Abstract
AIM
To determine the association of human antigen R (HuR) and inhibitors of apoptosis proteins (IAP1, IAP2) and prognosis in pancreatic cancer.
METHODS
Protein and mRNA expression levels of IAP1, IAP2 and HuR in pancreatic ductal adenocarcinoma (PDAC) were compared with normal pancreatic tissue. The correlations among IAP1/IAP2 and HuR as well as their respective correlations with clinicopathological parameters were analyzed. The Kaplan-Meier method and log-rank tests were used for survival analysis. Immunoprecipitation assay was performed to demonstrate HuR binding to IAP1, IAP2 mRNA. PANC1 cells were transfected with either anti-HuR siRNA or control siRNA for 72 h and quantitative reverse transcription polymerase chain reaction (RT-PCR), western blot analysis was carried out.
RESULTS
RT-PCR analysis revealed that HuR, IAP1, IAP2 mRNA expression were accordingly 3.3-fold, 5.5-fold and 8.4 higher in the PDAC when compared to normal pancreas (P < 0.05). Expression of IAP1 was positively strongly correlated with HuR expression (P < 0.05, r = 0.783). Western blot analysis confirmed RT-PCR results. High IAP1 expression, tumor resection status, T stage, lymph-node metastases, tumor differentiation grade, perineural and lymphatic invasion were identified as significant factors for shorter survival in PDAC patients (P < 0.05). Immunohistological analysis showed that HuR was mainly expressed in the ductal cancer cell’s nucleus and less so in cytoplasm. RNA immunoprecipitation analysis confirmed IAP1 and IAP2 post-transcriptional regulation by HuR protein. Following siHuR transfection, IAP1 mRNA and protein levels were decreased, however IAP2 expression levels were increased.
CONCLUSION
HuR mediated overexpression of IAP1 significantly correlates with poor outcomes and early progression of pancreatic cancer. Further studies are needed to assess the underlying mechanisms.
Keywords: Pancreatic cancer, Inhibitors of apoptosis proteins, Human antigen R, Post-transcriptional regulation
Core tip: We report fundamental knowledge about the direct interaction of RNA stabilizing protein human antigen R (HuR) and inhibitor of apoptosis proteins (IAP1, IAP2). Results suggest that upregulation of IAP1 in pancreatic cancer is significantly related to poor outcomes. Furthermore, HuR plays important role in post-transcriptional regulation of these molecules: HuR protein binds with IAP1, IAP2 and after HuR silencing, IAP1 protein and mRNA expression is downregulated and IAP2 is upregulated. These results demonstrate, that HuR regulates expression of IAP1 and IAP2 in pancreatic cancer cells and that IAP1 may be an important factor facilitating carcinogenic properties of HuR.
INTRODUCTION
With the aging of humanity there have been growing cases of pancreatic ductal adenocarcinoma (PDAC)[1]. The results of treatment of this cancer, however, remain one of the worst[2]. The pancreatic cancer is characterized by its aggressive course, biodiversity, strong chemoresistancy and often is diagnosed late, leading to poor outcomes[2]. With improving biotechnology sciences, there have been evolving faster and more efficient assessment of the individual patient’s tumor cytoprotective and oncogenic mechanisms[3].
Inhibitor of apoptosis proteins (IAPs) are the protein’s family characterized by the presence of one or more baculoviral IAP repeats (BIRs)[4]. The human genome contains eight IAP-encoding genes: neuronal apoptosis inhibitory protein (BIRC1), cellular IAP1 (cIAP1, BIRC2), cellular IAP2 (cIAP2, BIRC3), X chromosome-linked IAP (XIAP, BIRC4), survivin (BIRC5), apollon (BIRC6), melanoma IAP (BIRC7) and IAP-like protein 2 (BIRC8)[5]. IAPs are involved in various cellular functions, including regulation of apoptosis, cell cycle, and intracellular signal transduction[6]. Several IAP family members such as IAP1 and IAP2 function predominantly in regulating apoptosis and they can prevent apoptosis through various mechanisms, including caspase inhibition or participation in survival signaling pathways[7]. Furthermore, IAPs have altered activity in numerous cancer types (lympholeukemia, hepatic, esophageal, ovarian, pancreatic cancer)[8]. Overexpression of IAP in tumor cells inhibits cell death induced by a variety of apoptotic stimuli and induces resistance to chemotherapy[9]. Elevated IAPs expression is associated with poor response to a treatment, a worse overall survival and more aggressive course in the PDAC, however, underlying mechanisms are still unknown[10,11].
Normally, post- transcriptional regulation plays a critical role in the process of cell proliferation and apoptosis, but in cancer cells, this control might be impaired by changes in the expression of RNA binding proteins[12]. A key regulator of post-transcriptional gene regulation is human antigen R (HuR or ELAVL1), the member of embryonic lethal, abnormal vision in drosophila-like (ELAVL) family[13]. Mechanistically, HuR regulates mRNA cargos that typically contain U- or AU-rich sequences in the 3’-untranslated region (UTR)[14]. Increasing evidence support HuR as the first RNA- binding protein shown to play a critical role in both carcinogenesis and cancer progression by functioning as either an oncogene or a tumor suppressor that regulates the expression of various target genes[15]. Consequently, elevated HuR levels have been linked to both increased PDAC cell survival and poor clinico-pathologic features by supporting an antiapoptotic and pro-survival gene-expression network[16]. Additionally, a deregulated HuR pathway may be relevant to cancer biology and may possibly promote the abnormal expression of several proteins[17]. Recent research has shown that hyper-expression of HuR increases the stability of XIAP mRNA and determines the increased resistance and vitality of tumor cells[18,19]. Furthermore, cytoplasmic expression of HuR was associated with IAP2 expression in oral squamous cell carcinoma cells[20].
However, little is known about the role of ARE-binding protein HuR in the regulation of IAP1 and IAP2 expression and/or function in pancreatic cancer cells. Therefore, the aim of our study was to assess the relevance of the IAP1 and IAP2 regulation by mRNA stabilizing protein HuR signaling pathway in PDAC.
MATERIALS AND METHODS
Human pancreatic cancer tissues and data collection
Pancreatic carcinoma tissues were obtained from 61 patients undergoing a partial pancreatodeduodenectomy (Whipple resection) between 2011 - 2016 in the Department of Surgery at the Hospital of the Lithuanian University of Health Sciences. None of the patients received neo-adjuvant chemotherapy. All samples of pancreatic carcinoma were located in the head of the pancreas. The diagnosis of PDAC was confirmed by pathology. Normal pancreatic tissue samples were obtained through an organ donor program from 9 individuals who were free of pancreatic cancer. All normal tissue samples were obtained from the head of the pancreas to ensure comparability with the tumor samples. For qRT-PCR analysis freshly removed tissue samples were placed in RNALater (Ambion; Huntingdon, United Kingdom), whereas tissues for protein extraction were snap frozen in liquid nitrogen in the operating room upon surgical removal and maintained at -80 °C until use. Clinical data and histopathogical features from the same patient group were analyzed. The last follow up of patients’ survival was performed in August, 2017. Ethical approval was issued by the Ethics Committee of the Lithuanian University of Health Sciences (No. BE-2-10). Consent for the use of surgical tissue specimens and clinical data for research purposes was obtained from all the patients or their representatives.
Immunohistochemistry
The antibodies against HuR 1:300 [mouse monoclonal (Invitrogen)] and IAP1, IAP2 1:100 [rabbit monoclonal (Abcam)] were used for immunohistological analysis of tissues from healthy donors (n = 5) and PDAC patients (n = 20). Standard staining protocols were used. Paraffin-embedded tumor’s section was dewaxed with xylene and rehydrated by using alcohol solutions at different concentrations. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxide in methanol. To block the nonspecific binding, slides were treated with non-immune normal rabbit/mouse serum (Dako) for 1 h. All primary antibodies were incubated on slides for 24 h at 4 °C. After washing in TBST, slides were incubated in goat anti-rabbit, horseradish peroxidase conjugated secondary antibody (1:1000; Thermo Scientific). Immunohistochemistry was developed using the DAKO Envision+ system (Dako) and counterstained with hematoxylin.
Western blot analysis
Whole cells were lysed using the RIPA lysis buffer with protease inhibitors (Roche) and centrifuged at 10000 × g for 10 min. The supernatants were assayed for protein concentration with a BCA protein assay kit (Thermo Scientific). Protein samples were heated at 97 °C for 5 min before loading and 50 μg of the samples were subjected to 4%-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to poly-vinylidene fluoride (PVDF) membranes for 50 min at 20 V. The membranes were blocked with a blocking buffer (Invitrogen) for 30 min at room temperature and incubated overnight at 4 °C with primary antibodies. The following primary antibodies were used: 1:1000 mouse monoclonal anti-HuR from Abcam (ab186430), 1:5000 rabbit monoclonal anti-IAP1 from Abcam (ab108361), 1:1000 rabbit monoclonal anti-IAP2 from Abcam (ab32059), and 1:10000 mouse monoclonal anti-GAPDH from Ambion (AM4300). The membranes were washed and incubated with the appropriate peroxidase-conjugated secondary antibody (Invitrogen; anti-mouse or anti-rabbit) for 30 min, washed and incubated with a chemiluminescence substrate/detection kit (Invitrogen). Results were analyzed with an automated documenting system (Biorad).
RNA extraction and reverse transcription PCR
Total RNA extraction was performed from tissues and using PureLink RNA easy kit (Ambion) and TRI reagents (Zymo), according to the manufacturer’s protocol without DNA’se treatment. Purified RNA was quantified and assessed for purity by UV spectrophotometry (NanoDrop). Complementary DNA (cDNA) was generated from 2 μg of RNA with High Capacity RNA-to-cDNA Kit (Applied Biosystems). The amplification of specific RNA was performed in a 20 μL reaction mixture containing 2 μL of cDNA template, 1 × PCR master mix and the primers. The PCR primers used for detection of HuR, IAP1 and IAP2 were from Invitrogen: HuR: FW GTGAACTACGTGACCGCGAA; REV GACTGGAGCCTCAAGCCG; IAP1 (BIRC2): FW CGGCTAACGCTGGTCCTCG; REV AAATATCGCCGCCACCGAAA; IAP2 (BIRC3): FW TAAAAGGAAAGCACCAGTGCACAT; REV ATAACTCTTGGCAACCGAATCAAA.
Quantitative reverse transcription-PCR (qRT-PCR) analysis was performed using ABI 7500 fast Real-Time PCR system (Applied Biosystem). For normalization, GAPDH housekeeping gene was used. Relative quantification was performed using the 2- ∆∆Ct method.
Cell lines and growing conditions
Human pancreatic cancer cell line PANC-1 was obtained from ATCC and used for the analysis. Cells were grown in monolayers in sterile 25-cm2 capacity flask with 5-Ml RPMI-1640 medium (Gibco/Invitrogen) supplemented with 10% FBS (Gibco/Invitrogen) and 1% penicillin / streptomycin solution (Gibco/Invitrogen). Standard cells growing conditions were used -37 °C temperature, 5% CO2 - 95% air atmosphere, humidity. PANC-1 was cultured from a 56-year-old Caucasian male with an adenocarcinoma in the head of the pancreas, which invaded the duodenal wall. Metastases in one peripancreatic lymph node were discovered during a pancreaticoduodenectomy.
RNA immunoprecipitation
RNA immunoprecipitation experiments were performed with PANC-1 cells (10 × 106 cells/ flask) using anti-HuR antibodies according to the protocol provided with the kit (Merck, Millipore; catalog 17-701). Purified RNA was quantified and assessed for purity by UV spectrophotometry (NanoDrop). cDNA was generated with a High Capacity RNA-to-cDNA Kit (Applied Biosystems). Real-time polymerase chain reaction (qRT-PCR) was performed as described above with 9 μL of cDNA template per reaction to determine relative expression of IAP1, IAP2.
Transfection
HuR siRNA were purchased from Ambion (United States). siHuR sequences: Sense: UUAUCCGGUUUGACAtt; Antisense sequence: UGUCAAACCGGAUAAACGCaa.
Transfection was performed when cell cultures had reached 70%-80% confluence in 6-well plates. Lipofectamine RNAiMax (Gibco/Invitrogen) was used according to the manufacturer’s instructions for all transfections with OptiMem medium (Gibco/ Invitrogen). All experiments included two groups of control cells: untreated control and a control treated with the siRNA negative control. Transfection efficiency was assessed using Block-iT Alexa Fluor Red reagents (Invitrogen). Silencing efficiency was evaluated by Western blot analysis. Transfection of HuR siRNA was performed for 72 h. All assays were performed after 72 h of transfection (RT-PCR, Western Blot).
Statistical analysis
Statistical review of the study was performed by a biomedical statistician. SPSS 23.0 software (SPSS Company, Chicago, IL, United States) was used. The data are presented as means ± SE and median. As the hypothesis of normal distribution of data was rejected by the Shapiro-Wilks test, nonparametric statistical tests were used. The Mann-Whitney test was used for comparison of mRNA expression levels between groups. The correlations among HuR, IAP1and IAP2 were evaluated by the Spearman’s rank correlation coefficient. Additionally, their respective correlations with clinico-pathological parameters were investigated using a chi-square test or Fisher’s exact test. Survival rates were summarized using the Kaplan-Meier method and the log-rank test was performed to compare differences in survival between groups. For the survival analysis, patients were stratified into groups according to the mRNA expression of IAP1 and IAP2. The low group represents the lower expression then the mean value, and high represents higher values. Cox proportional hazard model was applied to identify prognostic factors that were independently associated with survival. A log-rank test was used. Statistical significance was defined as P < 0.05 (two-tailed P value).
RESULTS
Characteristics of the patients
The median patients’ age was 68 (range 44-87). The male/female ratio was 0.74. Pathological evaluation revealed that majority of patients had T3 stage (90.2%) and moderate cell differentiation G2 (57.4%) tumors. Regional metastatic lymph-nodes (N1) and lymphatic invasion (L1) were detected in 82.0% of cases. Additionally, microvascular invasion (V1) was detected in 80.3% and perineural invasion in 85.2% of cases. The median patients’ follow-up time was 37.8 mo and the median survival was 23.1 mo. All descriptive data of the studied group are summarized in Table 1.
Table 1.
Characteristics of the patients after pancreatoduodenectomy for pancreatic cancer n (%)
| Variable | No. of cases (n = 61) |
| Gender | |
| Male | 26 (42.6) |
| Female | 35 (57.4) |
| Age, yr (median) | 68 |
| T stage | |
| T1 | 3 (4.9) |
| T2 | 3 (4.9) |
| T3 | 55 (90.2) |
| T4 | 0 (0) |
| N status | |
| N0 | 11 (18.0) |
| N1 | 50 (82.0) |
| Lymphatic invasion | |
| L0 | 11 (18.0) |
| L1 | 50 (82.0) |
| Microvascular invasion | |
| V0 | 12 (19.7) |
| V1 | 49 (80.3) |
| Perineural invasion | |
| No | 9 (14.8) |
| Yes | 52 (85.2) |
| Differentiation grade | |
| G1 | 9 (14.8) |
| G2 | 35 (57.4) |
| G3 | 16 (26.2) |
| G4 | 1 (1.6) |
| Resection status | |
| R0 | 56 (91.8) |
| R1 | 5 (8.2) |
| Median survival (mo) | 23.10 |
IAP1, IAP2 is upregulated in human PDAC tissue
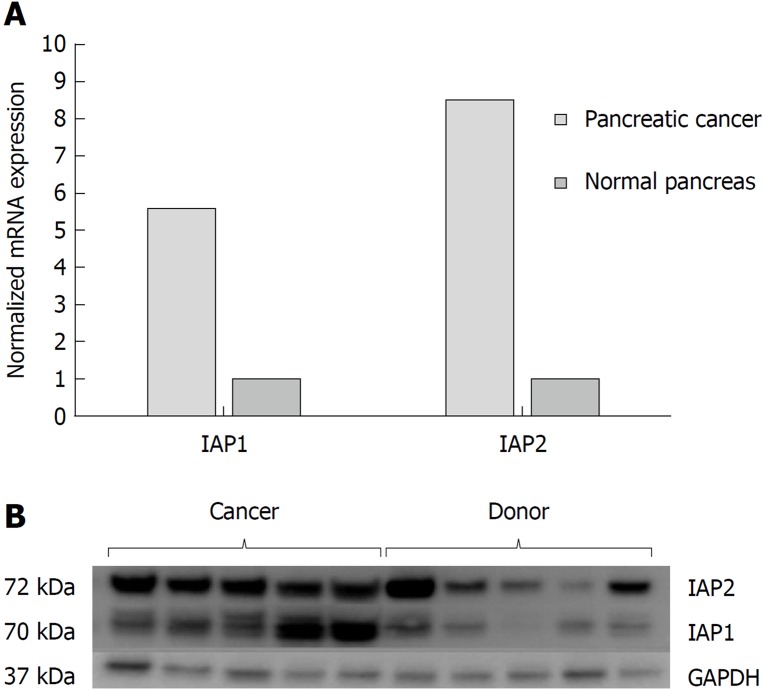
The RT-PCR analysis (Figure 1A) revealed that IAP1, IAP2 mRNA expression were accordingly 5.5-fold and 8.4 higher in the PDAC when compared to normal pancreatic tissue (P < 0.05). Western blot analysis also confirmed higher protein levels of IAP1 and IAP2 in the pancreatic cancer tissues, when compared to the normal pancreas (Figure 1B).
Figure 1.
Pancreatic cancer specimens displayed increased Inhibitors of apoptosis proteins expression analysis in cancer tissues. mRNA and protein expression of inhibitors of apoptosis proteins in normal tissues (n = 9) and pancreatic cancer (n = 61) were evaluated by A: quantitative reverse transcription polymerase chain reaction; and B: western blot analysis. IAP1: Inhibitor of apoptosis protein 1; IAP2: Inhibitor of apoptosis protein 2; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
However, due to lack of specificity of IAP1 and IAP2 antibodies to non-specific binding, there were inconclusive expression of IAP1 and IAP2 on immunohistological examination. Similar data is reported in the Human Protein Atlas[21] database: approximately half of the pancreatic tissue samples are reported to have low or undetectable levels of IAP1 and all the samples negative for IAP2 on immunohistological examination.
High expression of IAP1 is a significant predictive marker of worse outcome
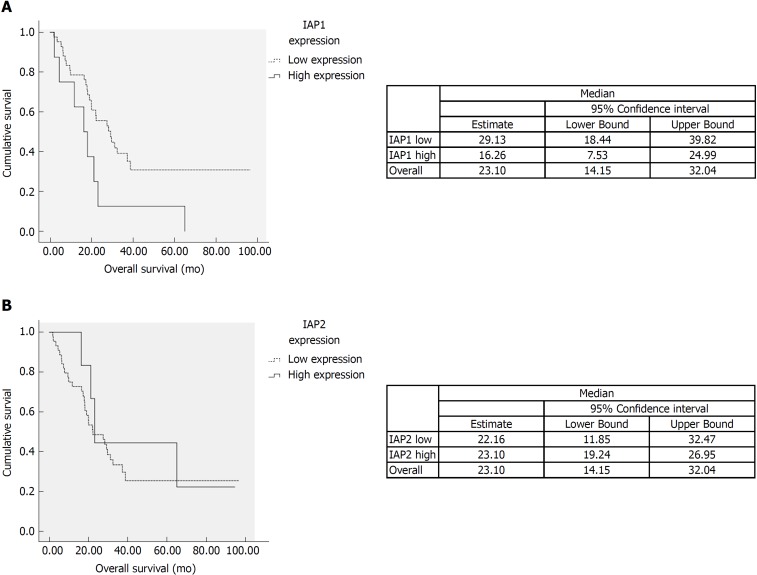
We analyzed the correlation among expression of IAP1, IAP2 and histopathological variables. IAP1 mRNA expression was correlated with lymphatic (P < 0.05) and perineural invasion (P < 0.05). However, no significant association was observed between IAP2 expression and histopathological features of the tumor. Additionally, univariate analysis was performed on different histopathological factors that might have influenced survival (Table 2). Tumor resection status, T stage, lymph-node metastases, tumor differentiation grade, perineural and lymphatic invasion were assessed as significant factors (P < 0.05) (Table 2) along with mRNA IAP1 expression. In multivariate analysis, IAP1 expression [hazard ratio (HR) = 5.51, 95%CI: 1.95-15.59, P = 0.001], tumor differentiation (HR = 2.73, 95%CI: 1.17-6.37, P = 0.02) and resection status (HR = 2.87, 95%CI: 1.31-6.29, P = 0.008) were revealed as the independent factors that had the negative impact on survival of PDAC patients (Table 3). Survival analysis revealed that patients with lower IAP1 expression were doing better in tested cohort group (Figure 2A). The median survival of patients with low expression of IAP1 was 29.1 mo while with high expression of IAP1 was 16.6 mo (P = 0.022, Log-rank test) (Figure 2A). However, there was no difference in survival of patients having either high or low IAP2 mRNA expression (Figure 2B).
Table 2.
Univariate analysis of histopathological features (log-rank)
| Variable | P value | |
| Resection status | R0/R1-R2 | 0.005 |
| T stage | T1-T2/T3-T4 | 0.146 |
| Lymph-nodes | N0/N1 | 0.046 |
| Tumor differentiation | G1-G2/G3-G4 | 0.027 |
| Perineural invasion | Yes/No | 0.010 |
| Micro-vessel infiltration | Yes/No | 0.073 |
| Lymphatic invasion | Yes/No | 0.039 |
| IAP1 expression | High/low | 0.022 |
| IAP2 expression | High/low | 0.624 |
IAP1: Inhibitor of apoptosis protein 1; IAP2: Inhibitor of apoptosis protein 2.
Table 3.
Multivariate analysis for overall survival
| Variables |
Overall Survival |
||
| HR | 95%CI | P value | |
| Tumor differentiation, G1-G2/G3-G4 | 2.73 | 1.17-6.37 | 0.02 |
| Resection status, R0/R1-R2 | 2.87 | 1.31-6.29 | 0.008 |
| IAP1 expression, high/low | 5.51 | 1.95-15.59 | 0.001 |
CI: Confidence interval; HR: Hazard ratio; IAP1: Inhibitor of apoptosis protein 1.
Figure 2.
Survival analysis. A: The survival time of patients with the low expression of inhibitor of apoptosis protein 1 tended to be longer than those with high expression (P < 0.05). B: There was no difference of survival among high and low inhibitor of apoptosis protein 2 (IAP2) mRNA expression. IAP1: Inhibitor of apoptosis protein 1; IAP2: Inhibitor of apoptosis protein 2.
HuR expression and correlation with IAP1 and IAP2
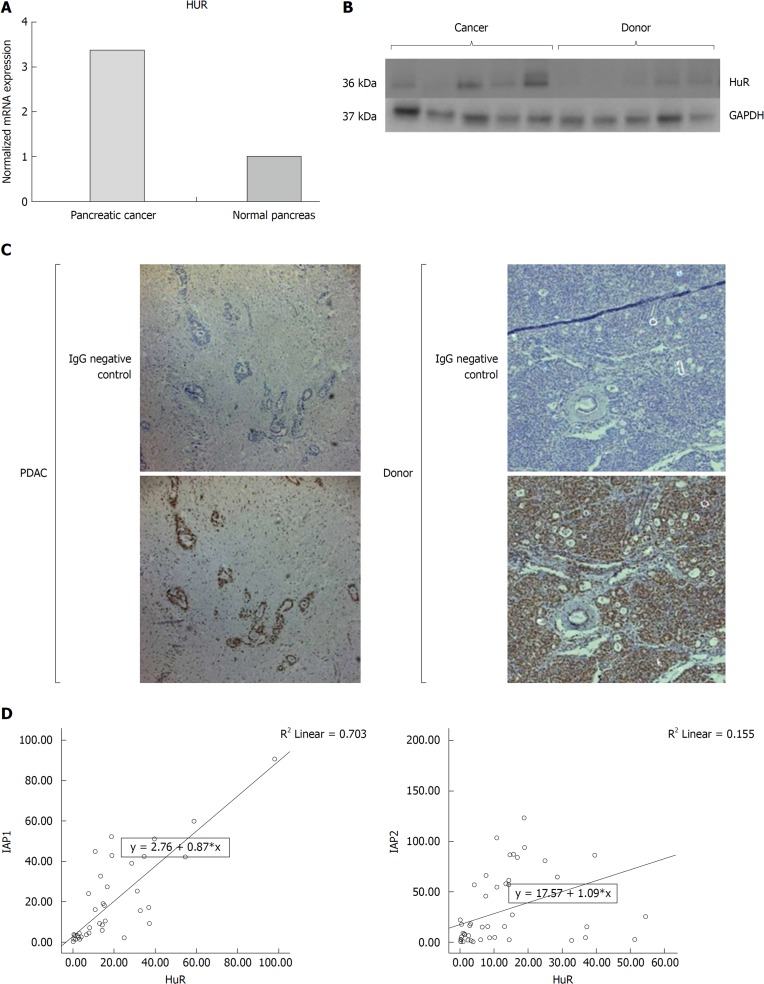
The RT-PCR analysis (Figure 3) revealed that HuR expression was higher in the PDAC when compared to normal pancreatic tissue (P < 0.05) (Figure 3A), along with upregulated protein levels of HuR in PDAC (Figure 3B). Immunohistological analysis revealed that HuR protein was highly expressed in donor pancreas acinar and islets cell’s nucleus and less in cytoplasm, but was not detectable in ductal epithelium cells. In the tissue of PDAC HuR was positive in the stromal nuclei, but not in the cytoplasm. HuR was mainly positive in the ductal cancer cell’s nucleus and less in cytoplasm (Figure 3C). Interestingly, mRNA expression of IAP1 was strongly positively correlated with HuR expression (P < 0.05, r = 0.783), but only the weak correlation with IAP2 was noticed (P < 0.05, r = 0.155) (Figure 3D). This is why further studies was conducted to reveal functional relation of HuR and IAPs.
Figure 3.
Pancreatic cancer specimens displayed increased human antigen R expression analysis in cancer tissues. mRNA and protein expression of human antigen R (HuR) were upregulated by A: quantitative reverse transcription polymerase chain reaction; and B: western blot analysis. C: Immunohistochemistry showed that HuR was mainly positive in the ductal cancer cell’s nucleus and less in cytoplasm. D: Expressions of inhibitors of apoptosis proteins were correlated with HuR expression. IAP1: Inhibitor of apoptosis protein 1; IAP2: Inhibitor of apoptosis protein 2; HuR: Human antigen R; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; PDAC: Pancreatic ductal adenocarcinoma.
HuR protein binds to IAP1 and IAP2 mRNA in pancreatic cancer cells
The RNA-immunoprecipitation analysis confirmed that HuR protein binds to IAP1 and IAP2 mRNA transcripts in extracts taken from untreated PANC1 cells, thus showing that HuR is directly involved in IAP1 and IAP2 regulation mechanisms. The Western blot analysis showed HuR and GAPDH protein levels in whole cells lysates. Magnetic beads with anti-HuR antibody and protein precipitates showed clear HuR signals, while GAPDH was undetectable. Magnetic beads with anti-IgG were used as negative controls for immunoprecipitation; thus, both HuR and GAPDH were undetectable (Figure 4). Total RNA bound to the precipitated HuR proteins obtained from the PANC-1 cells was isolated using the phenol-extraction method and analyzed by qRT-PCR using IAP1 and IAP2 primers. qRT-PCR revealed strong IAP1 and IAP2 mRNA expression (Ct 27.7 and Ct 30.2).
Figure 4.
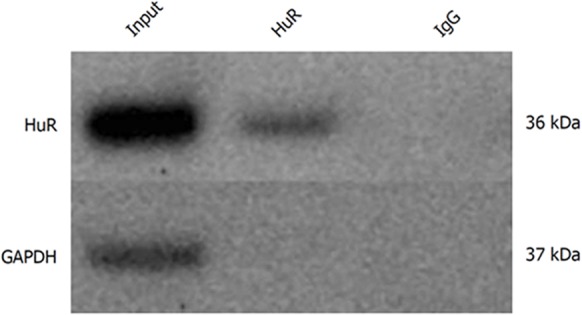
Human antigen R protein binds to inhibitors of apoptosis proteins mRNA in pancreatic cancer cells. Human antigen R (HuR) and Glyceraldehyde-3-phosphate dehydrogenase protein levels in whole cell lysates (input), magnetic beads with anti-HuR antibody and protein precipitates (HuR), and magnetic beads with anti-IgG and protein precipitates (IgG). HuR: Human antigen R; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
HuR silencing is associated with IAP1 and IAP2 mRNA and protein expression regulation in pancreatic cancer cells
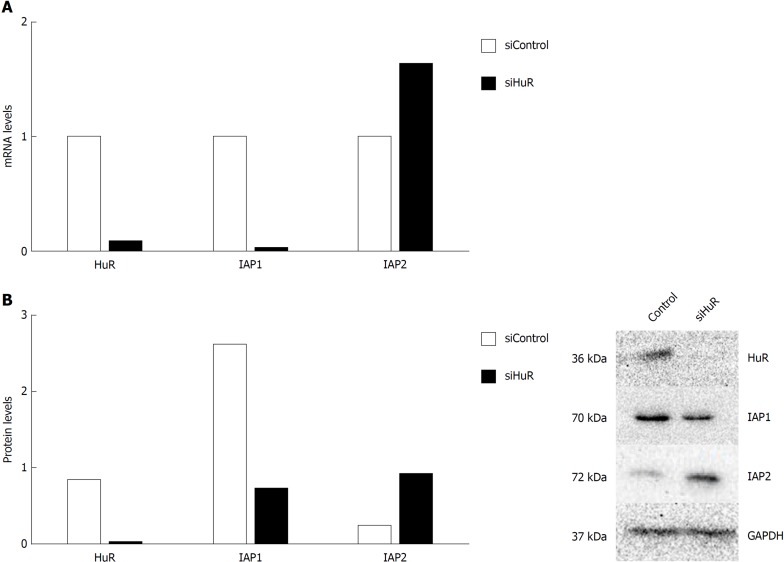
To demonstrate that HuR silencing has an effect on IAP1 and IAP2 mRNA expression, pancreatic cancer cell lines were transfected with anti-HuR siRNA’s. QRT-PCR and Western blot analysis revealed the decreased expression of HuR protein and mRNA in PANC-1 cell line after transfection. Moreover, expression of IAP1 protein and mRNA was decreased too, but IAP2 expression was increased (Figure 5).
Figure 5.
Human antigen R silencing is associated with inhibitors of apoptosis proteins mRNA and protein expression regulation in pancreatic cancer cells. Human antigen R (HuR) silencing decreased inhibitor of apoptosis protein 1 (IAP1) and increased inhibitors of apoptosis protein 2 (IAP2) mRNA expression (A) and decreased IAP1 and increased IAP2 protein expression (B). IAP1: Inhibitor of apoptosis protein 1; IAP2: Inhibitor of apoptosis protein 2; HuR: Human antigen R; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
DISCUSSION
Despite the discovery of multiple genomic alterations that give rise to PDAC, attempts to exploit these lesions for either early detection or treatment have so far been unsuccessful in the clinical setting[13].
There is a growing body of evidence that anti-apoptotic molecules IAP1, IAP2 are involved in the pathogenesis of various human malignancies, including pancreatic cancer[11,22,23]. However, IAP1 and IAP2 function in sustaining tumor growth and preventing apoptosis, remains unclear.
In this study, we analyzed the possible role of the HuR mediated post-transcriptional regulation of the IAP1 and IAP2 in the cohort of patients with typical PDAC and in PANC-1 cancer cell line in vitro. RNA-immunoprecipitation analysis in PANC-1 cell line confirmed that HuR protein binds with IAP1 and IAP2 mRNA and thus, plays an important role in post-transcriptional regulation of these molecules. Moreover, we supposed that the upregulation of these anti-apoptotic molecules IAP1 and IAP2 is mediated by the mRNA binding protein HuR. After HuR silencing expression of IAP1 protein and mRNA was down-regulated as expected. Surprisingly, IAP2 acted differently, when HuR is silenced, IAP2 mRNA and proteins levels were upregulated. These finding contradict in part with the study of Cha et al[20], where oral cancer cells were transfected with HuR siRNA, HuR and IAP2 expression were reduced. However, it might be due to different tumor’s features. On other hand, it is well established that mRNA stabilizing proteins could exert opposite effects for different target molecules[23] and could act by functioning as either an oncogene or a tumor suppressor[15], that might have happened with HuR and IAP2 regulation. However, as the mechanism underlying HuR and IAP2 mediated carcinogenesis is still unclear, more studies should be done in the future.
It is already known that IAPs are abnormally expressed in pancreatic cancer and their levels correlate with resistance to chemotherapy[11,24], that we were also able to confirm in our study. IAP1 and IAP2 mRNA levels were 5.5-fold and 8.4 higher in the PDAC when compared to normal pancreatic tissue, as well as protein levels were demonstrated to be induced in pancreatic cancer. Esposito et al. suggests that over-expression of IAP2 shows an early event in the progression of pancreatic cancer and it might contribute to the deregulation of the apoptotic signals that potentially influences patients’ survival[25]. Zender et al[26] have demonstrated that importance of genetic amplification of IAP1 can both promote tumorigenesis and sustain tumor growth in a mouse model of liver cancer. That may confirm our findings, where upregulation of IAP1 in PDAC patients was significantly related to poor outcome. Furthermore, IAP1 mRNA expression showed correlation with lymphatic and perineural invasion. Ponnelle et al[22] found a significant association between nuclear expression of IAP2 and a strong lymphoid stromal reaction. This reaction could indicate an important immune response towards tumor cells that try to escape cytotoxic cells by an ineffective upregulation of anti-apoptotic IAP family members. Nevertheless, this hypothesis cannot be clarified as long as the nuclear function of these IAPs remains unknown[22]. Esposito et al[25] suggest that a subcellular localization of IAP1 and IAP2 is required to exert their anti- apoptotic functions and is important synergistic effect of these two proteins in the inhibition of apoptosis in pancreatic cancer, which potentially influences patients’ survival. However, our immunohistological analysis of IAP1 and IAP2 was unsuccessful. The aberrant expression of IAP1 and IAP2 protein were noticed in most of pancreatic donor and cancer tissue samples. Many authors report the same problem with these proteins, speculating it can be due to lack of specificity of IAP1 and IAP2 antibodies or non-specific binding. This could reflect the phenomenon that has already been described by several studies that IAP1 is a nuclear protein and it translocate to the cytosol in response to various apoptotic signals thereby regulating caspases and exerting most of its antiapoptotic functions[7,25]. The co-expression of IAP1 and IAP2 in the cytoplasm of the cancer cells has been reported as an important synergistic effect of these two proteins in the inhibition of apoptosis in pancreatic cancer, which potentially influences patients’ survival[27].
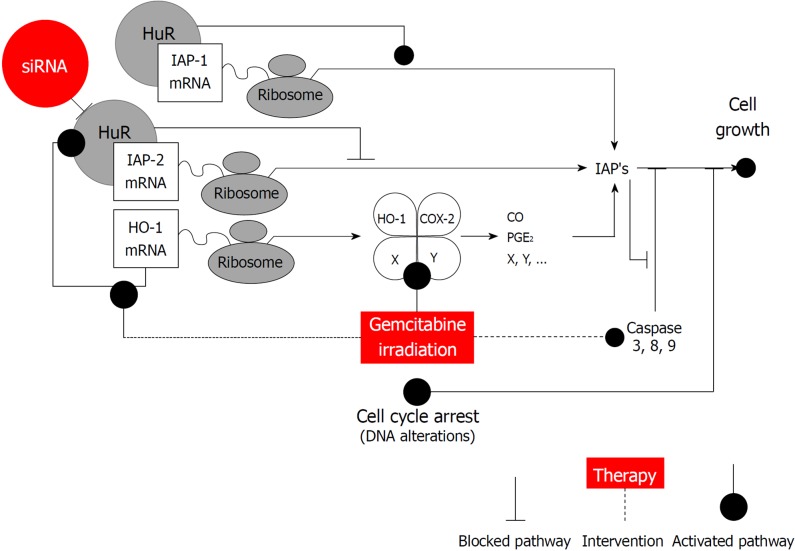
In this respect, it is interesting to note that HuR was proposed to orchestrate an antiapoptotic cellular program by several authors[18,23,28]. Indeed, numerous reports have established a link between elevated levels of HuR and several tumors including pancreatic cancer, indicating that dysregulation of mRNA stability might be involved in the malignant phenotype of these tumors[13,16,17,29,30]. For the most part HuR enhances expression of the antiapoptotic molecules and represses expression of the proapoptotic molecules[18]. Recent findings established that HuR is a critical regulator of pancreatic cancer cell metabolism and survival[16]. This study extends our previous work showing that epigenetic regulation of iRNA stabilizing proteins HuR is very important for heme oxygenase-1 (HO-1) / cyclooxygenase-2 (COX-2) cytoprotective mechanisms[16,31]. Based on our findings and possible HuR regulation mechanisms, we have constructed a scheme (Figure 6), that may explain the role of HuR and IAPs in pancreatic cancer. HuR can bind to IAPs and HO-1 and stabilize them, leading to an increased cell growth. However, when HuR is silenced, IAP2 overexpression is altered that could be modulated by HO-1 and a production of the COX-2 metabolites: prostaglandin E2 (PGE2) and carbon monoxide (CO). Additionally, the inhibitors IAPs inhibit caspase activation and/or activity.
Figure 6.
Human antigen R is an important regulator of pancreatic cancer cell growth and survival. Human antigen R (HuR) can function through the regulation of the stability or translation of target mRNAs that encode multiple cancer-related proteins as inhibitors of apoptosis proteins (IAPs), heme oxygenase-1 (HO-1), cyclooxygenase-2 (COX-2). HuR can bind to IAPs and HO-1 and stabilize them, leading to an increased cell growth. However, when HuR is silenced, IAP2 overexpression is altered that could be modulated by HO-1 and a production of the COX-2 metabolites: prostaglandin E2 and carbon monoxide. Additionally, IAPs inhibit caspase activation and/or activity. IAPs: Inhibitors of apoptosis proteins; HuR: Human antigen R; HO-1: Heme oxygenase-1; COX-2: Cyclooxygenase-2; PGE2: Prostaglandin E2; CO: Carbon monoxide.
In our study, HuR at mRNA and protein levels were upregulated in the PDAC. Our immunohistological analysis revealed that HuR protein was mainly positive in the ductal cancer cell’s nucleus and less in cytoplasm. It is already well established that HuR translocation from the nucleus to the cytoplasm in response to various stress stimuli (chemotherapy, hypoxia, oxidative stress, heat shock, nutrient deprivation) is associated with carcinogenesis and poor clinical outcomes in various cancers[13,15,16,32]. Furthermore, cytoplasmic HuR expression was demonstrated to induce and increase in IAP2 expression in oral squamous cell carcinoma (OSCC) by Cha et al[20]. However, there were inconclusive expression of IAP1 and IAP2 on immunohistological examination, as similar data is reported in the Human Protein Atlas[21] database.
In our presented study, we were able to distinguish that IAP1 and IAP2 are direct targets of HuR. It has therefore been suggested that IAP1 and IAP2 might regulate apoptosis indirectly, by influencing signaling pathways elicited by the Tumor necrosis factor (TNF) receptor superfamily[27,33]. Cells in which IAP1 was deleted, became sensitive to apoptosis induced by exogenous TNFa, suggesting novel uses of these compounds in treating cancer[27].
In conclusion, new fundamental knowledge about the direct interaction of RNA stabilizing protein HuR and IAPs was achieved. The results suggest that HuR regulates the expression of IAP1 and IAP2 in pancreatic cancer cells and that IAP1 may be one of the important factors that facilitate the carcinogenic properties of HuR. Moreover, upregulation of IAP1 in pancreatic cancer is significantly related with poor outcome. However, more data is needed to analyze the mechanism of response to chemotherapy treatment in the pancreatic cancer cell lines and in vitro underlying HuR and IAPs interaction.
Limitations of the study
Even though, as a part of retrospective analysis of patient’s data we were able to collect over 5 year’s survival rates, relatively small number of patients could act as a limitation of a study. Furthermore, it would be useful to investigate and compare the expression of HuR, IAP1 and IAP2 in PDA tissue obtained both from gemcitabine (GEM) treated and non-treated patients in order to fully understand the underlying mechanism and the role of HuR mediated post-transcriptional regulation for the exceptional resistance of pancreatic cancer to the conventional treatment. However, it is not routine practice to give neoadjuvant chemotherapy with GEM (or any other chemotherapeutic drug) prior to pancreatoduodenal resection in our hospital. Therefore, it is not possible to obtain pancreatic cancer tissue samples after GEM treatment for research purposes in our institution. Other weaknesses of the study would be unsuccessful immunohistochemistry of IAP1 and IAP2 and that the cell’s transfection experiment was done only in 1 pancreatic cancer cell line and further investigation must be carried out.
ARTICLE HIGHLIGHTS
Research background
Inhibitors of apoptosis proteins (IAPs) are involved in regulating mitosis and inhibiting cells from undergoing apoptosis. They have altered activity in numerous cancer types including pancreatic ductal adenocarcinoma (PDAC) and are implicated in progression of the disease, resistance to chemotherapy, worse outcome and prognosis. Recent research has shown that hyper-expression of mRNA stabilizing protein human antigen R (HuR) increases the stability of IAPs mRNA and determines the increased resistance and vitality of tumor cells. However, epigenetic regulation of IAP and its mechanisms to apoptotic potential and proliferation in pancreatic cancer cells is still unclear. Therefore, we conducted the study to determine the association of HuR mediated regulation and IAP1, IAP2 expression with clinicopathological parameters and prognosis of PDAC. In our study, we report new fundamental knowledge about the direct interaction of RNA stabilizing protein HuR and IAPs. Our results suggest that upregulation of IAP1 in pancreatic cancer is significantly related with poor outcomes. Furthermore, HuR plays important role in post-transcriptional regulation of these molecules: HuR protein binds with IAP1 and IAP2 mRNA and after HuR silencing, expression of IAP1 protein and mRNA is downregulated and IAP2 is upregulated. These results demonstrate for the first time that HuR regulates expression of IAP1 and IAP2 in pancreatic cancer cells and that IAP1 may be an important factor that facilitates carcinogenic properties of HuR.
Research motivation
The idea of the study was to investigate the significance of tumor apoptosis inhibition to prognosis of the disease analyzing interactions between IAPs and HuR and to determine the regulation within pancreatic cancer cells in epigenetic level. These findings lead to a better opportunity to predict the course of disease by choosing more individualized treatment with a specific biological therapy. The identification and analysis of these mechanisms will allow us to better understand the value of tumor’s factors changes in regulation of apoptotic potential in pancreatic cancer.
Research objectives
The main objective of our study was to investigate the importance of dysregulation of pancreatic cancer ‘s apoptotic potential IAPs to the prognosis of the disease. The major driving force of this study was that there is little known about the role of ARE-binding protein HuR in the regulation of IAPs expression and function in pancreatic cancer cells. Therefore, the aim of our study was to assess the relevance of the IAP1 and IAP2 regulation by mRNA stabilizing protein HuR signaling pathway in pancreatic cancer. The identification and analysis of these mechanisms will allow us to better understand the value of tumor’s factors changes in regulation of apoptotic potential in pancreatic cancer. Additionally, these investigated agents (IAPs and HuR) and their interaction could be useful for the future research as possible new therapeutic targets in the treatment of human pancreatic cancer.
Research methods
For this research project, we have conducted a study that included 61 patients undergoing a partial pancreatodeduodenectomy (Whipple resection) between 2011 - 2016 in the Department of Surgery at the Hospital of the Lithuanian University of Health Sciences for pancreatic cancer. The protein and mRNA expression levels of IAP1, IAP2 and HuR in PDAC were compared with normal pancreatic tissue and correlations with clinicopathological parameters and survival rates were analyzed. Furthermore, in vitro cells culture‘s experiments were performed to check possible epigenetic regulation.
Research results
Our results suggest that upregulation of IAP1 in pancreatic cancer is significantly related with poor patient’s outcomes. Furthermore, HuR plays important role in post-transcriptional regulation of these molecules. To our knowledge these results demonstrate for the first time about the direct interaction of RNA stabilizing protein HuR and IAPs in pancreatic cancer cells and that IAP1 may be an important factor that facilitates carcinogenic properties of HuR. Even though, as a part of retrospective analysis of patient’s data we were able to collect over 5 year’s survival rates, relatively small number of patients could act as a limitation of a study. Furthermore, it would be useful to investigate and compare the expression of HuR, IAP1 and IAP2 in PDA tissue obtained both from gemcitabine (GEM) treated and non-treated patients in order to fully understand the underlying mechanism and the role of HuR mediated post-transcriptional regulation for the exceptional resistance of pancreatic cancer to the conventional treatment. Other problems of the study that should be solved in the future studies are unsuccessful immunohistochemistry of IAP1 and IAP2 and that the cell’s transfection experiment should be done in more than 1 pancreatic cancer cell line.
Research conclusions
In our presented study, we were able to distinguish that IAP1 and IAP2 are direct targets of HuR. New fundamental knowledge about the direct interaction of RNA stabilizing protein HuR and IAPs was achieved. The results suggest that HuR regulates the expression of IAP1 and IAP2 in pancreatic cancer cells and that IAP1 may be one of the important factors that facilitate the carcinogenic properties of HuR. Moreover, upregulation of IAP1 in pancreatic cancer is significantly related with poor outcome. Additionally, these investigated agents (IAPs and HuR) and their interaction could be useful for the future research as possible new therapeutic targets in the treatment of human pancreatic cancer.
Research perspectives
We have to acknowledge that this study showed the importance of dysregulation of pancreatic cancer apoptotic potential to the prognosis of the disease. However, further research is needed to analyze the mechanism of response to chemotherapy treatment in the pancreatic cancer cell lines and in vitro underlying HuR and IAPs interaction. Furthermore, it would be useful to investigate and compare the expression of HuR, IAP1 and IAP2 in PDAC tissue obtained both from GEM treated and non-treated patients and cell’s transfection experiments in order to fully understand the underlying mechanism and the role of HuR mediated post-transcriptional regulation for the exceptional resistance of pancreatic cancer to the conventional treatment.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lithuania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: Ethical approval was issued by the Ethics Committee of the Lithuanian University of Health Sciences, No. BE-2-10. Consent for the use of surgical tissue specimens for the research purposes was obtained from all the patients or their representatives.
Conflict-of-interest statement: All authors have nothing to disclose.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: We’ve read the ARRIVE guidelines and prepared the manuscript accordingly.
Peer-review started: October 18, 2018
First decision: November 1, 2018
Article in press: November 16, 2018
P- Reviewer: Jung YD, Sun XT, Xiao MB S- Editor: Ma RY L- Editor: A E- Editor: Yin SY
Contributor Information
Ausra Lukosiute-Urboniene, Institute for Digestive System Research, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania; Department of Pediatric Surgery, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania. ausra.urboniene2@lsmuni.lt.
Aldona Jasukaitiene, Institute for Digestive System Research, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania.
Giedre Silkuniene, Institute for Digestive System Research, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania.
Vidmantas Barauskas, Department of Pediatric Surgery, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania.
Antanas Gulbinas, Institute for Digestive System Research, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania; Department of Surgery, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania.
Zilvinas Dambrauskas, Institute for Digestive System Research, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania; Department of Surgery, Lithuanian University of Health Sciences, Kaunas 50161, Lithuania.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 3.Cowley MJ, Chang DK, Pajic M, Johns AL, Waddell N, Grimmond SM, Biankin AV. Understanding pancreatic cancer genomes. J Hepatobiliary Pancreat Sci. 2013;20:549–556. doi: 10.1007/s00534-013-0610-6. [DOI] [PubMed] [Google Scholar]
- 4.Vischioni B, van der Valk P, Span SW, Kruyt FA, Rodriguez JA, Giaccone G. Expression and localization of inhibitor of apoptosis proteins in normal human tissues. Hum Pathol. 2006;37:78–86. doi: 10.1016/j.humpath.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10:169–177. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- 6.Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568–8580. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- 7.Samuel T, Okada K, Hyer M, Welsh K, Zapata JM, Reed JC. cIAP1 Localizes to the nuclear compartment and modulates the cell cycle. Cancer Res. 2005;65:210–218. [PubMed] [Google Scholar]
- 8.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira CG, van der Valk P, Span SW, Ludwig I, Smit EF, Kruyt FA, Pinedo HM, van Tinteren H, Giaccone G. Expression of X-linked inhibitor of apoptosis as a novel prognostic marker in radically resected non-small cell lung cancer patients. Clin Cancer Res. 2001;7:2468–2474. [PubMed] [Google Scholar]
- 10.Valenzuela MM, Ferguson Bennit HR, Gonda A, Diaz Osterman CJ, Hibma A, Khan S, Wall NR. Exosomes Secreted from Human Cancer Cell Lines Contain Inhibitors of Apoptosis (IAP) Cancer Microenviron. 2015;8:65–73. doi: 10.1007/s12307-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer. 2007;120:2344–2352. doi: 10.1002/ijc.22554. [DOI] [PubMed] [Google Scholar]
- 12.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimbo M, Blanco FF, Huang YH, Telonis AG, Screnci BA, Cosma GL, Alexeev V, Gonye GE, Yeo CJ, Sawicki JA, Winter JM, Brody JR. Targeting the mRNA-binding protein HuR impairs malignant characteristics of pancreatic ductal adenocarcinoma cells. Oncotarget. 2015;6:27312–27331. doi: 10.18632/oncotarget.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkhart RA, Pineda DM, Chand SN, Romeo C, Londin ER, Karoly ED, Cozzitorto JA, Rigoutsos I, Yeo CJ, Brody JR, Winter JM. HuR is a post-transcriptional regulator of core metabolic enzymes in pancreatic cancer. RNA Biol. 2013;10:1312–1323. doi: 10.4161/rna.25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci. 2013;14:10015–10041. doi: 10.3390/ijms140510015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakstaite A, Maziukiene A, Silkuniene G, Kmieliute K, Gulbinas A, Dambrauskas Z. HuR mediated post-transcriptional regulation as a new potential adjuvant therapeutic target in chemotherapy for pancreatic cancer. World J Gastroenterol. 2015;21:13004–13019. doi: 10.3748/wjg.v21.i46.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costantino CL, Witkiewicz AK, Kuwano Y, Cozzitorto JA, Kennedy EP, Dasgupta A, Keen JC, Yeo CJ, Gorospe M, Brody JR. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69:4567–4572. doi: 10.1158/0008-5472.CAN-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durie D, Lewis SM, Liwak U, Kisilewicz M, Gorospe M, Holcik M. RNA-binding protein HuR mediates cytoprotection through stimulation of XIAP translation. Oncogene. 2011;30:1460–1469. doi: 10.1038/onc.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GY, Lim SJ, Kim YW. Expression of HuR, COX-2, and survivin in lung cancers; cytoplasmic HuR stabilizes cyclooxygenase-2 in squamous cell carcinomas. Mod Pathol. 2011;24:1336–1347. doi: 10.1038/modpathol.2011.90. [DOI] [PubMed] [Google Scholar]
- 20.Cha JD, Kim HK, Cha IH. Cytoplasmic HuR expression: correlation with cellular inhibitors of apoptosis protein-2 expression and clinicopathologic factors in oral squamous cell carcinoma cells. Head Neck. 2014;36:1168–1175. doi: 10.1002/hed.23431. [DOI] [PubMed] [Google Scholar]
- 21.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 22.Ponnelle T, Chapusot C, Martin L, Bonithon-Kopp C, Bouvier AM, Plenchette S, Rageot D, Faivre J, Solary E, Piard F. Subcellular expression of c-IAP1 and c-IAP2 in colorectal cancers: relationships with clinicopathological features and prognosis. Pathol Res Pract. 2003;199:723–731. doi: 10.1078/0344-0338-00488. [DOI] [PubMed] [Google Scholar]
- 23.Donahue JM, Chang ET, Xiao L, Wang PY, Rao JN, Turner DJ, Wang JY, Battafarano RJ. The RNA-binding protein HuR stabilizes survivin mRNA in human oesophageal epithelial cells. Biochem J. 2011;437:89–96. doi: 10.1042/BJ20110028. [DOI] [PubMed] [Google Scholar]
- 24.Xie H, Jiang W, Xiao SY, Liu X. High expression of survivin is prognostic of shorter survival but not predictive of adjuvant gemcitabine benefit in patients with resected pancreatic adenocarcinoma. J Histochem Cytochem. 2013;61:148–155. doi: 10.1369/0022155412468137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito I, Kleeff J, Abiatari I, Shi X, Giese N, Bergmann F, Roth W, Friess H, Schirmacher P. Overexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancer. J Clin Pathol. 2007;60:885–895. doi: 10.1136/jcp.2006.038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zou T, Rao JN, Liu L, Xiao L, Wang PY, Cui YH, Gorospe M, Wang JY. Stabilization of XIAP mRNA through the RNA binding protein HuR regulated by cellular polyamines. Nucleic Acids Res. 2009;37:7623–7637. doi: 10.1093/nar/gkp755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatarian T, Jiang W, Leiby BE, Grigoli A, Jimbo M, Dabbish N, Neoptolemos JP, Greenhalf W, Costello E, Ghaneh P, Halloran C, Palmer D, Buchler M, Yeo CJ, Winter JM, Brody JR. Cytoplasmic HuR Status Predicts Disease-free Survival in Resected Pancreatic Cancer: A Post-hoc Analysis From the International Phase III ESPAC-3 Clinical Trial. Ann Surg. 2018;267:364–369. doi: 10.1097/SLA.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maréchal R, Van Laethem JL. HuR modulates gemcitabine efficacy: new perspectives in pancreatic cancer treatment. Expert Rev Anticancer Ther. 2009;9:1439–1441. doi: 10.1586/era.09.119. [DOI] [PubMed] [Google Scholar]
- 31.Jakstaite A, Maziukiene A, Silkuniene G, Kmieliute K, Dauksa A, Paskauskas S, Gulbinas A, Dambrauskas Z. Upregulation of cugbp2 increases response of pancreatic cancer cells to chemotherapy. Langenbecks Arch Surg. 2016;401:99–111. doi: 10.1007/s00423-015-1364-1. [DOI] [PubMed] [Google Scholar]
- 32.Richards NG, Rittenhouse DW, Freydin B, Cozzitorto JA, Grenda D, Rui H, Gonye G, Kennedy EP, Yeo CJ, Brody JR, Witkiewicz AK. HuR status is a powerful marker for prognosis and response to gemcitabine-based chemotherapy for resected pancreatic ductal adenocarcinoma patients. Ann Surg. 2010;252:499–505; discussion 505-6. doi: 10.1097/SLA.0b013e3181f1fd44. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa F, Friess H, Kleeff J, Xu ZW, Zimmermann A, Sheikh MS, Büchler MW. Effects and expression of TRAIL and its apoptosis-promoting receptors in human pancreatic cancer. Cancer Lett. 2001;163:71–81. doi: 10.1016/s0304-3835(00)00660-1. [DOI] [PubMed] [Google Scholar]