Abstract
Diabetic kidney disease (DKD), one of the most common and severe microvascular complications of diabetes, is the leading cause of chronic kidney disease and end-stage kidney disease worldwide. Since the development of renin-angiotensin system inhibition nearly three decades ago, no new therapeutic agents have received regulatory approval for treatment of DKD. Glucagon-like peptide-1 (GLP-1) receptor agonists, a class of newer antihyperglycemic agents, have shown promise for prevention of DKD onset and progression. This perspective summarizes clinical and experimental observations to give insight into biological mechanisms beyond glycemic control, such as natriuresis and anti-inflammatory actions, for preservation of kidney function in patients with diabetes.
Keywords: albuminuria, anti-inflammatory therapy, diabetes, end-stage renal disease
INTRODUCTION
Diabetic kidney disease (DKD) afflicts ~30% of patients with type 1 diabetes and 40% of patients with type 2 diabetes (31). This microvascular complication of diabetes now stands as the leading cause of chronic kidney disease and end-stage kidney disease in the world (5, 38). The relentless increase in DKD prevalence is largely attributable to continued growth of the diabetic population, which is projected to reach a size of 624 million people globally by the year 2040 (13). Almost all of the excess risk for all-cause and cardiovascular-related mortality among patients with diabetes occurs in those with DKD (1). The age-standardized rate of end-stage kidney disease has exhibited a nominal decline, just 28%, compared with ~50–70% declines for other diabetes complications (myocardial infarction, stroke, and lower limb amputation) between the years 1990 and 2012 (12). Notably, the number of deaths attributed to end stage kidney disease from diabetes increased by 94% over the same time period. This dramatic increase in mortality is presumably due to the rapidly expanding number of patients living with diabetes as well as a lack of effective therapies for DKD (19).
New treatments for prevention and treatment of DKD represent a pressing unmet medical need. Since the development of renin-angiotensin system inhibition nearly three decades ago, no new therapeutic agents have received regulatory approval for DKD treatment. Recently, several clinical trials have reported that a class of newer antihyperglycemic agents, the glucagon like peptide-1 (GLP-1) receptor agonists, prevent onset of macroalbuminuria and reduce decline in estimated glomerular filtration rate (eGFR) in patients with type 2 diabetes (21–23, 26). This Perspective describes the clinical trial data that provided these observations, discusses posited mechanisms for protection of the diabetic kidney by GLP-1 receptor agonists, and outlines important unanswered questions (8, 14, 40).
FROM THE PATIENT-SIDE: CLINICAL EFFECTS ON THE DIABETIC KIDNEY
Clinical benefits of the GLP-1 receptor agonists on DKD were identified in data resulting from clinical trials for cardiovascular safety that followed U.S. Food and Drug Administration approvals for treatment of hyperglycemia in type 2 diabetes. These studies showed that significantly fewer participants treated with semaglutide, liraglutide, or lixisenatide developed new or worsening DKD compared with those receiving placebo (22, 23, 26). This effect was largely driven by reducing risk of onset of new macroalbuminuria (urine albumin-to-creatinine ratio >300 mg/g) by 22–36% (21–23, 26). For liraglutide, the reduction in risk of death and major cardiovascular events, primarily atherosclerotic complications, was even greater in the subset of participants with eGFR <60 ml·min−1·1.73 m−2 than in the overall study population (23). However, kidney disease outcomes in these trials were not stratified by level of kidney function (22, 26).
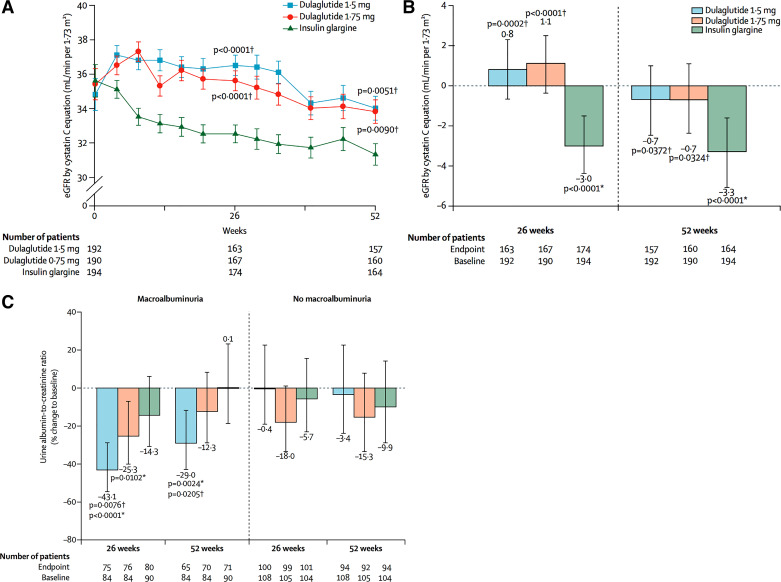
A recently completed clinical trial of patients with type 2 diabetes and moderate-to-severe chronic kidney disease (CKD) revealed that treatment with dulaglutide, compared with active treatment with insulin glargine as basal therapy, prevented decline in eGFR over 1 yr from a mean of loss of approximately −3 to −1 ml·min−1·1.73 m−2, respectively (Table 1; Fig. 1A). This difference, if sustained over time, would have a substantial clinical impact to delay kidney disease progression. Notably, all groups achieved similar reductions in hemoglobin A1C (HbA1c) and blood pressure was comparable between treatment groups, supporting the postulate that GLP-1 receptor agonists have a benefit on kidney function in people with diabetes and advanced CKD independent of benefits on glycemia or blood pressure.
Table 1.
Secondary kidney outcomes in clinical trials of glucagon like peptide-1 receptor agonists in patients with type 2 diabetes
| Name of the Study | Agent | Intervention | Study Population | Kidney Outcomes | Results |
|---|---|---|---|---|---|
| Clinical trial for glycemic control in moderate-to-severe chronic kidney disease | |||||
| AWARD-7* (n = 576) | Dulaglutide | Dulaglutide 0.75 and 1.5 mg vs. insulin glargine | Type 2 diabetes7.5% ≥ HbA1c ≤ 10.5%15 ≥ eGFR ≤ 6 0 ml·min−1·1.73 m−2Baseline means ± SD eGFR: 35 ± 0.6 ml·min−1·1.73 m−2Baseline median (interquartile range): UACR (mg/g): 209 (39–965) | eGFR and UACR change from baseline | eGFR in dulaglutide 1.5 mg (−1.1 ml·min−1·1.73 m−2, P < 0.05), and 0.75 mg (−1.5 ml·min−1·1.73 m−2 m2, P < 0.05) groups vs. eGFR in insulin glargine group (−2.9 ml·min−1·1.73 m−2 V, P < 0.0001) |
| In those with UACR >300 mg/g, eGFR decline was less with dulaglutide (1.5 mg: −0.5 ml·min−1·1.73 m−2, P < 0.05 vs. insulin; 0.75 mg: −0.7 ml·min−1·1.73 m−2, P < 0.05 vs. insulin; insulin: −5.5 ml·min−1·1.73 m−2) | |||||
| UACR reduction in dulaglutide 1.5 mg −29% (−34, −11.5), and −12.3% (−29,8.5) in 0.75 mg vs. insulin −13% (27.1, −39); P = 0.020 and P = 0.363, respectively) | |||||
| Clinical trials for cardiovascular safety | |||||
| LEADER† (n = 9,340) | Liraglutide | 1.8 mg (or the maximum tolerated dose) vs. placebo | Type 2 diabetes | New-onset albuminuria, doubling of sCr and CrCl <45 ml·min−1·1.73 m−2; RRT; death due to kidney disease | 1.9 events/100 patient-year in placebo group vs. 1.5 events/100 patient-year in liraglutide group (P = 0.003) |
| HbA1c >7% | |||||
| Prevalent CVD | |||||
| Baseline eGFR: n/N (%): | |||||
| eGFR >90 ml·min−1·1.73 m−2: | |||||
| placebo [1,655/4,672 (35.4)]; liraglutide [1,620/4,668 (34.7)] | |||||
| eGFR 60 ≤ 90 ml·min−1·1.73 m−2: placebo [1,975/4,672 (41.4)]; liraglutide [1,932/4,668 (42.3)] | |||||
| eGFR 30 ≤ 60 ml·min−1·1.73 m−2: placebo [935/4,672 (20.0)]; liraglutide [999/4,668 (21.4)] | |||||
| eGFR <30 ml·min−1·1.73 m−2: | |||||
| placebo [107/4,672 (2.3)]; | |||||
| liraglutide [117/4,668 (2.5)] | |||||
| Microalbuminuria or proteinuria at baseline: n/N (%): | |||||
| placebo: 558/4,672 (11.9%); | |||||
| liraglutide: 501/4,668 (10.7%) | |||||
| ELIXA‡ (n = 6,068) | Lixisenatide | 10–20 μg of lixisenatide vs. placebo | Type 2 diabetes5.5% ≥ HbA1c ≤ 11% | Proportional change in UACR from baseline to 108 wk | 24 vs. 34% reduction in UACR in placebo group vs. lixisenatide group (P = 0.004) |
| Recent acute coronary syndrome | |||||
| Baseline means ± SD eGFR: | |||||
| placebo: 75.2 ± 21.4 ml·min−1·1.73 m−2; lixisenatide: 76.7 ± 21.3 ml·min−1·1.73 m−2 | |||||
| Baseline median (interquartile range) UACR (mg/g): | |||||
| placebo: 10.4 (5.9–32.6); lixisenatide: 10.0 (6.0 −28.0) | |||||
| SUSTAIN-6¶ (n = 3,297) | Semaglutide | 0.5 mg vs. 1.0 mg of semaglutide vs. placebo | Type 2 diabetesHbA1c >7% | New or worsening nephropathy (persistent UACR >300 mg/g; doubling of sCr or eGFR <45 ml·min−1·1.73 m−2; RRT) | 6.1% with composite outcome in placebo group vs. 3.8% in semaglutide group (P = 0.005) |
| Age >50 yr with established CVD or CKD stages 3–5 | |||||
| Age >60 yr with CVD risk factors | |||||
| Baseline eGFR n/N (%): | |||||
| eGFR >90: 990/3,297 (30) | |||||
| eGFR 60 ≤ 90: 1,368/3,297 (41.5) | |||||
| eGFR 30 ≤ 60: 832/3,297 (25.2) | |||||
| eGFR 15 ≤ 30: 95/3,297 (2.9) | |||||
| eGFR <15: 12/3,297 (0.4) | |||||
UAC, urine albumin-to-creatinine ratio (with albumin measured in mg/g); CKD, chronic kidney disease, CVD, cardiovascular disease; sCr, serum creatinine; CrCl, creatinine clearance; RRT, renal replacement therapy; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate in ml·min−1·1.73 m−2.
A Randomized, Open-Label, Parallel-Arm Study Comparing the Effect of Once-weekly Dulaglutide With Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes and Moderate or Severe Chronic Kidney Disease (AWARD-7) (36).
Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) (21, 22).
Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome (ELIXA) (26).
Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes (SUSTAIN-6) (23).
Fig. 1.
Values estimated by chronic kidney disease epidemiology collaboration equation by cystatin C or creatinine. A: data presented as estimated glomerular filtration rate (eGFR) values by geometric least squares mean from log-transformed analysis. B: data presented as actual untransformed change from baseline in eGFR values [least squares mean, 95% confidence interval (CI)]. C: actual untransformed data macroalbuminuria status at baseline, with values presented as LSM (95% CI). *Versus baseline. †Versus insulin glargine. [Modified from Tuttle et al. (36) with permission from Elsevier.]
Nevertheless, GLP-1 receptor agonists may also provide indirect protection of the kidney by better control of hyperglycemia, hypertension, and excess body weight. First, addition of a GLP-1 receptor agonist to background antihyperglycemic therapy led to greater reductions in HbA1C, ranging from −0.3 to −1.9%, compared with control groups in the clinical trials for cardiovascular safety (20, 22, 23, 26). Second, GLP-1 receptor agonists also produce a reduction in blood pressure. In patients with type 2 diabetes and preserved kidney function, treatment with liraglutide, semaglutide, or dulaglutide lowered systolic blood pressure in the range of 2–5 mmHg compared with placebo or active antihyperglycemic comparators (2, 9, 35). A proposed mechanism for reduction in blood pressure is natriuresis. Greater absolute, fractional, and proximal tubular excretion of sodium have been observed in studies of acute GLP-1 receptor agonist administration in patients with type 2 diabetes and normal kidney function (33, 37). However, in diabetic patients with moderate-to-severe CKD, the blood pressure-lowering effect of GLP-1 receptor agonists appears absent or reduced, perhaps due to impaired natriuretic responsiveness (2, 11, 36, 41). Third, GLP-1 receptor agonists promote weight loss of 3 kg on average compared with other treatments for hyperglycemia in type 2 diabetes (27, 41). Reductions in waist circumference have also been observed with GLP-1 receptor agonist treatment (34). Moreover, the weight loss effect is present in patients with type 2 diabetes and CKD (7, 36). The precise mechanisms for weight loss remain to be determined but likely include central and peripheral neural pathways, reduction of appetite and increase in satiety by GLP-1 receptor activation (39).
TO THE BENCH-SIDE: UNRAVELING BIOLOGICAL MECHANISMS
Induction of natriuresis and anti-inflammatory mediators, effects downstream of GLP-1 receptor activation, may underpin protective mechanisms in the diabetic kidney. GLP-1 receptors are expressed in the kidney as well as in the pancreas, heart, intestine, lung, liver, thyroid, and vascular smooth muscle (28). The exact distribution of GLP-1 receptors in the parenchymal cells of the kidney is not well established and exhibits notable species differences (6, 16, 18, 28). In the Wistar rat, microdissection studies of the kidney have identified GLP-1 receptor mRNA in the glomerulus and proximal convoluted tubule (6). In a separate study of the Wistar rat, GLP-1 receptors were reported, via autoradiography for protein, in the afferent arteriole and in renin-producing cells of the macula densa but not in resident glomerular cells (16). There is immunohistochemical evidence for the GLP-1 receptor protein in the glomerulus of the db/db mouse model (C57BLKS/J-db/db) of type 2 diabetes (25). However, GLP-1 receptor protein in monkey kidneys was observed only in vascular smooth muscle cells and in the macula densa (28). Currently, there are very limited data on GLP-1 receptor distribution in human kidneys, although some early reports from mRNA expression and immunostaining indicate that they may be found in the kidney cortex and arterial walls (18, 28).
Treatment with a GLP-1 receptor agonist, exenatide, has been shown to stimulate production of cAMP and to activate the Na+/H+ exchanger 3 based on in vitro studies in a proximal tubular cell line (LLC-PK1), thus inferring a potential mechanism for GLP-1-induced natriuresis (4). In subsequent in vivo studies, GLP-1 administration to spontaneously hypertensive rats led to natriuresis, increases in urinary cAMP (a measure cAMP activation in the kidney), and vasorelaxation (6, 30). Exenatide increased sodium excretion in C57BLKS/J db/db mice but not in a comparator group with GLP-1 receptor knockout (29). As a bridge to clinical translation, natriuretic effects of GLP-1 receptor agonists have been observed in human physiology studies. In healthy men, infusion of a synthetic GLP-1 (7–36) amide increased sodium clearance by ~40% with stable glomerular filtration rate (32). Similarly, liraglutide increased sodium clearance without changing glomerular filtration rate in patients with type 2 diabetes (33).
GLP-1 receptor agonists reduce markers of kidney-level inflammation in rodent models of diabetes and systemic inflammation in humans. In the kidney of the C57BKS-db/db type 2 diabetic mouse, treatment with exenatide for 4–8 wk reduced glomerular macrophage infiltration and produced a dose-related reduction in 24-h urine albumin excretion (25). In a streptozotocin-induced Sprague-Dawley rat model of type 1 diabetes, exendin-4 reduced albuminuria, mesangial matrix expansion, and expression of mRNA of inflammatory markers including cluster of differentiation 14 and intracellular adhesion molecule (17). As a clinical correlate to systemic inflammation, dulaglutide lowered serum levels of C-reactive protein (CRP) by ~1 mg/l in patients with type 2 diabetes (9). Addition of exenatide to metformin reduced levels of serum CRP by a mean of 0.5 mg/l in another study of type 2 diabetes (3). A meta-analysis of randomized controlled trials with GLP-1 receptor agonist treatment in patients with type 2 diabetes found an ~2 mg/l reduction in serum CRP (24).
The precise mechanisms underlying the anti-inflammatory effects of GLP-1 receptor agonists remain to be elucidated. One candidate mechanism is via reduction of oxidative stress. In the KK/Ta-Akita mouse model of type 1 diabetes, liraglutide treatment reduced albuminuria and nicotinamide adenine dinucleotide phosphate oxidase activity in the kidney without altering glycemic control (10). Protein kinase A activity and cAMP were also elevated in the kidney in response to liraglutide therapy. In a streptozotocin model of type 1 diabetes in rats, liraglutide attenuated oxidative stress, expression of transforming growth factor-β and fibronectin in the kidney, and albuminuria via protein kinase A-mediated inhibition of renal nicotinamide adenine dinucleotide phosphate oxidases (15). Corresponding in vitro experiments in mesangial cell culture suggested that this effect was mediated by increased production of protein kinase A and cAMP (15). A conceptual model of hypothesized mechanisms for activation of natriuretic and antioxidant mechanisms by GLP-1 receptor agonists is shown in Fig. 2.
Fig. 2.
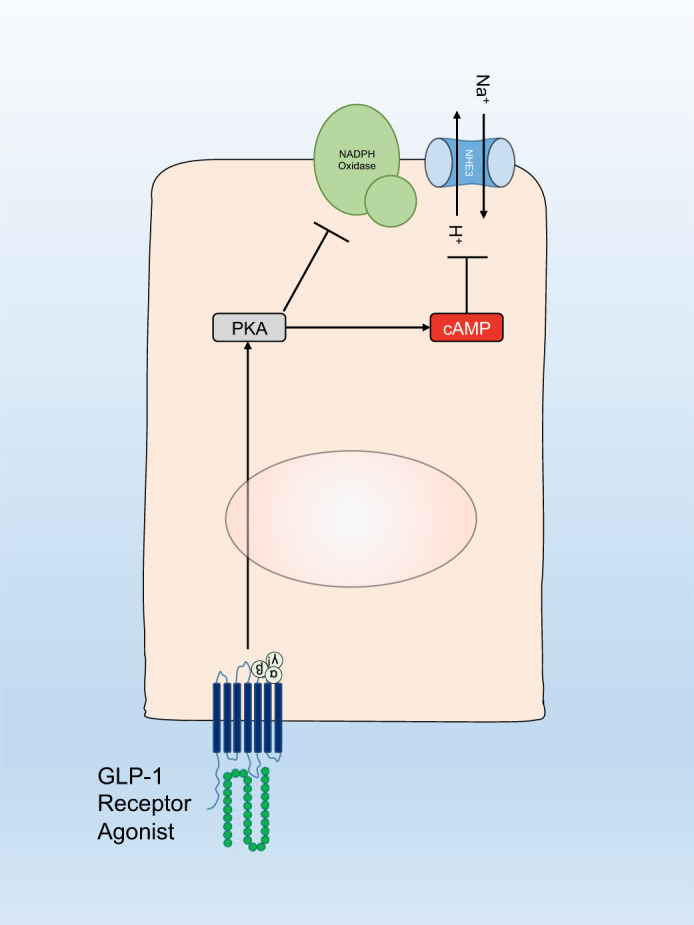
Conceptual model of hypothesized mechanisms for activation of natriuretic and antioxidant mechanisms by glucagon-like peptide 1 (GLP-1) receptor agonists. GLP-1 receptor agonists inhibit Na+/H+ exchanger 3 (NHE3) via a protein kinase A (PKA) and cyclic adenosine monophosphate (cAMP) dependent pathway to induce natriuresis. Antioxidant effects of GLP-1 receptor agonists occur through activation of protein kinase A and inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.
CONCLUSIONS
DKD poses an accelerating public health threat worldwide. Progress toward better treatments to prevent development and progression of DKD are urgently needed. In patients with, or at risk of DKD, emerging evidence from both clinical trials and experimental models points to GLP-1 receptor agonists as promising agents with clinical efficacy and a favorable safety profile. However, to optimize therapeutic application of GLP-1 receptor agonists there are pivotal questions that remain to be addressed. Specific human cell types and kidney structures that contain GLP-1 receptors, as well as associated anti-inflammatory pathways and antioxidant mechanisms, need to be delineated for a more robust understanding of underlying mechanisms for albuminuria-lowering and preservation of kidney function. Other key, yet unanswered, questions about GLP-1 receptor agonists include precise mechanisms for blood pressure reduction, weight loss, and natriuretic effects across various stages of kidney disease severity.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5UM1-DK-100846-03, 1U54-DK-083912, and U2C-DK-114886 (to K. R. Tuttle); National Center for Advancing Translational Sciences Grant 4UL1TR000423-10/WESC8883 (to K. R. Tuttle); and Janssen Research and Development Grant.
DICLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.P.D., R.Z.A., and K.R.T. prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
REFERENCES
- 1.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 24: 302–308, 2013. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bode B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res Clin Pract 97: 27–42, 2012. doi: 10.1016/j.diabres.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Bunck MC, Diamant M, Eliasson B, Cornér A, Shaginian RM, Heine RJ, Taskinen MR, Yki-Järvinen H, Smith U. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care 33: 1734–1737, 2010. doi: 10.2337/dc09-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009. doi: 10.1152/ajprenal.00082.2009. [DOI] [PubMed] [Google Scholar]
- 5.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 5: 2–7, 2015. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301: F355–F363, 2011. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, Bosch-Traberg H, Syrén A, Umpierrez GE. Efficacy and safety of Liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 39: 222–230, 2016. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 8.Degn KB, Brock B, Juhl CB, Djurhuus CB, Grubert J, Kim D, Han J, Taylor K, Fineman M, Schmitz O. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes 53: 2397–2403, 2004. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- 9.Ferdinand KC, White WB, Calhoun DA, Lonn EM, Sager PT, Brunelle R, Jiang HH, Threlkeld RJ, Robertson KE, Geiger MJ. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension 64: 731–737, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03062. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ, Seino Y, Yamada Y. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 85: 579–589, 2014. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 11.Gallwitz B, Vaag A, Falahati A, Madsbad S. Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int J Clin Pract 64: 267–276, 2010. doi: 10.1111/j.1742-1241.2009.02265.x. [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Li Y, Wang J, Rios Burrows N, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 13.IDF Diabetes Atlas Group Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res Clin Pract 109: 461–465, 2015. doi: 10.1016/j.diabres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, Vilsbøll T. Preserved inhibitory potency of GLP-1 on glucagon secretion in type 2 diabetes mellitus. J Clin Endocrinol Metab 94: 4679–4687, 2009. doi: 10.1210/jc.2009-0921. [DOI] [PubMed] [Google Scholar]
- 15.Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R, Yokomizo H, Hirata E, Sonoda N, Takayanagi R. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism 61: 1422–1434, 2012. doi: 10.1016/j.metabol.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Jensen EP, Poulsen SS, Kissow H, Holstein-Rathlou NH, Deacon CF, Jensen BL, Holst JJ, Sorensen CM. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol 308: F867–F877, 2015. doi: 10.1152/ajprenal.00527.2014. [DOI] [PubMed] [Google Scholar]
- 17.Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, Sato C, Ogawa D, Makino H. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54: 965–978, 2011. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 18.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med 48: 736–743, 2007. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 19.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, , et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012. [Erratum in Lancet 381: 628, 2013]. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 18: 317–332, 2016. doi: 10.1111/dom.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB. Liraglutide and renal outcomes in type 2 diabetes (Letter). N Engl J Med 377: 2197–2198, 2017. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 22.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311–322, 2016. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834–1844, 2016. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 24.Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications 31: 1237–1242, 2017. doi: 10.1016/j.jdiacomp.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD, Chang YS. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 18: 1227–1238, 2007. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators . Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 373: 2247–2257, 2015. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 27.Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS One 10: e0126769, 2015. doi: 10.1371/journal.pone.0126769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290, 2014. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 29.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–F971, 2012. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronn J, Jensen EP, Wewer Albrechtsen NJ, Holst JJ, Sorensen CM. Glucagon-like peptide-1 acutely affects renal blood flow and urinary flow rate in spontaneously hypertensive rats despite significantly reduced renal expression of GLP-1 receptors. Physiol Rep 5: e13503, 2017. doi: 10.14814/phy2.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 67, Suppl 1: S1–S305, 2016. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 98: E664–E671, 2013. doi: 10.1210/jc.2012-3855. [DOI] [PubMed] [Google Scholar]
- 33.Skov J, Pedersen M, Holst JJ, Madsen B, Goetze JP, Rittig S, Jonassen T, Frøkiaer J, Dejgaard A, Christiansen JS. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: a randomized clinical trial. Diabetes Obes Metab 18: 581–589, 2016. doi: 10.1111/dom.12651. [DOI] [PubMed] [Google Scholar]
- 34.Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Ji L, Zhan S. Effect of GLP-1 receptor agonists on waist circumference among type 2 diabetes patients: a systematic review and network meta-analysis. Endocrine 48: 794–803, 2015. doi: 10.1007/s12020-014-0373-0. [DOI] [PubMed] [Google Scholar]
- 35.Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, Zhang Y, Quan X, Ji L, Zhan S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res Clin Pract 110: 26–37, 2015. doi: 10.1016/j.diabres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Tuttle KR, Lakshmanana MC, Rayner B, Busch RS, Zimmerman AG, Woodward DB, Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 6: 605–617, 2018. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 37.Tonneijck L, Smits MM, Muskiet MHA, Hoekstra T, Kramer MHH, Danser AHJ, Diamant M, Joles JA, van Raalte DH. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia 59: 1412–1421, 2016. doi: 10.1007/s00125-016-3938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 37: 2864–2883, 2014. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 221: T1–T16, 2014. doi: 10.1530/JOE-13-0414. [DOI] [PubMed] [Google Scholar]
- 40.Villanueva-Peñacarrillo ML, Alcántara AI, Clemente F, Delgado E, Valverde I. Potent glycogenic effect of GLP-1(7-36)amide in rat skeletal muscle. Diabetologia 37: 1163–1166, 1994. doi: 10.1007/BF00418382. [DOI] [PubMed] [Google Scholar]
- 41.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344: d7771, 2012. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]