Abstract
Alternaria alternata is a fungal allergen associated with severe asthma and asthma exacerbations. Similarly to other asthma-associated allergens, Alternaria secretes a serine-like trypsin protease(s) that is thought to act through the G protein-coupled receptor protease-activated receptor-2 (PAR2) to induce asthma symptoms. However, specific mechanisms underlying Alternaria-induced PAR2 activation and signaling remain ill-defined. We sought to determine whether Alternaria-induced PAR2 signaling contributed to asthma symptoms via a PAR2/β-arrestin signaling axis, identify the protease activity responsible for PAR2 signaling, and determine whether protease activity was sufficient for Alternaria-induced asthma symptoms in animal models. We initially used in vitro models to demonstrate Alternaria-induced PAR2/β-arrestin-2 signaling. Alternaria filtrates were then used to sensitize and challenge wild-type, PAR2−/− and β-arrestin-2−/− mice in vivo. Intranasal administration of Alternaria filtrate resulted in a protease-dependent increase of airway inflammation and mucin production in wild-type but not PAR2−/− or β-arrestin-2−/− mice. Protease was isolated from Alternaria preparations, and select in vitro and in vivo experiments were repeated to evaluate sufficiency of the isolated Alternaria protease to induce asthma phenotype. Administration of a single isolated serine protease from Alternaria, Alternaria alkaline serine protease (AASP), was sufficient to fully activate PAR2 signaling and induce β-arrestin-2−/−-dependent eosinophil and lymphocyte recruitment in vivo. In conclusion, Alternaria filtrates induce airway inflammation and mucus hyperplasia largely via AASP using the PAR2/β-arrestin signaling axis. Thus, β-arrestin-biased PAR2 antagonists represent novel therapeutic targets for treating aeroallergen-induced asthma.
Keywords: airway inflammation, allergen-induced asthma, alternaria alternata, β-arrestin-signaling, biased G protein-coupled receptor signaling, protease-activated receptor-2
INTRODUCTION
Fungal exposure has long been linked to respiratory disease, including allergy and asthma (65). Alternaria alternata is a ubiquitous fungal allergen that has been associated with severe asthma and has been identified as a risk factor for the development and persistence of asthma (27, 36, 51, 55), Although most reports of Alternaria-associated asthma focus on outdoor exposure, including an increase in asthma exacerbations during thunderstorms (e.g., see Ref. 51), indoor exposure to Alternaria has also been associated with increased asthma symptoms (55). More than a dozen IgE-reacting proteins have been isolated from Alternaria, including the major antigen Alt a 1 (10, 22); however these proteins alone do not sufficiently explain the contribution of Alternaria to asthma symptoms and disease.
In addition to allergenic proteins, Alternaria secretes proteases that contribute to airway inflammation (8, 58). These heretofore unidentified proteases are thought to act through the G protein-coupled receptor (GPCR) protease-activated-receptor-2 (PAR2) to induce airway inflammation. That PAR2 is activated by trypsin-like serine proteases associated with allergy-inducing pathogens is not limited to Alternaria and also includes asthma-associated allergens from cockroach and house dust mites (e.g., see Refs. 1, 44, and 50). PAR2 in the airway, presumably activated by either endogenous or allergen-derived proteases, has been implicated in the pathogenesis of allergic asthma. PAR2 activation is associated with an increase in allergen-induced leukocyte lung infiltration, which is reduced in PAR2-null animals (5, 15, 16, 44). We have shown that direct activation of PAR2 using peptidomimetic agonists in ovalbumin-sensitized mice can cause inflammatory cell infiltrate, epithelial thickening, and heightened mucus production via a β-arrestin-2-dependent pathway (40). Interestingly, other studies have additionally implicated PAR2 signaling in bronchodilation independent of the PAR2/β-arrestin-2 signaling axis and via prostanoid release from PAR2-activated bronchial epithelial cells (14, 29, 49). These data suggest that many of the detrimental effects of PAR2 activation in the setting of allergic lung inflammation are β-arrestin dependent, whereas other putative protective effects can occur independent of β-arrestin signaling (40).
Our previous studies of filtrate products derived from Alternaria cultures demonstrated that they promote PAR2 signaling via a serine protease-mediated stimulus (as measured with Ca2+ signaling downstream of a G protein-coupled pathway) and can induce inflammation in vivo (8). Others have shown that Alternaria-dependent protease activity in vivo leads to an increase in airway IL-33 and subsequent asthma exacerbations (58). However, the identity and numbers of Alternaria filtrate serine proteases that can activate PAR2 have not been elucidated, and their mechanisms for regulating airway inflammation and remodeling have not been determined. We hypothesize that a serine protease-mediated PAR2-β-arrestin signaling axis plays a key role in Alternaria-induced asthma symptoms in vivo and that this lung inflammation can be largely mimicked by the isolated protease(s). To test these hypotheses, we first sought to determine whether Alternaria could activate the PAR2-β-arrestin-2 signaling axis. We next sought to determine whether the protease/PAR2/β-arrestin-2 signaling pathway was necessary to induce asthma phenotype in Alternaria filtrate sensitized and challenged wild-type, PAR2−/− or β-arrestin-2−/− mice. Finally, we set out to isolate and characterize the trypsin-like serine protease(s) produced by Alternaria and to evaluate the ability of the enzyme(s) so identified to activate PAR2 to stimulate Ca2+ signaling and PAR2-β-arrestin interactions as well as asthma symptoms in our mouse models.
MATERIALS AND METHODS
Materials.
All chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Alternaria preparations.
Alternaria preparations used in cellular and animal experiments (cat. no. XPM1D3A2.5; Greer Laboratories) were suspended in Hanks’ balanced saline solution buffered with 10 mM HEPES, pH 7.4. Two Alternaria preparations were used for isolation and sequencing of the single serine protease identified by the activity-based probe (below) and for isolation of a purified enzyme preparation for cell function studies: 1) the crude buffer extract of freeze-dried defatted Alternaria powder from above and 2) a culture filtrate from Strain ATCC 11680 (cat. no. XPM1C3A25; Greer Laboratories). The culture filtrate was used directly and the crude defatted Alternaria allergen reconstituted in 25 mM Tris·HCl buffer, pH 7.4, as a stock solution containing ∼1.7 U/mg trypsin-like activity using the QAR-AMC substrate assay (Bachem, I1550; see below). Aliquots of the Tris buffer solution were used for activity-based probe (ABP) labeling and for isolation of the purified protease fractions, as described below.
Other reagents.
The calcium-sensitive indicator Fluo-4 NW was from Life Technologies (Carlsbad, CA). Ion exchange columns were from Bio-Rad (Hercules, CA; Bio-Scale Mini Macro-Prep High Q Cartridge). Antibodies and their catalog numbers were as follows: anti-phospho-cofilin (no. 3311; Cell Signaling Technology), anti-phospho-cofilin-PE-Cy5.5 (no. bs-3099R-Cy5.5; BIOSS), anti-total cofilin (no. 612144; BD Biosciences), anti-CCR3-PE (no. FAB729P; R & D Systems), anti-CD3-APC-780 (no.47-0032-80; eBioscience); anti-CD4-APC (no. 17-0042-81; eBioscience), anti-CD8-PerCP-Cy5.5 (no. 551162; BD PharMingen), anti-CD19-PE (no. 12-0193-82; eBiosciences), anti-CD45-PerCP-Cy5.5 (no. 550994, Clone 30-F11; BD PharMingen), anti-CD45-FITC (no. 11-0451-81; eBioscience), anti-GR1-APC (no. 17-5931-81; eBioscience), F4/80, and APC-eFluor 780 (no. 47-4801-82; eBioscience). NOVEX 4–20% polyacrylamide gels were from Life Technologies.
Animal studies.
All animal procedures were in accordance with the guidelines on the use and care of laboratory animals set by the National Institutes of Health and approved by the Institutional Animal Care and Use Committees at the University of California Riverside (Riverside, CA) and the University of Calgary (Calgary, AB, Canada). β-Arrestin-2−/− mice on a C57BL/6 background were provided by Robert Lefkowitz (Duke University Medical Center). PAR2−/− mice were provided by Dr. Robin Plevin (University of Strathclyde, Glasgow, Scotland) and were developed by KOWA Pharmaceuticals. WT C57BL/6 (wild-type) mice were from The Jackson Laboratory. All animals were bred in-house. Animals were between 6 wk and 1 yr of age, and animal study groups were matched by age and sex. Specific numbers in each group are listed in the text.
Bioluminescence resonance energy transfer measurements of recruitment of β-arrestin-2.
Bioluminescence resonance energy transfer (BRET), in which the energy emitted when luciferase (Luc) oxidizes its substrate will excite an acceptor fluorophore [yellow fluorescence protein (YFP)] only if the two proteins containing the luciferase and YFP tags are in very close proximity (usually 2–6 nm), provides a means for monitoring direct actions in live cells. Briefly, cells transfected with PAR2-YFP and luciferase-tagged β-arrestin-2 were plated in 96-well microplates, and either vehicle or agonist is added along with the Luc substrate coelenterazine. Readings were collected using a Multilabel Reader Tristar9640 from Berthold, and emission was detected at 480 nm (Luc) and 535 nm (YFP). Net BRET was determined from the ratio of emission in the YFP channel to the emission in the luciferase channel (E535/E488) minus the donor-only (β-arrestin-luc) control values. To eliminate the possibility that observed signals represented nonspecific interactions, we included a β-arrestin-Luc + YFP in every experiment, which gave values similar to donor-only controls. Dose curves, ranging from 6.5 to 650 µg/ml Alternaria filtrate, were used to evaluate agonist EC50s; kinetic curves were performed with a single dose of agonist (65 μg/ml) and BRET values monitored over 20 min. For acceptor/donor ratio assays, β-arrestin-Luc (donor) concentration was held constant at 0.5 μg, and PAR2-YFP concentration was varied from 0.5 to 1.5 μg. BRET values were determined after 15 min of incubation with 65 μg/ml Alternaria filtrate.
Preparation of spleen lymphocytes for migration and signaling assays.
To isolate lymphocytes, age- and sex-matched wild-type and β-arrestin-2−/− mice were euthanized and spleens immediately removed, minced, homogenized in 10 ml of PBS, and filtered through sterile nylon mesh. Cells were passed through 70-μm filters, pelleted at 250 g for 5 min, washed three times in modified Hanks’ balanced saline solution (without Ca2+ and Mg2+), and resuspended in RPMI complete media for subsequent experiments. Cytospin analysis and flow cytometry revealed the prep was >80% lymphocytes.
Transwell migration assay.
After RBC lysis, ∼105 cells (isolated as described above) were plated onto collagen-coated membrane inserts with 5-μm pores and treated with Alternaria filtrate (65 μg/ml) or 100 nM 2fAP. After 1 h, nonmigratory cells were removed from the top of the filter, and migration was analyzed two ways. In the first procedure, cells attached to the underside of the filter were stained with crystal violet, and stained cells and migrated lymphocytes (identified by cell morphology) were counted using a bright-field compound microscope in four fields of vision. Migrated cells were then expressed as a fold change over baseline migration to saline control. Saline solution here and throughout in vitro and in vivo experiments was Hanks’ balanced saline solution buffered with 10 mM HEPES, pH 7.4 (HBSS). In the second procedure, migrated cells were gently scraped into 2 ml of FACS buffer, and CD4+ T cells were confirmed by flow cytometry using anti-CD3 and anti-CD4, antibodies. Migrated CD4T-cells were expressed as the number of cells per milliliter.
Determination of phospho-cofilin in cultured leukocytes.
Cells (105) were plated on 24-well culture dishes and grown overnight in serum supplemented RPMI when media was replaced with serum free media. Cells were allowed to recover from media exchange for 1 h, after which trypsin or Alternaria filtrate (65 μg/ml) was added for 0–60 min. Cells were lysed in 1× Laemmli sample buffer, sonicated, boiled, and analyzed by 15% SDS-PAGE, followed by Western blotting with anti-phospho-cofilin (rabbit, 1:1,000) and anti-total cofilin (mouse, 1:1,000) antibodies. Secondary antibodies anti-rabbit IR-800 (no. 611-732-127; Rockland) and anti-mouse Alexa 680 (no. A-21059; Invitrogen) were added at 1:30,000 and bands visualized on a LICOR Odyssey Infrared Imaging system. Band intensities were quantified with the LICOR Odyssey software, and phospho-cofilin levels were normalized to total cofilin levels for each blot.
Mouse inflammation models and measurement of airway inflammation.
Paired 6-wk-old wild-type, β-arrestin-2−/−, and PAR2−/− mice were exposed to either 25 μl of HBSS alone (negative saline control) or 0.4 mg/kg of Alternaria filtrate (in 25 μl HBSS), administered intranasally three times over an 8-day period (on days 1, 5, and 8). For trypsin inhibitor experiments, 232 pmol of soybean trypsin inhibitor (SBTI) were included with the Alternaria filtrate during each intranasal administration. For studies with purified AASP, we used 1.5 and 0.075 units of AASP in 25 μl of HBSS, administered intranasally on the same schedule, as was done for the Alternaria. Mice were euthanized on day 9, and lungs were lavaged with 5 ml of PBS. Bronchioalveolar lavage fluid (BALF) was centrifuged, and supernatants were used for analysis of cytokine production. Cell pellets were resuspended in PBS, subjected to NH4Cl lysis (0.83% ammonium chloride for 2 min at 4°C) to remove red blood cells (RBCs), and resuspended in 1 ml of PBS. Cell numbers were determined by hemocytometer, and samples were then divided into 2 (200 μl was used for cytospins, and 300 μl was used for flow cytometry). Lungs were simultaneously harvested and fixed for histological analysis.
Hematoxylin and eosin stain, Alcian blue stain, and quantification.
Staining was performed on paraffin-embedded sections and analyzed as described in previous studies (40). Briefly, for general histological analysis, lung sections were stained with hematoxylin and eosin (H & E) and histological grading based on infiltration of white blood cells and perivascular thickness. Separate sections were stained with Alcian blue (to stain mucin) and fast nuclear red (to identify cell nuclei). Quantification of inflammation and mucin staining was performed by histological grading, as previously described (40). For histological grading of inflammation, a score of 1 to 4 was given, with 1 being no inflammation present, 2 being one ring of leukocytes around the vasculature and mild infiltration, 3 being two to three rings and moderate infiltration, and 4 being four or more rings and numerous leukocytes throughout the epithelial tissue. Epithelial thickness was quantified using National Institutes of Health (NIH) ImageJ to measure the distance from the basolateral surface of the epithelial cell to the muscular layer. Mucin-positive cells were quantified by calculating the percentage of total epithelial cells per millimeter that stained positive with Alcian blue. Because Alcian blue can also stain basal lamina matrix proteins, staining at the basal lamina was not considered positive. Mucin staining was calculated using a user-defined NIH ImageJ macro; images were inverted, and total red channel density (corresponding to blue by eye) per 100 cells was determined.
Cytospins and differential cell counts.
Briefly, 100 μl of BALF was spun on to glass slides using Shandon CytoSpin, and cells were stained using a Hema 3 stain kit (Fisher Scientific), following manufacturer’s protocol. Differential counts were obtained by counting 200 cells/slide and categorized based on morphological criteria, as described in our previous study (40).
Flow cytometry.
BALF cell pellets were resuspended in 1 ml of FACS buffer and stained with a panel of antibodies (group A: anti-CD45, anti-CD3, anti-CCR3, and anti-GR1; group B: anti-CD4, anti-CD19, anti-CD8, anti-CD4 and anti-F4/80), as described in our previous studies using a BD FacScan, and analyzed with FlowJo version 6.1 (40). From CD45-positive population, FSC/SSC was used to identify granulocytes (high FSC/SSC), macrophages (high-FSC/mid-SSC) and lymphocytes (high-FSC/low-SSC). From the granulocyte population, eosinophils (CCR3+) and neutrophils (GR1) were further identified. T-lymphocytes were further confirmed as CD3+ and either CD4+ or CD8+. B cells were identified as the CD19-positive group. Macrophages were further confirmed as F4/80+. For in vivo cofilin dephosphorylation assays, resuspended BALF cells were fixed, permeabilized, and incubated with PE-Cy5.5-conjugated anti-phospho-cofilin and FITC-conjugated anti-CD45. Cells were first gated on CD45 staining to identify leukocytes, and granulocyte and lymphocytes were identified based on FSC/SSC. The population described as “granulocytes” in Fig. 8 was previously shown to be comprised primarily of eosinophils, and to a lesser extent, neutrophils and the population described as “lymphocytes” were primarily CD3 positive (see Fig. 7). Phospho-cofilin levels were then analyzed in granulocyte and lymphocyte populations. One sample from each treatment group received PE-Cy5.5 IgG isotype as a negative control (no. bs-0295P-Cy5.5; BIOSS). The number of cells with a mean fluorescence level above 5 × 103 and the mean fluorescence intensity in each treatment group were determined.
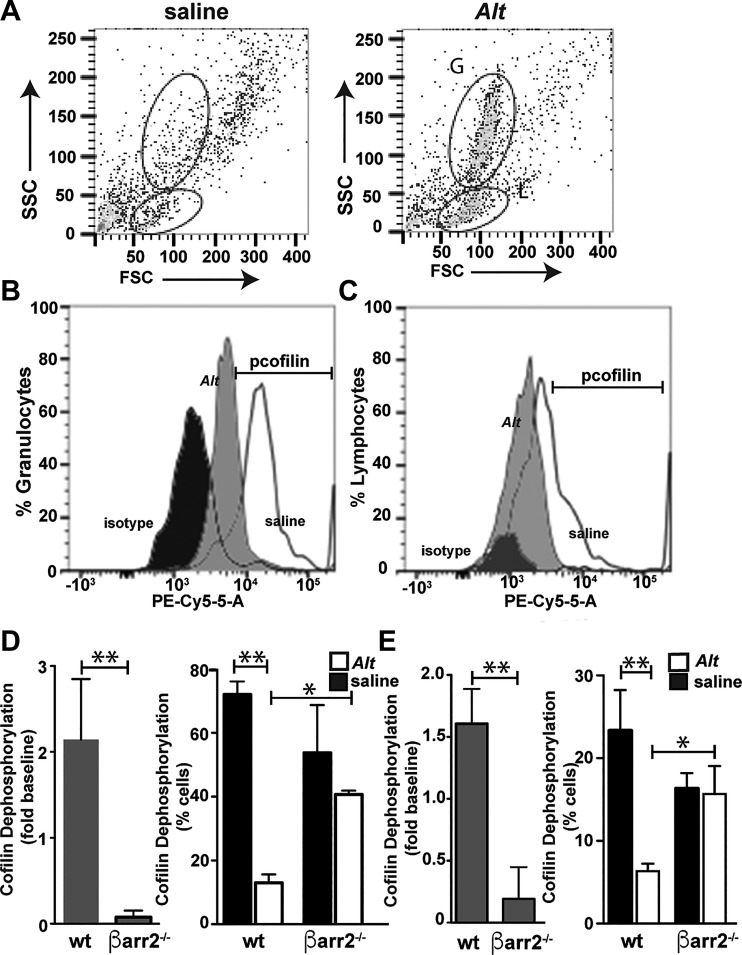
Fig. 8.
Alternaria (Alt) induces cofilin dephosphorylation in granulocytes and lymphocytes in vivo. Cells were recovered from the bronchioalveolar lavage fluid (BALF) isolated from Alt-treated wild-type (WT) and β-arrestin-2−/− mice. Cells were incubated with anti-CD45 and anti-phospho (p)-cofilin antibodies and analyzed by flow cytometry. Representative dot plot of CD45+ cells analyzed for forward (FSC) and side scatter (SSC). A: granulocytes (G) and lymphocyte (L) populations are indicated. B and C: histogram analysis of p-cofilin fluorescence in the granulocyte (B) and lymphocyte (C) population after treatment with Alt (gray) or Hanks’ balanced saline solution control (white). Isotype saline-treated controls (black) are included to show background fluorescence levels. The positive p-cofilin gate is indicated by the bracket. D and E: overall cofilin dephosphorylation (left) and %cells (right) showing reduced cofilin dephosphorylation are shown for granulocytes (D) and lymphocytes (E). Each group includes data from ≥4 and ≤7 mice. Significant differences between bracketed Alt-treated mice are denoted as follows: *P ≤ 0.05 or **P ≤ 0.01. Alt-dependent cofilin dephosphorylation in vivo was dependent on β-arrestin expression.
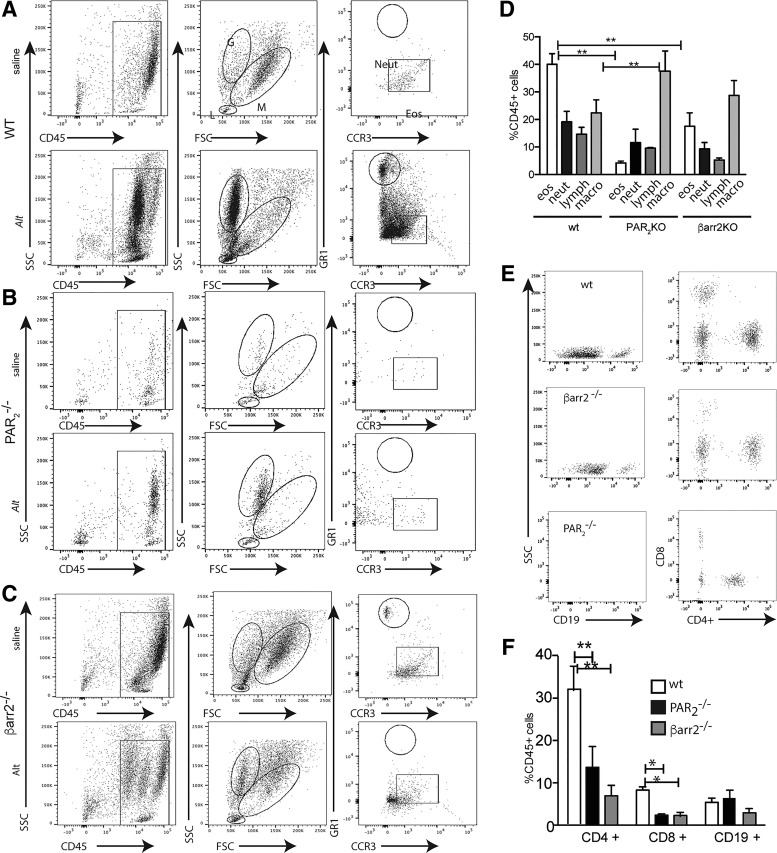
Fig. 7.
Alternaria treatment increases the percentage of eosinophils (eos) and CD4+ lymphocytes (lymph) in the bronchioalveolar lavage fluid (BALF) by a protease-activated receptor-2 (PAR2)/β-arrestin-2-dependent mechanism. A–C: representative histograms showing the gating strategy for identification of BALF cell types in wild-type (WT; A), PAR2−/− (B), and β-arrestin-2−/− (C) mice. D: %CD45+ cells that are scored as eosinophils [high side scatter (SSC), mid-foward scatter (FSC), CCR3+], neutrophils (neut; high SSC, high FSC, GR1+), lymphocytes (low SSC, mid-FSC, CD3+) or macrophages (macro; high SSC/high FSC) by flow cytometric analysis of BALF from Alt-treated mice. E and F: %CD45+ cells from Alt-treated mice that are CD4+ (Th2), CD8+ (Natural Killer cells) or CD19+ (B cells). Each group includes cell isolations from at least 4 and up to 7 mice. Significant differences between bracketed Alt-treated mice are denoted by *P ≤ 0.05 or **P ≤ 0.01.
Quantifying serine protease.
Alternaria enzyme samples were quantified in terms of their “trypsin-like” equivalents, using a microtiter plate fluorogenic assay with Glu-Ala-Arg-aminomethylcoumarin (QAR-AMC; I-1550; Bachem, Bubendorf, Switzerland) as substrate. High-specific-activity trypsin was used as a standard (catalog no. T0303, 16,000 U/mg, Type IX-S porcine trypsin; Sigma). The filtrate suspended in Hanks’ buffered saline solution (HBSS) with a protein content of ∼650 μg/ml bovine albumin equivalents routinely had a trypsin-like enzyme activity equivalent to 1.0 - 2.5 U/ml (as detected with the QAR substrate). Alternaria enzyme samples were also tested for serine and cysteine cathespin substrate, Phenylalanine-Arginine-aminomethylcoumarin (FR; I-1160; Bachem) and urokinase substrate Glycine-Glycine-Arginine-minomethylcoumarin (GGR; 230914; Calbiochem).
Activity-based probe labeling of serine proteases for mass spectral sequencing.
The serine protease activity-based probe (ABP) Biotin-Pro-Lys-diphenylphosphonate was synthesized and graciously provided for our studies by Dr. Brendan Gilmore, Queens University School of Pharmacy, Belfast, UK. Covalent labeling of enzymes in the Alternaria filtrates and crude cellular antigen, as well as in chromatographic column fractions isolated from these sources, was achieved essentially as described previously (28, 50), with minor modifications. The Alternaria samples were diluted to a final concentration of 1.5 U/ml and incubated with 100 µM of the ABP in a 10-µl reaction volume consisting of: 50 mM Tris·HCl, pH 8, 0.1% NP40, and 1.5 mM CaCl2 for 2 h at room temperature to biotinylate the enzymes. The reaction was terminated with the addition of Laemmli sample buffer, SDS-PAGE analysis (4–20%), followed by Western blotting with horseradish peroxidase-conjugated streptavidin. Additionally, the total protein in the fractions was visualized by staining gels with SYPRO Ruby fluorescent protein stain (Life Technologies) and monitoring UV-activated fluorescence in a Kodak Image Station 4000 MM Pro gel doc apparatus.
For isolation of the biotinylated enzymes for mass spectral sequencing, volumes corresponding to 7.5–15 U total of trypsin-like activity (estimated to be ∼0.5 to 1 µg of enzyme) were incubated with 100 μM of the ABP. The ABP-bound proteins were pulled down with Avidin-Sepharose beads for 1 h, washed five times with 2 M urea, and analyzed by SDS-PAGE (4–20%) and protein with Coomassie Blue stain (Bio-Rad). Bands in the molecular weight range of the ABP-labeled allergen enzyme (20–25 kDa) were excised from the gel and digested with chymotrypsin (10 μg/ml) at 37°C overnight. Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) analysis of in-gel chymotrypsin digested-proteins was carried out using a LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an Advion nanomate ESI source (Advion, Ithaca, NY) following ZipTip (Millipore, Billerica, MA), as described previously (52, 56). Data-dependent scanning was performed by the Xcalibur version 2.2 SP1.48 software using a survey mass scan at 60,000 resolution in the Orbitrap analyzer scanning m/z 400–2,000, followed by collision-induced dissociation (CID) tandem mass spectrometry (MS/MS) of the six most intense ions in the linear ion trap analyzer (4). The MS/MS spectra of visible protein bands processed for LC-MS/MS as above were searched against a custom database made of 1) NCBI proteins that contained the word “trypsin” in their annotation entries and 2) proteins that contained the word “trypsin” found at the Alternaria genome database. This custom protein database contained 4,623 entries. Variable modifications considered during the search included methionine oxidation (15.995 Da) and cysteine carbamidomethylation (57.021 Da) as well as adduction of lysine or cysteine residues by 4HNE (156.115 Da). Proteins were identified at 95% confidence, with XCorr scores as determined by a reversed database search.
Ion exchange chromatography for isolation of Alternaria serine proteases.
Proteases in the Alternaria culture filtrate and in the reconstituted Alternaria cellular antigen extract were subjected to anion and cation column chromatography. In brief, enzyme-containing preparations were dialyzed for 2 h at 4°C against 20 mM Tris·HCl buffer, pH 7.2, containing 10 mM NaCl. Dialyzed samples (1-ml volumes at 7.0–7.2 U/ml) were then applied to a 5-ml High Q ANION exchange column (40 × 12.6 mm) with a fast-phase liquid chromatography (FPLC) system (Pharmacia; Stockholm, Sweden) and 1-ml fractions were collected at a flow rate of 1 ml/min using a gradient/step NaCl elution protocol ranging from 10 mM to 1 M salt. The eluted fractions were monitored for both protein (absorption at 280 nm and SDS-PAGE) and trypsin activity (QAR substrate). Each fraction containing serine protease activity was analyzed by ABP assay, as described above.
Calcium signaling assay.
Kirsten virus-transformed rat kidney cells (KNRK), which do not express functional endogenous PAR2, were transfected with COOH terminally tagged YFP-rat-PAR2 (3), seeded at 50% confluence in 96-well black cell culture plate (BD Falcon, Franklin Lakes, NJ), and grown overnight. Cells were loaded for 30 min at 37°C with the no-wash Ca2+-sensing dye Fluo-4 NW (25 μg/ml) in Ca2+ assay buffer [HEPES-fortified Hank’s buffered saline, pH 7.4 (Gibco): 10 mM HEPES, 1.5 mM CaCl2, 1.5 mM MgCl2] along with 2.5 mM probenecid (Invitrogen, Carlsbad, CA). The plates were loaded into the Victor X4 2030 Multilabel plate reader (Perkin-Elmer, Waltham, MA), and agonists were applied using the plate reader sample injection system. Cells in the plate were excited at 480 nm, and kinetic traces of fluorescence emission at 530 nM, representing increased cytoplasmic Ca2+ levels, were recorded every 0.5 s for 1 min. Fluorescence levels were normalized to the signal generated by 2 µM Ca2+ ionophore A23187 (Sigma, St. Louis MO).
Measurement of Alternaria enzyme-stimulated bronchorelaxation.
After euthanasia, second-order bronchioles were excised and trimmed for mounting in a wire myograph for bioassay measurements at resting tension in a 37°C Krebs buffer bath. After 60 min of equilibration at ~0.5 g of resting tension, tissues were treated with 50 mM KCl (to test responsiveness), washed, and precontracted with 500 nM carbachol. After stable force was achieved (100% on the waveforms in response to carbachol), tissues were treated with substance P (SP), a neurokinin-1 receptor agonist, to demonstrate viable Gαq signaling. Tissues were washed, followed by treatment with Alternaria enzyme (0.6 U/ml). The relaxation response was recorded as done previously (40), with relaxation expressed as a percentage reduction in tension (%) relative to the tension observed with carbachol.
Data and statistical analysis.
All graphs and statistical analyses were performed using Microsoft Excel 2011 or GraphPad Prism 6.0. Experiments were performed a minimum of three times; specific numbers are indicated in the figure legends. Statistical significance was determined using one-way ANOVA and Tukey t-tests (to compare between treatment groups).
RESULTS
Alternaria-activated PAR2 signaling invokes β-arrestin signaling.
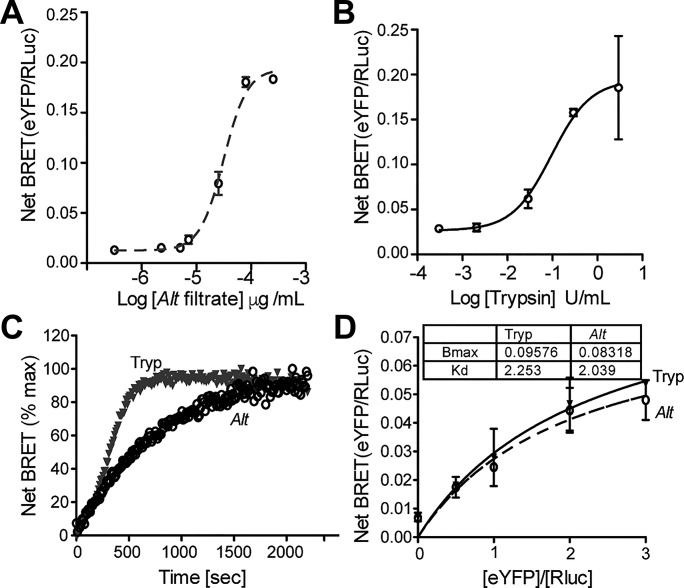
Existing cellular studies demonstrating Alternaria-PAR2 activation have relied on the PAR2/Gq/Ca2+ signaling as a cellular readout (8, 39). We have shown using the PAR2-activating peptide 2-furoyl-LIGRL-Orn-NH2 (2fAP) that PAR2-stimulated leukocyte cell migration, activation of cofilin, and subsequent actin reorganization and airway inflammation were dependent upon PAR2/β-arrestin-2 signaling (71, 72). To determine whether Alternaria could induce full PAR2 signaling (i.e., G protein and β-arrestin signaling), we first used bioluminescence resonance energy transfer (BRET), a cellular assay to examine the recruitment of luciferase-tagged β-arrestin-2 to PAR2-YFP, in response to receptor activation by Alternaria filtrate or purified trypsin (Fig. 1A) (38). Concentration-effect curves (0–650 μg/ml protein tested) showed that Alternaria filtrate stimulated BRET with an EC50 of 65 μg/ml protein (Fig. 1A). Comparison experiments using the PAR2-activating serine protease trypsin (0.00045–4.5 U tested in a 200-μl reaction) resulted in an EC50 of 2.0 U/ml (Fig. 1B). Because β-arrestin interactions are dependent upon receptor conformation, this steric requirement can translate into differences in the apparent rate of recruitment, the length of receptor/β-arrestin interactions, and/or differences in the apparent affinity of β-arrestin for PAR2. Differences in the apparent affinity of β-arrestin for PAR2 can be assessed by determining the acceptor/donor ratio at which BRET50 is obtained. Time-based capture of BRET shows that the kinetics of recruitment were slightly more rapid in response to trypsin than with Alternaria filtrates (Fig. 1C). However, both maximal BRET values (BRETmax) and half-maximal BRET values (BRET50) for β-arrestin-2/PAR2 interactions were similar in response to either 65 μg/ml Alternaria filtrate or 2.0 U/ml trypsin (Fig. 1D).
Fig. 1.
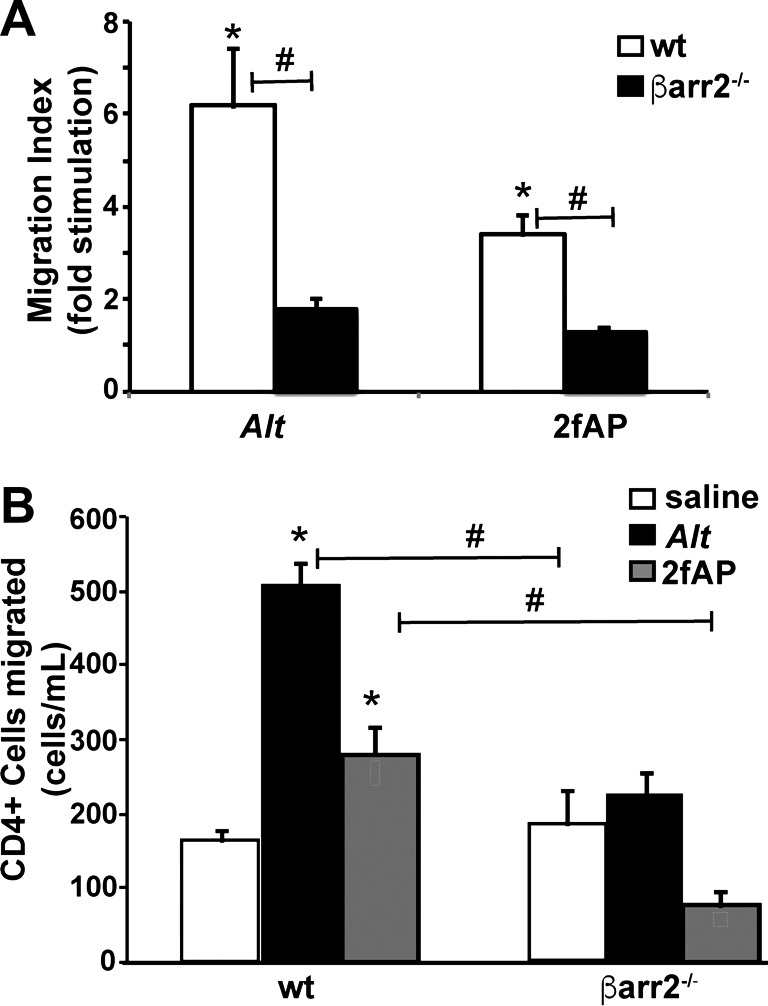
Alternaria promotes recruitment of β-arrestins to protease-activated receptor-2 (PAR2). A and B: bioluminescence resonance energy transfer (BRET) was measured upon addition of increasing concentrations of Alternaria (Alt) filtrate (A) or trypsin control (B). C: the time to reach maximal BRET signal was determined in response to Alt or trypsin. D: half-maximal (BRET50) and maximal BRET values (BRETmax) were determined for recruitment of β-arrestin-2 to PAR2 in response to 65 μg/ml Alt or 2 U/ml of trypsin. Alt triggered β-arrestin recruitment, similar to trypsin controls, albeit with a slightly reduced time constant; n ≥ 3 for all experimental time points; see materials and methods for experimental details.
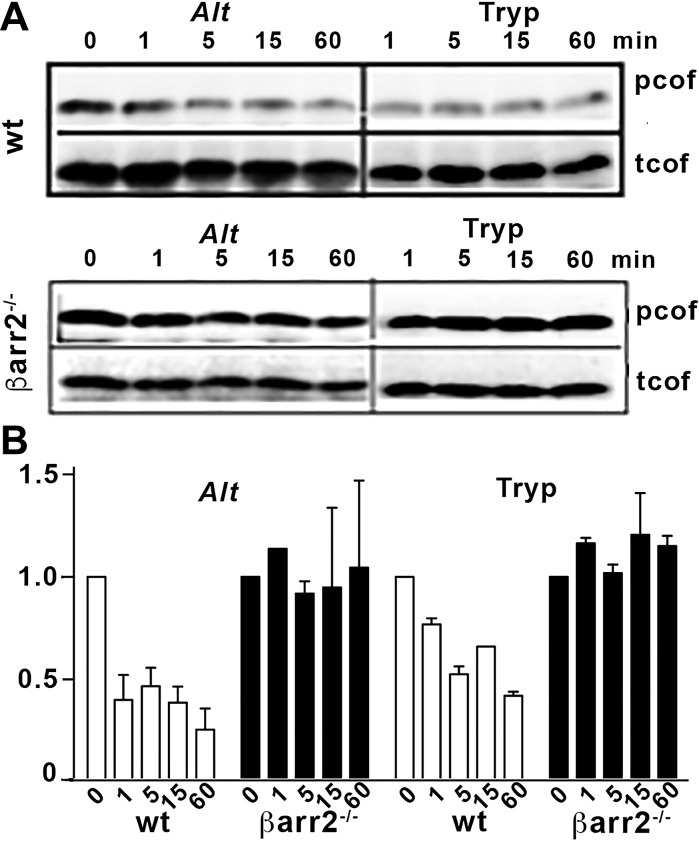
We have demonstrated that 2fAP-activated PAR2 promotes dephosphorylation and activation of cofilin by a β-arrestin-dependent, G protein-independent mechanism (71). We used this cellular assay to verify activation of Alternaria-induced, β-arrestin-dependent PAR2 signaling. Naïve lymphocytes isolated from spleens of wild-type or β-arrestin-2−/− mice were treated with Alternaria filtrate or trypsin (as a positive enzyme control) for 0–60 min. Using cytospin preparations, we determined that the preparation was predominantly lymphocytes, and there were no differences in basal numbers of lymphocytes between wild-type and β-arrestin-2−/− mice (not shown). Lysates were analyzed by SDS-PAGE, followed by Western blotting with anti-phospho(p)-cofilin and anti-total(t)-cofilin antibodies (Fig. 2, A and B). Both Alternaria filtrate and trypsin promoted cofilin dephosphorylation (≤45% of control values). This dephosphorylation response was abolished in cells prepared from β-arrestin-2−/− mice. We next performed Transwell chemotaxis assays with immune cells isolated from spleens of wild-type or β-arrestin-2−/− mice. Addition of Alternaria filtrate or 2fAP to the bottom chamber promoted an increase in wild-type cell migration compared with HBSS controls (6.1 ± 1.1-fold and 2.5 ± 0.3-fold recruitment, respectively; Fig. 3A). Migrated cells were collected from the Transwell filters and further analyzed by flow cytometry using CD4+ staining to specifically quantify T cell migration. Controls using saline without PAR2 activators resulted in recruitment of 165 ± 23 CD4+ cells/ml (Fig. 3B). Addition of Alternaria filtrates (510 ± 54 CD4+ cells/ml) or 2fAP (290 ± 48 CD4+ cells/ml) significantly increased the numbers of recruited T cells (Fig. 3B) when compared with the saline controls. In contrast, there was no increased migration of CD4+ T cells from β-arrestin-2−/− mice to Alternaria filtrate or 2fAP (Fig. 3, A and B). Taken together, we conclude that Alternaria filtrates promote protease-dependent PAR2 signaling through β-arrestin pathways. Coupled with previous results with Ca2+ signaling responses (8, 39), Alternaria filtrates can fully activate PAR2 signaling.
Fig. 2.
Alternaria promotes β-arrestin dependent activation of cofilin. Adherent cells from spleens of wild-type (WT) and β-arrestin-2−/− (β-arr2−/−) mice were treated with 65 μg/ml Alternaria (Alt) or 2 U/ml trypsin (Tryp; control). A: Western blots of cell lysates detecting phosphorylated (p) or total (t) cofilin (cof) are shown. B: quantification of normalized p-cofilin levels (fold change from untreated cells); n = 3 for each experiment, and data are shown as means ± SE. Alt activated cofilin is similar to the trypsin control.
Fig. 3.
Alternaria promotes β-arrestin-dependent lymphocyte migration. Lymphocytes were seeded onto Transwell filters, and basal medium was supplemented with Hanks’ balanced saline solution (HBSS), Alternaria (Alt), or the protease-activated receptor-2 (PAR2) agonist 2-furoyl-LIGRL-Orn-NH2 (2fAP). After 1 h, migrated cells were stained with Crystal Violet and counted (A) or recovered into 0.5 ml of FACS buffer, incubated with antibody to CD4, and analyzed by flow cytometry (B); n = 3 for each experiment, and data are shown as means ± SE. *Significant difference between HBSS and Alt-treated groups; #significant difference between bracketed groups (P < 0.01). Both Alt- and 2fAP-induced lymphocyte migration was abrogated in β-arrestin2−/− mice.
Protease-dependent, Alternaria-induced airway inflammation and goblet cell hyperplasia in vivo requires PAR2 and β-arrestin-2.
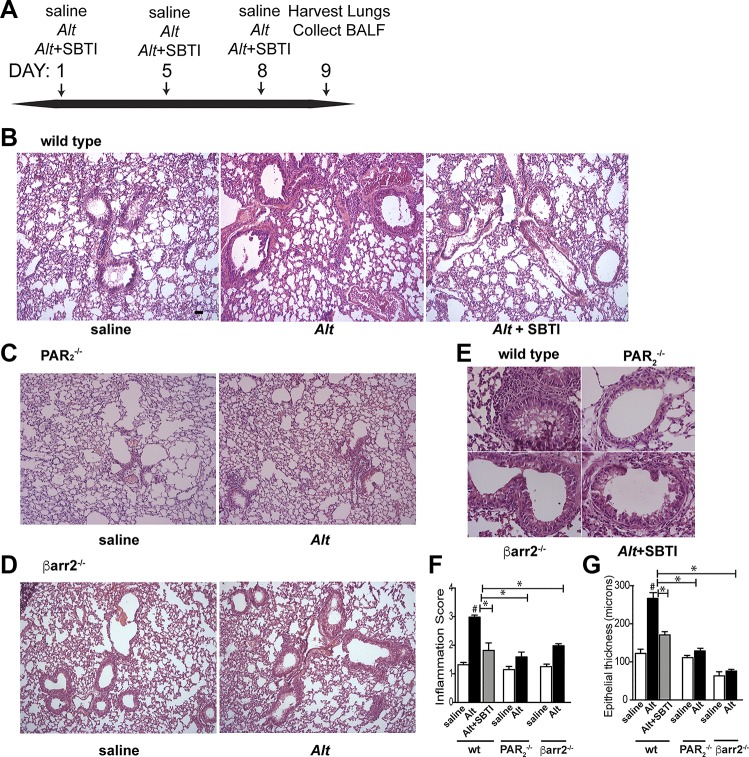
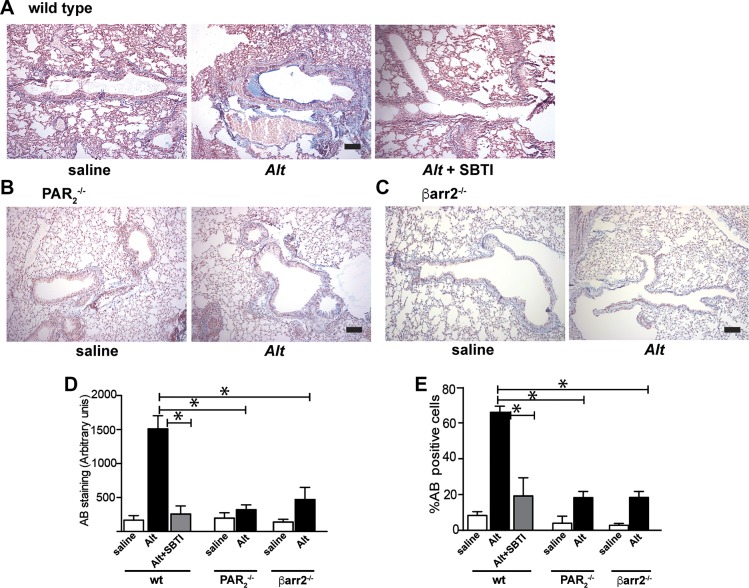
To determine the role of the PAR2/β-arrestin signaling axis in Alternaria-induced inflammation, Alternaria filtrate was administered to mice intranasally on days 1, 4, and 8 (Fig. 4A). Lung tissue and bronchoalveolar lavage fluid (BALF) were collected 24–48 h after the final administration for analysis. Epithelial damage and cellular inflammation were first assessed by examination of hematoxylin and eosin- (H & E)-stained lungs (Fig. 4, B–F). In wild-type mice, Alternaria filtrate induced inflammation that was largely reduced if SBTI was administered with the filtrate (Fig. 4, B–F). Alternaria filtrate treatment of PAR2−/− or β-arrestin-2−/− mice, however, resulted in only minor inflammatory response (Fig. 4, C, D, and F). Examination of bronchial epithelium in the exposed samples indicated that Alternaria promoted a twofold increase in epithelial thickness in the wild-type mice that was significantly abated by SBTI in the wild types as well and not observed in either PAR2−/− or β-arrestin-2−/− mice (Fig. 4G). We next examined the effect of Alternaria filtrate exposure on both the amount of mucin produced and the number of goblet cells in wild-type, PAR2−/−, and β-arrestin-2−/− mice using Alcian blue (AB) to mark acidic proteoglycans present in mucin and goblet cells (Fig. 5, A–C). Both increased mucus staining and goblet cell hyperplasia in response to Alternaria were abolished when Alternaria was administered with SBTI and were not observed in either the PAR2−/− or β-arrestin-2−/− mice. Semiquantification of the AB-stained lungs reveals a 15-fold increase in mucin staining (Fig. 5D) and a ninefold increase in mucin-positive goblet cells (Fig. 5E) in response to Alternaria exposure in wild-type mice. In contrast, Alternaria exposure of wild-type mice in the presence of SBTI resulted in AB staining and goblet cell numbers that were not different from the saline-treated controls (Fig. 5, D and E). Similarly, Alternaria filtrate exposure of PAR2−/− or β-arrestin-2−/− mice only slightly increased mucin staining and goblet cell number. Taken together, these data strongly support that Alternaria filtrate-mediated airway inflammation and mucus hyperplasia requires proteolytic activation of PAR2 and subsequent signaling through β-arrestins to induce asthma symptoms.
Fig. 4.
Alternaria induces protease-activated receptor-2 (PAR2) and β-arrestin-2-dependent inflammation of the airway epithelium. A: timeline of Alternaria filtrate (Alt) exposure included 4 mice/treatment group, which was repeated 3 times. B–D: representative images (×10) of hematoxylin and eosin-stained lungs after Hanks’ balanced saline solution control, Alt, or Alt + soybean trypsin inhibitor (SBTI) in wild-type (B), PAR2−/− (C), or β-arrestin-2−/− mice (D). Scale bar in B = 100 μm and is representative of images in B–D. E: higher magnification of lungs from Alt-treated mice are shown for emphasis of differences in inflammation. F and G: histological grading of slides, determined from 10–15 images quantified/mouse. Each group includes data from ≥4 and ≤8 mice. Significant differences between Alt-treated and saline controls or between bracketed Alt-treated groups are indicated as follows: *P ≤ 0.05. Alt-induced inflammation is reduced by protease and requires expression of PAR2 and β-arrestin-2; #P < 0.01, significant difference from saline-treated control.
Fig. 5.
Alternaria-induced mucus production and goblet cell hyperplasia require protease-activated receptor-2 (PAR2) and β-arrestin-2. A–C: representative images of Alcian blue (AB)-stained paraffin-embedded lung sections from wild-type (A), PAR2−/− (B), and β-arrestin-2−/− (C) mice (×10 mag). D and E: quantification of mucin staining (D) and %goblet cells (AB positive/10 mm2 section of epithelium; E). Each group includes data from ≥4 and ≤8 mice. Significant differences between Alt-treated and saline controls or between bracketed Alt-treated groups are indicated as follows: *P ≤ 0.01. Alt-induced mucus enhancement is reduced by protease and requires expression of PAR2 and β-arrestin-2.
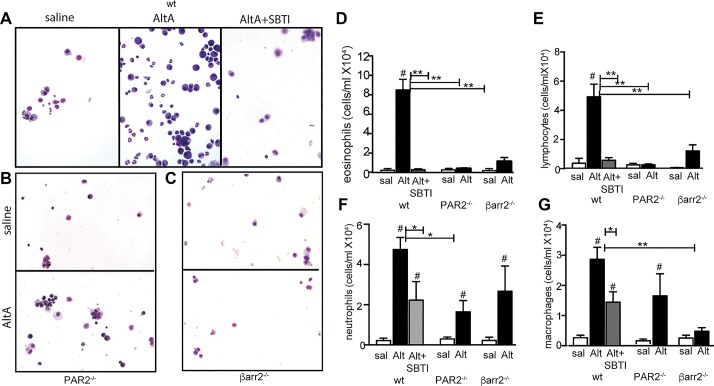
A major hallmark of airway inflammation, and one that was apparent in histological analyses above, is the infiltration of leukocytes into the lung. Both the total number of leukocytes present and the relative distributions of each cell type are indicative of the inflammatory response. Alternaria-induced cell infiltration into the airways can be seen in BALF cytospin preparations from wild-type mice and is markedly reduced when SBTI is included in the intranasal challenges (Fig. 6A). Examination of cytospin preparations from PAR2−/− and β-arrestin-2−/− mice also show reduced cell recruitment when comparing Alternaria filtrate exposure to HBSS-exposed controls (Fig. 6, B and C). Differential counts of cytospins (Fig. 6, D–G) revealed that exposure of wild-type mice to Alternaria filtrate increases eosinophil concentration in the BALF almost 150-fold in the wild-type animals (600 cell/ml in saline-treated mice to 88,800 cells/ml in the Alternaria-treated mice; Fig. 6D). Similar, albeit less dramatic, increases were observed for lymphocyte concentrations (14.5-fold change from 3,400 to 49,300 cells/ml; Fig. 6E), neutrophil concentrations (41-fold change from 1,110 to 45,100 cells/ml; Fig. 6F), and macrophage concentrations (11-fold change from 2,600 to 28,600 cells/ml; Fig. 6G). When compared with the Alternaria-treated wild type, both Alternaria filtrate/SBTI-treated wild type mice and Alternaria filtrate-treated PAR2−/− mice showed similar reductions in airway inflammation. Eosinophil BALF concentrations (1,600 cell/ml in the wild-type Alternaria/SBTI-treated animals and 7,800 cells/ml in the Alternaria-treated PAR2−/− animals) and lymphocyte BALF concentrations (3,800 and 2,500 cells/ml, respectively) were reduced by >90% (Fig. 6D). Neutrophil BALF concentrations (16,200 cells/ml in Alternaria/SBTI animals and 14,900 cells/ml in Alternaria-treated PAR2−/− animals) were reduced by ∼65% (Fig. 6E). Macrophage BALF concentrations (14,400 cells/ml in Alternaria/SBTI-treated animals and 13,400 cells/ml in Alternaria-treated PAR2−/− animals) were reduced by ∼50% (Fig. 6F). Whereas airway inflammation was also significantly reduced in the Alternaria-treated β-arrestin-2−/− mice, the pattern of inflammation reduction was distinct. Eosinophil BALF concentration in Alternaria-treated β-arrestin-2−/− mice (11,400 cells/ml) was reduced by 87% compared with Alternaria-treated wild-type mice (Fig. 6D); lymphocytes (12,200 cells/ml) and neutrophils (10,700 cells/ml) were reduced by ∼75% compared with Alternaria-treated wild type (Fig. 6, E and F), and macrophage recruitment (4,800 cells/ml) was decreased by 83% in β-arrestin-2−/− mice (Fig. 6G). In summary, Alternaria-induced eosinophil and lymphocyte recruitment was largely limited by protease inhibition, loss of PAR2 signaling, or β-arrestin signaling, whereas macrophage recruitment and, to a lesser extent, neutrophil recruitment were more sensitive to the β-arrestin signaling pathway. These data suggest an interplay between multiple signaling pathways for full Alternaria-induced inflammation.
Fig. 6.
Alternaria (Alt)-induced airway leukocyte migration requires protease-activated receptor-2 (PAR2) and β-arrestin-2. A–C: representative Giemsa-stained cytospins from bronchioalveolar lavage fluid (BALF) of wild-type (A), PAR2−/− (B), and β-arrestin-2−/− (C) mice. D–G: differential counts of eosinophils (D), lymphocytes (E), neutrophils (F), and macrophages (G) in BALF cytospins; n = 8 animals with 4 slides/treatment and 2 fields of vision/slide. #Significant differences between matching saline (sal)-treated controls. Significant difference between bracketed Alt-treated groups are indicated as follows: *P ≤ 0.05; **P ≤ 0.01. Alt-induced eosinophil and lymphocyte recruitments were abolished, and neutrophil recruitment reduced by protease inhibition or loss of PAR2 or β-arrestin expression. Alt-induced macrophage recruitment was partially reduced by protease inhibition and loss of PAR2 signaling but completely abolished by absence of β-arrestin. SBTI, soybean trypsin inhibitor.
To better determine any signaling-biased differential inflammatory recruitment, we subjected the BALFs collected from Alternaria-exposed animals as described above to flow cytometry (Fig. 7, A–D). In saline control-treated wild-type mice, <10% of the live cells in the BALF were CD45+. The CD45+ cells in saline control-treated BALF were comprised of 0.3% eosinophils [high forward scatter (FSC)/high side scatter (SSC)/CCR3+], 2.2% neutrophils (high FSC/high SSC/GR1+), 2.5% lymphocytes (high FSC/low SSC, CD3+), and 35% alveolar macrophages (high FSC/mid SSC, F4/80+). In contrast, BALF from Alternaria-treated wild-type mice displayed an inflammatory phenotype that included the following CD45+ cell distribution: 38% eosinophils, 19% neutrophils, 14% lymphocytes, and 24% macrophages (Fig. 7D). Although Alternaria also increased the relative amounts of eosinophils, neutrophils, and lymphocytes in PAR2−/− mice and β-arrestin-2−/− mice when compared with HBSS control, the specific values were significantly different from those observed in Alternaria-treated wild type mice. In PAR2−/− mice, Alternaria-treatment resulted in CD45+ cells in the BALF that were comprised of 4% eosinophils, 14% neutrophils, 10% lymphocytes, and 37% macrophage, whereas in β-arrestin-2−/− animals, the BALF cell composition was 19% eosinophils, 10% neutrophils, 6% lymphocytes, and 29% macrophages (Fig. 7D). Furthermore, flow cytometric analysis of the individual lymphocyte populations demonstrated that CD4+ T cells were the primary lymphocyte subtype recruited to the airways (Fig. 7, E and F). These data suggest that Alternaria-induced PAR2/β-arrestin signaling is important for increasing inflammation of the airways and also mediates recruitment of specific cell types to the airways.
To demonstrate the role of Alternaria-induced, β-arrestin-dependent PAR2 signaling in vivo, we employed a phospho-flow assay, using Cy5.5-conjugated anti-phospho-cofilin. We determined cofilin dephosphorylation in BALF cells after induction of lung inflammation with Alternaria filtrate. Granulocyte and lymphocyte populations were identified from the CD45+ cells by SSC versus FSC (Fig. 8A), and the mean Cy5.5 fluorescence for each population was determined by histogram analysis (Fig. 8, B and C). Alternaria treatment of wild-type mice resulted in leftward shift in mean fluorescence intensity from that observed in HBSS control-treated mice in both granulocytes (Fig. 8B) and lymphocytes (Fig. 8C), consistent with a 2.1-fold increase in cofilin dephosphorylation in granulocytes (Fig. 8D, left) and a 1.6-fold increase in lymphocytes (Fig. 8E, left). No significant cofilin dephosphorylation was observed in β-arrestin-2−/− mice in either cell type (Fig. 8, C and D). The percentage of wild-type granulocytes (Fig. 8D, right) and lymphocytes (Fig. 8E, right) in the BALF that stained positive for phospho-cofilin was also significantly reduced after Alternaria treatment. These data demonstrate for the first time that allergen treatment activates the PAR2/β-arrestin signaling axis in the invading inflammatory cells during airway inflammation in vivo. As expected, in β-arrestin-2−/− mice, cofilin dephosphorylation was diminished (e.g., decreased overall p-cofilin and decreased numbers of p-cofilin-positive cells). No differences in total cofilin levels were observed in BALF cells from each treatment group. Thus, Alternaria promotes robust β-arrestin-dependent dephosphorylation of cofilin in vivo.
Purification and sequence identification of a PAR2-activating serine protease from Alternaria alternata.
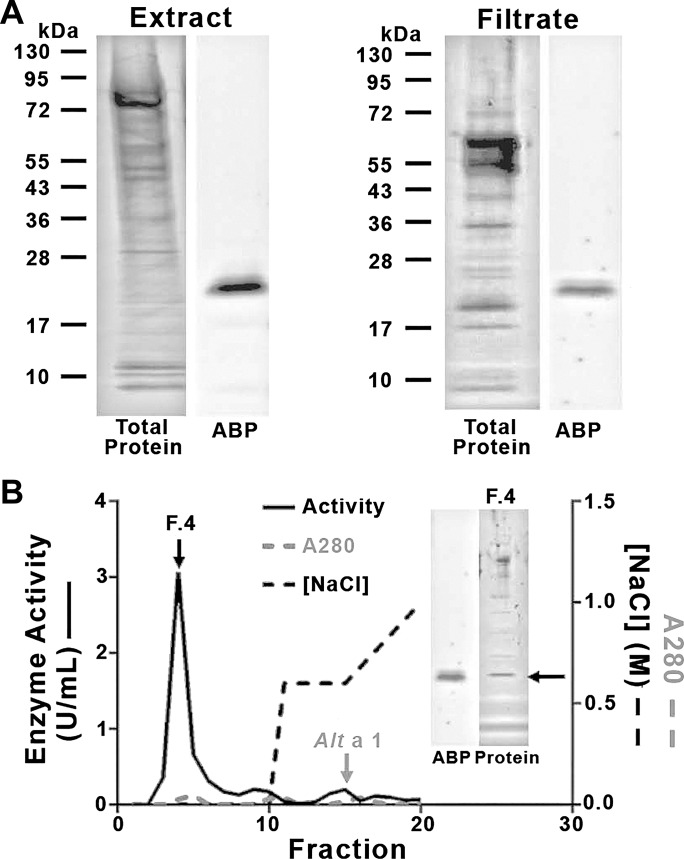
Our data above, similar to previous studies (8, 58), shows that both Alternaria filtrate-induced lung inflammation in vivo and PAR2 activation in cultured cells were inhibited by serine protease inhibitors [including soybean trypsin inhibitors (SBTI) and 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF)], implying the action of a serine protease. Airway inflammation in response to Alternaria has been reported using several commercial and noncommercial sources, including spores, filtrate, and cellular extract. Using a biotinylated, serine protease-selective activity-based probe (ABP) (46, 50), we found that both the unfractionated, defatted Alternaria cellular extract and the filtrate (see materials and methods) contained only a single biotinylated enzyme with an apparent molecular mass of ∼24 kDa (Fig. 9A). Consistent with the protease capture experiments, labeling of the enzyme with the ABP was abrogated by treating the samples with SBTI. The results suggest that a single serine protease from Alternaria is responsible for proteolysis at the canonical PAR2 cleavage site, leading to receptor activation.
Fig. 9.
Alternaria extract or filtrates contain a single protease-activated receptor-2 (PAR2)-activating protease. A: crude extract (left) or culture filtrate (right) was labeled with the biotinylated activity-based probe (ABP), as outlined in materials and methods. Total proteins are visualized in gel (left) or after Western blot detection of biotinylated enzymes (ABP; right). B: filtrate protein was applied to the High-Q anion exchange column; nonadsorbed fractions (1–10) and those eluted with a 2-step NaCl gradient (dashed black line) were collected. Fractions were monitored for enzyme activity (U/ml; left y-axis, solid black lines) and UV absorption (A280; gray lines, right ordinate). Alt a 1 was detected with ELISA. Protein in fraction 4 was run out on a gel as in A (inset at right and compared with ABP Western blot of whole filtrate from A, left). A single serine protease was labeled, isolated, and separated from the Alt a 1 antigen.
To isolate the active enzyme for biochemical characterization and sequencing, the filtrate was subjected to High Q-anion exchange chromatography. Serine protease activity was isolated from elution fraction 4, free from many of the other contaminating proteins and well separated from the Alt a 1 antigen (Fig. 9B). Similar purification of a single protease was achieved by cation exchange chromatography, and the same enzyme was isolated from crude Alternaria cellular extract using a DEAE column (not shown). Although the Alternaria enzyme is sensitive to inhibition by SBTI (Ki ∼25 pM), it displays biochemical characteristics distinct from those of mammalian trypsin in terms of relative Km for a variety of peptide substrates and optimal pH for activity (pH 10 for Alternaria protease compared with pH 8 for trypsin; Table 1). Sequencing of the ABP-labeled enzyme from the Alternaria culture filtrate and crude cellular extract yielded the same peptide sequence (Table 2). The peptide sequences obtained by mass spectral analysis matched the predicted amino acid sequence of an Alternaria enzyme found in the Est database. The sequence is entirely in keeping with a trypsin-like serine protease containing a serine-histidine-aspartic acid catalytic triad with a key ABP-targeted serine. We conclude that both the Alternaria culture filtrate and the cellular extract contain the same singular serine protease, with catalytic properties quite distinct from those of mammalian trypsin. We refer to this newly identified protease as Alternaria alkaline serine protease (AASP).
Table 1.
Biochemical characteristics of AASP
| Substrate for Km, μM |
Inhibitor (Ki) |
||||
|---|---|---|---|---|---|
| QAR (trypsin) | FVR (thrombin) | GGR (urokinase) | SBTI, pM | TLCK, nM | |
| Alt | 16 ± 2 | 366 ± 57 | 98 ± 31 | 18 ± 4 | 12,879 ± 842 |
| Trypsin | 6 ± 0.6 | 24 ± 5 | 1054 ± 211 | 4 ± 0.4 | 2 ± 0.2 |
AASP, Alternaria alkaline serine protease; Alt, Alternaria; FR, FR- aminomethylcoumarin; GGR, GGR-aminomethylcoumarin QAR, QAR-aminomethylcoumarin; SBTI, soybean trypsin inhibitor; TLCK, Nα-p-tosyl-l-lysine-chloromethyl ketone. Km values were determined for partially purified AASP (Alt) and purified trypsin with 3 substrates: QAR (trypsin specific), FVR (thrombin specific), and GGR (urokinase specific). Ki values to trypsin-specific inhibitor SBTI and chymotrypsin-specific inhibitor TLCK were determined; n = 3, Km and Ki values are graphed as means ± SE.
Table 2.
AASP amino acid sequence
| AASP sequence |
| MRFQSIIAIALPALVLAAPTPQDPDYEFPEDAPADDIVGGTTASAGEFPFIVSLQRSGSHFCGGSLLDSTTVITAAHCSVSSVIGSVSGLRVRAGSLKSSGGTLVGVSSVTVHPSYRSSGQDFDVAIWKLSTAVPTSSTIGYATLPASGSDPAAGSTATVAGWGALTEGGSSPSTLYKVSVPIVSRTECRSSYGTSAITNNMFCAGYTTGGRDSCQGDSGGPIVNSAKTLIGLVSWGNGCAQPNFPGVYARTAALLSFINSV |
| 99% Confidence |
| IVSLQRSGSHF |
| RVRAGSLNKSSGGTLVGVSSVTVHPSY |
| NKSSGGTLVGVSSVTVHPSY |
| VGVSSVTVHPSY |
| RSSGQDFDVAIW |
| KLSTAVPTSSTIGY |
| ATLPASGSDPAAGSTATVAGW |
| GALTEGGSSPSTL |
| GALTEGGSSPSTLY |
| KVSVPIVSRTECRSSY |
| GTSAITNNMF |
| GTSAITNNMFCAGY |
| TTGGRDSCQGDSGGPIVNSAKTL |
| GNGCAQPNFPGVY |
| SFINSV |
| 95% Confidence |
| IGLVSW |
| ARTAALL |
AASP, Alternaria alkaline serine protease. The AASP sequences obtained by mass spectral procedures, as outlined in materials and methods, were deconvoluted by comparison with the translated amino acid sequence of an Alternaria-expressed sequence tag library, also as outlined in materials and methods. Sequences that were identified with 99% confidence (underlined) and 95% confidence (boldface) are noted in the table and are shown below.
Activation of PAR2 signaling by AASP.
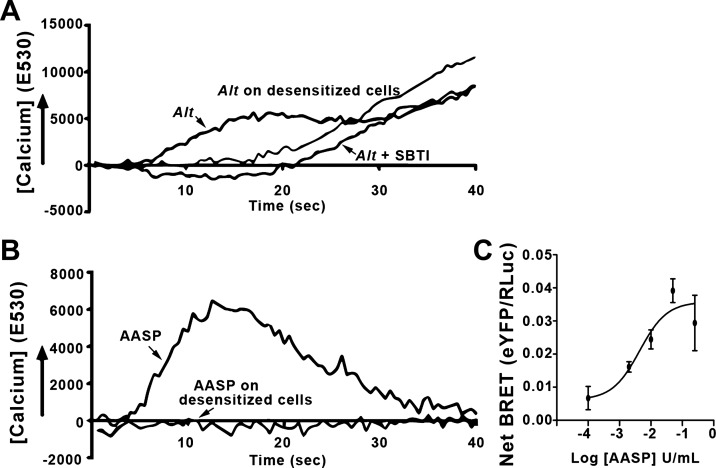
We first evaluated the ability of AASP from either of the chromatographic preparations noted above to promote Ca2+ mobilization, a hallmark of the canonical PAR2/G protein signaling. For comparison, we used the Alternaria filtrate to induce a Ca2+ change in PAR2-transfected KNRK cells (Fig. 10A). The primary Ca2+ response was eliminated by SBTI and by desensitization of PAR2 with preactivation by the PAR2 agonist 2fAP (8, 32), consistent with a protease-dependent, PAR2-specific Ca2+ activation. It is notable in this preparation that a secondary Ca2+ response appeared to be PAR2 independent. We next tested the partially purified AASP for PAR2-dependent Ca2+ signaling (fraction 4 from High Q column; Fig. 10B). AASP induced the typical, rapid Ca2+ transient in the PAR2-expressing KNRK cells associated with PAR2 activation (19). The Ca2+ transient was fully eliminated in desensitized cells, confirming PAR2-dependent Ca2+ mobilization. To examine whether AASP could induce β-arrestin recruitment, we repeated the BRET experiments described in Fig. 1 (see Fig. 10C). The EC50 for AASP-induced β-arrestin recruitment (2.5 U/ml) was similar to that observed for trypsin (Fig. 1C). In summary, the isolated AASP was sufficient to fully activate PAR2 Ca2+ and β-arrestin signaling in our cellular assays.
Fig. 10.
Alternaria (Alt) alkaline serine protease (AASP) induces protease-activated receptor-2 (PAR2)-dependent Ca2+ and β-arrestin signaling. A and B: Kirsten virus-transformed rat kidney cells (KNRK) cells transfected with PAR2 were loaded with the Ca2+ sensitive dye Fluo-4 and treated with Alt filtrate (A) or AASP from High-Q column fraction F4 (B) from Fig. 9B. Alt filtrate induced a protease-sensitive Ca2+ transient that required functional PAR2, as both soybean trypsin inhibitor (SBTI) and desensitization prevented the primary Ca2+ signaling. AASP induced a Ca2+ transient that required functional PAR2 as desensitization eliminated the Ca2+ signal. C: bioluminescence resonance energy transfer (BRET) was measured upon addition of increasing concentrations of AASP, as described in Fig. 1C. Half-maximal BRET value (BRET50) for AASP (2.0 U/ml) was similar to what was determined for trypsin (2.5 U/ml). AASP is sufficient to fully induce signaling pathways via PAR2; n ≥ 3 for all experiments (A and B) and experimental time points (C).
AASP-induced, PAR2-mediated inflammation.
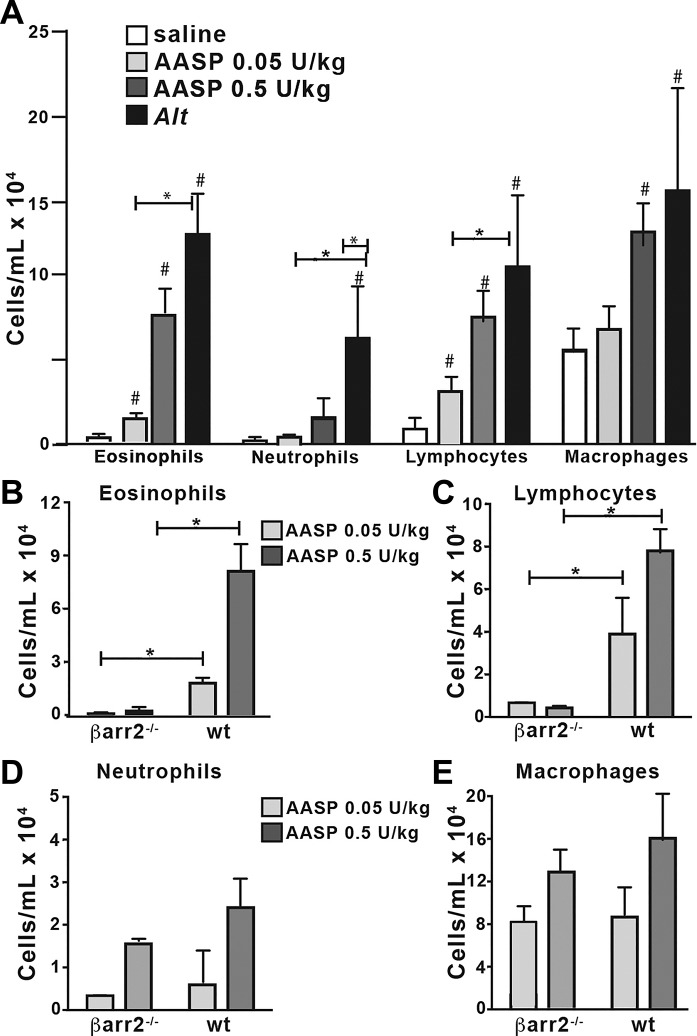
We next tested whether localized activation of PAR2 by AASP was sufficient to replicate some or all of the inflammatory effects of the Alternaria extract. Wild-type and β-arrestin-2−/− mice were intranasally exposed to either AASP with 0.5 U/kg trypsin-like activity (equivalent to the trypsin-like activity measured in Alternaria filtrates above) or the reduced 0.05 U trypsin-like activity in 25 µl saline. BALF was collected and analyzed for the presence of immune cell infiltrates. Although both doses of the purified AASP induced recruitment of eosinophils and lymphocytes to the airways of wild type mice (Fig. 11A), neutrophils and macrophages were recruited only by the higher dose and were <50% of that observed with total Alternaria filtrate. Significantly, both eosinophil and lymphocyte recruitment were abrogated in the β-arrestin-2−/− mice independent of AASP dose. We conclude that AASP is sufficient to promote acute inflammation in the airway.
Fig. 11.
Alternaria (Alt) alkaline serine protease (AASP) promotes β-arrestin-2-dependent recruitment of eosinophils and lymphocytes to the airways. Two doses of AASP were administered intranasally to wild-type (WT) or β-arrestin-2−/− mice (n ≥ 3). Negative controls (saline) and positive controls (Alt) were included. Bronchioalveolar lavage fluid (BALF) was collected and differential counts performed. Quantification of cell types in WT mice. A: AASP exposure elicited airway inflammation of all cell types, with the exception of neutrophils. B–E: direct comparison between WT and β-arrestin-2−/− mice of eosinophil (B), lymphocyte (C), neutrophil (D), and macrophage (E) recruitment following exposure of high and low doses of AASP. Both eosinophil and lymphocyte recruitment require β-arrestin-2 expression. P ≤ 0.05, *significant differences between bracketed experiments; #significant differences from saline control.
AASP-stimulated, prostaglandin-dependent bronchorelaxation.
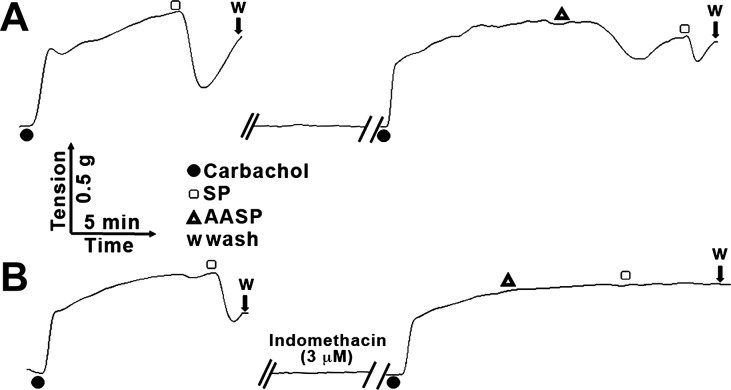
In addition to the β-arrestin-2-dependent inflammatory response to PAR2 activation observed in our work with the ovalbumin sensitized/2fAP challenged mouse model of asthma, we also observed epithelium-dependent, prostanoid-mediated bronchorelaxation following PAR2 activation (40). We thus evaluated whether AASP was sufficient to cause bronchorelaxation using a tracheal ring assay (Fig. 12). Following carbachol-induced contraction, the tracheal rings were first subjected to substance P as a control bronchorelaxant. After washout, the experiment was repeated with AASP and a similar bronchorelaxation observed (Fig. 12A). This bronchorelaxation was blocked by the cyclooxygenase inhibitor indomethacin (Fig. 12B). These data are consistent with AASP being sufficient to initiate the “protective” bronchorelaxation response due to the epithelial cell-generated production of cyclooxygenase-produced agonists (presumably, prostaglandins).
Fig. 12.
Alternaria alkaline serine protease (AASP)-mediated bronchorelaxation. Second-order mouse bronchiolar ring segments were first contracted with 1 μM carbachol and then exposed to substance P (SP; 20 nM) to demonstrate a prostanoid-generated indomethacin-blocked epithelium-dependent relaxation. Following wash with buffer (A) or buffer with 3 μM indomethacin (B), carbachol was reapplied, followed by AASP (0.6 U/ml) and a 2nd SP wash. AASP induced an indomethacin-sensitive bronchorelaxation. n ≥ 3 for all experiments.
DISCUSSION
Alternaria alternata is a prominent fungal allergen that has been associated with asthma development and cases of severe asthma (10, 27, 51). Extensive work with cellular and animal models has implicated serine-protease(s) from Alternaria and their activation of protease-activated receptor-2 (PAR2) in airway inflammation. PAR2 signals through a canonical Gq pathway that leads to activation of phosphatidylinositol 3-kinases (PI3K), LIM kinases (LIMK), nuclear mitogen-activated kinases (MAPK), prostaglandin E2 (PGE2) synthesis and release, and proliferation (18, 69, 71). PAR2 can also signal independent of G proteins via recruitment of β-arrestins and subsequent activation of membrane-retained MAPK (i.e., extracellular signal-regulated kinase 1 and 2), cofilin, and inhibition of PI3K and LIMK, all of which lead to reorganization of the actin cytoskeleton and chemotaxis in multiple cell types, including neutrophils and lymphocytes (24, 69, 71, 72). We have demonstrated that in vivo activation of airway PAR2 with a synthetic peptide agonist (2fAP) promotes leukocyte recruitment, epithelial thickening, and mucus production that requires β-arrestin-2, whereas bronchodilation and PGE2-mediated smooth muscle relaxation occur independent of β-arrestin-2 signaling (40). These findings were consistent with the in vitro pathways mediated by each signaling arm. Given that PAR2 is upregulated in both human respiratory epithelium and peripheral blood monocytes in asthmatics (33, 57), PAR2 signaling is of great interest in asthma development and severity. Previous reports on Alternaria-induced signaling in cellular and asthma models have relied on the use of protease inhibitors and/or the monitoring of Ca2+ signaling to detail specific roles for PAR2 (8, 39, 58). It is now apparent that both small molecular ligands (61) and proteases (53) can activate PAR2 independently of Ca2+ signaling. Here, we expand these observations to a more physiologically relevant model and show that proteolytic activation of PAR2 is sufficient to promote the recruitment of eosinophils and lymphocytes, epithelial thickening, and mucous accumulation observed with exposure to Alternaria. Furthermore, we have isolated and characterized a single serine protease from Alternaria, Alternaria alkaline serine protease (AASP), that is sufficient to induce inflammation in the mouse models. These inflammatory responses require the presence of β-arrestin-2, strongly suggesting that PAR2 signaling through β-arrestin is sufficient to promote airway inflammation. Alternaria and isolated AASP are capable of promoting both G protein-dependent signals (as demonstrated by Ca2+ mobilization) and β-arrestin-dependent signals (as demonstrated by β-arrestin recruitment to PAR2 and activation of cofilin). The slightly different immune cell recruitment induced by Alternaria filtrates and AASP confirms that roles for alternate proteins/antigens from Alternaria are required for full airway inflammation; nonetheless, the protease alone is able to promote significant inflammation.
While PAR2−/− mouse models have been used to evaluate the role for house dust mite- (15) or cockroach-induced asthma (16, 45), examination of β-arrestin-2 signaling arm for PAR2 activation has provided further clues in understanding the mechanisms that underlie allergic asthma. That GPCR/β-arrestin signaling pathways are important mediators of inflammation, and airway hyperresponsiveness is corroborated by other studies in ovalbumin-induced and cigarette smoke-induced allergic asthma animal models (11, 68) and by studies showing that some of the adverse effects associated with β2-adrenergic receptor activation are β-arrestin dependent (20, 64). Our studies suggested that PAR2-stimulated, β-arrestin-dependent cell migration is a major contributing factor to the cellular inflammation observed during asthma. These findings led to the prediction that β-arrestin-dependent activation of cofilin might be increased in the Alternaria-induced asthma model, a theory that is supported by the observation here that the cells recruited to the BALF by administration of Alternaria have more active (dephosphorylated) cofilin compared with cells found in the BALF in control mice. Furthermore, the observation that Alternaria-induced dephosphorylation of cofilin is not observed in β-arrestin-2−/− mice suggests that the PAR2-β-arrestin signaling axis is associated with the inflammatory process induced by Alternaria in vivo. Whether T cells directly encounter Alternaria proteases or whether the PAR2 response in vivo is a result of other serine proteases and chemokines released in response to PAR2 is still unclear. However, both Alternaria and 2fAP-mediated PAR2 activation promote barrier damage in cultured airway epithelial cells (Refs. 25 and 35 and Nichols HL and DeFea KA, unpublished observations), raising the possibility that some of the apically acting proteases might gain access to the serosa. Nonetheless, these studies are the first to demonstrate activation of β-arrestin-dependent PAR2 signaling by a ubiquitous allergen and the first to show an in vivo correlation of β-arrestin-dependent cofilin activation and leukocyte migration with lung inflammation.
Although many of the parameters of lung inflammation were similar in the Alternaria-asthma model we used herein and the ovalbumin/2fAP asthma model used in previous studies (40), there were some notable differences. First, we see a striking increase in neutrophil infiltration in the Alternaria asthma model. Although airway eosinophilia often characterizes allergic asthma, it is widely recognized that neutrophils are found in patients with acute severe asthma (17, 43). It has been shown in BALB/C mice that Alternaria induces a neutrophilic response in STAT6−/− animals rather than the more typical eosinophilic response (66). In that report, the authors postulated that neutrophil recruitment is a normal response that is exacerbated when mechanisms for eosinophil migration are eliminated. In our study, several lines of evidence suggest that neutrophil infiltration was slightly less dependent upon PAR2/β-arrestin-2 signaling and trypsin-like protease activity than were eosinophils and leukocytes. First, whereas eosinophil and CD4+-T cell infiltration into the airway was reduced by 80–90% in PAR2−/− and β-arrestin-2−/− or by pretreatment with SBTI, neutrophils were only reduced by 50%. Second, the purified AASP failed to promote significant neutrophil recruitment to the airways. These findings suggest a more complex signaling network at play, perhaps involving direct activation of immune cells by PAR2 agonists as well as release of chemokines that then act to recruit additional cells. Because AASP was insufficient to promote the neutrophilia observed with exposure to complete Alternaria extract, the recruitment of different cell types is likely mediated by nonproteolytic components of the Alternaria allergen. Taken together with our previous evidence that IgE production is not dependent upon the β-arrestin pathway but that PAR2 contributes to the process, these data suggest that AASP may mediate the localized PAR2 exacerbation of asthma in the airways via PAR2/β-arrestin signaling, whereas the systemic responses may be mediated by other components.
A main finding of our study is that the asthma allergen Alternaria alternata contains a single trypsin-like serine protease, AASP, that can activate PAR2 to promote airway inflammation via a β-arrestin-dependent mechanism. Serine proteases are a recurring theme among asthma-associated allergens (2), including those from other molds (13), house dust mite (59, 63), and cockroach (42, 50, 60). The protease described here differs considerably from the recently described vacuolar serine protease from Alternaria, Alt a 15 (21). Similarly to the Alternaria filtrate, AASP was able to activate PAR2 and initiate both Ca2+ and β-arrestin signaling. However, a close examination of the Ca2+ experiments shows a secondary Ca2+ signal invoked by the Alternaria filtrate that is absent in the AASP experiments. It has been shown that Alternaria exposure of epithelial cells can induce metabolite release, including ATP (41, 54, 58), and such metabolites could extend the Ca2+ signal. However, the Ca2+ signal induced by AASP was more similar to what is typically seen by specific PAR2-activating peptides (19). As noted above, AASP was sufficient for sensitization and initiation of airway inflammation, albeit with the notable change in neutrophil cell recruitment when compared with the Alternaria-induced asthma model. From these findings, we conclude that AASP is itself a potent asthma allergen that defines much but not all of the Alternaria-induced asthma response.
Although we and others have demonstrated a major role for PAR2 in the progression of airway inflammation, PAR2 activation has also been reported to orchestrate protective effects in the airway by promoting prostaglandin E2 (PGE2)-mediated bronchiolar smooth muscle relaxation, leading to bronchodilation (14, 29, 34, 40). We observed a similar indomethacin-sensitive bronchorelaxing action of AASP, indicating the “dual” effect of PAR2 activation on the airways, on the one hand promoting the influx of inflammatory cells and on the other triggering what might be considered as a “protective effect.” Thus, chronically, the overall effect of PAR2 activation may enhance the pathogenicity of the allergen and promote irreversible airway remodeling, which worsens the pathology. That said, the acute action of the allergen protease activating PAR2 on the airway epithelium to enhance bronchorelaxation represents an intrinsic beneficial action of PAR2 stimulation.
Triggered by infection, environmental allergens, or other stimuli, asthma remains poorly understood and difficult to manage due to the heterogeneity of the disease. In addition, there are concerns surrounding the side effects from long-term use of the most frequently prescribed asthma medications that represent different therapeutic classes currently used clinically as FDA-approved therapies. Treatments that have been the mainstay of asthma medications for almost 50 yr include corticosteroids (inflammation reduction), short-acting β2-agonists (fast response bronchorelaxant), long-acting β2-agonists (bronchorelaxant for preventative management), mast cell stabilizers (inflammatory control), anticholinergics (supplemental bronchorelaxant), and leukotrienes (supplemental anti-allergen and anti-inflammatory) (37, 47). Although asthma treatments have been largely unchanged, it is an unfortunate reality that asthma remains uncontrolled in more than half the patients receiving standard asthma medication (48). Given its role(s) in asthma sensitization and exacerbation, PAR2 represents a viable target for drug intervention in asthma. Enhancing this view are the findings that PAR2-neutralizing antibodies were recently shown to reduce asthma symptoms in acute and chronic allergic asthma mouse models (6, 7). There are only a handful of PAR2 antagonists that have been proposed (reviewed in Ref. 70). These include K-14585 (26, 31), GB88 (30, 61, 62), C391 (9), two small molecules isolated from a large screening library (AC-55541 and AC-264613) (23), and two recently described PAR2 allosteric antagonists (AZ8838 and AZ3451) (12). To our knowledge, none of these antagonists have been tested in asthma animal models. C391 is distinct from other antagonists in that it requires minimal pre-incubation for inhibition of peptide activation of PAR2-dependent Ca2+ signaling, it acts as an antagonist for both Ca2+ and β-arrestin signaling pathways, and it is the only known antagonist shown to be effective in blocking both natural protease and peptidomimetic activation of PAR2 in vitro (Ref. 9 and Yee MC, DeFea KA, and Boitano S, unpublished results). When considering the biphasic effects with respect to PAR2 signaling in the airway (i.e., inflammation and bronchorelaxation), there is a growing interest in developing both PAR2-signaling biased agonists and antagonists as putative therapeutic agents for asthma (2, 67). Because our previous studies suggest that the “protective effects” of PAR2 activation are independent of β-arrestin signaling (40), we suggest that it will be of therapeutic value to develop biased PAR2 antagonists that target the β-arrestin-dependent PAR2 signaling pathway, but which do not affect the β-arrestin-independent PAR2 signaling pathways that may be of benefit in the setting of asthma induced by PAR2-activating household allergens such as Alternaria.
GRANTS
Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by National Institute of Environmental Health Sciences Grant ES-06694 to the Southwest Environmental Health Sciences Center, National Institutes of Health (NIH)/National Cancer Institute Grant CA-023074 to the University of Arizona Cancer Center and by the BIO5 Institute of the University of Arizona. The Thermo Fisher LTQ Orbitrap Velos mass spectrometer was provided by grant 1S10-RR-028868-01 from NIH/National Center for Research Resources. This work was supported in part by R01-AI-083403 (to M. O. Daines), R01-NS-073664 and R21-AI-140257 (to S. Boitano), RNS072298A (to E. H. Wilson), and grants to M. D. Hollenberg from the Canadian Institutes of Health Research and the Lung Association of Alberta and Northwest Territories.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.Y., H.L.N., D.P., M.S., K.P., and K.L. performed experiments; M.C.Y., H.L.N., D.P., M.S., K.P., K.L., E.H.W., M.O.D., M.D.H., and K.A.D. analyzed data; M.C.Y., H.L.N., D.P., E.H.W., M.O.D., M.D.H., S.B., and K.A.D. interpreted results of experiments; M.C.Y., H.L.N., D.P., M.S., K.P., K.L., E.H.W., M.D.H., S.B., and K.A.D. prepared figures; M.C.Y., S.B., and K.A.D. drafted manuscript; M.C.Y., H.L.N., D.P., M.S., K.P., K.L., E.H.W., M.O.D., M.D.H., S.B., and K.A.D. approved final version of manuscript; S.B. and K.A.D. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert J. Lefkowitz (Duke University) for β-arrestin-2−/− mice; Dr. Robin Plevin (U. Strathclyde), for PAR2−/− mice; Dr. Brendan Gilmore, Queens University School of Pharmacy, Belfast, UK, for providing the serine protease activity-based probe reagent; and Dr. Julie Ledford for critical reading of the manuscript.
REFERENCES
- 1.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem 281: 6910–6923, 2006. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 2.Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, Hooper JD. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther 130: 248–282, 2011. doi: 10.1016/j.pharmthera.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ani B, Hansen KK, Hollenberg MD. Proteinase-activated receptor-2: key role of amino-terminal dipeptide residues of the tethered ligand for receptor activation. Mol Pharmacol 65: 149–156, 2004. doi: 10.1124/mol.65.1.149. [DOI] [PubMed] [Google Scholar]
- 4.Andon NL, Hollingworth S, Koller A, Greenland AJ, Yates JR 3rd, Haynes PA. Proteomic characterization of wheat amyloplasts using identification of proteins by tandem mass spectrometry. Proteomics 2: 1156–1168, 2002. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, El Mays T, Gilmore BF, Walker B, Gordon JR, Hollenberg MD, Vliagoftis H. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol 186: 3164–3172, 2011. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- 6.Asaduzzaman M, Davidson C, Nahirney D, Fiteih Y, Puttagunta L, Vliagoftis H. Protease-Activated Receptor-2 blockade inhibits changes seen in a chronic murine asthma model. Allergy 73: 416–420, 2018. doi: 10.1111/all.13313. [DOI] [PubMed] [Google Scholar]
- 7.Asaduzzaman M, Nadeem A, Arizmendi N, Davidson C, Nichols HL, Abel M, Ionescu LI, Puttagunta L, Thebaud B, Gordon J, DeFea K, Hollenberg MD, Vliagoftis H. Functional inhibition of PAR2 alleviates allergen-induced airway hyperresponsiveness and inflammation. Clin Exp Allergy 45: 1844–1855, 2015. doi: 10.1111/cea.12628. [DOI] [PubMed] [Google Scholar]
- 8.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol 300: L605–L614, 2011. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boitano S, Hoffman J, Flynn AN, Asiedu MN, Tillu DV, Zhang Z, Sherwood CL, Rivas CM, DeFea KA, Vagner J, Price TJ. The novel PAR2 ligand C391 blocks multiple PAR2 signalling pathways in vitro and in vivo. Br J Pharmacol 172: 4535–4545, 2015. doi: 10.1111/bph.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol 113: 227–234, 2004. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Hegde A, Choi YH, Theriot BS, Premont RT, Chen W, Walker JK. Genetic deletion of β-arrestin-2 and the mitigation of established airway hyperresponsiveness in a murine asthma model. Am J Respir Cell Mol Biol 53: 346–354, 2015. doi: 10.1165/rcmb.2014-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng RKY, Fiez-Vandal C, Schlenker O, Edman K, Aggeler B, Brown DG, Brown GA, Cooke RM, Dumelin CE, Doré AS, Geschwindner S, Grebner C, Hermansson NO, Jazayeri A, Johansson P, Leong L, Prihandoko R, Rappas M, Soutter H, Snijder A, Sundström L, Tehan B, Thornton P, Troast D, Wiggin G, Zhukov A, Marshall FH, Dekker N. Structural insight into allosteric modulation of protease-activated receptor 2. Nature 545: 112–115, 2017. doi: 10.1038/nature22309. [DOI] [PubMed] [Google Scholar]
- 13.Chiu LL, Perng DW, Yu CH, Su SN, Chow LP. Mold allergen, pen C 13, induces IL-8 expression in human airway epithelial cells by activating protease-activated receptor 1 and 2. J Immunol 178: 5237–5244, 2007. doi: 10.4049/jimmunol.178.8.5237. [DOI] [PubMed] [Google Scholar]
- 14.Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, Henry PJ, Carr MJ, Hamilton JR, Moffatt JD. A protective role for protease-activated receptors in the airways. Nature 398: 156–160, 1999. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 15.Davidson CE, Asaduzzaman M, Arizmendi NG, Polley D, Wu Y, Gordon JR, Hollenberg MD, Cameron L, Vliagoftis H. Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model. Clin Exp Allergy 43: 1274–1285, 2013. doi: 10.1111/cea.12185. [DOI] [PubMed] [Google Scholar]
- 16.Day SB, Zhou P, Ledford JR, Page K. German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2. J Innate Immun 2: 495–504, 2010. doi: 10.1159/000317195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vooght V, Smulders S, Haenen S, Belmans J, Opdenakker G, Verbeken E, Nemery B, Hoet PH, Vanoirbeek JA. Neutrophil and eosinophil granulocytes as key players in a mouse model of chemical-induced asthma. Toxicol Sci 131: 406–418, 2013. doi: 10.1093/toxsci/kfs308. [DOI] [PubMed] [Google Scholar]
- 18.DeFea KA, Zalevsky J, Thoma MS, Déry O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 148: 1267–1281, 2000. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn AN, Tillu DV, Asiedu MN, Hoffman J, Vagner J, Price TJ, Boitano S. The protease-activated receptor-2-specific agonists 2-aminothiazol-4-yl-LIGRL-NH2 and 6-aminonicotinyl-LIGRL-NH2 stimulate multiple signaling pathways to induce physiological responses in vitro and in vivo. J Biol Chem 286: 19076–19088, 2011. doi: 10.1074/jbc.M110.185264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forkuo GS, Kim H, Thanawala VJ, Al-Sawalha N, Valdez D, Joshi R, Parra S, Pera T, Gonnella PA, Knoll BJ, Walker JK, Penn RB, Bond RA. Phosphodiesterase 4 Inhibitors Attenuate the Asthma Phenotype Produced by β2-Adrenoceptor Agonists in Phenylethanolamine N-Methyltransferase-Knockout Mice. Am J Respir Cell Mol Biol 55: 234–242, 2016. doi: 10.1165/rcmb.2015-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriel MF, Postigo I, Gutiérrez-Rodríguez A, Suñén E, Guisantes JA, Fernández J, Tomaz CT, Martínez J. Alt a 15 is a new cross-reactive minor allergen of Alternaria alternata. Immunobiology 221: 153–160, 2016. doi: 10.1016/j.imbio.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Gabriel MF, Postigo I, Tomaz CT, Martínez J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ Int 89-90: 71–80, 2016. doi: 10.1016/j.envint.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Gardell LR, Ma JN, Seitzberg JG, Knapp AE, Schiffer HH, Tabatabaei A, Davis CN, Owens M, Clemons B, Wong KK, Lund B, Nash NR, Gao Y, Lameh J, Schmelzer K, Olsson R, Burstein ES. Identification and characterization of novel small-molecule protease-activated receptor 2 agonists. J Pharmacol Exp Ther 327: 799–808, 2008. doi: 10.1124/jpet.108.142570. [DOI] [PubMed] [Google Scholar]
- 24.Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem 278: 34418–34426, 2003. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 25.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol 134: 509–520, 2014. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goh FG, Ng PY, Nilsson M, Kanke T, Plevin R. Dual effect of the novel peptide antagonist K-14585 on proteinase-activated receptor-2-mediated signalling. Br J Pharmacol 158: 1695–1704, 2009. doi: 10.1111/j.1476-5381.2009.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med 155: 1356–1361, 1997. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 28.Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, Wallace JL, Hollenberg MD, Vergnolle N. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc Natl Acad Sci USA 102: 8363–8368, 2005. doi: 10.1073/pnas.0409535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry PJ. The protease-activated receptor2 (PAR2)-prostaglandin E2-prostanoid EP receptor axis: a potential bronchoprotective unit in the respiratory tract? Eur J Pharmacol 533: 156–170, 2006. doi: 10.1016/j.ejphar.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Hollenberg MD, Mihara K, Polley D, Suen JY, Han A, Fairlie DP, Ramachandran R. Biased signalling and proteinase-activated receptors (PARs): targeting inflammatory disease. Br J Pharmacol 171: 1180–1194, 2014. doi: 10.1111/bph.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanke T, Ishiwata H, Kabeya M, Saka M, Doi T, Hattori Y, Kawabata A, Plevin R. Binding of a highly potent protease-activated receptor-2 (PAR2) activating peptide, [3H]2-furoyl-LIGRL-NH2, to human PAR2. Br J Pharmacol 145: 255–263, 2005. doi: 10.1038/sj.bjp.0706189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawabata A, Saifeddine M, Al-Ani B, Leblond L, Hollenberg MD. Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1-targeted ligands. J Pharmacol Exp Ther 288: 358–370, 1999. [PubMed] [Google Scholar]
- 33.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol 108: 797–803, 2001. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 34.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 18: 684–692, 2012. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 35.Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, Dennison PW, Shamji BW, Edwards MJ, Holgate ST, Howarth PH, Davies DE. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PLoS One 8: e71278, 2013. doi: 10.1371/journal.pone.0071278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lombardi C, Savi E, Ridolo E, Passalacqua G, Canonica GW. Is allergic sensitization relevant in severe asthma? Which allergens may be culprit? World Allergy Organ J 10: 2, 2017. doi: 10.1186/s40413-016-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez FD, Vercelli D. Asthma. Lancet 382: 1360–1372, 2013. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 38.Marullo S, Bouvier M. Resonance energy transfer approaches in molecular pharmacology and beyond. Trends Pharmacol Sci 28: 362–365, 2007. doi: 10.1016/j.tips.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol 158, Suppl 1: 19–29, 2012. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols HL, Saffeddine M, Theriot BS, Hegde A, Polley D, El-Mays T, Vliagoftis H, Hollenberg MD, Wilson EH, Walker JK, DeFea KA. β-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA 109: 16660–16665, 2012. doi: 10.1073/pnas.1208881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H. ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J Physiol 591: 4595–4609, 2013. doi: 10.1113/jphysiol.2013.254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ock MS, Kim BJ, Kim SM, Byun KH. Cloning and expression of trypsin-encoding cDNA from Blattella germanica and its possibility as an allergen. Korean J Parasitol 43: 101–110, 2005. doi: 10.3347/kjp.2005.43.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordoñez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med 161: 1185–1190, 2000. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 44.Page K. Role of cockroach proteases in allergic disease. Curr Allergy Asthma Rep 12: 448–455, 2012. doi: 10.1007/s11882-012-0276-1. [DOI] [PubMed] [Google Scholar]
- 45.Page K, Ledford JR, Zhou P, Dienger K, Wills-Karp M. Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respir Res 11: 62, 2010. doi: 10.1186/1465-9921-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan Z, Jeffery DA, Chehade K, Beltman J, Clark JM, Grothaus P, Bogyo M, Baruch A. Development of activity-based probes for trypsin-family serine proteases. Bioorg Med Chem Lett 16: 2882–2885, 2006. doi: 10.1016/j.bmcl.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Pera T, Penn RB. Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther 164: 82–96, 2016. doi: 10.1016/j.pharmthera.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol 119: 1454–1461, 2007. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Peters T, Henry PJ. Protease-activated receptors and prostaglandins in inflammatory lung disease. Br J Pharmacol 158: 1017–1033, 2009. doi: 10.1111/j.1476-5381.2009.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polley DJ, Mihara K, Ramachandran R, Vliagoftis H, Renaux B, Saifeddine M, Daines MO, Boitano S, Hollenberg MD. Cockroach allergen serine proteinases: Isolation, sequencing and signalling via proteinase-activated receptor-2. Clin Exp Allergy 47: 946–960, 2017. doi: 10.1111/cea.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol 120: 610–617, 2007. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 52.Qian WJ, Liu T, Monroe ME, Strittmatter EF, Jacobs JM, Kangas LJ, Petritis K, Camp DG II, Smith RD. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: the human proteome. J Proteome Res 4: 53–62, 2005. doi: 10.1021/pr0498638. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandran R, Mihara K, Chung H, Renaux B, Lau CS, Muruve DA, DeFea KA, Bouvier M, Hollenberg MD. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2). J Biol Chem 286: 24638–24648, 2011. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramu S, Menzel M, Bjermer L, Andersson C, Akbarshahi H, Uller L. Allergens produce serine proteases-dependent distinct release of metabolite DAMPs in human bronchial epithelial cells. Clin Exp Allergy 48: 156–166, 2018. doi: 10.1111/cea.13071. [DOI] [PubMed] [Google Scholar]
- 55.Salo PM, Arbes SJ Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol 118: 892–898, 2006. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858, 1996. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 57.Shrestha Palikhe N, Nahirney D, Laratta C, Gandhi VD, Vethanayagam D, Bhutani M, Mayers I, Cameron L, Vliagoftis H. Increased Protease-Activated Receptor-2 (PAR-2) Expression on CD14++CD16+ Peripheral Blood Monocytes of Patients with Severe Asthma. PLoS One 10: e0144500, 2015. doi: 10.1371/journal.pone.0144500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol 134: 583–592.e6, 2014. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart GA, Thompson PJ, Simpson RJ. Protease antigens from house dust mite. Lancet 334: 154–155, 1989. doi: 10.1016/S0140-6736(89)90203-1. [DOI] [PubMed] [Google Scholar]
- 60.Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy 63: 768–776, 2008. doi: 10.1111/j.1398-9995.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 61.Suen JY, Cotterell A, Lohman RJ, Lim J, Han A, Yau MK, Liu L, Cooper MA, Vesey DA, Fairlie DP. Pathway-selective antagonism of proteinase activated receptor 2. Br J Pharmacol 171: 4112–4124, 2014. doi: 10.1111/bph.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suen JY, Gardiner B, Grimmond S, Fairlie DP. Profiling gene expression induced by protease-activated receptor 2 (PAR2) activation in human kidney cells. PLoS One 5: e13809, 2010. doi: 10.1371/journal.pone.0013809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol 167: 1014–1021, 2001. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 64.Thanawala VJ, Forkuo GS, Stallaert W, Leff P, Bouvier M, Bond R. Ligand bias prevents class equality among beta-blockers. Curr Opin Pharmacol 16: 50–57, 2014. doi: 10.1016/j.coph.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res 7: 205–220, 2015. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 Regulates the Development of Eosinophilic versus Neutrophilic Asthma in Response to Alternaria alternata. J Immunol 197: 4541–4551, 2016. doi: 10.4049/jimmunol.1600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker JK, DeFea KA. Role for β-arrestin in mediating paradoxical β2AR and PAR2 signaling in asthma. Curr Opin Pharmacol 16: 142–147, 2014. doi: 10.1016/j.coph.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 112: 566–574, 2003. doi: 10.1172/JCI200317265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry 45: 9374–9385, 2006. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- 70.Yau MK, Lim J, Liu L, Fairlie DP. Protease activated receptor 2 (PAR2) modulators: a patent review (2010-2015). Expert Opin Ther Pat 26: 471–483, 2016. doi: 10.1517/13543776.2016.1154540. [DOI] [PubMed] [Google Scholar]
- 71.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282: 20634–20646, 2007. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 72.Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem 285: 14318–14329, 2010. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]