Abstract
Sensory information from one leg has been known to elicit reflex responses in the contralateral leg, known as “crossed reflexes,” and these have been investigated extensively in cats and humans. Furthermore, experiments with mice have shown commissural pathways in detail by using in vitro and in vivo physiological approaches combined with genetics. However, the relationship between these commissural pathways discovered in mice and crossed reflex pathways described in cats and humans is not known. In this study, we analyzed the crossed reflex in mice by using in vivo electromyographic recording techniques combined with peripheral nerve stimulation protocols to provide a detailed description of the crossed reflex pathways. We show that excitatory crossed reflexes are mediated by both proprioceptive and cutaneous afferent activation. In addition, we provide evidence for a short-latency inhibitory crossed reflex pathway likely mediated by cutaneous feedback. Furthermore, the short-latency crossed inhibition is downregulated in the knee extensor muscle and the ankle flexor muscle during locomotion. In conclusion, this article provides an analysis of excitatory and inhibitory crossed reflex pathways during resting and locomoting mice in vivo. The data presented in this article pave the way for future research aimed at understanding crossed reflexes using genetics in mice.
NEW & NOTEWORTHY We describe for the first time excitatory and inhibitory crossed reflex pathways in mouse spinal cord in vivo and show that the inhibitory pathways are modulated during walking. This is a first step toward an understanding of crossed reflexes and their function during walking using in vivo recording techniques combined with mouse genetics.
Keywords: crossed reflex, locomotion, mice, sensory feedback
INTRODUCTION
Coordinated leg movement during locomotion is generated by the patterned activation of multiple motor neuron pools (locomotor pattern) that drives the orchestrated contraction of multiple leg muscles both within and between legs. The locomotor pattern of individual legs is driven by a network of interconnected sets of premotor interneurons in the spinal cord (central pattern generator, CPG) and the sensory feedback, mediated by cutaneous and proprioceptive inputs, from the periphery (McCrea 2001; Pearson 2004; Rossignol et al. 2006). Sensory feedback refines the spatiotemporal features of motor output during locomotion (Akay et al. 2014; Böhm and Wyart 2016; McCrea 2001), and removal of sensory feedback severely impairs locomotion (Akay et al. 2014). These data suggest that certain aspects of the locomotor pattern can be generated by the CPG, but sensory feedback is necessary for a functional locomotor pattern because it occurs during intact locomotion.
Reflex pathways that convey sensory information from one leg to the contralateral leg (crossed reflex pathways) were first described more than a century ago (Sherrington 1910). By using the cat as the animal model, it has been established that commissural interneurons (CINs), whose cell bodies are located in lamina VIII of the spinal cord, synapse with the spinal circuitry on the contralateral side (Jankowska 2008; Jankowska and Edgley 2010). These CINs are thought to be important in left-right coordination during locomotion (Matsuyama et al. 2004a, 2004b) and have been shown to transmit sensory information to contralateral motor neurons (Aggelopoulos and Edgley 1995; Arya et al. 1991; Edgley et al. 2003; Jankowska et al. 2005; Sherrington 1910). Proprioceptive sensory afferents (group I and II) and cutaneous afferents mediate crossed reflex responses in flexor and extensor muscles in anesthetized and awake cats during locomotion (Aggelopoulos et al. 1996; Arya et al. 1991; Edgley and Aggelopoulos 2006; Duysens and Loeb 1980; Gauthier and Rossignol 1981; Hurteau et al. 2018; Perl 1957).
Mice have become important for the investigation of neuronal mechanisms involved in locomotion because of the potential for genetic manipulation of their neural circuits underlying locomotion (Garcia-Campmany et al. 2010; Goulding 2009; Jessell 2000). Specifically, it has been shown that the commissural pathways involve genetically distinct classes of CINs that are important in left-right coordination during locomotion (Lanuza et al. 2004; Talpalar et al. 2013; Zhang et al. 2008). As in the CINs identified in cats, the cell bodies of some of these CINs in mice are also located in lamina VIII of the spinal cord (Lanuza et al. 2004; Lu et al. 2015; Zhang et al. 2008). Yet, because of the technical limitations of measuring crossed reflexes in mice in vivo, the role of these CINs in mice in crossed reflexes is not known. Crossed reflexes have been shown in rodents with the use of in vitro spinal cord preparations, where sensory afferents were activated on one side of the spinal cord and motor neuron responses were observed on the contralateral side, but only to a limited degree (Bagust and Kerkut 1987; Jiang et al. 1999). More recently, crossed reflexes have been recorded in vivo in decerebrate and immobilized adult mice, initiated by a moderately strong toe pinch (Nakanishi and Whelan 2012), demonstrating that activation of sensory afferents on one side of the body can induce motor activity on the contralateral side. Insights into the structure of the spinal circuitry underlying crossed reflexes are still obscure. Mutant mice with genetically modified spinal circuitry could resolve this obscurity. This article provides the first detailed description of crossed reflex responses in fully awake mice during resting and walking on a treadmill.
To describe the crossed reflex in awake adult mice, we recorded the electromyogram (EMG) activity from up to five hindlimb muscles while we stimulated peripheral nerves in the contralateral hind leg to activate sensory afferent fibers. We show that crossed reflex actions include flexor and extensor muscle activation mediated by both proprioceptive and cutaneous afferents. Furthermore, our data suggest that inhibitory as well as excitatory crossed reflex pathways can be measured using the techniques presented. Finally, we provide evidence that the inhibitory crossed reflex pathway is subject to modulation when the animal walks. These experiments lay the groundwork in the mouse model to identify specific CIN pathways involved in crossed reflexes and the role of these crossed reflex pathways during motor behavior.
METHODS
Experiments were conducted on 2- to 4-mo-old wild-type (WT) C57BL/6 mice of both sexes. None of the mice were trained on the treadmill before the experiments. All studies were performed according to the guidelines of the Canadian Council on Animal Care and approved by the local councils on animal care of Dalhousie University.
Construction of the electrodes.
The electrodes were made using multistranded, Teflon-coated annealed stainless steel wire (catalog no. 793200; A-M Systems). The construction of the EMG electrode and nerve cuff was previously described in detail (Akay 2014; Akay et al. 2006; Pearson et al. 2005). One or two nerve cuff electrodes and six EMG recording electrodes were attached to the headpiece pin connector (female, part no. SAM1153-12; DigiKey Electronics, Thief River Falls, MN) and covered with epoxy (5 Minute epoxy gel; Devcon).
Electrode implantation surgeries.
All surgeries were performed in aseptic conditions on a warm water-circulated heating pad maintained at 42°C. Each mouse underwent an electrode implantation surgery as previously described (Akay 2014). Briefly, the animals were anesthetized with isoflurane (5% for inductions, 2% for maintenance of anesthesia), ophthalmic eye ointment was applied to the eyes, and their skin was sterilized with three-part skin scrub using hibitane, alcohol, and povidone-iodine. Before each surgery, buprenorphine (0.03 mg/kg) and ketoprofen (5 mg/kg) were injected subcutaneously as analgesics while the animals were still under anesthesia. Additional buprenorphine injections were performed at 12-h intervals for 48 h.
A set of six bipolar EMG electrodes and one or two nerve stimulation cuffs were implanted in all experimental mice (Akay et al. 2006; Pearson et al. 2005) as follows: small incisions were made on the shaved areas (neck and both hind legs), and each bipolar EMG electrode and the nerve cuff electrodes were led under the skin from the neck incision to the leg incisions, and the headpiece connector was stitched to the skin around the neck incision. The EMG recording electrodes were implanted into the right (ipsilateral) hip flexor (iliopsoas, Ipr), knee flexor (semitendinosus, Str) and extensor (vastus lateralis, VLr), and ankle flexor (tibialis anterior, TAr) and extensor (gastrocnemius, Gsr), as well as the left ankle extensor (gastrocnemius, Gsl). Nerve stimulation electrodes were chronically implanted in the left leg to activate contralateral proprioceptive and cutaneous feedback (tibial nerve) or predominantly cutaneous afferents (sural nerve), as well as the right leg to activate ipsilateral cutaneous afferents (sural nerve). Anesthesia was discontinued, and mice were placed in a heated cage for 3 days before being returned to a regular mouse rack. Food mash and hydrogel were provided for the first 3 days after the surgery. Any handling of the mouse was avoided until the animal was fully recovered, and the first recording session started at least 10 days after electrode implantation surgeries.
In total, 19 WT mice received electrode implantation surgeries. In nine mice, a cuff electrode was implanted on the tibial nerve, and in another nine mice, the cuff electrode was implanted on the sural nerve of the left hind leg. In five mice of each group, an additional cuff electrode was implanted on the sural nerve of the right leg. In one mouse, cuff electrodes were implanted on both the tibial and the sural nerve of the left hind leg.
Crossed reflex recording sessions.
After animals fully recovered (~10 days) from electrode implantation surgeries, crossed reflexes were recorded as follows: under brief anesthesia with isoflurane, a wire to connect the headpiece connector with the amplifier and the stimulation insulation units (ISO-FLEX; A.M.P.I., Jerusalem, Israel or DS4; Digitimer, Welwyn Garden City, UK) was attached to the mouse. Anesthesia was discontinued, and the mouse was placed on a mouse treadmill (model 802; custom-built in the workshop of the Zoological Institute, University of Cologne, Germany). The electrodes were connected to an amplifier (model 102; custom-built in the workshop of the Zoological Institute, University of Cologne, Germany) and a stimulus isolation unit. After the animal fully recovered from anesthesia (at least 5 min), the minimal (threshold) current that was necessary to elicit a local reflex response was determined to ensure afferent activation. This was done by injecting single impulses lasting 0.2 ms into the tibial nerve (average ± SD threshold current: 113 ± 49 µA; range: 65–200 µA; see insets in Fig. 1B) or double impulses lasting 0.2 ms with 2-ms intervals into the sural nerve (average ± SD threshold current: 518 ± 375 µA; range: 170–1250 µA; insets in Fig. 1C). Following the determination of threshold currents, the crossed reflex experiments were performed by injecting five current impulses lasting 0.2 ms at 500 Hz into the tibial or the sural nerve, set at either 1.2 times the threshold current (1.2× threshold) or five times the threshold current (5× threshold). The 1.2× threshold stimulation likely activates primary proprioceptive afferents (group Ia and Ib), whereas 5× threshold stimulation activates group Ia, Ib, and the group II proprioceptive afferents, as well as low-threshold cutaneous afferents (group II) as previously described in mice (Schomburg et al. 2013; Steffens et al. 2012).
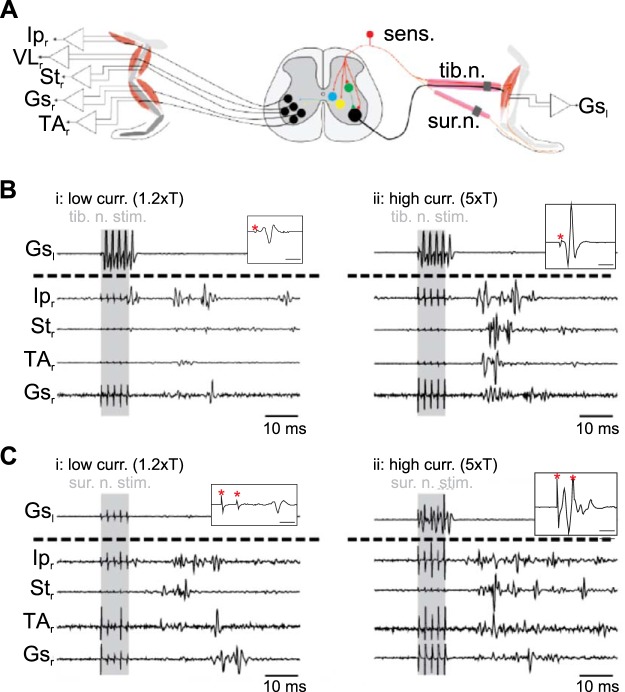
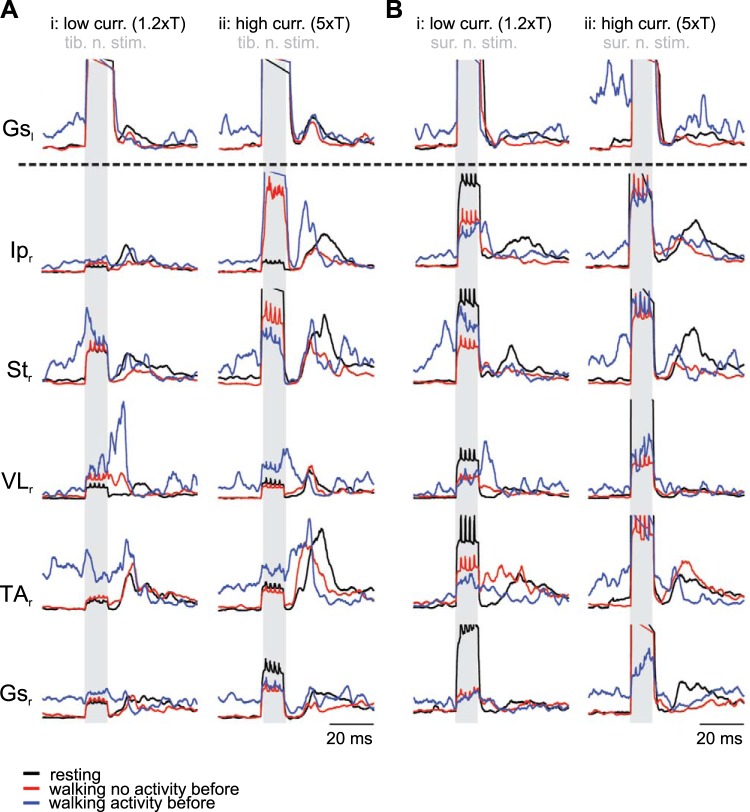
Fig. 1.
Schematic of experimental design used to investigate crossed reflex in vivo. A: experimental design used to investigate crossed reflex in vivo. B and C: examples of electromyographic recording at low (low curr. 1.2×T; i)- and high (high curr. 5×T; ii)-current stimulation from one mouse after stimulation of tibial nerve (tib. n. stim.; B) or sural nerve (sur. n. stim.; C). Shaded areas indicate stimulation. Insets in B and C show examples of the left gastrocnemius (Gsl) response to stimulation of the left tibial nerve with a single impulse (B) or the left sural nerve with double impulses (C). Time bars in insets indicate 2 ms, and red asterisks indicate stimulus artifact. Ipr, right iliopsoas; VLr, right vastus lateralis; Str, right semitendinosus; Gsr, right gastrocnemius; TAr, right tibialis anterior.
EMG signals from the five muscles of the right leg and the gastrocnemius muscle of the left leg were simultaneously recorded (sampling rate: 9.803 kHz) while the sural nerve or the tibial nerve of the left leg was electrically stimulated with five brief impulses (impulse duration: 0.2 ms, frequency: 500 Hz) using the ISO-FLEX (A.M.P.I.) and DS4 (Digitimer) stimulation insulation units. In some experiments, the right sural nerve was also stimulated in combination with contralateral tibial or sural nerve stimulation. Recordings of the crossed reflexes were performed while the mice were resting in the treadmill or moving at 0.2 m/s constant speed. The EMG signals were amplified (gain 100), bandpass filtered from 400 Hz to 20 kHz, and stored on the computer using Power1410 interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK). The filter settings were determined empirically to limit noise in freely behaving animals.
Statistical analysis.
All graphical representations of data were made using GraphPad Prism 5 and processed using Illustrator CS5 (Adobe). All data are means ± SD. One-to-one statistical comparisons of the data were done with the t-test or Mann-Whitney test using GraphPad Prism 5. Comparisons involving multiple averages were performed with an ANOVA test for nonparametric data sets (Kruskal-Wallis test). All statistical tests were two-tailed, and differences were considered statistically significant when the P value was <0.05.
RESULTS
Crossed reflex motor activity in flexor and extensor muscles.
First, we investigated the role of proprioceptive and cutaneous afferents in crossed reflexes in the mouse. We used in vivo electrophysiological techniques (Akay 2014) on WT mice in which we stimulated the tibial (proprioceptive and cutaneous) and sural (cutaneous only) nerves of the left leg while simultaneously recording flexor and extensor muscles of the right leg (Fig. 1A). Low-current stimulation (1.2× threshold) of the left tibial nerve predominantly activated group Ia (from muscle spindles) and Ib (from Golgi tendon organs) proprioceptive afferents (Jack 1978) and evoked motor responses in either flexor or extensor muscles of the right leg (Fig. 1Bi). High-current stimulation (5× threshold) of the left tibial nerve activated proprioceptive and cutaneous afferents evoked stronger motor responses simultaneously in right flexor and extensor muscles (Fig. 1Bii). Similarly, activation of cutaneous afferents by sural nerve stimulation induced simultaneous flexor and extensor muscle activity. Low-amplitude motor responses could be recorded at low-current sural nerve stimulation (1.2× threshold; Fig. 1Ci). Similar to tibial nerve stimulation, an increase of the current intensity to 5× threshold (high-current stimulation) evoked higher amplitude motor responses simultaneously in all investigated flexor and extensor muscles (Fig. 1Cii). Stimulation of either the tibial nerve or the sural nerve elicited crossed reflex responses in all recorded muscles and was more consistent when stimulation strength was set to 5× threshold. In neither of the stimulations was increased activity of the mice or vocalizing observed, suggesting pain receptors were not activated (Bui et al. 2013).
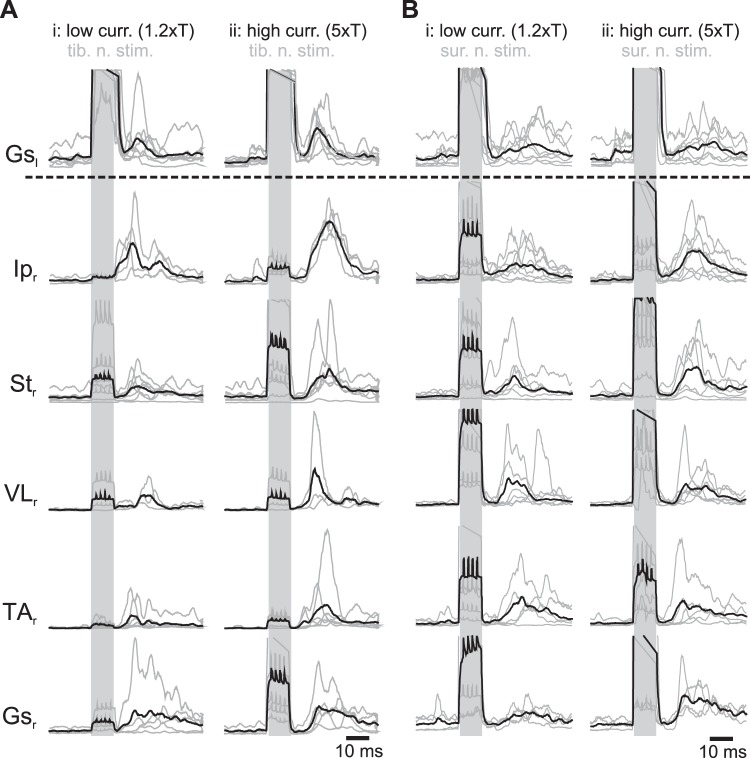
We further investigated the crossed reflex responses by analyzing the rectified EMG signals and averaging them over multiple trials (Fig. 2, A and B). The average EMG traces from all the trials performed at one current stimulation allowed us to determine if proprioceptive and cutaneous sensory afferents could evoke reliable motor activity in flexors and extensors of different joints in the contralateral leg. Our results show that crossed reflex responses in every recorded muscle could be elicited regardless of low- or high-current stimulation (Fig. 2). High-current stimulation (Fig. 2, Aii and Bii) evoked higher amplitude crossed reflex responses than low-current stimulation (Fig. 2, Ai and Bi).
Fig. 2.
Average crossed reflex motor responses. A and B: average crossed reflex responses from different muscles after low (low curr. 1.2×T; i)- and high (high curr. 5×T; ii)-current stimulation of tibial nerve from 7 animals (tib. n. stim.; A) or sural nerve stimulation from 8 animals (sur. n. stim.; B). Gray lines are averages of 20–40 stimulations in each animal. Black line corresponds to the average across all animals. Shaded areas indicate stimulation. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius.
In summary, our results show that stimulation of proprioceptive (low-current tibial nerve stimulation) and cutaneous (sural nerve stimulation) afferents evokes excitatory crossed reflex motor responses across hindlimb flexor and extensor muscles in mice in vivo.
Temporal characteristics of muscle activation pattern during crossed reflex.
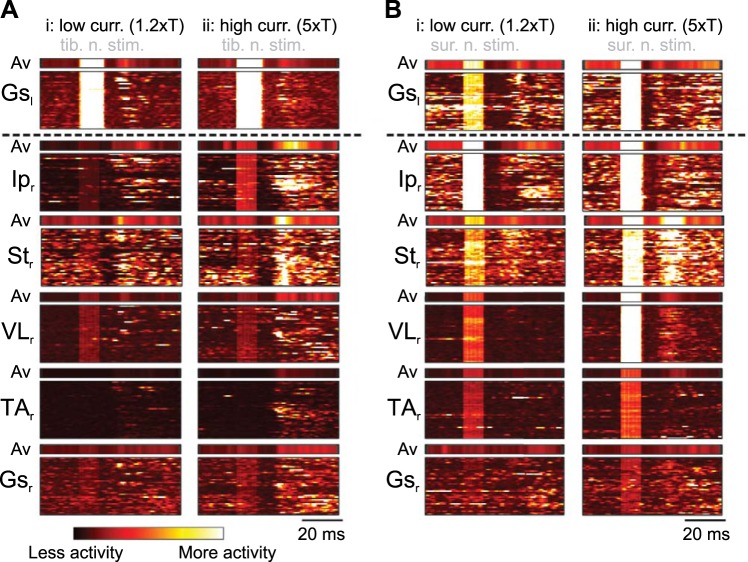
We next aimed to describe the overall activation pattern of the different groups of muscles in response to stimulation of the contralateral sensory afferents. The muscle activation patterns in response to contralateral tibial nerve and sural nerve stimulations are illustrated as heat diagrams in Fig. 3, A and B, respectively. At low- and high-current stimulation, replicable motor responses were evoked in the flexor and extensor muscles, confirming the result shown above (Fig. 2). We also detected a 10-ms activity gap between nerve stimulation offset and first signs of muscle activation following both the tibial and the sural nerve stimulation. This gap was visible as a darker phase in the color-coded map, before the activation of the motor responses, lasting for ~10 ms after the stimulation. These data suggest that reliable and stereotyped crossed reflex responses can be elicited by stimulating tibial nerve or sural nerves.
Fig. 3.
Muscle responses in ipsilateral and contralateral muscles are consistent across individual trials. A and B: heat diagram shows the muscle activity during low (low curr. 1.2×T; i)- and high (high curr. 5×T; ii)-current stimulation of the contralateral tibial nerve (tib. n. stim.; A) and the sural nerve (sur. n. stim.; B) from 1 representative animal for each nerve stimulation experiment. Muscle responses to each of 40 nerve stimulations from 1 experiment are staggered on the vertical axis as a function of time. Brighter colors indicate higher muscle activity. Concentrated brighter areas indicate consistent muscle activity to nerve stimulation, whereas dark areas indicate no activity. Average muscle activity of all trials (Av) is shown above each diagram. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius.
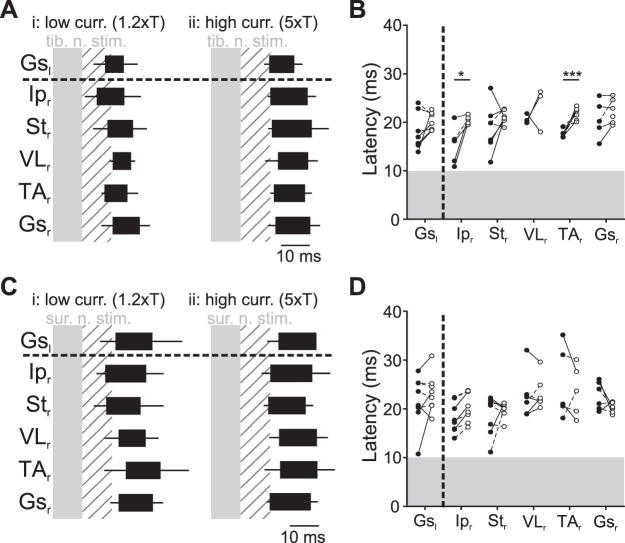
We further analyzed the delays of on- and offsets of EMG activities from all recorded muscle from the onset of the contralateral nerve stimulation (Fig. 4). Under low-current tibial nerve stimulation, even though not statistically different, the onset of the Ipr activity occurred on average first, followed by the distal flexor muscles (Str and TAr) and, finally, the extensor muscles (VLr and Gsr; Fig. 4Ai). However, during high-current tibial nerve stimulation, all muscle activity onsets were nearly synchronized. Comparing the onset latencies of different muscles during low- and high-current stimulation revealed that the onset latencies of the Ipr and TAr muscle activity, but not the extensor muscle activities, increased under high-current stimulation (Fig. 4B). This suggests two possibilities: at high-current tibial nerve stimulation, either the excitatory crossed reflex is delayed relative to low-current stimulation, or cutaneous afferents have a crossed inhibitory influence mediated by cutaneous afferents.
Fig. 4.
Crossed reflex muscle activation pattern. A: pattern of muscle activation at low (low curr. 1.2×T; i)- and high (high curr. 5×T; ii)-current stimulation following tibial nerve stimulation (tib. n. stim.) summarizing data from 7 experiments. B: average latency of the different muscles at low (●)- and high (○)-current stimulation following tibial nerve stimulation in individual animals. Latencies were measured from the start of the stimulation to the first visually detectable action potentials in the electromyographic recordings. Statistical significance between individual averages is indicated by a solid line, whereas dashed lines indicate no statistical difference. *P < 0.05; ***P < 0.001. C: pattern of muscle activation at low- and high-current stimulation following sural nerve stimulation (sur. n. stim.) summarizing data from 8 experiments. D: pattern of muscle activation at low- and high-current stimulation following sural nerve stimulation. Shaded areas represent the stimulation. Hatched areas represent 10-ms period after the stimulation. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius.
When we stimulated the sural nerve, the activation of the recorded muscles was more synchronous even at low-current stimulation, with some subtle differences (Fig. 4C). When we compared the onsets of EMG activities of low- and high-current left sural nerve stimulation, there was no statistical significance (Fig. 4D). These data suggest that high-current tibial nerve stimulation and low- or high-current sural nerve stimulation, activating more cutaneous afferent fibers, tend to activate all recorded muscles simultaneously. Our results regarding muscle activity onset synchronization raised two possibilities: either the excitatory crossed reflex could be delayed, or there is an inhibitory crossed reflex pathway mediated by cutaneous afferent fibers.
Short-latency inhibition in crossed and local reflex.
We next sought to differentiate between the two possibilities described above. We used a paired stimulation protocol where peripheral nerves in both legs were stimulated with different delays. Either tibial nerve or sural nerve was implanted in the left leg to evoke crossed reflex motor responses transduced by proprioceptive or cutaneous afferents, respectively. Furthermore, in addition to the five EMG recording electrodes implanted in the five muscles of the right leg, we implanted an additional nerve stimulation electrode on the right sural nerve to evoke local cutaneous reflex (Figs. 5 and 6). We predicted that if there is an inhibitory crossed reflex pathway, we should detect decreased activity with a constant delay after contralateral nerve stimulation regardless of the presence of EMG activity initiated by local reflexes.
Fig. 5.
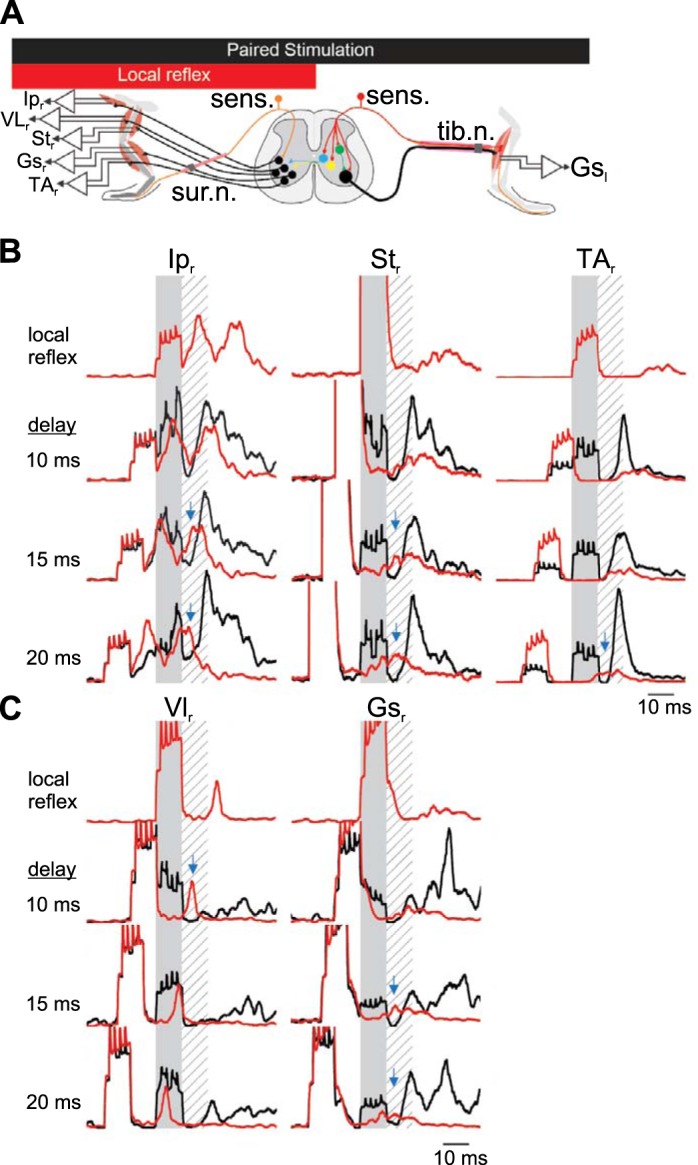
Short-latency inhibition in crossed reflex initiated by tibial nerve stimulation. A: schematic representation of the paired stimulation of both legs at different delays to provide evidence of an inhibitory period if there is a suppression of the motor responses from one leg following the stimulation of the other leg. Data are from 40 stimulations from 1 animal, representative of 4 experiments. B and C: examples of electromyographic (EMG) recording in the contralateral flexor muscles (B) or the extensor muscles (C) after stimulation of the contralateral tibial nerve. Shaded areas represent stimulation of the contralateral tibial nerve to evoke a crossed reflex. Hatched areas represent the silent period detected previously. Red traces represent average EMG response to local reflex activation initiated by ipsilateral sural nerve stimulation. Black lines indicate the EMG response when the ipsilateral sural nerve is stimulated with the contralateral tibial nerve with a delay indicated at left of each set of recordings. Blue arrows represent the suppression of the local cutaneous reflex by contralateral sensory stimulation. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius; sens., sensory stimulation; tib. n., tibial nerve; sur. n., sural nerve.
Fig. 6.
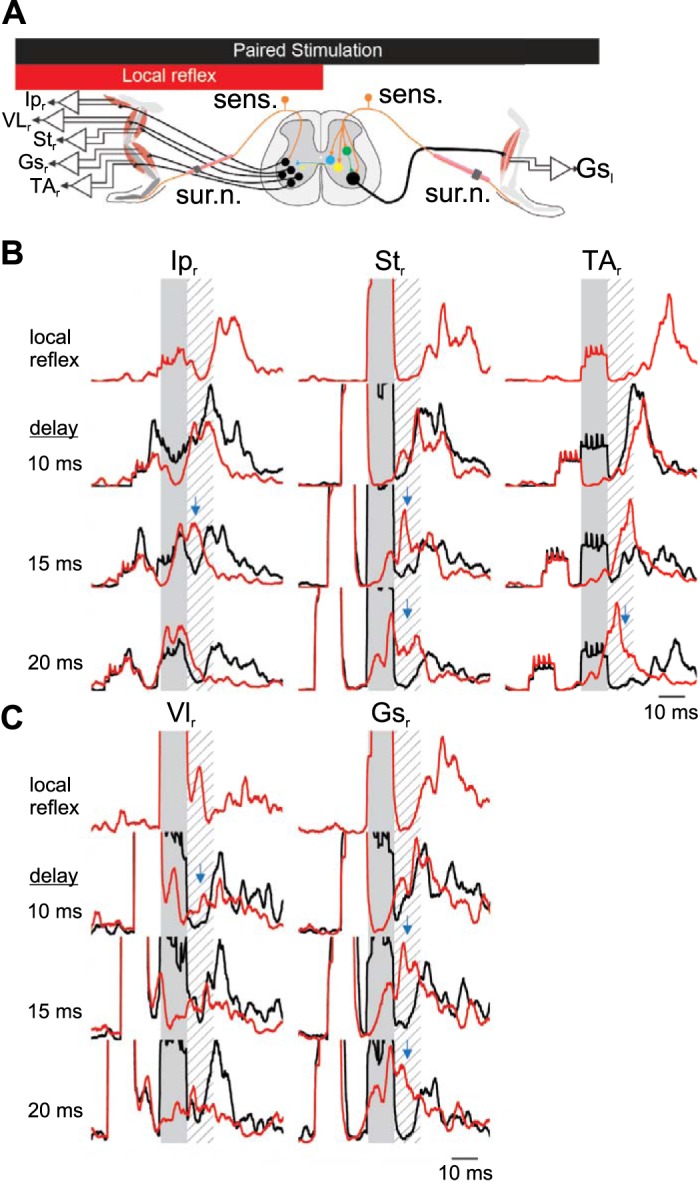
Short-latency inhibition in crossed reflex initiated by sural nerve stimulation. A: schematic representation of the paired stimulation of both legs at different delays to provide evidence of an inhibitory period if there is a suppression of the motor responses from one leg following the stimulation of the other leg. Averages are calculated using 40 stimulations from 1 animal, representative of 4 experiments. B and C: examples of electromyographic (EMG) recordings in the contralateral flexor muscles (B) or the extensor muscles (C) after stimulation of the contralateral sural nerve. Shaded areas represent stimulation of the contralateral tibial nerve to evoke a crossed reflex. Hatched areas represent the silent period detected previously. Red traces represent average EMG response to local reflex activation initiated by ipsilateral sural nerve stimulation. Black lines indicate the EMG response when the ipsilateral sural nerve is stimulated with the contralateral sural nerve with a delay indicated at left of each set of recordings. Blue arrows represent the suppression of local cutaneous reflex by contralateral sensory stimulation. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius; sens., sensory stimulation; sur. n., sural nerve.
First, we stimulated the left tibial nerve with high-current simultaneously with the right sural nerve at varying delays (Fig. 5A). Figure 5, B and C, show the average EMG responses from the recorded flexor and extensor muscles, respectively, in response to crossed and local reflexes with varying delays between these two stimulations. Immediately after stimulation of the left tibial nerve (Fig. 5, B and C), we could detect a period of decreased EMG activity regardless of the presence of the local reflex response initiated by right sural nerve stimulation. This response could be detected consistently in all recorded flexor (Fig. 5B) and extensor (Fig. 5C) muscles. This finding suggested that, apart from the excitatory crossed reflex pathway indicated by muscle activation, an early inhibitory crossed reflex pathway is also present in mice in vivo.
Similar results were obtained when we stimulated the left sural nerve to initiate the crossed reflex response (Fig. 6A). Stimulation of the left sural nerve with high current also initiated a period of decreased EMG activity regardless of the delay between the right sural nerve stimulation and the left sural nerve stimulation (Fig. 6, B and C). The decreased EMG activity was present in all recorded flexor (Fig. 6B) and extensor (Fig. 6C) muscle recordings. These observations suggest that high-current activation of afferent fibers initiate crossed reflex inhibitory responses that are likely induced by cutaneous afferent activation.
In summary, our results show that sensory information is transmitted to the contralateral motor neurons through inhibitory as well as excitatory pathways following tibial or sural nerve stimulation. Furthermore, this inhibitory pathway affects the activity of all recorded muscles of the contralateral leg, as does the excitatory pathway.
Crossed reflex responses during locomotion.
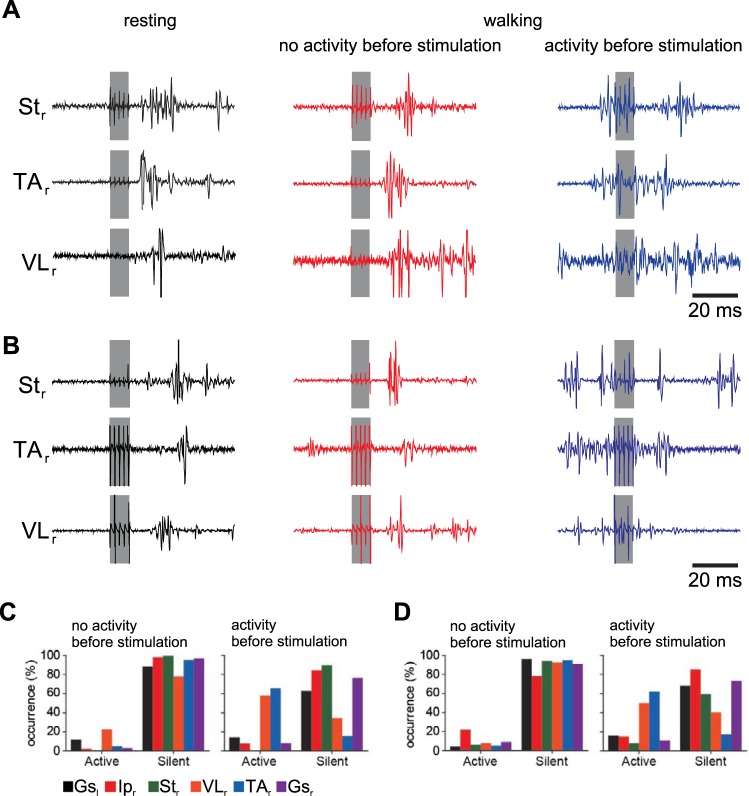
Do crossed reflex responses occur the same way when the animal is moving compared with when the animal is resting, as described above? To address this question, we performed contralateral tibial or sural nerve stimulation during locomotion on treadmill and recorded the EMG activity from the contralateral muscles (Fig. 7). Our data revealed that the crossed reflex response during locomotion depended on the timing of the nerve stimulation relative to the activity of the muscle before the stimulation, that is, whether the stimulation occurred when the muscle was active before the stimulation or inactive before the stimulation (Fig. 7). Therefore, we separated the data into two groups: 1) the muscle was inactive when the contralateral nerve was stimulated (red traces in Figs. 7A, 7B, 8A, and 8B); and 2) the muscle was active at the time the stimulation of the contralateral nerve occurred (blue traces in Figs. 7A, 7B, 8A, and 8B). When the VLr and TAr muscles were inactive before nerve stimulation, we consistently detected the same 10-ms delay that we had detected while the mice were at rest. However, when the contralateral nerve stimulation occurred while the VLr and TAr muscles were active, the 10-ms silent period was absent (Figs. 7 and 8). In contrast, the 10-ms delay was always present in the Ipr, Str, and Gsr activity profile, regardless of whether the muscle was already active before nerve stimulation (Figs. 7 and 8). These data indicate that the inhibitory crossed reflex pathway is downregulated during locomotion selectively for VLr and TAr muscles depending on activity before the contralateral nerve stimulation.
Fig. 7.
Crossed reflex response in right vastus lateralis (VLr) and right tibialis anterior (TAr) depends on muscle activity status before nerve stimulation during walking. A and B: electromyographic recording of the contralateral semitendinosus and the contralateral tibialis anterior in resting (black traces) and the two different states during walking: when the muscle was not active (red traces) or active (blue traces) before the tibial nerve (A) or the sural nerve (B) stimulation. C and D: relative counts (percentages) of muscle responses, as active or silent within the first 10 ms after stimulation of tibial nerve in 7 experiments (C) or the sural nerve in 5 experiments (D). Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; Gsr, right gastrocnemius.
Fig. 8.
Muscle responses in crossed reflex during walking. A and B: electromyographic recording in the contralateral muscles comparing the motor activity while the animal is resting (black traces) or walking when the muscle was not active (red traces) or active (blue traces) before tibial nerve (A) or sural nerve (B) stimulation. Traces are pooled averages from 7 mice for the tibial nerve stimulation and 5 mice for the sural nerve stimulation. Shaded background indicates nerve stimulation. Gsl, left gastrocnemius; Ipr, right iliopsoas; Str, right semitendinosus; VLr, right vastus lateralis; TAr, right tibialis anterior; Gsr, right gastrocnemius.
DISCUSSION
In this article, we present a detailed analysis of the crossed reflex responses in awake mice in vivo during resting and locomotion. Regardless of whether we stimulated peripheral nerves to activate proprioceptive or cutaneous afferents, activation of all muscles on the contralateral side was observed (Figs. 1–4). This indicates a widespread excitatory crossed reflex pathway to all motor neuron pools innervating the recorded muscles in this study. A more detailed investigation of the muscle responses indicates the presence of a short-latency inhibitory crossed reflex pathway (Figs. 3 and 4). To provide evidence for the inhibitory crossed reflex pathway, we demonstrated crossed reflex action in the presence of muscle activation, caused by local reflex activation through ipsilateral nerve stimulation (Figs. 5 and 6). Finally, we have shown that the inhibitory crossed reflex pathway controlling the activity of knee extensor (VL) and ankle flexor (TA) is downregulated during locomotion, whereas it is not changed for other recorded muscles (Figs. 7 and 8). This study represents the first comprehensive analysis of crossed reflexes in mice in vivo. We show the existence of a common crossed inhibitory pathway in mice that is modulated selectively for the VL and TA muscles during locomotion.
Non-nociceptive sensory afferent activation initiates crossed reflex responses.
In this article, we provide evidence that proprioceptive as well as cutaneous afferent stimulation initiates crossed reflex responses. Our results demonstrate that low-current tibial nerve stimulation, which mainly activates proprioceptive afferents, evokes crossed reflex responses in all recorded muscles. High-current electrical stimulation of the tibial nerve, which recruits additional cutaneous afferents, also causes activation of all contralateral muscles, but in a more synchronized manner. Furthermore, the sural nerve carries predominantly cutaneous afferents innervating the skin on the posterior site of the leg. In rodents, the sural nerve also carries a small amount of motor and proprioceptive afferent fibers to and from the flexor digiti minimi muscle in the foot (Peyronnard and Charron 1982; Steffens et al. 2012). The reflex response observed in this study through sural nerve stimulation is most likely due to cutaneous afferent activation, but the contribution of efferent and proprioceptive afferent activation to and from the flexor digiti minimi muscle cannot be excluded. Motor fiber activation is unlikely to be a contributor, because the delays considered in this study are too short for motor fibers to elicit toe muscle contractions that would elicit feedback in the EMG activity pattern. The contribution of proprioceptive afferent activation from the flexor digiti minimi muscle is a possibility that we cannot entirely exclude.
The highest current used in this study to activate afferent fiber was five times the threshold current to initiate a local reflex response. This was well below the current needed to activate nociceptive afferents, determined by vocalization (Bui et al. 2013), which typically occurs around 8–10 times the threshold current. Therefore, our results provided evidence that cutaneous afferents that initiated crossed reflex responses were non-nociceptive cutaneous afferents. These results suggest that proprioceptive as well as non-nociceptive cutaneous afferent activation initiates crossed reflex responses. Overall, these results are in accordance with the results of previous research on cats and humans that muscle afferents (Aggelopoulos and Edgley 1995; Arya et al. 1991; Gervasio et al. 2017; Jankowska and Edgley 2010; Stubbs and Mrachacz-Kersting 2009; Stubbs et al. 2011) as well as cutaneous afferents (Edgley and Aggelopoulos 2006; Gauthier and Rossignol 1981; Perl 1957; Stubbs and Mrachacz-Kersting 2009) mediate crossed reflex responses.
Inhibitory crossed reflex responses initiated by cutaneous afferent activation.
Two observations provide evidence for an inhibitory crossed reflex action initiated by non-nociceptive cutaneous afferent activation. First, stimulation of the tibial nerve, although not statistically significant, initiated a muscle activation sequence starting with the most proximal flexor muscle (Ipr), followed by the distal flexors (Str and TAr) and, finally, the two extensor muscles (VLr and Gsr) on the contralateral side. The onsets of the VLr and Gsr occurred ~10 ms after the stimulation offset (hatched area in Fig. 4Ai). When the stimulation strength was increased to additionally recruit cutaneous afferents, the muscle activation sequence was changed to simultaneous activation at ~10 ms after stimulation offset (hatched area in Fig. 4Aii). Stimulation of the sural nerve, regardless of stimulation strength, elicited an activation pattern resembling high-current tibial nerve stimulation. This finding suggests the possibility that cutaneous afferent activation induces an inhibitory crossed reflex pathway with inhibition of muscle activity lasting ~10 ms. Synchronization of muscle activity onsets with cutaneous afferent activation suggested the existence of an inhibitory crossed reflex pathway as previously described in cats (Aggelopoulos et al. 1996; Arya et al. 1991; Edgley and Aggelopoulos 2006).
The second observation supporting a crossed inhibitory pathway initiated by cutaneous afferents came from the experiments where the local reflex was suppressed by the crossed reflex. Ipsilateral cutaneous afferent stimulation was paired with contralateral afferent stimulation at different delays, which allowed us to detect a short-latency inhibition for ~10 ms (hatched areas in Figs. 5 and 6) initiated by contralateral cutaneous afferent stimulation. Our data do not provide evidence for a direct inhibitory influence that involve inhibitory synaptic input to the motor neurons. One alternative possibility is that the decreased EMG activity reflects motor neurons entering a refractory period following short-latency excitation, which is measured as reduced activity. Indeed, the Ip activity shown in Fig. 5B indicates increased activity before activity reduction within the 10-ms period. However, this short-latency activation was clearly absent in the TA, VL, and Gs, which does not support the idea of refractory period involvement, but our current data do not allow a definite exclusion of this possibility. Nevertheless, our data support previous findings of inhibitory crossed reflex pathways initiated by cutaneous afferents in cats (Edgley and Aggelopoulos 2006) and humans (Gervasio et al. 2017). Together, the two observations provide a strong indication that non-nociceptive cutaneous afferent activation initiates inhibitory crossed reflexes that last for ~10 ms.
Crossed reflex during locomotion.
Our data suggest that the crossed inhibitory reflex is downregulated when the animal walks selectively for the knee extensor (VL) and the ankle flexor (TA) muscles. We recorded the muscle activity response to contralateral afferent activation while the animals were moving on a treadmill at constant speed at 0.2 m/s. Stimulation of the contralateral nerves initiated muscle responses that closely resembled the responses in resting mice, except for the VLr and the TAr muscles, which depended on whether these muscles were active before nerve stimulation. That is, when VLr (mainly active during stance) and TAr (mainly active during swing) were inactive before the nerve stimulation, their responses were almost identical as during resting. In contrast, when these muscles were active, we did not observe the silent 10-ms latency period, indicating reduced strength of crossed inhibition relative to crossed excitation. Previous experiments in cats also observed a downregulated inhibitory crossed reflexes selectively for the VLr muscle during locomotion (Frigon and Rossignol 2008). The authors assumed that the absence of crossed inhibitory influence in the VLr was due to the more rostral location of the motor neuron pool in the spinal cord compared with the other muscle. However, we have shown that the inhibitory crossed reflex influence is not only downregulated in the VLr (motor neuron cell bodies located between lumbar spinal segments 1–3) but also in the TAr, whose motor neuron pool is located more caudally (lumbar spinal segments 3–4) (McHanwell and Biscoe 1981). The relative location of the motor neuron pool innervating the Ip in the mouse has not been demonstrated, but its location in the cat is even more rostral than the one innervating VL (Vanderhorst and Holstege 1997). Our results demonstrate that the inhibition of the Ip muscle is not modulated during locomotion as it is for the VL and TA. Therefore, our data do not support the view that the absence of the state-dependent modulation of inhibitory crossed reflex response is due to motor neuron pool location.
Crossed reflex responses during locomotion can switch from activation of extensor muscles to activation of flexor muscles depending on the phase of the step cycle (Duysens et al. 1980; Gervasio et al. 2013), which contributes to the dynamic stability during locomotion (Gervasio et al. 2015). Separating the contralateral afferent stimulation into phases in which Ipr was active (approximating swing phase) and phases in which Ipr was not active (approximating stance phase) did not reveal any conclusive phase dependency in our experiments (data not shown). However, clear and consistent differences in muscle response in VLr and TAr were observed when the separation was made on the basis of muscle activity. We observed that the inhibitory crossed reflex pathway was selectively downregulated for the VLr and TAr muscles when the muscles were active before stimulation. The short-latency inhibition was replaced by a very short-latency excitation during locomotion. The discrepancy between our results with the previous findings from cat experiments (Duysens et al. 1980; Frigon and Rossignol 2008; Gauthier and Rossignol 1981; Hurteau et al. 2017) may indicate differences in mechanisms across species. That is, whereas the nervous system of the cat takes stance or swing phase as a reference for reflex reversal, mice might prefer individual muscle activity. In addition, differences in these mechanisms might relate to the much smaller size and the much higher stepping frequency of mice relative to cats. Nevertheless, this is a speculation, and further investigation is required for a more definite conclusion.
In this article, we provide a detailed description of the motor output in one leg when sensory afferents in the contralateral leg are stimulated in mice in vivo. Our results demonstrate that crossed reflexes are mediated by cutaneous and proprioceptive afferents and can be evoked in vivo in freely moving mice. Furthermore, crossed reflex pathways involve a short-latency inhibitory component in flexor and extensor muscles that can be modulated to evoke appropriate motor responses according to the behavioral context of the contralateral leg. This short-latency inhibitory response is followed by an excitatory motor response in flexor and extensor muscles. These results will serve as the groundwork in our efforts to identify the involvement of genetically distinct classes of commissural interneurons (Lanuza et al. 2004; Talpalar et al. 2013; Zhang et al. 2008) involved in crossed reflexes. These insights will be important for understanding how sensory pathways transfer sensory information to the contralateral side of the spinal cord, and how sensory information modulates left-right coordination during locomotion.
GRANTS
This work was funded by the Dalhousie Medical Research Foundation and Natural Sciences and Engineering Research Council of Canada Grant RGPIN-2015-03871 (to T. Akay).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.D.L. conception and design of research; O.D.L. performed experiments; O.D.L. and T.A. analyzed data; O.D.L. and T.A. interpreted results of experiments; O.D.L. and T.A. prepared figures; O.D.L. and T.A. drafted manuscript; O.D.L. and T.A. edited and revised manuscript; O.D.L. and T.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brenda Ross for technical assistance. We thank Dr. Ying Zhang and Dr. Eiman Azim for helpful comments and suggestions on the manuscript, and Tyler Wells for proofreading the manuscript.
REFERENCES
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci 16: 723–729, 1996. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett 185: 60–64, 1995. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Akay T. Long-term measurement of muscle denervation and locomotor behavior in individual wild-type and ALS model mice. J Neurophysiol 111: 694–703, 2014. doi: 10.1152/jn.00507.2013. [DOI] [PubMed] [Google Scholar]
- Akay T, Acharya HJ, Fouad K, Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96: 642–651, 2006. doi: 10.1152/jn.00174.2006. [DOI] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci USA 111: 16877–16882, 2014. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol 444: 117–131, 1991. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagust J, Kerkut GA. Crossed reflex activity in an entire, isolated, spinal cord preparation taken from juvenile rodents. Brain Res 411: 397–399, 1987. doi: 10.1016/0006-8993(87)91094-8. [DOI] [PubMed] [Google Scholar]
- Böhm UL, Wyart C. Spinal sensory circuits in motion. Curr Opin Neurobiol 41: 38–43, 2016. doi: 10.1016/j.conb.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J, Loeb GE. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol 44: 1024–1037, 1980. doi: 10.1152/jn.1980.44.5.1024. [DOI] [PubMed] [Google Scholar]
- Duysens J, Loeb GE, Weston BJ. Crossed flexor reflex responses and their reversal in freely walking cats. Brain Res 197: 538–542, 1980. doi: 10.1016/0006-8993(80)91143-9. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Aggelopoulos NC. Short latency crossed inhibitory reflex actions evoked from cutaneous afferents. Exp Brain Res 171: 541–550, 2006. doi: 10.1007/s00221-005-0302-9. [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from feline group II muscle afferents. J Physiol 552: 961–974, 2003. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Short-latency crossed inhibitory responses in extensor muscles during locomotion in the cat. J Neurophysiol 99: 989–998, 2008. doi: 10.1152/jn.01274.2007. [DOI] [PubMed] [Google Scholar]
- Garcia-Campmany L, Stam FJ, Goulding M. From circuits to behaviour: motor networks in vertebrates. Curr Opin Neurobiol 20: 116–125, 2010. doi: 10.1016/j.conb.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L, Rossignol S. Contralateral hindlimb responses to cutaneous stimulation during locomotion in high decerebrate cats. Brain Res 207: 303–320, 1981. doi: 10.1016/0006-8993(81)90366-8. [DOI] [PubMed] [Google Scholar]
- Gervasio S, Farina D, Sinkjær T, Mrachacz-Kersting N. Crossed reflex reversal during human locomotion. J Neurophysiol 109: 2335–2344, 2013. doi: 10.1152/jn.01086.2012. [DOI] [PubMed] [Google Scholar]
- Gervasio S, Kersting UG, Farina D, Mrachacz-Kersting N. The effect of crossed reflex responses on dynamic stability during locomotion. J Neurophysiol 114: 1034–1040, 2015. doi: 10.1152/jn.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasio S, Voigt M, Kersting UG, Farina D, Sinkjær T, Mrachacz-Kersting N. Sensory feedback in interlimb coordination: contralateral afferent contribution to the short-latency crossed response during human walking. PLoS One 12: e0168557, 2017. doi: 10.1371/journal.pone.0168557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Chraibi A, Desrochers E, Telonio A, Frigon A. Nonlinear modulation of cutaneous reflexes with increasing speed of locomotion in spinal cats. J Neurosci 37: 3896–3912, 2017. doi: 10.1523/JNEUROSCI.3042-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau MF, Thibaudier Y, Dambreville C, Danner SM, Rybak IA, Frigon A. Intralimb and interlimb cutaneous reflexes during locomotion in the intact cat. J Neurosci 38: 4104–4122, 2018. doi: 10.1523/JNEUROSCI.3288-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJ. Some methods for selective activation of muscle afferent fibres. In: Studies in Neurophysiology: Presented to A. K. McIntyre, edited by Porter R. London: Cambridge University Press, 1978, p. 155–176. [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Brain Res Rev 57: 46–55, 2008. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010. doi: 10.1111/j.1460-9568.2010.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol 565: 645–658, 2005. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1: 20–29, 2000. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999. doi: 10.1016/S0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42: 375–386, 2004. doi: 10.1016/S0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lu DC, Niu T, Alaynick WA. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci 8: 25, 2015. doi: 10.3389/fnmol.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res 143: 239–249, 2004a. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: morphology and discharge patterns during fictive locomotion. J Comp Neurol 474: 546–561, 2004b. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol 533: 41–50, 2001. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci 293: 477–508, 1981. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- Nakanishi ST, Whelan PJ. A decerebrate adult mouse model for examining the sensorimotor control of locomotion. J Neurophysiol 107: 500–515, 2012. doi: 10.1152/jn.00699.2011. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Acharya H, Fouad K. A new electrode configuration for recording electromyographic activity in behaving mice. J Neurosci Methods 148: 36–42, 2005. doi: 10.1016/j.jneumeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Perl ER. Crossed reflexes of cutaneous origin. Am J Physiol 188: 609–615, 1957. doi: 10.1152/ajplegacy.1957.188.3.609. [DOI] [PubMed] [Google Scholar]
- Peyronnard JM, Charron L. Motor and sensory neurons of the rat sural nerve: a horseradish peroxidase study. Muscle Nerve 5: 654–660, 1982. doi: 10.1002/mus.880050811. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev 86: 89–154, 2006. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Kalezic I, Dibaj P, Steffens H. Reflex transmission to lumbar α-motoneurones in the mouse similar and different to those in the cat. Neurosci Res 76: 133–140, 2013. doi: 10.1016/j.neures.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens H, Dibaj P, Schomburg ED. In vivo measurement of conduction velocities in afferent and efferent nerve fibre groups in mice. Physiol Res 61: 203–214, 2012. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Mrachacz-Kersting N. Short-latency crossed inhibitory responses in the human soleus muscle. J Neurophysiol 102: 3596–3605, 2009. doi: 10.1152/jn.00667.2009. [DOI] [PubMed] [Google Scholar]
- Stubbs PW, Nielsen JF, Sinkjær T, Mrachacz-Kersting N. Phase modulation of the short-latency crossed spinal response in the human soleus muscle. J Neurophysiol 105: 503–511, 2011. doi: 10.1152/jn.00786.2010. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature 500: 85–88, 2013. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VGJM, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60: 84–96, 2008. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]