Abstract
Colonoscopy is a widely used method for diagnosing and treating colonic disease. The number of colonoscopies is increasing worldwide, and concerns about associated adverse events are growing. Large-scale studies using big data for post-colonoscopy complications have been reported. A colon perforation is a severe complication with a relatively high mortality rate. The perforation rate, as reported in large studies (≥ 50,000 colonoscopies) published since 2000, ranges from 0.005-0.085%. The trend in the overall perforation rate in the past 15 years has not changed significantly. Bleeding is a more common adverse event than perforation. Recent large studies (≥ 50,000 colonoscopies) have reported post-colonoscopy bleeding occurring in 0.001-0.687% of cases. Most studies about adverse events related to colonoscopy were performed in the West, and relatively few studies have been conducted in the East. The incidence of post-colonoscopy complications increases in elderly patients or patients with inflammatory bowel diseases. It is important to use a unified definition and refined data to overcome the limitations of previous studies. In addition, a structured training program for endoscopists and a systematic national management program are needed to reduce post-colonoscopy complications. In this review, we discuss the current trends in colonoscopy related to adverse events, as well as the challenges to be addressed through future research.
Keywords: Colonoscopy, Adverse events, Perforation, Bleeding, Post-colonoscopy
Core tip: Although colonoscopy-related adverse events rarely occur and the need for colonoscopy is increasing, the proportion of subjects with risk factors is increasing. Recently, the perforation rate, as reported in large studies, ranges from 0.005-0.085%. The perforation probability after colonoscopy does not decrease over time in either the West or the East. Other studies have reported post-colonoscopy bleeding occurring in 0.001-0.687% of cases. In this review article, we discuss the current trends in post-colonoscopy complications, as well as the challenges to be addressed through future research.
INTRODUCTION
Colonoscopy is a widely used method for diagnosing and treating colonic disease. As colorectal cancer (CRC) screenings and surveillance increase worldwide, the number of colonoscopies required is also steadily increasing[1]. In addition, the number of colonoscopies is also mounting in both patients with comorbidities as well as elderly patients due to increasing life expectancy[2-4]. Although colonoscopy-related adverse events rarely occur, the need for colonoscopy is increasing. As the proportion of subjects with risk factors increases, it is essential to identify and reduce these adverse events[5].
Recently proposed colonoscopy quality indicators have suggested appropriate performance targets for adverse events. According to the American Society for Gastrointestinal Endoscopy (ASGE)/American College of Gastroenterology Task Force on Quality in Endoscopy, post-polypectomy bleeding occurs in < 1% of cases[6]. The incidence of perforations is < 1:500 for all examinations and < 1:1000 for screenings[6]. The European Society of Gastrointestinal Endoscopy (ESGE) also recommends appropriate rates for colonoscopy-related adverse events. ESGE proposed a rate of < 5% for bleeding and < 1:1000 for perforations[7]. However, the proportion of adverse events associated with colonoscopy varies widely in practice. The incidence rate of adverse events changes according to the characteristics of the patient or the endoscopist, the type of procedure, and time trends[5,8-10].
Detecting neoplastic lesions during a colonoscopy prevents CRC[11]. For this reason, the role of colonoscopy in population screening tests (even in asymptomatic and previously healthy populations) is becoming increasingly important. It therefore must be recognized that colonoscopy-related adverse events could even be harmful to healthy people. In particular, complications, such as perforations or massive bleeding, may seriously affect the patient. It is therefore important to know exactly how the adverse event occurs in actual clinical practice and how it develops. Based on recent large-scale studies, this review will discuss trends in the occurrence of adverse events (perforation, bleeding, and others) related to colonoscopy, regional differences, and oversight of complications at the national level.
PERFORATION TIME TRENDS AND REGIONAL DIFFERENCES
A colon perforation is a well-known adverse event resulting from colonoscopy. It happens very rarely, but it is a feared adverse event with high morbidity and considerable mortality[12,13]. Generally, colonoscopy perforation was defined as intraperitoneal fat or viscera seen during the colonoscopy, or the presence of radiographic abnormalities (intra-abdominal free air on X-rays, or localized or diffuse release of gas or intestinal fluid into the peritoneum on computed tomography (CT) scans)[5,9,14]. Colonoscopy perforations may occur by several mechanisms, such as mechanical trauma, barotrauma, thermal energy and removal of a tissue lesion[14]. Iqbal et al[13] classified the injury characteristics, based on the mechanism of perforation, into thermal, polypectomy and blunt. Blunt injury is caused by direct trauma or torque from the endoscope; this mechanism results in the largest perforations. The cecum is the most frequent site of perforation due to thermal energy and polypectomy, as well as the rectosigmoid colon due to blunt injuries[13]. The outcome varies depending on the type of perforation. In particular, blunt injuries have larger perforations and a higher rate of fecal diversion than polypectomy injury and, therefore, a worse prognosis[13]. In addition, immediate detection of perforation results in less intraperitoneal contamination than delayed detection. In general, perforations detected during or immediately after colonoscopy have a better prognosis than those whose detection is delayed, and less frequently require surgical treatment[8,13,15]. In totality, the recto-sigmoid colon has emerged as the most frequent site of perforation[13]. Bielawska et al[16] demonstrated that increasing age, increasing American Society of Anesthesia (ASA) class, female gender, hospital setting, therapeutic colonoscopy and removing polyps > 10 mm are factors significantly associated with an increased risk of early perforation. In particular, perforations in elderly patients can lead to a high proportion of fatal consequences. Therefore, endoscopists should keep in mind that colonoscopy can be a major drawback to these patients[16,17].
Surgery plays an important role in the treatment of post-colonoscopy perforation. Recent advances in endoscopic techniques have enabled treatment of < 10 mm immediately detected colonoscopyrelated perforations in patients with good bowel preparation and stable vital signs[18]. The ESGE recommends the use of through-the-scope endoclips for small perforations and over-the-scope clips (OTSC) for larger ones[19]. In addition, electrocautery injury may induce colon perforations, which can be closed by endoscopic clipping, particularly during endoscopic submucosal dissection (ESD)[20,21]. According to systematic reviews, the OTSC method is effective for treating diagnostic and therapeutic colon perforations[22,23]. Furthermore, endoscopic band ligation is a salvage technique for the treatment of iatrogenic colonic perforation after failure of endo-clipping[24].
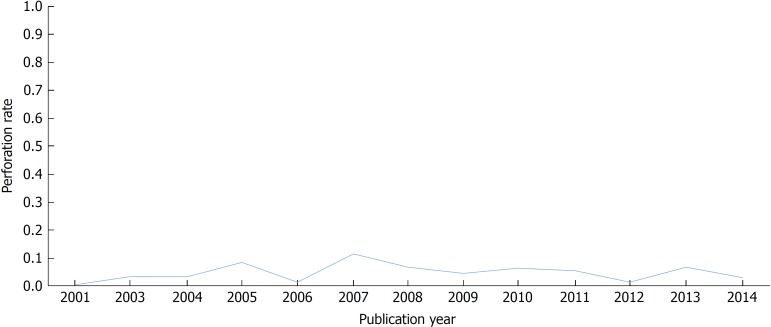
Large-scale studies about colonoscopy perforations were less reported before 2000. Araghizadeh et al[25] showed that 31 perforations occurred in 34,620 colonoscopies (0.09%) over 30 years. Two other studies also showed that the overall rate of perforation is < 0.1%[26,27]. However, another large population-based cohort study demonstrated 108 perforations after 39,286 colonoscopies (0.20%), and reported that the risk of perforation during a colonoscopy is roughly double compared with sigmoidoscopy[28]. Many large-scale studies on colonoscopy perforations have been published since 2000. In particular, recent studies have assessed the occurrence of colonoscopy perforations using big data analyses[16,29,30]. A large study based on comprehensive French health insurance data, which included nearly one million colonoscopies, suggested that the estimated perforation rate was 0.04% (424/947,061)[29]. That study indicated that a patient’s elderly status, resecting a large polyp (> 10 mm), and emergency colonoscopy are associated with a risk of perforation. Another study also reported similar perforation rates, such as 0.02% (192/1,144,900)[16]. Pox et al[30] stated the results of 2,821,392 colonoscopies conducted over 6 years in Germany. To date, this cohort is the largest database of screening colonoscopies worldwide. The overall perforation rate in that cohort was 0.016% (439/2,821,392), including 279 (0.46/1000) patients with polypectomy and 160 patients without polypectomy (0.12/1000)[30]. The most significant risk factor for adverse events was a polypectomy. The frequencies of colon perforations in some larger studies (sample sizes > 50,000 cases) published since 2000 are shown in Table 1. The perforation rate tends to remain stable and not to change (Figure 1). The results of our review are consistent with a recent large-scale meta-analysis by Reumkens et al[5] showing that the overall perforation rate was 0.05% and the trends in the past 15 years did not show any significant change.
Table 1.
Summary of calculated incidence rates for perforations related to colonoscopy from recent studies with sample sizes > 50,000 cases n (%)
| Ref. | Duration of enrollment | Study design (data source) | Publication year | Country | Colonoscopies performed | Perforation rate (%) |
| Sieg et al[27] | 1998-1999 | Prospective study | 2001 | Germany | 82416 | 4 (0.005) |
| Iqbal et al[76] | 1994-2000 | Retrospective study | 2005 | United States | 78702 | 72 (0.084) |
| Rabeneck et al[77] | 2002-2003 | Population-based cohort study | 2008 | Canada | 97091 | 54 (0.056) |
| Iqbal et al[13] | 1980-2006 | Retrospective review | 2008 | United States | 258248 | 180 (0.070) |
| Bokemeyer et al[45] | 2003-2006 | Study based on German online registry | 2009 | Germany | 269144 | 55 (0.020) |
| Crispin et al[45] | 2006 | Study based on compulsory health insurance (CHI) | 2009 | Germany | 236087 | 69 (0.029) |
| Warren et al[47] | 2001-2005 | Population-based, matched cohort study. | 2009 | United States | 53220 | 33 (0.062) |
| Arora et al[72] | 1995-2005 | Population-based cohort study | 2009 | United States | 277434 | 228 (0.082) |
| Rabeneck et al[78] | 2002-2003 | Population-based cohort study | 2011 | Canada | 67632 | 37 (0.055) |
| Pox et al[30] | 2003-2008 | Prospective cross-sectional study | 2012 | Germany | 2821392 | 439 (0.016) |
| Hamdani et al[73] | 2002-2010 | Retrospective cross-sectional study | 2013 | United States | 80118 | 50 (0.062) |
| Samalavicius et al[79] | 2007-2011 | Retrospective multicenter study | 2013 | Lithuania | 56882 | 40 (0.070) |
| Blotière et al[29] | 2010 | Study based on comprehensive French health insurance data (SNIIRAM) | 2014 | France | 947061 | 424 (0.045) |
| Rutter et al[52] | 2006-2012 | Study based on English National Health Service Bowel Cancer Screening Program (NHSBCSP) | 2014 | United Kingdom | 130831 | 20 (0.015) |
| Zafar et al[48] | 2007-2008 | Health Insurance Portability and Accountability Act compliant study | 2014 | United States | 54039 | 46 (0.085) |
| Bielawska et al[16] | 2000-2011 | Prospectively collected data from the Clinical Outcomes Research Initiative (CORI) National Endoscopic Database | 2014 | Canada | 1144900 | 192 (0.017) |
| Shi et al[34] | 2000-2012 | Retrospective study | 2014 | China | 110785 | 14 (0.012) |
Figure 1.
Yearly trends of perforation rates.
Most of the studies about post-colonoscopy perforations were performed in the West, whereas relatively few studies have been conducted in the East[31]. In addition, the numbers of subjects participating in the studies were significantly lower than in the West. Teoh et al[31] showed that the overall perforation rate is 0.113%, and revealed a decreasing trend between 1998 and 2005 in China. Unlike other studies, interestingly, perforation in this study occurred more in diagnostic colonoscopies than in therapeutic colonoscopies. In Taiwan, lower colonoscopy-related perforation rates have been reported at 0-0.065%[32,33]. A recent retrospective study in China reported on 110,785 colonoscopies analyzed during a 12 year period. As a result, 14 perforations (0.012%) occurred[34]. We used data from the National Health Insurance Service (NHIS) of the Republic of Korea to identify the incidence rate of colonoscopy perforations in 2011 (unpublished data). According to our results, 14 patients had perforations among 31,177 colonoscopies (0.045%). Taken together, colonoscopy-related perforation rates are not significantly different between the East and West. More importantly, the perforation probability after colonoscopy does not decrease over time in either the West or the East.
BLEEDING TIME TRENDS AND REGIONAL DIFFERENCES
Bleeding is one of the most common complications of colonoscopy, accounting for 0.3-6.1% of cases[35,36]. The definition of post-colonoscopy bleeding was somewhat different among studies: lower GI bleeding after colonoscopy with/without polypectomy requiring a transfusion of packed red blood cells, hospitalization, emergency room visit, or need for repeat colonoscopy in the setting of hematochezia[5,9,10,37]. Generally, immediate bleeding was defined as that occurring within 1 d after an endoscopic procedure, and delayed bleeding as that occurring from 24 h to 14 d after an endoscopic procedure[8,37,38]. Bleeding after a diagnostic endoscopy is very rare. If it occurs, it is typically associated with biopsy. This may occur when the blood vessel structure is directly biopsied, especially in patients with abnormal blood coagulation function[39]. It is also rarely seen in cases of severe mechanical friction due to the endoscope. According to Kavic et al[39], the incidence of hemorrhage during a diagnostic colonoscopy is only 0.03% (26/101,397), most of which occurs after the biopsy.
Bleeding after a polypectomy is known to occur more frequently and can be divided into immediate bleeding and delayed bleeding according to the time of onset[35,36]. The post-polypectomy bleeding rate (0.98%) is significantly higher compared with when a polypectomy is not performed (0.06%) (P < 0.001)[5]. The mechanism of post-polypectomy bleeding varies depending on polyp morphology. In the case of pedunculated polyps, a large feeding vessel usually passes through the stalk. Insufficient electrocoagulation during stalk cutting with a snare may cause pulsatile bleeding[40]. In the case of sessile polyps, the polypectomy section is usually deep and wide, which may result in insufficient electrocoagulation of the interior, resulting in bleeding from the internal margin of the section. In addition, exposed vessels are often located in the submucosal layer, which may increase the risk of delayed bleeding[41,42]. The number, size, morphology, and histology of polyps, as well as cardiovascular disease, are risk factors for post-polypectomy bleeding[8]. Shalman et al[43] reported that use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) does not increase the risk of post-polypectomy bleeding. A recent meta-analysis showed that aspirin and NSAIDs are risk factors for delayed, but not immediate, post-polypectomy bleeding[44]. Table 2 summarizes the risk factors associated with post-polypectomy bleeding.
Table 2.
| Patient-related factors | Polyp-related factors | Procedure-related factors |
| Old age | Polyp size | Cutting mode |
| Anticoagulants | Morphology of polyps | Bowel preparation |
| Cardiovascular disease | Histology | Inadvertent cold polypectomy |
| Chronic vascular disease | Number of resected polyps | Endoscopist’s experience |
| Clopidogrel and concomitant aspirin/nonsteroidal anti-inflammatory drugs | Resection method Use of prophylactic hemostasis |
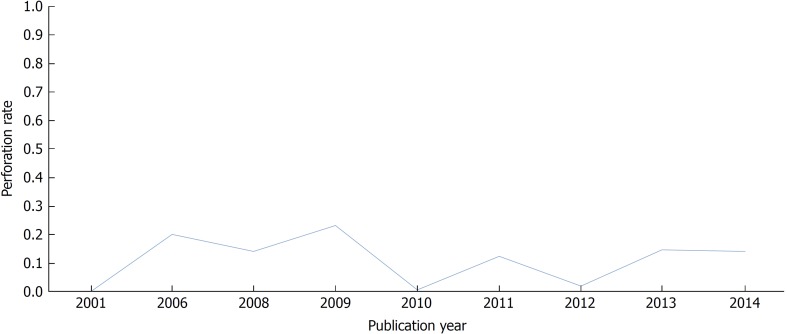
Relatively few large-scale studies on bleeding have been reported, particularly before 2000, because it is difficult to define meaningful bleeding compared with a perforation in a large data set. Sieg et al[27] published the incidence of bleeding in 82,416 colonoscopies conducted in Germany from 1998 to 1999. The rate of significant hemorrhage in that prospective study was 0.001%. This is a very low complication rate. That study only targeted outpatients, and most of the endoscopies were performed by highly skilled endoscopists, thus leading to a low incidence rate of bleeding. Two large studies of more than 200,000 patients in Germany were reported in 2009[45,46]. A total of 269,144 colonoscopies, among screening colonoscopies performed on asymptomatic patients, were collected in an online registry[45]. In that study, 0.164% (442/269,144) of cases reported bleeding, and the incidence of bleeding increased to 0.8% when a polypectomy was performed. However, most bleeding was treated by endoscopy, and surgical treatment was rarely required. Another study on outpatients reported a bleeding rate of 0.220% (520/236,087)[46]. It has also been demonstrated that male gender, increasing age, non-screening purposes, additional procedures (biopsy or polypectomy), and the absence of sedation/analgesia are risk factors for colonoscopy-related bleeding[46]. Recent studies in the United States have shown a higher bleeding incidence rate[47,48]. In a population-based, matched cohort study, serious gastrointestinal events, such as bleeding or transfusion necessity, occurred in 340 of the 53,220 colonoscopies (0.639%)[47]. The risk for bleeding was more than four times higher for the polypectomy group (8.7/1000 colonoscopies) than the screening group (2.1/1000 colonoscopies)[47]. In another study, lower gastrointestinal bleeding occurred in 371 patients among 54,039 asymptomatic patients (0.687%)[48]. However, in that study, bleeding risk was estimated to be higher than in other studies because all enrolled patients were elderly (> 66 years). Table 3 shows the frequency of post-colonoscopy bleeding in some larger studies (sample sizes > 50,000 cases) published since 2000. Figure 2 indicates the current trends in post-colonoscopy bleeding, with bleeding rates of 0.001-0.233%.
Table 3.
Summary of calculated incidence rates for bleeding related to colonoscopy from recent studies with sample sizes > 50,000 cases n (%)
| Ref. | Duration of enrollment | Study design (data source) | Publication year | Country | Colonoscopies performed | Bleeding |
| Sieg et al[27] | 1998-1999 | Prospective study | 2001 | Germany | 82416 | 1 (0.001) |
| Rabeneck et al[77] | 2002-2003 | Population-based cohort study | 2008 | Canada | 97091 | 137 (0.141) |
| Bokemeyer et al[45] | 2003-2006 | Study based on German online registry | 2009 | Germany | 269144 | 442 (0.164) |
| Crispin et al[46] | 2006 | Study based on compulsory health insurance (CHI) | 2009 | Germany | 236087 | 520 (0.220) |
| Warren et al[47] | 2001-2005 | Population-based, matched cohort study | 2009 | United States | 53220 | 340 (0.639) |
| Rabeneck et al[78] | 2002-2003 | Population-based cohort study | 2011 | Canada | 67632 | 83 (0.123) |
| Pox et al[30] | 2003-2008 | Prospective cross-sectional study | 2012 | Germany | 2821392 | 573 (0.020) |
| Blotière et al[29] | 2010 | Study based on comprehensive French health insurance data (SNIIRAM) | 2014 | France | 947061 | 933 (0.099) |
| Rutter et al[52] | 2006-2012 | Study based on English National Health Service Bowel Cancer Screening Program (NHSBCSP) | 2014 | United Kingdom | 130831 | 291 (0.222) |
| Zafar et al[48] | 2007-2008 | Health Insurance Portability and Accountability Act compliant study | 2014 | United States | 54039 | 371 (0.687) |
Figure 2.
Yearly trends of bleeding rates.
Similar to perforations, studies on complications associated with bleeding have rarely been performed in the East. Several studies have recently been published, however the number of patients is smaller than those in the West[37,40,49-51]. It is also difficult to generalize because these studies targeted patients in specific situations. Ng et al[49] reported no adverse events (bleeding or perforation) in 4,539 colonoscopies. Studies on bleeding have been published in Korea[37,40,50,51], and all of them report rates > 1%. Kim et al[51] showed that a total of 9,336 colonic polyps were removed, and 262 (2.806%) polyps present with immediate post-polypectomy bleeding. In this study, age ≥ 65 years, concurrent cardiovascular or renal disease, use of anticoagulation, large polyp (≥ 1 cm), morphology of polyp, poor bowel preparation, cutting mode, and inadvertent cutting were significant risk factors for post-polypectomy bleeding[51]. However, those studies were only conducted on post-polypectomy bleeding. It is thus inappropriate to apply this incidence rate directly to screening colonoscopies in the East. Using our unpublished data mentioned above, we confirmed an overall incidence rate of bleeding in colonoscopies of 1.119% (349/31,177) in Korea. In our study, we analyzed the data using NHIS codes. Therefore, the incidence of bleeding was higher because all minor bleeding events were included. Recent Western studies have reported bleeding rates in 0.020-0.687% of colonoscopies[29,30,48,52], and these results were two times less than those published in the East. It is unclear whether this difference is a real gap or a result of limitations in studies published in the East. In the future, well-designed large prospective studies on colonoscopy-related bleeding are needed in the East.
ADVERSE EVENTS IN SPECIFIC SITUATIONS AND MORTALITY
Post-polypectomy syndrome (PPS) is defined as the progress of abdominal pain, leukocytosis, fever, and localized peritonitis without radiographic evidence of colonic perforation[53]. PPS arises after colonoscopic polypectomy with electrocoagulation. The incidence of PPS is reported to vary from 0.003-0.1%[54]. However, PPS after ESD occurs in about 9% of cases, which is higher than that after polypectomy or endoscopic mucosal resection[55]. The risk factors for PPS are hypertension, large lesions, and non-polypoid lesions[56]. The protective effects of submucosal injections against PPS is unclear[57]. Generally, PPS should be conservatively managed with medical therapy (NPO status, IV fluids, and broad-spectrum antibiotics), because the prognosis is good in the majority of cases. In rare cases, however, surgical treatment may be necessary if there is a clear perforation with diffuse peritoneal signs[55].
The rate of bacteremia related to colonoscopy was 0-25%, and it was not associated with infectious complications[58]. Only one study has evaluated the risk of bacteremia after colonoscopy in non-bleeding cirrhotic patients[59]. Llach et al[60] reported that 6 of 58 cirrhotic patients who underwent colonoscopy were culture-positive. All detected organisms were members of the normal skin flora, and all patients were asymptomatic. This result demonstrates that colonoscopy does not induce bacteremia in cirrhotic patients, and that routine use of prophylactic antibiotics is not required[60]. Very rarely, continuous ambulatory peritoneal dialysis after colonoscopy with or without polypectomy may occur[61,62]. The International Society for Peritoneal Dialysis guidelines suggest antibiotic prophylaxis prior to colonoscopy. However, this recommendation is not supported by randomized controlled studies[63]. Bowel preparation (particularly with oral sodium phosphate (OSP)) may induce disorders of renal function and electrolytes[64]. In a large nationwide study, the adjusted OR for acute renal failure associated with the use of OSP was 3.7 (95%CI: 2.37-5.67) within 1 wk. Other studies have also reported that hyperphosphatemia occurs in small individuals (including lowrisk and well-hydrated patients) after administration of standard doses of OSP, and that this is related to body weight[65,66]. In South Korea, prescribing OSP for bowel preparation is illegal. We therefore strongly recommend not using OSP for the purpose of bowel preparation.
The number of elderly patients is rising significantly. Therefore, the need for colonoscopies will increase in the aged population. However, few studies have been conducted on the safety aspects of elderly patients related to colonoscopy. Lippert et al[67] showed that colonoscopy is safe and feasible in elderly patients, although the complication rate (perforation rate: 0.408% (3/735), bleeding rate: 0.136% (1/735)) increases slightly in elderly patients compared with the generally recognized complication rates in younger patients. Another study evaluated the complication rates of colonoscopy in patients ≥ 90 years old compared with those 75-79 years of age[3]. The group of patients > 90 years showed a higher overall complication rate, and most of the complications were cardiopulmonary events. However, the yield for advanced neoplasia and cancer increased significantly compared to the 75-79 year old patients. Therefore, it is more important to judge gains and losses due to colonoscopy in extremely elderly patients. According to a meta-analysis, the incidence rates for perforation and bleeding in patients ≥ 65 years old are 1.0/1000 colonoscopies and 6.3/1000 colonoscopies, respectively[9]. In particular, octogenarians have a greater chance of a perforation (1.5/1000 colonoscopies). Endoscopists must know precisely how many colonoscopy-related adverse events actually occur in elderly patients. Ultimately, practitioners must balance the risks, benefits, and costs of colonoscopy in elderly patients.
Inflammatory bowel diseases (IBDs) are chronic and progressive inflammatory diseases. Colonoscopy is a key modality for diagnosis, differential diagnosis, treatment, and predicting the prognosis of IBDs[68]. A systematic review study indicated that major complications were reported in 2% of patients with IBD who underwent therapeutic endoscopy[69]. Navaneethan et al[70] conducted a population-based study designed to estimate the risk of post-colonoscopy perforations in patients with IBD. The perforation rate was 1% (344/33,732) in the IBD group and 0.6% (3,658/578,458) in the control group (P = 0.0001). The risk of perforations was also significantly higher in the IBD group than that of the control group (adjusted odds ratio (OR): 1.83; 95% confidence interval (CI): 1.40-2.38).
The overall risk of colonoscopy-related complications increases when sedative drugs are used[71]. In that study, use of anesthesia was related to a 13% increase in the risk of short-term complications (ORs of hemorrhage: 1.28 (CI: 1.27-1.30) and of abdominal pain: 1.07 (CI: 1.05-1.08)). The risk of perforation increases only in patients undergoing a polypectomy (OR: 1.26 (CI: 1.09-1.52))[71]. However, it is unclear whether anesthesia is the cause of colonoscopy-related complications. Another study showed that non-gastroenterologist endoscopists are a risk factor for early colonoscopy perforations[16]. In this large study, non-gastroenterologists had higher rates of perforations than in colonoscopies conducted by gastroenterologists (OR: 2.00 (CI: 1.30-3.08)). Unlike a previous study[71], the use of sedative drugs did not significantly increase the perforation risk[16].
The incidence of mortality after a colonoscopy is very small. In a 2016 review, the mortality rate ranged from 0.007-0.07%[8]. In another meta-analysis, the mortality rate was 2.9/100,000 (95%CI: 1.1-5.5), and mortality rates have remained stable for the past 15 years[5]. However, these rates include all indications, so they cannot be applied equally to screening and surveillance colonoscopies. The most frequent colonoscopy-related complication that causes mortality is a perforation. The overall mortality rate was 25.6% among those who underwent surgical treatment after a colonoscopy perforation[31]. Another study reported a mortality rate of 7%[13]. Strong predictors of mortality are ASA class ≥ 3 and the presence of anti-platelet therapy[31].
DISCUSSION
Large-scale studies can provide more comprehensive information on post-colonoscopy complications. In singleinstitution studies, the number of subjects is small and only specific indications, such as polypectomy, are evaluated. However, population-based research using national data has the advantage of enabling unbiased conclusions to be reached.
We summarized the incidence of post-colonoscopy complications according to colonoscopy indication and procedure (Tables 4 and 5). Table 4 shows the post-colonoscopy perforation rates stratified by colonoscopy indication and procedure type. The rate of perforation in screening/surveillance colonoscopy was 0.010-0.067%. However, the rate of perforation in symptomatic/diagnostic colonoscopy was 0.022-0.268%. Arora et al[72] reported the incidence and risk of colonic perforation according to colonoscopy indication. In this study, 22% of all colonoscopies were conducted for screening purposes (58,457/269,712). The identification of diarrhea and obstruction as indications for a colonoscopy was related to a higher incidence of perforation (0.140% and 0.374%, respectively) compared with screening colonoscopy (0.067%)[72]. Another study involved a subgroup analysis according to colonoscopy indication[16]. Of the total of 1,144,443 colonoscopies, 544,474 were for screening or surveillance. The perforation rate was 0.011% in the screening/surveillance group and 0.022% in the symptomatic/surveillance group. ASA class IV/V was most significantly associated with an increased risk of perforation in the screening/surveillance group[16]. Hamdani et al[73] showed that the incidence of perforation in a diagnostic colonoscopy group was 20-fold that of the screening colonoscopy group. A recent large-scale study analyzing health insurance data showed that the risk of perforation is significantly increased for emergency colonoscopy (OR: 4.63, CI: 3.52-6.10)[29]. The aforementioned large-scale studies showed that the incidence of complications is lower for screening/surveillance colonoscopies than for other indications. A recent meta-analysis also indicated that the incidence of complications varies according to indication[5]. In general, screening or surveillance populations tend to be less likely to require additional procedures because they have a higher percentage of health status.
Table 4.
Perforation rates per colonoscopy indication and procedure type from recent studies with sample sizes > 50,000 cases (%)
| Ref. |
Indication |
Procedure |
||
| Screening/surveillance | Symptomatic/diagnostic | Without polypectomy | With polypectomy | |
| Sieg et al[27] | - | - | 0.005 | 0.063 |
| Crispin et al[46] | 0.040 | 0.030 | - | - |
| Warren et al[47] | 0.056 | 0.050 | 0.052 | 0.070 |
| Arora et al[72] | 0.067 | 0.086 | 0.077 | 0.077 |
| Pox et al[30] | 0.016 | - | 0.012 | 0.046 |
| Hamdani et al[73] | 0.010 | 0.268 | 0.010 | 0.037 |
| Rutter et al[52] | 0.063 | - | 0.031 | 0.091 |
| Bielawska et al[16] | 0.011 | 0.022 | - | - |
Table 5.
Bleeding rates per colonoscopy indication and procedure type from recent studies with sample sizes > 50,000 cases (%)
Unlike perforation, few large-scale studies have assessed the incidence of bleeding by colonoscopy indication (Table 5). Two studies together analyzed more than 50,000 colonoscopies and reported the incidence of bleeding according to colonoscopy indication. Crispin et al[46] reported similar rates of bleeding in screening and symptomatic colonoscopy groups (0.240% vs 0.210%); however, the OR was higher in the symptomatic group (1 (reference) vs 1.312 (1.042-1.655)). Warren et al[47] showed that the rate of bleeding after colonoscopy was higher in the diagnostic group than the screening group (0.206% vs 0.375%). The risk per 1000 persons of post-colonoscopy bleeding was also similar (2.1 vs 3.7). In another meta-analysis, the symptomatic group had a higher bleeding rate than the screening/surveillance group (2.4 (0.9-4.6) vs 4.6 (0.1-15.8), P < 0.001)[5].
Polypectomy also affects the incidence of perforation. According to six large-scale studies, the rate of perforation for polypectomy was 0.037-0.091%, compared to 0.005-0.077% for colonoscopy without polypectomy (Table 4)[27,30,47,52,72,73]. During polypectomy, perforation may occur due to grabbing of deep colonic wall layers or excessive thermal injury. The rate of complications during colonoscopy screening differs significantly depending on whether polypectomy was performed[30]. Polypectomy has a marked effect on the incidence of bleeding (Table 5). The rate of post-colonoscopy bleeding in the non-polypectomy group was 0.001-0.336%, compared to 0.092-1.136% in the polypectomy group (Table 5). Polypectomy is itself a risk factor for bleeding. In addition, the polyp size, morphology, and number (risk factors for post-polypectomy bleeding) exert a synergistic effect on the risk of bleeding.
To date, diverse risk factors for colonoscopic perforation and bleeding have been identified. Patient-related factors (old age, female gender, multiple comorbidities, large polyps) and the need for additional intervention such as polypectomy are among these risk factors[14,16]. Three studies have evaluated the risk factors for post-colonoscopy bleeding[5,8,9], which are listed in Table 6. Polypectomy, polyp size, and old age are common risk factors for post-colonoscopy perforation and bleeding in several studies.
Table 6.
Summary of major risk factors for perforation and bleeding related to colonoscopy from recent studies with sample sizes > 50,000 cases (%)
| Ref. | Risk factors for perforation | Risk factors for bleeding |
| Rabeneck et al[77] | Comorbidity score ≥ 3 (OR: 3.73, 95%CI: 1.59-8.77), Polypectomy (OR: 2.96, 95%CI: 2.31-3.80), Old age (OR: 2.06, 95%CI: 1.79-2.37) | Polypectomy (OR: 10.32, 95%CI: 6.52-16.34), Old age (OR: 1.61, 95%CI: 1.20-2.16) |
| Crispin et al[46] | Polyp size: 0.5-1 cm (OR: 11.93, 95%CI: 3.02-47.13), Polyp size: 1-3 cm (OR: 28.12, 95%CI: 7.82-101.09), Polyp size > 3 cm (OR: 31.49, 95%CI: 6.37-155.66), Polypectomy (OR: 2.27, 95%CI: 1.39-3.70), Old age (OR: 1.00, 95%CI: 1.00-1.00) | Polyp size: 0.5-1 cm (OR: 5.25, 95%CI: 3.42-8.06), Polyp size: 1-3 cm (OR: 16.84, 95%CI: 11.14-25.46), Polyp size > 3 cm (OR: 27.52, 95%CI: 17.20-44.05), Polypectomy (OR: 60.21, 95%CI: 35.90-100.99), Biopsy (OR: 8.88, 95%CI: 5.06-15.59), Colonoscopy in patients with symptoms (OR: 1.31, 95%CI: 1.04-1.67), Pedunculated polyp (OR: 1.55, 95%CI: 1.26-1.90), Number of polyps: 2-4 (OR: 1.26, 95%CI: 1.06-1.50), Old age (OR: 1.00, 95%CI: 1.00-1.00) |
| Arora et al[72] | Colonoscopy indication (obstruction) (OR: 5.09, 95%CI: 3.17-8.20), Colonoscopy procedure1 (OR: 6.12, 95%CI: 3.16-11.83), Comorbidity score ≥ 2 (OR: 1.52, 95%CI: 1.12-2.06), Old age (OR: 1.01, 95%CI: 1.00-1.02) | - |
| Pox et al[30] | Polypectomy | Polypectomy |
| Hamdani et al[73] | Colonoscopy indication: Crohn’s disease (OR: 5.16, 95%CI: 1.79-14.88), Colonoscopy indication: abdominal pain (OR: 5.79, 95%CI: 2.64-12.74), Colonoscopy indication : Diagnostic (OR: 15.33, 95%CI: 7.79-30.18), Inpatient (OR: 11.05, 95%CI: 5.14-23.75), ICU patient (OR: 5.83, 95%CI: 2.80-12.14), Low albumin (≤ 4.0) (OR: 3.58, 95%CI: 1.72-7.47), Old age (OR: 1.03, 95%CI: 1.01-1.05) | - |
| Samalavicius et al[79] | Low-volume practice center | - |
| Blotière et al[29] | Age: 60-69 (OR: 2.91, 95%CI: 1.66-5.10), Age: 70-79 (OR: 5.38, 95%CI: 3.08-9.40), Age ≥ 80 (OR: 7.51, 95%CI: 4.20-13.45), Emergency colonoscopy (OR: 4.63, 95%CI: 3.52-6.10), Polyp size ≥ 1 cm (OR: 2.72, 95%CI: 2.05-3.60) | Age: 60-69 (OR: 1.70, 95%CI: 1.18-2.43), Age: 70-79 (OR: 2.55, 95%CI: 1.77-3.66), Age ≥ 80 (OR: 3.23, 95%CI: 2.21-4.73), Emergency colonoscopy (OR: 5.99, 95%CI: 5.01-7.15), Polyp size ≥ 1 cm (OR: 5.12, 95%CI: 4.33-6.04), Chronic disease (OR: 1.76, 95%CI: 1.53-2.02), Gender (male) (OR: 1.64, 95%CI: 1.43-1.87) |
| Rutter et al[52] | Polypectomy, Location of polyp (cecum) (OR: 5.60, 95%CI: 1.37-22.83) | Polypectomy, Location of polyp (cecum) (OR: 13.50, 95%CI: 3.93-46.42), Increasing polyp size (OR: 4.92, 95%CI: 2.84-8.51) |
| Bielawska et al[16] | Age: 60-74 (OR: 2.69, 95%CI: 1.83–3.98), Age ≥ 75 (OR: 5.63, 95%CI: 3.73-8.49), Gender (female) (OR: 2.00, 95%CI: 1.43-2.80), ASA class III (OR: 2.14, 95%CI: 1.22-3.75), ASA class IV/V (OR: 7.20, 95%CI: 2.41-21.50), Hospital setting: university (OR: 2.83, 95%CI: 1.85-4.31), Hospital setting: VA/military (OR: 3.74, 95%CI: 2.37-5.89), Any therapy (OR: 3.93, 95%CI: 2.05-7.56), Polyp size ≥ 1 cm (OR: 4.14, 95%CI: 2.58-6.65), Endoscopy specialty: surgery or unknown (OR: 2.00, 95%CI: 1.30-3.08) | - |
Colonoscopy procedure includes treatment of foreign-body, submucosal injection, hemostasis, endoscopic ultrasound, transmural or intramural aspiration or biopsy.
CHALLENGES
Many clinical and national studies have identified adverse events during colonoscopy procedures. Although the rate of adverse events is low, it should not be underestimated. In particular, perforations are associated with high rates of morbidity and mortality. They can also cause serious conditions in healthy people. Another concern is the occurrence of a chain effect caused by the complication. An adverse event not only affects the patient the moment it occurs, but also afterwards. A rise in the complication rate is expected to increase the frequency of hospitalization, follow-up, and total costs. In addition, as more colonoscopies are performed, the number of procedures, such as polypectomy, will also increase, which will increase the probability of complications. These impacts may be greater at the national level. Therefore, it is important to accurately understand the occurrence of colonoscopy-related complications and to improve the rates in routine clinical practice.
Population-based cohort studies and large data-based studies are expected to provide more realistic information on colonoscopy complications. However, some points to be overcome in the future should be discussed. First, there is a likelihood that adverse events are under-reported, as reports of complications may be self-reported by the practitioner. In some cases, an adverse event may not be recorded correctly because the occurrence of complications can be disputed by the practitioner. In addition, if complications are not listed in the health insurance data, they will not be included in the overall incidence calculation. Therefore, the actual incidence of colonoscopy-related adverse events may be higher than reported. Second, many different colonoscopy indications have been revealed in different studies. Although there is a clear difference in the incidence rates of adverse events between screening/surveillance and polypectomy, the indications are not clearly distinguished in each study. In particular, it is often difficult to clearly distinguish these indications in large-scale research using big data because many data sources are mixed. Third, it is difficult to evaluate the mortality of each complication because various treatment methods have been applied for the same complication. Finally, most studies did not apply consistent definitions for an adverse event. This review article is limited by the use of different definitions of immediate and delayed bleeding among the included studies. In particular, there is a significant difference among studies that define post-colonoscopy complications with regard to when they occurred after the procedure (≤ 7 d, ≤ 14 d, or ≤ 30 d).
The exact study of post-colonoscopy complications is very difficult. To do this, a transparent reporting or monitoring system should be introduced. Therefore, the ASGE and ESGE guidelines recommend adverse events related to colonoscopy reporting as a longitudinal quality indicator of colonoscopy[74,75]. More importantly, accurate record of predictive factors associated with post-colonoscopy complications, unified checking systems after inspection, unit-specific web-based input systems for monitoring of short-term and long-term complications (depending on region, country or insurance company), and prospectively data accumulation systems should be established. In addition, a large-scale prospective study is needed to improve the prevention of post-colonoscopy complication through these monitoring systems.
CONCLUSION
In this review, we have highlighted recent global trends in colonoscopy-related adverse events. Despite the increasing number of colonoscopy trials solving technical difficulties, there has been no significant change in the incidence of post-colonoscopy complications (particularly perforations and bleeding), and a small number of complications are constantly occurring. It is crucial to use a consistent definition and refined data worldwide to identify and compare adverse events in the future. Future studies should use a uniform definition for post-colonoscopy complications. When complications occur, standardized systems and recording methods are required to enable structured monitoring and reviewing (type of complication, cause of occurrence, and treatment method). In addition, the recording and monitoring systems mentioned in the preceding challenges section should also be actively introduced. There is an inevitable part where adverse events like perforation can occur stochastically, regardless of the experience of the procedure. We should therefore share responsibility for these complications. These methods will improve quality indicators of the practitioner and help to institute a systematic national management program. In addition, introducing structured training programs for endoscopists is needed. If these processes are well established, we will be able to set a better national policy direction for colonoscopy, improve clinical outcomes and, ultimately, reduce patient risk in the future[76-84].
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: No author has any personal or financial conflict of interest.
Peer-review started: September 28, 2018
First decision: October 26, 2018
Article in press: November 30, 2018
P- Reviewer: Arya V, Cadoni S, Rerknimitr R S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Su Young Kim, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju 26426, South Korea.
Hyun-Soo Kim, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju 26426, South Korea. hyskim@yonsei.ac.kr.
Hong Jun Park, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju 26426, South Korea.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Singh H, Demers AA, Xue L, Turner D, Bernstein CN. Time trends in colon cancer incidence and distribution and lower gastrointestinal endoscopy utilization in Manitoba. Am J Gastroenterol. 2008;103:1249–1256. doi: 10.1111/j.1572-0241.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 3.Cha JM, Kozarek RA, La Selva D, Gluck M, Ross A, Chiorean M, Koch J, Lin OS. Risks and Benefits of Colonoscopy in Patients 90 Years or Older, Compared With Younger Patients. Clin Gastroenterol Hepatol. 2016;14:80–86.e1. doi: 10.1016/j.cgh.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Pan A, Schlup M, Lubcke R, Chou A, Schultz M. The role of aspirin in post-polypectomy bleeding--a retrospective survey. BMC Gastroenterol. 2012;12:138. doi: 10.1186/1471-230X-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post-Colonoscopy Complications: A Systematic Review, Time Trends, and Meta-Analysis of Population-Based Studies. Am J Gastroenterol. 2016;111:1092–1101. doi: 10.1038/ajg.2016.234. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 7.Rembacken B, Hassan C, Riemann JF, Chilton A, Rutter M, Dumonceau JM, Omar M, Ponchon T. Quality in screening colonoscopy: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) Endoscopy. 2012;44:957–968. doi: 10.1055/s-0032-1325686. [DOI] [PubMed] [Google Scholar]
- 8.Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30:705–718. doi: 10.1016/j.bpg.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc. 2011;74:885–896. doi: 10.1016/j.gie.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeer NC, Snijders HS, Holman FA, Liefers GJ, Bastiaannet E, van de Velde CJ, Peeters KC. Colorectal cancer screening: Systematic review of screen-related morbidity and mortality. Cancer Treat Rev. 2017;54:87–98. doi: 10.1016/j.ctrv.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai SL, Chen T, Yao LQ, Zhong YS. Management of iatrogenic colorectal perforation: From surgery to endoscopy. World J Gastrointest Endosc. 2015;7:819–823. doi: 10.4253/wjge.v7.i8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal CW, Cullinane DC, Schiller HJ, Sawyer MD, Zietlow SP, Farley DR. Surgical management and outcomes of 165 colonoscopic perforations from a single institution. Arch Surg. 2008;143:701–706; discussion 706-707. doi: 10.1001/archsurg.143.7.701. [DOI] [PubMed] [Google Scholar]
- 14.Rai V, Mishra N. Colonoscopic Perforations. Clin Colon Rectal Surg. 2018;31:41–46. doi: 10.1055/s-0037-1602179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JY, Choi PW, Jung SM, Kim NH. The Outcomes of Management for Colonoscopic Perforation: A 12-Year Experience at a Single Institute. Ann Coloproctol. 2016;32:175–183. doi: 10.3393/ac.2016.32.5.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielawska B, Day AG, Lieberman DA, Hookey LC. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clin Gastroenterol Hepatol. 2014;12:85–92. doi: 10.1016/j.cgh.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, Warren JL, Klabunde CN. Association of polypectomy techniques, endoscopist volume, and facility type with colonoscopy complications. Gastrointest Endosc. 2013;77:436–446. doi: 10.1016/j.gie.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taku K, Sano Y, Fu KI, Saito Y. Iatrogenic perforation at therapeutic colonoscopy: should the endoscopist attempt closure using endoclips or transfer immediately to surgery? Endoscopy. 2006;38:428. doi: 10.1055/s-2006-925248. [DOI] [PubMed] [Google Scholar]
- 19.Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP, Fockens P, Hassan C. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693–711. doi: 10.1055/s-0034-1377531. [DOI] [PubMed] [Google Scholar]
- 20.Parodi A, Repici A, Pedroni A, Blanchi S, Conio M. Endoscopic management of GI perforations with a new over-the-scope clip device (with videos) Gastrointest Endosc. 2010;72:881–886. doi: 10.1016/j.gie.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573–578. doi: 10.1055/s-0030-1256339. [DOI] [PubMed] [Google Scholar]
- 22.Singhal S, Changela K, Papafragkakis H, Anand S, Krishnaiah M, Duddempudi S. Over the scope clip: technique and expanding clinical applications. J Clin Gastroenterol. 2013;47:749–756. doi: 10.1097/MCG.0b013e318296ecb9. [DOI] [PubMed] [Google Scholar]
- 23.Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258–2274. doi: 10.1007/s00464-012-2754-x. [DOI] [PubMed] [Google Scholar]
- 24.Han JH, Park S, Youn S. Endoscopic closure of colon perforation with band ligation; salvage technique after endoclip failure. Clin Gastroenterol Hepatol. 2011;9:e54–e55. doi: 10.1016/j.cgh.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 25.Araghizadeh FY, Timmcke AE, Opelka FG, Hicks TC, Beck DE. Colonoscopic perforations. Dis Colon Rectum. 2001;44:713–716. doi: 10.1007/BF02234572. [DOI] [PubMed] [Google Scholar]
- 26.Korman LY, Overholt BF, Box T, Winker CK. Perforation during colonoscopy in endoscopic ambulatory surgical centers. Gastrointest Endosc. 2003;58:554–557. doi: 10.1067/s0016-5107(03)01890-x. [DOI] [PubMed] [Google Scholar]
- 27.Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc. 2001;53:620–627. doi: 10.1067/mge.2001.114422. [DOI] [PubMed] [Google Scholar]
- 28.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95:230–236. doi: 10.1093/jnci/95.3.230. [DOI] [PubMed] [Google Scholar]
- 29.Blotière PO, Weill A, Ricordeau P, Alla F, Allemand H. Perforations and haemorrhages after colonoscopy in 2010: a study based on comprehensive French health insurance data (SNIIRAM) Clin Res Hepatol Gastroenterol. 2014;38:112–117. doi: 10.1016/j.clinre.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460–1467.e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Teoh AY, Poon CM, Lee JF, Leong HT, Ng SS, Sung JJ, Lau JY. Outcomes and predictors of mortality and stoma formation in surgical management of colonoscopic perforations: a multicenter review. Arch Surg. 2009;144:9–13. doi: 10.1001/archsurg.2008.503. [DOI] [PubMed] [Google Scholar]
- 32.Mai CM, Wen CC, Wen SH, Hsu KF, Wu CC, Jao SW, Hsiao CW. Iatrogenic colonic perforation by colonoscopy: a fatal complication for patients with a high anesthetic risk. Int J Colorectal Dis. 2010;25:449–454. doi: 10.1007/s00384-009-0822-z. [DOI] [PubMed] [Google Scholar]
- 33.Chiu HM, Lee YC, Tu CH, Chen CC, Tseng PH, Liang JT, Shun CT, Lin JT, Wu MS. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol. 2013;11:832–838.e1-e2. doi: 10.1016/j.cgh.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Shi X, Shan Y, Yu E, Fu C, Meng R, Zhang W, Wang H, Liu L, Hao L, Wang H, Lin M, Xu H, Xu X, Gong H, Lou Z, He H, Xing J, Gao X, Cai B. Lower rate of colonoscopic perforation: 110,785 patients of colonoscopy performed by colorectal surgeons in a large teaching hospital in China. Surg Endosc. 2014;28:2309–2316. doi: 10.1007/s00464-014-3458-1. [DOI] [PubMed] [Google Scholar]
- 35.Sorbi D, Norton I, Conio M, Balm R, Zinsmeister A, Gostout CJ. Postpolypectomy lower GI bleeding: descriptive analysis. Gastrointest Endosc. 2000;51:690–696. doi: 10.1067/mge.2000.105773. [DOI] [PubMed] [Google Scholar]
- 36.ASGE Standards of Practice Committee. Fisher DA, Maple JT, Ben-Menachem T, Cash BD, Decker GA, Early DS, Evans JA, Fanelli RD, Fukami N, Hwang JH, Jain R, Jue TL, Khan KM, Malpas PM, Sharaf RN. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745–752. doi: 10.1016/j.gie.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Choung BS, Kim SH, Ahn DS, Kwon DH, Koh KH, Sohn JY, Park WS, Kim IH, Lee SO, Lee ST, Kim SW. Incidence and risk factors of delayed postpolypectomy bleeding: a retrospective cohort study. J Clin Gastroenterol. 2014;48:784–789. doi: 10.1097/MCG.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 38.Kwon MJ, Kim YS, Bae SI, Park YI, Lee KJ, Min JH, Jo SY, Kim MY, Jung HJ, Jeong SY, Yoon WJ, Kim JN, Moon JS. Risk factors for delayed post-polypectomy bleeding. Intest Res. 2015;13:160–165. doi: 10.5217/ir.2015.13.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavic SM, Basson MD. Complications of endoscopy. Am J Surg. 2001;181:319–332. doi: 10.1016/s0002-9610(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Lee HJ, Ahn JW, Cheung DY, Kim JI, Park SH, Kim JK. Risk factors for delayed post-polypectomy hemorrhage: a case-control study. J Gastroenterol Hepatol. 2013;28:645–649. doi: 10.1111/jgh.12132. [DOI] [PubMed] [Google Scholar]
- 41.Rosen L, Bub DS, Reed JF 3rd, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126–1131. doi: 10.1007/BF02052261. [DOI] [PubMed] [Google Scholar]
- 42.Heldwein W, Dollhopf M, Rösch T, Meining A, Schmidtsdorff G, Hasford J, Hermanek P, Burlefinger R, Birkner B, Schmitt W, Munich Gastroenterology Group The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116–1122. doi: 10.1055/s-2005-870512. [DOI] [PubMed] [Google Scholar]
- 43.Shalman D, Gerson LB. Systematic review with meta-analysis: the risk of gastrointestinal haemorrhage post-polypectomy in patients receiving anti-platelet, anti-coagulant and/or thienopyridine medications. Aliment Pharmacol Ther. 2015;42:949–956. doi: 10.1111/apt.13367. [DOI] [PubMed] [Google Scholar]
- 44.Pigò F, Bertani H, Grande G, Federica A, Vavassori S, Conigliaro RL. Post-polypectomy bleeding after colonoscopy on uninterrupted aspirin/non steroideal antiflammatory drugs: Systematic review and meta-analysis. Dig Liver Dis. 2018;50:20–26. doi: 10.1016/j.dld.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Bokemeyer B, Bock H, Hüppe D, Düffelmeyer M, Rambow A, Tacke W, Koop H. Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269000 cases. Eur J Gastroenterol Hepatol. 2009;21:650–655. doi: 10.1097/meg.0b013e32830b8acf. [DOI] [PubMed] [Google Scholar]
- 46.Crispin A, Birkner B, Munte A, Nusko G, Mansmann U. Process quality and incidence of acute complications in a series of more than 230,000 outpatient colonoscopies. Endoscopy. 2009;41:1018–1025. doi: 10.1055/s-0029-1215214. [DOI] [PubMed] [Google Scholar]
- 47.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, Ransohoff DF. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–857, W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 48.Zafar HM, Harhay MO, Yang J, Armstron K. Adverse events Following Computed Tomographic Colonography compared to Optical Colonoscopy in the Elderly. Prev Med Rep. 2014;1:3–8. doi: 10.1016/j.pmedr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng SC, Ching JY, Chan V, Wong MC, Suen BY, Hirai HW, Lam TY, Lau JY, Ng SS, Wu JC, Chan FK, Sung JJ. Diagnostic accuracy of faecal immunochemical test for screening individuals with a family history of colorectal cancer. Aliment Pharmacol Ther. 2013;38:835–841. doi: 10.1111/apt.12446. [DOI] [PubMed] [Google Scholar]
- 50.Moon HS, Park SW, Kim DH, Kang SH, Sung JK, Jeong HY. Only the size of resected polyps is an independent risk factor for delayed postpolypectomy hemorrhage: a 10-year single-center case-control study. Ann Coloproctol. 2014;30:182–185. doi: 10.3393/ac.2014.30.4.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Kim TI, Kim WH, Kim YH, Kim HJ, Yang SK, Myung SJ, Byeon JS, Lee MS, Chung IK, Jung SA, Jeen YT, Choi JH, Choi KY, Choi H, Han DS, Song JS. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am J Gastroenterol. 2006;101:1333–1341. doi: 10.1111/j.1572-0241.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- 52.Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90–97. doi: 10.1055/s-0033-1344987. [DOI] [PubMed] [Google Scholar]
- 53.Dib J Jr. Post-Polypectomy Syndrome. Am J Gastroenterol. 2017;112:390. doi: 10.1038/ajg.2016.475. [DOI] [PubMed] [Google Scholar]
- 54.Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am. 2010;20:659–671. doi: 10.1016/j.giec.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Hirasawa K, Sato C, Makazu M, Kaneko H, Kobayashi R, Kokawa A, Maeda S. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World J Gastrointest Endosc. 2015;7:1055–1061. doi: 10.4253/wjge.v7.i12.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH, Shin JE. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202–207. doi: 10.1055/s-0032-1326104. [DOI] [PubMed] [Google Scholar]
- 57.Sethi A, Song LM. Adverse events related to colonic endoscopic mucosal resection and polypectomy. Gastrointest Endosc Clin N Am. 2015;25:55–69. doi: 10.1016/j.giec.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 58.ASGE Standards of Practice Committee. Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81–89. doi: 10.1016/j.gie.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Llach J, Elizalde JI, Bordas JM, Gines A, Almela M, Sans M, Mondelo F, Pique JM. Prospective assessment of the risk of bacteremia in cirrhotic patients undergoing lower intestinal endoscopy. Gastrointest Endosc. 1999;49:214–217. doi: 10.1016/s0016-5107(99)70489-x. [DOI] [PubMed] [Google Scholar]
- 61.Lin YC, Lin WP, Huang JY, Lee SY. Polymicrobial peritonitis following colonoscopic polypectomy in a peritoneal dialysis patient. Intern Med. 2012;51:1841–1843. doi: 10.2169/internalmedicine.51.7485. [DOI] [PubMed] [Google Scholar]
- 62.Wu HH, Li IJ, Weng CH, Lee CC, Chen YC, Chang MY, Fang JT, Hung CC, Yang CW, Tian YC. Prophylactic antibiotics for endoscopy-associated peritonitis in peritoneal dialysis patients. PLoS One. 2013;8:e71532. doi: 10.1371/journal.pone.0071532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, Price V, Ramalakshmi S, Szeto CC. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31:614–630. doi: 10.3747/pdi.2011.00057. [DOI] [PubMed] [Google Scholar]
- 64.Florentin M, Liamis G, Elisaf MS. Colonoscopy preparation-induced disorders in renal function and electrolytes. World J Gastrointest Pharmacol Ther. 2014;5:50–54. doi: 10.4292/wjgpt.v5.i2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casais MN, Rosa-Diez G, Pérez S, Mansilla EN, Bravo S, Bonofiglio FC. Hyperphosphatemia after sodium phosphate laxatives in low risk patients: prospective study. World J Gastroenterol. 2009;15:5960–5965. doi: 10.3748/wjg.15.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehrenpreis ED. Increased serum phosphate levels and calcium fluxes are seen in smaller individuals after a single dose of sodium phosphate colon cleansing solution: a pharmacokinetic analysis. Aliment Pharmacol Ther. 2009;29:1202–1211. doi: 10.1111/j.1365-2036.2009.03987.x. [DOI] [PubMed] [Google Scholar]
- 67.Lippert E, Herfarth HH, Grunert N, Endlicher E, Klebl F. Gastrointestinal endoscopy in patients aged 75 years and older: risks, complications, and findings--a retrospective study. Int J Colorectal Dis. 2015;30:363–366. doi: 10.1007/s00384-014-2088-3. [DOI] [PubMed] [Google Scholar]
- 68.Terheggen G, Lanyi B, Schanz S, Hoffmann RM, Böhm SK, Leifeld L, Pohl C, Kruis W. Safety, feasibility, and tolerability of ileocolonoscopy in inflammatory bowel disease. Endoscopy. 2008;40:656–663. doi: 10.1055/s-2008-1077445. [DOI] [PubMed] [Google Scholar]
- 69.Hassan C, Zullo A, De Francesco V, Ierardi E, Giustini M, Pitidis A, Taggi F, Winn S, Morini S. Systematic review: Endoscopic dilatation in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:1457–1464. doi: 10.1111/j.1365-2036.2007.03532.x. [DOI] [PubMed] [Google Scholar]
- 70.Navaneethan U, Parasa S, Venkatesh PG, Trikudanathan G, Shen B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011;5:189–195. doi: 10.1016/j.crohns.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology. 2016;150:888–894; quiz e18. doi: 10.1053/j.gastro.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–664. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Hamdani U, Naeem R, Haider F, Bansal P, Komar M, Diehl DL, Kirchner HL. Risk factors for colonoscopic perforation: a population-based study of 80118 cases. World J Gastroenterol. 2013;19:3596–3601. doi: 10.3748/wjg.v19.i23.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, Lieb JG 2nd, Park WG, Rizk MK, Sawhney MS, Shaheen NJ, Wani S, Weinberg DS. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 75.Kaminski MF, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees CJ, Dekker E, Hoff G, Jover R, Suchanek S, Ferlitsch M, Anderson J, Roesch T, Hultcranz R, Racz I, Kuipers EJ, Garborg K, East JE, Rupinski M, Seip B, Bennett C, Senore C, Minozzi S, Bisschops R, Domagk D, Valori R, Spada C, Hassan C, Dinis-Ribeiro M, Rutter MD. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378–397. doi: 10.1055/s-0043-103411. [DOI] [PubMed] [Google Scholar]
- 76.Iqbal CW, Chun YS, Farley DR. Colonoscopic perforations: a retrospective review. J Gastrointest Surg. 2005;9:1229–1235: discussion 1236. doi: 10.1016/j.gassur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, Wai E, Goldwasser M, Sutradhar R, Stukel TA. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906, 1906.e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 78.Rabeneck L, Saskin R, Paszat LF. Onset and clinical course of bleeding and perforation after outpatient colonoscopy: a population-based study. Gastrointest Endosc. 2011;73:520–523. doi: 10.1016/j.gie.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 79.Samalavicius NE, Kazanavicius D, Lunevicius R, Poskus T, Valantinas J, Stanaitis J, Grigaliunas A, Gradauskas A, Venskutonis D, Samuolis R, Sniuolis P, Gajauskas M, Kaselis N, Leipus R, Radziunas G. Incidence, risk, management, and outcomes of iatrogenic full-thickness large bowel injury associated with 56,882 colonoscopies in 14 Lithuanian hospitals. Surg Endosc. 2013;27:1628–1635. doi: 10.1007/s00464-012-2642-4. [DOI] [PubMed] [Google Scholar]
- 80.Consolo P, Luigiano C, Strangio G, Scaffidi MG, Giacobbe G, Di Giuseppe G, Zirilli A, Familiari L. Efficacy, risk factors and complications of endoscopic polypectomy: ten year experience at a single center. World J Gastroenterol. 2008;14:2364–2369. doi: 10.3748/wjg.14.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh M, Mehta N, Murthy UK, Kaul V, Arif A, Newman N. Postpolypectomy bleeding in patients undergoing colonoscopy on uninterrupted clopidogrel therapy. Gastrointest Endosc. 2010;71:998–1005. doi: 10.1016/j.gie.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 82.Luigiano C, Ferrara F, Ghersi S, Fabbri C, Cennamo V, Landi P, Polifemo AM, Billi P, Bassi M, Consolo P, Alibrandi A, D’Imperio N. Endoclip-assisted resection of large pedunculated colorectal polyps: technical aspects and outcome. Dig Dis Sci. 2010;55:1726–1731. doi: 10.1007/s10620-009-0905-2. [DOI] [PubMed] [Google Scholar]
- 83.Bae GH, Jung JT, Kwon JG, Kim EY, Park JH, Seo JH, Kim JY. [Risk factors of delayed bleeding after colonoscopic polypectomy: case-control study] Korean J Gastroenterol. 2012;59:423–427. doi: 10.4166/kjg.2012.59.6.423. [DOI] [PubMed] [Google Scholar]
- 84.Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N, Sano K, Graham DY. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417–423. doi: 10.1016/j.gie.2013.08.040. [DOI] [PubMed] [Google Scholar]