Abstract
Pericytes are key regulators of the microvasculature through their close interactions with the endothelium. However, pericytes play additional roles in tissue homeostasis and repair, in part by transitioning into myofibroblasts. Accumulation of myofibroblasts is a hallmark of fibrotic diseases such as idiopathic pulmonary fibrosis (IPF). To understand the contribution and role of pericytes in human lung fibrosis, we isolated these cells from non-IPF control and IPF lung tissues based on expression of platelet-derived growth factor receptor-β (PDGFR-β), a common marker of pericytes. When cultured in a specialized growth medium, PDGFR-β+ cells retain the morphology and marker profile typical of pericytes. We found that IPF pericytes migrated more rapidly and invaded a basement membrane matrix more readily than control pericytes. Exposure of cells to transforming growth factor-β, a major fibrosis-inducing cytokine, increased expression of α-smooth muscle actin and extracellular matrix genes in both control and IPF pericytes. Given that pericytes are uniquely positioned in vivo to respond to danger signals of both systemic and tissue origin, we stimulated human lung pericytes with agonists having pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). Both control and IPF lung pericytes increased expression of proinflammatory chemokines in response to specific PAMPs and DAMPs released from necrotic cells. Our results suggest that control and IPF lung pericytes are poised to react to tissue damage, as well as microbial and fibrotic stimuli. However, IPF pericytes are primed for migration and matrix invasion, features that may contribute to the function of these cells in lung fibrosis.
Keywords: human, fibrosis, immunity, lung, pericyte
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic, ultimately fatal lung disease with a median survival of 2–5 yr after diagnosis and a 5-yr survival rate from 20 to 40% (25, 35). A key feature of IPF is the presence of myofibroblasts: contractile cells that deposit excessive extracellular matrix (ECM) and synthesize profibrotic mediators that perpetuate the disorder. The origin of myofibroblasts in IPF has been a subject of intense interest and controversy, with resident and perivascular fibroblasts, pericytes, epithelial, endothelial and bone marrow-derived cells all being ascribed a progenitor role (reviewed in Refs. 18, 19). We have focused on the contribution of pericytes to the myofibroblastic pool in the fibrotic lung. Pericytes are required for the development and maturation of small blood vessels, primarily capillaries, and regulation of vascular permeability and contractility, as well as remodeling during angiogenesis (reviewed in Ref. 1). In addition to their anatomical location and shared basement membrane with the endothelium, pericytes have been identified by a number of markers. Some commonly recognized markers include platelet-derived growth factor receptor-β (PDGFR-β), the Tie2 ligand angiopoietin-1 (Ang-1), CD146 (also known as MCAM or MUC18), the proteoglycan neural glial antigen-2 (NG2, or chondroitin sulfate proteoglycan 4), and the ganglioside 3G5 (37). Some of these markers are thought to be reflective of the functional milieu in the pericyte-endothelium microenvironment. For example, signaling through PDGFR-β is critical for pericyte recruitment during angiogenesis and for maturation of microvessels (1). Ang-1 expression by pericytes may maintain endothelial integrity in the mature microvasculature in a paracrine manner through Tie2 receptor signaling in endothelial cells (1). However, expression of these markers in pericytes is not uniform across different organs and their expression may alter during injury and repair.
Because pericytes interface between the circulating blood and the interstitial space, they have been postulated to play additional roles in tissue homeostasis and repair. Using a strategy based on expression of the transcription factor FoxD1 in pericyte precursors, we and others showed that pericytes contribute to the myofibroblast population in bleomycin-induced lung injury (21) and in kidney fibrosis resulting from ureteral obstruction (20). Furthermore, mouse lung pericytes treated in vitro with transforming growth factor-β (TGF-β) transition into myofibroblastic cells, as defined by increased expression of collagen-1α1, connective tissue growth factor (CTGF), and α-smooth muscle actin (α-SMA) (23). NG2+ cells in IPF lungs were recently shown to colocalize with α-SMA (40), supporting pericytes as myofibroblast progenitors in human tissue.
In addition to their role as myofibroblast precursors, pericytes from rodent lungs, as well as human and mouse brain, express functional Toll-like receptors (TLRs) and may act as immune surveillance cells in vivo (14–16, 22, 24, 26, 33). Our previous work showed that stimulation of mouse lung pericytes in vitro with lipopolysaccharide (LPS) and other classical pathogen-associated molecular pattern (PAMPs) results in the synthesis of multiple chemokines and cell adhesion molecules (22). TLRs and cytosolic pattern recognition receptors such as the NOD-like receptors (NLRs) also recognize damage-associated molecular patterns (DAMPs) released from injured or dying cells and degraded ECM. Activation of pericytes by sterile inflammation results in upregulation of chemokines and cell adhesion molecules that guide interstitial trafficking of extravasated leukocytes (34, 42). Leaf et al. (28) showed that in kidney pericytes, immune and fibrotic responses to tissue injury both converge on the TLR adaptor protein myeloid differentiation primary response 88 (MyD88). Sustained inflammatory signaling in response to PAMPs or DAMPs has been postulated as one possible contribution to the pathogenesis of progressive fibrosis in IPF. However, the response of human lung pericytes to these inflammatory cues has not yet been fully characterized.
To define the repertoire of human lung pericyte responses in injury, repair, and fibrogenesis, we isolated these cells from lung tissues of patients with and without diagnosed IPF. Given that PDGFR-β is expressed constitutively on pericytes (11), we chose to use this marker for cell selection, and assessed the expression of other common pericyte markers. We compared the migration and invasive abilities of control and IPF pericytes. We also compared the responses of control and IPF pericytes to immune and fibrotic stimulants in vitro.
MATERIALS AND METHODS
Isolation and culture of PDGFR-β+ pericytes and fibroblasts from human lung.
All tissues were obtained with approval of the Medical University of South Carolina Institutional Review Board for Human Research. Non-IPF lung samples consisted of excess tissue obtained from clinically indicated lobectomies in collaboration with the Hollings Cancer Center Cancer Biorepository or tissues from failed donors obtained through an agreement with the University of Pittsburgh. IPF samples were obtained from surgical lung transplant explants or autopsy. Lung tissue samples were either previously cryopreserved as described (2) or were fresh (with cold ischemia time up to 36 h). Characteristics of the lung donors are outlined in Table 1.
Table 1.
Patient characteristics
| Non-IPF Controls |
IPF |
|||
|---|---|---|---|---|
| Diagnosis | Age, yr | Sex | Age, yr | Sex |
| Epileptic seizure | 19 | M | 50–59 | M |
| Lung adenocarcinoma | 40–50 | M | 50–59 | M |
| Accident | 52 | M | 50–59 | M |
| Stroke | 52 | M | 50–59 | M |
| Stroke | 52 | M | 56 | M |
| Stroke | 64 | M | 56 | M |
| Anoxia | 38 | F | 56 | M |
| Lung adenocarcinoma | 50–60 | F | 65 | M |
| Data not available | NA | NA | 50–59 | F |
| Data not available | NA | NA | 57 | F |
| 60–69 | F | |||
NA, not available; M, male subjects; F, female subjects.
Tissue was minced and digested in buffer (HBSS with 10 mM HEPES, pH 7.4, 3 mM CaCl2, 3 mM MgCl2, and 2× antibiotic/antimycotic) containing dispase (5 U/ml) and collagenase I (300 U/ml), with vigorous shaking at 37°C for 1 to 2 h. The suspension was filtered (70 μm), centrifuged (350 × g, 10 min), and washed with DMEM + 10% FBS. The dissociated cells were either plated on 0.2% gelatin-coated dishes in a specialized, low-serum (2% FBS) growth medium for pericytes (Pericyte Medium; cat. no. 1201; ScienCell, Carlsbad, CA) or in DMEM containing 10% FBS and penicillin/streptomycin (for fibroblasts). After expansion of the cells in Pericyte Medium (generally for 7–14 days), cells were detached with Accutase (BD Biosciences, San Jose, CA) and labeled with anti-CD45, anti-CD31 (PECAM), and anti-CD326 (EpCAM) magnetic microbeads (Miltenyi) to deplete leukocytes, endothelial cells, and epithelial cells, respectively. Labeled cells were passed over a magnetized column and the flow-through cells were collected for labeling with PE-conjugated anti-PDGFR-β (clone REA363; Miltenyi). This step was followed by incubation with anti-PE magnetic microbeads (Miltenyi) and passage through a magnetized column. Retained cells (PDGFR-β+) were eluted and maintained in Pericyte Medium. Cells were cultured for up to eight passages.
Cell culture on Matrigel.
After being thawed on ice, Matrigel (Corning) was dispensed into wells of a 48-well tissue culture plate (125 µl/well) and allowed to solidify at 37°C for at least 30 min. Pericytes were plated at a density of 2 × 104 cells per well in Pericyte Medium. After 6 h, images were captured using a Nikon inverted phase-contrast microscope equipped with a Nikon DS-L1 camera.
Immunocytochemistry.
Cells grown on chamber slides coated with 0.2% gelatin were fixed with 4% PFA for 15 min at room temperature and then washed with DPBS. After being blocked with 1% BSA for 15 min at room temperature, cells were incubated overnight at 4°C with a rabbit monoclonal PDGFR-β antibody (clone Y92; Abcam), a rabbit polyclonal NG2 antibody (H-300; Santa Cruz Biotechnology) or a mouse monoclonal CD31 antibody (clone JC/70A; Novus). Human microvascular endothelial cells (Lonza) were used as a positive control for CD31 staining. Cells were washed with DPBS and incubated for 1–2 h at room temperature with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody or Alexa Fluor 555-conjugated goat anti-mouse secondary antibody (Invitrogen), diluted 1:500 in DPBS. After being washed with DPBS, chambers were removed and the slides were coverslipped using Vectashield HardSet Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired using a Zeiss Observer.Z1 microscope with Zen 2 software.
Flow cytometry.
Cells were detached with Accutase, washed and resuspended in FACs buffer (Dulbecco’s phosphate-buffered saline, 2% BSA, and 0.75 mM NaN3), and added into a round-bottom 96-well plate. After CD16/CD32 was blocked with Human Fc Block (BD Biosciences), cells were stained with the following antibodies: CD44/HCAM-APC (BD Biosciences), CD73/ecto-5′-NT-APC (Miltenyi Biotec), CD90/Thy1-APC (Life Technologies), CD140a/PDGFRα-PerCP-Cy5.5 (BD Biosciences), and CD146/MCAM-PE-Cy7 (BD Biosciences), diluted according to the manufacturer’s instructions. Cells were analyzed using a Guava 8HT flow cytometer and InCyte Analysis Version 3.1 (EMD Millipore, Billerica, MA).
Cell stimulation.
PAMP molecules were purchased from InvivoGen (San Diego, CA). DAMP molecules included high mobility group box 1 protein (HMGB1; R&D Systems, Minneapolis, MN), heat shock protein-60 (HSP-60; Enzo Life Sciences, Farmingdale, NY), and ATP (Sigma-Aldrich, St. Louis, MO). Uric acid (Sigma-Aldrich) crystals were prepared as described (9). Necrotic cell lysate was prepared from normal human lung PDGFR-β-negative cells by subjecting 107 cells/ml to five successive freeze/thaw cycles. After the cells were centrifuged at 13,000 g, the supernatant was removed and stored at −30°C. The supernatant was diluted 1:20 in assay medium for addition to cells. PAMPs and DAMPs were used at the concentrations listed in Table 2. Active TGF-β1 was purchased from R&D Systems and used at 10 ng/ml. The TGF-β receptor (ALK5) inhibitor SB431542 was obtained from Sigma and used at 10 µM.
Table 2.
PAMPs and DAMPs used in this study
| Ligand | Concentration | Receptor(s) |
|---|---|---|
| Lipopolysaccharide (LPS) | 100 ng/ml | TLR4 |
| Pam3CSK4 (triacylated lipoproteins) | 300 ng/ml | TLR1/2 |
| FSL-1 (diacylated lipoproteins) | 100 ng/ml | TLR2/6 |
| Recombinant flagellin | 100 ng/ml | TLR5 |
| CpG oligodeoxynucleotides | 5 µM | TLR9 |
| High mobility group box 1 protein (HMGB1) | 1 µg/ml | TLR2, TLR4, TLR9, RAGE |
| Heat shock protein-60 (HSP-60) | 5 µg/ml | TLR2, TLR4 |
| ATP | 500 µM | P2X7, NLRP3 |
| Monosodium urate crystals | 100 µg/ml | NLRP3 |
| Necrotic cell lysate (NCL) | 3 × 105 cells | NLRP3 |
TLR, Toll-like receptor; RAGE, receptor for advanced glycation end products; P2X7, purinergic receptor; NLRP3, NOD-, LRR-, and pyrin domain-containing 3 protein.
For stimulation, cells were plated in 12-well gelatin-coated tissue culture dishes at a density of 2–6 × 104 cells/well in complete Pericyte Medium. After cells reached 70–80% confluency, they were either used directly in experiments or were washed with PBS and serum and growth factor starved overnight in basal Pericyte Medium supplemented with 0.1% BSA, 10 nM hydrocortisone and insulin-transferrin-selenium (ThermoFisher Scientific), hereafter referred to as “serum-free medium.” At the appropriate time points after stimulation, conditioned media were collected and cells were harvested for RNA isolation and analysis as described below.
Quantitative RT-PCR.
Total RNA was isolated by standard kits in conjunction with DNase treatment as needed and was reverse transcribed to cDNA using Applied Biosystems High-Capacity RNA-to-cDNA Kit (ThermoFisher Scientific) or iScript Reverse Transcription SuperMix (Bio-Rad, Hercules, CA). Real-time PCR was done using ABI TaqMan Gene Expression Assays (ThermoFisher Scientific) on ThermoFisher StepOnePlus and Bio-Rad CFX96/CFX384 instruments. Quantification of relative gene expression was assessed after normalizing to HPRT or B2M. Predesigned primers and probes included the following: ABCG2 (Hs01053790_m1), ACTA2 (Hs00909449_m1), B2M (Hs00187842_M1), CCL2 (Hs00234140_m1), COL1A1 (Hs00164004_m1), CTGF (Hs01026927_g1), CXCL1 (Hs00605382_gH), CXCL8 (Hs00174103_m1), GLI1 (Hs00171790_m1), HPRT (Hs02800695_m1), ICAM1 (Hs00164932_m1), MYH11 (Hs00224610_m1), RGS5 (Hs01591223_s1), S100A4 (Hs00243202_m1), and TBX18 (Hs01385457_m1). Relative expression was calculated in MS Excel using the 2−ΔΔCT method.
ELISA and Luminex analysis.
Soluble levels of CXCL1 and CXCL8 in conditioned media from cells stimulated by PAMPs for 18 h were measured by ELISA (R&D Systems). Levels of secreted CCL2, CX3CL1, CXCL8, IL-6, matrix metalloproteinase-2 (MMP-2), and VEGF in conditioned media from cells treated 72 h with either vehicle or TGF-β (10 ng/ml) were determined using a Luminex magnetic bead-based multiplex kit from R&D Systems and a MagPix instrument (Millipore).
Western blotting.
Cells were lysed in 2× sample buffer (125 mM Tris·Cl, pH 6.8, 10% glycerol, 3% SDS, 715 mM β-mercaptoethanol, and 0.01% bromophenol blue) and analyzed by immunoblotting after blocking membranes in PBS containing 0.05% Tween-20 and 5% nonfat milk (Bio-Rad). The following primary antibodies were used at 1:1,000: Col1a1 (cat. no. sc-8784R; Santa Cruz Biotechnology), α-SMA (A5228; Sigma), and GAPDH (cat. no. sc-25778; Santa Cruz Biotechnology). Blots were developed using peroxidase conjugated donkey anti-rabbit or goat anti-mouse secondary antibody at 1:10,000 and the ECL 2 Western Blotting Substrate Kit (Pierce). Blots were imaged using a FluorChem R instrument (ProteinSimple, San Jose, CA).
Migration and invasion assays.
Migration was assessed using an in vitro scratch “wound” assay. Cells were grown to confluence in 24-well gelatin-coated dishes and then serum starved overnight. Linear scratch wounds were applied in each well using a 200-µl pipet tip and wells were washed three times with PBS. Cells were cultured in serum-free medium containing PDGF-BB (50 ng/ml), TGF-β (10 ng/ml), or diluent (1 mg/ml BSA in 4 mM HCl). Photomicrographs were taken using the ×4 objective on a Nikon phase contrast microscope at 0, 8, and 24 h. A line drawn on the bottom of each well provided a landmark for proper orientation at each time point. The area of wound closure was quantified using National Institutes of Health ImageJ software (version 1.50i), and data are expressed as percent migration compared with the 0-h time point.
We used the CytoSelect 24-Well Cell Invasion Assay (Basement Membrane, Colorimetric) from Cell Biolabs (cat. no. CBA-110) to assess cell invasion. Cells were incubated overnight in serum-free medium before detaching and seeding in matrix-coated transwells at 5 × 105 cells per ml. The bottom chamber contained Pericyte Medium supplemented with 10% FBS. After a 24-h incubation, cells remaining in the top chamber were removed with moistened cotton swabs and transwells were fixed and stained with Cell Stain Solution from the kit. Five fields of each transwell were randomly chosen for image capture at ×100 magnification. Cells in each field were counted using National Institutes of Health ImageJ and added to obtain the total number of migrated cells.
Proliferation assay.
Cells were seeded in 96-well gelatin-coated dishes at 5 × 103 cells/well, using complete Pericyte Medium. After allowing 3–4 h for attachment, cells were washed with PBS and incubated overnight in serum-free medium. The next day, medium was removed and complete Pericyte Medium or serum-free medium containing FGF-2 (Sigma-Aldrich) at 10 ng/ml or PDGF-BB (R&D Systems) at 50 ng/ml was added along with bromodeoxyuridine (BrdU) reagent (Cell Proliferation ELISA Kit; Roche). Cells incubated in serum-free medium served as controls, and wells without cells were used as blanks. After 72 h, a fixation/denaturation solution was added and BrdU was detected colorimetrically using an anti-BrdU-peroxidase conjugate and substrate, followed by addition of H2SO4 to quench the reaction. Absorbance was measured at 450 nm using a reference wavelength of 690 nm. After the blank wells were subtracted from the absorbance values, proliferation was quantified as percentage of growth relative to that in serum-free medium.
Statistics.
All experiments were performed with at least three independent cell isolates. An unpaired two-tailed Student’s t-test, using Welch’s correction if variances were unequal, was used to compare IPF data to control for each treatment. Statistical significance was set at P ≤ 0.05. Prism version 5.0b (GraphPad Software, San Diego, CA) was used for statistical analysis.
RESULTS
Isolation of human lung PDGFR-β+ cells.
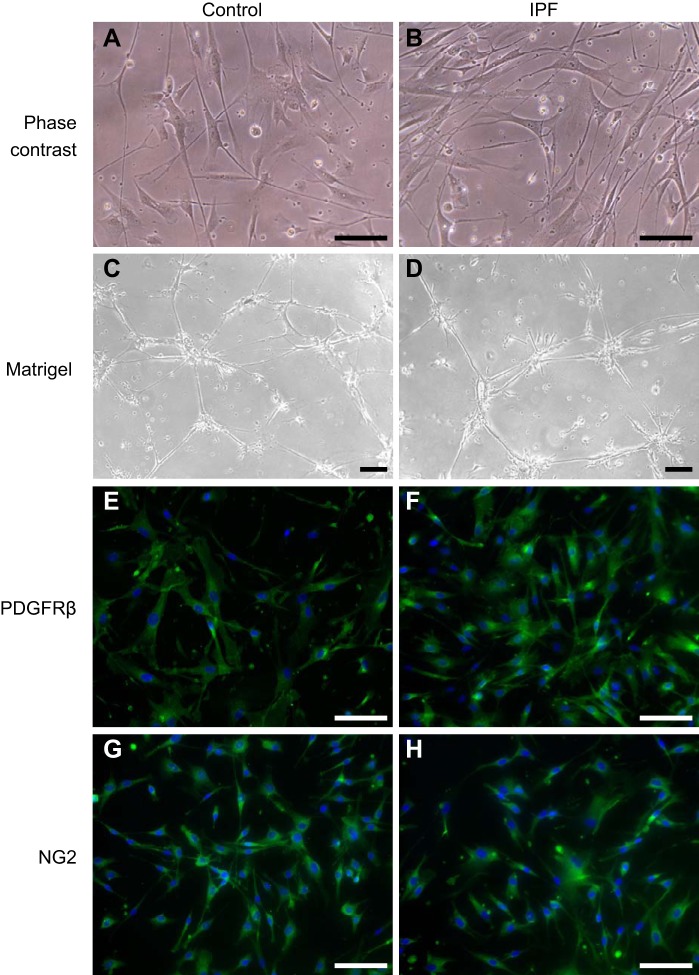
Cells isolated based on PDGFR-β positivity were maintained in a commercial low-serum medium designed to enhance the growth of pericytes and to allow retention of their characteristic markers through multiple passages. PDGFR-β+ cells from non-IPF control and IPF human lungs appear grossly similar by phase contrast microscopy (Fig. 1, A and B), with both types having a spindle-shaped or stellate morphology and elongated, multibranching cellular processes that are typical of pericytes in general and also those from the lung (3, 48). In addition, at confluence the PDGFR-β+ pericytes tend to align in a parallel fashion (data not shown), as was reported for isolated brain pericytes grown in the same defined medium (39). When plated on Matrigel, PDGFR-β+ cells from both control and IPF lungs assemble into primitive networks composed of nodes of cells from which cellular processes project and connect with other nodes (Fig. 1, C and D). Such behavior on Matrigel was previously demonstrated for human pericytes (3, 4).
Fig. 1.
Cell morphology and characteristics of isolated human lung platelet-derived growth factor receptor-β-positive (PDGFR-β+) pericytes. Representative PDGFR-β+ cells from nonidiopathic pulmonary fibrosis (IPF) controls and IPF lungs were assessed by phase contrast light microscopy after plating on plastic (A and B) or Matrigel (C and D). Pericytes were also assessed by immunofluorescence for PDGFR-β (E and F) and neural glial antigen-2 (NG2; G and H). Scale bar = 500 µm in A, B, and E–H and 100 µm in C and D.
Expression of cell-surface pericyte markers by human lung PDGFR-β+ cells.
Our cells retain PDGFR-β expression in culture; are positive for NG2, another widely used marker of pericytes; and are negative for CD31 (Fig. 1, E–H, and data not shown). We analyzed other cell-surface markers by flow cytometry after passage 3 (Fig. 2 and Table 3). The majority of both control and IPF PDGFR-β+ cells have high levels of the mesenchymal markers CD73 and CD90, as well as the hyaluronan receptor CD44, as was previously found for human lung pericytes isolated on the basis of dual CD73 and CD90 positivity (5). In addition, PDGFR-β+ cells from human lungs show little expression of CD146, consistent with findings by Bichsel et al. (5) for their isolated CD73+CD90+ pericytes. However, a greater percentage of IPF PDGFR-β+ cells are CD146+ as compared with controls (Table 3), although this percentage is quite small. Control and IPF cells also differ in their cell-surface abundance of PDGFR-α, with IPF cells showing a lower mean fluorescence intensity than control (and close to significantly lower percent of positive cells). PDGFR-α is generally considered a marker of fibroblasts, although it has been detected on a subset of mouse and human lung pericytes (5, 21).
Fig. 2.
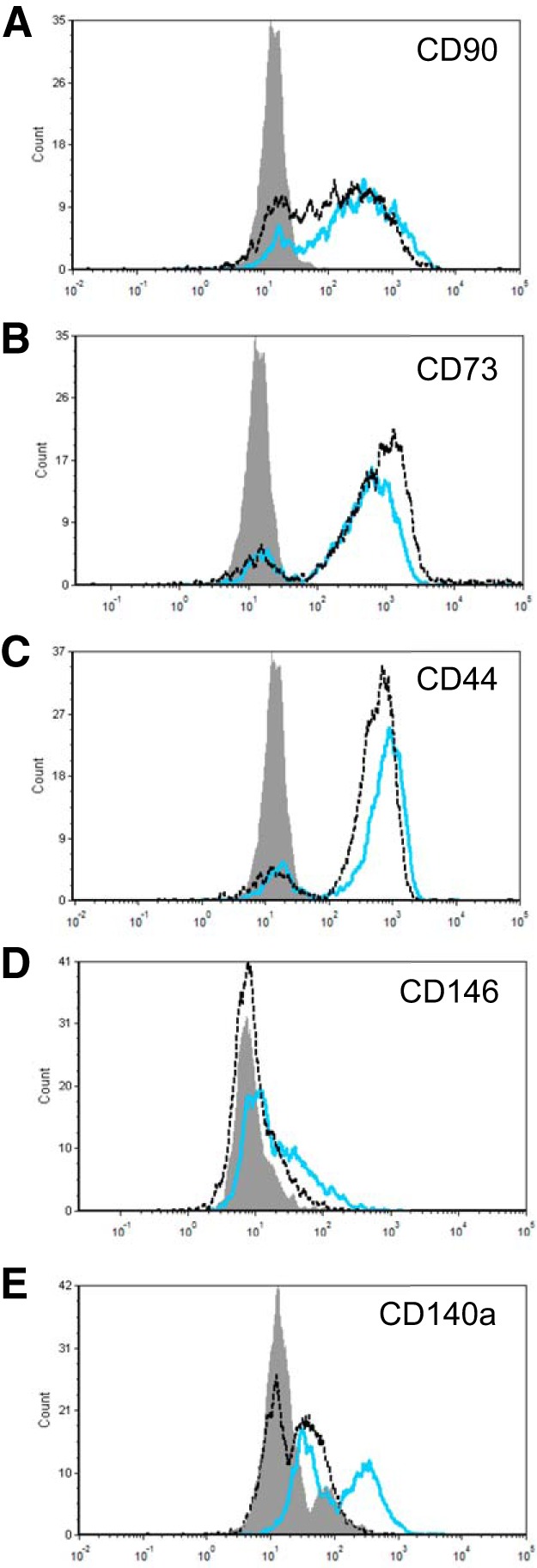
Representative histograms of cell-surface marker staining on control and idiopathic pulmonary fibrosis (IPF) platelet-derived growth factor receptor-β (PDGFR-β)-positive pericytes. Non-IPF (blue line) and IPF (dashed black line) pericytes were stained for CD90 (A), CD73 (B), CD44 (C), CD146 (D), and CD140a (E; PDGFR-α) and analyzed by flow cytometry. Negative controls are shown in gray.
Table 3.
Characterization of PDGFR-β+ cells by flow cytometry
| %Positive Cells |
MFI |
|||||
|---|---|---|---|---|---|---|
| Cell-Surface Marker | Control (n = 3) | IPF (n = 3) | P Value | Control (n = 3) | IPF (n = 3) | P Value |
| CD90 (Thy1) | 97 ± 2 | 89 ± 5 | 0.19 | 533 ± 134 | 448 ± 118 | 0.65 |
| CD73 (ecto-5′-NT) | 99 ± 0.5 | 98 ± 0.1 | 0.17 | 830 ± 129 | 1176 ± 77 | 0.08 |
| CD44 (HCAM) | 99 ± 0.3 | 98 ± 0.4 | 0.26 | 587 ± 121 | 642 ± 39 | 0.69 |
| CD146 (MCAM) | 4 ± 1 | 12 ± 2 | 0.03 | 147 ± 44 | 51 ± 10 | 0.11 |
| CD140a (PDGFR-α) | 26 ± 6 | 9 ± 3 | 0.06 | 377 ± 35 | 131 ± 6 | 0.002 |
Values are means ± SE. MFI, mean fluorescence intensity; Thy1, thymus cell antigen 1; ecto-5′-NT, ecto-5′-nucleotidase; HCAM, homing cell adhesion molecule; MCAM, melanoma cell adhesion molecule; PDGFR-α, platelet-derived growth factor receptor-α; IPF, idiopathic pulmonary fibrosis.
Expression of other pericyte and mesenchymal cell markers by quantitative RT-PCR.
We used quantitative RT-PCR to analyze our control and IPF pericyte isolates for the expression of other pericyte markers and markers indicative of perivascular mesenchymal stem cells (MSCs), as well as additional mesenchymal markers. Expression of genes was classified as low if Ct values were between 30 and 34 and absent if Ct values were ≥35 (Table 4). Based on these parameters, we found no expression of RGS5, a marker of embryonic and angiogenic pericytes (6). However, TBX18, a transcription factor recently described as a marker of both pericytes and vascular smooth muscle cells (17), is expressed at low levels in our PDGFR-β+ lung cells (Table 4). Expression of this gene has been shown to be downregulated in vitro relative to cells in vivo (17), so it is possible that mRNA levels of TBX18 are higher in lung pericytes before isolation. With regard to mesenchymal stem cell genes, there was no expression of the transcription factor Gli1 and the ATP-binding cassette transporter ABCG2, both markers of perivascular mesenchymal stem cells that contribute to the myofibroblast pool during fibrotic remodeling in the lung (27, 29). Finally, by comparing gene expression relative to fibroblasts from non-IPF control lungs, with “fibroblasts” being defined as cells that grew from the initial lung digest in DMEM containing 10% serum, we found that both control and IPF pericytes express markedly lower levels of fibroblast specific protein-1 (S100A4) and α-SMA (ACTA2) and are negative for the heavy chain of smooth muscle myosin (MYH11) (data not shown), in agreement with previous reports for pericytes (10, 48). Taken together, our data demonstrate that the population of PDGFR-β+ cells that we isolated is consistent with pericytes and either does not include or has a very low level of the MSCs that have been described in the lung (27, 29).
Table 4.
Expression of pericyte and mesenchymal stem cell markers by RT-PCR
| Ct Values for Indicated Genes |
|||||
|---|---|---|---|---|---|
| Pericyte Isolates | B2M | RGS5 | GLI1 | ABCG2 | TBX18 |
| Control 1 | 22.2 | 35.3 | ND | 39.2 | 33.5 |
| Control 2 | 22.4 | 35.3 | ND | 35.8 | 32.7 |
| Control 3 | 22.3 | 35.8 | 38.7 | 38.4 | 32.5 |
| IPF 1 | 22.0 | ND | ND | ND | 33.2 |
| IPF 2 | 21.0 | ND | ND | ND | 31.9 |
| IPF 3 | 22.4 | ND | ND | ND | 30.6 |
IPF, idiopathic pulmonary fibrosis; ND, not detected.
Pericytes from IPF lung are more migratory than cells from non-IPF controls.
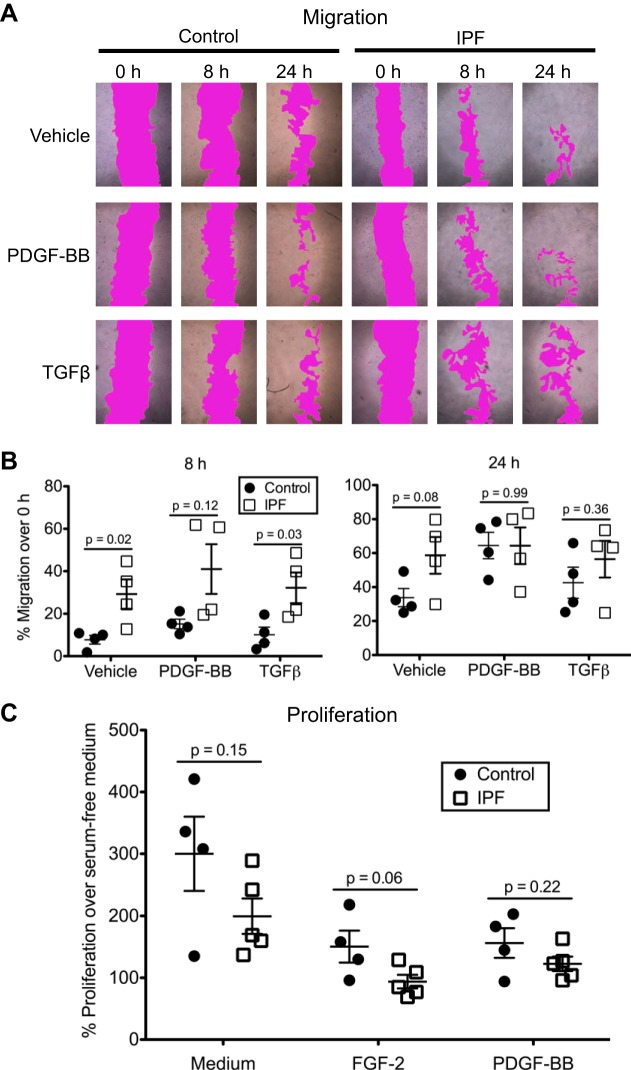
Migration of pericytes is an intrinsic property that is important in their role as modulators of angiogenesis. In addition, detachment and migration of pericytes away from the endothelium are key features during the transition of these cells to myofibroblasts. We asked if PDGFR-β+ pericytes from IPF lungs differ from controls in their migratory behavior. To address this question, we assessed the ability of pericytes to close a scratch wound made in the monolayer either in the absence of growth factors or in the presence of PDGF-BB or TGF-β (Fig. 3A). Both factors are involved in pericyte recruitment (47). Interestingly, even in the absence of exogenous growth factors, IPF pericytes exhibited significantly greater migration compared with control pericytes at 8 and 24 h (Fig. 3B). Addition of TGF-β did not significantly enhance wound closure for either group over that observed with vehicle. Both control and IPF pericytes migrated more rapidly in response to PDGF-BB compared with TGF-β (Fig. 3B). Although the PDGF-treated IPF pericytes again trended toward increased migration compared with the controls, this difference was not statistically significant (P = 0.12). By 24 h, both control and IPF pericytes had similarly migrated into the wound area.
Fig. 3.
Migratory and proliferative properties of control and idiopathic pulmonary fibrosis (IPF) platelet-derived growth factor receptor-β (PDGFR-β)-positive pericytes. A: closure of scratch “wounds” in the monolayer by one control isolate and one IPF isolate without stimulation (vehicle) and in the presence of PDGF-BB (50 ng/ml) or transforming growth factor-β (TGF-β; 10 ng/ml). Area of the scratch wound is shown with magenta false coloring to enhance visualization. B: quantification of wound closure (%migration compared with 0-h time point) for 4 control and 4 IPF isolates. Shown are the means (lines) ± SE. Control and IPF data were compared at each condition by an unpaired, two-tailed Student’s t-test using Welch’s correction if variances were significantly different. C: proliferation of PDGFR-β+ cells measured by bromodeoxyuridine (BrdU) incorporation over 72 h in complete pericyte medium and in serum-free medium containing FGF-2 (10 ng/ml) or PDGF-BB (50 ng/ml). Each isolate was compared with its own control (incubated in serum-free medium containing vehicle) to determine the %proliferation. Each treatment group was analyzed by an unpaired two-tailed Student’s t-test. Shown are the means (lines) ± SE.
To rule out proliferation as a factor contributing to the differences in scratch wound closure, we measured BrdU incorporation in control and IPF pericytes after a 72-h incubation in complete Pericyte Medium or serum-free medium supplemented individually with FGF-2 and PDGF-BB, both growth factors that are known to induce pericyte proliferation (31, 47). As expected, pericytes proliferated most robustly in complete medium (Fig. 3C), which contained FGF-2, IGF-1, and EGF, in addition to 2% serum. Although there was no statistically significant difference in proliferation between control and IPF pericytes, the IPF cells tended to propagate more slowly than controls, especially in response to FGF-2 (Fig. 3C). Therefore, we conclude that proliferation did not contribute to the accelerated wound closure by IPF pericytes in the scratch assay.
To determine if the ability of IPF pericytes to migrate in a more physiological context differs from controls, we used an assay designed to quantitatively measure cell invasion through a Matrigel-coated filter. Twenty-four hours after seeding control and IPF pericytes on the filters, we found that more IPF cells had migrated through the matrix than control cells (Fig. 4A) in response to serum added to the bottom chamber. In addition, emergence of each IPF cell body was more complete than that seen with the control pericytes (Fig. 4B).
Fig. 4.
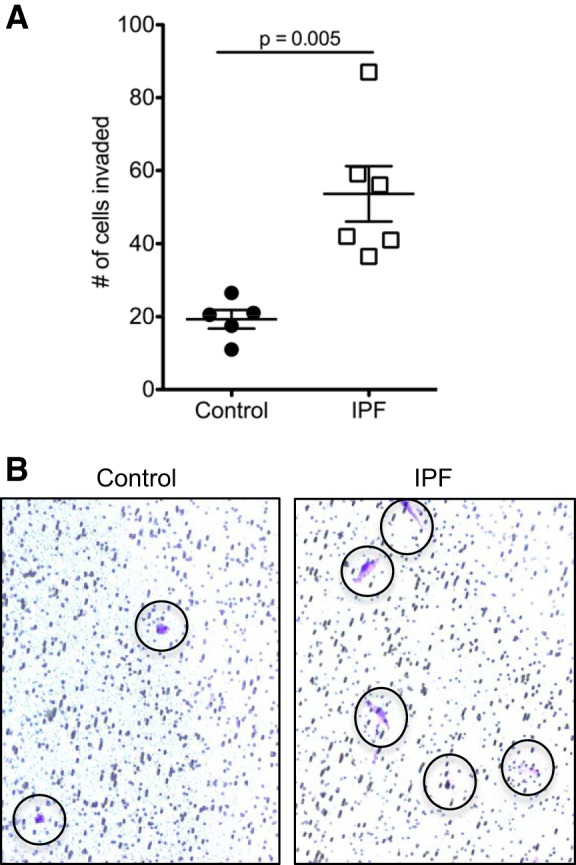
Invasion of Matrigel by control and idiopathic pulmonary fibrosis (IPF) pericytes. A: control and IPF pericytes were compared in their ability to invade a polycarbonate filter coated with basement membrane matrix (Matrigel) in response to 10% FBS after 24 h. Shown are the means (lines) ± SE. Data were analyzed using an unpaired two-tailed Student’s t-test with Welch’s correction. B: representative images of cells that have invaded the matrix and migrated to the other side of the filter (cells stained blue and indicated by the circles) are shown.
Human lung PDGFR-β+ pericytes express proinflammatory chemokines in response to stimulation by PAMPs and DAMPs in vitro.
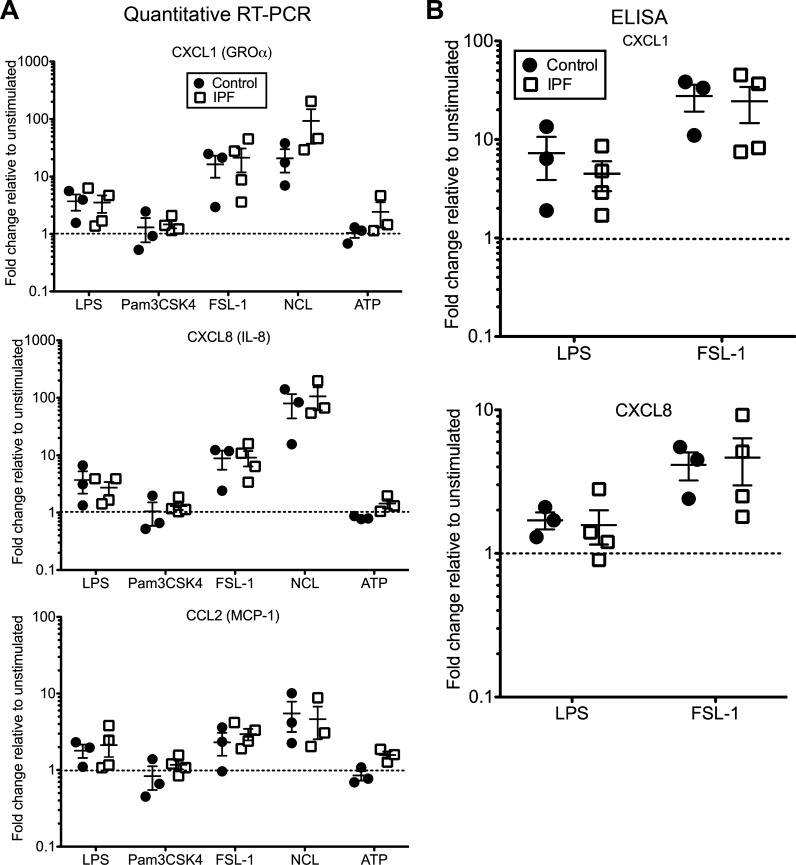
Pericytes are increasingly being recognized as cells important in immunomodulation and tissue repair (30, 43). Isolated pericytes from rodent lungs, as well as human and mouse brain, kidney and placenta, elaborate a number of chemokines and cell adhesion molecules in response to stimulation by PAMPs and DAMPs (14–16, 24, 26, 28, 33, 42). We tested if human lung pericytes react similarly to a panel of these stimuli (Table 2) and if IPF cells differ from nonfibrotic controls in their responses. Of the typical pathogen-derived TLR ligands, FSL-1, a ligand that mimics diacylated lipoproteins and signals through the TLR2/6 heterodimer, produces the most robust response in terms of chemokine expression from human lung pericytes (Fig. 5A). Expression of CXCL1 (also known as GROα or KC) and CXCL8 (IL-8) was increased in all pericyte isolates examined, with more variability in CCL2 (MCP-1) expression. LPS, a TLR4 ligand, also elicits an average two- to fivefold increase in chemokine expression (Fig. 5A). Similar patterns of expression were observed for other chemokines, including CXCL2, as well as ICAM-1 (data not shown). Using ELISAs, we verified that the levels of CXCL1 and CXCL8 secreted into the conditioned medium were increased in the majority of isolates, particularly in response to FSL-1 (Fig. 5B). By contrast, there was little to no augmentation of chemokine expression with the TLR1/2 ligand Pam3CSK4 (Fig. 5A), as well as TLR5 and TLR9 ligands (data not shown). Our data show that pericytes express functional TLR2, TLR4, and TLR6 and that both control and IPF pericytes have essentially the same magnitude of response to ligands for these receptors.
Fig. 5.
Response of control and idiopathic pulmonary fibrosis (IPF) pericytes to pathogen-associated molecular patterns and damage-associated molecular patterns in vitro. A: pericytes from control and IPF lungs were treated for 18–24 h with vehicle (unstimulated) or the indicated agonists at the concentrations listed in Table 2. RNA was isolated to assess chemokine expression, with data presented as fold change in expression for each isolate compared with its own vehicle (set to 1 and indicated by the dotted lines) after normalizing to B2M. Shown are the means (lines) ± SE. NCL, necrotic cell lysate. B: conditioned media were collected after 18–24 h stimulation for analysis by ELISAs for CXCL1 and CXCL8. Data are presented as fold change in levels relative to unstimulated (set to 1 and indicated by the dotted lines) for each isolate. Shown are the means (lines) ± SE.
Incubation with HMGB1 and HSP-60, intracellular DAMPs that are released by necrotic cells and signal through TLR2 and TLR4 (Table 2), had little effect on chemokine expression and secretion, with the strongest level of induction less than twofold (data not shown). We then assessed agonists that target the inflammasome component NOD-, LRR-, and pyrin domain-containing 3 protein (NLRP3), including ATP, monosodium urate crystals, and necrotic cell lysate (NCL). Of these, only NCL had a significant effect on mRNA levels of the chemokines CXCL1, CXCL8, and CCL2, with a response exceeding even that of FSL-1 (Fig. 5A). There were no significant differences in the response of control and IPF pericytes to NCL.
Both non-IPF and IPF pericytes are responsive to the profibrotic cytokine TGF-β.
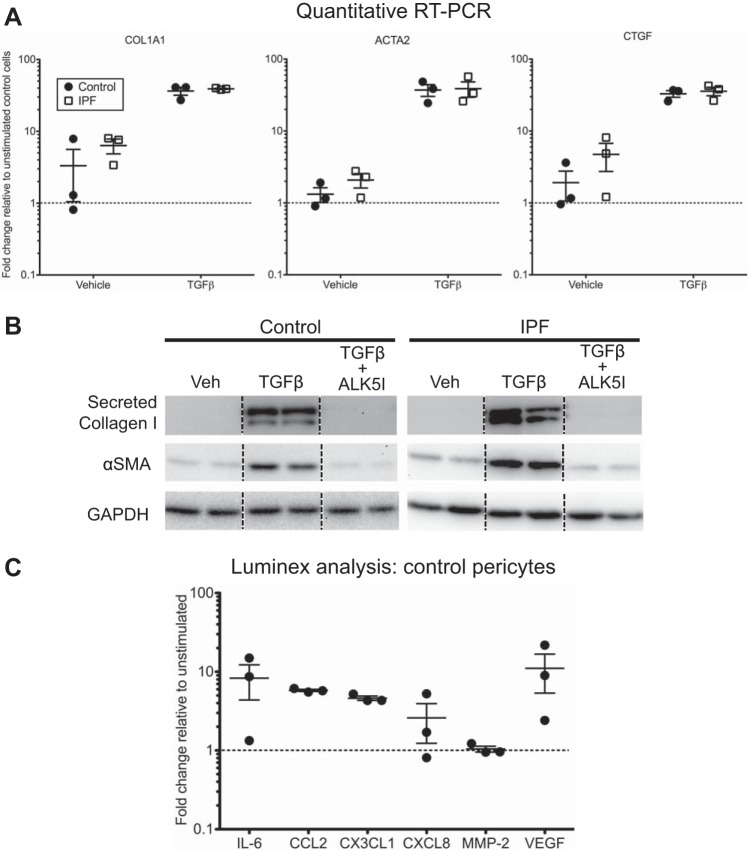
Activation of mouse and human pericytes from multiple organs with TGF-β, a canonical profibrotic cytokine, leads to the myofibroblastic differentiation of these cells (5, 28, 31, 36, 40, 44, 46). Other studies established that human lung pericytes express α-SMA protein and assemble it into contractile fibers when treated in vitro with TGF-β (5, 36). We set out to compare the expression of α-SMA and fibrotic ECM genes in IPF pericytes relative to cells from non-IPF lungs and to determine if IPF pericytes differ from controls in their response to TGF-β. We found that at baseline IPF pericytes do not have significantly elevated expression of α-SMA, collagen I, or CTGF relative to control pericytes (Fig. 6A). Treatment of the cells with TGF-β for 48 h caused a robust increase in expression of the same genes and at a similar level for both control and IPF pericytes (Fig. 6A). Note that one of the three control isolates had higher baseline mRNA levels of these genes than the two used to calculate relative expression, demonstrating the variability often observed with human samples. Immunoblotting of conditioned media or whole cell lysates confirmed increased secretion of collagen and expression of α-SMA protein with TGF-β and also showed that inclusion of the TGF-β receptor inhibitor SB431542 abolished this increase (Fig. 6B).
Fig. 6.
Response of control and idiopathic pulmonary fibrosis (IPF) lung pericytes to transforming growth factor-β (TGF-β) in vitro. A: pericytes from control and IPF lungs were treated for 48 h with vehicle or TGF-β at 10 ng/ml. After RNA isolation, expression of collagen I (COL1A1), α-smooth muscle actin (α-SMA; ACTA2), and connective tissue growth factor (CTGF) was assessed by quantitative RT-PCR. Levels of expression were determined relative to that in normal cells (2 isolates) treated with vehicle alone (set to 1 and denoted by the dotted lines). B: pericytes from control and IPF lungs were treated for 72 h with vehicle (Veh), TGF-β at 10 ng/ml, or TGF-β at 10 ng/ml and 10 µM of the TGF-β receptor inhibitor SB431542 (ALK5I). Conditioned media were collected and cell lysates were prepared for immunoblotting. A representative control and IPF pericyte isolate are shown, with duplicate lanes shown for the treatment of each isolate. The dotted lines designate noncontiguous lanes from the same gel. C: pericytes from control lungs were treated for 72 h with vehicle or TGF-β at 10 ng/ml and conditioned media were collected for analysis by a Luminex magnetic bead-based multiplex kit. Data are displayed relative to vehicle controls for each isolate.
In addition to promoting a profibrotic phenotype, TGF-β governs expression of inflammatory and proangiogenic mediators in human brain pericytes (38, 44). For example, levels of secreted IL-6 and MMP-2 are increased after exposure of pericytes to TGF-β (38, 44). We used a Luminex panel to quantify levels of soluble mediators expressed by lung pericytes after a 72-h treatment with active TGF-β. We focused on mediators that had been shown to be altered in brain pericytes by TGF-β in a recent study (38). Consistent with this study, we found that conditioned media from lung pericytes showed an increase in IL-6 levels with TGF-β stimulation, although the levels varied among the isolates (Fig. 6C). However, levels of CCL2 and CX3CL1 were elevated four to sixfold in conditioned media from all three isolates we assessed, whereas these chemokines were decreased in brain pericytes (38). CXCL8 levels also varied among the isolates, ranging from no change to increased fivefold, and MMP-2 was essentially not altered by TGF-β (Fig. 6C). Of note, we found that VEGF was secreted at higher levels in response to TGF-β, as compared with vehicle alone. By contrast, TGF-β did not have a significant effect on the production of Ang-1 (data not shown), a pericyte-derived protein important in cross talk with endothelial cells.
DISCUSSION
In this study, we used the canonical pericyte marker PDGFR-β to isolate and compare the characteristics of lung pericytes from patients with IPF to that of cells from non-IPF control subjects. The morphological properties and marker profiles of the isolated PDGFR-β+ cells led us to conclude that these cells are most consistent with pericytes rather than other major populations of lung stromal cells such as fibroblasts, smooth muscle cells, or myofibroblasts. We also did not find compelling evidence for MSC identity in the PDGFR-β+ isolates. Our experiments further highlight the functional plasticity of human lung PDGFR-β+ stromal cells/pericytes, whether IPF or non-IPF, as immune modulators, injury responders, and potential myofibroblast progenitors.
Pericytic cells have been isolated from human lung samples using antibodies against the 3G5 antigen (36, 48), NG2 (3), and CD73/CD90 (5). Our study confirms expression of the major pericyte markers, such as PDGR-β and NG2. However, we found little expression of CD146 by flow cytometry (Fig. 2 and Table 3) and gene expression analysis (data not shown), similar to the report by Bichsel et al. (5) but in contrast to the study by Yuan et al. (48). The low positivity for CD146 is surprising as it is a typical marker of pericytes from other organs, such as placenta and brain (11). The finding that IPF pericytes have a higher percentage of CD146+ cells than the controls is of unknown significance. Conflicting findings of CD146 expression in published studies may be explained by heterogeneity in the human lung pericyte population, similar to the CNS (32). Subpopulations of human lung pericytes may vary in the expression of CD146. The use of different cell surface markers to purify and expand human lung pericytes in culture might lead to enrichment of subpopulations of pericytes that are CD146+ or CD146−, accounting for the differences in pericyte CD146 expression observed in published reports. It has become clear that pericytes are highly plastic and heterogeneous cells, whose origin even within a single organ may differ (13).
We also found that PDGFR-β+ pericytes have a subset of cells that are positive for PDGFR-α (Fig. 2 and Table 3), with IPF pericytes having both a lower percentage of positive cells and a lower staining intensity for this marker than the control cells. Another group previously demonstrated that a majority of NG2+ cells from non-IPF human lung are also PDGFR-α+ (5). In addition, in our experiments using transgenic mice to fate map pericytes, we determined that the uninjured lung contains a small population of collagen-producing cells that are positive for both PDGF receptors (21). Taken together, these findings raise the intriguing possibility that there is a subpopulation of pericytes with a specific role in tissue maintenance and repair in the lung. Although PDGFR-α signaling is generally associated with a fibrogenic phenotype (7), the receptor is downregulated on lung fibroblasts that have been treated with TGF-β in vitro (8). It may be that the fibrotic microenvironment promotes a similar decrease in cell-surface PDGFR-α in a subset of IPF pericytes.
Culture of pericytes in medium containing 10% serum causes upregulation of α-SMA and other fibrosis-associated genes in these cells (39, 41, 44, 45). Therefore, the commercial medium that we and others have used for cultivation of human lung pericytes contains only 2% fetal bovine serum and has a supplement with growth factors (FGF-2, IGF-1, and EGF) that are conducive for maintaining a pericytic phenotype. This medium favors growth of pericytes even without using a marker-based technique for purifying them (3, 39). FGF-2 is known to suppress α-SMA expression in cultured pericytes (31, 44). Compared with lung fibroblasts, our cells express little α-SMA and fibroblast-specific protein-1. Taken together, these findings suggest that our approach did not select for activated resident fibroblasts or myofibroblasts that may express PDGFR-β (7). In addition, we ruled out the possibility that we had isolated smooth muscle cells because our cells lacked expression of the smooth muscle myosin heavy chain. Finally, our results also indicate that lung PDGFR-β+ pericytes do not contain a substantial population of MSCs, based on the lack of Gli1 and ABCG2 expression (Table 3). Indeed, ABCG2+ MSCs are believed to be precursors of NG2+ pericytes in the lung (29). The absence of RGS5, an important mediator of G protein signaling in pericytes during vascular development and remodeling, is consistent with our PDGFR-β+ pericytes having a mature phenotype.
IPF pericytes are indistinguishable from non-IPF when viewed by two-dimensional phase-contrast microscopy (Fig. 1). On Matrigel, both types of pericytes spontaneously organize into clusters of cells connected by cell extensions that are reminiscent of primitive tube-like structures (Fig. 1). Thus, like endothelial cells, pericytes on a basement membrane matrix have an innate tendency to assemble into nascent vessels. However, our data showed an enhanced mobility of pericytes from IPF lungs over non-IPF in both two- and three-dimensional assays (Figs. 3 and 4). These findings raise the intriguing possibility that pericytes from IPF lung are primed to migrate through ECM. The function of pericytes may be altered in the pathological microenvironment of IPF lungs, with enhanced migratory potential as one feature of their phenotype. Whether other recognized functions of pericytes in homeostasis are also altered in the setting of IPF, such as their angiogenic potential and maintenance of endothelial integrity, remains unexplored. Future studies employing coculture models with endothelial cells will lead to greater insights into functional perturbations within the pericyte-endothelial niche in the setting of IPF. Unlike fibroblasts in IPF, pericytes from diseased lungs do not appear to be more proliferative than non-IPF control cells; our data showed that the IPF cells actually trend toward lower levels of proliferation in response to serum, FGF-2, or PDGF-BB (Fig. 3). Study of additional samples may help clarify this trend.
Challenge of PDGFR-β+ lung pericytes with PAMPs and DAMPs to mimic pathogenic signals received during tissue injury caused upregulation of a panel of proinflammatory molecules (Fig. 5). By testing multiple agonists for specific TLRs, we determined that human lung pericytes primarily express TLR2, TLR4, and TLR6 and have a generally stronger response to a TLR2/6 ligand compared with TLR4. Compared with the response of mouse lung pericytes, however, the magnitude of chemokine induction is much lower (by 5- to 65-fold, depending on the specific chemokine) (22). This may reflect a species-specific difference or a difference in the age of donors: mice are typically used at 8–12 wk of age (equivalent to ~20 human years) for experiments, whereas the human subjects in our study had a median age range of 51–59 (Table 1). Aging may reduce the immune response in these cells. Human lung pericytes also have a narrower repertoire of TLRs than mouse cells and do not produce TNF-α with stimulation (unpublished data and Ref. 16). Despite lung pericytes having functional TLRs for the DAMPs HMGB1 and HSP-60, these agonists surprisingly had little effect on chemokine expression. Moreover, other investigators observed at most an approximately twofold increase in CXCL8 mRNA with stimulation of human brain pericytes with HMGB1 (16), suggesting these molecules are not strong immune inducers in pericytes. ATP and uric acid crystals also do not have a notable effect on lung pericytes. By contrast, necrotic cell lysate triggers assembly of the NLRP3 inflammasome and robust induction of chemokine expression (Fig. 5). Finally, although TLR signaling in kidney pericytes has been linked to fibrogenesis (28), we did not detect increased expression of fibrotic genes with stimulation of human lung pericytes by PAMPs or necrotic cell lysate (data not shown).
We previously demonstrated that mouse lung pericytes are a source of myofibroblasts (21). Our findings confirm that human lung PDGFR-β+ pericytes also respond to TGF-β by upregulation of profibrotic genes (Fig. 6), in agreement with other studies (5, 36, 40). There were no significant differences in expression of these genes between non-IPF and IPF pericytes in the absence of TGF-β, arguing against the possibility that we isolated myofibroblasts from IPF lungs by our selection strategy. One limitation of our study is that we cannot determine whether myofibroblasts in IPF are originally derived from pericytes and cannot define the specific contributions of pericytes to this disease. The effect of TGF-β on pericyte function in vivo is complex. Secretion and activation of latent TGF-β by endothelial cells result in pericyte upregulation of VEGF, which in turn fosters endothelial survival and stability (12). We demonstrated that treatment of human lung pericytes with TGF-β increases VEGF levels in conditioned media (Fig. 6). Although MMP-2 is another permeability factor that is upregulated in brain pericytes by TGF-β (38), we did not see increased secretion of MMP-2 from lung pericytes (Fig. 6). This could be due to differences in the assays used to assess soluble mediators and/or organ-specific pericyte responses.
In conclusion, we show that while isolated PDGFR-β+ pericytes from IPF lungs share many transcriptional and morphological similarities with pericytes from non-IPF lungs, they exhibit an increased propensity to migrate and invade ECM. These functional alterations in IPF pericytes may have implications for the pathogenesis of this poorly understood disease. Although more work is needed to understand the lung pericyte-endothelial niche in health and disease, our data add to accumulating evidence that pericytes play an important role in immunologic and fibrogenic processes in the lung.
GRANTS
This work was supported in part by National Heart, Lung and Blood Institute Grant R01-HL-133751 (to C. Feghali-Bostwick and L. M. Schnapp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.L.W., J.P.H., C.F.H., and L.M.S. conceived and designed research; C.L.W., S.E.S., and J.P.H. performed experiments; C.L.W., S.E.S., J.P.H., C.F.H., and L.M.S. analyzed data and interpreted results of experiments; C.L.W. prepared figures and drafted manuscript; C.L.W., S.E.S., C.F.-B., C.F.H., and L.M.S. edited and revised manuscript; C.L.W., S.E.S., J.P.H., C.F.-B., C.F.H., and L.M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lindsey Felton, Andraia Li, Seth Bollenbecker, Sarah Falta, Charles Reese, and David Bastian for valuable contributions to the study. We also thank Drs. John Baatz and Demetri Spyropoulos at the Medical University of South Carolina and Dr. Joseph Pilewski at the University of Pittsburgh for generously sharing lung tissues.
REFERENCES
- 1.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Baatz JE, Newton DA, Riemer EC, Denlinger CE, Jones EE, Drake RR, Spyropoulos DD. Cryopreservation of viable human lung tissue for versatile post-thaw analyses and culture. In Vivo 28: 411–423, 2014. [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley RG, Rouleau C, Morgenbesser SD, Weber W, Cook BP, Shankara S, Madden SL, Teicher BA. Pericytes from human non-small cell lung carcinomas: an attractive target for anti-angiogenic therapy. Microvasc Res 71: 163–174, 2006. doi: 10.1016/j.mvr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res 65: 9741–9750, 2005. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- 5.Bichsel CA, Hall SR, Schmid RA, Guenat OT, Geiser T. Primary human lung pericytes support and stabilize in vitro perfusable microvessels. Tissue Eng Part A 21: 2166–2176, 2015. doi: 10.1089/ten.tea.2014.0545. [DOI] [PubMed] [Google Scholar]
- 6.Bondjers C, Kalén M, Hellström M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729, 2003. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JC, Badgett A, Lindroos PM, Osornio-Vargas AR. Transforming growth factor beta 1 downregulates the platelet-derived growth factor alpha-receptor subtype on human lung fibroblasts in vitro. Am J Respir Cell Mol Biol 13: 496–505, 1995. doi: 10.1165/ajrcmb.13.4.7546780. [DOI] [PubMed] [Google Scholar]
- 9.Chen DP, Wong CK, Tam LS, Li EK, Lam CW. Activation of human fibroblast-like synoviocytes by uric acid crystals in rheumatoid arthritis. Cell Mol Immunol 8: 469–478, 2011. doi: 10.1038/cmi.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36: 642–654, 2008. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol 264: 275–288, 2003. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, de Almeida VM, de Paula Guerra DA, Pinheiro Dos Santos GS, Mintz A, Delbono O, Birbrair A. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol 427: 6–11, 2017. doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman DA, Jiang Y, Tyburski JG, Wilson RF, Steffes CP. Cytokine production in lipopolysaccharide-exposed rat lung pericytes. J Trauma 62: 89–93, 2007. doi: 10.1097/TA.0b013e31802dd712. [DOI] [PubMed] [Google Scholar]
- 15.Gaceb A, Özen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab 38: 45–57, 2018. doi: 10.1177/0271678X17719645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289: 2457–2468, 2014. doi: 10.1074/jbc.M113.521161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20: 345–359.e5, 2017. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B. Myofibroblasts. Exp Eye Res 142: 56–70, 2016. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung CF, Mittelsteadt KL, Brauer R, McKinney BL, Hallstrand TS, Parks WC, Chen P, Schnapp LM, Liles WC, Duffield JS, Altemeier WA. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol 312: L556–L567, 2017. doi: 10.1152/ajplung.00349.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung CF, Wilson CL, Chow YH, Schnapp LM. Role of integrin alpha8 in murine model of lung fibrosis. PLoS One 13: e0197937, 2018. doi: 10.1371/journal.pone.0197937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, Mee EW, Faull RL, Dragunow M. A role for human brain pericytes in neuroinflammation. J Neuroinflammation 11: 104, 2014. doi: 10.1186/1742-2094-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 3: 285–292, 2006. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation 8: 139, 2011. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leaf IA, Nakagawa S, Johnson BG, Cha JJ, Mittelsteadt K, Guckian KM, Gomez IG, Altemeier WA, Duffield JS. Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. J Clin Invest 127: 321–334, 2017. doi: 10.1172/JCI87532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marriott S, Baskir RS, Gaskill C, Menon S, Carrier EJ, Williams J, Talati M, Helm K, Alford CE, Kropski JA, Loyd J, Wheeler L, Johnson J, Austin E, Nozik-Grayck E, Meyrick B, West JD, Klemm DJ, Majka SM. ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol 307: C684–C698, 2014. doi: 10.1152/ajpcell.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro R, Compte M, Álvarez-Vallina L, Sanz L. Immune regulation by pericytes: modulating innate and adaptive immunity. Front Immunol 7: 480, 2016. doi: 10.3389/fimmu.2016.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci 44: 4994–5005, 2003. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 32.Park TI, Feisst V, Brooks AE, Rustenhoven J, Monzo HJ, Feng SX, Mee EW, Bergin PS, Oldfield R, Graham ES, Curtis MA, Faull RL, Dunbar PR, Dragunow M. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci Rep 6: 26587, 2016. doi: 10.1038/srep26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res 1550: 1–8, 2014. doi: 10.1016/j.brainres.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209: 1219–1234, 2012. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmüller P, Humbert M, Guignabert C. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 129: 1586–1597, 2014. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 37.Rowley JE, Johnson JR. Pericytes in chronic lung disease. Int Arch Allergy Immunol 164: 178–188, 2014. doi: 10.1159/000365051. [DOI] [PubMed] [Google Scholar]
- 38.Rustenhoven J, Aalderink M, Scotter EL, Oldfield RL, Bergin PS, Mee EW, Graham ES, Faull RL, Curtis MA, Park TI, Dragunow M. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation 13: 37, 2016. doi: 10.1186/s12974-016-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rustenhoven J, Smyth LC, Jansson D, Schweder P, Aalderink M, Scotter EL, Mee EW, Faull RLM, Park TI, Dragunow M. Modelling physiological and pathological conditions to study pericyte biology in brain function and dysfunction. BMC Neurosci 19: 6, 2018. doi: 10.1186/s12868-018-0405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sava P, Ramanathan A, Dobronyi A, Peng X, Sun H, Ledesma-Mendoza A, Herzog EL, Gonzalez AL. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight 2: 96352, 2017. doi: 10.1172/jci.insight.96352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiwen X, Rajkumar V, Denton CP, Leask A, Abraham DJ. Pericytes display increased CCN2 expression upon culturing. J Cell Commun Signal 3: 61–64, 2009. doi: 10.1007/s12079-009-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Brühl ML, Gärtner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 14: 41–51, 2013. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 43.Stark K, Pekayvaz K, Massberg S. Role of pericytes in vascular immunosurveillance. Front Biosci (Landmark Ed) 23: 767–781, 2018. doi: 10.2741/4615. [DOI] [PubMed] [Google Scholar]
- 44.Thanabalasundaram G, Schneidewind J, Pieper C, Galla HJ. The impact of pericytes on the blood-brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol 43: 1284–1293, 2011. doi: 10.1016/j.biocel.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Tigges U, Welser-Alves JV, Boroujerdi A, Milner R. A novel and simple method for culturing pericytes from mouse brain. Microvasc Res 84: 74–80, 2012. doi: 10.1016/j.mvr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verbeek MM, Otte-Höller I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol 144: 372–382, 1994. [PMC free article] [PubMed] [Google Scholar]
- 47.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 312: 623–629, 2006. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Yuan K, Orcholski ME, Panaroni C, Shuffle EM, Huang NF, Jiang X, Tian W, Vladar EK, Wang L, Nicolls MR, Wu JY, de Jesus Perez VA. Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol 185: 69–84, 2015. doi: 10.1016/j.ajpath.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]