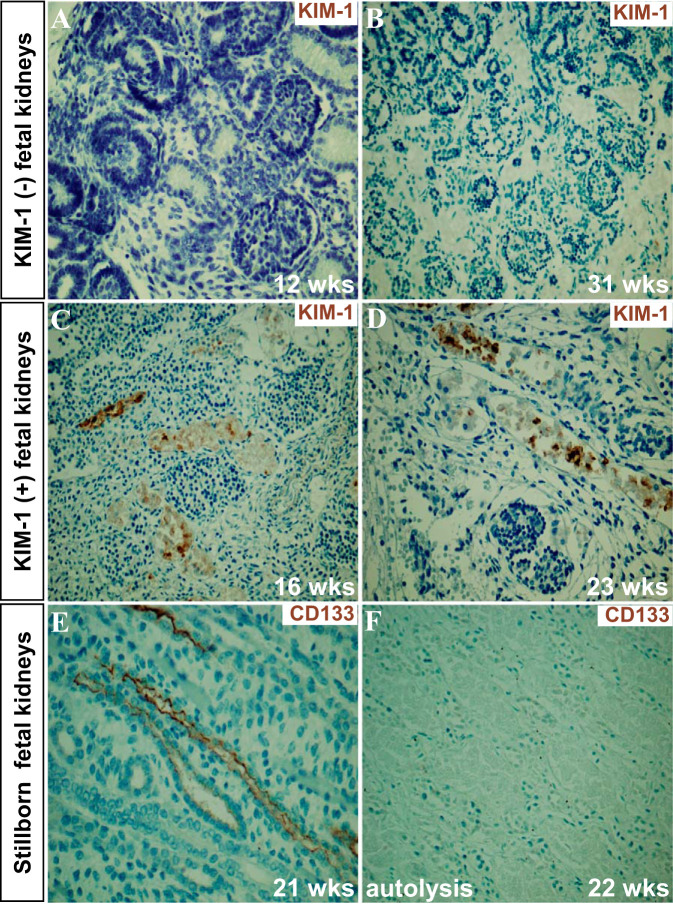
In stillborn and deceased liveborn cases, the ages at birth ranged from 15 to 40 gestational weeks (mean age 24.75 wk; n = 52, 30 stillborn and 22 liveborn). KIM-1 was expressed in 40/52 (77%) of the fetal/neonatal kidneys: with scores of 1+ in 25 cases, 2+ in 14 cases, and 3+ in 1 case. Normal fetal kidneys from 12–31 wk of gestation do not express detectable KIM-1 [Fig. 3, A (34) and B]. In contrast, KIM-1-positive staining was present as early as 16 wk of gestation in the kidney of a non-cohort spontaneously aborted fetus and in many of the cohort stillborn fetal kidneys (Fig. 3, C and D). Autolysis was present in 29 kidneys (29/52, 56%) and ranged from 1+ in 8 cases, 2+ in 7 cases, and 3+ in 14 cases. In addition, in 5 of the 30 stillborn cases there was only necrotic kidney tissue present with no recognizable glomeruli, tubule or vascular structures. Twenty-three cases (23/52, 44%) did not show autolysis. There was no significant correlation between KIM-1 scores and autolysis scores (r = 0.20 and P = 0.15). Autolysis scores were inversely correlated with CD133 expression scores along fetal renal tubules (Fig. 3, E and F; correlation r = 0.70 and P < 0.0001). CD133 would be expected to be present in the developing nephron (2, 19). Our data suggested that the absence of CD133 in the fetal tubules is related to autolysis and loss of antigenicity. Clinical evidence for systemic hypoxia (presence of aspirated amniotic fluid contents in stillborns or need for intubation or presence of aspirated amniotic fluid contents primary or lung atelectasis in liveborns) was present by chart review in 30/52 (58%) cases (Table 3). Among the 30 cases where evidence for hypoxia was available, KIM-1 expression was found in 73% of cases, including 5 stillborns whose kidney structures were not identifiable (Table 3). If one excludes those stillborns whose kidneys were so necrotic that there were no recognizable renal structures, then KIM-1 was expressed in 88% of cases with documented clinical evidence for hypoxia (22 of 25).
Fig. 3.
The expression of Kidney injury molecule-1 (KIM-1) and CD133 in fetal kidneys. A and B: KIM-1 is not expressed in two fetal kidneys at 12 and 31 wk of development. C and D: the expression of KIM-1 was seen on the apical surface of proximal tubules in fetal kidneys at 16 and 23 wk of gestational age respectively. E and F: the expression of CD133 was seen on the surface of developing renal tubules without autolysis (week 21), whereas it was undetectable when autolysis was prominent (week 22). Scale bar = 50 µm (magnifications: ×200 in A–F).
Table 3.
The correlation of KIM-1 expression and hypoxia in pediatric autopsy kidneys
| Stillborn (%) | Liveborn (%) | Total (%) | |
|---|---|---|---|
| KIM-1 positive | 22/30 (73%) | 18/22 (81%) | 40/52 (77%) |
| CC of antemortem hypoxia | 12/30 (40%) | 18/22 (81%) | 30/52 (58%) |
| KIM-1 positive/CC: antemortem hypoxia | 7/12 (58%) | 15/18 (83%) | 22/30 (73%) |
| KIM-1 positive/CC: antemortem hypoxia; kidney tissue where tubules are identifiable | 7/7 (100%) | 15/18 (83%) | 22/25 (88%) |
KIM-1, kidney injury molecule-1; CC, clinical correlates: indication of antemortem hypoxia included either the presence of aspirated amniotic fluid contents (squames in pulmonary alveoli) in stillborns or need for intubation in liveborn patients.
KIM-1 scores and major autopsy diagnoses of 22 liveborn cases are listed in Table 4. The period of neonate survival ranged from 20 min to 35 days. The dominant autopsy finding in liveborns with intubation history was respiratory distress syndrome with diffuse alveolar membrane formation in fetal lungs (cases 1−12), and the major autopsy finding in liveborns without a history of intubation was aspiration of amniotic fluid (cases 13–22). None of the liveborn kidneys had significant autolysis. KIM-1 protein expression was found to be detectable in 18 out of these 22 cases. We found no evidence for extramedullary hematopoiesis in either fetal or adult kidneys.
Table 4.
KIM-1 scores in liveborns with and without intubation history
| Case No. | Time of Death after Birth | Gestational Age at Birth | KIM-1 Scores | Major Autopsy Diagnoses |
|---|---|---|---|---|
| With intubation history | ||||
| 1 | 17 h | 22 4/7 wk | 1+ | Respiratory distress syndrome |
| 2 | 12 h | 23 4/7 wk | 0 | Respiratory distress syndrome |
| 3 | 4 h | 23 3/4 wk | 0 | Respiratory distress syndrome, intraventricular hemorrhage in the brain |
| 4 | 8 days | 32 6/7 wk | 2+ | Multiple organs congenital abnormalities, encephalocele |
| 5 | 35 days | 25 6/7 wk | 2+ | Extensive brain infarction |
| 6 | 24 h | 27 wk | 2+ | Respiratory distress syndrome |
| 7 | 24 h | 27 6/7 wk | 0 | Respiratory distress syndrome |
| 8 | 41 days | 33 wk | 2+ | Dandy-Walker anomaly, acute bronchopneumonia |
| 9 | 14 days | 22 4/7 wk | 1+ | Respiratory distress syndrome |
| 10 | 24 h | 29 5/7 wk | 1+ | Respiratory distress syndrome |
| 11 | 26 days | 24 6/7 wk | 1+ | Respiratory distress syndrome |
| 12 | 2 h | 27 3/7 | 1+ | Immature sacrococcygeal teratoma |
| No intubation history | ||||
| 13 | 1.5 h | 21 4/7 wk | 1+ | Amniotic fluid aspiration |
| 14 | 0.2 h | 19 5/7 wk | 1+ | Amniotic fluid aspiration |
| 15 | 1 h | 18 wk | 1+ | Amniotic fluid aspiration |
| 16 | 3 h | 19 2/7 wk | 1+ | Amniotic fluid aspiration |
| 17 | 0.3 h | 19 wk | 2+ | Amniotic fluid aspiration |
| 18 | 1 h | 20 wk | 2+ | Primary lung atelectasis |
| 19 | 3 h | 23 wk | 2+ | Undetermined |
| 20 | 1 h | 21 5/7 wk | 1+ | Induced abortion due to hypertension |
| 21 | 1.5 h | 15 wk | 0 | Undetermined |
| 22 | 1.5 h | 21 wk | 1+ | Induced abortion due to fetal congenital malformation |
Seven of 12 (58%) intubated fetuses lived less than 48 h, and no fetus without intubation lived longer than 3 h. None of the liveborns had significant autolysis in the kidneys. Respiratory distress syndrome is characterized by diffuse hyaline membrane formation in the fetal lungs. KIM-1, kidney injury molecule-1.
DISCUSSION
In autopsy cases, kidneys are particularly vulnerable to postmortem autolysis, which makes pathologic diagnosis of antemortem acute tubular injury more difficult (8, 12). Currently, the pathologist relies on the rare presence of mitotic figures in proximal tubular cells. However, signs of regeneration (mitotic figures) may not be present. In the face of these uncertainties, it is often important to identify antemortem acute tubular injury in autopsy cases, since acute tubular injury can contribute to the patient’s death and might provide evidence for unexpected nephrotoxicity.
KIM-1 is undetectable in normal kidney tissue but is increased dramatically in the injured proximal tubule where the protein is inserted into the apical membrane (10, 18, 27, 34). Our animal and cell culture studies have demonstrated that KIM-1 acts as a phosphatidylserine receptor on renal epithelial cells during tubular injury, converting the normal proximal tubule cell into a phagocyte that facilitates the clearance of dead cells in the lumen (16). KIM-1, also named T-cell immunoglobulin and mucin-domain-containing molecule-1 (TIM-1), has been reported to be present in T cells (particularly Th2 cells) and regulatory B cells, but at very low levels (21, 30). KIM-1 upregulation in proximal tubules is associated with activation of oxidative stress and STAT3 (1) and persistent expression of KIM-1 in renal tubules may contribute to development of chronic kidney disease (15, 31). The monoclonal antibody against KIM-1 used in the current study does not stain T lymphocytes, other types of inflammatory cells, or any other normal tissues in humans (34). The ectodomain of KIM-1 is shed into the urine where it can be detected easily in adult and pediatric patients with acute kidney injury (3, 4, 7, 27, 34). In the urine and kidney tissue, KIM-1 is a sensitive and specific marker for kidney injury (7, 10, 11, 18). Due to its high degree of stability at room temperatures, KIM-1 can also be detected in autopsy kidney by using our anti-KIM-1 antibody. In our study, we found that the intensity of expression of KIM-1 in adult autopsy cases reflected the extent of impaired kidney function just before death and was independent of postmortem autolysis. By comparison the detection of CD133 is highly dependent upon the absence of postmortem autolysis (Fig. 3).
The significant correlation between postmortem KIM-1 expression and antemortem serum creatinine in the adult cases indicates that the expression of KIM-1 is a sensitive marker for antemortem tubular injury. Differential susceptibility of segments (S1 to S3) of proximal tubules has been found in rodent kidneys with enhanced vulnerability of the S3 segment located in the outer stripe of the medulla (9, 28, 29, 33). We found increased KIM-1 intensity in the S3 segment cells when compared with S1 segment cells, consistent with increased susceptibility of the S3 cells to acute injury in human kidneys. This vulnerability of the S3 segment may result from limited capacity to undergo anaerobic glycolysis (5) and its perfusion with low oxygen containing capillary blood. In contrast, S1 segments of proximal tubules are located in the better-oxygenated cortex. Thus our data indicate that human and rodent kidneys share similarities in vulnerability of different regions of proximal tubules (13, 14, 18, 33). The cause of death in adults in this study was often multifactorial.
Our focus in this article was to identify premortem acute proximal tubule injury distinguishable even in the setting of chronic tubular injury and the presence of autolysis.
Metanephric nephrons are recognizable before the 14th week of gestation in humans (24). The second phase of nephrogenesis starts from the 14th to 22nd week of gestation with the formation of nephron arcades. The last period of nephrogenesis occurs from the 22nd to 36nd week of gestation and is characterized by adding several more nephrons in each nephron arcade (24). KIM-1 is not detectable in the developing kidneys of rodents (17), and was not expressed in 12 and 31 wk aborted human kidneys (34). KIM-1 was, however, detectable as early as 16 wk in the kidney of another fetus.
There are several limitations in studying fetal and newborn kidneys. First, serum creatinine values were not available for correlation with kidney injury. Even if available, the creatinine of the stillborn child or neonate in the first days of life would be of limited value in evaluating renal function and tubular injury. Early after birth, cord blood creatinine reflects the mother’s renal function rather than the fetus, and the serum creatinine values on the following days are variable until the infant achieves a new steady state (6, 26). With the ongoing nephrogenesis in fetal kidneys, we were not able to determine differential susceptibility of segments of fetal proximal tubules.
Hypoxia-ischemia is known to be one of the most common causes of acute kidney injury in the neonatal period, accounting for 30–40% of cases in one study (25). In addition to identifying squamous cells in the pulmonary alveoli during autopsy as evidence of fetal hypoxia (23), positive KIM-1 staining in fetal and neonatal kidneys may be another reliable index to indicate kidney hypoxia in pediatric autopsy cases.
The expression of KIM-1 was seen in 88% of fetal/neonatal kidneys with discernable proximal tubules among the pediatric cases with evidence for hypoxia (Table 3 and Table 4).
In summary, the expression of KIM-1 in adult and fetal autopsy kidneys is a measure of antemortem kidney injury and is detectable even when there is extensive postmortem autolysis. KIM-1 levels in adults correlate with antemortem levels of creatinine and correlate significantly with clinical indexes of hypoxia in the fetus or newborn. Thus KIM-1 can serve as a sensitive and reliable marker for antemortem proximal tubular injury in adults, fetuses, or newborns. To our knowledge, this is the first description of a tool for determining antemortem kidney injury on autopsy in the presence of kidney autolysis. KIM-1 analysis of autopsy adult kidney tissue also reveals preferential vulnerability of S3 segments of proximal tubules to acute injury in humans.
GRANTS
This work was supported by a Geisinger intramural grant (to P. L. Zhang) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-039773, DK-072381, and DK-038452 (to J. V. Bonventre).
DISCLOSURES
J. V. Bonventre is a co-inventor of KIM-1 patents that are assigned to Partners HealthCare and licensed by Partners to Johnson & Johnson, Sekisui Medical, Biogen Idec, Astute, and a number of research reagent companies. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.V.B., W.Y., and P.L.Z. conceived and designed research; W.Y. and P.L.Z. performed experiments; W.Y., P.L.Z., J.M., F.L., and J.V.B. analyzed data; W.Y., P.L.Z., J.M., F.L., and J.V.B. interpreted results of experiments; W.Y., P.L.Z., F.L., and J.V.B. prepared figures; W.Y., P.L.Z., and J.V.B. drafted manuscript; W.Y., P.L.Z., and J.V.B. edited and revised manuscript; W.Y., P.L.Z., and J.V.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate excellent technical support from Thomas M. Blasick and Sharon K. Hicks. We thank Drs. Michael Ferguson and Helmut Rennke for reading the manuscript and providing helpful comments.
REFERENCES
- 1.Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, Vaidya VS. A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am Soc Nephrol 25: 105–118, 2014. doi: 10.1681/ASN.2013020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 3.Askenazi DJ, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, Mehta RL, Ambalavanan N. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res 70: 302–306, 2011. doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, Liwo A, Devarajan P, Ambalavanan N. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr 159: 907–912.e1, 2011. doi: 10.1016/j.jpeds.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 248: F522–F526, 1985. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- 6.Bökenkamp A, Dieterich C, Dressler F, Mühlhaus K, Gembruch U, Bald R, Kirschstein M. Fetal serum concentrations of cystatin C and beta2-microglobulin as predictors of postnatal kidney function. Am J Obstet Gynecol 185: 468–475, 2001. doi: 10.1067/mob.2001.115283. [DOI] [PubMed] [Google Scholar]
- 7.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 8.Borczuk AC, Berman JW, Factor SM. Distribution of endothelin immunoreactivity in human kidney correlates with antemortem acute renal failure: a possible postmortem immunohistochemical test. Hum Pathol 28: 193–199, 1997. doi: 10.1016/S0046-8177(97)90106-4. [DOI] [PubMed] [Google Scholar]
- 9.Brezis M, Rosen S. Hypoxia of the renal medulla–its implications for disease. N Engl J Med 332: 647–655, 1995. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 10.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera GA, Joseph L, Gu X, Hough A, Barlogie B. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med 128: 875–879, 2004. doi:. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108: 9226–9231, 2011. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035, 2013. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286: F552–F563, 2004. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 19.Ivanova L, Hiatt MJ, Yoder MC, Tarantal AF, Matsell DG. Ontogeny of CD24 in the human kidney. Kidney Int 77: 1123–1131, 2010. doi: 10.1038/ki.2010.39. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen NO, Jorgensen F. Ultrastructural observations on the pars descendens of the proximal tubule in the kidney of the male rat. Z Zellforsch Mikrosk Anat 136: 479–499, 1973. doi: 10.1007/BF00307365. [DOI] [PubMed] [Google Scholar]
- 21.Khademi M, Illés Z, Gielen AW, Marta M, Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris RA, Hafler DA, Kuchroo VK, Olsson T, Piehl F, Wallström E. T Cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J Immunol 172: 7169–7176, 2004. doi: 10.4049/jimmunol.172.11.7169. [DOI] [PubMed] [Google Scholar]
- 22.Lee CY, Shin S, Lee J, Seo HH, Lim KH, Kim H, Choi JW, Kim SW, Lee S, Lim S, Hwang KC. MicroRNA-mediated down-regulation of apoptosis signal-regulating kinase 1 (ASK1) attenuates the apoptosis of human mesenchymal stem cells (MSCs) transplanted into infarcted heart. Int J Mol Sci 17: 1752, 2016. doi: 10.3390/ijms17101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhavan M, Sivasankaran P, Ratnakar C, Chandra K. Epithelial squames in fetal lungs as an index of respiratory insufficiency. Indian J Pediatr 39: 65–67, 1972. doi: 10.1007/BF02756584. [DOI] [PubMed] [Google Scholar]
- 24.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet 8: 791–802, 2007. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 25.Sweetman DU, Riordan M, Molloy EJ. Management of renal dysfunction following term perinatal hypoxia-ischaemia. Acta Paediatr 102: 233–241, 2013. doi: 10.1111/apa.12116. [DOI] [PubMed] [Google Scholar]
- 26.Treiber M, Gorenjak M, Pecovnik Balon B. Serum cystatin-C as a marker of acute kidney injury in the newborn after perinatal hypoxia/asphyxia. Ther Apher Dial 18: 57–67, 2014. doi: 10.1111/1744-9987.12054. [DOI] [PubMed] [Google Scholar]
- 27.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 28.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int 14: 31–49, 1978. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam MA, Jones DB, Rennke HG, Sandstrom D, Patel Y. Mechanism of proximal tubule brush border loss and regeneration following mild renal ischemia. Lab Invest 45: 355–365, 1981. [PubMed] [Google Scholar]
- 30.Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, Bonventre JV, Kuchroo VK. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc Natl Acad Sci USA 109: 12105–12110, 2012. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin W, Naini SM, Chen G, Hentschel DM, Humphreys BD, Bonventre JV. Mammalian target of rapamycin mediates kidney injury molecule 1-dependent tubule injury in a surrogate model. J Am Soc Nephrol 27: 1943–1957, 2016. doi: 10.1681/ASN.2015050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang PL, Hafron JM. Progenitor/stem cells in renal regeneration and mass lesions. Int Urol Nephrol 46: 2227–2236, 2014. doi: 10.1007/s11255-014-0821-z. [DOI] [PubMed] [Google Scholar]
- 33.Zhang PL, Lun M, Schworer CM, Blasick TM, Masker KK, Jones JB, Carey DJ. Heat shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci 38: 57–64, 2008. [PubMed] [Google Scholar]
- 34.Zhang PL, Rothblum LI, Han WK, Blasick TM, Potdar S, Bonventre JV. Kidney injury molecule-1 expression in transplant biopsies is a sensitive measure of cell injury. Kidney Int 73: 608–614, 2008. doi: 10.1038/sj.ki.5002697. [DOI] [PMC free article] [PubMed] [Google Scholar]