Abstract
Activation of GABAA receptors on sensory axons produces a primary afferent depolarization (PAD) that modulates sensory transmission in the spinal cord. While axoaxonic synaptic contacts of GABAergic interneurons onto afferent terminals have been extensively studied, less is known about the function of extrasynaptic GABA receptors on afferents. Thus, we examined extrasynaptic α5GABAA receptors on low-threshold proprioceptive (group Ia) and cutaneous afferents. Afferents were impaled with intracellular electrodes and filled with neurobiotin in the sacrocaudal spinal cord of rats. Confocal microscopy was used to reconstruct the afferents and locate immunolabelled α5GABAA receptors. In all afferents α5GABAA receptors were found throughout the extensive central axon arbors. They were most densely located at branch points near sodium channel nodes, including in the dorsal horn. Unexpectedly, proprioceptive afferent terminals on motoneurons had a relative lack of α5GABAA receptors. When recording intracellularly from these afferents, blocking α5GABAA receptors (with L655708, gabazine, or bicuculline) hyperpolarized the afferents, as did blocking neuronal activity with tetrodotoxin, indicating a tonic GABA tone and tonic PAD. This tonic PAD was increased by repeatedly stimulating the dorsal root at low rates and remained elevated for many seconds after the stimulation. It is puzzling that tonic PAD arises from α5GABAA receptors located far from the afferent terminal where they can have relatively little effect on terminal presynaptic inhibition. However, consistent with the nodal location of α5GABAA receptors, we find tonic PAD helps produce sodium spikes that propagate antidromically out the dorsal roots, and we suggest that it may well be involved in assisting spike transmission in general.
NEW & NOTEWORTHY GABAergic neurons are well known to form synaptic contacts on proprioceptive afferent terminals innervating motoneurons and to cause presynaptic inhibition. However, the particular GABA receptors involved are unknown. Here, we examined the distribution of extrasynaptic α5GABAA receptors on proprioceptive Ia afferents. Unexpectedly, these receptors were found preferentially near nodal sodium channels throughout the afferent and were largely absent from afferent terminals. These receptors produced a tonic afferent depolarization that modulated sodium spikes, consistent with their location.
Keywords: antidromic action potential, branch point failure, dorsal root, dorsal root reflex, extrasynaptic, GABA, intracellular recording, primary afferent depolarization, spinal cord
INTRODUCTION
GABAergic interneurons directly innervate the axons of sensory afferents in the spinal cord, producing a characteristic primary afferent depolarization (PAD) that modulates sensory transmission (Alvarez 1998; Eccles et al. 1962a, 1962b; Lidierth and Wall 1998; Rudomin and Schmidt 1999; Russo et al. 2000). PAD is caused by GABAA receptor-mediated chloride currents that are paradoxically depolarizing (reversal potential near −20 mV) because of the unusually high intracellular chloride content in adult sensory afferents (Gallagher et al. 1978; Sung et al. 2000). GABAergic control of PAD has numerous origins. Classically, stimulation of low-threshold proprioceptive afferents themselves is well known to evoke a fairly rapid PAD lasting <100 ms (phasic PAD) that is widely distributed across many primary afferents (Barron and Matthews 1938; Eccles et al. 1962b; Rudomin and Schmidt 1999; Wall 1958; Willis 1999), enabling sensory input to directly control sensory transmission. This phasic PAD results from proprioceptive or cutaneous afferents disynaptically activating specialized GABAergic neurons (GABApre), which, in turn, synapse back onto proprioceptive afferents, forming the classical trisynaptic loop (Bardoni et al. 2013; Betley et al. 2009; Delgado-Lezama et al. 2013; Eccles et al. 1962b; Engelman and MacDermott 2004; Hochman et al. 2010; Hughes et al. 2005; Jankowska et al. 1981; Rudomin 1999). Interestingly, proprioceptive afferents activate GABApre neurons in about lamina V, whereas cutaneous afferents activate different more dorsally located GABAergic neurons to generate PAD (Jankowska et al. 1981). Also, phasic PAD is highly specifically organized, even with individual collaterals of the same afferent receiving different GABAergic input (Eguibar et al. 1997; Lomelí et al. 1998). PAD is also activated by central circuits, including descending supraspinal systems (Andersen et al. 1964; Enríquez et al. 1996; Rudomin and Schmidt 1999), and the spinal pattern generating neurons for locomotion (Dubuc and Rossignol 1989; Rossignol et al. 1998), although much less is known about how these circuits control PAD. Furthermore, a slower or steady tonic PAD has been suggested (Delgado-Lezama et al. 2013; Lomelí et al. 1998; Rudomin et al. 2004a, 2004b; Wall 1998), likely mediated by extrasynaptic GABA receptors and dorsally located GABA spillover (Russo et al. 2000), although the details remain uncertain. In the present paper we focus on the origin and role of tonic PAD.
Classically, PAD has been closely associated with presynaptic inhibition of afferents in the stretch reflex, reducing monosynaptic excitatory postsynaptic potentials (EPSPs) on motoneurons. Presynaptic inhibition was first discovered when it was noticed that the extensor motoneuron monosynaptic reflex was profoundly inhibited by a brief flexor nerve conditioning stimulation (Eccles et al. 1961a; Frank and Fortes 1957; Willis 1999). Early on, Eccles et al. (1963) noticed that this inhibition lasted for longer than expected for glycinergic postsynaptic reciprocal inhibition (also flexor related), and instead it was modulated by GABA related drugs, as was PAD itself, suggesting a link between PAD and presynaptic inhibition. It took many decades before definitive evidence emerged that this inhibition was mediated by specialized GABAergic neurons that contact afferents terminals (GABApre neurons) activating GABAA receptors and associated PAD (Alvarez 1998; Betley et al. 2009; Curtis et al. 1977; Fink et al. 2014; Graham and Redman 1994). This PAD is thought to inhibit transmitter release by shunting or inactivating spikes invading the terminals or interfering with terminal calcium currents (Bardoni et al. 2013; Cattaert and El Manira 1999; Curtis 1998; Rudomin and Schmidt 1999).
However, even for this classic form of presynaptic inhibition, many contradictions remain. For example, the GABAA receptor-mediated classic phasic PAD is much too short (<100 ms) to account for the long-lasting inhibition produced by the conditioning flexor nerve stimulation (>500 ms) (Curtis 1998; Curtis and Lacey 1994; Eccles et al. 1963; Redman 1998), suggesting a role for longer-lasting tonic PAD or GABAB receptors. Also, the location of GABAA receptors (and related PAD generation) is uncertain, since they have proven difficult to find on afferent terminals (Alvarez et al. 1996; Betley et al. 2009), suggesting that they may be more dorsal, perhaps acting at branch points rather than at terminals (Howland et al. 1955; Wall 1998). Even the GABAergic synaptic contacts on proprioceptive afferent terminals are ambiguous, usually simultaneously contacting motoneurons (in triadic contacts) (Hughes et al. 2005; Pierce and Mendell 1993), making it difficult to distinguish pre- and postsynaptic inhibition without a better understanding of the location of GABAA receptors on afferents and motoneurons (Canto-Bustos et al. 2017; Curtis 1998; Delgado-Lezama et al. 2013; Stuart and Redman 1992).
PAD also has excitatory actions, mostly related to sodium spike initiation rather than modulating terminal transmitter release (Willis 1999). Phasic PAD evoked by dorsal root stimulation can be so large that it directly excites afferents, producing outright spikes in many proprioceptive afferents (Eccles et al. 1961b; Rudomin and Schmidt 1999). These spikes can propagate antidromically and are thus readily observed when recording from adjacent dorsal roots, producing the dorsal root reflex (DRR) (Eccles et al. 1961b; Willis 1999). These PAD-evoked spikes can also propagate orthodromically to afferent terminals to excite motoneurons (Bos et al. 2011; Duchen 1986; Eccles et al. 1961b; Verdier et al. 2003; Willis 1999). PAD-evoked spikes occur under normal physiological conditions, including during locomotion or mastication (Eccles et al. 1961b; Rossignol et al. 1998; Verdier et al. 2003; Willis 1999), and become more prominent with injury or disease (Eccles et al. 1961b; Fink et al. 2014). For example, they occur during arthritis and somehow interact with receptors on peripheral vasculature to cause neurogenic inflammation, swelling and pain (Willis 1999). Furthermore, PAD has long been known to lower the threshold for directly activating the group Ia afferent terminals with a microelectrode (Delgado-Lezama et al. 2013; Eccles et al. 1962b; Rudomin 1999; Wall 1958), suggesting that it should help nodal spike transmission, even though it may also inhibit terminal transmitter release. Finally, in some cases spontaneous transmitter release from central neuron terminals (of calyx of Held and spinal interneurons) has been demonstrated to be enhanced by GABAergic and glycinergic-mediated presynaptic depolarization, similar to PAD (Awatramani et al. 2005; Engelman and MacDermott 2004; Jang et al. 2002; Turecek and Trussell 2001). Taken together these results suggest that, in addition to presynaptic inhibition, ongoing PAD may be excitatory to synaptic transmission by helping spike propagation, facilitating low-level transmitter release and enhancing motoneuron activity.
Many afferents, including proprioceptive Ia afferents, become very complex once they enter the spinal cord with extensive branching, large en passant boutons and enlarged nodal regions, the latter on large heavily myelinated branches, especially at branch points throughout the afferent (Nicol and Walmsley 1991). These enlarged nodal regions have synaptic specializations, prompting Walmsley to call them en passant boutons. Regardless of the terminology, these bouton-like nodes clearly have a dual function of 1) acting like normal sodium channel nodes and 2) receiving synaptic contacts, quite different from the classic small Ia afferent terminal boutons connecting to motoneurons (Nicol and Walmsley 1991; Pierce and Mendell 1993; Vincent et al. 2017; Walmsley et al. 1995). When Walmsley and others (Nicol and Walmsley 1991; Pierce and Mendell 1993; Verdier et al. 2004; Walmsley et al. 1995) discovered that these large bouton-like nodes receive presynaptic GABAergic bouton contacts (P boutons), speculation arose that this GABAergic innervation might block sodium spike transmission in the afferent (Howland et al. 1955; Rudomin and Schmidt 1999; Verdier et al. 2003; Wall and McMahon 1994; Walmsley et al. 1995), likely at vulnerable branch points where spikes theoretically easily fail (Goldstein and Rall 1974; Lüscher et al. 1983; Verdier et al. 2004; Wall and McMahon 1994). However, computer simulations subsequently showed that a shunt from GABAA receptors likely only has local effects on the node/bouton with GABA innervation and does not influence orthodromic spike propagation to more distal nodes and boutons on proprioceptive afferents (Graham and Redman 1994; Walmsley et al. 1995) (although see Verdier et al. 2004). Nevertheless, this leaves open the question of what are the synaptic specializations and GABA at nodes doing?
Possibly GABA receptors modulate the function of these nodes, en passant boutons, and branch points on afferents, but we do not know whether they even express GABA receptors; indeed, we know surprisingly little about the distribution of GABA receptors on proprioceptive afferents outside of the terminals branches. The major synaptic β subunits of GABAA receptors and the binding protein gephyrin appear to be lacking in many afferent terminals (Alvarez et al. 1996; Betley et al. 2009; Lorenzo et al. 2014). Thus, we have begun to characterize the GABA receptor distribution throughout the central afferent branches, both dorsally and ventrally. Here we report on the distribution of the GABA receptor containing the α5 subunit (α5GABAA), which is expressed in dorsal root ganglion cells and the dorsal horn (Delgado-Lezama et al. 2013; Paul et al. 2012; Perez-Sanchez et al. 2017), but its spatial distribution on afferents is unknown. This α5GABAA receptor is generally found extrasynaptically (Caraiscos et al. 2004; Farrant and Nusser 2005; Olsen and Sieghart 2009), and thus has largely escaped detection in previous proprioceptive afferent studies that almost exclusively looked for synaptic axoaxonic contacts (P-boutons) (Fyffe et al. 1986; Smith et al. 2017; Walmsley et al. 1995) and used presynaptic labels like GAD65 and VGAT (Betley et al. 2009; Fink et al. 2014; Smith et al. 2017); although see Alvarez 1998 and Lorenzo et al. 2014. We unexpectedly found that α5GABAA receptors are largely absent from the ventral terminals of proprioceptive afferents, but instead are widely distributed in the afferents at most nodes and branch points and are particularly densely distributed in the dorsal horn. Thus, we focused our analysis on GABAergic functions of dorsal origin.
Understanding the function of GABA and PAD initiated in the dorsal horn has been hampered by the sheer density and complexity of GABAergic neurons involved in gating sensory transmission through the dorsal horn (Bardoni et al. 2013; Braz et al. 2014; Lidierth and Wall 1998; Melzack and Wall 1965; Price et al. 2005; Todd 2015; Wall 1998). Particularly problematic are the findings that presynaptic GABAergic transmission in the dorsal horn is often mediated by diffuse extrasynaptic spillover of GABA (Lorenzo et al. 2014), and dorsal afferent branches often lack synaptic contacts from GABAergic P-boutons (Alvarez 1998), contrary to the simpler synaptic GABAergic innervation of afferents in the ventral horn via numerous P-boutons (Alvarez 1998; Betley et al. 2009; Hughes et al. 2005; Pierce and Mendell 1993; Smith et al. 2017). For example, Russo et al. (2000) found that a barrage of sensory activity (including C fiber activity) produces highly specific spillover of GABA and glutamate in the dorsal horn that evokes a PAD in nearby primary sensory afferents by local microcircuits, without the need for spike-mediated transmitter release. GABAergic neuronal circuits in the superficial dorsal horn (Hughes et al. 2012; Lidierth and Wall 1998; Russo et al. 2000; Todd 2015) generate a pronounced PAD in cutaneous afferents, which are themselves entirely confined to the dorsal horn (Alvarez 1998; Fyffe et al. 1986). This PAD is readily triggered by cutaneous stimulation and is characteristically slower and larger than PAD evoked by proprioceptive stimulation in proprioceptive or cutaneous afferents, consistent with different neuronal mechanisms (Eccles and Krnjevic 1959; Lidierth and Wall 1998). Taken together, these findings open the possibility that many sensory afferents, perhaps even proprioceptive group Ia afferents, may be modulated by GABA spillover in the dorsal horn, provided they have dorsally located GABA receptors. Extrasynaptic GABA receptors are well equipped to respond to such GABA spillover due to their high affinity to GABA, long single channel opening time, and unique ability to not desensitize to GABA, unlike other GABAA receptors (Caraiscos et al. 2004; Delgado-Lezama et al. 2013; Farrant and Nusser 2005; Olsen and Sieghart 2009). These extrasynaptic receptors should produce a very long-lasting PAD (tonic PAD), which modulates afferent transmission similar to that suggested by Wall and others (Delgado-Lezama et al. 2013; Loeza-Alcocer et al. 2013; Wall 1998), although this has not been directly investigated. Thus, in the present paper, we made intracellular recordings from low-threshold proprioceptive afferents to directly evaluate the role of α5GABAA receptors in mediating tonic PAD.
METHODS
Low-threshold primary sensory afferents, including proprioceptive group Ia afferents, were recorded and filled with neurobiotin in the whole sacrocaudal spinal cord of adult female Sprague-Dawley rats (2–5 mo old; >200 g) maintained in vitro. All experiments were approved by the Health Sciences University of Alberta Animal Care and Use Committee.
In vitro preparation.
Under urethane anesthesia (1.8 g/kg, with a maximum dose of 0.45 g), a laminectomy was performed, and then the entire spinal cord caudal to the lumbar level was rapidly removed and immersed in oxygenated modified artificial cerebrospinal fluid (mACSF), as detailed previously (Harvey et al. 2006; Murray et al. 2010, 2011). The sacral S3 and S4 and caudal Ca1 dorsal roots were freed from each other in preparation for recording or stimulating, and any remaining roots were cut away. For preparations involving intracellular recordings, rather than root recordings, the cord was stabilized by gluing it to a rigid mesh paper with a trace amount of cyanoacrylate, either with the dorsal side upwards or left side upwards (ventral or right side glued to paper, respectively), the latter only used to record afferents and PAD deep in the dorsal or ventral horns. After 0.5–1.5 h in mACSF (at 20°C) the cord was transferred to a recording chamber containing normal ACSF (nACSF) saturated with carbogen (95% O2-5% CO2), and maintained near 21–23°C with a flow rate of 5 ml/min.
Intracellular recording.
Recording from fine afferent collaterals in the spinal cord, without damaging them or disturbing their intracellular milieu, required specialized ultrasharp intracellular electrodes modified from those we developed for motoneuron recording (Harvey et al. 2006). That is, glass capillary tubes (1.5 mm and 0.86 mm outer and inner diameters, respectively; with filament; 603000 A-M Systems; Sequim, WA) were pulled with a Sutter P-87 puller (Flaming-Brown; Sutter Instrument, Novato, CA) set to make bee stinger-shaped electrodes with a short, relatively wide final shaft (~1 mm) that tapered slowly from 30 to 3 µm over its length and then abruptly tapered to a final tip over the final 20 µm length. The tip was subsequently beveled to a 100-nm hypodermic-shaped point, as verified with electron microscope images (Harvey et al. 2006). The very small tip and wide shaft gave a combination of ease of penetrating axons in dense adult connective tissue, and good current-passing capabilities to both control the potential and fill the axons with neurobiotin. Prior to beveling, electrodes were filled through their tips with 2 M K-acetate mixed with varying proportions of 2 M KCl (to make intracellular Cl− concentrations ranging of 0, 100, 500, and 100 mM) or 500 mM KCl in 0.1 Trizma buffer with 5–10% neurobiotin (Vector Laboratories, Birmingame, CA). They were then beveled from an initial resistance of 40–150 MΩ to 30–40 MΩ using a rotary beveller (Sutter BV-10). GABAergic chloride-mediated potentials (PAD) were the same with different concentrations of KCl, without passing large amounts of negative current, indicating that the ultrasharp tips impeded fluid exchange between the electrode and intracellular milieu.
Electrodes were advanced into afferents with a stepper motor (model 666, Kopf; 10 µm steps at maximal speed), usually at the boundary between the dorsal columns and gray matter, but also deeper in the dorsal horn. Occasionally, we were able to penetrate proprioceptive group Ia afferents in the ventral horn (likely at boutons or larger branches near terminals). All intracellular recordings were made with an Axoclamp2B amplifier (Axon Instruments and Molecular Devices, San Jose, CA) and sampled at 30 kHz (Clampex and Clampfit; Molecular Devices). Sometimes recordings were made in discontinuous single electrode voltage clamp (gain 0.8–2.5nA/mV; for Ca PICs) or discontinuous current clamp (switching rate 7 kHz) modes, as indicated.
Dorsal root stimulation, afferent identification and recording.
Dorsal roots were mounted on silver-silver chloride wires above the nACSF of the recording chamber and covered with grease (3:1 mixture of petroleum jelly and mineral oil, surrounded by a high vacuum synthetic grease barrier) for monopolar stimulation. The dorsal roots were stimulated with a current pulse (0.1 ms) with varying intensities, expressed as a multiple of the afferent volley threshold of ~0.003 mA. When the intracellular recording electrode was in the dorsal horn, an extracellular field corresponding to the group Ia afferent volley was observed as the first event after root stimulation. This occurred with a latency of 0.5–1.0 ms, depending on the root length (which was kept as long as possible, 10–20 mm) and corresponding to a conduction velocity of ~16–24 m/s, as previously described for room temperature in vitro conduction (Li et al. 2004).
Upon penetration, afferents were identified with direct orthodromic spikes evoked from dorsal root stimulation. We focused on the lowest threshold (T) afferents, including proprioceptive group Ia afferents and cutaneous afferents (Aβ), identified by their direct response to dorsal root stimulation, very low threshold (<1.5 × T, for group I and <3 × T for others), short latency (group Ia coincident with onset of afferent volley), and antidromic response to ventral horn afferent terminal microstimulation (~10 µA stimulation via tungsten microelectrode to activate Ia afferent terminals; tested in some afferents). Good quality afferents used for analysis rested near −70 mV, had a spike that peaked at about +10 mV (overshoot) and had brief AHP (10 ms). After recording electrophysiological properties, including PAD, afferents were sometimes filled with neurobiotin by passing a very large 2–4 nA current with 90% duty cycle (900 ms on, 100 ms off) for 10–20 min. The identity of group Ia proprioceptive afferents were then confirmed anatomically by their unique extensive innervation of motoneurons.
We also recorded from the central ends of dorsal roots cut within a few millimeters of their entry in to the spinal cord, to give the compound potential from all afferents in the root (dorsal roots potential, DRP), which has previously been shown to correspond to PAD, although it is attenuated compared with the intracellular recordings of PAD. We initially mounted the freshly cut roots onto a silver-silver chloride wire just above the bath and covered them in grease. This yielded good measurements of phasic and tonic PAD (DRP) evoked by root stimulation over a <1 s time scale, which were high-pass filtered at 0.1 Hz (10 s time constant) to remove drift. However, this method was not adequate to quantify changes in spontaneous tonic PAD over a time scale of minutes when we added GABA receptor blockers, because the DRP signals are much smaller than intracellular PAD signals. Thus, at this low signal level slow drift in the electrode-liquid interface at the root is problematic. To rectify this problem, we filled one end of a 1.5-mm glass capillary tube (without filament; model 628500, A-M Systems) with ACSF mixed with 1.5% agar, mounted the root onto the agar at the end of the tube, and again sealed the root in grease just above the bath. The other end of the tube was filled with 1 M NaCl and a silver-silver chloride recording wire was inserted into this fluid for a highly conductive liquid-metal interface. This yielded relatively steady DC recordings of the dorsal root potential (DRP, tonic PAD), measured with an Axoclamp2B amplifier (Axon Instruments, Molecular Devices).
Drugs and solutions.
Two kinds of ACSF were used in in vitro experiments: mACSF in the dissection dish before recording and nACSF in the recording chamber. mACSF was composed of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 D-glucose, and 1 kynurenic acid. nACSF was composed of (in mM) 122 NaCl, 25 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, 0.5 NaH2PO4, and 12 D-glucose. Both types of ACSF were saturated with carbogen (95% O2-5% CO2) and maintained at pH 7.4. The drugs added to the nACSF were gabazine (SR95531), bicuculline, APV, bumetanide, L655708, and CNQX (all from Tocris, Minneapolis, MN), and tetrodotoxin (TTX; TRC, Toronto, Canada). All drugs were first dissolved as a 10–50 mM stock in water before final dilution in ACSF applied to the cord in vitro, with the exception of bicuculline and L655708, which were first dissolved in minimal amounts of DMSO (final concentration in ACSF 0.02%; by itself, DMSO had no effect on in vitro DRP in vehicle controls).
Tissue fixation and sectioning.
After afferents were injected with neurobiotin the spinal cord was left in the recording chamber in oxygenated nACSF for an additional 4–6 h. Then the spinal cord was immersed in 4% paraformaldehyde (in phosphate buffer) for 20–22 h at 4°C, cryoprotected in 30% sucrose in phosphate buffer for 24–48 h, embedded in OCT (Sakura Finetek, Torrance, CA), frozen at −60°C with 2-methylbutane, cut on a cryostat NX70 (Fisher Scientific) in sagittal or transverse 25-µm sections, and mounted on slides. Slides were frozen until further use.
Immunolabelling.
The tissue sections on slides were first rinsed with phosphate-buffered saline (PBS; 100 mM, 10 min) and then again with PBS containing 0.3% Triton X-100 (TBS-TX, 10-min rinses used for all TBS-TX rinses). For the sodium channel antibody, we additionally incubated slides three times for 10 min each with a solution of 0.2% sodium borohydride (NaBH4; Fisher, S678-10) in PB, followed by a PBS rinse (4×, 5 min). Next, for all tissue, nonspecific binding was blocked with a 1-h incubation in PBS-Tx with 10% normal goat serum (NGS; Vector, S-1000). Sections were then incubated for at least 20 h at room temperature with a combination of the following primary antibodies in PBS-TX: rabbit anti-α5 GABAA receptor subunit (1:200; Acris Antibodies TA338505 Origene Tech, Rockville, MD), mouse anti-Neurofilament 200 (NF200) (1:2,000; N0142, Sigma-Aldrich, St. Louis, MO), guinea pig anti-VGLUT1 (1:1,000; AB5905, Abcam, Cambridge, UK), and mouse anti-Pan sodium channel (1:500; S8809, Sigma-Aldrich). The latter is a pan-sodium antibody, labeling an intracellular peptide sequence common to all known vertebrate sodium channels. The following day, tissue was rinsed with PBS-TX (3×, 10 min/each) and incubated with fluorescent secondary antibodies. The secondary antibodies used included: goat anti-rabbit AF555 (1:200; A270399, ThermoFisher Scientific, Waltham, MA), goat anti-mouse Alexa Fluor 647 (1:500; A21235, ThermoFisher Scientific), goat anti-mouse Alexa Fluor 488 (1:500; A11001, ThermoFisher Scientific), goat anti-guinea pig Alexa Fluor 647 (1:500; A21450, ThermoFisher Scientific), streptavidin-conjugated Alexa Fluor 488 (1:200; 016–540–084, Jackson ImmunoResearch, West Grove, PA) or streptavidin-conjugated Alexa Fluor 647 (1:200; 016–170–084, Jackson ImmunoResearch) in PBS-TX, applied on slides for 2 h at room temperature. The latter streptavidin antibodies were used to label neurobiotin-filled afferents. After rinsing with PBS-TX (2×, 10 min/each) and PBS (2×, 10 min/each), the slides were covered with Fluoromount-G (00-4958-02, ThermoFisher Scientific) and coverslips (no. 1.5, 0.175 mm, 12-544-E; Fisher Scientific, Pittsburg, PA).
Standard negative controls in which the primary antibody was either omitted or blocked with its antigen (quenching), were used to confirm the selectivity of the antibody staining, and no specific staining was observed in these controls. For antibody quenching, the peptides used to generate the antibodies (AAP34984, Aviva Systems Biology, San Diego, CA) were mixed with the antibodies at a 10:1 ratio and incubated for 20 h and 4°C. This mixture was then used instead of the antibody in the above staining procedure.
Image acquisition.
Image acquisition was performed by epifluorescence (Leica DM 6000 B) and confocal (Leica TCS SP8 Confocal System) microscopy for low-magnification imaging and high-magnification 3D reconstruction, respectively. All the confocal images were taken with a ×63 (1.4 NA) oil immersion objective lens and 0.1-µm optical sections that were collected into a z-stack over 10–20 µm. Excitation and recording wavelengths were set to optimize the selectivity of imaging the fluorescent secondary antibodies. The same parameters of laser intensity, gain and pinhole size was used to take pictures for each animal, including the negative control. Low-power complete sagittal sections were imaged with an epifluorescence ×10 objective lens using the Tilescan option in Leica Application Suite X software (Leica Microsystems CMS, Mannheim, Germany). Sequential low-power images were used to reconstruct the afferent extent over the whole spinal cord, using CorelDraw (Ottawa, Canada), and to identify locations where high-power images were taken.
3D reconstruction of afferents and localization of α5GABAA receptors.
The fluorescently labeled afferents (neurobiotin), GABA receptors, VGLUT1, NF200, and sodium channels were analyzed by 3D confocal reconstruction software in the Leica Application Suite X (Leica Microsystems CMS). To be very conservative in avoiding nonspecific antibody staining, a threshold was set for each fluorescence signal at a minimal level where no background staining was observed in control tissue with the primary antibody omitted, less 10%. Signals above this threshold were rendered in 3D for each antibody. Any GABA receptor or Na+ channel expression within the volume of the neurobiotin-filled axon (binary mask set by threshold) was labeled yellow and white, respectively, and the density in the afferents quantified using the same Leica software. We also examined raw image stacks of the neurobiotin afferents and receptors, to confirm that the automatically 3D reconstructed and identified receptors labeled within the afferent (yellow) corresponded to manually identified receptors colocalized with neurobiotin (Fig. 1). This was repeated for a minimum of 10 examples for each condition, and in all cases, the 3D identified and manually identified receptors and channels were identical. Many receptors and channels lay outside the afferent, and near the afferent these were difficult to manually identify without the 3D reconstruction software, making the 3D reconstruction the only practical method to fully quantify the receptors over the entire afferent. We also optimized the reconstruction of the neurobiotin-filled afferents following the methods of Fenrich (Fenrich et al. 2014), including brightening and widening the image edges slightly (1 voxel, 0.001 µm3) when necessary to join broken segments of the afferent in the final afferent reconstruction, to account for the a priori knowledge that afferents are continuous and neurobiotin signals tend to be weaker at the membrane (image edges) and in fine processes.
Fig. 1.
Localization of GABA receptors on 3D reconstructed afferents. A, left: neurobiotin-filled afferent (green) and immunolabelled α5GABAA receptors (red) imaged sequentially at 0.1-µm increments in depth with a laser-scanning confocal microscope and displayed as maximum intensity projection across the image stack. Right: same image with the afferent and receptors reconstructed in 3D, and any receptor within the afferent volume was labeled yellow rather than green, whereas receptors not on the afferent remained red. Amplified images are shown from insets in the middle panels. Arrows show a large cluster of α5GABAA receptors on the afferent. The lower panel shows the afferent from a side view or slightly rotated lateral view, again showing receptor clusters within the afferent in yellow. B, top: images stacks from the same afferent, with NF200 immunolabelling shown additionally to provide a background stain of other axons in the spinal cord, with presynaptic contacts approaching the afferent in the zone where the receptors are located. Bottom: sequential images from the same stack as in A, showing the receptor cluster on the afferent at the arrow.
Data analysis.
Data were analyzed in Clampfit 8.0 (Axon Instruments/Molecular Devices) and Sigmaplot (Jandel Scientific, San Rafael, CA) and shown as mean ± SD (the latter used to quantify variability). A Student’s t-test or ANOVA (as appropriate) was used to test for statistical differences, with a significance level of P < 0.05. Power of tests was computed with α = 0.05. A Kolmogorov-Smirnov test for normality was applied to each data set, with a P < 0.05 level set for significance. Most data sets were found to be normally distributed, as is required for a t-test. For those that were not normal, a Wilcoxon signed rank test was used with P < 0.05.
RESULTS
Proprioceptive group Ia afferent reconstruction in the adult rat spinal cord.
When we advanced an intracellular electrode vertically downward into the dorsal horn of the adult rat spinal cord (usually at the lateral edge of the dorsal columns) large primary sensory afferents were readily penetrated in the gray matter just below the dorsal columns (Fig. 2, A and B). We focused on the lowest threshold (T) afferents, including proprioceptive afferents (group I) and cutaneous afferents (Aβ), identified by their low threshold, rapid response to dorsal root stimulation and post hoc anatomical reconstruction, as detailed in Methods. Afferents with stable penetrations were filled with neurobiotin and reconstructed in 3D with laser-scanning confocal microscopy (Fig. 2A). We found that best axon recordings (lasting >30 min and resting near −70 mV) were made from the collateral branches that dove vertically into the gray matter from the dorsal columns (primary collaterals detailed below and in Fig. 2B). This suggests that the vertically oriented electrode penetrated these moderately large branches while running nearly parallel to them, effectively cannulating the axon by running parallel to the axon branch.
Fig. 2.
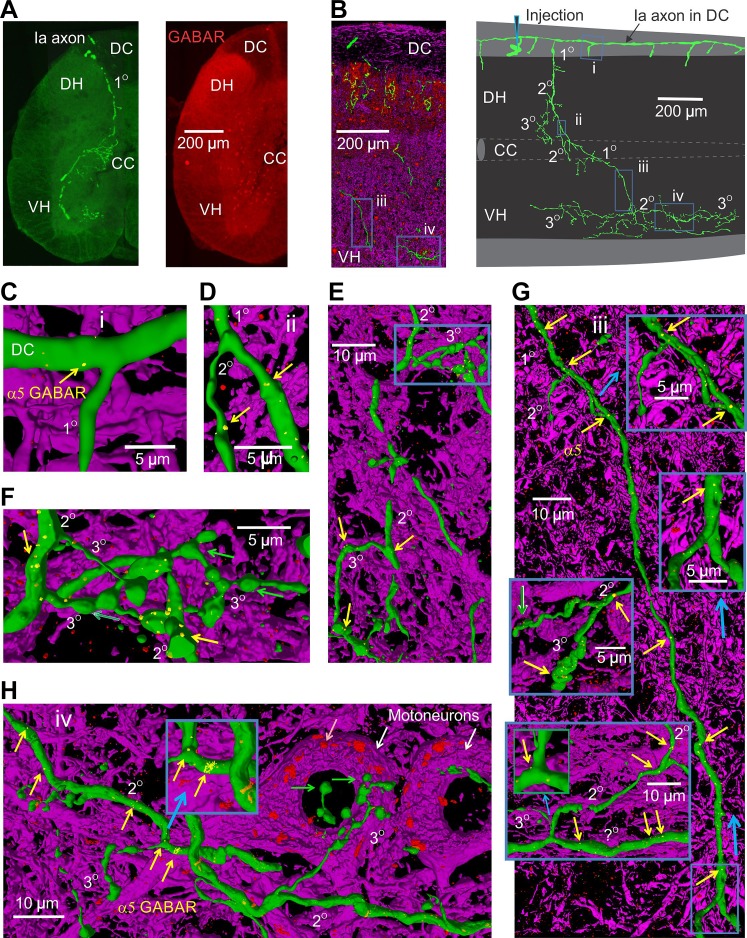
Distribution of α5GABAA receptors on proprioceptive group Ia afferent. A, left: low-power image of a transverse section of the spinal cord with neurobiotin-filled group Ia afferent (green) lying in the plane of the section, with many collateral branches arising from the afferent in the deep dorsal horn (DH) above the central canal (CC) and in the ventral horn (VH). Right, same section with immunolabelling for α5GABAA receptors (red). B: sagittal section through the spinal cord showing fragments of the afferent as it traverses from the dorsal columns to the ventral horn. Neurofilament labeling counterstain (purple). Right, fully reconstructed primary axon collateral branching off from the dorsal columns and descending to the ventral horn, reconstructed from sequential sagittal sections. Boxes (i, ii, iii, and iv) indicate regions expanded below. The axon was penetrated at the boundary of the dorsal columns (DC) and the gray matter (injection). C: high-power image of the dorsal columns axon of the afferent forming a descending primary branch (1°; primary branch point) reconstructed in 3D. α5GABAA receptors colocalized with afferent are labeled yellow, and others labeled red (using methods of Fig. 1). Neurofilament is labeled purple. Note GABA receptor cluster near branch point (arrow). D: secondary branch point (from 1° to 2° collaterals), again with GABA receptors nearby (yellow). E and F: terminal branches and boutons in the dorsal horn, with some GABA receptor labeling near the branch points (yellow arrows). Terminal boutons sometimes lacked GABA receptors (green arrow). Inset in E expanded in F. G: long descending collateral from the central canal to the ventral horn, with GABA receptors at branch points, as well as nodes in long unbranched sections (yellow). Insets on right show expanded regions at branch points. Inset on left shows peculiar branch from small secondary to much larger ventral axon (ventral columns), again with GABA receptors nearby. H: afferent terminal branches (3°) and boutons on motoneurons lacking GABA receptors (green arrows), but larger secondary branch points containing receptor clusters (yellow arrows). Motoneurons (white arrows) contained large α5GABAA receptor clusters (red, pink arrow).
Dorsal horn.
To start, we examined proprioceptive group Ia afferents, identified post hoc by their unique extensive connections to motoneurons (Fig. 2, A and B; n = 8; unlike group Ib afferents) (Scheibel and Schiebel 1969; Vincent et al. 2017). These afferents projected from the dorsal root medially to the dorsal columns where they formed a T-junction and sent large diameter (3.7 ± 1.2 µm) axons both rostrally and caudally to form the dorsal columns. Numerous moderately large diameter collateral branches (2.0 ± 0.7 µm) arose from the dorsal columns at regular intervals (230 ± 110 µm; Fig. 2B) and projected ventrally into the gray matter toward the motoneurons (termed primary branches, abbreviated 1° branches in Fig. 2, A–C). We focused on these primary branches, fully reconstructing n = 20 of them with all their subsequent branches. These primary branches formed second order collateral branches in the deep dorsal horn and intermediate zone (laminae IV-VII; dorsal secondary branches, 2° in Fig. 2, A and D; 1.2 ± 0.3 µm, n = 42 measurements) at 59 ± 32-µm intervals, which themselves branched to form fine dorsal terminal branches (tertiary or higher order branches, 3° in Fig. 2, E and F) at 36 ± 19-µm intervals, characterized by chains of large en passant and terminal boutons (1–3 µm) connected by thin axon segments (0.5 ± 0.2 µm, n = 25).
Ventral horn.
Ventral to the central canal, the primary collaterals of the group Ia afferent continued to be moderately large (2.0 ± 0.4 µm) as they projected to the motor nucleus (Fig. 2G) and formed numerous long ventral secondary branches (1.3 ± 0.4 µm diameter; n = 24 measures) at 61 ± 20-µm intervals. From these secondary branches, many fine terminal branches arose (ventral terminal branches, 3° in Fig. 2H), at 21 ± 15-µm intervals. These terminal branches densely innervated motoneurons with large en passant and terminal boutons separated by fine axons (0.4 ± 0.2 µm diameter, n = 9 measurements), similar to cat afferents (Burke and Glenn 1996; Walmsley et al. 1995). Occasionally, the secondary branches bifurcated into two much larger axons (3.4 ± 1.1 µm), which projected rostocaudally at the most ventral border of gray matter and ventral columns (termed ventral column branch; Fig. 2G, bottom inset) from which terminal collaterals arose, as in Scheibel and Schiebel 1969.
α5GABAA receptors preferentially innervate large branch points of proprioceptive afferents.
Immunolabelling for the α5 subunit of the GABAA receptor (red; Fig. 2) was widely distributed throughout the spinal cord but were most densely distributed dorsally (Fig. 2A). When we specifically examined colocalization of the α5GABAA receptor immunoreactivity with neurobiotin-filled proprioceptive group Ia afferents (3D reconstructed afferents, green), we found that α5GABAA receptors were widely distributed on the afferent. Typically, these receptors appeared as clusters of punctate dots spaced at regular intervals, especially at nodes (detailed below) on primary and secondary branches and the dorsal columns (receptors yellow; Fig. 2, C–H; clusters 0.5 ± 0.2 µm, n = 89). Interestingly, these receptor clusters were consistently very close to branch points, including where the dorsal columns formed the primary branch (clusters on average 10.0 ± 9.0 µm from the branch point; n = 22 clusters evaluated), and where primary and secondary branches formed secondary and terminal branches (clusters 15.2 ± 4.4 µm and 5.4 ± 6.3 µm from branch points, respectively; n = 35 each; significantly less than midpoint between branch points; P < 0.05). These receptors were also on the peculiar ventral column branches (Fig. 2G, lower inset). In the dorsal horn some en passant and terminal boutons also expressed α5GABAA receptors, especially near where these branches arose from the larger diameter secondary branches (Fig. 2, E and F, yellow arrows), although many boutons lacked this receptor (Fig. 2F, green arrows). Unexpectedly, in the ventral horn terminal branches and their boutons connecting to motoneurons often lacked α5GABAA receptors (Fig. 2H, green arrows), with the nearest receptors located in large clusters on the second-order axons (Fig. 2H, inset).
Quantitative comparison of α5GABAA receptors in each portion of the axon revealed that these receptors were most densely expressed in the second order ventral and dorsal collaterals (receptors occupying 0.18 ± 0.09 and 0.17 ± 0.06% of total collateral volume, respectively), and tended to be slightly less densely expressed in first-order dorsal and ventral branches (65 ± 44% and 77 ± 49% of daughter secondary branch density) and dorsal terminal branches (82 ± 66% of parent secondary collateral density). In contrast, ventral terminal branches had a much lower receptor density (16 ± 22% of parent secondary branch density; P < 0.05, n = 30 collaterals tested in each condition), consistent with the observations that many terminals completely lacked this receptor. Dorsal column axons had a low density as well (6 ± 5% of daughter secondary branch density, due to their large size).
Cutaneous afferents.
Other low-threshold large diameter afferents were also filled with neurobiotin, likely including proprioceptive group Ib and II afferents and cutaneous Aβ afferents. Most of these afferents were anatomically similar to cutaneous Aβ afferents (Fyffe et al. 1986; Scheibel and Schiebel 1969), which are prominent in this region of the cord innervating the tail. We thus focused on these putative cutaneous afferents (n = 12). Consistent with Aβ afferent morphology, these afferents did not project to motoneurons and were instead confined to the superficial dorsal horn (Fig. 3A). They had large dorsal column axons which branched frequently to form moderately large axons that projected downward into the dorsal gray matter (primary branches, as in Ia; Fig. 3B). From these primary collaterals secondary and tertiary (terminal) branches were formed, the latter containing chains of en passant boutons connected by very fine axons (Fig. 3, C and D). Unlike group Ia afferents (Fig. 2), these chains of boutons had a compact curly branching structure, reminiscent of those previously reported for Aβ cutaneous terminal branches (Fig. 3A) (Fyffe et al. 1986; Scheibel and Schiebel 1969).
Fig. 3.
Distribution of α5GABAA receptors on putative low-threshold cutaneous afferent. A: low-power image of a low-threshold afferent traveling in dorsal columns (DC) with short primary collaterals descending into the superficial dorsal horn (DH) and forming compact terminal clusters (putative cutaneous afferent; neurobiotin labeled green). CC, central canal; VH, ventral horn. B: branch point from the DC axon to primary collateral (1°), with α5GABAA receptors nearby (yellow, arrows), reconstructed in 3D. Receptors outside afferent are red. Neurofilament NF200 is purple. C: secondary (2°) collaterals and tertiary terminal branches (3°) densely covered with α5GABAA receptors (yellow arrows), including near branch points and on boutons (arrows). Green arrows show terminals lacking GABA receptors. Inset on left expanded at right. D: secondary to terminal branch point with multiple GABA receptor clusters (yellow at yellow arrows), and GABA receptors on some but not all terminals. Lower panel, same collateral, but with neurofilament labeling and many GABA receptors not in axon shown (red).
These cutaneous afferents also expressed α5GABAA receptors throughout their arbor. Like in Ia afferents, large GABA receptor clusters were often near branch points (within on average 7.1 ± 6.8 µm of branches; n = 77 clusters evaluated), including at dorsal column branch points (Fig. 3B), and branch points where tertiary terminal branches were formed from secondary axon branches (Fig. 3C, inset; Fig. 3D, top arrow). En passant and terminal boutons also had α5GABAA receptors, exhibiting a similar uneven distribution to that in dorsal Ia afferent boutons, with some boutons lacking receptors (Fig. 3C, green arrow) and others having many receptors (Fig. 3C, lower yellow arrows). Unlike in group Ia afferents, the densest α5GABAA receptors expression was on terminal branches (0.78 ± 0.66% of total volume), and the density progressively decreased as the branch size increased (density 70%, 33%, and 1% of terminal branch density in secondary, primary, and dorsal column branches, respectively, n = 30 branches each). Interestingly, maximum receptor density in these afferents was 4.3 times that in Ia afferents, consistent with the very large PAD observed in cutaneous afferents (Lidierth and Wall 1998).
Distribution of α5GABAA receptors outside of afferents.
The α5GABAA receptor was also distributed widely throughout the spinal cord, not just in afferents. There was a particularly strong receptor expression in the dorsal horn (Fig. 2A) (Perez-Sanchez et al. 2017) and in motoneurons (large NF200 positive cells with red clusters of α5GABAA receptors in Fig. 2H) (Canto-Bustos et al. 2017).
Relationship of α5GABAA receptors to nodes and sodium channels.
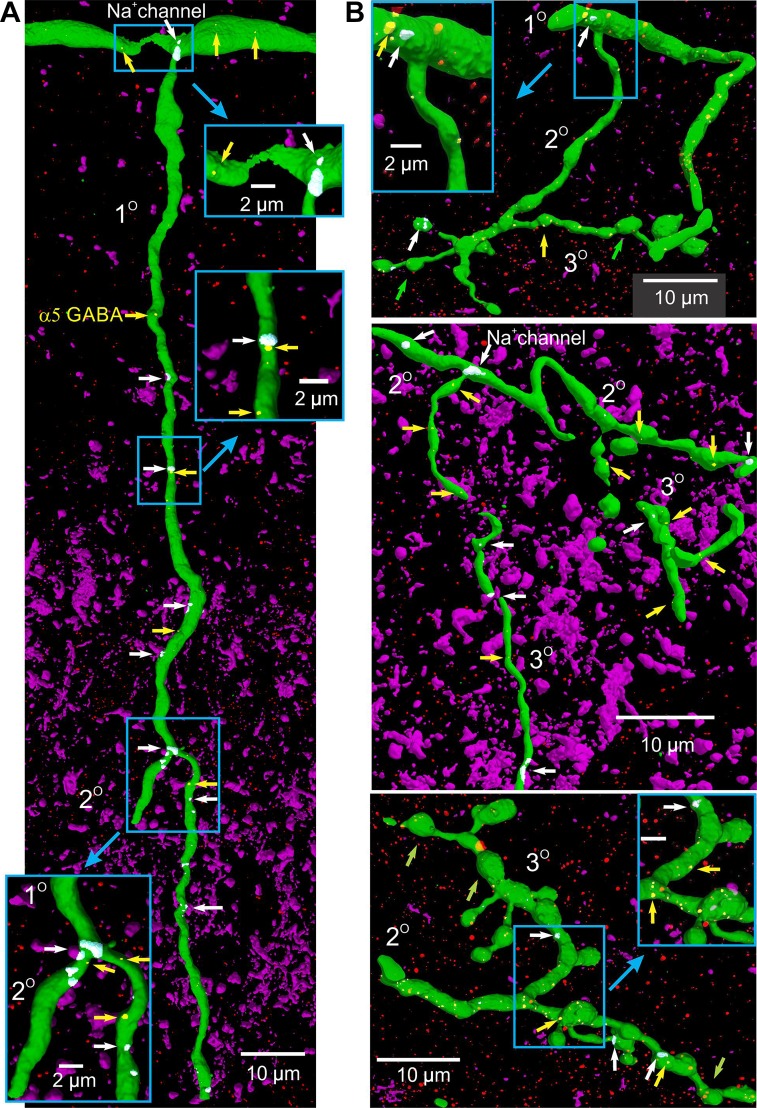
We next used a pan-sodium channel antibody to examine the relationship of GABA receptors to nodes in proprioceptive and cutaneous afferents. Dense bands or clusters of sodium channels were located on all afferent axons at regular intervals, corresponding to the nodes. On long unbranched sections of these afferents (dorsal columns and primary branches) these channel clusters (nodes) were at 53 ± 20 and 45 ± 26-µm intervals in proprioceptive and cutaneous afferents, respectively (n = 35 each). Sodium clusters invariably also occurred at all the major branch points in both propriospinal and cutaneous afferents, including where the primary branches arose off the dorsal columns (Fig. 4A, top inset), as well as at branch points where the secondary and tertiary (terminal) branches were formed (Fig. 4, A and B). Importantly, sodium channels were often absent from terminal branches and boutons (Fig. 4B, green arrow), and only occurred, if at all, on one or two boutons along a chain of many boutons (Fig. 4B, middle panel, white arrows; top panel, left white arrow).
Fig. 4.
Distribution of sodium channels on afferents, relative to α5GABAA receptors. A: large dorsal column branch of a proprioceptive Ia afferent (horizontal, top) branching to primary collateral (1°) that descended into the gray matter and formed a secondary branch (2°). Afferent labeled with neurobiotin (green). The sodium channels labeled with a pan-sodium antibody and colocalized on the afferent are indicated in white (by white arrows, channels outside afferent not shown). Sodium channel immunolabelling occurred in clusters at branch point nodes and at other nodes on long unbranched sections of the afferent. Sodium α5GABAA receptor clusters on the afferent (yellow arrows) were expressed near the sodium channel nodes (receptors outside node red). Nodes expanded in box insets. NF200, purple. B: branch points in primary and secondary collateral with sodium channel clusters in cutaneous afferent. Terminal branches (3°) and boutons typically lacked sodium channels, with a few exceptions in long or complex terminal branches (white arrows). Terminals lacking GABA receptors are marked with green arrows.
Interestingly, the dense sodium channel clusters and α5GABAA receptor clusters on proprioceptive afferents were consistently found very close to each other on major primary and secondary branches of all afferents (on average 4.9 ± 5.4 µm apart; n = 27 cluster pairs evaluated, significantly less than half the internodal distance, P < 0.05), as well as in the dorsal columns (Fig. 4, insets) 10.4 ± 7.2 µm apart, n = 14, P < 0.05), suggesting that α5GABAA receptors have a role in regulating sodium spikes. These GABA receptors and sodium channels were only dissociated in the dorsal terminal boutons where sodium channel clusters were generally absent, whereas dorsal terminal boutons had α5GABAA expression (in cutaneous and proprioceptive afferents). Functionally this allows GABA to modulate the spike’s action on dorsal horn transmitter release, presumably by shunting the passive sodium current reaching the terminals (as in crayfish) (Cattaert et al. 1992). In contrast, proprioceptive group Ia afferent terminal branches (and boutons) in the ventral horn generally lacked both sodium and α5GABAA clusters.
VGLUT1 and NF200 immunolabelling.
We labeled some cords with a vesicular glutamate transporter VGLUT1 antibody known to label afferent terminal glutamate release sites (Betley et al. 2009; Todd 2015). As expected, we found VGLUT1 labeling to be mostly confined to afferent terminal boutons and absent from other locations, including most branch points and nodes (not shown). We also used heavy neurofilament staining (NF200, purple in Figs. 1–4) to show the location of other axons and neurons in the cord, as many, but not all, neurons express this marker (including motoneurons, as mentioned above). NF200 was expressed in group Ia afferents themselves in between nodes in the dorsal columns and dorsal root, but disappeared at the nodes (not shown), consistent with (Bush and Gordon-Weeks 1994), making it also useful for confirmation of the location of nodes. Once the group I afferents entered the gray matter, NF200 was largely absent, presumably because of the frequent branches and nodes. This distribution of NF200 made it not very useful for observing the overall bundles of group Ia afferents that projected to the ventral horn, except to show locations where many nodes aligned, corresponding to where GABA inputs and receptors clustered (black holes among purple NF200 in Fig. 2G, near arrows).
Origin of primary afferent depolarization.
While we advanced the electrode just below the dorsal columns into the gray matter, as detailed above, we stimulated the low-threshold afferents in the dorsal roots to characterize the afferents we approached. When the electrode was still extracellular to the afferents this stimulation always produced a small extracellular field with the same components Lloyd defined in cats many years ago (Lloyd and McIntyre 1949). First there was a very fast negative afferent volley (Fig. 5A, downward field, black and gray) with the earliest component corresponding to spikes in the lowest threshold proprioceptive group Ia afferents entering the cord (Hubbard et al. 1969). Penetration of a proprioceptive afferent at this location indeed revealed a fast-action potential (spike; red) when the root containing the axon was stimulated suprathreshold (Fig. 5A). Following the afferent volley there was a second fast negative extracellular field corresponding to the transient synaptic activation of first order neurons in the dorsal horn (synaptic field, Fig. 5B, black trace). Penetrating a nearby interneuron showed an EPSP at this latency (not shown) (Eccles and Krnjevic 1959). Both the extracellular afferent volley and the synaptic field were also visible during intracellular recording from a proprioceptive afferent when the stimulation was reduced to just subthreshold to initiate a spike (Fig. 5B, red). This extracellular field contamination was largely removed by subtracting the intracellular and extracellular traces to yield the true transmembrane potential in the afferent (Fig. 5B, green) (Eccles and Krnjevic 1959).
Fig. 5.
Distribution of primary afferent depolarization (PAD) in the spinal cord. A: extracellular recording (EC; black) of the group Ia afferent volley (field) in lamina I near the dorsal columns (DC) in response to S4 dorsal root (DR) stimulation (1.2 × threshold (T), for volley), expanded 10× in gray. Intracellular (IC) spike (red) in response to the same DR stimulation after penetrating a nearby proprioceptive group Ia afferent (S4), demonstrating that extracellular fields are negative when there is a nearby positive intracellular event. Afferent resting at −72 mV. B: same cell as in A, but on longer time scale and stimulation set lower (1.1 × T), subthreshold to direct orthodromic spike in the same afferent. Intracellular recording (red) shows a PAD in response to the DR stimulation. Extracellular recording (black) shows the afferent volley field, followed by a second field corresponding to the synaptic excitation of local interneurons in the dorsal horn, and finally a slow long-lasting negative field corresponding to PAD (expanded in gray, 7×; truncated at vertical line). Subtraction of the EC from the IC recordings gives the actual transmembrane potential (green). C: representative intracellular (red) and extracellular (black) recordings from different laminae in the spinal cord during dorsal root stimulation (~2.0 × T), with peak PAD observed in the deep dorsal horn (positive red, IC and negative black, EC), including both an early, fast phasic PAD and later tonic PAD (n = 22). In the ventral horn, only a transient 20 ms long PAD was seen in the ventral afferent terminals recorded intracellularly (Lamina IX, red, n = 4), and extracellularly there was no negative PAD field (n = 20/20). Scale 0.5 mV for dorsal EC fields, 0.1 mV for ventral EC fields and 2 mV for all IC recordings.
In both the raw intracellular afferent recordings (red) and the corrected transmembrane potential estimates (green), the dominant response to dorsal root stimulation was a long depolarization lasting at least 100 ms, corresponding to PAD (Fig. 5, B and C) (Eccles et al. 1962a). When recording extracellularly the compound effect of PAD from nearby afferents produced a long negative field, which we refer to as the PAD field, corresponding to the final component of the Lloyds extracellular field description (Fig. 5, B and C, black and gray) (Eccles et al. 1962a; Lloyd and McIntyre 1949). While small, this PAD field has proven to be a useful tool to judge the overall PAD generated in nearby afferents (Eccles et al. 1962a, 1962b), covarying with PAD (Fig. 5C) and eliminated by the GABAA receptor antagonist bicuculline, as detailed below. Importantly, both the intracellular and extracellularly recorded PAD (Fig. 5C, left) generally had an early large component (<100 ms, termed phasic PAD, corresponding to classical PAD of Eccles et al. 1962a, 1962b) and a smaller but long-lasting component (termed tonic PAD or specifically stimulus-evoked tonic PAD; lasting >100 ms and usually up to 300 ms or more as detailed in later in Fig. 10).
Fig. 10.
Spatial and temporal summation of tonic primary afferent depolarization (PAD), including facilitation by cutaneous afferents. A: intracellular recording from proprioceptive group I afferent (Ca1 afferent), resting at −83 mV (dotted line). Stimulating the adjacent S4 dorsal root (DR) at group I intensity [1.5 × T (T, afferent volley threshold, blue)] evoked a phasic PAD with a small tonic PAD. Increasing the DR stimulation to additionally recruit low- (2.5 × T, Aβ; red) and high-threshold (6 × T, black) cutaneous afferents progressively increased the tonic PAD (starting 20 ms later, relative to group-I-evoked PAD, shown in light blue for reference), without increasing the early portion of phasic PAD. Each plot is an average of 10 responses with DR stimulation delivered at 1-s intervals. B: repeated stimulation of the DR at 2.5 × T (in same afferent as in A) facilitated tonic PAD shown on a longer time scale but decreases phasic PAD that rides on top of tonic PAD (first few phasic PADs expanded in insets, black). Temporal facilitation of tonic PAD was maximal at the 2 Hz DR stimulation rate and decreased with rate, mostly gone by 10 s interstimulus intervals. Similar facilitation also occurred for smaller and larger DR stimuli [1.5 × T and 6 × T], being larger with larger stimuli (not shown). C: PAD recorded from the dorsal root (DRP) in response to stimulating an adjacent DR at low-threshold cutaneous intensity (2.5 × T, red). The tonic PAD component is increased by turning the stimulation up to C fiber intensity (100 × T; 5 sweeps at 10-s intervals averaged). Repeated C fiber stimulation to compute averages displayed increased the dorsal root reflex (DRR) by tonically depolarizing the root (tonic PAD; not shown). D: Another proprioceptive group I axon recorded intracellularly, showing the same slow buildup of tonic PAD with repeated 1 Hz DR stimulation at group I and C fiber intensity, lasting a minute poststimuli for the latter. E: tonic PAD facilitated by repeated DR stimulation was consistently blocked by L655708 (0.1 µM; n = 6/6).
To discover the spatial location where PAD was generated, we advanced the electrode into the cord along the proprioceptive afferent pathway while evoking PAD by stimulating low-threshold afferents (Fig. 5C; 2 × T, afferent volley threshold), as did Eccles (Eccles et al. 1962a, 1962b). Interestingly, the extracellular PAD field (inward current sink, negative field) reached a maximum in laminae II-V of the dorsal horn, and by far dominated the neuronal responses in this region. Indeed, when we penetrated proprioceptive group I afferents, here the intracellularly recorded PAD was large compared with other regions and included phasic and tonic PAD components (n = 22/22 intracellular and extracellular; Fig. 5C). However, the extracellular PAD field decreased to zero at the level of the central canal (lamina VII), and then reversed to a very small positive field in the ventral horn (lamina VII-IX; Fig. 5C, right), although still with the same time course as PAD (n = 20/20). This indicates that there is little active PAD generated at the ventral terminals in the motor nucleus, relative to in PAD generation in the dorsal horn. The later small positive ventral field (in Lamina IX) corresponds to passive outward current in proprioceptive afferents terminal branches arising from axially propagated currents generated by GABA receptors (PAD) at a distant location (in dorsal horn), as detailed by source-sink analysis (Hubbard et al. 1969). Thus, surprisingly little PAD appeared to be generated in afferent branches in the ventral horn.
To confirm the relative lack of PAD in the ventral horn, we repeatedly attempted to make direct intracellular recordings from proprioceptive Ia afferent terminal branches in the ventral horn. While very rare, we succeeded occasionally in making stable afferent terminal recordings. In these afferent terminals dorsal root stimulation only produced a very brief PAD (~20 ms depolarization; n = 4/4; red plot on right of Fig. 5C; lamina IX), much shorter than the phasic PAD (~100 ms) recorded in proprioceptive afferent branches penetrated in the dorsal horn, and no tonic PAD was present (Fig. 5C, left). Taken together with our extracellular recordings, these results suggest that in low-threshold proprioceptive afferents tonic (and phasic) PAD is mostly generated by receptors in the dorsal horn, consistent with our observations of the highest density of α5GABAA receptors in the dorsal horn and the relative lack of receptors on ventral Ia afferent terminals. This PAD must be passively attenuated by the time it reaches ventral terminals, due to the large electrotonic distance.
PAD in low-threshold afferents is mediated by GABAA receptors and a depolarized chloride reversal potential.
Intracellular recordings made from large low-threshold afferents, including proprioceptive group Ia afferents, consistently demonstrated a classical phasic PAD (<100 ms) that occurred with stimulation of either the dorsal root containing the afferent itself, an adjacent ipsilateral root or even a contralateral dorsal root (Fig. 6, A–B and F), indicating a widespread spatial origin to PAD (82% or n = 320/390 afferents recorded exhibited PAD evoked from one or more roots). Regardless of the root stimulated, the PAD arose ~2–4 ms after the afferent volley (Figs. 5B and 6B), consistent with a trisynaptic origin (Jankowska et al. 1981). The phasic PAD peaked at ~20 - 40 ms latency, after which it decayed over the next 100 ms, although again there was a long-lasting tonic PAD component >100 ms, (tonic PAD, Figs. 6, B and G and 5C; quantified in Fig. 10 later). No obvious differences were seen between PAD in proprioceptive group Ia and other low-threshold large afferents (e.g., Aβ), and so these were grouped together for analysis. Blocking GABAA receptors with bicuculline or gabazine eliminated the phasic PAD and most of the tonic PAD (Fig. 6, E and F; and the PAD field) as expected (Willis 1999). However, a very small and slowly rising PAD remained (lasting up to 1 s), which is likely glutamate mediated and involving NMDA receptors (APV sensitive, not shown; as in Russo et al. 2000), as detailed below.
Fig. 6.
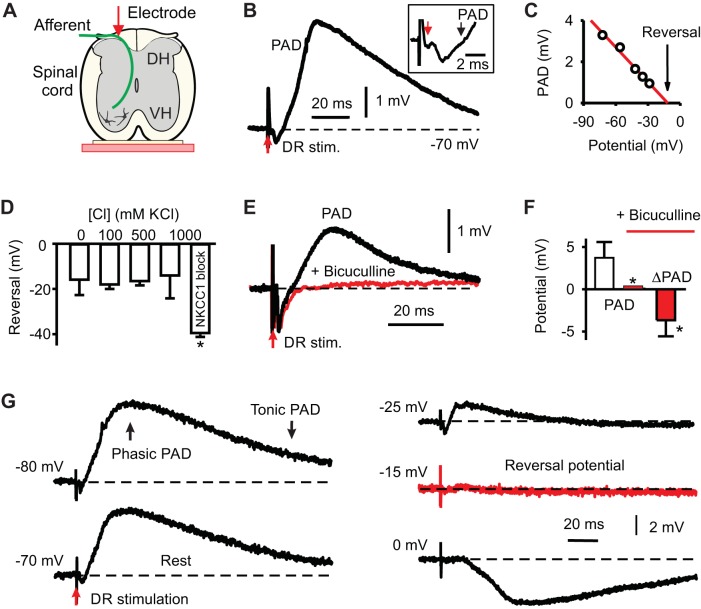
Phasic primary afferent depolarization (PAD) mediated by GABAA receptor chloride currents. A: intracellular recording of group I afferent in the dorsal horn (DH) from S4 dorsal root (DR). VH, ventral horn. B: PAD evoked by stimulation of adjacent Ca1 dorsal root. Inset: onset of PAD, at black arrow. Afferent volley at red arrow. Resting potential at dotted line, −67 mV. C: PAD amplitude variation with initial holding potential, with reversal potential near −15 mV. D: reversal potential increased by blocking NKCC1 chloride pump with bumetanide (50 µM). Reversal potential unchanged by varying intracellular chloride, without injecting current, proving that the ultrasharp electrode tips (50 nm opening) do not allow mixing of the electrode and cellular contents; n = 5 per group. E–F: high-dose bicuculline (50 µM) or gabazine (30 µM) blocked most of PAD (n = 18 and 4, respectively), leaving only a small and very slow component. G: PAD in a group Ia afferent held at different potentials with a bias current [iCa1 afferent, with contralateral Ca1 DR stimulation at 2 × threshold (T)]. Fast phasic PAD and sustained tonic PAD indicated. Variability shown with standard deviation (SD) error bars. *Significantly changed, P < 0.05.
The stimulus-evoked phasic and tonic PAD in the afferents had a reversal potential of about −15 mV (Fig. 6, C, D, and G), consistent with PAD resulting from GABAergic chloride currents that reverse at high potentials in adult afferents due to high intracellular chloride concentrations (as detailed in the Introduction). Indeed, blocking the NKCC1 pump (that causes this high intracellular chloride concentration) decreased the reversal potential (Fig. 6D). This very high PAD chloride reversal potential explains the strong depolarizing action of GABAA receptors in mediating PAD, especially at or below the typical resting potential of −70 mV observed in these axons. Often we could depolarize the afferent to near the PAD reversal potential, but the electrode could not pass adequate current to further depolarize the afferent, and thus the reversal potential was computed by extrapolation as in Fig. 6C. Possibly a glutamate or even gap junction component to PAD made reversing PAD difficult, although these are likely small at rest in relation to the main GABAA component of PAD (Fig. 6E).
Changing the electrode intracellular chloride concentration did not change the PAD reversal potential, indicating that little electrode medium entered the axon under resting conditions (Fig. 6D). This is consistent with the very fine tips used for recording not disturbing the axon intracellular environment (before we injected large long currents to fill the axon with neurobiotin).
α5GABAA receptors produce a tonic PAD in low-threshold afferents.
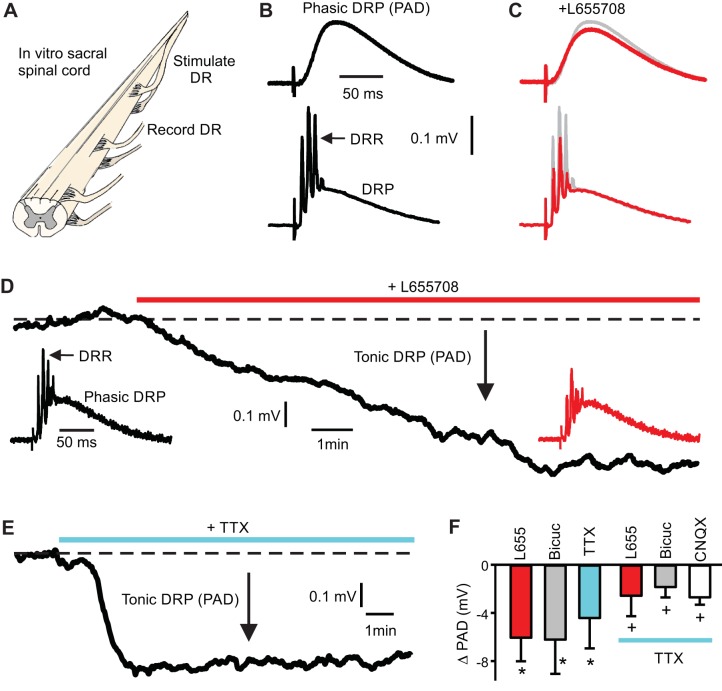
Blocking all GABAA receptors with bicuculline hyperpolarized all afferents, including the lowest threshold proprioceptive afferents (~10 mV; Fig. 7, A and C), indicating that there is a spontaneous tonic GABA activation that tonically depolarizes the afferents (termed spontaneous tonic PAD; here PAD is spontaneous, as opposed to stimulus-evoked tonic PAD detailed earlier). Application of the selective α5GABAA receptor blocker L655708 likewise hyperpolarized afferents by a similar amount (~7 mV; Fig. 7, B and C), and at the same time decreased the tonic, but not phasic, component of the PAD evoked by dorsal root stimulation (Fig. 7C). This suggests that α5GABAA receptors specifically produce tonic PAD and not the early phasic PAD. The conductance of the axon also dropped as the membrane hyperpolarized (Fig. 7B, 1/Rm), consistent with a block of GABAergic chloride currents, and thus ruling out recording drift.
Fig. 7.

Tonic primary afferent depolarization (PAD) is mediated by α5GABAA receptors. A: intracellular recording from group I afferent (S4). Blocking all GABAA with high-dose bicuculline (50 µM) eliminated a spontaneous tonic PAD, hyperpolarizing the afferent. Resting potential at dotted line, −74 mV. B: selectively blocking α5GABAA receptors with L655708 (0.1–0.2 µM), likewise, hyperpolarized group I afferents (different afferent), and increased input resistance (Rm measured with −0.1 nA pulse; loss of shunt). C: on average, bicuculline decreased both spontaneous tonic PAD (n = 5) and dorsal root-evoked phasic PAD (n = 22), with a larger reduction in tonic PAD, indicating that spontaneous tonic PAD is large in relation to phasic PAD. In contrast, L655708 only blocked tonic PAD and not phasic PAD (n = 5). Error bars SD; *significantly different, P < 0.05.
Tonic PAD is mediated partly by nonspike-mediated GABA receptor activity or spillover.
To more efficiently quantify the average function of PAD, we measured the compound effect of PAD in all afferents by recording the dorsal root potential (DRP) from the cut ends of short dorsal roots (Willis 1999) using a specialized agar bridge electrode to record steady DC potentials without substantial drift (Fig. 8). In this arrangement, stimulation of one dorsal root (S4 or Ca1) evoked a phasic DRP (Fig. 8B) on all roots, including ipsilateral and contralateral dorsal roots (S3, S4, and Ca1). This phasic DRP was mediated by phasic PAD and GABA (Wall 1958), because it had an identical time course to phasic PAD (Fig. 6) and was blocked by bicuculline (n = 36/36). The DRP was an order of magnitude smaller in absolute potential than the average phasic PAD recorded intracellularly (Figs. 6B and 8; 0.4 vs. 3.7 on average), consistent with electrotonic attenuation of the PAD axially along the dorsal root leading to the recording electrode (Lloyd and McIntyre 1949). Under these conditions we were able to efficiently screen the action of drugs on the primary sensory afferent potential, inferring tonic PAD from the tonic changes in the DRP (by multiplying by 3.7/phasic DRP; or a factor of ~9).
Fig. 8.
Extrasynaptic α5GABAA receptors contribute to a tonic primary afferent depolarization (PAD), assist antidromic spikes, and are partly activated by a TTX-resistant GABA leak. A: extracellular recording from cut central end of an S4 dorsal root (DR) in grease very close to the cord, to observe the compound potentials from many afferents. B: dorsal root stimulation [Ca DR; 2 × threshold (T)] evoked a dorsal root potential (DRP) corresponding to phasic PAD in Fig. 5. Sometimes this PAD appeared alone (top), but more often it appeared with a compound action potential riding on the DRP (bottom), which correspond to spikes evoked by PAD (dorsal root reflex; DRR). C–D: blocking α5GABAA receptors with L655708 (0.1 µM) did not change phasic PAD (DRP) but hyperpolarized the afferents (reduced tonic DRP; tonic PAD) and reduced the DRR (n = 7). E: blocking spike-mediated synaptic activity with high-dose tetrodotoxin (TTX; 2 µM; n = 9) also hyperpolarized the afferents. F: overall, blocking GABA receptors with L655708, bicuculline (50 µM, n = 5) or gabazine (30 µM, n = 3) reduced tonic PAD computed from the reduction in DRP, as did TTX. Bicuculline and gabazine data combined (and abbreviated Bicuc). Both drugs also eliminated the phasic PAD (DRP). After TTX application, subsequent application of L655708, bicuculline or glutamate receptor blockers (CNQX and APV; 10 µM and 50 µM; abbreviated CNQX) further reduced tonic PAD in the presence of TTX (n = 9, 5 and 5, respectively). Error bars SD; *significantly different, P < 0.05.
As expected, L655708 and bicuculline each hyperpolarized the afferents recorded in the root (Fig. 8, D and F), consistent again with the presence of a spontaneous GABA tone and associated tonic PAD blocked by these drugs (tonic DRP), like with direct intracellular recording (Fig. 7). When we applied a high-dose of TTX alone (2 µM), to block all spike-mediated transmission, then afferents likewise hyperpolarized (but to a lesser extent; tonic DRP reduced; Fig. 8, E and F), indicating that spike-mediated GABA release played a role in activating tonic PAD and the extrasynaptic GABA receptors. After TTX, subsequent application of L655708, bicuculline or gabazine further hyperpolarized the afferents (tonic DRP), although significantly less than before TTX (Fig. 8F), thus suggesting a tonic PAD and GABA tone mediated by nonspike-mediated transmission or constitutive GABA receptor activity, consistent with Russo et al. (2000). Application of CNQX and APV after TTX application likewise hyperpolarized afferents (Fig. 8F), suggesting nonspike-mediated release of glutamate in maintaining the tonic GABA tone, also as suggested by Russo et al. 2000.
Antidromic spikes are insecure and depolarization dependent.
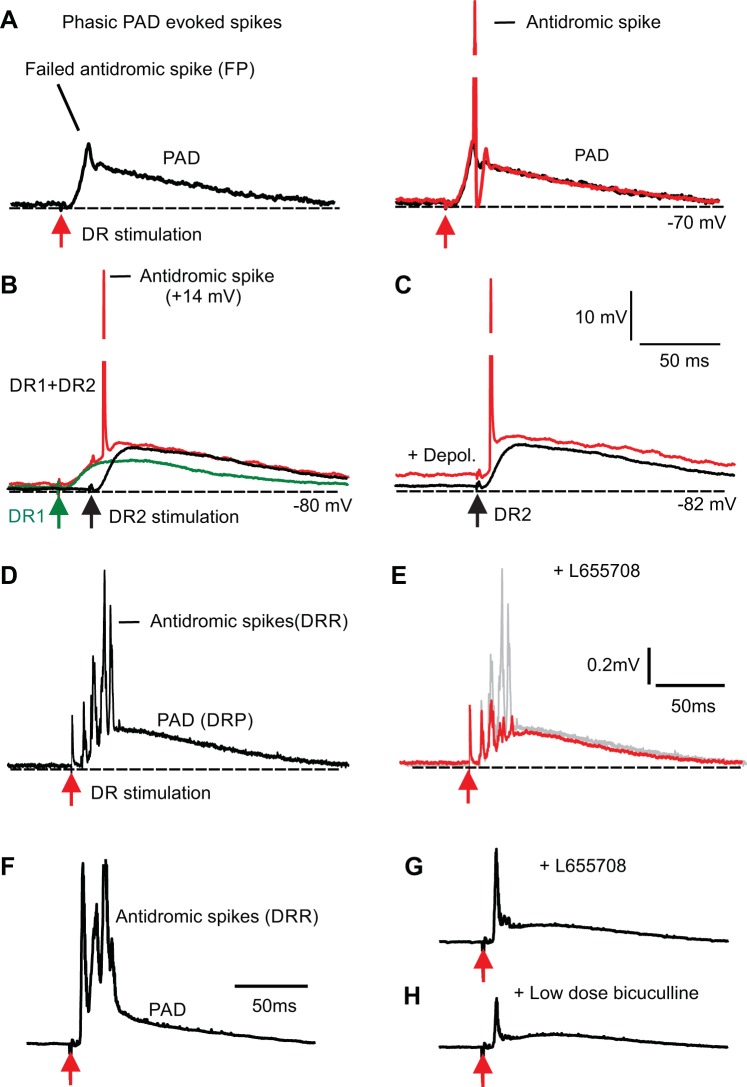
Considering that Delgado’s group (Loeza-Alcocer et al. 2013) suggested that tonic PAD might have a paradoxical action of facilitating spikes, especially antidromically propagating spikes, we evaluated the effect of tonic PAD on the dorsal root reflex (DRR). As mentioned in the Introduction, the DRR is evoked by phasic PAD from one dorsal root stimulation being so large that it activates spikes in many afferents, which propagate antidromically out the dorsal roots. This causes a DRR, which we usually observed on dorsal roots recorded while stimulating adjacent roots, including roots contralateral to the stimulated dorsal roots (Figs. 8, B and D, and 9, D and F; n = 24/30), with a central latency of 6–7 ms.
Fig. 9.
Antidromic spikes are facilitated by depolarization, including tonic primary afferent depolarization (PAD). A: intracellular recording from Ia afferent with (red; Ca1 afferent) and without an antidromic spike triggered by phasic PAD evoked by S4 dorsal root stimulation [2 × threshold (T)]. This spike is insecurely propagated, often failing spontaneously, and leaving only a small failed spike (failure potential). Resting potential (dotted line, −70 mV). Spike peaked at +15 mV overshoot but is truncated. B: two separate phasic PADs evoked in an Ia afferent (Ca1 root afferent) by stimulating an adjacent dorsal root [(DR); S4, black; 2 × T] or a contralateral dorsal root (Ca1, green; 2 × T). Neither stimuli alone evoked an antidromic spike, but the extra combined potential from the two stimuli together evoked an antidromic spike (red). C: spontaneous depolarization of the afferent facilitates an antidromic spike evoked by PAD in an axon without an antidromic spike initially (phasic PAD evoked by S4 DR stimulation, 2 × T). D–E: antidromic spikes (dorsal root reflex; DRR) recorded on dorsal root (black and gray) are reduced by blocking tonic PAD with L655708 (0.1 µM). F–H: Another example of antidromic spikes reduced by L655708 and then further reduced by bicuculline (10 µM).
To better understand the origin of the DRR, we recorded intracellularly from proprioceptive group I afferents in the superficial dorsal horn or dorsal columns. As expected, we sometimes directly observed antidromic spikes produced by the fast-rising phase of PAD at a minimum central latency of 6 ms (6 to 12 ms range) after dorsal root stimulation (Fig. 9A). Interestingly, these antidromic spikes were prone to failure (insecure), with spikes occurring in only 9% of axons (n = 10/112 examined), and when they did occur they spontaneously failed most of the time (Fig. 9A; occurring on 41 ± 17% of stimuli; n = 10). However, when the antidromic spike was not present or failed intermittently there was usually a small failed spike visible (failure potential in 79% of axons with PAD; n = 89/112; Fig. 9A), indicating that a spike was actually reliably initiated by PAD but failed to propagate to the electrode located in the superficial dorsal horn, leaving only a passively attenuated spike visible (failure potential, with size depending on the electrotonic distance to failure).
The antidromic spike failure and variability depended on the potential of the afferent, with full spikes occurring more frequently at more depolarized levels (Fig. 9C; spontaneous depolarizations) and the summation of two phasic PADs evoked by separate root stimuli causing a spike, whereas individually they did not (Fig. 9B; n = 5/6). This suggests that any spontaneous depolarization from tonic PAD should also assist in antidromic spike propagation. Indeed, we found that selectively blocking tonic PAD with L655708 to hyperpolarize afferents caused a decrease in antidromic spikes recorded on dorsal roots (DRR; Figs. 9, D–G and 8, B–D; n = 10/10 DRR decreased). This occurred in the absence of a reduction in the phasic PAD (Figs. 7C, 8, C and D, and 9E), ruling out changes in phasic PAD reducing spiking, consistent with the hyperpolarization from L655708, making these spikes less easy to initiate. Low-dose bicuculline further reduced the antidromic spikes (Fig. 9H; n = 11/11), with relatively little reduction in phasic PAD. Finally, complete block of PAD with high-dose bicuculline eliminated the DRR (and DRP; n = 11/11; not shown) as expected. In contrast, when tonic PAD was increased by C fiber stimulation (described below in Fig. 10C, n = 5/5) there was an increase in the incidence of antidromic spikes evoked by subsequent phasic PAD from low-threshold dorsal root stimulation.
Spatial summation of different afferents facilitating phasic and tonic PAD.
We noticed that progressively activating additional afferents by gradually increasing the dorsal root stimulation intensity increased phasic PAD (in 15/15 low-threshold proprioceptive afferents tested; Fig. 10, A and C). The largest portion of phasic PAD was activated by low stimulation currents at proprioceptive group I intensity (1.5 × T), but it was further increased by recruiting slightly higher threshold cutaneous afferents (Aβ or group II; with stimuli at 3 × T). Additional stimulation at 6 × T and 100 × T further increased PAD and associated DRR (Fig. 10, A and C). This is consistent with a spatial summation of most afferents, including C fibers, in PAD generation.
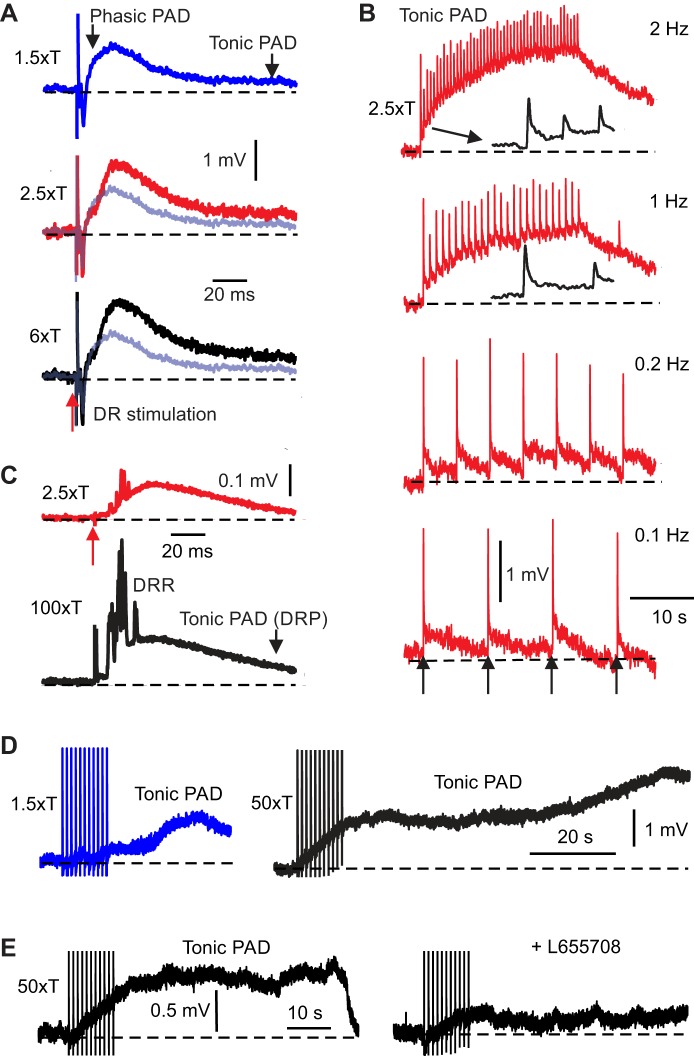
These same dorsal root stimuli also produced a tonic PAD that lasted much longer than phasic PAD, as detailed above (100–1,000 ms; Fig. 10, A and C; n = 12/15), and this stimulus-evoked tonic PAD likewise increased in size with increasing intensity. However, in contrast to phasic PAD, increasing the stimulation to cutaneous afferent intensities (2.5 × T; Fig. 10A, red) and C fiber intensities (100 × T; Fig. 10C, black) yielded the largest increases in tonic PAD, with increases beginning late at ~20 ms poststimulation (Fig. 10A) and lasting for over a second (Fig. 10B, black insets). This suggests that tonic PAD starts slowly (20 ms) and lasts for seconds, and thus arises from a different neuronal circuit than phasic PAD, with a likely cutaneous origin (similar to in turtle, Fig. 1 of Russo et al. 2000) and is perhaps related to the PAD neurons of Jankowska et al. (1981). The increased DRR with C fiber stimulation (Fig. 10C) likely resulted from a sustained depolarization built up from the repeated trials to produce the average responses shown in Fig. 10 C (see below).
Temporal summation of tonic PAD with afferent stimulation, mediated by α5GABAA receptors.
The evoked tonic PAD in low-threshold proprioceptive afferents was markedly increased when the dorsal root stimulation was repeated at short intervals (2 Hz stimulation train, 2.5 × T; top Fig. 10B). This temporal summation of tonic PAD occurred at intervals up to 5 s (Fig. 10B, 0.2 Hz train; n = 8/10 afferents). In contrast, phasic PAD decreased with repeated stimulation at these intervals (Fig. 10B, inset, black traces). Importantly, the large tonic PAD that built up during repeated stimulation continued for 10–20 s after the stimulation train (after effect; Fig. 10B, right, n = 8/8), suggesting that brief bursts of sensory activity can potently modulate GABA and PAD for many seconds. Only at intervals of 10 s or longer (<0.1 Hz) did the tonic PAD cease to build up (summate) with repetition (Fig. 10B, bottom). This temporal summation started immediately in most afferents (Fig. 10B; n = 5/8), whereas in other afferents the summation of tonic PAD with repetition was delayed until after the stimulation train ceased (Fig. 10D; n = 3/8; during this delay afferents transiently hyperpolarized). However, in all cases a similar duration of tonic PAD occurred after the stimulation train ceased (10–20 s; Fig. 10, B and D).
More intense stimulation trains, to activate all fibers including the C fibers (50 × T, 1 Hz), likewise evoked a tonic PAD that summated with repetition. However, in this case, the buildup in PAD always started immediately and was larger and much longer lasting, continuing for up to a minute after a 10-s long stimulation train (Fig. 10, D and E; 1 Hz train; n = 12/12 axons tested), suggesting a long-lasting C fiber mediated component.
Afferent-evoked tonic PAD was blocked with the α5GABAA receptor antagonist L655708 (Fig. 10E; n = 6/6), like the spontaneous tonic PAD discussed above. Together these results suggest that GABA tone and an associated α5GABAA receptor activation gradually builds with time during repeated afferent stimulation and only slowly subsides afterward, consistent with extrasynaptic GABA spillover as Russo suggests (Russo et al. 2000).
Dorsally located neuronal circuits generate a tonic PAD mediated by extrasynaptic α5GABAA.
To further evaluate the origin of PAD evoked by sensory afferents (especially C fibers), we replicated in rats the turtle experiments of Russo (Russo et al. 2000), which take advantage of TTX-resistant C fibers. Specifically, we bathed the whole cord in low-dose TTX (100–200 nM) to block all spike transmission in the cord and roots except for TTX-resistant C fiber activity, and again we assessed PAD via dorsal root recordings (DRP; Fig. 11A). Prior to TTX, both low-threshold stimulation (group I) and higher intensity stimulation (100 × T; including C fiber activation) evoked a large phasic PAD (DRP) that peaked rapidly, within 20 ms, and was associated with PAD evoked spiking (DRR; Fig. 11B). As described above, there was also a tonic PAD that lasted well over 100 ms and was larger for the higher intensity stimulation, consistent with the involvement of C fibers (Fig. 11B). Low-dose TTX application readily blocked the fast phasic PAD, spike-mediated DRR and the tonic PAD evoked by low-intensity stimulation (top Fig. 11C). In contrast, the same TTX application spared the tonic PAD evoked by high-intensity C fiber stimulation, presumably because it did not block TTX-resistant sodium channels on C fibers. This allowed us to view the tonic PAD in isolation, which as expected peaked slowly (at 100-ms latency) and lasted for seconds (Fig. 11C). Across all roots tested with this TTX dose there was consistently a tonic PAD (DRP) initiated by C fiber stimulation (n = 6/6), indicating that local nonspiking microcircuits near the C fiber terminals in the superficial dorsal horn mediate a tonic PAD (Alvarez 1998; Russo et al. 2000). Furthermore, application of L655708 in these rats reduced this tonic PAD (Fig. 11D, n = 6/6), indicating that these microcircuits evoke at least part of the α5GABAA receptor-mediated tonic PAD. Likely the remaining tonic PAD in TTX and L655708 has a glutamate mediated component (Russo et al. 2000).
Fig. 11.
Local GABAergic microcircuits in the dorsal horn contribute to tonic primary afferent depolarization (PAD). A: in vitro rat spinal cord in low-dose tetrodotoxin (TTX) to block all spike transmission, except in TTX-resistant C fibers. B: prior to TTX, dorsal roots stimulation at both low and high intensity [2.5 × and 100 × threshold (T)] evoked a dorsal root reflex (DRP; PAD related) and PAD-evoked spikes [dorsal root reflex (DRR)], with both early phasic and late tonic PAD components. C: bath application of TTX (100 nM) blocked the PAD evoked by low-threshold stimulation, but there remained a delayed slow C fiber-mediated tonic PAD evoked by high-intensity stimulation (100 × T). PAD evoked spikes (DRR) were blocked by TTX, confirming the TTX block of spike transmission in low-threshold afferents (n = 6/6). D: application of the α5GABAA receptor antagonist L655708 consistently inhibited the C fiber-mediated tonic PAD (n = 6/6). Top trace: amplified from part C.
DISCUSSION
Our results demonstrate that large low-threshold proprioceptive and cutaneous afferents of adult rats express extrasynaptic α5GABAA receptors throughout most of their extensively branched axonal structure in the spinal cord. These receptors are specifically localized at branch points near sodium channel nodes, including a dense expression in the dorsal horn far from proprioceptive afferent terminals on motoneurons. As previously described, proprioceptive group Ia afferents are heavily myelinated as they traverse through the spinal cord gray matter, with myelin only lost at terminal branches (Nicol and Walmsley 1991; Pierce and Mendell 1993; Walmsley et al. 1995). Furthermore, branch points in Ia afferents are invariably associated with intermyelin nodes where there are always sodium channels (Fig. 4) (Nicol and Walmsley 1991). Thus, our finding that α5GABAA receptors are preferentially associated with branch points and nodes is consistent with previous observations of Walmsey (Walmsley et al. 1995) that intermyelin nodes have synaptic specializations (Walmsey and colleagues termed these en passant boutons, but these are perhaps better called nodal boutons to avoid confusion with terminal boutons). Roughly speaking, nodes, branch points, and Walmsley’s large en passant boutons all appear to be the same thing in proprioceptive group Ia afferents, and this is where α5GABAA receptors are mostly located. Previous studies of synaptic GABAergic contacts on proprioceptive group I afferents have occasionally revealed contacts far from the terminals (Cattaert and El Manira 1999; Lamotte d’Incamps et al. 1998; Verdier et al. 2004; Walmsley et al. 1995), although by far the bulk of previous studies have focused only on contacts on afferent terminals (Burke and Glenn 1996; Fink et al. 2014; Fyffe et al. 1986; Hughes et al. 2005, 2012; Pierce and Mendell 1993; Smith et al. 2017; Walmsley et al. 1995) due to the shear complexity of innervation elsewhere. Thus, our previous impression of GABAergic innervation of afferents has been biased to the terminals.
Perhaps our most surprising finding is that proprioceptive group Ia afferent terminal collaterals and boutons on motoneurons have relatively little expression of α5GABAA receptors, with many boutons not having any receptors. However, in retrospect, other laboratories have also found that the major synaptic GABAA receptor β2 and β3 subunits appear to be lacking from proprioceptive afferent terminals on motoneurons (Alvarez et al. 1996; Betley et al. 2009). There is no doubt that GABAergic P-boutons innervate these afferent terminals (Alvarez 1998; Betley et al. 2009; Fink et al. 2014; Hughes et al. 2005; Pierce and Mendell 1993; Smith et al. 2017), but it now is even more uncertain what receptors this GABA acts on. Possibly other GABAA receptor subunits that we have not tested are at the terminal, or instead GABAB receptors play a major role in presynaptic terminal inhibition, as demonstrated in cat and neonatal rat (Curtis 1998; Lev-Tov et al. 1988; Pinco and Lev-Tov 1993; Redman 1998).
We find that functionally the α5GABAA receptors generate a spontaneous tonic depolarization of afferents (tonic PAD), which slowly increases with repeated dorsal root stimulation and persists even after synaptic transmission is blocked, suggesting that it is caused by a extrasynaptic leakage of GABA (GABA tone), in localized microcircuits (Lorenzo et al. 2014; Russo et al. 2000) although constitutive α5GABAA receptor activity could also contribute (Street et al. 2004). Consistent with the dominant dorsal expression of the α5GABAA receptors, extracellular and intracellular recordings from low-threshold proprioceptive and cutaneous afferents indicate that both tonic and phasic PAD is on average generated dorsally in the spinal cord. We find that surprisingly little tonic or phasic PAD is generated at ventral proprioceptive afferent terminals (our direct intracellular recording afferent terminals on motoneurons reveal only a small, short 20 ms long PAD; Fig. 5C), and there is no lamina IX extracellular PAD field, although this is consistent with a relative lack of ventral terminal GABAA receptors in general (Alvarez et al. 1996; Betley et al. 2009; Bohlhalter et al. 1994). Using extracellular recordings in cats, Eccles’ group also found phasic PAD from cutaneous afferents to be generated dorsally (Eccles et al. 1962a), but PAD from proprioceptive afferents to be generated ventrally (Eccles et al. 1962b). However, closer inspection of Eccles et al. (1962b) reveals that in the cat, proprioceptive PAD fields are generated in the intermediate and ventral lamina (VI and VII) as much as 1–2 mm from the motoneurons and are thus at a similar distance from motoneurons that we find in rat, taking into account the larger spinal cord in cat. Finally, the afferent stimulation that most strongly facilitates the α5GABAA-mediated tonic PAD involves low- and high-threshold cutaneous afferents that terminate exclusively in the superficial dorsal horn (Alvarez et al. 1996; Fyffe et al. 1986; Scheibel and Schiebel 1969), further supporting the notion that dorsally derived GABA predominately generates tonic PAD and modulates afferent transmission through the dorsal horn, even for Ia afferents.
Our finding that PAD is not generated in afferent terminals must be reconciled with indirect measures of terminal PAD using the Wall afferent excitability test (Wall 1958). In this excitability test the afferent terminals are stimulated directly with a microelectrode and the threshold to evoke an antidromic response is measured. Using this method Rudomin’s group (Rudomin and Schmidt 1999) and others (Loeza-Alcocer et al. 2013; Wall 1958) found that various afferent stimuli that evoke PAD or DRPs produce a change in afferent terminal excitability with a similar time course to PAD measured dorsally (DRPs, ~100 ms) and thus assumed that this excitability is an effective measure of PAD in the ventral afferent terminals on motoneurons. However, this assumption is now thrown into question considering our new results showing that ventral terminal PAD can be very small and brief (<20 ms) and terminals on motoneurons often lack GABAA receptors. Perhaps terminal excitability is also influenced by GABAB receptors, as well as GABAA receptors, a suggestion consistent the extensive work from Curtis’s laboratory showing that GABAB receptors alter spikes evoked at the afferent terminals (Curtis 1998; Curtis and Lacey 1994; Curtis et al. 1977). This issue clearly needs further study, including determining whether proprioceptive afferent terminals have GABAB receptors.
Uncertain role of α5GABAA in presynaptic inhibition.
From a classical standpoint, the spatial distribution of the α5GABAA receptors is puzzling because these receptors are not preferentially located on Ia afferent terminals and thus are not well positioned to help with terminal presynaptic inhibition. Presynaptic inhibition is thought to be mediated in large part by the shunting action of GABA receptors at the afferent terminal (Curtis 1998), which reduces the height of the action potential that invades the terminal and thereby reduces calcium current activation and associated transmitter release (Cattaert and El Manira 1999; Delgado-Lezama et al. 2013; Engelman and MacDermott 2004; Hochman et al. 2010; Rudomin and Schmidt 1999; Willis 1999). Based on computer simulation studies, this shunting can theoretically only be effective if it is placed close to the terminal, somewhere between the last node that generates a sodium spike and the terminal (Cattaert and El Manira 1999; Graham and Redman 1994; Walmsley et al. 1995). If this shunting instead originates at nodes further away it can mostly just reduce the height of the spike from those distal nodes and not influence the downstream spikes that are regeneratively fully reactivated by successive nodes, unless of course the shunt causes outright failure of the node it is nearby, an unlikely event (Graham and Redman 1994 and Walmsley et al. 1995) without a lot of applied GABA (Verdier et al. 2003).
Na+ channels are located at branch points and not terminals, like GABA receptors.
We found that as expected, proprioceptive Ia afferents (and cutaneous afferents) are covered with dense bands of sodium channels (nodes), spaced at ~50-µm intervals and similar to the intermodal spacing reported in cat (Graham and Redman 1994). Importantly, we also found that the last sodium channel node before the terminals is located at the last major secondary branch point, and that terminal branches themselves have few Na+ channels, unlike in small diameter afferents (Black et al. 2012). Thus, the final action potential effect on the terminal boutons must be by passive current flow (over ~100 µm, similar to the likely space constant of these axons), allowing the spike to be readily attenuated by any shunting action of terminal GABA, like in crayfish (Cattaert and El Manira 1999). This makes it even more puzzling why the GABA receptors are not located on the passive terminals, and are instead at nodes, where their GABAergic currents are readily swamped by much larger Na+ currents.
Spike facilitation by α5GABAA mediated tonic PAD.
A clue to the function of α5GABAA receptors comes from their preferential dense clustering on large axon segments near branch points and nodes (on Ia afferents), mirroring the distribution of Na+ channels. We suggest that these GABAA receptors and associated tonic PAD assist with spike propagation and, in particular, help antidromic spike propagation. Branch points of axons are theoretically where propagating action potentials most likely fail, and more specifically, failure is likely if the parent branch diameter (d0) is smaller than the geometric sum of the downstream daughter branches (d1 and d2; d03/2 < d13/2 + d23/2; termed the 3/2 power law) making it difficult for the incoming sodium current to depolarize the daughter branches sufficiently (Goldstein and Rall 1974; Henneman et al. 1984; Verdier et al. 2003; Walmsley et al. 1995). Failure in large afferents occurs sometimes with normal orthodromic spike propagation on very long axons (Henneman et al. 1984; Wall and McMahon 1994) but is especially likely with antidromic spike propagation because the spike is propagating from small collaterals to larger axon segments, breaking the 3/2 power law. Consistent with this, we find that while dorsal root-evoked phasic PAD reliably initiates spikes in Ia proprioceptive afferents (on the rising phase of PAD), these do not reliably propagate antidromically to the dorsal columns (of course, the ones that do propagate contribute to the DRR; the remaining spikes only produce a failure potential; Fig. 9A). Likely, these spikes are initiated deep in the spinal cord (where PAD is maximal; Fig. 5) but fail to propagate through the many branch points as they travel antidromically to the dorsal columns, leaving only a small failed spike visible (failure potential in Fig. 9A). We demonstrate that this failure is depolarization dependent. In particular, increasing tonic PAD (with C fiber stimulation) facilitates these antidromic spikes, and blocking tonic PAD does the opposite, consistent with previous findings that the summated phasic PAD from two dorsal root stimuli also increases antidromic spikes (Eccles et al. 1961b; Loeza-Alcocer et al. 2013). Thus, tonic PAD and associated α5GABAA receptors generally facilitate spike transmission, likely by simply bringing failed spikes closer to the sodium threshold at locations where branch point failures occur. Conceptually, if an antidromic spike from just one collateral of a proprioceptive afferent reaches the dorsal columns, it is very likely to propagate both antidromically and orthodromically up and down the dorsal columns, causing additional orthodromic activation of other collaterals of the same axon, which further excites the afferents and the motoneurons (Duchen 1986). Thus, tonic PAD not only facilitates antidromic spikes but likely also increases overall motoneuron activity.
Whether or not nodal and branch point α5GABAA receptors more generally help normal orthodromic propagation of spikes arising from the periphery remains an open question, although this seems plausible because these spikes sometimes fail to propagate over the entire afferent (Henneman et al. 1984; Wall and McMahon 1994) and we have observed some unusual branching patterns likely to generate spike failure (ventral columns; Fig. 2). It remains to be reconciled why Wall and others (Verdier et al. 2003; Wall 1998) found that GABA can contribute to branch points failure (antidromic and orthodromic), whereas we suggest that GABA can prevent branch point failure of spike propagation. However, these two GABA functions may not be mutually exclusive, and rather just depend on the amount of GABA and how secure the spikes are. Very high levels of GABA (like Verdier et al. 2003 puffed on the cord; or in anesthetized animals studied by Wall, 1998; Eccles et al. 1963) can theoretically block secure orthodromic spike transmission through a branch point (Graham and Redman 1994). In contrast, lower more physiological GABA levels cannot influence spike transmission (Walmsley et al. 1995) but may well help initiate a spike that otherwise fails at branch points (insecure spikes). Further investigation is needed to determine more clearly when branch point failure occurs (Henneman et al. 1984 indicates it is common, rather than rare) and whether GABA and tonic PAD can help prevent this failure.
Origins of GABA that mediates tonic PAD.
We found that all sizes of sensory afferents are capable of activating tonic PAD, but activation from repeated low- and high-threshold cutaneous stimulation (especially C fibers) is the most effective method of producing a very large and long-lasting tonic PAD. Thus, possibly the same dorsally located neurons that Jankowska (Jankowska et al. 1981) previously described to mediate cutaneous evoked PAD contribute to tonic PAD. Based on its long-time scale of action, this tonic PAD is likely mediated by extrasynaptic spillover of GABA that accumulates with repeated intense stimulation. Indeed, even when all central spike transmission is blocked (with a low-dose of TTX), the TTX-resistant C fibers are still able to evoke a slow tonic PAD (Fig. 11). These results demonstrate that glutamate and GABA spillover from nonspiking microcircuits in the superficial dorsal horn must at least, in part, contribute to PAD in adult rats, as Russo (Russo et al. 2000) discuss extensively for the turtle spinal cord, and also as in microcircuits of crayfish (Watson et al. 2005).
Spillover of GABA and microcircuits driven PAD by no means indicates that the regulation of GABA and PAD is nonspecific. Instead, it is likely that circuits controlling dorsal GABA and PAD are closely related to the fine-tuned circuits that gate pain transmission (Bardoni et al. 2013; Braz et al. 2014; Lidierth and Wall 1998; Melzack and Wall 1965; Price et al. 2005; Todd 2015; Wall 1998). Especially interesting are circuits involved in central sensitization of pain following nociceptive stimulation, which increase in activity and connectivity following C fiber stimulation (Contreras-Hernández et al. 2018), like tonic PAD. Likely, other forms of neuronal activity, such as during locomotion (Rossignol et al. 1998), may cause similar spillover and similar buildup of tonic PAD, although this remains to be shown. While we find that tonic PAD is reduced by blocking neuronal activity with TTX, there remains a portion that is further blocked by GABA receptor antagonists after high-dose TTX application, suggesting that there is a tonic GABA receptor tone, although we do not know its origin (neuron, astrocytic or constitutive). Regardless of the details, these results indicate that PAD in proprioceptive, and cutaneous afferents is, in part, generated in the dorsal horn by local circuits far from the ventral terminals.
C fiber activation of tonic PAD.
C fibers are unusual sensory afferents because they not only have sensory receptors in the periphery, but are also covered with sensory TRP channels both centrally and peripherally, allowing them to respond to central and peripheral changes in many modalities, including temperature, pH, oxygen, and mechanical stress, depending on the channel present (TRPV1, TRPM8, TRPA1) (Szallasi and Sheta 2012; Takahashi et al. 2011; Takashima et al. 2007). This leads to a steady low-level barrage of C fiber activity, which can increase even further with injury (Hulse et al. 2010). Thus, it is reasonable to suppose that part of the spontaneous tonic PAD we observe (L655708-sensitive potential) results from spontaneous C fiber activity or even spontaneous transmitter release from C fibers, given the strong effect of C fiber stimulation on evoking tonic PAD. Interestingly, serotonin inhibits C fiber activity (Amrutkar et al. 2012; Zhao et al. 2016) and thus may modulate reflexes by reducing tonic PAD and afferent transmission on large afferents.
Summary and clinical implications.
For many years, we have known that rapidly released synaptic GABA and its associated fast phasic PAD plays a major role in modulating sensory transmission to motoneurons on a rapid time scale correlated with the early part of presynaptic inhibition (Barron and Matthews 1938; Curtis 1998). The present study demonstrates that a slower and much more persistent α5GABAA receptor-mediated PAD is present spontaneously, builds up over many seconds to minutes with sensory stimulation, and results from extrasynaptic GABA spillover. This causes a tonic PAD that modulates afferent transmission quite differently than expected. The α5GABAA receptor activity is in large part dorsally located on proprioceptive Ia afferents where it cannot easily influence ventral terminal presynaptic inhibition, and instead helps facilitate the propagation of afferent spikes, in particular, facilitating spikes evoked by rapid synaptically evoked phasic PAD. Since PAD-evoked afferent spikes (DRR) contribute to motoneuron reflexes (Duchen 1986), peripheral receptor sensitization and allodynia after nerve injury (Willis 1999), inhibiting α5GABAA receptors and modulating tonic PAD might prove to have clinical utility. This approach has particular appeal when treating motoneuron-related weakness with spinal cord or nerve injury, because α5GABAA receptors are expressed strongly on motoneurons (Fig. 2) (Canto-Bustos et al. 2017) and blocking them can increase motoneuron excitability (Canto-Bustos et al. 2017; Delgado-Lezama et al. 2013). More generally, considering that α5GABAA receptors help with spike initiation, they may well assist in normal orthodromic sensory transmission to motoneurons, facilitating the monosynaptic reflex. While this remains to be verified, it is intriguing that afferent spike propagation to motoneurons has been suggested to routinely fail at branch points (Henneman et al. 1984), and thus tonic PAD and α5GABAA receptors might help prevent this failure, ultimately increasing sensory transmission to motoneurons, in contrast to presynaptic inhibition.
GRANTS
Research was supported by the Canadian Institutes of Health Research (MOP 14697) and the National Institutes of Health (NIH, R01NS47567).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.M.L.-O., Y.L., S.L., S.B., R.S., K.F., K.K.F., and D.J.B. performed experiments; A.M.L.-O., Y.L., S.L., R.S., K.K.F., and D.J.B. analyzed data; A.M.L.-O., Y.L., S.L., S.B., K.K.F., and D.J.B. interpreted results of experiments; A.M.L.-O., Y.L., S.L., S.B., and D.J.B. prepared figures; A.M.L.-O., Y.L., K.K.F., and D.J.B. edited and revised manuscript; A.M.L.-O., Y.L., S.L., S.B., K.F., K.K.F., and D.J.B. approved final version of manuscript; D.J.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Leo Sanelli for technical assistance and Tia Bennett and Monica Gorassini for editing the document.
REFERENCES
- Alvarez FJ. Anatomical basis for presynaptic inhibition of primary sensory fibers. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM,. New York: Oxford University Press, 1998, p. 13–41. [Google Scholar]
- Alvarez FJ, Taylor-Blake B, Fyffe RE, De Blas AL, Light AR. Distribution of immunoreactivity for the β2 and β3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp Neurol 365: 392–412, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Amrutkar DV, Ploug KB, Hay-Schmidt A, Porreca F, Olesen J, Jansen-Olesen I. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 153: 830–838, 2012. doi: 10.1016/j.pain.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Slow potential waves produced in the cuneate nucleus by cutaneous volleys and by cortical stimulation. J Neurophysiol 27: 78–91, 1964. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron 48: 109–121, 2005. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Takazawa T, Tong CK, Choudhury P, Scherrer G, Macdermott AB. Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn. Ann N Y Acad Sci 1279: 90–96, 2013. doi: 10.1111/nyas.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DH, Matthews BH. The interpretation of potential changes in the spinal cord. J Physiol 92: 276–321, 1938. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Wright CV, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 139: 161–174, 2009. doi: 10.1016/j.cell.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Frézel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 8: 82, 2012. doi: 10.1186/1744-8069-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Mohler H, Fritschy JM. Inhibitory neurotransmission in rat spinal cord: co-localization of glycine- and GABAA-receptors at GABAergic synaptic contacts demonstrated by triple immunofluorescence staining. Brain Res 642: 59–69, 1994. doi: 10.1016/0006-8993(94)90905-9. [DOI] [PubMed] [Google Scholar]
- Bos R, Brocard F, Vinay L. Primary afferent terminals acting as excitatory interneurons contribute to spontaneous motor activities in the immature spinal cord. J Neurosci 31: 10184–10188, 2011. doi: 10.1523/JNEUROSCI.0068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82: 522–536, 2014. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol 372: 465–485, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Bush MS, Gordon-Weeks PR. Distribution and expression of developmentally regulated phosphorylation epitopes on MAP 1B and neurofilament proteins in the developing rat spinal cord. J Neurocytol 23: 682–698, 1994. doi: 10.1007/BF01181643. [DOI] [PubMed] [Google Scholar]
- Canto-Bustos M, Loeza-Alcocer E, Cuellar CA, Osuna P, Elias-Viñas D, Granados-Soto V, Manjarrez E, Felix R, Delgado-Lezama R. Tonically active α5GABAA receptors reduce motoneuron excitability and decrease the monosynaptic reflex. Front Cell Neurosci 11: 283, 2017. doi: 10.3389/fncel.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662–3667, 2004. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaert D, El Manira A. Shunting versus inactivation: analysis of presynaptic inhibitory mechanisms in primary afferents of the crayfish. J Neurosci 19: 6079–6089, 1999. doi: 10.1523/JNEUROSCI.19-14-06079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaert D, el Manira A, Clarac F. Direct evidence for presynaptic inhibitory mechanisms in crayfish sensory afferents. J Neurophysiol 67: 610–624, 1992. doi: 10.1152/jn.1992.67.3.610. [DOI] [PubMed] [Google Scholar]
- Contreras-Hernández E, Chávez D, Hernández E, Velázquez E, Reyes P, Béjar J, Martín M, Cortés U, Glusman S, Rudomin P. Supraspinal modulation of neuronal synchronization by nociceptive stimulation induces an enduring reorganization of dorsal horn neuronal connectivity. J Physiol 596: 1747–1776, 2018. doi: 10.1113/JP275228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR. Two types of inhibition in the spinal cord. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM. New York: Oxford University Press, 1998, p. 150–161. [Google Scholar]
- Curtis DR, Lacey G. GABA-B receptor-mediated spinal inhibition. Neuroreport 5: 540–542, 1994. doi: 10.1097/00001756-199401000-00002. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Lodge D, Brand SJ. GABA and spinal afferent terminal excitability in the cat. Brain Res 130: 360–363, 1977. doi: 10.1016/0006-8993(77)90283-9. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Loeza-Alcocer E, Andrés C, Aguilar J, Guertin PA, Felix R. Extrasynaptic GABAA receptors in the brainstem and spinal cord: structure and function. Curr Pharm Des 19: 4485–4497, 2013. doi: 10.2174/1381612811319240013. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Rossignol S. The effects of 4-aminopyridine on the cat spinal cord: rhythmic antidromic discharges recorded from the dorsal roots. Brain Res 491: 335–348, 1989. doi: 10.1016/0006-8993(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Excitation of mouse motoneurones by GABA-mediated primary afferent depolarization. Brain Res 379: 182–187, 1986. doi: 10.1016/0006-8993(86)90274-X. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol 159: 147–166, 1961a. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Kostyuk PG, Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol 161: 237–257, 1962a. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Kozak W, Magni F. Dorsal root reflexes of muscle group I afferent fibres. J Physiol 159: 128–146, 1961b. doi: 10.1113/jphysiol.1961.sp006797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Krnjevic K. Potential changes recorded inside primary afferent fibres within the spinal cord. J Physiol 149: 250–273, 1959. doi: 10.1113/jphysiol.1959.sp006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol 160: 62–93, 1962b. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological studies on presynaptic inhibition. J Physiol 168: 500–530, 1963. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguibar JR, Quevedo J, Rudomin P. Selective cortical and segmental control of primary afferent depolarization of single muscle afferents in the cat spinal cord. Exp Brain Res 113: 411–430, 1997. doi: 10.1007/PL00005595. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci 5: 135–145, 2004. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Enríquez M, Jiménez I, Rudomin P. Segmental and supraspinal control of synaptic effectiveness of functionally identified muscle afferents in the cat. Exp Brain Res 107: 391–404, 1996. doi: 10.1007/BF00230421. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fenrich KK, Zhao EY, Wei Y, Garg A, Rose PK. Isolating specific cell and tissue compartments from 3D images for quantitative regional distribution analysis using novel computer algorithms. J Neurosci Methods 226: 42–56, 2014. doi: 10.1016/j.jneumeth.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Fortes MGF. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Fed Proc 16: 39–40, 1957. [Google Scholar]
- Fyffe RE, Cheema SS, Rustioni A. Intracellular staining study of the feline cuneate nucleus. I. Terminal patterns of primary afferent fibers. J Neurophysiol 56: 1268–1283, 1986. doi: 10.1152/jn.1986.56.5.1268. [DOI] [PubMed] [Google Scholar]
- Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol 275: 263–282, 1978. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SS, Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J 14: 731–757, 1974. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B, Redman S. A simulation of action potentials in synaptic boutons during presynaptic inhibition. J Neurophysiol 71: 538–549, 1994. doi: 10.1152/jn.1994.71.2.538. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Lüscher HR, Mathis J. Simultaneously active and inactive synapses of single Ia fibres on cat spinal motoneurones. J Physiol 352: 147–161, 1984. doi: 10.1113/jphysiol.1984.sp015283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Shreckengost J, Kimura H, Quevedo J. Presynaptic inhibition of primary afferents by depolarization: observations supporting nontraditional mechanisms. Ann N Y Acad Sci 1198: 140–152, 2010. doi: 10.1111/j.1749-6632.2010.05436.x. [DOI] [PubMed] [Google Scholar]
- Howland B, Lettvin JY, McCULLOCH WS, Pitts W, Wall PD. Reflex inhibition by dorsal root interaction. J Neurophysiol 18: 1–17, 1955. doi: 10.1152/jn.1955.18.1.1. [DOI] [PubMed] [Google Scholar]
- Hubbard JI, Llinas R, Quastel DMJ. Electrophysiological Analysis of Synaptic Transmission. London: Arnold, 1969. [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabó G, Erdélyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci USA 102: 9038–9043, 2005. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Graham BA. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol 590: 3927–3951, 2012. doi: 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse R, Wynick D, Donaldson LF. Intact cutaneous C fibre afferent properties in mechanical and cold neuropathic allodynia. Eur J Pain 14: 565.e1–565.e10, 2010. doi: 10.1016/j.ejpain.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Jeong HJ, Katsurabayashi S, Akaike N. Functional roles of presynaptic GABAA receptors on glycinergic nerve terminals in the rat spinal cord. J Physiol 541: 423–434, 2002. doi: 10.1113/jphysiol.2001.016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, McCrea D, Rudomín P, Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol 46: 506–516, 1981. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- Lamotte d’Incamps B, Destombes J, Thiesson D, Hellio R, Lasserre X, Kouchtir-Devanne N, Jami L, Zytnicki D. Indications for GABA-immunoreactive axo-axonic contacts on the intraspinal arborization of a Ib fiber in cat: a confocal microscope study. J Neurosci 18: 10030–10036, 1998. doi: 10.1523/JNEUROSCI.18-23-10030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A, Meyers DE, Burke RE. Activation of type B gamma-aminobutyric acid receptors in the intact mammalian spinal cord mimics the effects of reduced presynaptic Ca2+ influx. Proc Natl Acad Sci USA 85: 5330–5334, 1988. doi: 10.1073/pnas.85.14.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91: 2236–2246, 2004. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Lidierth M, Wall PD. Dorsal horn cells connected to the lissauer tract and their relation to the dorsal root potential in the rat. J Neurophysiol 80: 667–679, 1998. doi: 10.1152/jn.1998.80.2.667. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC, McIntyre AK. On the origins of dorsal root potentials. J Gen Physiol 32: 409–443, 1949. doi: 10.1085/jgp.32.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeza-Alcocer E, Canto-Bustos M, Aguilar J, González-Ramírez R, Felix R, Delgado-Lezama R. α5GABAA receptors mediate primary afferent fiber tonic excitability in the turtle spinal cord. J Neurophysiol 110: 2175–2184, 2013. doi: 10.1152/jn.00330.2013. [DOI] [PubMed] [Google Scholar]
- Lomelí J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature 395: 600–604, 1998. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, De Koninck Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABAAA receptors. J Neurosci 34: 8300–8317, 2014. doi: 10.1523/JNEUROSCI.0159-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher HR, Ruenzel P, Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol 49: 269–289, 1983. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Stephens MJ, Rank M, D’Amico J, Harvey PJ, Li X, Harris RL, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiol 106: 925–943, 2011. doi: 10.1152/jn.01011.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. A serial section electron microscope study of an identified Ia afferent collateral in the cat spinal cord. J Comp Neurol 314: 257–277, 1991. doi: 10.1002/cne.903140205. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56: 141–148, 2009. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J, Zeilhofer HU, Fritschy JM. Selective distribution of GABAA receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J Comp Neurol 520: 3895–3911, 2012. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- Perez-Sanchez J, Lorenzo LE, Lecker I, Zurek AA, Labrakakis C, Bridgwater EM, Orser BA, De Koninck Y, Bonin RP. α5GABAA Receptors mediate tonic inhibition in the spinal cord dorsal horn and contribute to the resolution of hyperalgesia. J Neurosci Res 95: 1307–1318, 2017. doi: 10.1002/jnr.23981. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Mendell LM. Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci 13: 4748–4763, 1993. doi: 10.1523/JNEUROSCI.13-11-04748.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Synaptic excitation of alpha-motoneurons by dorsal root afferents in the neonatal rat spinal cord. J Neurophysiol 70: 406–417, 1993. doi: 10.1152/jn.1993.70.1.406. [DOI] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem 5: 547–555, 2005. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman SJ. The relative contributions of GABAA and GABAB receptors to presynaptic inhibition of group Ia EPSPs. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM. New York: Oxford University Press, 1998, p. 162–177. [Google Scholar]
- Rossignol S, Beloozerova I, Gossard JP, Dubuc R. Presynaptc mechanisms during locomotion. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM. New York: Oxford University Press, 1998, p. 385–397. [Google Scholar]
- Rudomin P. Presynaptic selection of afferent inflow in the spinal cord. J Physiol Paris 93: 329–347, 1999. doi: 10.1016/S0928-4257(00)80061-3. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Lomelí J, Quevedo J. Differential modulation of primary afferent depolarization of segmental and ascending intraspinal collaterals of single muscle afferents in the cat spinal cord. Exp Brain Res 156: 377–391, 2004a. doi: 10.1007/s00221-003-1788-7. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Lomelí J, Quevedo J. Tonic differential supraspinal modulation of PAD and PAH of segmental and ascending intraspinal collaterals of single group I muscle afferents in the cat spinal cord. Exp Brain Res 159: 239–250, 2004b. doi: 10.1007/s00221-004-1953-7. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Russo RE, Delgado-Lezama R, Hounsgaard J. Dorsal root potential produced by a TTX-insensitive micro-circuitry in the turtle spinal cord. J Physiol 528: 115–122, 2000. doi: 10.1111/j.1469-7793.2000.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Schiebel AB. Terminal patterns in cat spinal cord III. Primary afferent collaterals. Brain Res 13: 417–443, 1969. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Smith CC, Paton JFR, Chakrabarty S, Ichiyama RM. Descending systems direct development of key spinal motor circuits. J Neurosci 37: 6372–6387, 2017. doi: 10.1523/JNEUROSCI.0149-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street LJ, Sternfeld F, Jelley RA, Reeve AJ, Carling RW, Moore KW, McKernan RM, Sohal B, Cook S, Pike A, Dawson GR, Bromidge FA, Wafford KA, Seabrook GR, Thompson SA, Marshall G, Pillai GV, Castro JL, Atack JR, MacLeod AM. Synthesis and biological evaluation of 3-heterocyclyl-7,8,9,10-tetrahydro-(7,10-ethano)-1,2,4-triazolo[3,4-a]phthalazines and analogues as subtype-selective inverse agonists for the GABAAα5 benzodiazepine binding site. J Med Chem 47: 3642–3657, 2004. doi: 10.1021/jm0407613. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J Physiol 447: 675–692, 1992. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci 20: 7531–7538, 2000. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Sheta M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expert Opin Investig Drugs 21: 1351–1369, 2012. doi: 10.1517/13543784.2012.704021. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 7: 701–711, 2011. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157, 2007. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ. Plasticity of inhibition in the spinal cord. Handb Exp Pharmacol 227: 171–190, 2015. doi: 10.1007/978-3-662-46450-2_9. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature 411: 587–590, 2001. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci 23: 2002–2007, 2003. doi: 10.1523/JNEUROSCI.23-06-02002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier D, Lund JP, Kolta A. Synaptic inputs to trigeminal primary afferent neurons cause firing and modulate intrinsic oscillatory activity. J Neurophysiol 92: 2444–2455, 2004. doi: 10.1152/jn.00279.2004. [DOI] [PubMed] [Google Scholar]
- Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T, Cope TC. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol 118: 2687–2701, 2017. doi: 10.1152/jn.00497.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol 142: 1–21, 1958. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD. Some unanswered questions about the mechanims and function of presynaptic inhibition. In: Presynaptic Inhibition and Neuron Control, edited by Rudmon P, Romo R, Mendell LM. New York: Oxford University Press, 1998, p. 228–241. [Google Scholar]
- Wall PD, McMahon SB. Long range afferents in rat spinal cord. III. Failure of impulse transmission in axons and relief of the failure after rhizotomy of dorsal roots. Philos Trans R Soc Lond B Biol Sci 343: 211–223, 1994. doi: 10.1098/rstb.1994.0022. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Graham B, Nicol MJ. Serial E-M and simulation study of presynaptic inhibition along a group Ia collateral in the spinal cord. J Neurophysiol 74: 616–623, 1995. doi: 10.1152/jn.1995.74.2.616. [DOI] [PubMed] [Google Scholar]
- Watson A, Le Bon-Jego M, Cattaert D. Central inhibitory microcircuits controlling spike propagation into sensory terminals. J Comp Neurol 484: 234–248, 2005. doi: 10.1002/cne.20474. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124: 395–421, 1999. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Qin W, Cao J, Zhang Y, Ni J, Sun Y, Jiang X, Tao J. Serotonin type-1D receptor stimulation of A-type K+ channel decreases membrane excitability through the protein kinase A- and B-Raf-dependent p38 MAPK pathways in mouse trigeminal ganglion neurons. Cell Signal 28: 979–988, 2016. doi: 10.1016/j.cellsig.2016.05.004. [DOI] [PubMed] [Google Scholar]