Abstract
Disruption of the monoaminergic system, e.g. by sleep deprivation (SD), seems to promote certain diseases. Assessment of monoamine levels over the circadian cycle, during different sleep stages and during SD is instrumental to understand the molecular dynamics during and after SD. To provide a complete overview of all available evidence, we performed a systematic review. A comprehensive search was performed for microdialysis and certain monoamines (dopamine, serotonin, noradrenaline, adrenaline), certain monoamine metabolites (3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxyindoleacetic acid (5-HIAA)) and a precursor (5-hydroxytryptophan (5-HTP)) in PubMed and EMBASE. After screening of the search results by two independent reviewers, 94 publications were included. All results were tabulated and described qualitatively. Network-meta analyses (NMAs) were performed to compare noradrenaline and serotonin concentrations between sleep stages. We further present experimental monoamine data from the medial prefrontal cortical (mPFC). Monoamine levels varied with brain region and circadian cycle. During sleep, monoamine levels generally decreased compared to wake. These qualitative observations were supported by the NMAs: noradrenaline and serotonin levels decreased from wakefulness to slow wave sleep and decreased further during Rapid Eye Movement sleep. In contrast, monoamine levels generally increased during SD, and sometimes remained high even during subsequent recovery. Decreases during or after SD were only reported for serotonin. In our experiment, SD did not affect any of the mPFC monoamine levels. Concluding, monoamine levels vary over the light-dark cycle and between sleep stages. SD modifies the patterns, with effects sometimes lasting beyond the SD period.
Keywords: Systematic review, network meta-analysis, microdialysis, monoamines, circadian rhythm, sleep deprivation
Introduction
Circadian rhythms (CRs) and sleep are influenced by multiple external (e.g. light) and internal (e.g. accumulation of hypnogenic substances) factors [1]. Our relationship to light cues and rhythms has become disturbed in our industrialized 24/7 society. This resulted in increased prevalences of CR desynchrony and impaired sleep, which in turn severely impact health [2].
Unusual working hours can result in low sleep quality, consumption of stimulants and/or hypnotics, and stress. These factors could all contribute to sleep-deprivation associated disorders (e.g. insomnia and mental illnesses) [3,4,5,6,7].
Sleep deprivation (SD) induces severe cognitive impairments such as loss of attention, increased reaction times, impaired multitasking and planning, slurred speech, impaired memory, and poor emotion regulation [8,9,10]. Mainly the loss of attention affects safety e.g. in driving [11] and dangerous work [12,13]. Furthermore, SD and CR disruption are associated with diseases such as obesity, sleep apnoea, diabetes, cardiovascular diseases, and depression [2,14].
While the behavioural impact of SD is well-known, our knowledge of the responsible underlying neurological mechanism is still limited. Sleep is embedded in a complex network of interconnected brain regions using multiple neurotransmitters and neuromodulators, within which the monoaminergic pathways seem responsible for sleep-wake modulation [15,16]. In addition, monoamines play a role in certain cognitive processes that are disrupted by SD [17,18,19]. Therefore, it is important to provide a complete overview of how the release of these monoamines is related to circadian rhythms and how it is affected by sleep deprivation.
A well-established way to study the release of neurotransmitters is to measure them with microdialysis. Microdialysis is a versatile technique to study the extracellular space in vivo, based on the simple principle of diffusion [20]. A probe with a semi-permeable membrane is placed in a region of interest. When the probe is perfused continuously with an isotonic solution, substances in the extracellular space (e.g. neurotransmitters and neuromodulators) will diffuse through the membrane into the perfusion fluid, which is collected for analysis. The concentrations in the perfusate reflect neuronal release and are dependent on neuronal activity [21,22]. Microdialysis allows for measurements on a minutes-hours timescale for several compounds simultaneously [23,24].
Because monoamines seem to be involved in many of the functions disrupted by SD, we performed a systematic review of in vivo extracellular concentration of several monoamines [i.e. dopamine (DA), serotonin (5-HT), noradrenaline (NA), adrenaline (ADRE)) and related compounds (i.e. 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxytryptophan (5-HTP), and 5-hydroxyindoleacetic acid (5-HIAA)) in relation to 1.) CRs, 2.) naturally occurring sleep stages, and 3.) SD. We excluded the monoamine histamine from this review, because we included it in a comparable review on amino acids [25], aligned with our primary data.
Systematic reviews (SRs) provide all available evidence on a subject in a complete and organized manner (i.e. transparent and reproducible methodology) [26]. Even though numerous excellent narrative reviews exist on microdialysis (e.g. [27]), SRs combining microdialysis and monoamine measurements are scarce. The two examples we are aware of describe the enhancement of monoamine levels by ethanol administration [28] and serotonin neurotransmission after administration of selective serotonin reuptake inhibitors [29]. Other SRs on the microdialysis technique addressed measurements of amino acids [30], acetylcholine [31], and adenosine [32].
The research questions for this SR were to determine whether and how monoamine concentrations are influenced by 1) CRs; 2) naturally occurring sleep-wake stages, and 3) SD. We provide qualitative descriptions of the overall trends and quantitative comparison of monoamine levels between wake-sleep stages.
In addition to our SR, we present data from an unpublished study of the medial prefrontal cortex (mPFC) before, during and after SD. These data are the first published on several monoamines during SD in the mPFC. Our microdialysis experiment was designed in line with our preceding behavioural work, showing that SD affects certain but not all mPFC related cognitive tasks in rats [33,34].
Material and Methods
In this method section we first describe our systematic literature review, and then our experimental data collection. We wrote a protocol for the review before starting the selection of publications. The protocol was posted to the SyRCLE website (www.SYRCLE.nl) on 20 October 2017 [35].
Systematic review
Search and selection
Our extensive search strategy consisted of three components: “circadian rhythm, sleep, and sleep deprivation”, “neurotransmitters and metabolites” and “microdialysis”. The full search strategy is provided in our protocol [35]. We searched PubMed and EMBASE on 18 September 2017. Duplicates and triplicates were manually removed.
Screening was conducted in EROS (Early Review Organising Software; Institute of Clinical Effectiveness and Health Policy, Buenos Aires, Argentina) by two independent reviewers (JMLM and CHCL for title abstract screening, and JMLM, CHCL or EJMA for full text screening). Discrepancies were discussed among reviewers until consensus was reached. We excluded publications on other techniques than microdialysis, extracerebral and in vitro microdialysis, and other substances than dopamine, noradrenaline, adrenaline, serotonin, 5-HTP, DOPAC and 5-HIAA. During full text screening we further excluded publications not describing sleep-related conditions and/or prolonged baseline for CRs. Sleep-related conditions comprised SD, naturally occurring sleep-wake stages and models for sleep disorders. Prolonged baseline was defined as “an uninterrupted and undisturbed period of at least 6h within which one light-dark transfer occurs”. Publications were included regardless of species, year of publication, language and type of experiment. We only included peer-reviewed publications.
We excluded publications on CRs shifts, and, deviating from our protocol, those on sleep disordered animals (e.g. [36]) because of the limited number of studies retrieved by our searches. Further deviating from our protocol, we retrieved additional references by checking the reference lists of all reviews encountered during full text screening. Listed references with “release” or words starting with “dialys*” in their title and those otherwise deemed relevant by the review authors were retrieved for screening.
Data extraction and quality assessment
Data were extracted on study design (e.g. independent or dependent groups), animal model (e.g. strain, sex), microdialysis technique (e.g. flow rate, perfusion medium) and outcome measurements (type of monoamine, concentration or % of baseline). From one publication, we extracted as much as possible without knowledge of Japanese [37]. All other included publications were in English.
Microdialysis experimental methods are heterogeneous due to the versatility of the technique. For example, separate experimental groups can be used for different interventions or brain regions, but within-subject cross-over designs and simultaneous measurements in several brain regions are also common. When publications described separate experimental groups, the groups were treated as independent experiments, from hereon called “studies” (indicated by upper case letters in the reference_ID, in contrast to publications from the same authors and same year, which are indicated by lower case letters). If measurements were simultaneously performed in several brain regions within the same animals (k = 34 studies), the brain regions were treated as the experimental unit (instead of the animal), also called “studies”. We only included “studies” meeting our inclusion criteria.
For publications using seals (k = 4), each seal was treated as an independent observation, unless only pooled data were presented. This was deemed necessary because of the low number of animals included per study, and the high diversity in the probe numbers and locations per animal.
Extracted data were tabulated in Excel. Outcomes were sorted by monoamine (dopamine, serotonin, noradrenaline, adrenaline, DOPAC, and 5-HIAA) and review question (CRs, sleep, and SD; results section 3.3–3.5). Study characteristics were tabulated per review question (appendix 1–3).
As a crude measure of study (reporting) quality, we calculated percentages of reported study characteristics for our sample of studies. We created a list of characteristics based on the SYRCLE risk of bias tool [38], adapted it to sleep studies as partially described before [39], and added specific elements for microdialysis studies, as described in appendix 4.
Network meta-analysis and meta-analysis
Data were copied from texts and tables, or, if no numerical data were provided, extracted from figures with a graphical ruler (Universal graphic ruler, v3.8.6498). Concentrations were converted into nmol/L (nM) if necessary.
In our protocol we only specified that a meta-analysis would be designed and performed if at least 2 included articles measured the same monoamine in the same condition. As sufficient data were available on serotonin and noradrenaline during the naturally occurring sleep stages (Slow Wave Sleep (SWS) and Rapid Eye Movement (REM) sleep) and wakefulness, we decided to conduct two network meta-analyses (NMAs). Five studies provided concentrations for SWS and wake only, because the REM sleep episodes were too short to collect a full dialysate sample, or because REM was not observed.
If two values were given for the same stage, we conservatively assumed a correlation of 1 between them and included the mathematical average in the NMAs [40]. If the number of animals was only provided as a range throughout the experiment, the median was used for the NMAs.
Seal studies were excluded from the NMAs because of their previously described complex designs, besides the uniqueness of seals’ sleep patterns comprising unilateral sleep. The study of Zeitzer et al [41] was excluded from the NMAs because of its low power; repeated measurements were performed over one night in one human. The study of Bellesi et al [42] was excluded as we could not calculate the actual monoamine concentrations from the provided percentages.
Analyses were conducted in R, version 3.4.3 (2017-11-30) – “Kite-Eating Tree” using the netmeta package. We used the netmeta function with random effect models and standardised mean differences.
To verify the NMA results, we conducted four regular meta-analyses (MAs), using the metacont function and forest plots from the meta and metafor packages, hakn = TRUE.
Experiment: Prefrontal cortex primary data before, during and after SD in rats
Methods for this experiment have been described previously [43]. Shortly, 11 Wistar rats were implanted with custom-made concentric microdialysis probes (4mm membrane length) in the medial prefrontal cortex (mPFC) at an angle of 12° (AP+3.0mm; L ± 1.8; V–5.5; relative to Bregma). Rats recovered for approximately one-week post-surgery before the start of the experiment. They were connected to the microdialysis tubing and placed into separate compartments of SD devices. Artificial cerebrospinal fluid (145 mmol/l NaCl, 1.2 mmol/l CaCl2, 2.7 mmol/l KCl, 1.0 mmol/l MgCl2) was perfused through the probe at a flow rate of 3 μl/min. Rats habituated to the experimental set up for 12h. After this period, 24h of baseline measurements were followed by 12h of SD during the light phase (modelling a sleepless night in humans) and 16h of recovery. The experiment was approved by the experimental animal committee of the Royal Netherlands Academy of Arts and Sciences and performed in accordance with European guidelines and Dutch legislation (Wet Op de Dierproeven, 1996).
Dialysates were collected in one-hour samples (180 μL) in 300μL plastic vials (7431100, Aurora Borealis) placed in a refrigerated fraction collector (6°C; CMA 470, Aurora Borealis). Samples were transferred to ice and split into 8 fractions. After the experiment, fractions were stored at –80°C. The fractions used for monoamine measurements (20 μL) were transported on dry ice from Amsterdam to Beerse. Monoamines were determined by Janssen Pharmaceutica, Research & Development (Beerse, Belgium), department of Neuroscience Systems Biology. Their standard protocol comprises HPLC-FD following subsequent derivatization with benzylamine and 1,2-diphenylethylenediamine, as described before by Fujino et al [44].
To prevent outliers affecting our results, we selected a non-parametric approach for data analysis. Monoamine median and interquartile ranges were calculated for each stage (light phase, dark phase, SD, and recovery) in Excel. Friedman’s ANOVA’s were performed in SPSS version 22 for each monoamine separately. If the Friedman’s test was significant, post-hoc Wilcoxon tests were performed to compare baseline light with baseline dark, and SD and recovery with the corresponding baseline period.
A first paper on the validation of our method for SD showed dialysate corticosterone concentrations [43]; a second paper showed adenosine concentrations from the same experiment [32]. A paper on amino acids is in progress.
Results
In this section, we present the results of our systematic review, followed by our experimental data.
For the review, we start with a description of the publications retrieved from the search and the selection process (Figure 1), we then describe the study characteristic of the included publications, and we finish with qualitative descriptions of the monoamine concentrations for CR (Tables 1, 2, 3, 4, 5, 6), naturally occurring sleep stages (Tables 7, 8, 9, 10, 11), and SD (Tables 12, 13, 14, 15, 16, 17). The section on sleep stages comprises the network meta-analyses of serotonin and noradrenaline (Figures 2–3).
Figure 1.
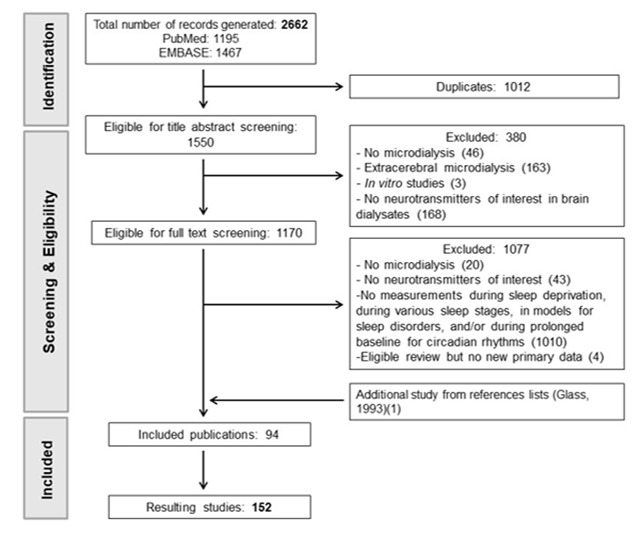
Flow scheme of screening process.
Table 1.
Circadian rhythms in dopamine levels.
| Dopamine-Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | Dopamine Levels |
| Dugovic et al (2009) [54] | Rats | 6h–18h | 6h | Prefrontal Cortex | Higher during DP, lower during DP |
| Barbier et al (2007) [55] | Rats | 6h–18h | 20h | Prefrontal Cortex | Fairly stable |
| Nakayama et al (1993) [56] | Rats | 8h–20h | 24h | Medial Prefrontal Cortex | Higher during DP, lower during LP No effect of extra 12h DP |
| Robinson et al (1991) [57] | Sheep | Natural cycle | 20h | Preoptic Area | Stable during DP, higher during LP |
| Alfinito et al (2009) [58] | Rats | 12:12 | 12h30 | Preoptic Area | Stable |
| Smith et al (1992) [46] | Rats | 7h–19h | 18h | Striatum | Higher during DP, lower during LP |
| Castaneda et al (2004) A1 [59] | Rats | 20h–8h | 30h | Striatum | Lower during DP, higher during LP |
| Castaneda et al (2004) B1 [59] | Rats | 6h DP–24h LP | 30h | Striatum | Higher at DP onset, then decrease and reach its lowest during LP |
| Hood et al (2010) [60] | Rats | 8h–20h | 24h | Striatum | Higher during DP, lower during LP |
| Sano et al (1992) A [47] | Rats, young animals | 6h–18h | 24h | Striatum | Higher during DP, lower during LP |
| Sano et al (1992) B [47] | Rats, old animals | 6h–18h | 24h | Striatum | Same pattern but levels are lower |
| Sano et al (1992) C [47] | Rats, enriched milieu | 6h–18h | 24h | Striatum | Stable |
| Sano et al (1992) D [47] | Rats, isolated | 6h–18h | 24h | Striatum | Stable |
| Decker et al (2005) [53] | Rats | 7h–19h | 48h | Striatum | A few spikes but mean is stable between DP and LP |
| De Marquez-Pardo et al (2000) [52] | Rats | 8h–20h | 24h | Neostriatum | Higher during DP, lower during LP |
| Ferris et al (2014) A [48] | Rats | ZT0–ZT12 | 36h | Caudate Putamen | Higher during DP, lower during LP |
| Ferris et al (2014) B [48] | Mice | ZT0–ZT12 | 38h | Caudate Putamen | Peak at DP onset, higher during DP, lower during LP |
| Ferris et al (2014) C [48] | Mice (DAT KO) | ZT0–ZT12 | 38h | Caudate Putamen | Stable |
| Paulson et al (1994) 1 [61] | Rats | 6h–20h | 20h | Caudate Nucleus | Higher during DP (double the NAC levels), lower during LP |
| Paulson et al (1996) 1 [62] | Rats | 6h–20h | 18h20 | Caudate Nucleus | Higher during DP, lower during LP |
| Murillo-Rodriguez et al (2013) [63] | Rats | 7h–19h | 6h | Nucleus Accumbens | Stable |
| Paulson et al (1994) 2 [61] | Rats | 6h–20h | 20h | Nucleus Accumbens | Stable |
| Paulson et al (1996) 2 [62] | Rats | 6h–20h | 18h20 | Nucleus Accumbens | Higher during DP, lower during LP |
| Castaneda et al (2004) A2 [59] | Rats | 20h–8h | 30h | Nucleus Accumbens | Lower during DP, higher during LP |
| Castaneda et al (2004) B2 [59] | Rats | 6h DP–24h LP | 30h | Nucleus Accumbens | Higher during DP, lower during LP |
| Verhagen et al (2009) [64] | Rats | 2h–14h | 36h | Lateral to Nucleus Accumbens Shell | Higher during DP, lower during LP |
| Fetissov et al (2000) 1 [65] | Rats | 6h–18h | 24h | Lateral Hypothalamus | Peak at DP onset, then start to decrease after 2h. Stay stable during LP |
| Fetissov et al (2000) 2 [65] | Rats | 6h–18h | 24h | Ventromedial Hypothalamus | Gradually decrease |
| Izumo et al (2012) [66] | Rats | 7h–19h | 15h | Central Nucleus of the Amygdala | Higher during DP, lower during LP (wide error bars) |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitude of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: L/D cycle: Light-Dark Cycle; LP: Light Phase; DP: Dark Phase; DAT KO: Dopamine Transporter Knock Out; ZT: Zeitgeber.
Table 2.
Circadian rhythms in DOPAC levels.
| DOPAC- Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | DOPAC Levels |
| Ferris et al (2014) A [48] | Rats | ZT0–ZT12 | 36h | Caudate Putamen | Higher during DP, lower during LP |
| Paulson et al (1994) [61] | Rats | 6h–20h | 20h | Caudate Nucleus | Higher during DP, lower/stable during LP |
| Paulson et al (1996) [62] | Rats | 6h–20h | 18h20 | Caudate Nucleus | Higher during DP, lower during LP |
| Castaneda et al (2004) A1 [59] | Rats | 20h–8h | 30h | Striatum | Higher during DP, lower during LP |
| Castaneda et al (2004) B1 [59] | Rats | 6h DP–24h LP | 30h | Striatum | Higher during DP, lower during LP |
| Hucke et al (1998) A [51] | Rats, nulliparous | 6h–18h | 8h | Striatum | Higher during DP, stable during LP |
| Hucke et al (1998) B [51] | Rats, primiparous | 6h–18h | 8h | Striatum | Higher during DP, stable during LP |
| Sano et al (1992) A [47] | Rats, young animals | 6h–18h | 24h | Striatum | Higher during DP, lower during LP. Highest values mid DP, lowest values mid LP |
| Sano et al (1992) B [47] | Rats, old animals | 6h–18h | 24h | Striatum | Smaller variation and level of DOPAC than young group |
| Sano et al (1992) C [47] | Rats, isolated | 6h–18h | 24h | Striatum | Stable |
| Sano et al (1992) D [47] | Rats, enriched environment | 6h–18h | 24h | Striatum | Higher levels than isolated, higher during DP, lower during LP |
| Smith et al (1992) [46] | Rats | 7h–19h | 18h | Striatum | Highest during LP, decrease gradually during the entire duration, reach lowest during DP |
| De Marquez-Prado et al (2000) [52] | Rats | 8h–20h | 24h | Neostriatum | Decrease during the entire duration (start at DP) |
| Castaneda et al (2004) A2 [59] | Rats | 20h–8h | 30h | Nucleus Accumbens | Higher during DP, lower during LP |
| Castaneda et al (2004) B2 [59] | Rats | 6h DP–24h LP | 30h | Nucleus Accumbens | Higher during DP, lower during LP. |
| Paulson et al (1994) [61] | Rats | 6h–20h | 20h | Nucleus Accumbens | Increase during LP to be the highest at DP onset. Stay stable during DP, lower during LP |
| Paulson et al (1996) [62] | Rats | 6h–20h | 18h20 | Nucleus Accumbens | Higher during DP, lower during LP |
| Verhagen et al (2009) [64] | Rats | 2h–14h | 36h | Lateral to Nucleus Accumbens Shell | Higher during DP, highest at the end of DP Lowest level mid-LP. High range of fluctuation. |
| Nakayama et al (1993) [56] | Rats | 8h–20h | 24h | Medial Prefrontal Cortex | Peak at DP onset and about 3/4 DP, decrease strongly between the 2 peaks. Decrease during LP |
| Luo et al (2014) [67] | Rats | ? | 24h | SCN | Higher during DP |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: DOPAC: 3,4-Dihydroxyphenylacetic acid; L/D Cycle: Light-Dark Cycle; LP: Light Phase; DP: Dark Phase; SCN: Suprachiasmatic Nucleus; ZT: Zeitgeber.
Table 3.
Circadian rhythms in serotonin levels.
| Serotonin-Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | Serotonin Levels |
| Huang et al (2008) [68] | Rats | 6h–18h | 72h | Pineal Gland | Peak at DP onset, then decrease at its lowest, before increasing again before LP. Stable during LP. |
| Sun et al (2002) [69] | Rats | 11h–1h | 312h | Pineal Gland | Increase strongly at DP onset, then decrease gradually, increase at the end. Stable during LP. |
| Sun et al (2003) [70] | Rats | 11h–1h | 132h | Pineal Gland | Peak at DP onset, gradual decrease during the rest of DP. Increase during LP. |
| Azekawa et al (1991) [71] | Rats | 7h–19h | 24h | Pineal Gland | Peak after DP onset followed by strong decrease until mid DP. Then increase until LP onset. Lower during LP |
| Liu et al (2005) [72] | Rats | 11h–23h | 72h | Pineal Gland | Peak 1h after DP onset, and 3h before LP. Nadir is seen at LP beginning, followed by a gradual increase until DP onset. |
| Liu et al (2006) A [73] | Rats (LEW) | 6h–18h | 120h | Pineal Gland | Higher after DP onset, followed by a sharp decrease until the end of DP. Levels return to baseline level and stay stable during LP |
| Liu et al (2006) B [73] | Rats (SD) | 6h–18h | 24h | Pineal Gland | Higher after DP onset but shifted compared to LEW followed by a strong decrease until the end of DP. Return to baseline level and stay stable during LP |
| Liu et al (2006) C [73] | Rats (Wistar TG) | 6h–18h | 24h | Pineal Gland | Higher at about 1/3rd of DP, followed by a sharp decrease until the end of DP. Levels return to baseline level and stay stable during LP |
| Liu et al (2006) D [73] | Rats (PVG) | 6h–18h | 24h | Pineal Gland | Higher 1h after DP onset, followed by a decrease until the end of DP. Levels return to baseline level and stay stable during LP |
| Liu et al (2006) E [73] | Rats (LEW) | 6h–18h | 24h | Pineal Gland | Higher 3h–4h after DP onset, followed by decrease until the end of DP. Levels return to baseline level and stay stable during LP |
| Liu et al (2006) F [73] | Hamsters | 6h–18h | 24h | Pineal Gland | Peak at DP onset followed by an increase and fluctuations (less marked than in rats) |
| Garabette et al (2000) [74] | Rats | 7h–19h | 24h | Adjacent to SCN | Lower during DP. Higher during LP |
| Grossman et al (2000) A [75] | Hamsters | 5h–19h | 11h | Lateral Margin of SCN | Higher after DP onset. Stable during LP |
| Dudley et al (1998) A [76] | Hamsters | 7h–22h | 24h | Lateral Margin of the SCN | Peak at DP onset followed by gradual decrease. Stay stable during LP |
| Dudley et al (1998) B [76] | Hamsters | 7h–22h | 48h | Lateral Margin of the SCN | Peak 2h after DP onset, followed by gradual decrease. Stay stable during LP |
| Barassin et al (2002) [77] | Rats | 12:12 | 17h | SCN or in Between SCN Nuclei | Peak at DP onset followed by decrease. Lower during LP |
| Knoch et al (2004) [78] | Hamsters | 12:12 | 24h | SCN | Peak 2h after DP onset, followed by decrease. Lower during LP |
| Oshima et al (2003) [79] | Mice | 6h–18h | 24h | Hippocampus | Higher during DP, peak at onset and mid DP. Decrease during LP (but one peak mid LP) |
| Lopez-Rodriguez et al (2003) a [80] | Rats | 1h–13h | 24h | Posterior Hippocampus | Small peak at LP onset, yet fairly stable |
| Linthorst et al (1994) [81] | Rats | 7h30–19h30 | 11h | Hippocampus | Peak at DP onset. Fairly stable during LP |
| Yang et al (2013) A [49]* | Mice (SERT +/+) | 4h–16h | 20h | Ventral Hippocampus and Ventral Striatum | Peak 3h after DP onset followed by a decrease. LP and rest of DP stable |
| Yang et al (2013) B [49]* | Mice (SERT +/–) | 4h–16h | 20h | Ventral Hippocampus and Ventral Striatum | Smaller peak 3h after DP onset followed by a sudden sharp decrease. LP and rest of DP stable. Or peak at 3h + peak 3h before LP onset. Or overall fluctuation |
| Yang et al (2013) C [49]* | Mice (SERT –/–) | 4h–16h | 20h | Ventral Hippocampus and Ventral Striatum | Gradual decrease during both DP and LP |
| Kalen et al (1989) [82] | Rats | 12:12 | 24h | Caudal Hippocampus | Higher during DP, lower during LP |
| Penalva et al (2002) A [83] | Mice (CHR-R1 +/+) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP |
| Penalva et al (2002) B [83] | Mice (CHR-R1 +/–) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP |
| Penalva et al (2002) C [83] | Mice (CHR-R1 –/–) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP |
| Takahashi et al (1998) [84] | Rats | 7h–17h | 24h | Striatum | Higher during DP, stable during LP |
| Verhagen et al (2009) [64] | Rats | 2h–14h | 36h | Lateral to Nucleus Accumbens Shell | Higher during DP, lower during LP. Start increase 1h before DP onset, and reach its highest 5h after DP onset. Then decrease and reach nadir during mid-LP. |
| Izumo et al (2012) [66] | Rats | 7h–19h | 15h | Central Nucleus of the Amygdala | Peak at DP onset and mid DP followed each time by gradual decrease. Stable during LP |
| Smriga et al (2002) [85] | Rats | 7h–19h | 25h | Central Nucleus of the Amygdala | Peak at DP onset followed by gradual decrease. 1h before LP, increase to baseline level. Stable during LP |
| Dugovic et al (2009) [54] | Rats | 6h–18h | 6h | Prefrontal Cortex | Higher during DP, stable during LP |
| Barbier et al (2007) [55] | Rats | 6h–18h | 20h | Prefrontal Cortex | Stable |
| Jitsuki et al (2009) A [50] | Rats, male | 5h–19h | 24h | Medial Prefrontal Cortex | Fairly stable |
| Jitsuki et al (2009) B [50] | Rats, diestrous | 5h–19h | 24h | Medial Prefrontal Cortex | Higher during DP, lower during LP |
| Jitsuki et al (2009) C [50] | Rats, proestrous | 5h–19h | 24h | Medial Prefrontal Cortex | Higher during DP, lower during LP |
| Grossman et al (2004) [86] | Hamsters | 14:10 | 24h | Margin of Thalamic Intergeniculate Leaflet | Higher 1h after DP onset, higher during DP Lower during LP, nadir mid LP |
| Sayer et al (1999) [87] | Rats | 6h–18h | ? | Anterior Hypothalamus | Stable during DP, slightly higher during LP |
| Fetissov et al (2000) 1 [65] | Rats | 6h–18h | 24h | Lateral hypothalamus | Stable apart from one peak during LP |
| Fetissov et al (2000) 2 [65] | Rats | 6h–18h | 24h | Ventromedial Hypothalamus | Peak 1h–2h after DP onset, followed by a return to baseline. Stable during rest of DP and LP |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals. * These studies provided average monoamine concentrations pooled for several brain regions.
Abbreviations: L/D cycle: Light-Dark Cycle; LP: Light Phase; DP: Dark Phase; LEW: Lewis; SD: Sprague-Dawley; TG: Transgenic; SERT: Serotonin Transporter; SCN: Suprachiasmatic Nucleus.
Table 4.
Circadian rhythms in 5–HIAA levels.
| 5-HIAA – Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | 5–HIAA Levels |
| Glass et al (1993) a [88] | Hamsters | 8h–22h | 24h | Lateral margin of SCN | Peak at DP onset followed by a gradual decrease. Stable during LP, lower than DP |
| Luo et al (1999) A [89] | Hamsters, glucose intolerant | 8h30–22h30 9h–23h | 24h | Top of SCN | Increase during DP (peak 4h after DP onset), decreases during LP |
| Luo et al (1999) B [89] | Hamsters, glucose tolerant | 8h30–22h30 9h–23h | 24h | Top of SCN | Small peak during DP. Rather stable during LP |
| Barassin et al (2002) [77] | Rats | 12:12 | 17h | SCN or in Between SCN Nuclei | Peak 4–6h after DP onset followed by decrease |
| Glass et al (1993) b [90] | Hamsters | 7h–21h | 24h | SCN | Peak 2h after DP, increase during DP, decrease during LP |
| Glass et al (1992) A [91] | Hamsters | 8h–0h | 24h | SCN | Peak 2h after DP onset, return to baseline at LP onset. Stable during LP except a decrease at 19h |
| Glass et al (1992) B [91] | Hamsters | 8h–0h | 24h | Lateral Margin of the SCN | Peak 2h after DP onset, return to baseline at LP onset. Stable during LP except a decrease at 19h |
| Luo et al (2000) [92] | Hamsters | 0h–14h | 24h | SCN | Peak at DP onset and nadir 4h DP onset. Stable during the rest of sampling time. |
| Glass et al (1993) c [45] | Hamsters | 8h–0h | 24h | SCN | Peak at DP onset, increases during DP, decreases during LP |
| Castaneda et al (2004) A1 [59] | Rats | 20h–8h | 30h | Striatum | Slight increase during DP, slight decrease during LP |
| Castaneda et al (2004) B1 [59] | Rats | 6h DP–24h LP | 30h | Striatum | Lower during DP, higher during LP |
| Sano et al (1992) A [47] | Rats, young animals | 6h–18h | 24h | Striatum | Nadir at DP onset, then increase gradually until the end of DP. Start of LP decrease gradually. |
| Sano et al (1992) B [47] | Rats, old animals | 6h–18h | 24h | Striatum | Stable |
| Sano et al (1992) C [47] | Rats, enriched media | 6h–18h | 24h | Striatum | Stable |
| Sano et al (1992) D [47] | Rats, isolated | 6h–18h | 24h | Striatum | Stable |
| Smith et al (1992) [46] | Rats | 7h–19h | 18h | Striatum | Stable |
| Nakayama et al (2002) [93] | Rats | 8h–20h | 24h | Striatum | Higher during DP, lower during LP |
| Takahashi et al (1998) [84] | Rats | 7h–19h | 24h | Striatum | Higher during DP, lower during LP |
| Castaneda et al (2004) A2 [59] | Rats | 20h–8h | 30h | Nucleus Accumbens | Higher during DP, lower during LP |
| Castaneda et al (2004) B2 [59] | Rats | 6h DP–24h LP | 30h | Nucleus Accumbens | Inconsistent during DP (fluctuation up and down), lower during LP |
| Paulson et al (1994) 2 [61] | Rats | 6h–20h | 20h | Nucleus Accumbens | Higher during DP, lower during LP |
| Paulson et al (1996) [62] | Rats | 6h–20h | 18h20 | Nucleus Accumbens | Slightly higher during DP, lower during LP |
| Verhagen et al (2009) [64] | Rats | 2h–14h | 36h | Lateral to Nucleus Accumbens Shell | Higher during DP (peak around the end of DP), lower during LP |
| Paulson et al (1994) 1 [61] | Rats | 6h–20h | 20h | Caudate Nucleus | Higher during DP, lower during LP |
| Paulson et al (1996) [62] | Rats | 6h–20h | 18h20 | Dorsolateral Caudate Nucleus | Higher during DP, lower during LP |
| Oshima et al (2003) [79] | Mice | 6h–18h | 24h | Hippocampus | Higher during DP, lower during LP |
| Nakayama et al (2002) [93] | Rats | 8h–20h | 24h | Hippocampus | Stable |
| Linthorst et al (1994) [81] | Rats | 7h30–19h30 | 11h | Hippocampus | Fairly stable, higher at DP onset (slightly) |
| Kalen et al (1989) [82] | Rats | 12:12 | 24h | Caudal Hippocampus | Stable, apart from a peak at the end of DP |
| Penalva et al (2002) A [83] | Mice, (CHR–R1 +/+) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP |
| Penalva et al (2002) B [83] | Mice, (CHR–R1 +/–) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP |
| Penalva et al (2002) C [83] | Mice, (CHR–R1 –/–) | 6h–18h | 18h | Dorsal Hippocampus | Higher during DP, lower during LP (higher levels than other mice) |
| Glass et al (1992) C [91] | Hamsters | 8h–0h | 24h | Preoptic Area | Peak at DP onset followed by gradual decrease. Stable during LP |
| Ezrokhi et al (2014) A [94] | Rats (CTL) | 5h–19h | 24h | Ventromedial Hypothalamus | Gradual decrease (start at LP) |
| Ezrokhi et al (2014) B [94] | Rats (SHR), treated with vehicle | 5h–19h | 24h | Ventromedial Hypothalamus | Higher during DP, lower during LP |
| Luo et al (1998) A [95] | Hamsters, glucose tolerant | 8h30–22h30 | 25h | Ventromedial Hypothalamus | Lower level than intolerant group. Higher during DP with a peak at the end. Lower during LP |
| Luo et al (1998) B [95] | Hamsters, glucose intolerant | 8h30–22h30 | 25h | Ventromedial Hypothalamus | Higher level and more fluctuations than tolerant group. Levels increases during DP with a peak at the end. Lower levels during LP |
| Luo et al (1998) C [95] | Hamsters (CTL) | 8h30–22h30 | 25h | Ventromedial Hypothalamus | Fairly stable, higher during DP (slightly) |
| Stanley et al (1989) a [96] | Rats | 9h–21h | 24h | Paraventricular Nucleus | Peak 1h after DP onset followed by sudden decrease. Lower during LP |
| Glass et al (1992) D [91] | Hamsters | 8h–0h | 24h | Posterior Hypothalamus | Peak at DP onset followed by gradual decrease Stable during LP |
| Gonzales-Pina et al (2003) [97] | Rats | 12:12 | 24h | Dorsal Raphe | Higher during DP, lower during LP |
| Azekawa et al (1991) [71] | Rats | 7h–19h | 24h | Pineal gland | Peak at DP onset followed by strong decrease and then a gradual increase until the end of DP. Lower levels during LP |
| Nakayama et al (1993) [56] | Rats | 8h–20h | 24h | Medial Prefrontal Cortex | Higher during DP, lower during LP |
| Nakayama et al (2002) [93] | Rats | 8h–20h | 24h | Medial Prefrontal Cortex | Higher during DP, lower during LP |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: 5-HIAA: 5-hydroxyindoleacetic acid; L/D Cycle: Light-Dark Cycle; LP: Light Phase; DP: Dark Phase; SCN: Suprachiasmatic Nucleus; CTL: Control, SHR: Spontaneously Hypertensive Rats; CHR–R1: Corticotropin-Releasing Hormone Receptor 1.
Table 5.
Circadian rhythms in noradrenaline levels.
| Noradrenaline-Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | Noradrenaline Levels |
| Barbier et al (2007) [55] | Rats | 6h–18h | 20h | Prefrontal Cortex | Higher during DP, stable during LP |
| Dugovic et al (2009) [54] | Rats | 6h–18h | 6h | Prefrontal Cortex | Higher during DP, stable during LP |
| Robinson et al (1991) [57] | Sheep | natural | 20h | Preoptic Area | Decrease gradually |
| Alfinito et al (2009) [58] | Rats | 12:12 | 12h30 | Preoptic Area | Higher during DP, stable during LP |
| Mitome et al (1994) a [37] | Rats | 6h–18h | 52h | Paraventricular Nucleus | Higher during DP, lower during LP |
| Stanley et al (1989) b [98] | Rats | 9h–21h | 48h | Paraventricular Nucleus | Peak 1h after DP onset, followed by sudden decrease, until a second smaller peak at 3h before LP. Lower levels during LP |
| Mitome et al (1994) b [99] | Rats | 6h–18h | 54h | Paraventricular Nucleus | Higher during DP, lower during LP |
| Morien et al (1995) A [100]* | Rats | 7h–19h | 24h | Paraventricular Nucleus | Peak 1h and 8h after DP onset. Higher during DP, lower during LP |
| Smriga et al (2000) b [101] | Rats | 7h–19h | 24h | Lateral Hypothalamus | Gradual increase from baseline during LP Peak at DP onset followed by sudden decrease and return to baseline |
| Smriga et al (2000) a [102] | Rats | 7h–19h | 26h | Ventral Hypothalamus | Higher during DP, lower during LP |
| Kalen et al (1989) [82] | Rats | 12:12 | 24h | Caudal Hippocampus | Higher during DP, lower during LP |
| Drijfhout et al (1996) [103] | Rats | 6h–18h | 16h | Pineal gland | Peak 1–3h after DP onset, decrease 2h before LP Higher during DP, lower during LP |
| Morien et al (1995) B [100]* | Rats | 7h–19h | 24h | Septal Nuclei and the Ventromedial Thalamus | Stable |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals. *These studies provided average monoamine concentrations pooled for several brain regions.
Abbreviations: L/D Cycle: Light-Dark Cycle; LP: Light Phase; DP: Dark phase.
Table 6.
Circadian rhythms in adrenaline levels.
| Adrenaline-Circadian Rhythms | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | L/D Cycle | Duration | Brain Region | Adrenaline Levels |
| Robinson et al (1991) [57] | Sheep | Natural cycle | 20h | Preoptic Area | Stable |
Abbreviations: L/D Cycle: Light/Dark Cycle; DP: Dark-Phase; LP: Light-Phase.
Table 7.
Dopamine levels during naturally occurring sleep stages.
| Dopamine-Sleep | ||||
|---|---|---|---|---|
| Reference | Animals | L/D Cycle | Brain Region | Dopamine Levels |
| Orosco et al (1995) [104] | Rats | 6h–18h | PVN/VMN | 2 days measurement with different observation Day1: W and REM high, SWS lower/ Day2: W and REM low with SWS high |
| Nicolaidis et al (2001) A [105] | Rats | ? | PVN/VMN | Levels increases from SWS to REM and from REM to W. Levels decreases from W to SWS |
| Shouse et al (2000) a 1 [106] | Cats | ? | Amygdala | Stable during all stages (AW, QW, SWS and REM) |
| Shouse et al (2001) a 1 [107] | Cats | ? | Amygdala | Stable during wake and sleep |
| Shouse et al (2001) b 1 [108] | Cats | ? | Amygdala | Stable during wake and sleep |
| Shouse et al (2000) a 2 [106] | Cats | ? | Locus Coeruleus | Stable during all stages (AW, QW, SWS and REM) |
| Shouse et al (2001) a 1 [107] | Cats | ? | Locus Coeruleus | Stable during wake and sleep |
| Shouse et al (2001) b 2 [108] | Cats | ? | Locus Coeruleus | Stable during wake and sleep |
| Lena et al (2005) 1 [109] | Rats | 8h–20h | Medial Prefrontal Cortex | W: high level, SWS: low level, REM: in between |
| De Saint Hilaire (2000) [110] | Rats | 6h–18h | Prefrontal Cortex | W: lower, SWS: high. Higher in REM when followed by W |
| Nicolaidis et al (2001) B [105] | Rats | ? | Prefrontal Cortex | W to SWS: decrease, SWS to W: increases |
| Lena et al (2005) 2 [109] | Rats | 8h–20h | Nucleus Accumbens | W and REM: high, SWS: low |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: W: Wake; SWS: Slow Wave Sleep; REM: Rapid Eye Movements Sleep; PVN: Paraventricular Nucleus; VMN: Ventromedial Hypothalamic Nucleus.
Table 8.
DOPAC levels during naturally occurring sleep stages.
| DOPAC-Sleep | ||||
|---|---|---|---|---|
| Reference | Animals | L/D Cycle | Brain Region | DOPAC Levels |
| De Saint Hilaire (2000) [110] | Rats | 6h–18h | Prefrontal Cortex | Stable |
| Orosco et al (1995) [104] | Rats | 6h–18h | PVN/VMN | W: low, SWS: intermediate, REM: high |
| Nicolaidis et al (2001) A [105] | Rats | ? | PVN/VMN | From SWS to W: decrease, from SWS to REM: increase, from REM to W: increase |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: DOPAC: 3,4–Dihydroxyphenylacetic acid; W: Wake; SWS: Slow Wave Sleep; REM: Rapid Eye Movements Sleep; PVN: Paraventricular Nucleus; VMN: Ventromedial Hypothalamic Nucleus.
Table 9.
Serotonin levels during naturally occurring sleep stages.
| Serotonin-Sleep | ||||
|---|---|---|---|---|
| Reference | Animals | L/D Cycle | Brain Region | Serotonin Levels |
| Orosco et al (1995) [104] | Rats | 6h–18h | PVN/VMN | W: high, SWS: intermediate, REM: low |
| Nicolaidis et al (2001) A [105] | Rats | ? | PVN/VMN | W: high, SWS: low |
| Wilkinson et al (1991) [111] | Cats | ? | Preoptic Area/Anterior Hypothalamus | W: high, SWS: low |
| Python et al (2001) [112] | Rats | 8h–20h | Preoptic Area | W: high, SWS: intermediate, REM: low. SWS after REM showed no strong fluctuation, but when W after REM levels showed a strong increase |
| Shouse et al (2000) a 1 [106] | Cats | ? | Amygdala | W: high, SWS: intermediate, REM: low |
| Shouse et al (2001)a 1 [107] | Cats | ? | Amygdala | W: high, SWS: low |
| Shouse et al (2001) b 1 [108] | Cats | ? | Amygdala | W: high, SWS: intermediate, REM: low |
| Shouse et al (2000) a 2 [106] | Cats | ? | Locus Coeruleus | W: high, SWS: intermediate, REM: low |
| Shouse et al (2001) a 2 [107] | Cats | ? | Locus Coeruleus | W: high, SWS: low |
| Shouse et al (2001) b 1 [108] | Cats | ? | Locus Coeruleus | W: high, SWS: intermediate, REM: low |
| Park et al (1999) [113] | Rats | 7h–19h | Posterior Hippocampus | W: high, SWS and REM: low |
| Gronli et al (2007) [114] | Rats | 7h–19h | Hippocampus | W and SWS: high, REM: low |
| Bjorvatn et al (2002) A1 [115] | Rats | 6h–18h | Ventral Hippocampus | W: high, Sleep: low |
| Penalva et al (2003) A [116] | Rats | 7h30–19h30 | Dorsal Hippocampus | W: high, SWS: low, REM: low |
| Fiske et al (2006) 1 [117] | Rats | 6h–18h | Dorsal Raphe | W: high, SWS and REM: low |
| Fiske et al (2008) 1 [118] | Rats | 6h–18h | Dorsal Raphe | W: high, SWS and REM: low |
| Portas et al (1994) [119] | Cats | Constant light | Dorsal Raphe | W: high, SWS: intermediate, REM: low |
| Portas et al (1996) [120] | Cats | Constant light | Dorsal Raphe | W: high, SWS: intermediate, REM: low |
| Portas et al (1998) 1 [121] | Rats | 6h–18h | Dorsal Raphe | W: high, SWS: intermediate, REM: low |
| De Saint Hilaire et al (2000) [110] | Rats | 6h–18h | Prefrontal Cortex | W: high, SWS: intermediate, REM: low. Except, 5–HT increases in REM if followed by W |
| Nicolaidis et al (2001) B [105] | Rats | ? | Prefrontal Cortex | W increases before a SWS stage. From SWS to W decrease after a long SWS period. |
| Portas et al (1998) 2 [121] | Rats | 6h–18h | Frontal cortex | W: high, SWS: intermediate, REM: low |
| Mukaida et al (2007) [122] Ɨ | Rats | 7h–19h | Frontal cortex | W: high, SWS: lower |
| Fiske et al (2008) 2 [118] | Rats | 6h–18h | Frontal cortex | Stable |
| Bjorvatn et al (2002) A2 [115] | Rats | 6h–18h | Frontal cortex | W: high, Sleep: low |
| Zeitzer et al (2002) [41] | Human | L/D cycle of the season | Lateral Ventricle | W: high, SWS: intermediate, REM: low From stage 2 to REM: decrease/from REM to stage 2: increase |
| McCarley et al (2004) [123] | ? | ? | PPT | W: high, SWS: intermediate, REM: low |
| Strecker et al (1999) [124] | Cats | ? | PPT | W: high, SWS: intermediate, REM: low |
| Fiske et al (2006) 2 [117] | Rats | 6h–18h | Frontal cortex | W: high, SWS: low, REM: intermediate |
| Lapierre et al (2012) [125] | Seals | ? | Cortex | W: high, SWS: intermediate, REM: low |
| Lapierre et al (2013) a [126] | Seals | 8h–20h | Cerebral cortex | W: high, BSWS: the lowest, REM: low |
| Lyamin et al (2016) A [127]* | Seals | 8h–20h | Occipital cortex and Frontal cortex | W: high, SWS: intermediate, REM: low. Same decrease was seen in seals specific sleep stages (USWS (right and left), BSWS) |
| Blanco-Centurion et al (2001) A [128] | Rats | 8h–20h | Gigantocellular reticular nucleus | W: high, SWS: intermediate, REM: low |
| Iwakiri et al (1993) [129] | Cats | ? | Medial Pontine Reticular Formation | W: high, SWS: intermediate, REM: low |
| Lyamin et al (2016) C [127] | Seals | 8h–20h | Thalamus | W: high, SWS: intermediate, REM: low |
| Lyamin et al (2016) D [127] | Seals | 8h–20h | Caudate nucleus | W: high, SWS: intermediate, REM: low |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals. Ɨ Anaesthesia was applied during baseline: 6 L/min mixture of 25% oxygen and75% nitrogen. All the other studies measured natural sleep. *These studies provided average monoamine concentrations pooled for several brain regions.
Abbreviations: W: Wake; SWS: Slow Wave Sleep; REM: Rapid Eye Movements Sleep; PVN: Paraventricular Nucleus; VMN: Ventromedial Hypothalamic Nucleus; PPT: Pedunculopontine Tegmental Nucleus.
Table 10.
5-HIAA levels during naturally occurring sleep stages.
| 5-HIAA-Sleep | ||||
|---|---|---|---|---|
| Reference | Animals | L/D Cycle | Brain Region | 5-HIAA Levels |
| De Saint Hilaire (2000) [110] | Rats | 6h–18h | Prefrontal Cortex | Stable |
| Orosco et al (1995) [104] | Rats | 6h–18h | PVN/VMN | 1st day: W: intermediate, SWS: low, REM: high 2nd day: W: high, SWS: intermediate, REM: low |
| Nicolaidis et al (2001) A [105] | Rats | ? | PVN/VMN | W: High, Sleep: Lower. W is lower if preceded by REM. |
| Portas et al (1994) [119] | Cats | Constant light | Dorsal Raphe | Stable |
| Iwakiri et al (1993) [129] | Cats | ? | Medial Pontine Reticular Formation | Stable |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: 5-HIAA: 5-hydroxyindoleacetic acid; W: Wake; SWS: Slow Wave Sleep; REM: Rapid Eye Movements Sleep; PVN: Paraventricular Nucleus; VMN: Ventromedial Hypothalamic Nucleus.
Table 11.
Noradrenaline levels during naturally occurring sleep stages.
| Noradrenaline-Sleep | ||||
|---|---|---|---|---|
| Reference | Animals | L/D Cycle | Brain Region | Noradrenaline Levels |
| Orosco et al (1995) [104] | Rats | 6h–18h | PVN/VMN | 1st day: W: high, SWS: low, REM: intermediate 2nd day W: high, SWS: intermediate, REM: low |
| Nicolaidis et al (2001) A [105] | Rats | ? | PVN/VMN | W: high, SWS: intermediate, REM: low. If SWS followed by W or REM: increase, while if REM or W is followed by SWS: decrease |
| Lyamin et al (2016) B [127] | Seals | 8h–20h | Hypothalamus | W: high, SWS: intermediate, REM: low |
| Shouse et al (2000) a 1 [106] | Cats | ? | Amygdala | W: high, SWS: intermediate, REM: low |
| Shouse et al (2000) b 1 [130] | Cats | ? | Amygdala | W: high, SWS: low |
| Shouse et al (2001) a 1 [107] | Cats | ? | Amygdala | W: high, SWS: low |
| Shouse et al (2001) b 1 [108] | Cats | ? | Amygdala | W: high, SWS: intermediate, REM: low |
| Park et al (2002) [131] | Rats | 7h–19h | Amygdala | W: high, SWS: low, REM: lower |
| Shouse et al (2000) a 2 [106] | Cats | ? | Locus Coeruleus | W: high, SWS: intermediate, REM: low |
| Shouse et al (2000) b 2 [130] | Cats | ? | Locus Coeruleus | W: high, Sleep: low |
| Shouse et al (2001) a 2 [107] | Cats | ? | Locus Coeruleus | W: high, Sleep: low |
| Shouse et al (2001) b 2 [108] | Cats | ? | Locus Coeruleus | W: high, SWS: intermediate, REM: low |
| Bellesi et al (2016) A1 | Mice | 8h–20h | Medial Prefrontal Cortex | W: high, Sleep: low |
| Lena et al (2005) 1 [109] | Rats | 8h–20h | Medial Prefrontal Cortex | W: high, SWS: intermediate, REM: low |
| De Saint Hilaire et al (2000) [110] | Rats | 6h–18h | Prefrontal Cortex | W: lower, SWS: high (relatively stable) |
| Lena et al (2005) 2 [109] | Rats | 8h–20h | Nucleus Accumbens | W: high, SWS: intermediate, REM: low |
| Lapierre et al (2013) b [132] | Seals | ? | Cortex | W: high, SWS: intermediate, REM: low. |
| Lyamin et al (2016) A [127]* | Seals | 8h–20h | Occipital cortex, frontal cortex | W: high, SWS: intermediate, REM: low. Same decrease was seen in seals specific sleep stages (USWS (right and left), BSWS) |
| Bellesi et al (2016) A2 | Mice | 8h–20h | M1 | W: high, Sleep: low |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Fluctuations are described as “higher” and “lower” disregarding actual magnitudes of changes. Rows are sorted by brain region. Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals. * These studies provided average monoamine concentrations pooled for several brain regions.
Abbreviations: W: Wake; SWS: Slow Wave Sleep; REM: Rapid Eye Movements Sleep; PVN: Paraventricular Nucleus; VMN: Ventromedial Hypothalamic Nucleus; M1: Primary Motor Cortex.
Table 12.
Dopamine and sleep deprivation.
| Dopamine-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | Dopamine levels during/after SD |
| Murillo-Rodriguez et al (2016) [134] | Rats | -Stroking fur with paint brush -Light noise in the cage -Tapping -Placing object in the cage | 6h | Nucleus Accumbens | Increase after SD |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Abbreviation: SD: Sleep Deprivation.
Table 13.
DOPAC and sleep deprivation.
| DOPAC-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | DOPAC levels during/after SD |
| Zant et al (2010) [135] | Rats | Gentle handling | 6h | Basal Forebrain | Increase during SD, decrease to baseline levels during sleep recovery |
| Zant et al (2011) [136] | Rats | -Gentle handling including placing objects in the cage | 6h | Basal Forebrain | Increase during 3 first hours of SD, then plateau. It decreases to baseline levels during sleep recovery |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results.
Abbreviations: DOPAC: 3,4-Dihydroxyphenylacetic acid; SD: Sleep Deprivation.
Table 14.
Serotonin and sleep deprivation.
| Serotonin-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | Serotonin levels during/after SD |
| Bjorvatn et al (2002) B1 [115] | Rats | -Gentle sensory stimulation (knocking on the plexiglas door, opening the door, gentle handling) | 8h30 | Ventral hippocampus | Decrease during SD |
| Lopez-Rodriguez et al (2003) a [80] | Rats | Modified disk-over-water | 24h | Posterior Hippocampus | Increase during SD and remain high during recovery |
| Lopez-Rodriguez et al (2003) b [133] | Rats | Small platform (6cm) in tank filled with water (REM deprivation) | 24h but measurement for 11h | Posterior Hippocampus | Increase during SD and decrease below baseline during recovery |
| Penalva et al (2003) B [116] | Rats | -Introducing or removing objects -Shaking the cage slightly | 4h | Dorsal hippocampus | Increase during SD. During recovery time, levels are high during W and low during REM sleep. |
| Penalva et al (2003) C [116] | Rats | -Introducing or removing objects -Shaking the cage slightly | 4h | Dorsal hippocampus | Increase during SD. During recovery time, levels are high during W and low during REM sleep. |
| Bjorvatn et al (2002) B2 [115] | Rats | -Gentle sensory stimulation (knocking on the plexiglas door, opening the door, handling) | 8h30 | Frontal Cortex | Decrease during SD |
| Blanco-Centurion et al (2001) B [128] | Rats | Platform (6.5cm) surrounded by water (REM deprivation) | 92h | Gigantoreticular Cellular Nucleus | Decrease (factor 100) during SD and remain low during recovery |
| Grossman et al (2000) B [75] | Hamsters | -Continuous gentle handling -Light puffs of air | 3h (red dim light) | Lateral Margin of SCN | Increase during SD, decreases during recovery but slight increase at the end. |
| Grossman et al (2000) C [75] | Hamsters | -Continuous handling-Light puffs of air | 3h | Lateral Margin of SCN | Increase during SD, highest peak at the end of SD. Decreases to baseline levels during recovery |
| Murillo-Rodriguez et al (2016) [134] | Rats | -Stroking fur with paint brush-Light noise in the cage-Tapping-Placing object in the cage | 6h | Nucleus Accumbens | Increase after SD |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Rows are sorted by brain region.
Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: SD: Sleep Deprivation; SCN: Suprachiasmatic Nucleus.
Table 15.
5-HIAA and sleep deprivation.
| 5-HIAA-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | 5-HIAA levels during/after SD |
| Zant et al (2010) [135] | Rats | Gentle handling | 6h | Basal Forebrain | Increase during SD and return to baseline level during recovery |
| Zant et al (2011) [136] | Rats | -Gentle handling -Placing object in the cage | 6h | Basal Forebrain | Increase during SD and return to baseline level during recovery |
| Blanco-Centurion et al (2001) B [128] | Rats | Platform (6.5cm) surrounded by water (REM deprivation) | 92h | Gigantoreticular Cellular Nucleus | Decrease during SD, and increase during recovery |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Rows are sorted by brain region.
Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: SD: Sleep Deprivation; 5-HIAA: 5-Hydroxyindoleacetic acid.
Table 16.
Noradrenaline and sleep deprivation.
| Noradrenaline-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | Noradrenaline levels during/after SD |
| Bellesi et al (2016) B1 | Mice | -Exposure to novel objects | 6h | Medial Prefrontal Cortex | Increase during SD and slightly decrease at the end |
| Bellesi et al (2016) B2 | Mice | -Exposure to novel objects | 6h | M1 | Increase during SD |
| Murillo-Rodriguez et al (2016) [134] | Rats | -Stroking fur with paint brush -Light noise in the cage-Tapping-Placing object in the cage | 6h | Nucleus Accumbens | Increase after SD |
Each row represents one study (i.e. an experimental group within a publication) and a qualitative description of the results. Rows are sorted by brain region.
Lower case letters indicate separate publications from the same authors in the same year; upper cases letters represent separate groups within publications; numbers represent separate brain regions within animals.
Abbreviations: SD: Sleep Deprivation; M1: Primary Motor Cortex.
Table 17.
Adrenaline and sleep deprivation.
| Adrenaline-SD | |||||
|---|---|---|---|---|---|
| Reference_ID | Animals | SD Methods | Duration | Brain Region | Adrenaline levels during/after SD |
| Murillo-Rodriguez et al (2016) [134] | Rats | -Stroking fur with painting brush-Light noise during the cage-Tapping-Placing object in the cage | 6h | Nucleus Accumbens | Increase after SD |
Abbreviations: SD: Sleep Deprivation.
Figure 2.
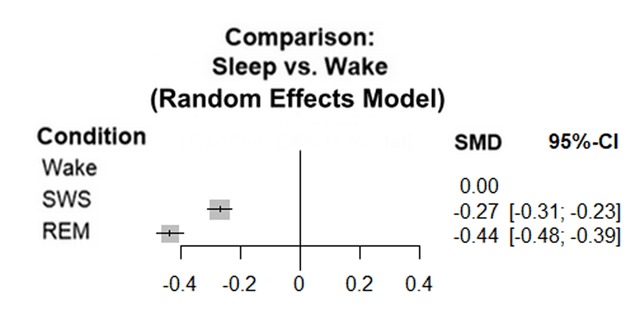
Network meta-analysis comparing serotonin levels during wakefulness, SWS and REM sleep.
This plot summarises the results of 26 studies; 19 had data for each stage, 7 had data only for wakefulness and SWS. For the overall effect, p < 0.0001. The analysis shows significant heterogeneity; Τ² = 0.0059; I² = 98.4%.
Abbreviations: SWS: Slow Wave Sleep; REM: Rapid Eye Movement sleep.
Figure 3.
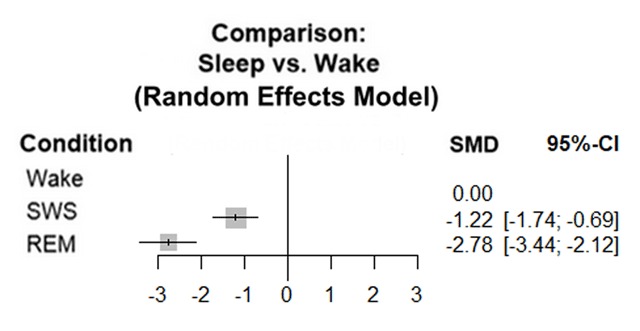
Network meta-analysis of noradrenaline levels during wakefulness, SWS and REM sleep.
This plot summarises the results of 13 studies; 8 had data for each stage, 5 had data only for wakefulness and SWS. For the overall effect p < 0.0001. The analysis shows significant heterogeneity; Τ² = 0.7835; I² = 99.5%.
Abbreviations: SWS: Slow Wave Sleep; REM: Rapid Eye Movement sleep.
For the experimental data on monoamine levels in mPFC, we tabulated concentrations (nmol/L) and statistics for all compounds (Table 18) and present the findings for DOPAC in a figure (Figure 4).
Table 18.
Summary of medians, interquartile ranges and Friedman’s ANOVA test statistics for each compound analysed during the different phases of the experiment.
| Analyte | Phase | Median (nmol/L) | IQR 25% | IQR 75% | Test statistics |
|---|---|---|---|---|---|
| 5-HT | Light | 1.56 | 1.17 | 6.88 | χ2(3) = 5.694 p = 0.127 |
| Dark | 0.92 | 0.61 | 2.21 | ||
| SD | 0.96 | 0.37 | 1.67 | ||
| Recovery | 1.19 | 0.44 | 4.60 | ||
| 5-HIAA | Light | 86.67 | 61.21 | 60.71 | χ2(3) = 6.60 p = 0.086 |
| Dark | 42.56 | 20.80 | 98.86 | ||
| SD | 103.32 | 72.68 | 55.56 | ||
| Recovery | 104.07 | 86.46 | 33.14 | ||
| 5-HTP | Light | 2.17 | 0.35 | 2.21 | χ2(3) = 4.92 p = 0.178 |
| Dark | 1.57 | 0.24 | 1.45 | ||
| SD | 2.43 | 0.46 | 0.95 | ||
| Recovery | 1.20 | 0.89 | 1.23 | ||
| DA | Light | 0.49 | 0.19 | 0.15 | χ2(3) = 5.40 p = 0.145 |
| Dark | 0.26 | 0.12 | 0.12 | ||
| SD | 0.34 | 0.10 | 0.22 | ||
| Recovery | 0.30 | 0.15 | 0.12 | ||
| DOPAC | Light | 2.10 | 0.97 | 2.47 | χ2(3) = 8.846 p = 0.037 |
| Dark | 1.70 | 0.67 | 3.35 | ||
| SD | 2.06 | 0.84 | 4.70 | ||
| Recovery | 1.33 | 0.36 | 1.36 | ||
| NA | Light | 0.28 | 0.14 | 1.00 | χ2(3) = 7.145 p = 0.067 |
| Dark | 0.47 | 0.300 | 0.38 | ||
| SD | 0.31 | 0.19 | 0.44 | ||
| Recovery | 0.11 | 0.01 | 0.09 | ||
| ADRE | Light | 0.18 | 0.06 | 0.04 | χ2(3) = 1.8 p = 0.615 |
| Dark | 0.21 | 0.10 | 0.06 | ||
| SD | 0.28 | 0.14 | 0.15 | ||
| Recovery | 0.23 | 0.11 | 0.09 | ||
Friedman’s ANOVA’s were performed to compare concentrations (nmol/L) between the different phases. For 5-HT, light phase n = 7, dark phase and SD n = 8, and recovery n = 9. For 5-HIAA, light phase, dark phase, and SD n = 11, recovery n = 10. For 5-HTP, light phase n = 9, dark phase, SD, and recovery n = 8. For dopamine, all phases n = 11. For DOPAC, light phase and recovery n = 9, dark phase n = 7, SD n = 8. For noradrenaline, all phase n = 11. For adrenaline, light phase, SD and recovery n = 10, dark phase n = 9. Numbers of observations vary because of missing samples (temporarily obstructed flow) and some concentrations being below HPLC detection limits.
Abbreviations: 5-HT: Serotonin; 5-HIAA: 5-Hydroxyindoleacetic Acid; 5-HTP: 5-Hydroxytryptophan; DA: Dopamine; DOPAC: 3,4-Dihydroxyphenylacetic acid; NA: Noradrenaline; ADRE: Adrenaline; IQR: Inter Quartile Range.
Figure 4.
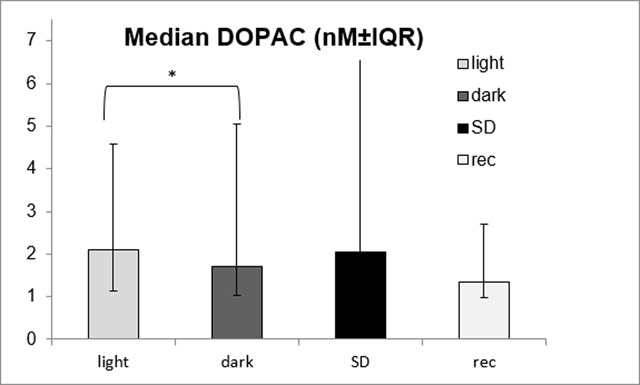
Median DOPAC dialysates concentrations in (nM) ± inter quartile range.
Light: 12h of baseline during the light phase; dark: 12h of baseline during the dark phase; SD: 12h of sleep deprivation during the light phase and rec: recovery for 12h. *Wilcoxon signed rank test: T = 0, p = 0.018.
Systematic review
Search and selection
Our search retrieved 2662 publications; 1195 from Medline and 1467 from EMBASE. After duplicate removal, 1550 publications remained for title abstract screening, and thereafter, 1170 for full text screening. From these, 94 were included. Screening of the reference lists only resulted in one additional publication [45]. The flow of included and excluded publications is presented in Figure 1.
Data were extracted from the 94 included publications, which could comprise multiple “studies” as detailed in the methods.
Study characteristics and quality assessment
The 94 included publications comprised 89 full papers, 4 conference abstracts, and 1 review containing otherwise unpublished data. Of the 89 full texts, 11 described 2 different experiments, 18 more than one experimental group and 16 simultaneous measurements within one animal. The 152 resulting studies describe CR experiments [93], sleep experiments [45] or SD experiments [14].
Animals
Species was reported for 151 studies (99.34%). 95 Studies (62.5%) were on rats; 54 on Wistars, 31 on Sprague-Dawleys, 2 on Lewis, 2 on Holtzman, 2 on lean Zucker, 1 on PVG and 1 on Spontaneously Hypertensive rats. Hamsters were used in 21 studies (13.72%); 15 used Syrians, 4 Siberians, and 1 Djungarian. Cats and mice were both used in 13 studies (8.5% each). The strain of mice was C57BL/6 (5) and mutant (7; for SERT, CHR-R1 and DAT genes). The remaining studies were on seals (7), sheep (1), and one human (1). The sex of the animals was reported in 135 studies (88.82%); most reported using males only (113), only few used females only (10) or both genders (12). Forty-one studies (26.8%) reported both the animals’ age and weight; 101 (66.45%) reported one of the two [64 studies only weight, 37 studies only age). Groups sizes were reported for 139 studies (91.45%) and varied from 1–133 animals.
Experimental set-up and microdialysis
Length of light and dark phase (L/D cycle) was reported for 133 studies (87.5%), actual clock times were specified for 129. Overall, 102 studies used a 12:12 LD cycle, 20 a 14:10 LD cycle, 5 a 16:10 LD cycle and 2 microdialyzed over 6h of dark and 24h of light.
Post-surgical recovery time was reported for 124 studies (81.58%) and ranged from 24h to 3 weeks. Probe length was reported for 135 studies (88.81%), and probe/membrane type for 134 (88.16%). Perfusion matrix (e.g. aCSF, Ringer) and perfusion rate (0,12–3 μL/min excluding one study [46] using slow perfusion [57 nl/min]) were reported in 149 studies (98.03%). Nine studies (7 CR studies and 2 SD studies) used reuptake inhibitors in the perfusion matrix; all of them for serotonin. Sample bin time was reported for 148 studies (97.37%) and fluctuated from 3 min to 2h. Probe recovery (2.4%–72 ± 3%) was reported for 55 studies (36.2%); 53 provided actual values, 2 reported methods to determine recovery without values. Histological verification of probe placement was described for 110 studies (72.37%) and one study verified probe placement by CT-scan [41].
Sample analysis was reported for 148 studies (97.37%); 146 using High Performance Liquid Chromatography (HPLC) and 2 using capillary electrophoresis. Monoamines were measured k = 86 studies for serotonin, k = 52 studies for 5-HIAA, k = 41 studies for dopamine, k = 35 studies for noradrenaline, k = 25 studies DOPAC, k = 2 studies for adrenaline. 5-HTP was not measured in our sample of studies.
General reporting quality
Approval by an ethical committee was reported for 80 studies (52.63%). Randomization of at least one study stage was reported for 13 studies (8.55%) and power analysis for only 4 studies (2.63%). Authors from 26 studies (17.11%) declared not having any conflicts of interests, authors from 2 clearly stated a conflict of interest. Funding source was mentioned for 98 studies (64.47%).
Monoamine measurements and circadian rhythms
CRs in monoamine concentrations as described in the included studies are described by monoamine in Tables 1, 2, 3, 4, 5, 6. Monoamine levels fluctuate over the dark and light phases. Patterns depend on the brain area and monoamine studied. We describe the findings with 3 general patterns: pattern 1: monoamine levels are higher during the dark phase and lower during the light phase; pattern 2: levels peak at or around dark phase onset; and pattern 3: levels remain stable during dark and light. Dopamine, DOPAC and noradrenaline mostly followed pattern 1. Serotonin and 5-HIAA mostly followed pattern 2 and occasionally pattern 1. Dopamine, noradrenaline and adrenaline sporadically followed pattern 3.
The patterns may differ by brain region; for some brain regions, a specific pattern was observed, while for others, patterns varied. For instance, pattern 1 was observed for dopamine and DOPAC levels in the caudate putamen, for DOPAC and 5-HIAA levels in the nucleus accumbens, and for serotonin, 5-HIAA, and noradrenaline levels in the hippocampus. In the striatum pattern 1 and 3 have been observed for dopamine and 5-HIAA; for DOPAC only pattern 1 has been described.
Likewise, pattern 2 was observed for serotonin levels in the amygdala and pineal gland, and for serotonin and 5-HIAA levels in the suprachiasmatic nucleus. In the preoptic area, pattern 2 was observed for 5-HIAA; pattern 3 for dopamine and adrenaline. The frontal cortex and the hypothalamus, including the paraventricular nucleus (PVN), showed consistent patterns. Other brain regions, such as the thalamus or the dorsal raphe, have only been investigated in one or two studies. In the thalamus, 5-HT levels seemed to follow pattern 1, while noradrenaline levels seemed to follow pattern 3. In the dorsal raphe, 5-HIAA levels seemed to follow pattern 1.
Besides brain region, other factors seemed to modify monoamine levels, e.g. the age of the animals [47], genetic factors [48,49], the environment [47] or the sex of the animals [50]. Furthermore, in female rats, the menstrual cycle also seemed to play a role [51].
Monoamine levels seemed to decrease or to lose rhythmicity with age; in older animals levels were lower than in younger animals [47]. Comparing studies with similar characteristics apart from the age of the animals, older animals (22–27 months) showed stable dopamine levels in the striatum, while younger animals (1–6 months) followed the fluctuating pattern 1 [47,52,53].
Monoamine measurements and naturally occurring sleep stages
The patterns in monoamine concentrations during naturally occurring sleep stages are described by monoamine in Tables 7, 8, 9, 10, 11.
Monoamine levels fluctuate between wakefulness and naturally occurring sleep stages (SWS and REM). Like in the preceding section, we describe the findings with 3 general patterns; pattern A: monoamine levels decrease from wakefulness to SWS and decrease further to REM; pattern B: monoamine levels increase from wakefulness to SWS and increase further to REM; and pattern C: levels remain stable during wakefulness and both sleep stages. Some studies do not follow these general patterns, as described below.
All monoamines have been shown to fluctuate according to pattern A in at least one brain region, except for adrenaline, which was not studied within our sample. Serotonin levels match pattern A in 11 of the 15 reported regions (PVN/VMN, amygdala, locus coeruleus, preoptic area, hippocampus, PPT, medial reticular pontine formation, cortex, thalamus, gigantocellular reticular nucleus, caudate nucleus). Cortex noradrenaline levels, and prefrontal/frontal cortex dopamine levels also followed Pattern A.
The patterns may again differ by brain region; for some regions a specific pattern was observed, while for others, patterns varied. For instance, noradrenaline and serotonin levels followed pattern A in the amygdala and locus coeruleus, while dopamine levels followed pattern C. Similarly, DOPAC, 5-HIAA, and noradrenaline levels followed pattern A in the PVN/VMN, while, DOPAC levels followed pattern B.
Measurement characteristics and study designs of the included studies were heterogeneous, which could explain observed inconsistencies. For example, in the dorsal raphe, serotonin levels seemed to follow either pattern A or a pattern where levels are high during wakefulness and SWS, and become lower during REM.
Our meta-analyses showed that serotonin and noradrenaline levels overall followed pattern A; they decreased from wakefulness to SWS and decreased further to REM sleep (Figures 2 and 3).
For serotonin, concentrations during SWS and REM both showed significant decreases compared to wake; p < 0.0001 (95 Confidence Interval (CI) SWS [–0.31; –0.23], REM [–0.48; –0.39]; I2 = 98.4%). Our sensitivity analyses confirmed the findings from the NMA; the overall effect for SWS versus wakefulness was –1.45 (SMD) with CI 95% [–2.07; –0.82], p < 0.01 and I2 = 66%. The overall effect for wakefulness versus REM was –1.61 (SMD) with CI 95% [–2.36; –0.86], p < 0.01 and I2 = 68% (appendix 5–6).
For noradrenaline, the concentrations during SWS and REM also showed significant decreases compared to wake; p < 0.0001 (CI 95% SWS [–1.74; –0.69], REM: [–3.44; –2.12]; I2 = 99.5%). Our sensitivity analyses again confirmed these findings; the overall effect for SWS versus wakefulness was –1.54 (SMD) with CI 95% [–2.19; –0.89], p = 0.01 and I2 = 53%. The overall effect for wakefulness versus REM was –2.58 (SMD) with CI 95% [–4.48; –0.69], p < 0.01 and I2 = 80% (appendix 7–8).
Monoamine measurements and sleep deprivation
SD alters dialysate monoamine concentrations. Most SD studies measured serotonin levels. Monoamine levels mainly increased during and/or after SD, except for serotonin which has been shown to both increase and decrease (Table 12, 13, 14, 15, 16, 17).
The increases in monoamine levels during SD seemed reversible. For instance, DOPAC and 5-HIAA levels in the basal forebrain, and 5-HT levels in SCN increased during SD but returned to baseline during recovery. However, recovery was not always observed; for instance, in the nucleus accumbens, dopamine, noradrenaline, and adrenaline levels all increased and in the posterior hippocampus serotonin levels remained elevated after SD. Besides, in the gigantocellular reticular nucleus, serotonin levels dropped with a factor 100 during SD, and they remained decreased during recovery (128). Similar patterns were observed for serotonin levels in the frontal cortex and in the hippocampus, albeit with a lower amplitude (115). The SD-induced changes in the hippocampus could be specific to parts of this brain region. Serotonin levels were observed to decrease during SD in the ventral hippocampus, while increases were observed in the posterior hippocampus. Findings during recovery were inconsistent; both a decrease and an increase compared to baseline were observed for serotonin (80, 133).
Medial prefrontal cortex experimental data
Monoamines levels remained fairly stable over baseline, as well as during SD and subsequent recovery (Table 18). Differences between the stages were not significant (p ≥ 0.07), except for DOPAC; χ2(3) = 8.486, p = 0.037 (Figure 4). The subsequent post-hoc tests showed a significant difference only between baseline light and dark, with a decrease in DOPAC levels from the light phase to the dark phase: T = 0, p = 0.018. The SD and recovery periods were not different from baseline (for SD versus light: T = 4, p = 0.091, for recovery versus light: T = 15, p = 0.886)
Discussion
This systematic review provides a full overview of the available evidence on monoamine levels in brain microdialysates in relation to CRs and sleep. To the best of our knowledge, this is the first systematic review on this subject. It includes all relevant studies retrieved by searches in two important databases. We are also the first to implement a network meta-analysis for the direct comparison of three sleep-wake stages. Other systematic reviews on sleep in animals have focussed on adenosine [137] and anxiety-related behaviour [39].
In this review, we showed that monoamine levels fluctuate with CRs and naturally occurring sleep stages. In line with their function as “arousal” transmitters, they generally decrease from wakefulness to SWS, and further decrease from SWS to REM [138]. For noradrenaline and serotonin, we confirmed this with meta-analyses.
Monoamines are thought to promote wakefulness via a network comprising the brainstem, thalamus, hypothalamus, basal forebrain, and cortex. The brainstem contains several wake-promoting nuclei: the locus coeruleus (noradrenaline), the dorsal and median raphe nuclei (serotonin), the ventral periaqueductal grey, the substantia nigra and the ventral tegmental area (dopamine). More specifically, monoamines were thought to inhibit sleep-promoting regions such as the ventrolateral optic area (VLPO) [15,16]. Recent evidence suggest that the monoaminergic pathways may not cause sleep promotion, but counteract unpredicted shifts in CRs or effects of stressors [139].
While our systematic review focusses on monoamines, these neuromodulators do not act in isolation. For instance, the SCN provides input to the above-mentioned brainstem nuclei to synchronize sleep-wake regulation with the environmental light-dark cycle. Glutamate and acetylcholine release in the SCN depends on input from the laterodorsal and pedunculopontine tegmental nuclei [140], and the SCN receives cholinergic input from the basal forebrain [141], which seem involved in phase-shifting activity patterns in response to changing light-dark rhythms.
We exclusively addressed the release of monoaminergic neurotransmitters. Neurotransmitters exert their actions via binding to receptors. It is important to also analyze patterns in the expression of these receptors. Circadian variations in receptor expression have been shown for e.g. adrenergic, muscarinergic, opioidergic, gabaergic, and dopaminergic receptors [142]. Besides, nicotinergic receptors seem to be involved in regulation of the sleep cycle [143]. A recent narrative review on the neurochemistry of wake and sleep regulation can put our findings into further perspective [144].
Our review shows that while monoamine fluctuations differed between brain regions and monoamines, overall the monoamine concentrations seem to be higher during the active dark phase than during the inactive light phase. However, fluctuations also vary with factors such as sex, age, BMI, genetic status, temperature, season, and humidity [145,146,147]. Several mechanisms could be involved, and evidence is present for sleep-related changes in monoamine synthesis [148,149], degradation [150,151,152,153,154], receptors and transporters [148,155] and binding [156,157].
The conclusions we can draw are limited by the overall amount of evidence; the number of studies per condition is low. Mainly SD studies and adrenaline studies are currently underrepresented in the literature (only 15 studies for SD and 2 studies for adrenaline). New literature has probably appeared since we performed our search in September 2017. As systematic reviews generally take over a year from start to completion [158], a lag time from search to publication is hard to avoid. We do not expect the relative and absolute number of additionally available studies since September 2017 to change our conclusions. At this stage; further primary studies are still warranted. An update of this SR in a few years from now should be more conclusive. Current systematic review efforts should first focus on e.g. cholinergic neurotransmission and on receptor expression in relation to circadian rhythms, sleep, and sleep deprivation.
Overall conclusions on monoamine neurochemistry in relation to sleep and wake are further limited by the variations in experimental designs between the included studies. Heterogeneity was observed for e.g. species, group size, brain region, experimental duration, L/D cycle, type of SD (reviewed by [159]), flow rate, perfusate, probe membrane type and probe type.
The SD method itself could also affect monoamine concentrations, for example via stress. SD can be stressful for the animals because of e.g. social isolation, humidity, and/or restricted or forced locomotion. In SD studies, it is challenging to implement appropriate controls. While some SD techniques are considered less stressful (e.g. gentle handling) than others (e.g. disk over water), they all are intrusive and probably stressful when chronic.
Several studies have analysed SD-induced stress in animals [39,43,160,161,162]. The stress induced by our experimental SD method seems to be minimal; we previously showed that corticosterone concentrations were not elevated above the normal circadian peak [43]. In line with this, the currently presented data show stable levels of adrenaline, and noradrenaline.
The overall risk of bias for the studies included in our review is difficult to estimate because of poor reporting of experimental procedures. For example, reporting of power calculations and randomisation was mostly absent. Many details of the microdialysis technique were reported well; sample time, perfusion rate, matrix type, and analysis technique were reported in more than 96% of the included studies, and probe length and membrane type were reported in more than 85%. However, reporting of recovery and verification of probe placement could have been better. The list of questions we developed for risk of bias assessment of microdialysis studies (appendix 4) seems to provide an adequate reflection of the technique-related modulating factors [163]. We recommend its use for future systematic reviews on the microdialysis technique.
We compared the noradrenaline and serotonin concentrations between the 3 sleep-wake stages usually distinguished in rodents (wake, SWS and REM) with NMAs. Only noradrenaline and serotonin were analysed, as the total number of studies of other monoamines was low, and the heterogeneity between study designs was considered too high for meta-analyses in general. The results of our NMAs were consistent with pairwise comparisons of sleep stages with classic meta-analysis techniques. NMAs have previously been used for clinical trials; to compare treatment effects for more than two treatments, and even to rank a series of treatments in efficacy without direct comparisons being available [164]. The NMA technique seems well-suited for systematic reviews comparing several sleep-wake stages; it allows for multiple comparisons and missing data, while taking variations at the study-level into account.
Our primary data suggest that monoamine levels in the medial prefrontal cortex (mPFC) are stable during and after SD. The mPFC is involved in several sleep-dependent processes such as attention, memory, incentive processing, decision making, and emotional regulation, which rely, at least partially, on the monoaminergic pathways [17,18,19,165,166]. The frontal cortex, and notably the mPFC, deactivate during sleep and SD [167,168]. Probably, other neurotransmitters and neuromodulators than the studied monoamines are involved.
Our systematic review provides a complete overview of the previously published SD- and CR-related monoamine data in the mPFC. Concerning CR, in rats, 7 CRs studies show that monoamine levels fluctuated with CRs. Dopamine, noradrenaline, 5-HT, and 5-HIAA levels augmented during the dark phase and decreased or remained stable during light phase. DOPAC levels were higher during the dark phase than during the light phase. Concerning SD, only 1 SD study in mice showed that noradrenaline levels increased during SD.
In contrast to these preceding studies, our primary data show no variation in SD- and CR-related mPFC monoamines, except for DOPAC levels, which were higher during baseline light than during baseline dark. The variability of our data is high, probably due to occasional blockages of the microdialysis set-up. However, in the same samples, we did find normal circadian curves and minimal effects of SD for corticosterone [43]. We used non-parametric analyses of the median values per 12h-phase to prevent the variability affecting our outcomes. The differences between the findings of preceding studies and our data could be caused by differences in experimental design (e.g. method of SD, probe size, flow rate, precise coordinates and probe angle). At this stage, the overall effects of CR and SD on mPFC monoamine levels remains unclear.
Sleep is required for several fundamental physiological processes, extending beyond the monoaminergic pathways. This paper shows that monoamines fluctuate with CRs, sleep stages, and SD. The monoamines are affected by several factors including e.g. brain region, species, sex, age, BMI, genetic status, temperature, season and humidity. Monoamines may not be part of the basic mechanism underlying the regulation of the sleep-wake cycle [134]. However, their involvement in sleep-wake regulation seems clear. Primary studies are still warranted to clarify how.
Additional Files
The additional files for this article can be found as follows:
Study characteristics of included publications on circadian rhythms.
Study characteristics of included publications on sleep stages.
Study characteristic of included publications on sleep deprivation.
Risk of bias – questions adapted to microdialysis studies.
Forest plot comparing serotonin concentration (nanomole/L) during wake and slow waves sleep.
Forest plot comparing serotonin concentration (nanomole/L) during wake and rapid-eye movement sleep.
Forest plot comparing noradrenaline concentration (nanomole/L) during wake and slow waves sleep.
Forest plot comparing noradrenaline concentration (nanomole/L) during wake and rapid-eye movement sleep.
Acknowledgements
The systematic review was funded by R2N, Federal State of Lower Saxony and the DFG (FOR2591, BL953/11-1). ZonMW provided funding for publication of our primary data (“more knowledge with less animals” scheme; project 40-42600-98-215). The microdialysis experiment was funded by the Netherlands Organisation for Scientific Research (NWO; 051-04-010 to Eus van Someren), the analysis of the monoamines by Janssen Pharmaceutica N.V., Beerse, Belgium.
The authors would like to thank Alice Tillema for her help with the systematic search optimisation, Rob de Vries for advice in protocol development and Mischa Schirris, Leslie Eggels and Mark Wuite for help in setting up and performing the microdialysis experiments.
Competing Interests
The authors have no competing interests to declare.
Author Contribution
RJ, MF and CL performed the microdialysis experiment. CN and PD performed the HPLC analyses. JM, PD and CL designed the systematic review protocol. JM, MA and CL screened the literature for inclusion. JM extracted the data from the included papers, CL performed a quality control on 5% of them. JM and CL analysed all data, which all authors interpreted. JM and CL wrote the manuscript, which all authors reviewed.
References
- 1.Foster, RG and Kreitzman, L. Rhythm of life: the biological clocks that control the dayly lives of every living thing Yale University Press, New Haven and London; 2005. [Google Scholar]
- 2.Touitou, Y, Reinberg, A and Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sciences. 2017; 173: 94–106. DOI: 10.1016/j.lfs.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Kecklund, G and Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ. 2016; 355: i5210 DOI: 10.1136/bmj.i5210 [DOI] [PubMed] [Google Scholar]
- 4.Kim, H, Jeong, G, Park, YK and Kang, SW. Sleep quality and nutritional intake in subjects with sleep issues according to perceived stress levels. J Lifestyle Med. 2018; 8(1): 42–9. DOI: 10.15280/jlm.2018.8.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin, JS, Laberge, L, Sasseville, A, Bérubé, M, Alain, S, Houle, J, et al. Day and night shift schedules are associated with lower sleep quality in Evening-types. Chronobiol Int. 2015; 32(5): 627–36. DOI: 10.3109/07420528.2015.1033425 [DOI] [PubMed] [Google Scholar]
- 6.Chung, KH, Li, CY, Kuo, SY, Sithole, T, Liu, WW and Chung, MH. Risk of psychiatric disorders in patients with chronic insomnia and sedative-hypnotic prescription: a nationwide population-based follow-up study. J Clin Sleep Med. 2015; 11(5): 543–51. DOI: 10.5664/jcsm.4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, B, Wang, Y, Cui, F, Huang, T, Sheng, P, Shi, T, et al. Association between insomnia and job stress: a meta-analysis. Sleep Breath; 2018. DOI: 10.1007/s11325-018-1682-y [DOI] [PubMed] [Google Scholar]
- 8.Waters, F and Bucks, RS. Neuropsychological effects of sleep loss: implication for neuropsychologists. J Int Neuropsychol Soc. 2011; 17(4): 571–86. DOI: 10.1017/S1355617711000610 [DOI] [PubMed] [Google Scholar]
- 9.Horne, J. Sleepfaring: A Journey Through the Science of Sleep Oxford University Press; 2007. [Google Scholar]
- 10.Abrams, RM. Sleep Deprivation. Obstet Gynecol Clin North Am. 2015; 42(3): 493–506. DOI: 10.1016/j.ogc.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 11.Shekari Soleimanloo, S, White, MJ, Garcia-Hansen, V and Smith, SS. The effects of sleep loss on young drivers’ performance: A systematic review. PLoS One. 2017; 12(8): e0184002 DOI: 10.1371/journal.pone.0184002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker, RS and Parker, P. The impact of sleep deprivation in military surgical teams: a systematic review. J R Army Med Corps. 2017; 163(3): 158–63. DOI: 10.1136/jramc-2016-000640 [DOI] [PubMed] [Google Scholar]
- 13.Costa, G. Chapter 24 – Sleep deprivation due to shift work In: Lotti, M and Bleecker, ML (Eds.), Handbook of Clinical Neurology. 2015; 131: 437–46. Elsevier; DOI: 10.1016/B978-0-444-62627-1.00023-8 [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem In: Colten, HR and Altevogt, BM (eds.), Washington (DC): National Academies Press (US); 2006. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 15.Murillo-Rodríguez, E, Arias-Carrión, O, Zavala-García, A, Sarro-Ramírez, A, Huitrón-Reséndiz, S and Arankowsky-Sandoval, G. Basic sleep mechanisms an integrative review. Cent Nerv Syst Agents Med Chem. 2012; 12: 38–54. DOI: 10.2174/187152412800229107 [DOI] [PubMed] [Google Scholar]
- 16.Luppi, PH and Fort, P. Neurochemistry of sleep: an overview of animal experimental work. Handbook of Clinical Neurology, Sleep Disorders, Part 1. 2011; 98: 173–90. 3rd series, chapter 11. DOI: 10.1016/B978-0-444-52006-7.00011-3 [DOI] [PubMed] [Google Scholar]
- 17.Takahashi, H. Monoamines and Decision-Making Under Risks In: Reuter, M and Montag, C (Eds.), Neuroeconomics. 2016; 85–95. Berlin, Heidelberg: Springer Berlin Heidelberg; DOI: 10.1007/978-3-642-35923-1_5 [DOI] [Google Scholar]
- 18.Puig, MV, Rose, J, Schmidt, R and Freund, N. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Front Neural Circuits. 2014; 8: 93 DOI: 10.3389/fncir.2014.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovheim, H. A new three-dimensional model for emotions and monoamine neurotransmitters. Med Hypotheses. 2012; 78(2): 341–8. DOI: 10.1016/j.mehy.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 20.Ungerstedt, U and Hallstrom, A. In vivo microdialysis– a new approach to the analysis of neurotransmitters in the brain. Life Sci. 1987; 41(7): 861–4. DOI: 10.1016/0024-3205(87)90181-0 [DOI] [PubMed] [Google Scholar]
- 21.Westerink, BH, Damsma, G, Rollema, H, De Vries, JB and Horn, AS. Scope and limitations of in vivo brain dialysis: a comparison of its application to various neurotransmitter systems. Life Sci. 1987; 41(15): 1763–76. DOI: 10.1016/0024-3205(87)90695-3 [DOI] [PubMed] [Google Scholar]
- 22.Di Chiara, G. In-vivo brain dialysis of neurotransmitters. Trends Pharmacol Sci. 1990; 11(3): 116–21. DOI: 10.1016/0165-6147(90)90197-G [DOI] [PubMed] [Google Scholar]
- 23.Anderzhanova, E and Wotjak, CT. Brain microdialysis and its applications in experimental neurochemistry. Cell Tissue Res. 2013; 354(1): 27–39. DOI: 10.1007/s00441-013-1709-4 [DOI] [PubMed] [Google Scholar]
- 24.Chefer, VI, Thompson, AC, Zapata, A and Shippenberg, TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009; Chapter 7: Unit 7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenaars, CH, Van Luijk, J, Freymann, J, van Ee, TJ, Zoer, B, Drinkenburg, WH, et al. Amino acids in microdialysates. http://www.syrcle.nl/. 2017; Protocol.
- 26.de Vries, RBM, Wever, KE, Avey, MT, Stephens, ML, Sena, ES and Leenaars, M. The Usefulness of Systematic Reviews of Animal Experiments for the Design of Preclinical and Clinical Studies. ILAR Journal. 2014; 55(3): 427–37. DOI: 10.1093/ilar/ilu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerink, BHC and Cremers, TIFH. Handbook of microdialysis – methods, applications and perspectives Amsterdam, the Netherlands: Elsevier – Academic Press; 2007. [Google Scholar]
- 28.Brand, I, Fliegel, S, Spanagel, R and Noori, HR. Global ethanol-induced enhancements of monoaminergic neurotransmission: a meta-analysis study. Alcohol Clin Exp Res. 2013; 37(12): 2048–57. DOI: 10.1111/acer.12207 [DOI] [PubMed] [Google Scholar]
- 29.Fritze, S, Spanagel, R and Noori, HR. Adaptive dynamics of the 5-HT systems following chronic administration of selective serotonin reuptake inhibitors: a meta-analysis. Journal of Neurochemistry. 2017; 142(5): 747–55. DOI: 10.1111/jnc.14114 [DOI] [PubMed] [Google Scholar]
- 30.Fliegel, S, Brand, I, Spanagel, R and Noori, HR. Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. n Silico Pharmacol. 2013; 1: 7 DOI: 10.1186/2193-9616-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noori, HR, Fliegel, S, Brand, I and Spanagel, R. The impact of acetylcholinesterase inhibitors on the extracellular acetylcholine concentrations in the adult rat brain: a meta-analysis. Synapse. 2012; 66(10): 893–901. DOI: 10.1002/syn.21581 [DOI] [PubMed] [Google Scholar]
- 32.Van der Mierden, S, Savelyev, SA, IntHout, J, De Vries, RBM and Leenaars, CHC. Intracerebral microdialysis of adenosine and AMP – a systematic review and meta-regression analysis J Neurochem. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenaars, CH, Joosten, RN, Kramer, M, Post, G, Eggels, L, Wuite, M, et al. Spatial reversal learning is robust to total sleep deprivation. Behav Brain Res. 2012; 230(1): 40–7. DOI: 10.1016/j.bbr.2012.01.047 [DOI] [PubMed] [Google Scholar]
- 34.Leenaars, CH, Joosten, RN, Zwart, A, Sandberg, H, Ruimschotel, E, Hanegraaf, MA, et al. Switch-task performance in rats is disturbed by 12 h of sleep deprivation but not by 12 h of sleep fragmentation. Sleep. 2012; 35(2): 211–21. DOI: 10.5665/sleep.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menon, JML, de Vries, RBM, Drinkenburg, WH and Leenaars, CH. Neurotransmitters and metabolites in brain microdialysates under sleep, circadian rhythms and sleep deprivation conditions – A systematic review. http://www.syrcle.nl/; 2017.
- 36.Wisor, JP, Nishino, S, Sora, I, Uhl, GH, Mignot, E and Edgar, DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001; 21(5): 1787–94. DOI: 10.1523/JNEUROSCI.21-05-01787.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitome, M. The central mechanism of feeding-associated circadian corticosterone rhythm in rats: analyses of paraventricular noradrenaline by in vivo microdialysis. [Japanese]. [Hokkaido igaku zasshi] The Hokkaido journal of medical science. 1994; 1: 120–35. [PubMed] [Google Scholar]
- 38.Hooijmans, CR, Rovers, MM, de Vries, RB, Leenaars, M, Ritskes-Hoitinga, M and Langendam, MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014; 14: 43 DOI: 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pires, GN, Bezerra, AG, Tufik, S and Andersen, ML. Effects of experimental sleep deprivation on anxiety-like behavior in animal research: systematic review and meta-analysis. Neurosci Biobehav Rev. 2016; 68: 575–89. DOI: 10.1016/j.neubiorev.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 40.Borenstein, M, Hedges, LV, Higgins, JPT and Rothstein, HR. Introduction to meta-analysis John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 41.Zeitzer, JM, Maidment, NT, Behnke, EJ, Ackerson, LC, Fried, I, Engel, J, Jr., et al. Ultradian sleep-cycle variation of serotonin in the human lateral ventricle. Neurology. 2002; 59(8): 1272–4. DOI: 10.1212/WNL.59.8.1272 [DOI] [PubMed] [Google Scholar]
- 42.Bellesi, M, Tononi, G, Cirelli, C and Serra, PA. Region-specific dissociation between cortical noradrenaline levels and the sleep/wake cycle. Sleep. 2016; 1: 143–54. DOI: 10.5665/sleep.5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leenaars, CH, Dematteis, M, Joosten, RN, Eggels, L, Sandberg, H, Schirris, M, et al. A new automated method for rat sleep deprivation with minimal confounding effects on corticosterone and locomotor activity. J Neurosci Methods. 2011; 196(1): 107–17. DOI: 10.1016/j.jneumeth.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 44.Fujino, K, Yoshitake, T, Kehr, J, Nohta, H and Yamaguchi, M. Simultaneous determination of 5-hydroxyindoles and catechols by high-performance liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J Chromatogr A. 2003; 1012(2): 169–77. DOI: 10.1016/S0021-9673(03)01180-4 [DOI] [PubMed] [Google Scholar]
- 45.Glass, JD, Hauser, UE and Randolph, WW. In: vivo microdialysis of 5-hydroxyindoleacetic acid and glutamic acid in the hamster suprachiasmatic nuclei. Am Zool. 1993; 33: 212–8. DOI: 10.1093/icb/33.2.212 [DOI] [Google Scholar]
- 46.Smith, AD, Olson, RJ and Justice, JB, Jr. Quantitative microdialysis of dopamine in the striatum: Effect of circadian variation. J Neurosci Methods. 1992; 1: 33–41. DOI: 10.1016/0165-0270(92)90111-P [DOI] [PubMed] [Google Scholar]
- 47.Sano, A, Aoi, K, Azekawa, T, Sei, H, Seno, H and Morita, Y. Diurnal monoamine variation in young and old rats: a microdialysis study. Journal of nutritional science and vitaminology. 1992; 577–80. DOI: 10.3177/jnsv.38.Special_577 [DOI] [PubMed] [Google Scholar]
- 48.Ferris, MJ, Espana, RA, Locke, JL, Konstantopoulos, JK, Rose, JH, Chen, R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proceedings of the National Academy of Sciences of the United States of America. 2014; 26: E2751–E9. DOI: 10.1073/pnas.1407935111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, H, Thompson, AB, McIntosh, BJ, Altieri, SC and Andrews, AM. Physiologically relevant changes in serotonin resolved by fast microdialysis. ACS Chemical Neuroscience. 2013; 5: 790–8. DOI: 10.1021/cn400072f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jitsuki, S, Kimura, F, Funabashi, T, Takahashi, T and Mitsushima, D. Sex-specific 24-h profile of extracellular serotonin levels in the medial prefrontal cortex. Brain Res. 2009; 1260: 30–7. DOI: 10.1016/j.brainres.2008.12.084 [DOI] [PubMed] [Google Scholar]
- 51.Hucke, EETS, Cruz-Casallas, PE, Florio, JC and Felicio, LF. Reproductive experience reduces striatal dopaminergic responses in freely moving female rats. NeuroReport. 1998; 16: 3589–93. DOI: 10.1097/00001756-199811160-00009 [DOI] [PubMed] [Google Scholar]
- 52.De Marquez Prado, B, Castaneda, TR, Galindo, A, Del Arco, A, Segovia, G, Reiter, RJ, et al. Melatonin disrupts circadian rhythms of glutamate and GABA in the neostriatum of the awake rat: A microdialysis study. Journal of Pineal Research. 2000; 4: 209–16. DOI: 10.1034/j.1600-0633.2002.290403.x [DOI] [PubMed] [Google Scholar]
- 53.Decker, MJ, Jones, KA, Solomon, IG, Keating, GL and Rye, DB. Reduced extracellular dopamine and increased responsiveness to novelty: Neurochemical and behavioral sequelae of intermittent hypoxia. Sleep. 2005; 2: 169–76. DOI: 10.1093/sleep/28.2.169 [DOI] [PubMed] [Google Scholar]
- 54.Dugovic, C, Shelton, JE, Aluisio, LE, Fraser, IC, Jiang, X, Sutton, SW, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. Journal of Pharmacology and Experimental Therapeutics. 2009; 1: 142–51. DOI: 10.1124/jpet.109.152009 [DOI] [PubMed] [Google Scholar]
- 55.Barbier, AJ, Aluisio, L, Lord, B, Qu, Y, Wilson, SJ, Boggs, JD, et al. Pharmacological characterization of JNJ-28583867, a histamine H3 receptor antagonist and serotonin reuptake inhibitor. Eur J Pharmacol. 2007; 576(1–3): 43–54. DOI: 10.1016/j.ejphar.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 56.Nakayama, K, Takeda, A, Hiyama, T, Yoshimuta, N and Ushijima, S. Diurnal rhythm of 5HIAA release determined by in vivo microdialysis in freely moving rats. Japanese Journal of Psychiatry and Neurology. 1993; 2: 491–3. DOI: 10.1111/j.1440-1819.1993.tb02164.x [DOI] [PubMed] [Google Scholar]
- 57.Robinson, JE, Kendrick, KM and Lambart, CE. Changes in the release of gamma-aminobutyric Acid and catecholamines in the preoptic/septal area prior to and during the preovulatory surge of luteinizing hormone in the ewe. J Neuroendocrinol. 1991; 4: 393–9. DOI: 10.1111/j.1365-2826.1991.tb00293.x [DOI] [PubMed] [Google Scholar]
- 58.Alfinito, PD, Chen, X, Mastroeni, R, Pawlyk, AC and Deecher, DC. Estradiol increases catecholamine levels in the hypothalamus of ovariectomized rats during the dark-phase. European Journal of Pharmacology. 2009; 1–3: 334–9. DOI: 10.1016/j.ejphar.2009.06.045 [DOI] [PubMed] [Google Scholar]
- 59.Castaneda, TR, Marquez De Prado, B, Prieto, D and Mora, F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: Modulation by light. Journal of Pineal Research. 2004; 3: 177–85. DOI: 10.1046/j.1600-079X.2003.00114.x [DOI] [PubMed] [Google Scholar]
- 60.Hood, S, Cassidy, P, Cossette, MP, Weigl, Y, Verwey, M, Robinson, B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010; 42: 14046–58. DOI: 10.1523/JNEUROSCI.2128-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulson, PE and Robinson, TE. Relationship between Circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on- line microdialysis. Behavioral Neuroscience. 1994; 3: 624–35. DOI: 10.1037/0735-7044.108.3.624 [DOI] [PubMed] [Google Scholar]
- 62.Paulson, PE and Robinson, TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology. 1996; 5: 325–37. DOI: 10.1016/0893-133X(95)00141-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murillo-Rodriguez, E, Palomero-Rivero, M, Millan-Aldaco, D and Di Marzo, V. The administration of endocannabinoid uptake inhibitors OMDM-2 or VDM-11 promotes sleep and decreases extracellular levels of dopamine in rats. Physiology and Behavior. 2013; 1: 88–95. DOI: 10.1016/j.physbeh.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 64.Verhagen, LAW, Luijendijk, MCM, Korte-Bouws, GAH, Korte, SM and Adan, RAH. Dopamine and serotonin release in the nucleus accumbens during starvation-induced hyperactivity. European Neuropsychopharmacology. 2009; 5: 309–16. DOI: 10.1016/j.euroneuro.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 65.Fetissov, SO, Meguid, MM, Chen, C and Miyata, G. Synchronized release of dopamine and serotonin in the medial and lateral hypothalamus of rats. Neuroscience. 2000; 3: 657–63. DOI: 10.1016/S0306-4522(00)00374-2 [DOI] [PubMed] [Google Scholar]
- 66.Izumo, N, Ishibashi, Y, Ohba, M, Morikawa, T and Manabe, T. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behavioural Brain Research. 2012; 1: 1–6. DOI: 10.1016/j.bbr.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 67.Luo, S, Zhang, Y, Ezrokhi, M, Trubitsyna, Y and Cincotta, AH. High-fat feeding abolishes the insulin-sensitizing peak in circadian dopamine activity at the biological clock. Diabetes. 2014; A470. [Google Scholar]
- 68.Huang, Z, Liu, T, Chattoraj, A, Ahmed, S, Wang, MM, Deng, J, et al. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. Journal of Pineal Research. 2008; 4: 506–14. DOI: 10.1111/j.1600-079X.2008.00627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun, X, Deng, J, Liu, T and Borjigin, J. Circadian 5-HT production regulated by adrenergic signaling. Proceedings of the National Academy of Sciences of the United States of America 2002; 7: 4686–91. DOI: 10.1073/pnas.062585499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun, X, Liu, T, Deng, J and Borjigin, J. Long-term in vivo pineal microdialysis. Journal of Pineal Research. 2003; 2: 118–24. DOI: 10.1034/j.1600-079X.2003.00064.x [DOI] [PubMed] [Google Scholar]
- 71.Azekawa, T, Sano, A, Sei, H and Morita, Y. Diurnal changes in pineal extracellular indoles of freely moving rats. Neuroscience Letters. 1991; 1: 93–6. DOI: 10.1016/0304-3940(91)90441-U [DOI] [PubMed] [Google Scholar]
- 72.Liu, T and Borjigin, J. Free-running rhythms of pineal circadian output. Journal of Biological Rhythms. 2005; 5: 430–40. DOI: 10.1177/0748730405277868 [DOI] [PubMed] [Google Scholar]
- 73.Liu, T and Borjigin, J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. Journal of Circadian Rhythms; 2006; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garabette, ML, Martin, KF and Redfern, PH. Circadian variation in the activity of the 5-HT(1B) autoreceptor in the region of the suprachiasmatic nucleus, measured by microdialysis in the conscious freely-moving rat. British Journal of Pharmacology. 2000; 8: 1569–76. DOI: 10.1038/sj.bjp.0703753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grossman, GH, Mistlberger, RE, Antle, MC, Ehlen, JC and Glass, JD. Sleep deprivation stimulates serotonin release in the suprachiasmatic nucleus. NeuroReport. 2000; 9: 1929–32. DOI: 10.1097/00001756-200006260-00024 [DOI] [PubMed] [Google Scholar]
- 76.Dudley, TE, DiNardo, LA and Glass, JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. Journal of Neuroscience. 1998; 13: 5045–52. DOI: 10.1523/JNEUROSCI.18-13-05045.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barassin, S, Raison, S, Saboureau, M, Bienvenu, C, Maitre, M, Malan, A, et al. Circadian tryptophan hydroxylase levels and serotonin release in the suprachiasmatic nucleus of the rat. European Journal of Neuroscience. 2002; 5: 833–40. DOI: 10.1046/j.1460-9568.2002.01928.x [DOI] [PubMed] [Google Scholar]
- 78.Knoch, ME, Gobes, SMH, Pavlovska, I, Su, C, Mistlberger, RE and Glass, JD. Short-term exposure to constant light promotes strong circadian phase-resetting responses to nonphotic stimuli in Syrian hamsters. European Journal of Neuroscience. 2004; 10: 2779–90. DOI: 10.1111/j.0953-816X.2004.03371.x [DOI] [PubMed] [Google Scholar]
- 79.Oshima, A, Flachskamm, C, Reul, JMHM, Holsboer, F and Linthorst, ACE. Altered Serotonergic Neurotransmission but Normal Hypothalamic-Pituitary- Adrenocortical Axis Activity in Mice Chronically Treated with the Corticotropin-Releasing Hormone Receptor Type I Antagonist NBI 30775. Neuropsychopharmacology. 2003; 12: 2148–59. DOI: 10.1038/sj.npp.1300267 [DOI] [PubMed] [Google Scholar]
- 80.Lopez-Rodriguez, F, Wilson, CL, Maidment, NT, Poland, RE and Engel, J, Jr. Total sleep deprivation increases extracellular serotonin in the rat hippocampus. Neuroscience. 2003; 2: 523–30. DOI: 10.1016/S0306-4522(03)00335-X [DOI] [PubMed] [Google Scholar]
- 81.Linthorst, ACE, Flachskamm, C, Holsboer, F and Reul, JMHM. Local administration of recombinant human interleukin-1beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary- adrenocortical axis activity, and body temperature. Endocrinology. 1994; 2: 520–32. DOI: 10.1210/endo.135.2.7518383 [DOI] [PubMed] [Google Scholar]
- 82.Kalen, P, Rosegren, E, Lindvall, O and Bjorklund, A. Hippocampal Noradrenaline and Serotonin Release over 24 Hours as Measured by the Dialysis Technique in Freely Moving Rats: Correlation to Behavioural Activity State, Effect of Handling and Tail-Pinch. Eur J Neurosci. 1989; 3: 181–8. DOI: 10.1111/j.1460-9568.1989.tb00786.x [DOI] [PubMed] [Google Scholar]
- 83.Pealva, RG, Flachskamm, C, Zimmermann, S, Wurst, W, Holsboer, F, Reul, JMHM, et al. Corticotropin-releasing hormone receptor type 1-deficiency enhances hippocampal serotonergic neurotransmission: An in vivo microdialysis study in mutant mice. Neuroscience. 2002; 2: 253–66. DOI: 10.1016/S0306-4522(01)00475-4 [DOI] [PubMed] [Google Scholar]
- 84.Takahashi, H, Takada, Y, Nagai, N, Urano, T and Takada, A. Extracellular serotonin in the striatum increased after immobilization stress only in the nighttime. Behavioural Brain Research. 1998; 1–2: 185–91. DOI: 10.1016/S0166-4328(97)00120-4 [DOI] [PubMed] [Google Scholar]
- 85.Smriga, M, Kameishi, M, Uneyama, H and Torii, K. Dietary L-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. Journal of Nutrition. 2002; 12: 3744–6. DOI: 10.1093/jn/132.12.3744 [DOI] [PubMed] [Google Scholar]
- 86.Grossman, GH, Farnbauchm, L and Glass, JD. Regulation of serotonin release in the Syrian hamster intergeniculate leaflet region. NeuroReport. 2004; 1: 103–6. DOI: 10.1097/00001756-200401190-00021 [DOI] [PubMed] [Google Scholar]
- 87.Sayer, TJO, Hannon, SD, Redfern, PH and Martin, KF. Diurnal variation in 5-HT(1B) autoreceptor function in the anterior hypothalamus in vivo: Effect of chronic antidepressant drug treatment. British Journal of Pharmacology. 1999; 8: 1777–84. DOI: 10.1038/sj.bjp.0702535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glass, JD, Hauser, UE, Blank, JL, Selim, M and Rea, MA. Differential timing of amino acid and 5-HIAA rhythms in suprachiasmatic hypothalamus. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1993; 334–3: R504–R11. [DOI] [PubMed] [Google Scholar]
- 89.Luo, S, Luo, J and Cincotta, AH. Suprachiasmatic nuclei monoamine metabolism of glucose tolerant versus intolerant hamsters. NeuroReport. 1999; 10: 2073–7. DOI: 10.1097/00001756-199907130-00015 [DOI] [PubMed] [Google Scholar]
- 90.Glass, JD, Hauser, UE, Randolph, W, Ferriera, S and Rea, MA. Suprachiasmatic nucleus neurochemistry in the conscious brain: correlation with circadian activity rhythms. Journal of biological rhythms. 1993; S47–52. [PubMed] [Google Scholar]
- 91.Glass, JD, Randolph, WW, Ferreira, SA, Rea, MA, Hauser, UE, Blank, JL, et al. Diurnal variation in 5-hydroxyindole-acetic acid output in the suprachiasmatic region of the siberian hamster assessed by in vivo microdialysis: Evidence for nocturnal activation of serotonin release. Neuroendocrinology. 1992; 4: 582–90. DOI: 10.1159/000126277 [DOI] [PubMed] [Google Scholar]
- 92.Luo, S, Luo, J and Cincotta, AH. Association of the antidiabetic effects of bromocriptine with a shift in the daily rhythm of monoamine metabolism within the suprachiasmatic nuclei of the Syrian hamster. Chronobiology International. 2000; 2: 155–72. DOI: 10.1081/CBI-100101040 [DOI] [PubMed] [Google Scholar]
- 93.Nakayama, K. Diurnal rhythm in extracellular levels of 5-hydroxyindoleacetic acid in the medial prefrontal cortex of freely moving rats: An in vivo microdialysis study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002; 7–8: 1383–8. DOI: 10.1016/S0278-5846(02)00304-4 [DOI] [PubMed] [Google Scholar]
- 94.Ezrokhi, M, Luo, S, Trubitsyna, Y and Cincotta, AH. Neuroendocrine and metabolic components of dopamine agonist amelioration of metabolic syndrome in SHR rats. Diabetology and Metabolic Syndrome. 2014; 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo, S, Meier, AH and Cincotta, AH. Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters. Neuroendocrinology. 1998; 1: 1–10. DOI: 10.1159/000054344 [DOI] [PubMed] [Google Scholar]
- 96.Stanley, BG, Schwartz, DH, Hernandez, L, Leibowitz, SF and Hoebel, BG. Patterns of extracellular 5-hydroxyindoleacetic acid (5-HIAA) in the paraventricular hypothalamus (PVN): Relation to circadian rhythm and deprivation-induced eating behavior. Pharmacology Biochemistry and Behavior. 1989; 1: 257–60. DOI: 10.1016/0091-3057(89)90459-0 [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez-Pina, R, Alfaro-Rodriguez, A and De Jesus Morales-Martinez, J. The Role of the Dorsal Raphe in the Sleep Disruptions Produced by Ozone Exposure. Proceedings of the Western Pharmacology Society. 2003; 116–20. [PubMed] [Google Scholar]
- 98.Stanley, BG, Schwartz, DH, Hernandez, L, Hoebel, BG and Leibowitz, SF. Patterns of extracellular norepinephrine in the paraventricular hypothalamus: Relationship to circadian rhythm and deprivation-induced eating behavior. Life Sciences. 1989; 4: 275–82. DOI: 10.1016/0024-3205(89)90136-7 [DOI] [PubMed] [Google Scholar]
- 99.Mitome, M, Honma, S, Yoshihara, T and Honma, KI. Prefeeding increase in paraventricular NE release is regulated by a feeding-associated rhythm in rats. American Journal of Physiology – Endocrinology and Metabolism. 1994; 429–4: E606–E11. [DOI] [PubMed] [Google Scholar]
- 100.Morien, A, Wellman, PJ and Fojt, J. Diurnal rhythms of paraventricular hypothalamic norepinephrine and food intake in rats. Pharmacology Biochemistry and Behavior. 1995; 1: 169–74. DOI: 10.1016/0091-3057(95)00084-A [DOI] [PubMed] [Google Scholar]
- 101.Smriga, M and Torii, K. Preferable Monosodium Glutamate and Sodium Chloride Solutions do not Affect Diurnal Norepinephrine Release in the Rat Lateral Hypothalamus. Nutr Neurosci. 2000; 5: 367–72. DOI: 10.1080/1028415X.2000.11747334 [DOI] [PubMed] [Google Scholar]
- 102.Smriga, M, Mori, M and Torii, K. Circadian release of hypothalamic norepinephrine in rats in vivo is depressed during early L-lysine deficiency. Journal of Nutrition. 2000; 6: 1641–3. DOI: 10.1093/jn/130.6.1641 [DOI] [PubMed] [Google Scholar]
- 103.Drijfhout, WJ, Van Der Linde, AG, Kooi, SE, Grol, CJ and Westerink, BHC. Norepinephrine release in the rat pineal gland: The input from the biological clock measured by in vivo microdialysis. Journal of Neurochemistry. 1996; 2: 748–55. DOI: 10.1046/j.1471-4159.1996.66020748.x [DOI] [PubMed] [Google Scholar]
- 104.Orosco, M, Rouch, C, De Saint-Hilaire, Z and Nicolaidis, S. Dynamic changes in hypothalamic monoamines during sleep/wake cycles assessed by parallel EEG and microdialysis in the rat. Journal of Sleep Research. 1995; 3: 144–9. DOI: 10.1111/j.1365-2869.1995.tb00163.x [DOI] [PubMed] [Google Scholar]
- 105.Nicolaidis, S, Gerozissis, K and Orosco, M. [Variations of hypothalamic and cortical prostaglandins and monoamines reveal transitions in arousal states: microdialysis study in the rat]. Rev Neurol (Paris). 2001; 11(Pt 2): S26–33. [PubMed] [Google Scholar]
- 106.Shouse, MN, Staba, RJ, Saquib, SF and Farber, PR. Monoamines and sleep: Microdialysis findings in pons and amygdala. Brain Research. 2000; 1–2: 181–9. DOI: 10.1016/S0006-8993(00)02013-8 [DOI] [PubMed] [Google Scholar]
- 107.Shouse, MN, Staba, RJ, Ko, PY, Saquib, SF and Farber, PR. Monoamines and seizures: Microdialysis findings in locus ceruleus and amygdala before and during amygdala kindling. Brain Research. 2001; 1: 176–92. DOI: 10.1016/S0006-8993(00)03292-3 [DOI] [PubMed] [Google Scholar]
- 108.Shouse, MN, Staba, RJ, Saquib, SF and Farber, PR. Long-lasting effects of feline amygdala kindling on monoamines, seizures and sleep. Brain Research. 2001; 1: 147–65. DOI: 10.1016/S0006-8993(00)03265-0 [DOI] [PubMed] [Google Scholar]
- 109.Lena, I, Parrot, S, Deschaux, O, Muffat-Joly, S, Sauvinet, V, Renaud, B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep-wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. Journal of Neuroscience Research. 2005; 6: 891–9. DOI: 10.1002/jnr.20602 [DOI] [PubMed] [Google Scholar]
- 110.De Saint Hilaire, Z, Orosco, M, Rouch, C, Python, A and Nicolaidis, S. Neuromodulation of the prefrontal cortex during sleep: A microdialysis study in rats. NeuroReport. 2000; 8: 1619–24. DOI: 10.1097/00001756-200006050-00005 [DOI] [PubMed] [Google Scholar]
- 111.Wilkinson, LO, Auerbach, SB and Jacobs, BL. Extracellular serotonin levels change with behavioral state but not with pyrogen-induced hyperthermia. Journal of Neuroscience. 1991; 9: 2732–41. DOI: 10.1523/JNEUROSCI.11-09-02732.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Python, A, Steimer, T, De Saint Hilaire, Z, Mikolajewski, R and Nicolaidis, S. Extracellular serotonin variations during vigilance states in the preoptic area of rats: A microdialysis study. Brain Research. 2001; 1–2: 49–54. DOI: 10.1016/S0006-8993(01)02477-5 [DOI] [PubMed] [Google Scholar]
- 113.Park, SP, Lopez-Rodriguez, F, Wilson, CL, Maidment, N, Matsumoto, Y and Engel, J, Jr. In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep-wakefulness. Brain Research. 1999; 2: 291–6. DOI: 10.1016/S0006-8993(99)01511-5 [DOI] [PubMed] [Google Scholar]
- 114.Gronli, J, Fiske, E, Murison, R, Bjorvatn, B, Sorensen, E, Ursin, R, et al. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behavioural Brain Research. 2007; 1: 42–51. DOI: 10.1016/j.bbr.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 115.Bjorvatn, B, Gronli, J, Hamre, F, Sorensen, E, Fiske, E, Bjorkum, A, et al. Effects of sleep deprivation on extracellular serotonin in hippocampus and frontal cortex of the rat. Neuroscience. 2002; 2: 323–30. DOI: 10.1016/S0306-4522(02)00181-1 [DOI] [PubMed] [Google Scholar]
- 116.Penalva, RG, Lancel, M, Flachskamm, C, Reul, JMHM, Holsboer, F and Linthorst, ACE. Effect of sleep and sleep deprivation on serotonergic neurotransmission in the hippocampus: A combined in vivo microdialysis/EEG study in rats. European Journal of Neuroscience. 2003; 9: 1896–906. DOI: 10.1046/j.1460-9568.2003.02612.x [DOI] [PubMed] [Google Scholar]
- 117.Fiske, E, Gronli, J, Bjorvatn, B, Ursin, R and Portas, CM. The effect of GABA (A) antagonist bicuculline on dorsal raphe nucleus and frontal cortex extracellular serotonin: A window on SWS and REM sleep modulation. Pharmacology Biochemistry and Behavior. 2006; 2: 314–21. DOI: 10.1016/j.pbb.2006.02.014 [DOI] [PubMed] [Google Scholar]
- 118.Fiske, E, Portas, CM, Gronli, J, Sorensen, E, Bjorvatn, B, Bjorkum, AA, et al. Increased extracellular 5-HT but no change in sleep after perfusion of a 5-HT1 A antagonist into the dorsal raphe nucleus of rats. Acta Physiologica. 2008; 1: 89–97. DOI: 10.1111/j.1748-1716.2007.01792.x [DOI] [PubMed] [Google Scholar]
- 119.Portas, CM and McCarley, RW. Behavioral state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: A microdialysis study in the freely moving cat. Brain Research. 1994; 2: 306–12. DOI: 10.1016/0006-8993(94)91132-0 [DOI] [PubMed] [Google Scholar]
- 120.Portas, CM, Thakkar, M, Rainnie, D and McCarley, RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. Journal of Neuroscience. 1996; 8: 2820–8. DOI: 10.1523/JNEUROSCI.16-08-02820.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Portas, CM, Bjorvatn, B, Fagerland, S, GrOnli, J, Mundal, V, Sørensen, E, Ursin, R, et al. On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience. 1998; 3: 807–14. DOI: 10.1016/S0306-4522(97)00438-7 [DOI] [PubMed] [Google Scholar]
- 122.Mukaida, K, Shichino, T, Koyanagi, S, Himukashi, S and Fukuda, K. Activity of the serotonergic system during isoflurane anesthesia. Anesthesia and Analgesia. 2007; 4: 836–9. DOI: 10.1213/01.ane.0000255200.42574.22 [DOI] [PubMed] [Google Scholar]
- 123.McCarley, RW. Mechanisms and models of REM sleep control. Archives Italiennes de Biologie. 2004; 4: 429–67. [PubMed] [Google Scholar]
- 124.Strecker, RE, Thakkar, MM, Porkka-Heiskanen, T, Dauphin, LJ, Bjorkum, AA and McCarley, RW. Behavioral state-related changes of extracellular serotonin concentration in the pedunculopontine tegmental nucleus: a microdialysis study in freely moving animals. Sleep research online: SRO. 1999; 2: 21–7. [PubMed] [Google Scholar]
- 125.Lapierre, JL, Kosenko, P, Kodama, T, Peever, J, Mukhametov, L, Lyamin, O, et al. Unlike acetylcholine, cortical serotonin release is not lateralized during asymmetrical slow wave sleep in the fur seal. Sleep. 2012; A26. [Google Scholar]
- 126.Lapierre, JL, Kosenko, PO, Kodama, T, Peever, JH, Mukhametov, LM, Lyamin, OI, et al. Symmetrical serotonin release during asymmetrical slow-wave sleep: Implications for the neurochemistry of sleep-waking states. Journal of Neuroscience. 2013; 6: 2555–61. DOI: 10.1523/JNEUROSCI.2603-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lyamin, OI, Lapierre, JL, Kosenko, PO, Kodama, T, Bhagwandin, A, Korneva, SM, et al. Monoamine release during unihemispheric sleep and unihemispheric waking in the fur seal. Sleep. 2016; 3: 625–36. DOI: 10.5665/sleep.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Blanco-Centurion, CA and Salin-Pascual, RJ. Extracellular serotonin levels in the medullary reticular formation during normal sleep and after REM sleep deprivation. Brain Research. 2001; 1–2: 128–36. DOI: 10.1016/S0006-8993(01)03209-7 [DOI] [PubMed] [Google Scholar]
- 129.Iwakiri, H. Extracellular levels of serotonin in the medial pontine reticular formation in relation to sleep-wake cycle in cats: A microdialysis study. Neuroscience Research. 1993; 2: 157–70. DOI: 10.1016/0168-0102(93)90018-L [DOI] [PubMed] [Google Scholar]
- 130.Shouse, MN, Farber, PR and Staba, RJ. Physiological basis: How NREM sleep components can promote and REM sleep components can suppress seizure discharge propagation. Clinical Neurophysiology. 2000; SUPPL. 2: S9–S18. DOI: 10.1016/S1388-2457(00)00397-7 [DOI] [PubMed] [Google Scholar]
- 131.Park, SP. In vivo microdialysis measures of extracellular norepinephrine in the rat amygdala during sleep-wakefulness. Journal of Korean medical science. 2002; 3: 395–9. DOI: 10.3346/jkms.2002.17.3.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lapierre, JL, Kosenko, P, Korneva, SM, Kodama, T, Peever, J, Mukhametov, L, et al. Cortical norepinephrine release is not lateralized during asymmetrical slow-wave sleep in the fur seal. Sleep. 2013; A59. [Google Scholar]
- 133.Lopez-Rodriguez, F, Wilson, C, Maidment, N, Poland, R, Chase, MH and Engel, J, Jr. Extracellular serotonin in the rat hippocampus during REM sleep deprivation. Sleep Research Online. 2003; 3: 115–22. [DOI] [PubMed] [Google Scholar]
- 134.Murillo-Rodriguez, E, Machado, S, Rocha, NB, Budde, H, Yuan, TF and Arias-Carrion, O. Revealing the role of the endocannabinoid system modulators, SR141716A, URB597 and VDM-11, in sleep homeostasis. Neuroscience. 2016; 433–49. DOI: 10.1016/j.neuroscience.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 135.Zant, JC, Rozov, S, Kostin, A, Panula, P and Porkka-Heiskanen, T. Dynamic changes in neurotransmitter levels in the basal forebrain during and after sleep deprivation. Journal of Sleep Research. 2010; 191. [Google Scholar]
- 136.Zant, JC, Leenaars, CHC, Kostin, A, Van Someren, EJW and Porkka-Heiskanen, T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Research. 2011; 40–8. DOI: 10.1016/j.brainres.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 137.Van der Mierden, S, Savelyev, SA, IntHout, J, De Vries, RBM and Leenaars, CHC. Intracerebral microdialysis of adenosine and AMP – a systematic review and meta-regression analysis (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kodama, T, Mallick, BN, Pandi-Perumal, SR, McCarley, RW and Morrison, AR. REM sleep: regulation and function 2011; 266–79. Chapter 27. Cambridge University Press. [Google Scholar]
- 139.Saper, CB and Fuller, PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017; 44: 186–92. DOI: 10.1016/j.conb.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abbott, SM, Arnold, JM, Chang, Q, Miao, H, Ota, N, Cecala, C, et al. Signals from the brainstem sleep/wake centers regulate behavioral timing via the circadian clock. PLoS One. 2013; 8(8): e70481 DOI: 10.1371/journal.pone.0070481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamakawa, GR, Basu, P, Cortese, F, MacDonnell, J, Whalley, D, Smith, VM, et al. The cholinergic forebrain arousal system acts directly on the circadian pacemaker. Proc Natl Acad Sci U S A 2016; 113(47): 13498–503. DOI: 10.1073/pnas.1610342113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wirz-Justice, A, Tobler, I, Kafka, MS, Naber, D, Marangos, PJ, Borbely, AA, et al. Sleep deprivation: effects on circadian rhythms of rat brain neurotransmitter receptors. Psychiatry Res. 1981; 5(1): 67–76. DOI: 10.1016/0165-1781(81)90062-7 [DOI] [PubMed] [Google Scholar]
- 143.Madrid-Lopez, N, Estrada, J, Diaz, J, Bassi, A, Delano, PH and Ocampo-Garces, A. The Sleep-Wake Cycle in the Nicotinic Alpha-9 Acetylcholine Receptor Subunit Knock-Out Mice. Front Cell Neurosci. 2017; 11: 302 DOI: 10.3389/fncel.2017.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scammell, TE, Arrigoni, E and Lipton, JO. Neural Circuitry of Wakefulness and Sleep. Neuron. 2017; 93(4): 747–65. DOI: 10.1016/j.neuron.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bailey, M and Silver, R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014; 35(1): 111–39. DOI: 10.1016/j.yfrne.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krizo, JA and Mintz, EM. Sex differences in behavioral circadian rhythms in laboratory rodents. Front Endocrinol (Lausanne). 2014; 5: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brewerton, TD, Putnam, KT, Lewine, RRJ and Risch, SC. Seasonality of cerebrospinal fluid monoamine metabolite concentrations and their associations with meteorological variables in humans. J Psychiatr Res. 2018; 99: 76–82. DOI: 10.1016/j.jpsychires.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Basheer, R, Magner, M, McCarley, RW and Shiromani, PJ. REM sleep deprivation increases the levels of tyrosine hydroxylase and norepinephrine transporter mRNA in the locus coeruleus. Mol Brain Res. 1998; 57: 235–40. DOI: 10.1016/S0169-328X(98)00088-6 [DOI] [PubMed] [Google Scholar]
- 149.Majumdar, S and Majumdar, BN. Increased levels of tyrosine hydroxylase and glutamic acid decarboxylase in locus coeruleus neurons after rapid eye movement sleep deprivation in rats. Neuroscience Letters. 2003; 338: 193–6. DOI: 10.1016/S0304-3940(02)01404-0 [DOI] [PubMed] [Google Scholar]
- 150.Perez, NM, Mattei, R and Benedito, MAC. Decreased activity of striatal monoamine oxidase B after rapid eye movement (REM) sleep deprivation in rats. Pharmacol Biochem Behav. 1998; 60(1): 33–7. DOI: 10.1016/S0091-3057(97)00556-X [DOI] [PubMed] [Google Scholar]
- 151.Medvedev, A. Tribulin and endogenous MAO-inhibitory regulation in vivo. NeuroToxicology. 2004; 25(1–2): 185–92. DOI: 10.1016/S0161-813X(03)00098-6 [DOI] [PubMed] [Google Scholar]
- 152.Perez, NM and Benedito, MAC. Activities of monoamine oxidase (MAO) A and B in discrete regions of rat brain after rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1997; 58(2): 605–8. DOI: 10.1016/S0091-3057(97)10002-8 [DOI] [PubMed] [Google Scholar]
- 153.Thakkar, M and Mallick, BN. Effect Of rapid eye movement sleep deprivation on rat brain monoamine oxidases. Neuroscience. 1993; 55(3): 677–83. DOI: 10.1016/0306-4522(93)90433-G [DOI] [PubMed] [Google Scholar]
- 154.Wang, Z, Chen, L, Zhang, L and Wang, X. Paradoxical sleep deprivation modulates depressive-like behaviors by regulating the MAOA levels in the amygdala and hippocampus. Brain Res. 2017; 1664: 17–24. DOI: 10.1016/j.brainres.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 155.Azizi, H, Hwang, J, Suen, V, Kang, NZ, Somvanshi, R, Tadavarty, R, et al. Sleep deprivation induces changes in 5-HT actions and 5-HT1A receptor expression in the rat hippocampus. Neurosci Lett. 2017; 655: 151–5. DOI: 10.1016/j.neulet.2017.06.053 [DOI] [PubMed] [Google Scholar]
- 156.Hipolide, DC, Moreira, KM, Barlow, KB, Wilson, AA, Nobrega, JN and Tufik, S. Distinct effects of sleep deprivation on binding to norepinephrine and serotonin transporters in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2005; 29(2): 297–303. DOI: 10.1016/j.pnpbp.2004.11.015 [DOI] [PubMed] [Google Scholar]
- 157.Hamdi, A, Brock, J, Ross, K and Prasad, C. Effects of rapid eye movement sleep deprivation on the properties of striatal dopaminergic system. Pharmacol Biochem Behav. 1993; 46: 863–6. DOI: 10.1016/0091-3057(93)90214-E [DOI] [PubMed] [Google Scholar]
- 158.Borah, R, Brown, AW, Capers, PL and Kaiser, KA. Analysis of the time and workers needed to conduct systematic reviews of medical interventions using data from the PROSPERO registry. BMJ Open. 2017; 7(2): e012545 DOI: 10.1136/bmjopen-2016-012545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mehta, R, Khan, S and Mallick, BN. Relevance of deprivation studies in understanding rapid eye movement sleep. Nat Sci Sleep. 2018; 10: 143–58. DOI: 10.2147/NSS.S140621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Villafuerte, G, Miguel-Puga, A, Rodriguez, EM, Machado, S, Manjarrez, E and Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev. 2015; 2015 DOI: 10.1155/2015/234952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Suchecki, D, Lobo, LL, Hipolide, DC and Tufik, S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. Journal of Sleep Research. 1998; 7: 276–81. DOI: 10.1046/j.1365-2869.1998.00122.x [DOI] [PubMed] [Google Scholar]
- 162.Coenen, AML and Van Luijtelaar, ELJM. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav. 1985; 35: 501–4. DOI: 10.1016/0031-9384(85)90130-1 [DOI] [PubMed] [Google Scholar]
- 163.de Lange, ECM. Recovery and Calibration Techniques. Toward Quantitative Microdialysis. 2013; 4: 13–33. [Google Scholar]
- 164.Tonin, FS, Rotta, I, Mendes, AM and Pontarolo, R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017; 15(1): 943 DOI: 10.18549/PharmPract.2017.01.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Milstein, JA, Lehmann, O, Theobald, DE, Dalley, JW and Robbins, TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl). 2007; 190(1): 51–63. DOI: 10.1007/s00213-006-0594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Robbins, TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000; 133(1): 130–8. DOI: 10.1007/s002210000407 [DOI] [PubMed] [Google Scholar]
- 167.Thomas, M, Sing, H, Belenky, G, Holcomb, H, Mayberg, H, Dannals, R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000; 9(4): 335–52. DOI: 10.1046/j.1365-2869.2000.00225.x [DOI] [PubMed] [Google Scholar]
- 168.Muzur, A, Pace-Schott, EF and Hobson, JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002; 6(11): 475–81. DOI: 10.1016/S1364-6613(02)01992-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study characteristics of included publications on circadian rhythms.
Study characteristics of included publications on sleep stages.
Study characteristic of included publications on sleep deprivation.
Risk of bias – questions adapted to microdialysis studies.
Forest plot comparing serotonin concentration (nanomole/L) during wake and slow waves sleep.
Forest plot comparing serotonin concentration (nanomole/L) during wake and rapid-eye movement sleep.
Forest plot comparing noradrenaline concentration (nanomole/L) during wake and slow waves sleep.
Forest plot comparing noradrenaline concentration (nanomole/L) during wake and rapid-eye movement sleep.


