Abstract
Parvalbumin-expressing (PV) GABAergic interneurons regulate local circuit dynamics. In terms of the excitation driving PV interneuron activity, the N-methyl-d-aspartate receptor (NMDAR)-mediated component onto PV interneurons tends to be smaller than that onto pyramidal neurons but makes a significant contribution to their physiology and development. In the visual cortex, PV interneurons mature during the critical period. We hypothesize that during the critical period, the NMDAR-mediated signaling and functional properties of glutamatergic synapses onto PV interneurons are developmentally regulated. We therefore compared the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)- and NMDAR-mediated synaptic responses before (postnatal days 15–20, P15–P20), during (P25–P40), and after (P50–P60) the visual critical period. AMPAR miniature excitatory postsynaptic currents (mEPSCs) showed a developmental decrease in frequency, whereas NMDAR mEPSCs were absent or showed extremely low frequencies throughout development. For evoked responses, we consistently saw a NMDAR-mediated component, suggesting pre- or postsynaptic differences between evoked and spontaneous neurotransmission. Evoked responses showed input-specific developmental changes. For intralaminar inputs, the NMDAR-mediated component significantly decreased with development. This resulted in adult intralaminar inputs almost exclusively mediated by AMPARs, suited for the computation of synaptic inputs with precise timing, and likely having NMDAR-independent forms of plasticity. In contrast, interlaminar inputs maintained a stable NMDAR-mediated component throughout development but had a shift in the AMPAR paired-pulse ratio from depression to facilitation. Adult interlaminar inputs with facilitating AMPAR responses and a substantial NMDAR component would favor temporal integration of synaptic responses and could be modulated by NMDAR-dependent forms of plasticity.
NEW & NOTEWORTHY We show for the first time input-specific developmental changes in the N-methyl-d-aspartate receptor component and short-term plasticity of the excitatory drive onto layers 2/3 parvalbumin-expressing (PV) interneurons in the visual cortex during the critical period. These developmental changes would lead to functionally distinct adult intralaminar and interlaminar glutamatergic inputs that would engage PV interneuron-mediated inhibition differently.
Keywords: AMPA receptors, NMDA receptors, PV interneurons
INTRODUCTION
Inhibitory GABAergic interneurons shape the activity of cortical microcircuits. The most common subtype of GABAergic interneurons express the Ca2+-binding protein parvalbumin (PV) (Gonchar et al. 2008; Markram et al. 2004). PV interneurons target the soma and axon initial segment of pyramidal neurons, strongly influencing pyramidal neuron activity and, accordingly, local network dynamics (Fino et al. 2013; Hu et al. 2014; Sohal et al. 2009). Dysfunction of PV interneurons contributes to disease states such as epilepsy and schizophrenia (Cammarota et al. 2013; Gonzalez-Burgos and Lewis 2012).
Excitatory inputs, mediated by glutamate, drive the activity of PV interneurons. Glutamatergic synapses onto PV interneurons often differ from those onto pyramidal neurons, having a small or undetectable N-methyl-d-aspartate (NMDA) receptor (NMDAR) component and a significant Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) component (Angulo et al. 1999; Geiger et al. 1997; Lalanne et al. 2016; Matta et al. 2013; Wang and Gao 2010). Although the NMDAR component onto PV interneurons can be small, its ablation from PV interneurons early in development but not in adulthood disrupts circuit oscillations and leads to behavioral deficits (Belforte et al. 2010; Korotkova et al. 2010). In PV interneurons, NMDARs can mediate Hebbian forms of plasticity in the hippocampus (Le Roux et al. 2013) and play a role in tonic (Povysheva and Johnson 2012) and presynaptic (Pafundo et al. 2018) modulation in the prefrontal cortex. Hence, NMDAR-mediated signaling onto PV interneurons plays important physiological roles and may be developmentally regulated.
During the critical period in primary visual cortex (V1), sensory experience drives the functional maturation of PV interneurons, including their intrinsic membrane properties (Lazarus and Huang 2011) and their innervation onto pyramidal neurons (Chattopadhyaya et al. 2004). Perineuronal nets are structural regulators of glutamatergic synapses onto PV interneurons that also develop during the critical period (Chang et al. 2010; Maya-Vetencourt and Pizzorusso 2013). Furthermore, glutamatergic synapses onto PV interneurons during the critical period show changes in presynaptic release probability (Miao et al. 2016), number of synapses (Miyamae et al. 2017), and Ca2+ permeability of AMPARs (Lu et al. 2014). The contribution of NMDARs to glutamatergic transmission onto PV interneurons during the critical period is unknown.
In hippocampal PV interneurons, the ratio of NMDAR to AMPAR varies in an input-specific manner, affecting synaptic integration and plasticity (Le Roux et al. 2013; Lei and McBain 2002; Sambandan et al. 2010). Cortical layer 2/3 PV interneurons receive their glutamatergic inputs primarily from intralaminar layer 2/3 and interlaminar layer 4/5 pyramidal neurons (Helmstaedter et al. 2008; Jiang et al. 2015; Reyes et al. 1998; Xu and Callaway 2009). Intralaminar inputs provide the local excitatory drive involved in PV interneuron-mediated feedback inhibition, whereas interlaminar inputs convey columnar and sensory information engaging PV interneurons in feedforward inhibition (Tremblay et al. 2016).
In this study, we hypothesized that the excitatory drive and the role of NMDARs in PV interneurons in the visual cortex mature during the visual critical period and that such changes could be input specific. To test this hypothesis, we studied AMPAR- and NMDAR-mediated synaptic responses onto PV interneurons in layers 2/3 of V1 during three developmental windows spanning the visual critical period in mice. We observed input-specific changes: with development, layer 2/3 intralaminar inputs show a progressive reduction in the relative NMDAR component, whereas for interlaminar inputs, presumably mainly from layer 5, the relative NMDAR component remains constant. On the other hand, only interlaminar inputs show developmental changes in short-term dynamics, transitioning from depression in juvenile stage to facilitation in adult stage. Thus adult intralaminar and interlaminar inputs would engage PV interneurons differently: intralaminar inputs dominated by the AMPAR component would have a short synaptic integration window and most likely NMDAR-independent forms of plasticity, whereas interlaminar inputs would be suited for temporal integration and a more prominent NMDAR component could serve as a substrate for plasticity.
MATERIALS AND METHODS
Animals
To identify and target PV interneurons, we crossed the Pv-Cre line (Hippenmeyer et al. 2005; Kuhlman and Huang 2008) with the LSL-tdTomato line [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J from Jackson Laboratories, donator: Allen Brain Institute]. The resulting offspring express the fluorescent protein tdTomato dependent on Cre-mediated recombination, allowing visualization of PV interneurons (Akgul and Wollmuth 2013). All animal procedures were approved by the institutional animal care and usage committee at Stony Brook University and were in concordance with the guidelines established by the National Institutes of Health.
Electrophysiological Recording
Solutions, slicing, and recording conditions of PV interneurons in layers 2/3 of primary visual cortex were largely carried out as previously described (Akgul and Wollmuth 2013). Briefly, the recording artificial cerebral spinal fluid (ACSF) consisted of (in mM) 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, and 1.2 MgCl2 and was saturated with 95% O2-5% CO2. The internal solution used for voltage-clamp experiments contained (in mM) 100 Cs-gluconate, 15 CsCl, 10 HEPES, 20 phosphocreatine, 4 Mg-ATP, 0.3 Na-GTP, 3 QX-314, and 10 BAPTA, pH 7.3 (CsOH). The junction potential for the internal solution relative to the external solution, measured in an initial set of experiments, was −15 mV and was compensated during data acquisition. For current-clamp experiments of presynaptic pyramidal neurons in paired recordings, we used an internal solution that contained (in mM) 135 K-gluconate, 4.3 KCl, 2 NaCl, 10 HEPES, 20 phosphocreatine, 4 Mg-ATP, and 0.3 Na-GTP, pH 7.3 (KOH). For both internal solutions, the osmolarity was adjusted to 300 mosM with sucrose. Biocytin (0.2%) was included in the internal solution for postfixation neuronal labeling of the interneurons.
Acute slice preparation.
Mice between postnatal day 15 (P15) and day 20 (P20) were anesthetized with isoflurane, decapitated, and sliced in the recording ACSF. Mice between P25 and P60 were anesthetized with ketamine (0.12 ml/100 g) and xylazine (0.05 ml/100 g) and then transcardially perfused, and the brains were sliced in ice-cold cutting ACSF that contained (in mM) 230 sucrose, 2.5 KCl, 10 glucose, 25 NaHCO3, 1.25 NaH2PO4, 0.5 CaCl2, 10 MgSO4, and 1.5 pyruvate, saturated with 95% O2-5% CO2.
Coronal slices were collected from each blocked hemisphere using a vibratome (Leica). Slice collection began 0.5–1 mm rostral to the caudal cortical surface and totaled four 300-µm slices per hemisphere. Slices from P15–P20 animals were directly incubated into recording ACSF. Slices from older animals (P25–P60) were initially collected in a mixture of 50% cutting ACSF and 50% recording ACSF for 15 min to reduce excitotoxicity and improve slice health and were subsequently placed in regular recording ACSF. In both cases, slices were incubated for 1 h at room temperature before recordings using the recording ACSF.
Electrophysiology.
An EPC 10/2 USB amplifier with PatchMaster software (HEKA Elektronik, Lambrecht, Germany) was used to record membrane potentials or currents at 32–34°C. Recordings were sampled at 10 kHz and low-pass filtered using a four-pole Bessel filter at 5 kHz.
On achieving the whole cell configuration, we measured the resting membrane potential (Vm) in current clamp. The amplifier mode was changed to voltage clamp and the baseline holding potential set to –70 mV. Input resistance (Rin) and series resistance (Rs) were constantly monitored throughout the experiment with a 5-mV hyperpolarizing pulse. Recordings included in analysis showed <30% change in Rin or Rs during the course of the experiment.
Experimental Procedures
Miniature EPSCs.
AMPAR-mediated mEPSCs were recorded at −70 mV in the standard ACSF (containing Mg2+) plus the Nav blocker tetrodotoxin (TTX; 1 µM) to block action potentials and the GABAA antagonist picrotoxin (50 µM) to block inhibitory synaptic responses. After recording AMPAR-mediated mEPSCs, we added the AMPAR antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 µM) and the NMDAR co-agonist glycine (10 µM) to the bath and held the neurons at +40 mV to record NMDAR-mediated mEPSCs. For selected recordings, we applied the NMDAR antagonist dl-2-amino-5-phosphonovaleric acid (APV; 50 µM) to confirm that mEPSCs were mediated by NMDARs.
Intralaminar evoked EPSCs.
We used paired recordings (pyramidal to interneuron) to measure monosynaptic glutamatergic inputs between pyramidal neurons and PV interneurons in layers 2/3 (Watanabe et al. 2005). We initially patched a PV interneuron and searched around for synaptically connected pyramidal neurons, which were identified by their triangular soma and an apical dendrite parallel to the plane of the interneuron. To test for connectivity, an interneuron and a pyramidal neuron were recorded in current clamp. A train of three action potentials at 10 Hz were evoked in the presynaptic pyramidal neuron by injecting suprathreshold current steps and looked for EPSCs in the PV interneuron. When an excitatory monosynaptic input was established, the AMPAR-mediated EPSC was recorded at −70 mV in the presence of picrotoxin (50 µM). Subsequently, DNQX (20 µM) and glycine (10 µM) were added to the ACSF, and the NMDAR-mediated EPSC was recorded at +40 mV. APV was occasionally used to confirm that the evoked EPSCs recorded under these conditions were mediated by NMDARs. The connection probability was consistent with previous publications (Gu et al. 2013; Watanabe et al. 2005) and did not change among the age groups tested (juvenile = 66%, n = 53; critical period = 65%, n = 77; and adult = 68%, n = 71).
Interlaminar evoked EPSCs.
Interlaminar EPSCs were evoked using electrodes pulled from borosilicate theta glass (Warner Instruments), filled with ACSF, and connected to an Iso Flex stimulation unit (A.M.P.I.) via silver wires. The stimulation electrode was placed in layer 5, directly underneath the PV interneuron being patched in layers 2/3. The intensity of the stimulation and the location of the stimulation electrode were adjusted to generate monosynaptic responses with minimal amplitude and low failure rates, also characterized by a single peak with a constant latency (≤3 ms). After stable responses were obtained, AMPAR- and NMDAR-mediated EPSCs were recorded as described for the intralaminar inputs.
Data Analysis
mEPSC analysis.
mEPSCs were digitally refiltered at 2 kHz before being analyzed using the MiniAnalysis program (Synaptosoft) (Akgul and Wollmuth 2013; Helm et al. 2013). For each trace, we measured the root mean square (RMS) noise at a stable segment of the recording and set the threshold of detection to at least two times RMS. Recordings with baseline RMS noise >5 pA were not included in analysis. Segments with high levels of noise that obscured the baseline were omitted, and event detection resumed when the baseline leveled. After automated detection, high-resolution windows of the recordings were visually inspected to remove false events and to test for possible positive events.
Evoked responses.
Paired recordings and evoked responses were analyzed using custom-written programs in Igor Pro (WaveMetrics, Lake Oswego, OR). An average of 100 or 30 consecutive traces of repetitive stimulation, for paired recordings and interlaminar stimulation, respectively, were used as a template for the analysis. We defined a baseline right before the start of each action potential or stimulation artifact and measured current peak amplitudes in individual traces at the time of the peak in the averaged response. This approach increases signal-to-noise ratio and increases detectability of events, especially at +40 mV, where the noise levels and detection of slow NMDAR currents becomes a limiting factor. AMPAR- and NMDAR-mediated currents were fitted with a double exponential function, and the weighted tau (wτ) was estimated according to the formula wτ = τ1·f1 + τ2·f2. Paired-pulse ratio (PPR) was estimated as the amplitude ratio of the second EPSC divided by the first EPSC (P2/P1), which we report throughout the article. We also calculated the PPR from the third EPSC to the first EPSC (P3/P1), but it always had the same short-term dynamics as P2/P1 (data not shown).
Biocytin Staining
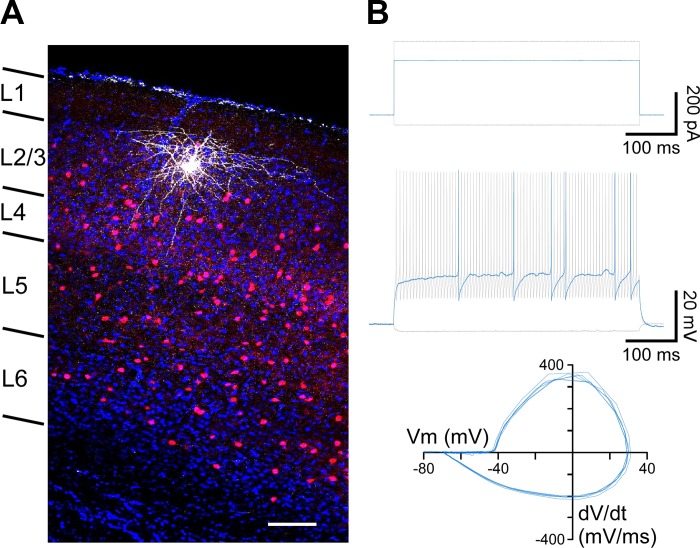
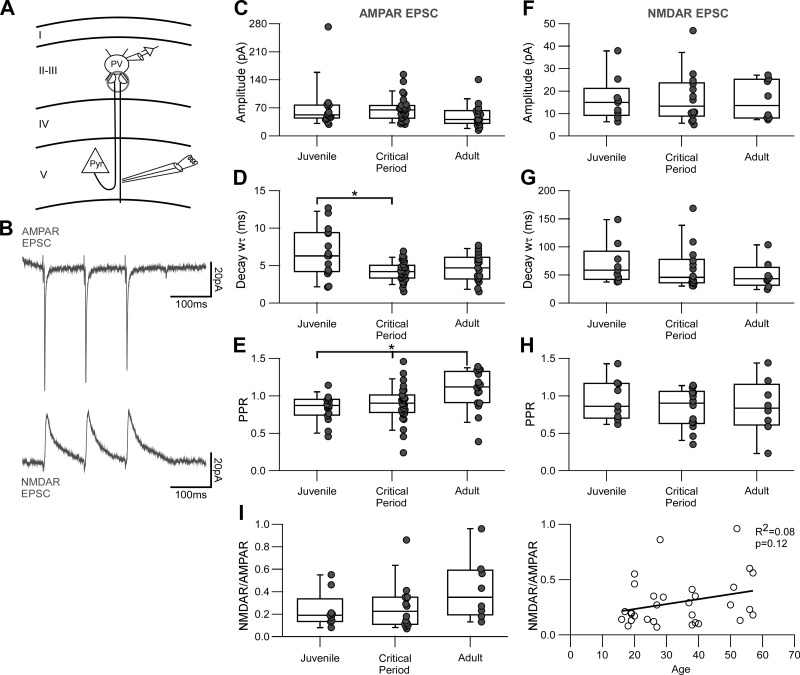
Post hoc labeling for a subset of recorded interneurons was achieved using biocytin staining. After recordings performed with an internal solution containing 0.2% biocytin, slices were fixed in 4% paraformaldehyde in PBS for up to a week and then permeabilized with 1% Triton-X in PBS for 1 h. Subsequently, slices were stained with DyLight649 streptavidin (1:2,000) in 0.1% Triton-X in PBS overnight at 4°C. Slices were washed three times for 5 min each in PBS and stained with DAPI before being mounted on a glass slide for microscopy. Labeled neurons were imaged using a confocal microscope and software (Olympus Fluoview). The location of recorded PV interneurons was confirmed to layers 2/3 of primary visual cortex, and their morphology corresponded to basket cells (Fig. 1A).
Fig. 1.
Identification of parvalbumin (PV)-expressing interneurons in primary visual cortex using PV-tdTomato mice. A: PV-expressing interneurons were identified by PV-tdTomato (red) expression. Confocal image (×40 magnification) of a recorded PV interneuron stained with biocytin (white), confirming post hoc its location in layers 2/3 of primary visual cortex. DAPI staining (blue) is shown for reference. Laminar distribution is indicated at left. Scale bar, 100 μm. B: the characteristic fast-spiking phenotype of PV interneurons was confirmed for a subset of recordings in current-clamp configuration. Top: applied current stimulation. The PV interneuron was held at Im = 0 pA, and successive pulses of current injection were applied from −50 pA (bottom gray trace) until rheobase (250 pA; blue trace) was reached and up to 350 pA (top gray trace). Middle: membrane voltage responses to the corresponding current injections in top panel (rheobase in blue). Bottom: phase plots of the action potentials evoked at rheobase. The action potentials displayed the characteristic fast kinetics and strong afterhyperpolarization of PV interneurons (Helm et al. 2013; Tricoire et al. 2011). Vm, membrane potential; dV/dt, change in voltage over time.
Statistical Analyses
Statistical analysis was done using Minitab software (Minitab, State College, PA). Data sets were tested for normality and homoscedasticity. In all our data sets, one of these tests was negative. We therefore conducted nonparametric tests and presented data as medians (interquartile range). We used the Kruskal-Wallis (KW) test at a significance level of 0.05, and multiple comparisons were done using the Mann-Whitney (MW) test with Bonferroni correction (for 3 groups, α = 0.016). Linear regressions were used to test for correlations between variables, and the R2 and P value for the linear model are reported. For mEPSCs, we also compared the distribution of events by randomly sampling 30 events from each cell and combined them for each subgroup. The Kolmogorov-Smirnov (KS) test (α = 0.01) was used to test for significance. For each set of experiments we report the number of cells and number of animals in the corresponding figure legends. We did not find animal-by-animal variation in the data.
RESULTS
In this study, we addressed how the NMDAR component and overall properties of glutamatergic inputs that drive PV interneuron activity in layers 2/3 are developmentally regulated during the visual critical period. We compared synaptic responses mediated by AMPARs and NMDARs in three developmental stages spanning the critical period: juvenile (J; P15–P20), critical period (CP; P25–P40), and adult (A; P50–P60).
Targeting PV Interneurons in Layers 2/3 of Primary Visual Cortex
We used PV-tdTomato mice to target fluorescently labeled PV interneurons (Akgul and Wollmuth 2013; Helm et al. 2013). Figure 1A shows a confocal image of a slice from this mouse line, showing the tdTomato signal and DAPI staining. In many instances, we confirmed the location of recorded PV interneurons post hoc to layers 2/3 of primary visual cortex by using biocytin (Fig. 1A). In most of our recordings, we used a Cs-based internal solution containing the Na+ channel blocker QX-314, precluding action potential generation. For an initial subset of recordings, we confirmed the characteristic fast-spiking phenotype of PV interneurons in current-clamp configuration (Fig. 1B) (Cauli et al. 1997; Goldberg et al. 2011; Tricoire et al. 2011). In Fig. 1B, blue traces show the corresponding action potentials (middle) and their phase plots (bottom) in response to rheobase stimulation (top). As illustrated by the phase plots, recorded PV interneurons had the characteristic fast action potentials with a strong afterhyperpolarization.
Developmental Changes of AMPAR- and NMDAR-Mediated mEPSCs During and After the Visual Critical Period
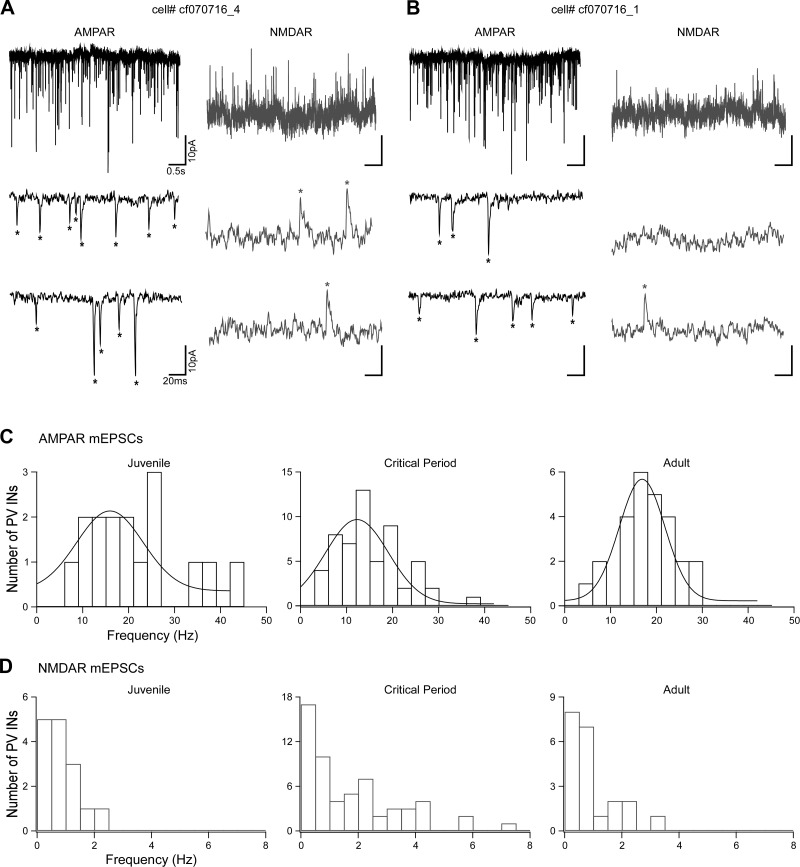
We initially analyzed global glutamatergic inputs onto PV interneurons by recording AMPAR- and NMDAR-mediated mEPSCs (Fig. 2). To isolate AMPAR-mediated mEPSCs, PV interneurons were held at −70 mV in the presence of picrotoxin, TTX, and extracellular Mg+2 (Fig. 2, A and B). Subsequently, to isolate NMDAR-mediated mEPSCs, we added DNQX to the bath and held the membrane potential at +40 mV (Fig. 2, A and B). The example recordings in Fig. 2, A and B, of 5-s (top trace) or 200-ms (bottom 2 traces) recordings are from two different interneurons from the same animal (P28). Both cells have similar AMPAR-mediated event frequencies (19.0 vs. 18.5 Hz, respectively) and very low and variable NMDAR-mediated event frequencies (2.4 vs. 0.7 Hz, respectively). Figure 2 shows the frequency distributions of the AMPAR (Fig. 2C) and NMDAR (Fig. 2D) components of spontaneous miniature events. Whereas the frequency of AMPAR-mediated mEPSCs follows a normal distribution, the NMDAR-mediated mEPSCs occurred at low frequencies, were skewed to the left, and displayed a high degree of variability, especially during the critical period.
Fig. 2.
AMPA receptor (AMPAR)- and NMDA receptor (NMDAR)-mediated miniature excitatory postsynaptic currents (mEPSCs) over development. A and B: AMPAR- and NMDAR-mediated mEPSCs from 2 different parvalbumin (PV)-expressing interneurons (INs) recorded from the same animal (postnatal day 28, P28). The neuron in B displays a very low frequency of NMDAR events compared with the one in A. Top left, representative AMPAR-mediated currents (5 s) recorded in voltage clamp (membrane potential = −70 mV) in the presence of extracellular Mg2+, tetrodotoxin, and picrotoxin. Bottom left, segments of the same current trace expanded to show 200 ms of recording. Top right, representative NMDAR-mediated currents (5 s) from the same neuron, recorded at +40 mV with added 6,7-dinitroquinoxaline-2,3-dione and glycine. Bottom right, segments of the same current trace expanded to show 200 ms of recording. Asterisks indicate identified individual events. Frequencies are AMPAR = 19.0 Hz, NMDAR = 2.4 Hz (A) and AMPAR = 18.5 Hz, NMDAR = 0.7 Hz (B). C: histogram distribution of AMPAR-mediated mEPSC frequencies (bin size = 3 Hz) over the 3 developmental stages: juvenile (J), P15–P20; critical period (CP), P25–P30/P35–P40; and adult (A), P50–P60. All the frequency distributions are well fitted to a normal distribution. Anderson-Darling test of normality, P values: J = 0.16, CP = 0.13, A = 0.94. At a 95% confidence level, P values <0.05 indicate a non-normal distribution. D: histogram distribution of NMDAR-mediated mEPSC frequencies (bin size = 0.5 Hz). The event frequencies are very low, and with the exception of juvenile, do not follow a normal distribution. Anderson-Darling test of normality, P values: J = 0.26, CP < 0.05, A < 0.05.
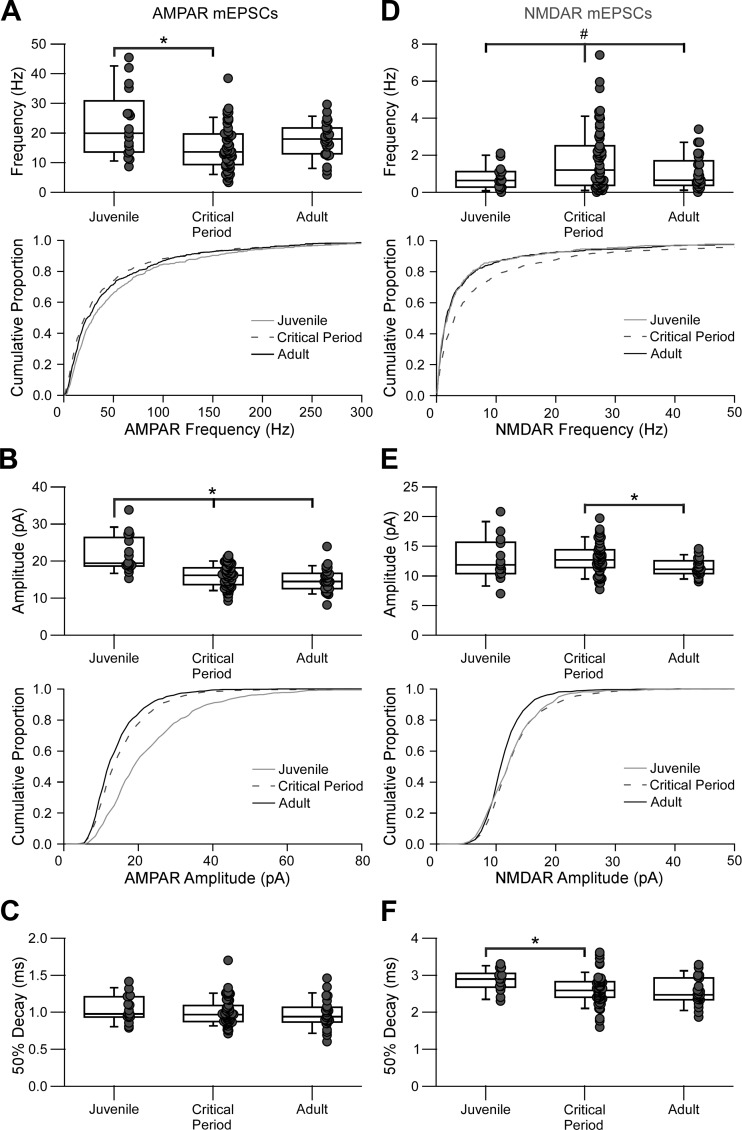
Figure 3 summarizes the results for AMPAR (Fig. 3, A–C) and NMDAR (Fig. 3, D–F) mEPSCs. Notably for the AMPAR-mediated mEPSCs and consistent with previous results (Akgul and Wollmuth 2013), the frequency of AMPA-mediated mEPSCs (Fig. 3A) was significantly decreased in the critical period compared with that in the juvenile period (J = 19.9 [12.7] Hz, CP = 13.6 [9.9] Hz, A = 17.9 [7.9] Hz; KW P < 0.05, multiple comparisons MW with Bonferroni correction P < 0.016). For NMDAR mEPSCs, the median frequency (Fig. 3D) did not change significantly (J = 0.63 [0.82] Hz, CP = 1.2 [2.1] Hz, A = 0.65 [1.07] Hz; KW P = 0.1), although the frequency variance was significantly higher during the critical period compared with both juvenile and adult (J = 0.38 Hz2, CP = 2.84 Hz2, A = 0.89 Hz2; Levene’s test P < 0.05).
Fig. 3.
Changes in AMPA receptor (AMPAR)- and NMDA receptor (NMDAR)-mediated miniature excitatory postsynaptic currents (mEPSCs) during the visual critical period. Summary data are shown for mEPSCs grouped by the 3 developmental stages: juvenile (J), P15–P20; critical period (CP), P25–P30/P35–P40; and adult (A), P50–P60. Each dot represents one recorded parvalbumin-expressing interneuron. The box plot indicates the median and interquartile range for each age group. *Significant difference between indicated groups [Kruskal-Wallis (KW), P < 0.05; multiple-comparisons Mann-Whitney (MW) with Bonferroni correction, P < 0.016]. #Significant difference in variance (Levene’s test, P < 0.05). A–C: AMPAR-mediated mEPSC summary plots showing frequency (A), amplitude (B), and decay kinetics (C). A: the frequency of events significantly decreased during the critical period. Top, box-dot plot comparing the 3 age groups (J = 19.9 [12.7] Hz, n = 17 cells, 9 mice; CP = 13.6 [9.9] Hz, n = 56 cells, 20 mice; A = 17.9 [7.9] Hz, n = 26 cells, 10 mice). Bottom, cumulative histograms for the same data displayed at top [CP ≠ J; Kolmogorov-Smirnov (KS) test, P < 0.01). B, top: box-dot plot showing the amplitude decrease during the critical period (J = 19.4 [6.6] pA, n = 17 cells; CP = 16.2 [4.4] pA, n = 56 cells; A = 14.5 [3.9] pA, n = 26 cells; KW P < 0.001; MW P < 0.016). Bottom, cumulative histograms for the same data displayed at top (J ≠ CP ≠ A; KS test, α < 0.01). C: the decay kinetics did not change significantly between any age group (J = 0.98 [0.27] ms, n = 17 cells; CP = 0.97 [0.20] ms, n = 56 cells; A = 0.94 [0.18] ms, n = 26 cells; KW, P = 0.46). D–F: NMDAR-mediated mEPSC summary plots showing frequency (D), amplitude (E), and decay kinetics (F). D, top: the median frequency did not change significantly (J = 0.63 [0.82] Hz; n = 15 cells, 8 mice; CP = 1.2 [2.1] Hz, n = 59 cells, 20 mice; A = 0.65 [1.07] Hz, n = 25 cells, 9 mice; KW, P = 0.1), although the frequency variance was significantly higher during the critical period compared with both juvenile and adult (J = 0.38 Hz2, CP = 2.84 Hz2, A = 0.89 Hz2; Levene’s test, P < 0.05). Bottom, cumulative histograms for the same data displayed at top (CP ≠ J, CP ≠ A; KS test, α < 0.01). E: the amplitude significantly decreased from the critical period to adult stages (J = 11.9 [4.5] pA, n = 14 cells; CP = 12.7 [2.9] pA, n = 55 cells; A = 11.1 [1.8] pA, n = 23 cells; KW P < 0.05; MW P < 0.016). Bottom, cumulative histograms for the same data displayed at top (A ≠ J, A ≠ CP; KS test, α < 0.01). F: decay kinetics decreased during the critical period (J = 2.90 [0.29] ms, n = 14 cells; CP = 2.60 [0.43] ms, n = 55 cells; A = 2.47 [0.46] ms, n = 23 cells; KW P < 0.05, MW P < 0.016).
The lower NMDAR event frequencies compared with AMPAR events could suggest that NMDARs contribute little to these synapses; marginal NMDAR contributions have been reported in these synapses in both the cortex (Angulo et al. 1999; Wang and Gao 2010) and the hippocampus (Lei and McBain 2002; Matta et al. 2013). Another possibility is that this approach is at the detection limit of NMDAR currents generated by spontaneous neurotransmitter release and that although NMDAR contributes to spontaneous synaptic transmission, we cannot reliably detect it. This discordance could also reflect different synaptic pools or heterogeneous release sites associated with NMDAR-lacking vs. NMDAR-containing synapses. Alternatively, it could reflect differences in the distribution of AMPARs and NMDARs relative to the location of release sites, whereby the AMPARs, being the dominant component of the synaptic responses, are located closer to the release sites and can sense single-vesicle release more effectively.
The NMDAR-Mediated Component of Intralaminar Inputs onto Layer 2/3 PV Interneurons Progressively Decreases with Development
mEPSCs are an index of the overall spontaneous glutamatergic input received by a PV interneuron but do not provide information about specific inputs. Pyramidal neurons in the same cortical layer are a major source of excitatory input onto neighboring PV interneurons (Dantzker and Callaway 2000; Jiang et al. 2015). We therefore characterized intralaminar inputs in V1 (layer 2/3 pyramidal neurons onto layer 2/3 PV interneurons) using paired whole cell recordings (Fig. 4) (Reyes et al. 1998).
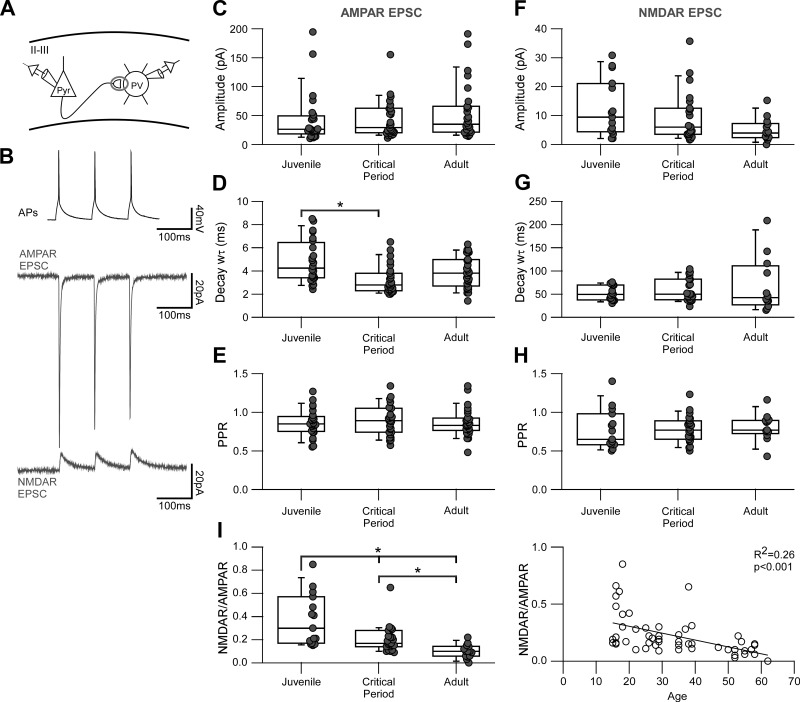
Fig. 4.
The NMDA receptor (NMDAR)-mediated component of intralaminar inputs onto layer 2/3 parvalbumin (PV)-expressing interneurons progressively decreases with development. A: schematic of the intralaminar paired recording configuration used to study evoked glutamatergic connections between pyramidal (Pyr) neurons and PV interneurons in layers 2/3 (II/III) of the primary visual cortex. B: sample traces of a paired recording between a pyramidal neuron and a PV interneuron. Top, 3 action potentials (APs) evoked in a pyramidal neuron at 10 Hz in current clamp. Middle, average AMPA receptor (AMPAR)-mediated excitatory postsynaptic currents (EPSCs; 100 sweeps) recorded at −70 mV in the presence of picrotoxin and extracellular Mg2+. Bottom, average NMDAR-mediated EPSCs (100 sweeps) recorded at +40 mV from the same cell with added 6,7-dinitroquinoxaline-2,3-dione. C–E: summary box-dot plots of intralaminar AMPAR-mediated EPSCs showing amplitude (C), decay kinetics (D), and paired-pulse ratio (PPR; E) grouped by the 3 developmental stages: juvenile (J), P15–P20; critical period (CP), P25–P30/P35–P40; and adult (A), P50–P60. Each dot represents one recorded PV interneuron. The box plot indicates the median and interquartile range for each age group. *Significant difference between indicated groups [Kruskal-Wallis (KW), P < 0.05; multiple-comparisons Mann-Whitney (MW) with Bonferroni correction, P < 0.016]. C: the median amplitude of unitary responses slightly increased over development, but these changes were not statistically significant (J = 26.4 [25.3] pA, n = 25 cells, 16 mice; CP = 29.4 [39.1] pA, n = 27 cells, 19 mice; A = 35.4 [43.7] pA, n = 28 cells, 18 mice; KW P = 0.49). D: the decay kinetics became significantly faster during the critical period with respect to juvenile (J = 4.3 [2.8] ms, n = 24 cells; CP = 2.8 [1.4] ms, n = 27 cells; A = 3.8 [2.1] ms, n = 28 cells; KW P < 0.05, MW P < 0.016). E: the PPR did not change significantly among any age group (J = 0.85 [0.17], n = 25 cells; CP = 0.89 [0.27], n = 27 cells; A = 0.83 [0.14], n = 28 cells; KW P = 0.78). F–H: summary box-dot plots of intralaminar NMDAR-mediated EPSCs showing amplitude (F), decay kinetics (G), and PPR (H). F: the amplitude became progressively smaller with age, but this change did not reach statistical significance (J = 9.5 [15.6] pA, n = 15 cells, 10 mice; CP = 6.0 [8.8] pA, n = 23 cells, 15 mice; A = 4.0 [4.4] pA, n = 14 cells, 11 mice; KW, P = 0.07). G: the decay kinetics did not change significantly over development (J = 49.5 [30.2] ms, n = 15 cells; CP = 50.5 [43.5] ms, n = 21 cells; A = 40.2 [30.4] ms, n = 12 cells; KW, P = 0.51). H: the PPR did not change significantly over development (J = 0.65 [0.33], n = 15 cells; CP = 0.77 [0.22], n = 23 cells; A = 0.77 [0.14], n = 13 cells; KW, P = 0.54). I, left: box-dot plots of NMDAR-to-AMPAR ratios (NMDAR/AMPAR) for intralaminar connections. There is a significant progressive decrease in the relative NMDAR component from juvenile into adulthood (J = 0.30 [0.34], n = 15 cells, 10 mice; CP = 0.17 [0.11], n = 23 cells, 15 mice; KW, P < 0.001, MW P < 0.016). Right, the progressive decrease in NMDAR/AMPAR is evidenced by a negative correlation between age and NMDAR/AMPAR. The P value and R2 of the linear fit are displayed in the graph.
Figure 4A is a schematic of a paired recording between a presynaptic pyramidal cell and a postsynaptic PV interneuron in layers 2/3 of primary visual cortex. We current-clamped a pyramidal neuron and evoked three action potentials at a frequency of 10 Hz while simultaneously recording from a synaptically connected and voltage-clamped PV interneuron. The connection probability was above 60% and did not change with development [J = 66% (n = 53), CP = 65% (n = 77), A = 68% (n = 71)].
Figure 4B shows example average traces of a connected pair displaying the action potentials in the pyramidal cell (top) along with the corresponding postsynaptic AMPAR (middle) and NMDAR (bottom) average current responses. We isolated the AMPAR component by holding the membrane potential at −70 mV in the presence of picrotoxin, and the NMDAR component by adding DNQX and recording at +40 mV.
Figure 4 shows summary data for the AMPAR (Fig. 4, C–E) and NMDAR (Fig. 4, F–H) components of the evoked EPSCs. There are two notable effects. First, the NMDAR-to-AMPAR ratio (Fig. 4I) showed a strong and robust progressive decrease in the relative NMDAR component (J = 0.30 [0.34], CP = 0.17 [0.11], A = 0.10 [0.08]; KW P < 0.001, MW P < 0.016). Second, the decay kinetics for AMPARs (Fig. 4D) became significantly faster during the critical period with respect to juvenile (J = 4.3 [2.8] ms, CP = 2.8 [1.4] ms, A = 3.8 [2.1] ms; KW P < 0.05, MW P < 0.016), whereas NMDARs did not (J = 49.5 [30.2] ms, CP = 50.5 [43.5] ms, A = 40.2 [30.4] ms; KW P = 0.51). We did not see any changes in the PPR for either the AMPAR (Fig. 4E) or NMDAR (Fig. 4H) currents, implying that presynaptic release at this input does not change during development.
In summary, intralaminar inputs rely less and less on NMDAR transmission with age such that for adult, synaptic transmission is dominated by AMPARs. Additionally, the faster AMPAR current kinetics during the critical period suggests a change in the subunit composition of the receptors, possibly to increasing numbers of Ca2+-permeable AMPARs (Geiger et al. 1995; Lalanne et al. 2016). These developmental changes would result in mature synaptic inputs with a short synaptic integration window, determined exclusively by AMPARs.
Interlaminar Inputs onto Layer 2/3 PV Interneurons Maintain a Constant NMDAR-Mediated Component But Show a Shift in Their Short-Term Plasticity with Development
In addition to local intralaminar inputs, cortical PV interneurons receive inputs from excitatory neurons located in other cortical and subcortical areas (Lu et al. 2014; Wall et al. 2016). Layers 2/3 receive a significant input from deeper layers, mostly layers 4 and 5 (Helmstaedter et al. 2008; Thomson and Lamy 2007; Xu and Callaway 2009). We therefore targeted interlaminar inputs onto layer 2/3 PV interneurons using extracellular stimulation.
To study interlaminar glutamatergic input to PV interneurons, we used minimal stimulation intensity (Fig. 5A), leading to minimal responses with a single peak and short delays (≤3 ms) (Fig. 5B). We recorded PV interneurons in layers 2/3 and placed a stimulation electrode in layer 5, directly underneath the recorded interneuron (Fig. 6A). Ideally, this stimulation paradigm would isolate putative monosynaptic responses mediated by single fibers, likely arising from layer 5 but also other ascending fibers projecting to layers 2/3. Figure 6B shows averaged AMPAR and NMDAR currents evoked in response to extracellular stimulation in layer 5. The interpulse interval and AMPAR and NMDAR current isolation protocols were the same as for the intralaminar input experiments.
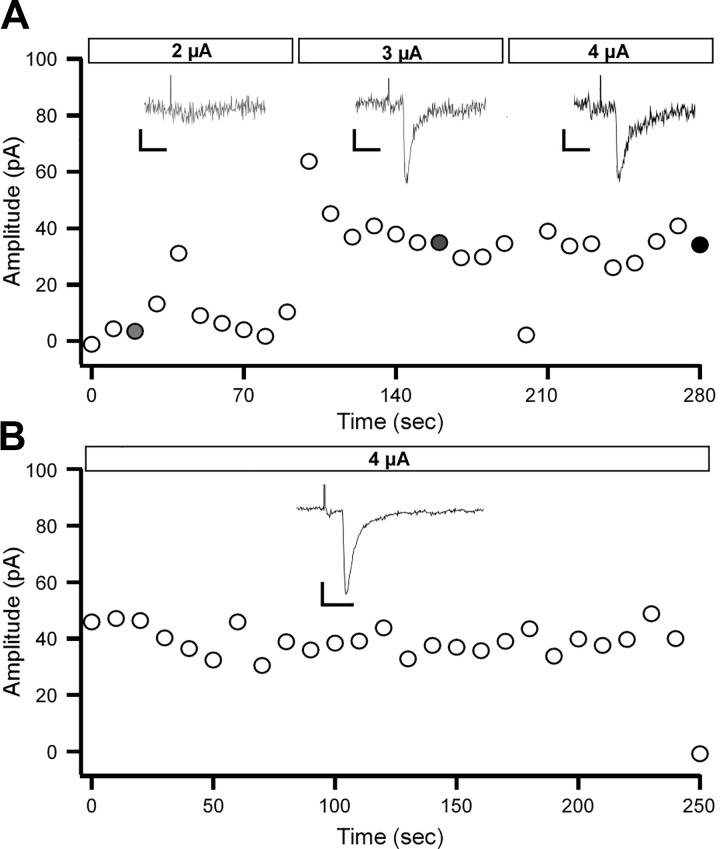
Fig. 5.
Isolation of minimally evoked glutamatergic responses in layer 2/3 parvalbumin (PV)-expressing interneurons. The extracellular stimulation electrode was placed in layer 5 during recordings from a PV interneuron in layers 2/3. The level of stimulation used was above threshold, adjusted until stable minimal synaptic responses with low failure rates were evoked. A: calibration plot of the stimulation used to evoke minimal responses. Horizontal bars at top represent 3 levels of stimulation used to evoke a postsynaptic response. Inset currents represent examples of responses to each stimulation level, indicated by the filled circles. Scale bars: 10 pA, 5 ms. B: current peak responses on repetitive stimulation at 4 μA. This stimulation level generates stable and reliable synaptic responses, with minimal failures. Inset current shows the average response for all data points displayed in the plot. Scale bars: 10 pA, 5 ms.
Fig. 6.
Interlaminar inputs onto layer 2/3 parvalbumin (PV)-expressing interneurons maintain a constant NMDA receptor (NMDAR)-mediated component but show a shift in their short-term plasticity with development. A: schematic of the extracellular stimulation used to assess interlaminar glutamatergic inputs onto layer 2/3 PV interneurons. The stimulation electrode was placed in layer 5 directly underneath a PV interneuron that was patched in layers 2/3 of the primary visual cortex. Pyr, pyramidal neuron. B: sample traces of synaptic responses evoked by extracellular stimulation. Three pulses of stimulation applied at 10 Hz generated putative monosynaptic responses with both AMPA receptor (AMPAR)- and NMDAR-mediated components. Top, average AMPAR-mediated excitatory postsynaptic currents (EPSCs; 30 sweeps) recorded at −70 mV in the presence of picrotoxin and extracellular Mg2+. Bottom, average NMDAR-mediated EPSCs (30 sweeps) recorded at +40 mV from the same cell with added 6,7-dinitroquinoxaline-2,3-dione. C–E: summary box-dot plots of interlaminar AMPAR-mediated EPSCs showing amplitude (C), decay kinetics (D), and paired-pulse ratio (PPR; E) grouped by the 3 developmental stages: juvenile (J), P15–P20; critical period (CP), P25–P30/P35–P40; and adult (A), P50–P60. Each dot represents one recorded PV interneuron. The box plot indicates the median and interquartile range for each age group. *Significant difference between indicated groups [Kruskal-Wallis (KW), P < 0.05; multiple-comparisons Mann-Whitney (MW) with Bonferroni correction, P < 0.016]. C: the median amplitude of the responses showed no statistical differences (J = 52.6 [29.9] pA, n = 15 cells, 7 mice; CP = 65.0 [28.1] pA, n = 28 cells, 17 mice; A = 40.7 [25.7] pA, n = 19 cells, 8 mice; KW, P = 0.054). D: the decay kinetics became faster during the critical period (J = 6.3 [5.0] ms, n = 15 cells; CP = 4.2 [1.5] ms, n = 28 cells; A = 4.4 [2.4] ms, n = 19 cells; KW, P < 0.05; MW, P < 0.016). E: the PPR shifted from depression into facilitation from juvenile into adulthood (J = 0.87 [0.19], n = 15 cells; CP = 0.90 [0.21], n = 28 cells; A = 1.15 [0.36], n = 19 cells; KW, P < 0.05; MW, P < 0.016). F–H: summary box-dot plots of interlaminar NMDAR-mediated EPSCs showing amplitude (F), decay kinetics (G), and PPR (H). F: there was no change in peak currents (J = 15.0 [6.7] pA, n = 9 cells, 7 mice; CP = 13.3 [13.1] pA, n = 14 cells, 11 mice; KW, P = 0.99). G: there was no change in the decay kinetics (J = 58.6 [32.5] ms, n = 9 cells; CP = 45.7 [36.7] ms, n = 14 cells; A = 43.5 [16.5] ms, n = 8 cells; KW, P = 0.28). H: there was no change in the short-term dynamics (J = 0.86 [0.44], n = 9 cells; CP = 0.90 [0.40], n = 14 cells; A = 0.83 [0.41], n = 8 cells; KW, P = 0.65). I, left: box-dot plots of NMDAR-to-AMPAR ratios (NMDAR/AMPAR) for interlaminar connections. There is no change in the relative NMDAR component from juvenile into adulthood (J = 0.19 [0.07], n = 9 cells, 7 mice; CP = 0.23 [0.23], n = 14 cells, 11 mice; A = 0.35 [0.36], n = 8 cells, 6 mice; KW, P = 0.19). Right, the plot between age and NMDAR/AMPAR shows a slight positive correlation. The P value and R2 of the linear fit are displayed in the graph.
We examined the AMPAR and NMDAR components of the synaptic responses evoked by extracellular stimulation before, during, and after the critical period (Fig. 6, C–I), comparing the same time windows that we used for intralaminar input data. As observed for intralaminar inputs, the decay kinetics of the AMPAR component significantly decreased during the critical period (Fig. 6D; J = 6.3 [5.0] ms, CP = 4.2 [1.5] ms, A = 4.4 [2.4] ms; KW P < 0.05, MW P < 0.016), whereas the NMDAR component showed no kinetic changes in decay (Fig. 6G; J = 58.6 [32.5] ms, CP = 45.7 [36.7] ms, A = 43.5 [16.5] ms; KW P = 0.28).
In contrast to the intralaminar inputs, the NMDAR-to-AMPAR ratio for interlaminar inputs did not change significantly over development (Fig. 6I; J = 0.19 [0.07], CP = 0.23 [0.23], A = 0.35 [0.36]; KW P = 0.19). We also observed a progressive increase in the PPR for the AMPAR EPSCs (Fig. 6E), transitioning from paired-pulse depression to facilitation in older animals (J = 0.87 [0.19], CP = 0.90 [0.21], A = 1.15 [0.36]; KW P < 0.05, MW P < 0.016). This change was not present for the NMDAR currents (Fig. 6H; J = 0.86 [0.44], CP = 0.90 [0.40], A = 0.83 [0.41]; KW P = 0.65).
In summary, the most notable developmental change for interlaminar glutamatergic inputs is a shift in PPR for the AMPAR component, from depression to facilitation. However, we did not observe this change for the NMDAR component. In contrast to intralaminar inputs, the relative NMDAR component of the synaptic response did not change with development and instead had a tendency to increase with age. Finally, interlaminar connections showed faster kinetics of the AMPAR-mediated responses during the critical period, as occurs for intralaminar inputs (Fig. 4D), suggesting a parallel change in the subunit composition of the AMPARs for both connections during the critical period.
Adult Intralaminar and Interlaminar Inputs Differ on Their Relative NMDAR-Mediated Component
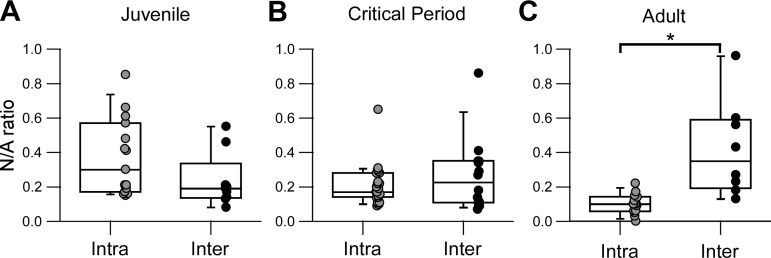
One of the main differences observed between the two inputs was the decrease or lack of developmental change in the relative NMDAR component, for intralaminar (Fig. 4I) and interlaminar (Fig. 6I) inputs, respectively. Because this parameter is normalized (Kauer and Malenka 2007), we directly compared it between the two inputs and among the three developmental groups (Fig. 7). There was no significant difference of this parameter before (Fig. 7A) or during the critical period (Fig. 7B). However, adult intralaminar inputs had a significantly lower NMDAR/AMPAR compared with interlaminar inputs (Fig. 7C). Thus the developmental changes observed during the critical period lead to adult intralaminar and interlaminar inputs with distinct profiles in their relative NMDAR contribution (Fig. 8).
Fig. 7.
Adult intralaminar and interlaminar inputs differ on their relative NMDAR-mediated component. A–C: summary box-dot plots compare NMDAR-to-AMPAR ratios (N/A) between intralaminar (Intra) and interlaminar (Inter) connections for juvenile (A), critical period (B), and adult (C) developmental stages. Each dot represents one recorded PV interneuron. The box plot indicates the median and interquartile range for each age group. *Significant difference between indicated groups [multiple-comparisons Mann-Whitney (MW) with Bonferroni correction, P < 0.016]. A and B: there is a no significant difference in N/A between the two connections for either juvenile (Intra = 0.30 [0.34], n = 15 cells, 10 mice; Inter = 0.19 [0.07], n = 9 cells, 7 mice; MW, P = 0.11) or critical period (Intra = 0.17 [0.11], n = 23 cells, 15 mice; Inter = 0.23 [0.23], n = 14 cells, 11 mice; MW, P = 0.63). C: in the adult stage, N/A ratio is significantly smaller for intralaminar compared with interlaminar connections (Intra = 0.10 [0.08], n = 14 cells, 11 mice; Inter = 0.35 [0.36], n = 8 cells, 6 mice; MW, P = 0.001).
Fig. 8.
Distinct properties of mature intralaminar and interlaminar excitatory inputs. Schematic shows the sources of glutamatergic inputs onto layer 2/3 parvalbumin (PV)-expressing interneurons and their synaptic properties in juvenile and adult stages. Middle, the most prominent sources of glutamatergic drive to layer 2/3 PV interneurons are intralaminar (layers 2/3) and interlaminar (layers 4/5) pyramidal neurons (Pyr). Vertical ticks underneath Pyr neurons represent the rate of action potential firing and the sparse coding of superficial layers compared with deeper layers that accentuates with development. Left, representative normalized AMPA receptor (AMPAR)- and NMDA receptor (NMDAR)-mediated currents for intra- and interlaminar juvenile inputs; both inputs display similar properties and therefore would have similar drive onto PV interneuron activity. Right, representative normalized AMPAR- and NMDAR-mediated currents for intra- and interlaminar adult inputs. A minimal contribution of NMDARs for intralaminar connections would ensure precise timing and engagement of feedback inhibition in the sparsely firing superficial pyramidal neurons. A substantial NMDAR component and facilitating dynamics would ensure temporal integration of interlaminar inputs and efficient recruitment of feedforward inhibition.
DISCUSSION
PV interneurons play a critical role in the development of the local circuits involved in visual processing (Davis et al. 2015; Gu et al. 2016; Levelt and Hübener 2012). In the present study, we addressed developmental changes in glutamatergic transmission onto layer 2/3 PV interneurons in V1 before, during, and after the visual critical period using both miniature and evoked currents. The main developmental changes are as follows: 1) For intralaminar inputs, a substantial and progressive decrease in the relative NMDAR component occurred (Fig. 4I) such that by adulthood, it was barely detectable. In contrast, for the interlaminar inputs, the NMDAR component remained robust throughout development (Fig. 6I). 2) Short-term plasticity in the AMPAR-mediated component of intralaminar inputs was stable and mainly depressing throughout development (Fig. 4E). In contrast, the short-term synaptic plasticity in interlaminar inputs converted from depression in juvenile stage to mostly facilitation in adult stage (Fig. 6E). 3) For both inputs, the AMPAR-mediated responses became more transient during the critical period (Figs. 4D and 6D).
Input-Specific Developmental Changes in the Relative NMDAR Component of Evoked Glutamatergic Responses onto PV Interneurons
Cortical PV interneurons in layers 2/3 receive significant glutamatergic input from local pyramidal neurons via intralaminar synaptic connectivity (Reyes et al. 1998; Watanabe et al. 2005; Yoshimura and Callaway 2005). The rest of the glutamatergic inputs come from deeper cortical layers, i.e., layer 4/5 interlaminar inputs (Helmstaedter et al. 2008; Xu and Callaway 2009), as well as from other cortical and subcortical regions (Ji et al. 2016; Lu et al. 2014; Wall et al. 2016).
When developmental changes in intralaminar and interlaminar glutamatergic inputs onto PV interneurons were compared, the major difference we found was that whereas intralaminar inputs had a marked progressive reduction on their relative NMDAR component during and after the critical period (J = 30%, CP = 17%, A = 10%), interlaminar inputs did not and instead tended to increase the contribution of NMDAR component in their glutamatergic synapses (J = 19%, CP = 23%, A = 35%).
On the basis of our data, we predict that both juvenile intralaminar and interlaminar inputs would have a comparably wide temporal integration window, conferred by the slow kinetics of a substantial NMDAR-mediated synaptic component. With development, intralaminar connections would progressively display a shorter integration window as the AMPAR component becomes dominant, whereas adult interlaminar connections would maintain properties similar to those observed in juvenile connections. Thus temporal precision of intralaminar inputs would increase with development, whereas interlaminar inputs would conserve temporal integration of the synaptic responses.
Developmental differences in the relative NMDAR component could also differentiate the two inputs in terms of calcium dynamics and synaptic plasticity. Such input-specific forms of plasticity, dependent on the relative synaptic contribution of NMDARs, occur in hippocampal PV interneurons (Le Roux et al. 2013; Lei and McBain 2002; Sambandan et al. 2010). Although NMDAR-dependent plasticity has only been reported in hippocampal PV interneurons (Le Roux et al. 2013), some forms of experience-dependent plasticity in vivo in the visual cortex depend on NMDAR signaling in PV interneurons (Kaplan et al. 2016). With a significant NMDAR-mediated Ca2+ entry, immature intralaminar inputs and interlaminar inputs across development could express similar forms of Hebbian plasticity.
GluA2-lacking, Ca2+-permeable AMPARs can mediate NMDAR-independent forms of plasticity in PV interneurons (Goldberg et al. 2003; Lu et al. 2007). Ca2+-permeable AMPARs are characterized by their fast kinetics (Geiger et al. 1995; Lalanne et al. 2016). We observed faster AMPAR responses in both intralaminar and interlaminar inputs during the visual critical period, consistent with a developmental switch in the subunit composition of the receptors (Okaty et al. 2009). Our results are also consistent with a previous study of layer 2/3 pyramidal to layer 2/3 PV interneuron connections in V1 that showed an increase in the Ca2+-permeable AMPAR-component during the critical period assessed pharmacologically (Lu et al. 2014). Considering the minimal contribution of NMDARs to mature intralaminar inputs in adult stages, this could represent the main source of Ca2+ entry to this input, with the potential expression of non-Hebbian forms of plasticity (Laezza and Dingledine 2004).
Input-Specific Developmental Changes in Short-Term Plasticity of Evoked Glutamatergic Responses onto PV Interneurons
Short-term plasticity allows the dynamic regulation of synaptic efficacies and constitutes an important mechanism for neuronal processing (Klug et al. 2012). Intralaminar AMPAR-mediated currents remained depressing throughout development (Fig. 4E), which would decrease responsiveness to higher frequency inputs, acting as low-pass filters. In contrast, for interlaminar inputs, AMPAR-mediated responses went from juvenile depressing to adult facilitating synaptic responses (Fig. 6E). Such changes are consistent with the optimization of interlaminar inputs for temporal integration with development.
Interestingly, interlaminar NMDAR-mediated responses do not display a shift in PPR (Fig. 6H) and do not follow facilitating dynamics of the AMPAR-mediated component. Differences in the PPR of AMPAR and NMDAR components have been described in synapses in the hippocampus and amygdala (Poncer and Malinow 2001; Zinebi et al. 2001). Although PPR is typically associated to presynaptic release probability, postsynaptic factors can also influence this parameter (Poncer and Malinow 2001; Rozov and Burnashev 1999). A possible explanation for this disparity is saturation NMDARs, because they have higher affinity for glutamate compared with AMPARs (Clements et al. 1992). If a facilitating response is produced by enhanced presynaptic glutamate release, it can be detected by unsaturated AMPARs. Scarce NMDARs, on the other hand, could be already saturated from the initial release and as a result would not be able to detect enhanced glutamate release in subsequent stimulations. Another possible explanation for this difference could be heterogeneity in the release sites. Such heterogeneity could arise as a result of distinct release sites or different synaptic contacts for AMPARs and NMDARs, each one with slightly different release probabilities; alternatively, this could be the result of the different distribution of AMPARs and NMDARs relative to the release sites (Franks et al. 2002). However, the exact nature of this phenomenon remains to be established.
We assume our extracellular stimulation is mainly targeting layer 5 inputs. The different properties between intralaminar and interlaminar connections indicate that the extracellular stimulation is indeed assessing different sets of fibers and not stimulating antidromic axon potentials from pyramidal layer 2/3 descending axons. Layer 4 dendritic and axonal projections rarely extend to our site of stimulation on layer 5 (Thomson and Lamy 2007), making them an unlikely source of excitation under our conditions. Other potential sources of excitatory drive to layer 2/3 PV interneurons that could be recruited with extracellular stimulation include long-range projections from contralateral visual cortex and other cortical regions (Lu et al. 2014) and ascending thalamocortical projections (Ji et al. 2016). We cannot rule out the possibility that these sources are included in the responses classified as interlaminar inputs. Nonetheless, a study assessing the NMDAR component at synapses onto PV interneurons in adult prefrontal cortex found the same minimal contribution NMDARs across multiple long-range inputs (Bogart and O’Donnell 2018), comparable to the intralaminar inputs we report presently. In addition, corticothalamic inputs onto PV interneurons have strongly depressing glutamatergic synapses across development (Cruikshank et al. 2010; Hull et al. 2009; Miao et al. 2016), in contrast to the facilitating dynamics of interlaminar adult connections found in this study. On the basis of these differences, we believe that our interlaminar inputs are for the most part homogenous layer 5 ascending fibers and not long-range projections.
Developmental Changes in Spontaneous Glutamatergic Transmission onto PV Interneurons
We also addressed the developmental changes in spontaneous glutamatergic transmission recording mEPSCs, which sample all glutamatergic inputs received by a neuron. The frequency of mEPSC reflects presynaptic release probability (Pr) and/or synapse number, and the amplitude of events represents postsynaptic factors, such as receptor number, receptor density, and/or sensitivity to released neurotransmitter (Kaeser and Regehr 2014).
We saw changes in the frequency and amplitude of AMPAR-mediated mEPSCs (Fig. 3, A–C), as shown previously (Akgul and Wollmuth 2013). The AMPAR-mediated mEPSC frequencies decreased during the critical period (Fig. 3A), suggesting a reduced Pr, a result consistent with developmental changes in short-term synaptic plasticity, at least for the interlaminar inputs (Fig. 6E). Alternatively, decreased mEPSC frequency with age could reflect a reduction in AMPAR-containing synapse number. This latter explanation is consistent with synaptic pruning as has been proposed for glutamatergic inputs onto pyramidal neurons during the critical period (Han et al. 2017; Zuo et al. 2005).
Another observation from our study is the lack of consistency in the developmental changes between spontaneous (mEPSCs) and evoked (intralaminar and interlaminar) glutamatergic transmission, and how the features observed in mEPSCs data are not predictive of those present in evoked responses. The major input to PV interneurons comes from local, intralaminar pyramidal neurons; therefore, this input would be expected to be highly represented in the mEPSCs. The absence of NMDAR-mediated mEPSCs in a subset of PV interneurons (Fig. 3D) contrasts with the intralaminar evoked NMDAR currents present in almost all interneurons recorded (Fig. 4F). However, the detectability of NMDAR mEPSCs represents a limiting factor considering their low signal-to-noise ratio. The progressive reduction in amplitude of evoked intralaminar NMDAR currents (Fig. 4I) would make detection of spontaneous events even harder for this input and is reflected in a decreased amplitude of NMDAR mEPSCs in adult stages (Fig. 3E).
Alternatively, a number of studies have suggested that the two forms of neurotransmission (spontaneous vs. evoked release) may be independent or represent different synaptic populations, playing different roles in brain function (Kaeser and Regehr 2014; Kavalali 2015; Walter et al. 2014). A dissociation between spontaneous and evoked glutamatergic transmission has been demonstrated at both the presynaptic (Abrahamsson et al. 2017; Sara et al. 2005) and postsynaptic levels, including different pools of AMPARs (Sara et al. 2011) and NMDARs (Atasoy et al. 2008; Townsend et al. 2003). Our results are in agreement with this notion and suggest that in PV interneurons, this dichotomy between spontaneous and evoked glutamatergic transmission is also present.
Implications of Developmental Changes of Glutamatergic Synapses onto PV Interneurons for Circuit Function and Closure of the Critical Period
In vivo studies in the visual cortex have shown the sparse coding of layers 2/3 (Rochefort et al. 2009), whereby pyramidal neuron firing of the superficial layers is lower compared with that in deeper cortical layers and is determined by the tone of inhibition (Petersen and Crochet 2013). Our data suggest that the maturational profile of intralaminar inputs onto PV interneurons is optimized for precise timing and coincidence detection of sparse layer 2/3 excitatory inputs, requiring a higher frequency threshold for temporal integration, whereas interlaminar inputs, conveying columnar sensory information, are suited for temporal integration of higher frequency inputs (Fig. 8). This scenario would result in an efficient, frequency-dependent engagement of PV feedforward inhibition, narrowing the response time of layer 2/3 pyramidal neurons. In addition, locally driven PV feedback and lateral inhibition would be time-locked to the local activity, further maintaining temporal precision.
The opening of the critical period for ocular dominance is accompanied by a substantial increase in the inhibitory tone and maturation of GABAergic transmission (Jiang et al. 2005), whereas the mechanism mediating the closure of the critical period has remained elusive. A characteristic feature of the end of the visual critical period is the recruitment of mature inhibitory circuits with temporal precision, driven in part by mature glutamatergic synapses onto inhibitory interneurons. Disruption of perineuronal nets, which regulate excitatory synapses onto PV interneurons, allows ocular dominance plasticity to be induced in adulthood well past the end of the normal critical period (Carulli et al. 2010). At the functional level, glutamatergic innervation onto PV interneurons influences excitation/inhibition balance in local microcircuits, relevant for the expression of cortical plasticity (Morishita et al. 2010). Therefore, the structural and functional maturation of excitatory synapses onto PV interneurons could serve as a potential substrate for the closure of the critical period.
Our results indicate that in the adult visual cortex, intralaminar and interlaminar inputs differ substantially in their NMDAR signaling. NMDARs can mediate dendritic supralinear summation of excitatory inputs onto PV interneurons and allow the separation of neuronal assemblies depending on the contribution of NMDARs at different inputs (Cornford et al. 2018). The nonlinear engagement of feedforward PV interneuron-mediated inhibition could segregate even further the activity of existing subnetworks of excitatory neurons present in layers 2/3 of primary visual cortex (Yoshimura et al. 2005). This kind of circuit configuration in mature cortex could play a role in the computation and segregation of different features of sensory information and processing.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke GrantR01 NS088479 (to L. P. Wollmuth), an American Pediatric Surgical Association Foundation Grant (to H. Hsieh), and intramural grants from the Departments of Surgery and Pediatrics (to H. Hsieh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.F. performed experiments; C.F. and L.P.W. analyzed data; C.F., H.H., and L.P.W. interpreted results of experiments; C.F. prepared figures; C.F. and L.P.W. drafted manuscript; C.F., H.H., and L.P.W. edited and revised manuscript; C.F., H.H., and L.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gulcan Akgul, Sana Fujimura, and Alexander Baez for technical assistance and Drs. Arianna Maffei, David Talmage, and Alexander Reyes for helpful discussions and/or comments on the manuscript.
REFERENCES
- Abrahamsson T, Chou CY, Li SY, Mancino A, Costa RP, Brock JA, Nuro E, Buchanan KA, Elgar D, Blackman AV, Tudor-Jones A, Oyrer J, Farmer WT, Murai KK, Sjöström PJ. Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron 96: 839–855.e5, 2017. doi: 10.1016/j.neuron.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Akgul G, Wollmuth LP. Synapse-associated protein 97 regulates the membrane properties of fast-spiking parvalbumin interneurons in the visual cortex. J Neurosci 33: 12739–12750, 2013. doi: 10.1523/JNEUROSCI.0040-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol 82: 1295–1302, 1999. doi: 10.1152/jn.1999.82.3.1295. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci 28: 10151–10166, 2008. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13: 76–83, 2010. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LJ, O’Donnell P. Multiple long-range inputs evoke NMDA currents in prefrontal cortex fast-spiking interneurons. Neuropsychopharmacology 43: 2101–2108, 2018. doi: 10.1038/s41386-018-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Losi G, Chiavegato A, Zonta M, Carmignoto G. Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J Physiol 591: 807–822, 2013. doi: 10.1113/jphysiol.2012.238154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133: 2331–2347, 2010. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci 17: 3894–3906, 1997. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci 13: 1090–1097, 2010. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 24: 9598–9611, 2004. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science 258: 1498–1501, 1992. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- Cornford JH, Mercier MS, Leite M, Magloire V, Häusser M, Kullmann DM. Dendritic NMDA receptors in parvalbumin neurons enable strong and stable neuronal assemblies (Preprint). bioRxiv, 2018. doi: 10.1101/279505. [DOI] [PMC free article] [PubMed]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci 3: 701–707, 2000. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, Gandhi SP. Inhibitory neuron transplantation into adult visual cortex creates a new critical period that rescues impaired vision. Neuron 86: 1055–1066, 2015. doi: 10.1016/j.neuron.2015.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist 19: 228–237, 2013. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks KM, Bartol TM Jr, Sejnowski TJ. A Monte Carlo model reveals independent signaling at central glutamatergic synapses. Biophys J 83: 2333–2348, 2002. doi: 10.1016/S0006-3495(02)75248-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Lübke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18: 1009–1023, 1997. doi: 10.1016/S0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15: 193–204, 1995. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Jeong HY, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb Cortex 21: 666–682, 2011. doi: 10.1093/cercor/bhq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron 40: 807–821, 2003. doi: 10.1016/S0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat 1: 3, 2008. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38: 950–957, 2012. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A, Quinlan EM. Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 79: 335–346, 2013. doi: 10.1016/j.neuron.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Tran T, Murase S, Borrell A, Kirkwood A, Quinlan EM. Neuregulin-dependent regulation of fast-spiking interneuron excitability controls the timing of the critical period. J Neurosci 36: 10285–10295, 2016. doi: 10.1523/JNEUROSCI.4242-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Cooke SF, Xu W. Experience-dependent equilibration of AMPAR-mediated synaptic transmission during the critical period. Cell Reports 18: 892–904, 2017. doi: 10.1016/j.celrep.2016.12.084. [DOI] [PubMed] [Google Scholar]
- Helm J, Akgul G, Wollmuth LP. Subgroups of parvalbumin-expressing interneurons in layers 2/3 of the visual cortex. J Neurophysiol 109: 1600–1613, 2013. doi: 10.1152/jn.00782.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. J Neurosci 28: 8273–8284, 2008. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3: e159, 2005. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345: 1255263, 2014. doi: 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci 29: 9127–9136, 2009. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XY, Zingg B, Mesik L, Xiao Z, Zhang LI, Tao HW. Thalamocortical innervation pattern in mouse auditory and visual cortex: laminar and cell-type specificity. Cereb Cortex 26: 2612–2625, 2016. doi: 10.1093/cercor/bhv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev 50: 126–133, 2005. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S, Tolias AS. Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350: aac9462, 2015. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Regehr WG. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu Rev Physiol 76: 333–363, 2014. doi: 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan ES, Cooke SF, Komorowski RW, Chubykin AA, Thomazeau A, Khibnik LA, Gavornik JP, Bear MF. Contrasting roles for parvalbumin-expressing inhibitory neurons in two forms of adult visual cortical plasticity. eLife 5: e11450, 2016. doi: 10.7554/eLife.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858, 2007. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 16: 5–16, 2015. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Klug A, Borst JG, Carlson BA, Kopp-Scheinpflug C, Klyachko VA, Xu-Friedman MA. How do short-term changes at synapses fine-tune information processing? J Neurosci 32: 14058–14063, 2012. doi: 10.1523/JNEUROSCI.3348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68: 557–569, 2010. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS One 3: e2005, 2008. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Dingledine R. Voltage-controlled plasticity at GluR2-deficient synapses onto hippocampal interneurons. J Neurophysiol 92: 3575–3581, 2004. doi: 10.1152/jn.00425.2004. [DOI] [PubMed] [Google Scholar]
- Lalanne T, Oyrer J, Mancino A, Gregor E, Chung A, Huynh L, Burwell S, Maheux J, Farrant M, Sjöström PJ. Synapse-specific expression of calcium-permeable AMPA receptors in neocortical layer 5. J Physiol 594: 837–861, 2016. doi: 10.1113/JP271394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus MS, Huang ZJ. Distinct maturation profiles of perisomatic and dendritic targeting GABAergic interneurons in the mouse primary visual cortex during the critical period of ocular dominance plasticity. J Neurophysiol 106: 775–787, 2011. doi: 10.1152/jn.00729.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux N, Cabezas C, Böhm UL, Poncer JC. Input-specific learning rules at excitatory synapses onto hippocampal parvalbumin-expressing interneurons. J Physiol 591: 1809–1822, 2013. doi: 10.1113/jphysiol.2012.245852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron 33: 921–933, 2002. doi: 10.1016/S0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci 35: 309–330, 2012. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- Lu J, Tucciarone J, Lin Y, Huang ZJ. Input-specific maturation of synaptic dynamics of parvalbumin interneurons in primary visual cortex. Proc Natl Acad Sci USA 111: 16895–16900, 2014. doi: 10.1073/pnas.1400694111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci 27: 9711–9720, 2007. doi: 10.1523/JNEUROSCI.2513-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 16: 1032–1041, 2013. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Pizzorusso T. Molecular mechanisms at the basis of plasticity in the developing visual cortex: epigenetic processes and gene programs. J Exp Neurosci 7: 75–83, 2013. doi: 10.4137/JEN.S12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q, Yao L, Rasch MJ, Ye Q, Li X, Zhang X. Selective maturation of temporal dynamics of intracortical excitatory transmission at the critical period onset. Cell Reports 16: 1677–1689, 2016. doi: 10.1016/j.celrep.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Miyamae T, Chen K, Lewis DA, Gonzalez-Burgos G. Distinct physiological maturation of parvalbumin-positive neuron subtypes in mouse prefrontal cortex. J Neurosci 37: 4883–4902, 2017. doi: 10.1523/JNEUROSCI.3325-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330: 1238–1240, 2010. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci 29: 7040–7052, 2009. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pafundo DE, Miyamae T, Lewis DA, Gonzalez-Burgos G. Presynaptic effects of N-methyl-d-aspartate receptors enhance parvalbumin cell-mediated inhibition of pyramidal cells in mouse prefrontal cortex. Biol Psychiatry 84: 460–470, 2018. doi: 10.1016/j.biopsych.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron 78: 28–48, 2013. doi: 10.1016/j.neuron.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Poncer JC, Malinow R. Postsynaptic conversion of silent synapses during LTP affects synaptic gain and transmission dynamics. Nat Neurosci 4: 989–996, 2001. doi: 10.1038/nn719. [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Johnson JW. Tonic NMDA receptor-mediated current in prefrontal cortical pyramidal cells and fast-spiking interneurons. J Neurophysiol 107: 2232–2243, 2012. doi: 10.1152/jn.01017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rochefort NL, Garaschuk O, Milos RI, Narushima M, Marandi N, Pichler B, Kovalchuk Y, Konnerth A. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc Natl Acad Sci USA 106: 15049–15054, 2009. doi: 10.1073/pnas.0907660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401: 594–598, 1999. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- Sambandan S, Sauer JF, Vida I, Bartos M. Associative plasticity at excitatory synapses facilitates recruitment of fast-spiking interneurons in the dentate gyrus. J Neurosci 30: 11826–11837, 2010. doi: 10.1523/JNEUROSCI.2012-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Bal M, Adachi M, Monteggia LM, Kavalali ET. Use-dependent AMPA receptor block reveals segregation of spontaneous and evoked glutamatergic neurotransmission. J Neurosci 31: 5378–5382, 2011. doi: 10.1523/JNEUROSCI.5234-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deák F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45: 563–573, 2005. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci 1: 19–42, 2007. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Yoshii A, Mishina M, Constantine-Paton M. Developmental loss of miniature N-methyl-d-aspartate receptor currents in NR2A knockout mice. Proc Natl Acad Sci USA 100: 1340–1345, 2003. doi: 10.1073/pnas.0335786100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91: 260–292, 2016. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Erkkila BE, Jeffries BW, Yuan X, McBain CJ. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci 31: 10948–10970, 2011. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Sorokin JM, Taniguchi H, Huang ZJ, Callaway EM. Brain-wide maps of synaptic input to cortical interneurons. J Neurosci 36: 4000–4009, 2016. doi: 10.1523/JNEUROSCI.3967-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter AM, Haucke V, Sigrist SJ. Neurotransmission: spontaneous and evoked release filing for divorce. Curr Biol 24: R192–R194, 2014. doi: 10.1016/j.cub.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol 588: 2823–2838, 2010. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Rozov A, Wollmuth LP. Target-specific regulation of synaptic amplitudes in the neocortex. J Neurosci 25: 1024–1033, 2005. doi: 10.1523/JNEUROSCI.3951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Callaway EM. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci 29: 70–85, 2009. doi: 10.1523/JNEUROSCI.4104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci 8: 1552–1559, 2005. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature 433: 868–873, 2005. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- Zinebi F, Russell RT, McKernan M, Shinnick-Gallagher P. Comparison of paired-pulse facilitation of AMPA and NMDA synaptic currents in the lateral amygdala. Synapse 42: 115–127, 2001. doi: 10.1002/syn.1107. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46: 181–189, 2005. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]