Abstract
Discovery of novel anticandidal agents with clarified mechanisms of action, could be a rationalist approach against diverse pathogenic fungal strains due to the rise of resistance to existing drugs. In support to this hypothesis, in this paper, a series of benzimidazole-oxadiazole compounds were synthesized and subjected to antifungal activity evaluation. In vitro activity assays indicated that some of the compounds exhibited moderate to potent antifungal activities against tested Candida species when compared positive control amphotericin B and ketoconazole. The most active compounds 4h and 4p were evaluated in terms of inhibitory activity upon ergosterol biosynthesis by an LC-MS-MS method and it was determined that they inhibited ergosterol synthesis concentration dependently. Docking studies examining interactions between most active compounds and lanosterol 14-α-demethylase also supported the in vitro results.
Keywords: benzimidazole; 1,3,4-oxadiazole; antifungal activity; ergosterol biosynthesis; cytotoxicity; molecular docking
1. Introduction
The treatment of fungal infections continues to be a thought-provoking problem worldwide. Such infections vary in severity from superficial to complex fungal infections and most commonly affect immunocompromised patients [1]. In the past the affected patients have used numerous antibiotics and synthetic drugs, mostly without precautions, which has led to antifungal resistance to prescribed agents, therefore, the high morbidity and mortality caused by fungi are still increasing serious threat [2]. Since current antifungals do not meet the growing requirements of managing with life threatening infections, medicinal chemists are highlighting the need for the search of novel antifungal drugs.
Nucleic acids, protein, sterols and cell wall are known targets of the current agents in antifungal therapy [3]. The introduction of polyene antifungals, such as amphotericin B, became a milestone in clinic, however, infusional toxicity, nephrotoxicity and electrolyte imbalances limited its usage [4,5]. Several decades later, azoles were discovered as a result of the continued search for new and less toxic antifungals. Ketoconazole, the first presented compound in the early 1980s for the oral treatment of systemic fungal infections [6]. Azoles block the 14-α-demethylation of lanosterol into ergosterol, which is a major component of fungal cytoplasmic membranes and a bioregulator of membrane asymmetry, fluidity and integrity [3]. This blocking occurs via the coordination of the nucleophilic nitrogen of the azole heterocyclic ring as the sixth ligand of the heme ferric ion in the active side of lanosterol 14-α-demethylase (CYP51) and the interaction of the azole drug side chains with the CYP51 polypeptide structure [7,8]. Catecholase is another specific enzyme type in fungi and it is also responsible for biologically essential functions. Thus, inhibition of catecholase may terminate the vital functions of the fungi [9,10,11]. In addition to inhibition of such vital fungal enzymes, some strategies including inhibition of candida biofilm formation [12,13] and treatment with organoselenium based agents [14,15,16,17] are recent antifungal treatment options.
In spite of the widespread use of these agents, the development of new azole antifungal drugs has been constantly required in the clinical therapy due to a number of clinically important limitations such as drug-resistant strains, serious side effects, narrow spectrum and toxicity [18,19]. The improvement of new hybrid molecules, which can be used either alone or as part of a combination therapy, is generally considered as a functional strategy to cope with the growing problem of antifungal resistance. The combination of two or more biologically important azole scaffolds could generate a new class of azole based antifungal agents. To this end, we designed some hybrid compounds bearing benzimidazole and 1,3,4-oxadiazole rings based upon the studies that reported the antifungal potentials of such compounds [20,21,22,23]. The electron rich nature of azoles provides easily to interact with various enzymes and receptors via noncovalent interactions. Benzimidazoles are an indispensable class of heterocyclic family and promising candidates for developing biologically active compounds due to their various pharmacological activities [24,25,26,27,28,29,30,31,32,33]. Comprehensive biochemical and pharmacological studies reported their large potentiality to inhibit the growth of fungal strains [34,35,36,37,38]. Particularly, in some studies 5-fluoro or 5-chloro substituted benzimidazoles displayed significant antifungal activity [39,40,41,42,43]. 1,3,4-Oxadiazole nucleus is also a fertile source of bioactivity in drug discovery because of its varied biological activities [44,45,46,47,48,49,50]. Recently, our research group has been actively involved in developing novel antifungal agents through modifying various azole compounds. Previously, we reported novel benzimidazole-triazole and benzimidazole-thiazole hybrid compounds some of which possessed significant ergosterol biosynthesis inhibition at 0.78 µg/mL and 1.56 µg/mL concentrations, respectively [8,51,52]. Taking the above points in consideration and in continuation of our studies, in current research, we clubbed benzimidazole and 1,3,4-oxadiazole rings to optimize the overall structure for better and promising antifungal efficacy and to evaluate the effect of the 1,3,4-oxadiazole ring on activity.
2. Results and Discussion
2.1. Chemistry
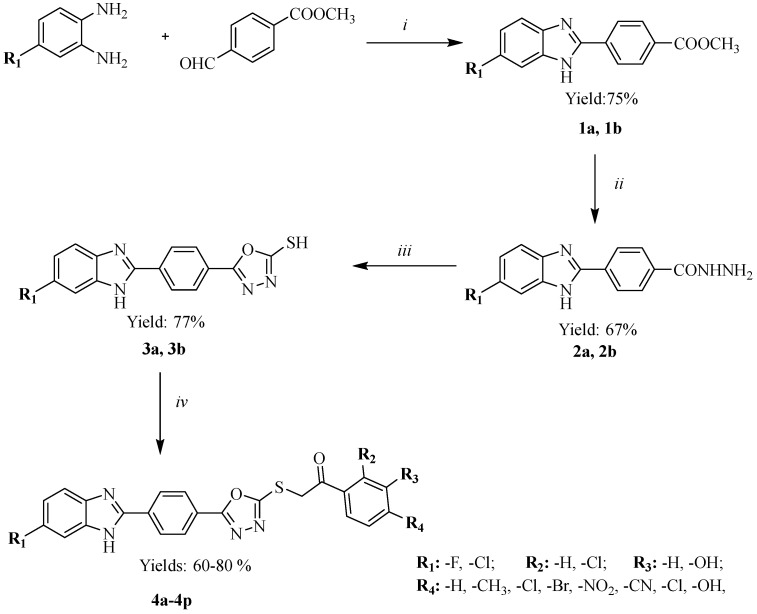
The reaction sequences followed for the synthesis of the final compounds 4a–4n are outlined in Scheme 1. Compounds 1 and 2 were synthesized according to reported methods [37]. The ring closure reaction of the corresponding hydrazide 2 with carbon disulfide in the presence of sodium hydroxide resulted in the formation of 5-(4-(1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazole-2-thiol (3), which was then reacted with suitable substituted phenacyl bromides to obtain the target compounds 4a–4p. The chemical structures of the compounds were elucidated via IR, 1H-NMR, 13C-NMR and HRMS spectroscopic methods. In the IR spectra of all compounds, the occurrence of N-H bonds were confirmed through bands in the region of 3325–3130 cm−1. The C=O bonds were confirmed through bands in the region of 1683–1658 cm−1.The characteristic C=N and C=C stretching vibrations were detected in between 1610–1462 cm−1. 1H-NMR spectra of compounds 4a–4p are presented in the Supplementary Materials (Figures S1–S16). CH2-protons attached to carbonyl group resonated as singlet at 5.05–5.27 ppm in the 1H-NMR spectra of compounds. The singlet NH signal of benzimidazole was appeared at over 13 ppm in compounds 4a–4p. The signals belonging to aromatic region was seen at 6.88–8.40 ppm. In the 13C-NMR spectra, the methylene carbons between the sulphur atom and carbonyl group was observed around 41 ppm just over the DMSO peaks. Two carbons on the oxadiazole ring were 163–165 ppm, while the carbonyl carbon was over 191 ppm. All other aromatic carbons were recorded between 96 ppm and 160 ppm. Also, carbon-fluorine coupling constants were observed for the first eight fluorinated derivatives. The HRMS spectra of compounds were found to be in full agreement with their molecular formula.
Scheme 1.
The synthetic protocol of the compounds. Reagents and conditions: (i) Na2S2O5, DMF, MWI, 10 min, (ii) NH2NH2xH2O, EtOH, MWI, 10 min, (iii) CS2/NaOH, EtOH, reflux, 8 h, (iv) appropriate phenacyl bromides, K2CO3, acetone, rt, 8 h.
2.2. Biological Activity Screening
2.2.1. Antifungal Activity
The in vitro antifungal activities of all synthesized derivatives 4a–4p were screened at between 1 mg/mL–1.95 µg/mL concentrations using various Candida strains including C. albicans (ATCC 90030), C. krusei (ATCC 6258) and C. parapsilopsis (ATCC 22019) following the protocol of the EUCAST [53]. Amphotericin B and ketoconazole were selected as positive controls. The results of antifungal potential of the compounds presented in Table 1 indicated that among all strains, C. albicans was the most susceptible to compounds. Compounds 4h and 4p showed comparable activity with reference drugs. Compounds 4h and 4p were determined to have MIC50 values of 1.95 µg/mL against C. albicans, while that of amphotericin B was 1.95 µg/mL and ketoconazole was 7.8 µg/mL, respectively. Compound 4p was the most potent derivative of the series, with MIC50 values of 1.95 µg/mL, 7.8 µg/mL and 31.25 µg/mL against C. albicans, C. krusei and C. parapsilopsis, respectively. Ketoconazole showed anticandidal activity with MIC50 values 7.8 µg/mL, 1.95 µg/mL and 1.95 µg/mL against C. albicans, C. krusei and C. parapsilopsis, respectively. The MIC50 values of amphotericin B were 1.95 µg/mL towards all Candida strains.
Table 1.
Some characteristics and MIC50 (μg/mL) values of compounds (4a–4s).
| Compound | R1 | R2 | R3 | R4 | C. albicans | C. krusei | C. parapsilopsis |
|---|---|---|---|---|---|---|---|
| 4a | F | H | H | H | >1000 | >1000 | >1000 |
| 4b | F | H | H | CH3 | >1000 | >1000 | >1000 |
| 4c | F | H | H | Cl | 1000 | 1000 | 1000 |
| 4d | F | H | H | Br | 500 | 1000 | >1000 |
| 4e | F | H | H | NO2 | >1000 | >1000 | >1000 |
| 4f | F | H | H | CN | >1000 | >1000 | >1000 |
| 4g | F | Cl | H | Cl | >1000 | >1000 | >1000 |
| 4h | F | H | OH | OH | 1.95 | 31.25 | 62.5 |
| 4i | Cl | H | H | H | 500 | >1000 | >1000 |
| 4j | Cl | H | H | CH3 | >1000 | >1000 | >1000 |
| 4k | Cl | H | H | Cl | >1000 | >1000 | >1000 |
| 4l | Cl | H | H | Br | 1000 | >1000 | >1000 |
| 4m | Cl | H | H | NO2 | 1000 | >1000 | >1000 |
| 4n | Cl | H | H | CN | >1000 | >1000 | >1000 |
| 4o | Cl | Cl | H | Cl | >1000 | >1000 | >1000 |
| 4p | Cl | H | OH | OH | 1.95 | 7.8 | 31.25 |
| Amphotericin B | - | - | - | - | 1.95 | 1.95 | 1.95 |
| Ketoconazole | - | - | - | - | 7.8 | 1.95 | 1.95 |
The synthesized compounds can be categorised as two classed based upon the presence of fluoro (compounds 4a–4h) or chloro (compounds 4i–4p) substituents at the C-5 position of the benzimidazole ring to investigate the structure-activity relationship of the compounds. The other factor providing the diversity of the compounds is the phenyl ring attached to the carbonyl moiety that carries different substituents at the C-2, C-3 or C-4 positions. The presence of a chloro substituent on the benzimidazole ring (compounds 4i–4p) is generally more beneficial than a fluoro substituent (compounds 4a–4h). In both analogues 4a–4h and 4i–4p, a bromo substituent at the C-4 position and a hydroxy substituent at the C-3 and C-4 positions of the phenyl ring enhance the activity. Compounds 4h and 4p in which two hydroxy groups are involved, forming a catechol moiety, proved to be the most active antifungal agent in the series. Catechol hydroxy groups, which are hydrogen bonding donors, are thus beneficial for high antifungal activity.
2.2.2. Cytotoxicity Test
High selectivity for fungal cells compared to mammalian cells is an essential parameter in the development of novel antifungal agents. Therefore, we evaluated the cytotoxic effects of the most active compounds 4h and 4p on NIH/3T3 cells. The results of this investigation show that tested compounds are nontoxic at their active concentrations against NIH/3T3 cells (IC50 ≥ 500 ± 7.09 µg/mL and 141.291 ± 10.48 µg/mL, respectively). These results provide support for the idea that compounds 4h and 4p came to the forefront on the search of a new and safe antifungal candidate.
2.2.3. Quantification of Ergosterol Level
The most widely used antifungal drugs (ketoconazole, fluconazole etc.) target CYP51 and inhibit ergosterol biosynthesis in fungal membrane. Based on this, ergosterol quantification was carried out to gain insight into the antifungal mechanism of action of the most active compounds 4h and 4p. The IC50 of these compounds and ketoconazole on ergosterol biosynthesis is outlined in Table 2.
Table 2.
IC50 of the compounds 4h, 4p and ketoconazole on ergosterol biosynthesis of C. albicans.
| Compound | IC50 (µg/mL) |
|---|---|
| 4h | 2.84 ± 0.16 |
| 4p | 3.28 ± 0.22 |
| Ketoconazole | 0.66 ± 0.07 |
A dose dependent decrease in ergosterol biosynthesis was detected with the treatment of tested compounds. This result reveals that the anticandidal effect of the tested compounds may arise from the inhibitory potential on ergosterol biosynthesis in a manner similar to that of the ketoconazole.
2.2.4. Molecular Docking Studies
According to antifungal activity, compounds 4h and 4p were found as the most potent derivatives in the series and their ergosterol inhibition potency was proven via ergosterol quantification assay. In order to evaluate in silico features of these compounds, docking studies were performed on 14 α-sterol demethylase, which is a key enzyme for ergosterol biosynthesis in fungi.
As mentioned in anticandidal activity results, compounds 4h and 4p were the most effective especially on C. albicans with MIC50 value of 1.95 µg/mL. Thus, X-ray crystal structure originating from C. albicans (PDB ID: 5FSA) [54] was retrieved from the Protein Data Bank server (www.pdb.org) and docking studies were performed by using this crystal structure.
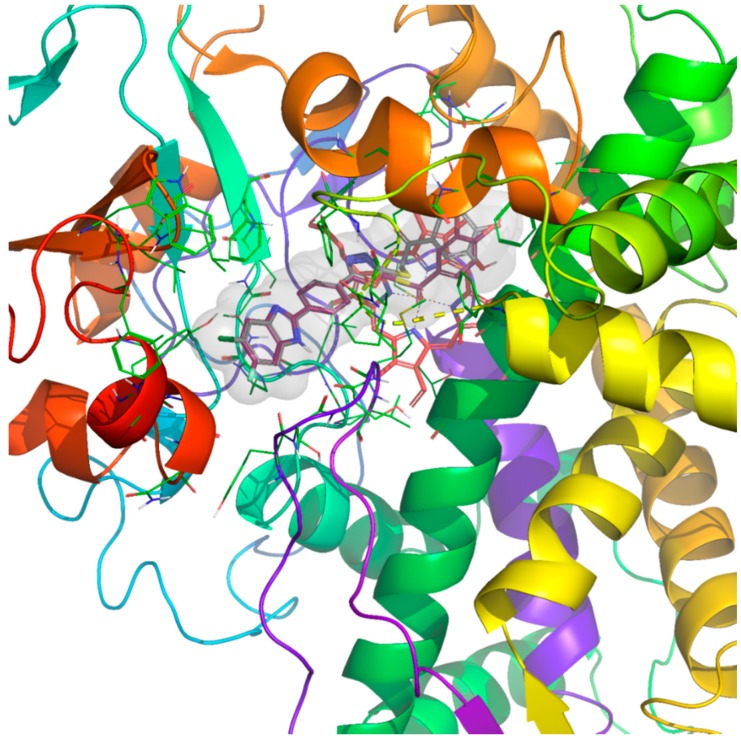
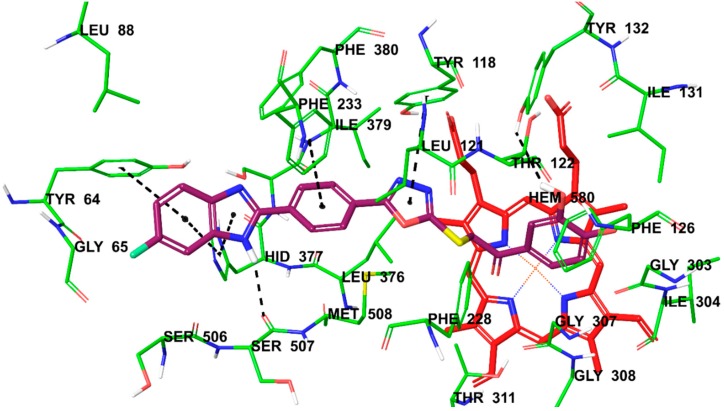
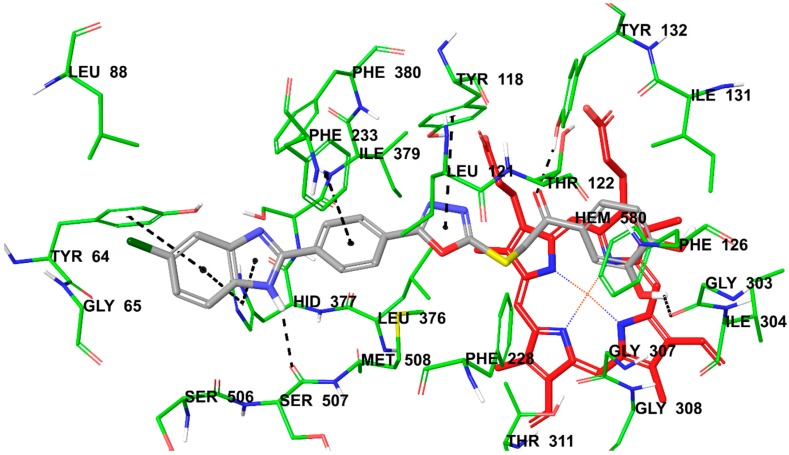
Figure 1, Figure 2 and Figure 3 present the docking poses of compounds 4h and 4p. It can be seen in these figures that compounds 4h and 4p show six common interactions. The fluoro or chloro substituted benzimidazole ring is especially important for hydrophobic binding profile. The benzo side of benzimidazole is in interaction with phenyl of Tyr64 by doing π–π interaction. Also, benzo and imidazole parts of benzimidazole create another π–π interaction with imidazole of Hid377 together. The NH moiety of benzimidazole is essential for polar interaction by forming a hydrogen bond with carbonyl of Ser507. Another common interaction for compounds 4h and 4p is related to phenyl between benzimidazole and oxadiazole rings in their structures. This phenyl establishes a π–π interaction with phenyl of Phe233. Furthermore, the last common interaction is another π–π interaction, which forms between oxadiazole ring and phenyl of Tyr118. The carbonyl moiety in compound 4p forms a hydrogen bond with hydroxyl of Tyr132.
Figure 1.
The superimpositions pose of active compounds 4h and 4p in the active region of 14 α-sterol demethylase (PDB code: 5FSA). The inhibitors 4h and 4p are colored with maroon and grey, respectively, and HEM with red.
Figure 2.
The interacting mode of compound 4h in the active region of 14 α-sterol demethylase (PDB code: 5FSA). The inhibitor is colored with maroon and HEM with red.
Figure 3.
The interacting mode of compound 4p in the active region of 14 α-sterol demethylase (PDB code: 5FSA). The inhibitor is colored with grey and HEM with red.
The only structural difference between compounds 4h and 4p is the presence of fluoro or chloro substitutions on benzimidazole ring, respectively. These compounds have 3,4-dihydroxyphenyl moiety unlike the other compounds in the series. The efficacy of these compounds is thought to be due in particular to hydroxyl functional groups. This idea has been proven by docking studies. According to Figure 2 and Figure 3, hydroxyl substituent at 3rd position of phenyl ring is in an interaction with hydroxyl of Tyr132 and carbonyl of Gly303 for compounds 4h and 4p, respectively. This means that 3rd position of phenyl ring may be important for inhibition of 14 α-sterol demethylase and ergosterol biosynthesis. Furthermore, the substituents capable of forming hydrogen bond such as hydroxyl moiety at this this position may be suggested for strong binding in the enzyme site. All these in silico findings help to explain the potent antifungal activity and ergosterol inhibition profiles of compounds 4h and 4p.
3. Materials and Methods
3.1. General Information
All chemicals employed in the synthetic procedures were purchased from Sigma-Aldrich (Sigma-Aldrich Corp., St. Louis, MO, USA) or Merck (Merck KGaA, Darmstadt, Germany). Melting points of the obtained compounds were determined by an MP90 digital melting point apparatus (Mettler Toledo, Columbus, OH, USA) and were uncorrected. 1H-NMR and 13C-NMR spectra of the synthesized compounds were registered by a Bruker 300 MHz and 75 MHz digital FT-NMR spectrometer (Bruker Bioscience, Billerica, MA, USA) in DMSO-d6, respectively. Splitting patterns were designated as follows: s: singlet; d: doublet; t: triplet; m: multiplet in the NMR spectra. Coupling constants (J) were reported as Hertz. The IR spectra were obtained on a Shimadzu, IR Prestige-21 (Shimadzu, Tokyo, Japan). M + 1 peaks were determined by Shimadzu LC/MS ITTOF system. All reactions were monitored by thin-layer chromatography (TLC) using Silica Gel 60 F254 TLC plates (Merck KGaA).
3.2. Chemistry
3.2.1. Synthesis of methyl 4-(5(6)-substituted-1H-benzimidazol-2-yl)benzoates 1a, 1b and 4-(5(6)-substituted-1H-benzimidazol-2-yl)benzohydrazides 2a, 2b
The starting compounds 1a, 1b, 2a and 2b were synthesized in accordance to the method described in the literature [51].
3.2.2. Synthesis of 5-[4-(5(6)-substituted-1H-benzimidazol-2-yl)phenyl]-1,3,4-oxadiazole-2-thiol derivatives 3a, 3b
Compounds 2a or 2b (0.031 mol) were dissolved in a solution of NaOH (1.48 g, 0.037 mol) in ethanol (150 mL). After addition of carbon disulfide (2.24 mL, 0.037 mol), the mixture was refluxed for 8 h. After this period, the solution was cooled and acidified to pH 4–5 with concentrated hydrochloric acid solution. The obtained solid was filtered, washed with water, dried and recrystallized from ethanol.
3.2.3. Synthesis of 2-[(5-(4-(5(6)-substituted-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-substitutedphenyl)ethan-1-ones 4a–4n
Compounds 3a or 3b (2 mmol) in acetone (40 mL) were stirred with an appropriate 4-substituted phenacyl bromide (2 mmol) and potassium carbonate (0.33 g, 2.4 mmol) at room temperature for 8 h. After the evaporation of acetone under reduced pressure, the product was washed with water, dried and crystallized from ethanol (96%).
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-phenyl-ethan-1-one (4a), Yield 71%, m.p. 262.4–265.7 °C. IR νmax (cm−1): 3205 (N-H), 3053 (aromatic C-H), 2960 (aliphatic C-H), 1674 (C=O), 1597–1469 (C=N, C=C), 1313–1001 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.22 (2H, s, CO-CH2), 7.05–7.15 (1H, m, aromatic-H), 7.34–7.76 (5H, m, aromatic-H), 8.08–8.13 (4H, m, aromatic-H), 8.34 (2H, d, J = 8.4 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.1, 98.4 (d, J = 29.6 Hz), 105.0 (d, J = 22.3 Hz), 111.6, 112.8 (d, J = 9.5 Hz), 120.7, 124.3, 127.5, 127.6, 127.7, 129.0, 129.4, 132.3 (d, J = 13.6 Hz), 134.5, 135.5, 153.6 (d, J = 225.9 Hz), 164.1, 165.2, 193.2. HRMS (m/z): [M + H]+ calcd for C23H15FN4O2S: 431.0973; found: 431.0954.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-methylphenyl)ethan-1-one (4b), Yield 71%, m.p. 240.1–242.7 °C. IR νmax (cm−1): 3305 (N-H), 3074 (aromatic C-H), 2995 (aliphatic C-H), 1672 (C=O), 1604–1498 (C=N, C=C), 1309–1031 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 2.42 (3H, s, CH3), 5.18 (2H, s, CO-CH2), 7.05–7.16 (1H, m, aromatic-H), 7.41 (2H, d, J = 8.0 Hz, aromatic-H), 7.48–7.59 (1H, m, aromatic-H), 7.69–7.73 (1H, m, aromatic-H), 7.99 (2H, d, J = 7.9 Hz, aromatic-H), 8.11–8.14 (2H, m, aromatic-H), 8.32–8.36 (2H, m, aromatic-H), 13.27 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 21.7, 41.0, 98.3 (d, J = 24.2 Hz), 105.0 (d, J = 24.7 Hz), 111.9, 112.7 (d, J = 8.0 Hz), 112.9, 120.7, 124.4, 127.5, 127.6, 129.1, 129.9, 131.2, 133.1 (d, J = 12.1 Hz), 145.1 (d, J = 3.0 Hz), 153.8 (d, J = 253.5 Hz), 164.2, 165.2, 192.6. HRMS (m/z): [M + H]+ calcd for C24H17FN4O2S: 445.1129; found: 445.1111.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-chlorophenyl) ethan-1-one (4c), Yield 67%, m.p. 253.6–255.3 °C. IR νmax (cm−1): 3273 (N-H), 3088 (aromatic C-H), 2954 (aliphatic C-H), 1672 (C=O), 1589–1473 (C=N, C=C), 1292–1091 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.20 (2H, s, CO-CH2), 7.07–7.14 (1H, m, aromatic-H), 7.43 (1H, br s, aromatic-H), 7.56–7.62 (1H, m, aromatic-H), 7.68 (2H, d, J = 8.6 Hz, aromatic-H), 8.08–8.13 (4H, m, aromatic-H), 8.34 (2H, d, J = 8.5 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.0, 98.7 (d, J = 23.8 Hz), 105.1 (d, J = 25.3 Hz), 111.9 (d, J = 9.6 Hz), 124.3, 127.5, 127.7, 129.3, 129.5, 130.9, 132.3 (d, J = 12.6 Hz), 133.3, 134.2, 139.4, 141.5, 155.9 (d, J = 268.9 Hz), 164.0, 165.3, 192.3. HRMS (m/z): [M + H]+ calcd for C23H14ClFN4O2S: 465.0583; found: 465.0561.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-bromophenyl)ethan-1-one (4d), Yield 60%, m.p. 245.4–247.9 °C. IR νmax (cm−1): 3261 (N-H), 3078 (aromatic C-H), 2937 (aliphatic C-H), 1681 (C=O), 1600–1473 (C=N, C=C), 1290–1076 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.27 (2H, s, CO-CH2), 7.06–7.13 (1H, m, aromatic-H), 7.42 (1H, br s, aromatic-H), 7.62 (1H, br s, aromatic-H), 8.12 (2H, d, J = 8.5 Hz, aromatic-H), 8.32 (4H, t, J = 8.6 Hz, aromatic-H), 8.40 (2H, d, J = 8.9 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.3, 96.9 (d, J = 28.5 Hz), 105.0 (d, J = 23.3 Hz), 111.6 (d, J = 11.6 Hz), 124.2, 124.4, 127.5, 127.7, 128.8, 130.4, 132.1 (d, J = 10.4 Hz), 133.3, 140.1, 142.0, 150.8, 156.4 (d, J = 256.3 Hz), 163.9, 165.3, 192.6. HRMS (m/z): [M + H]+ calcd for C23H14BrFN4O2S: 509.0078; found: 509.0062.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-nitro phenyl)ethan-1-one (4e), Yield 67%, m.p. 250.9–254.6 °C. IR νmax (cm−1): 3263 (N-H), 3026 (aromatic C-H), 2949 (aliphatic C-H), 1672 (C=O), 1585–1473 (C=N, C=C), 1294–1072 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.20 (2H, s, CO-CH2), 7.07–7.15 (1H, m, aromatic-H), 7.44 (1H, dd, J1 = 2.2 Hz, J2 = 9.4 Hz, aromatic-H), 7.62–7.66 (1H, m, aromatic-H), 7.82 (2H, d, J = 8.6 Hz, aromatic-H), 8.02 (2H, d, J = 8.6 Hz, aromatic-H), 8.13 (2H, d, J = 8.5 Hz, aromatic-H), 8.34 (2H, d, J = 8.5 Hz, aromatic-H), 13.27 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.0, 97.8 (d, J = 25.9 Hz), 106.2 (d, J = 25.9 Hz), 111.8 (d, J = 10.9 Hz), 124.3, 127.5, 127.7, 128.7, 130.0, 131.0, 131.3 (d, J = 10.4 Hz), 132.5, 133.2, 134.5, 141.3, 157.3 (d, J = 274.2 Hz), 164.0, 165.3, 192.5. HRMS (m/z): [M + H]+ calcd for C23H14FN5O4S: 476.0823; found: 476.0826.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-cyanophenyl) ethan-1-one (4f), Yield 64%, m.p. 243.8–247.7 °C. IR νmax (cm−1): 3264 (N-H), 3093 (aromatic C-H), 2946 (aliphatic C-H), 2229 (C≡N), 1681 (C=O), 1608–1467 (C=N, C=C), 1305–1080 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.23 (2H, s, CO-CH2), 7.07–7.13 (1H, m, aromatic-H), 7.37–7.73 (2H, m, aromatic-H), 8.10 (4H, t, J = 8.7 Hz, aromatic-H), 8.23 (2H, d, J = 8.5 Hz, aromatic-H), 8.33 (2H, d, J = 8.5 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.1, 98.4 (d, J = 27.3 Hz), 105.0 (d, J = 21.8 Hz), 113.0 (d, J = 9.7 Hz), 116.3, 118.5, 124.2, 127.5, 127.7, 129.6, 130.1, 131.1 (d, J = 11.5 Hz), 133.3, 133.4, 138.7, 141.0, 157.6 (d, J = 229.9 Hz), 163.9, 165.3, 192.5. HRMS (m/z): [M + H]+ calcd for C24H14FN5O2S: 456.0925; found: 456.0918.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(2,4-dichlorophenyl)ethan-1-one (4g), Yield 72%, m.p. 254.7–256.7 °C. IR νmax (cm−1): 3215 (N-H), 3086 (aromatic C-H), 2954 (aliphatic C-H), 1687 (C=O), 1585–1475 (C=N, C=C), 1303–1070 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.05 (2H, s, CO-CH2), 7.06–7.16 (1H, m, aromatic-H), 7.49–7.59 (1H, m, aromatic-H), 7.63–7.73 (2H, m, aromatic-H), 7.81 (1H, d, J = 2.0 Hz, aromatic-H), 7.96 (1H, d, J = 8.4 Hz, aromatic-H), 8.13 (2H, d, J = 8.4 Hz, aromatic-H), 8.35 (2H, d, J = 8.2 Hz, aromatic-H), 13.27 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 43.7, 98.9 (d, J = 24.9 Hz), 105.2 (d, J = 26.2 Hz), 111.7 (d, J = 10.5 Hz), 113.7, 125.0, 128.3, 128.4, 129.0, 130.5 (d, J = 13.8 Hz), 131.6, 132.8, 133.1, 134.1, 136.0, 138.5, 144.4, 157.2 (d, J = 261.7 Hz), 164.5, 166.1, 194.7. HRMS (m/z): [M + H]+ calcd for C23H13Cl2FN4O2S: 499.0193; found: 499.0170.
2-[(5-(4-(5(6)-Fluoro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(3,4-dihydroxyphenyl)-ethan-1-one (4h), Yield 62%, m.p. 234.7–236.9 °C. IR νmax (cm−1): 3234 (N-H, O-H), 3072 (aromatic C-H), 2927 (aliphatic C-H), 1660 (C=O), 1595–1467 (C=N, C=C), 1301–1080 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.08 (2H, s, CO-CH2), 6.88 (1H, d, J = 8.2 Hz, aromatic-H), 7.10 (1H, t, J = 8.9 Hz, aromatic-H), 7.43 (2H, s, aromatic-H), 7.51 (1H, d, J = 8.1 Hz, aromatic-H), 7.63 (1H, br s, aromatic-H), 8.11 (2H, d, J = 7.9 Hz, aromatic-H), 8.34 (2H, d, J = 7.9 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 40.9, 98.8 (d, J = 22.4 Hz), 103.9 (d, J = 24.9 Hz), 111.7 (d, J = 14.1 Hz), 115.6, 115.7, 122.7, 124.3, 127.2, 127.5, 127.7, 129.6, 130.6, 133.2, 144.6, 145.9, 152.2, 156.7 (d, J = 250.2 Hz), 164.4, 165.1, 191.1. HRMS (m/z): [M + H]+ calcd for C23H15FN4O4S: 463.0871; found: 463.0854.
2-[(5-(4-(5(6)-Chloro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-phenylethan-1-one (4i), Yield 74%, m.p. 157.2–159.1 °C. IR νmax (cm−1): 3130 (N-H), 3084 (aromatic C-H), 2929 (aliphatic C-H), 1678 (C=O), 1579-1471 (C=N, C=C), 1294–1074 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.22 (2H, s, CO-CH2), 7.26 (1H, d, J = 7.4 Hz, aromatic-H), 7.61–7.73 (5H, m, aromatic-H), 8.11 (2H, t, J = 8.5 Hz, aromatic-H), 8.35 (2H, d, J = 7.6 Hz, aromatic-H), 13.34 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.1, 111.8, 113.5, 118.5, 120.0, 122.6, 124.5, 126.8, 127.5, 127.8, 129.0, 129.4, 133.1, 134.5, 140.0, 142.3, 160.1, 165.2, 193.2. HRMS (m/z): [M + H]+ calcd for C23H15ClN4O2S: 447.0677; found: 447.0665.
2-((5-(4-(5(6)-Chloro-1H-benzo[d]imidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio)-1-(p-tolyl)ethan-1-one (4j), Yield 68%, m.p. 201.5–203.7 °C. IR νmax (cm−1): 3312 (N-H), 2938 (aliphatic C-H), 1674 (C=O), 1611–1474 (C=N, C=C), 1302–1024 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 2.41 (3H, s, CH3), 5.08 (2H, s, CH2), 7.23–7.29 (1H, m, aromatic-H), 7.40 (2H, d, J = 8.1 Hz, aromatic-H), 7.58 (1H, d, J = 8.6 Hz, aromatic-H), 7.70–7.76 (1H, m, aromatic-H), 7.98 (2H, d, J = 8.2 Hz, aromatic-H), 8.12 (2H, d, J = 8.5 Hz, aromatic-H), 8.35 (2H, d, J = 8.5 Hz, aromatic-H), 13.34 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 21.7, 41.1, 111.8, 116.4, 117.1, 120.9, 122.8, 124.5, 127.0, 127.5, 127.8, 129.1, 129.9, 133.0, 136.5, 140.6, 145.1, 162.1, 165.2, 192.6. HRMS (m/z): [M + H]+ calcd for C24H17ClN4O2S: 461.0834; found: 461.0818
2-((5-(4-(5(6)-Chloro-1H-benzo[d]imidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio)-1-(4-chlorophenyl) ethan-1-one (4k), Yield 78%, m.p. 229.4–231.2 °C. IR νmax (cm−1): 3215 (N-H), 3086 (aromatic C-H), 2954 (aliphatic C-H), 1684 (C=O), 1583–1476 (C=N, C=C), 1304–1070 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.10 (2H, s, CH2), 7.23–7.29 (1H, m, aromatic-H), 7.43 (1H, br s, aromatic-H), 7.56–7.62 (1H, m, aromatic-H), 7.62 (2H, d, J = 8.2 Hz, aromatic-H), 7.90 (2H, d, J = 8.2 Hz, aromatic-H), 8.12 (2H, d, J = 8.5 Hz, aromatic-H), 8.35 (2H, d, J = 8.5 Hz, aromatic-H), 13.26 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.0, 123.0, 125.8, 126.9, 127.1, 127.5, 127.7, 127.8, 128.7, 129.4, 130.7, 131.3, 131.5, 132.2, 133.0, 152.4, 161.0, 165.0, 190.1. HRMS (m/z): [M + H]+ calcd for C23H14Cl2N4O2S: 481.0287; found: 481.0274.
2-[(5-(4-(5(6)-Chloro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-bromophenyl)ethan-1-one (4l), Yield 65%, m.p. 205.3–207.6 °C. IR νmax (cm−1): 3290 (N-H), 3034 (aromatic C-H), 2951 (aliphatic C-H), 1672 (C=O), 1585–1473 (C=N, C=C), 1303–1070 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.19 (2H, s, CO-CH2), 7.25 (1H, d, J = 8.5 Hz, aromatic-H), 7.57–7.72 (2H, m, aromatic-H), 7.82 (2H, d, J = 8.6 Hz, aromatic-H), 8.01 (2H, d, J = 8.6 Hz, aromatic-H), 8.12 (2H, d, J = 8.6 Hz, aromatic-H), 8.34 (2H, d, J = 8.6 Hz, aromatic-H), 13.32 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.0, 113.5, 121.5, 124.0, 124.4, 127.5, 127.8, 128.5, 128.7, 130.5, 131.0, 132.5, 133.1, 134.5, 139.0, 141.6, 164.0, 165.2, 192.5. HRMS (m/z): [M + H]+ calcd for C23H14BrClN4O2S: 524.9782; found: 524.9762.
2-((5-(4-(5(6)-Chloro-1H-benzo[d]imidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio)-1-(4-nitrophenyl)ethan-1-one (4m), Yield 68%, m.p. 211.2–213.8 °C. IR νmax (cm−1): 3240 (N-H), 3062 (aromatic C-H), 2953 (aliphatic C-H), 1653 (C=O), 1525–1471 (C=N, C=C), 1344–1074 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.27 (2H, s, CH2), 7.23–7.29 (1H, m, aromatic-H), 7.58 (1H, d, J = 8.6 Hz, aromatic-H), 7.70–7.76 (1H, m, aromatic-H), 7.82 (2H, d, J = 8.2 Hz, aromatic-H), 7.99 (2H, d, J = 8.2 Hz, aromatic-H), 8.12 (2H, d, J = 8.5 Hz, aromatic-H), 8.35 (2H, d, J = 8.5 Hz, aromatic-H), 13.29 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.4, 112.5, 115.3, 120.1, 124.3, 124.4, 126.1, 127.5, 127.7, 127.8, 130.4, 131.3, 133.0, 140.1, 143.6, 150.7, 162.8, 165.3, 192.5. HRMS (m/z): [M + H]+ calcd for C23H14ClN5O4S: 492.0528; found: 492.0508.
2-[(5-(4-(5(6)-Chloro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(4-cyanophenyl) ethan-1-one (4n), Yield 71%, m.p. 160.3–162.3 °C. IR νmax (cm−1): 3246 (N-H), 3024 (aromatic C-H), 2949 (aliphatic C-H), 2242 (C≡N), 1681 (C=O), 1563–1471 (C=N, C=C), 1296–1055 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.24 (2H, s, CO-CH2), 7.26 (1H, d, J = 6.2 Hz, aromatic-H), 7.60–7.77 (2H, m, aromatic-H), 8.07–8.14 (4H, m, aromatic-H), 8.23 (2H, d, J = 8.6 Hz, aromatic-H), 8.35 (2H, d, J = 8.6 Hz, aromatic-H), 13.34 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 41.1, 116.3, 118.5, 118.9, 121.0, 123.0, 123.7, 124.4, 127.5, 127.8, 129.6, 133.1, 133.4, 138.7, 139.1, 142.8, 162.8, 163.9, 165.3, 192.8. HRMS (m/z): [M + H]+ calcd for C24H14ClN5O2S: 472.0629; found: 472.0608.
2-[(5-(4-(5(6)-Chloro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(2,4-dichlorophenyl)ethan-1-one (4o), Yield 80%, m.p. 253.7–256.8 °C. IR νmax (cm−1): 3215 (N-H), 3086 (aromatic C-H), 2954 (aliphatic C-H), 1683 (C=O), 1583–1475 (C=N, C=C), 1303–1070 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.05 (2H, s, CO-CH2), 7.26 (1H, br s, aromatic-H), 7.60–7.82 (4H, m, aromatic-H), 7.96 (1H, d, J = 8.3 Hz, aromatic-H), 8.13 (2H, d, J = 8.5 Hz, aromatic-H), 8.36 (2H, d, J = 8.6 Hz, aromatic-H), 13.35 (1H, s, NH). 13C-NMR (DMSO-d6, ppm) δ 42.9, 115.1, 119.1, 123.7, 124.4, 127.5, 127.8, 128.2, 128.8, 129.0, 130.8, 132.0, 132.2, 132.3, 133.1, 135.2, 137.7, 142.0, 163.8, 165.3, 194.0. HRMS (m/z): [M + H]+ calcd for C23H13Cl3N4O2S: 514.9898; found: 514.9881.
2-[(5-(4-(5(6)-Chloro-1H-benzimidazol-2-yl)phenyl)-1,3,4-oxadiazol-2-yl)thio]-1-(3,4-dihydroxyphenyl)-ethan-1-one (4p), Yield 68%, m.p. 152.4–155.8 °C. IR νmax (cm−1): 3240 (N-H, O-H), 3062 (aromatic C-H), 2895 (aliphatic C-H), 1658 (C=O), 1595–1471 (C=N, C=C), 1300–1024 (C-N, C-O). 1H-NMR (DMSO-d6, ppm) δ 5.08 (2H, s, CO-CH2), 6.88 (1H, d, J = 8.2 Hz, aromatic-H), 7.26 (1H, dd, J1 = 8.6 Hz, J2 = 2.0 Hz aromatic-H), 7.43 (1H, d, J = 2.1 Hz, aromatic-H), 7.51 (1H, dd, J1 = 8.3 Hz, J2 = 2.2 Hz, aromatic-H), 7.62–7.68 (2H, m, aromatic-H), 8.12 (2H, d, J = 8.6 Hz, aromatic-H), 8.35 (2H, d, J = 8.6 Hz, aromatic-H), 13.22 (1H, s, N-H). 13C-NMR (DMSO-d6, ppm) δ 40.9, 115.6, 115.7, 122.4, 122.7, 123.3, 124.5, 127.2, 127.5, 127.8, 129.0, 130.2, 133.0, 138.5, 140.6, 145.9, 151.8, 152.2, 163.4, 165.1, 191.1. HRMS (m/z): [M + H]+ calcd for C23H15ClN4O4S: 479.0575; found: 479.0563.
3.3. Antifungal Assays
Compounds 4a–4p were tested for their antifungal activity in concert with EUCAST definitive (EDef 7.1) method as reported in literature [53]. Amphotericin B was positive control. The in vitro growth inhibitory activity of the compounds were tested against C. albicans (ATCC 90030), C. krusei (ATCC 6258) and C. parapsilopsis (ATCC 22019). The results were obtained as MIC values and MIC readings were accomplished twice for each chemical agent.
The fungal strains were incubated at 37 °C overnight, then sustained in Roswell Park Memorial Institute (RPMI) medium. Inoculum density was adjusted to 0.5 McFarland turbidity standard by McFarland Densitometer (DEN-1B Mcfarland densitometer, Biosan, Riga, Latvia). The resulting inoculum suspension contains 0.5–2.5 × 105 cfu/mL for fungi. DMSO was used as solvent. Resazurin (20 µg/mL) was used to observe the fungal growth. MIC50 values were determined with a microplate reader at 590 nm excitation, 560 nm emission. Each experiment in antifungal assay was performed twice. The details of the anticandidal assay were reported in our previous study [55].
3.4. Cytotoxicity Assay
The cytotoxicity of the most active compounds (4h and 4p) was determined by MTT assay as previously reported [56]. NIH/3T3 mouse embryonic fibroblast cells (ATCC® CRL-1658™, London, UK) were incubated according to the supplier’s recommendations and seeded as 1 × 104 cells into each well of 96-well plates. The IC50 values were determined by plotting a dose-response curve of inhibition % versus tested concentrations of the compound.
3.5. Quantification of Ergosterol Level
Ergosterol level was determined using the extract of total sterols from C. albicans following the method described by Breivik and Owades [57] with arrangements as described in our previous work [51]. In order to calculate IC50 values against ergosterol biosynthesis, compounds and reference agent were tested at five different concentrations (0.49 µg/mL, 0.98 µg/mL, 1.95 µg/mL, 3.91 µg/mL and 7.81 µg/mL).
3.6. Molecular Docking Studies
Molecular docking studies were performed by using in silico procedure to define the binding modes of compounds 4h and 4p on 14 α-sterol demethylase enzyme active region. X-ray crystal structures of 14 α-sterol demethylase enzyme (PDB code: 5FSA) [54] were retrieved from the Protein Data Bank server (www.pdb.org). The docking procedure was applied using Schrödinger Maestro [58] interface as in previously described by our research group [8,52,59,60].
4. Conclusions
As part of our ongoing efforts to develop new antifungal heterocyclic compounds, in the current study, new benzimidazole-oxadiazole compounds 4a–4p were synthesized by a four-step methodology and evaluated for their anticandidal activity. Among all the compounds 4h and 4p demonstrated comparable inhibitory activity on the growth of tested Candida species as positive controls and were selected as the lead compounds. Two hydroxy groups at m- and p-positions (compounds 4h and 4p) potentiated the biological activity. MTT assays revealed that these compounds are nontoxic against three tested strains at their active concentrations. Inhibition of ergosterol biosynthesis on fungal membranes may be their mechanism of antifungal action. These findings suggest that 4h and 4p are promising lead compounds for the enhancement of new agents in the therapy of fungal infections. In conclusion, we believe that the obtained results add value to our study program in this field and will guide our future studies.
Supplementary Materials
The following are available online, Figures S1–S16: 1H-NMR spectra of compounds 4a–4p.
Author Contributions
Z.A.K. and. Y.Ö. conceived and designed the experiments; U.A.Ç. and D.O. performed the synthesis and microbiological test; Ö.A. and B.K.Ç. performed and analyzed the spectral data; S.L. performed the Cytotoxicity Assay; B.N.S. performed docking and ADME studies; and A.Ç.K. and A.S.K. wrote the paper.
Funding
This study was financially supported by Anadolu University Scientific Research Projects Commission, Project No. 1805S191.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1a, 1b, 2a, 2b, 3a, 3b, 4a–4p are available from the authors.
References
- 1.Pfaller M.A., Diekema D. Epidemiology of invasive Candidiasis: A persistent public health problem. J. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y.N., Bheemanaboina R.R.Y., Cai G.X., Zhou C.H. Novel purine benzimidazoles as antimicrobial agents by regulating ROS generation and targeting clinically resistant Staphylococcus aureus DNA groove. Bioorg. Med. Chem. Lett. 2018;28:1621–1628. doi: 10.1016/j.bmcl.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Kathiravan M.K., Salake A.B., Chothe A.S., Dudhe P.B., Watode R.P., Mukta M.S., Gadhwe S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Chavanet P., Clement C., Duong M., Buisson M., D’Athis P., Dumas M., Bonnin A., Portier H. Toxicity and efficacy of conventional amphotericin B deoxycholate versus escalating doses of amphotericin B deoxycholate—fat emulsion in HIV-infected patients with oral candidosis. Clin. Microbiol. Infect. 1997;3:455–461. doi: 10.1111/j.1469-0691.1997.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 5.Bicanic T., Bottomley C., Loyse A., Brouwer A.E., Muzoora C., Taseera K., Jackson A., Phulusa J., Hosseinipour M.C., van der Horst C., et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob. Agents Chemother. 2015;59:7224–7231. doi: 10.1128/AAC.01698-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maertens J.A. History of the development of azole derivatives. Clin. Microbiol. Infect. 2004;10:1–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 7.Warrilow A.G., Parker J.E., Kelly D.E., Kelly S.L. Azole affinity of sterol 14α-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob. Agents Chemother. 2013;57:1352–1360. doi: 10.1128/AAC.02067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Can N.Ö., Acar Çevik U., Sağlık B.N., Levent S., Korkut B., Özkay Y., Kaplancıklı Z.A., Koparal A.S. Synthesis, molecular docking studies, and antifungal activity evaluation of new benzimidazole-triazoles as potential lanosterol 14 α-demethylase inhibitors. J. Chem. 2017;2017:15. doi: 10.1155/2017/9387102. [DOI] [Google Scholar]
- 9.Saboury A.A., Zolghadri S., Haghbeen K., Moosavi-Movahedi A.A. The inhibitory effect of benzenethiol on the cresolase and catecholase activities of mushroom tyrosinase. J. Enzyme Inhib. Med. Chem. 2006;21:711–717. doi: 10.1080/14756360600810787. [DOI] [PubMed] [Google Scholar]
- 10.Jaenicke E., Decker H. Tyrosinases from crustaceans form hexamers. Biochem. J. 2003;371:515–523. doi: 10.1042/bj20021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Ferrer A., Rodríguez-López J.N., García-Cánovas F., García-Carmona F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1995;1247:1–11. doi: 10.1016/0167-4838(94)00204-T. [DOI] [PubMed] [Google Scholar]
- 12.Pu Y.U., Liu A., Zheng Y., Ye B.I.N. In vitro damage of Candida albicans biofilms by chitosan. Exp. Ther. Med. 2014;8:929–934. doi: 10.3892/etm.2014.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramage G., Saville S.P., Wickes B.L., López-Ribot J.L. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giurg M., Gołąb A., Suchodolski J., Kaleta R., Krasowska A., Piasecki E., Piętka-Ottlik M. Reaction of bis [(2-chlorocarbonyl) phenyl] diselenide with phenols, aminophenols, and other amines towards diphenyl diselenides with antimicrobial and antiviral properties. Molecules. 2017;22:974. doi: 10.3390/molecules22060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wójtowicz H., Kloc K., Maliszewska I., Młochowski J., Piętka M., Piasecki E. Azaanalogues of ebselen as antimicrobial and antiviral agents: Synthesis and properties. II Farm. 2004;59:863–868. doi: 10.1016/j.farmac.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Wójtowicz H., Chojnacka M., Młochowski J., Palus J., Syper L., Hudecova D., Uher M., Piasecki E., Rybka M. Functionalized alkyl and aryl diselenides as antimicrobial and antiviral agents: Synthesis and properties. II Farm. 2003;58:1235–1242. doi: 10.1016/j.farmac.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Piętka-Ottlik M., Burda-Grabowska M., Woźna M., Waleńska J., Kaleta R., Zaczyńska E., Piasecki E., Giurg M. Synthesis of new alkylated and methoxylated analogues of ebselen with antiviral and antimicrobial properties. Arkivoc. 2017:546–556. doi: 10.24820/ark.5550190.p009.797. [DOI] [Google Scholar]
- 18.Chandrika N.T., Shrestha S.K., Ngo H.X., Garneau-Tsodikova S. Synthesis and investigation of novel benzimidazole derivatives as antifungal agents. Bioorg. Med. Chem. 2016;24:3680–3686. doi: 10.1016/j.bmc.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaya Çavuşoğlu B., Yurttaş L., Cantürk Z. The synthesis, antifungal and apoptotic effects of triazole-oxadiazoles against Candida species. Eur. J. Med. Chem. 2018;144:255–261. doi: 10.1016/j.ejmech.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Desai N.C., Kotadiya G.M. Microwave-assisted synthesis of benzimidazole bearing 1,3,4-oxadiazole derivatives: Screening for their in vitro antimicrobial activity. Med. Chem. Res. 2014;23:4021–4033. doi: 10.1007/s00044-014-0978-0. [DOI] [Google Scholar]
- 21.Ansari K.F., Lal C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem. 2009;44:4028–4033. doi: 10.1016/j.ejmech.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Meshram G.A., Vala V.A. Synthesis, characterization, and antimicrobial activity of benzimidazole-derived chalcones containing 1,3,4-oxadiazole moiety. Chem. Heterocycl. Comp. 2015;51:44–50. doi: 10.1007/s10593-015-1653-1. [DOI] [Google Scholar]
- 23.Patel R.V., Patel P.K., Kumari P., Rajani D.P., Chikhalia K.H. Synthesis of benzimidazolyl-1,3,4-oxadiazol-2ylthio-N-phenyl (benzothiazolyl) acetamides as antibacterial, antifungal and antituberculosis agents. Eur. J. Med. Chem. 2012;53:41–51. doi: 10.1016/j.ejmech.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Gaballah S.T., El-Nezhawy A.O., Amer H., Ali M.M., Mahmoud A.E., Hofinger-Horvath A. Synthesis and Antiproliferative Activities of Benzimidazole-Based Sulfide and Sulfoxide Derivatives. Sci. Pharm. 2016;84:1–18. doi: 10.3797/scipharm.1507-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Tan C., Gao C., Zhang C., Luan X., Chen X., Liu H., Chen Y., Jiang Y. Discovery of benzimidazole derivatives as novel multi-target EGFR, VEGFR-2 and PDGFR kinase inhibitors. Bioorg. Med. Chem. 2011;19:4529–4535. doi: 10.1016/j.bmc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Kohara Y., Kubo K., Imamiya E., Wada T., Inada Y., Naka T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J. Med. Chem. 1996;39:5228–5235. doi: 10.1021/jm960547h. [DOI] [PubMed] [Google Scholar]
- 27.Can-Eke B., Puskullu M.O., Buyukbingol E., Iscan M. A study on the antioxidant capacities of some benzimidazoles in rat tissues. Chem. Biol. Interact. 1998;113:65–77. doi: 10.1016/S0009-2797(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu C., Wang Z., Denis D., Greig G., Lamontagne S., O’Neill G., Slipetz D., Wang J. Benzimidazoles as new potent and selective DP antagonists for the treatment of allergic rhinitis. Bioorg. Med. Chem. Lett. 2004;14:3195–3199. doi: 10.1016/j.bmcl.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Demirayak S., Abu Mohsen U., Caqri Karaburun A. Synthesis and anticancer and anti-HIV testing of some pyrazino [1,2-a] benzimidazole derivatives. Eur. J. Med. Chem. 2002;37:255–260. doi: 10.1016/S0223-5234(01)01313-7. [DOI] [PubMed] [Google Scholar]
- 30.Luis F.H., Campos A.H., Castillo R., Vazquez G.N., Arteche O.S., Hernandez M.H., Mulia L.Y. Synthesis and biological activity of 2-(trifluoromethyl)-1H-benzimidazole derivatives against some protozoa and Trichinella spiralis. Eur. J. Med. Chem. 2010;45:3135–3141. doi: 10.1016/j.ejmech.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 31.Achar K.C.S., Hosamani K.M., Seetharamareddy H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010;45:2048–2054. doi: 10.1016/j.ejmech.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Shingalapur R.V., Hosamani K.V., Keri R.S., Hugar M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010;45:1753–1759. doi: 10.1016/j.ejmech.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Anandarajagopal K., Tiwari R.N., Bothara K.G., Sunilson J.A.J., Dineshkumar C., Promwichit P. 2-Mercaptobenzimidazole derivatives: Synthesis and anticonvulsant activity. Adv. Appl. Sci. Res. 2010;1:132–138. [Google Scholar]
- 34.Vasantha K., Basavarajaswamy G., Vaishali Rai M., Boja P., Pai V.R., Shruthi N., Bhat M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015;25:1420–1426. doi: 10.1016/j.bmcl.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Fang X.J., Jeyakkumar P., Avula S.R., Zhou Q., Zhou C.H. Design, synthesis and biological evaluation of 5-fluorouracil-derived benzimidazoles as novel type of potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2016;26:2584–2588. doi: 10.1016/j.bmcl.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 36.El-Gohary N.S., Shaaban M.I. Synthesis, antimicrobial, antiquorum-sensing and antitumor activities of new benzimidazole analogs. Eur. J. Med. Chem. 2017;137:439–449. doi: 10.1016/j.ejmech.2017.05.064. [DOI] [PubMed] [Google Scholar]
- 37.El-Gohary N.S., Shaaban M.I. Synthesis and biological evaluation of a new series of benzimidazole derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Eur. J. Med. Chem. 2017;131:255–262. doi: 10.1016/j.ejmech.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H.Z., He S.C., Peng Y.J., Zhang H.J., Gopala L., Tangadanchu V.K.R., Gan L.L., Zhou C.H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017;136:165–183. doi: 10.1016/j.ejmech.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 39.Ouahrouch A., Ighachane H., Taourirte M., Engels J.W., Sedra M.H., Lazrek H.B. Benzimidazole-1,2,3-triazole Hybrid Molecules: Synthesis and Evaluation for Antibacterial/Antifungal Activity. Arch. Pharm. 2004;347:748–755. doi: 10.1002/ardp.201400142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patil D.N., Chaturvedi S.C., Kale D.L., Kakde R.B., Dhiker S.B. Synthesis and antimicrobial activity of some benzimidazole derivatives. Cont. J. Pharm. Sci. 2008;2:44–48. [Google Scholar]
- 41.Raghunath M., Viswanathan C.L. Benzimidazole-2-carbamic acid as a privileged scaffold for antifungal, anthelmintic and antitumor activity: A Review. Int. J. Pharm. Pharm. Sci. 2014;6:17–25. [Google Scholar]
- 42.He Y., Wu B., Yang J., Robinson D., Risen L., Ranken R., Byln L., Sheng Z., Swayze E.E. 2-Piperidin-4-yl-benzimidazoles with broad spectrum antibacterial activities. Bioorg. Med. Chem. Lett. 2003;13:3253–3256. doi: 10.1016/S0960-894X(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Chen Y.F., Yan W., Cao L.L., Ye Y.H. Synthesis and biological evaluation of benzimidazole phenylhydrazone derivatives as antifungal agents against phytopathogenic fungi. Molecules. 2016;21:1574. doi: 10.3390/molecules21111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husain A., Ahmad A., Alam M.M., Ajmal M., Ahuja P. Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]-1-(biphenyl-4-yl) propan-1-ones as safer antiinflammatory and analgesic agents. Eur. J. Med. Chem. 2009;44:3798–3804. doi: 10.1016/j.ejmech.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Padmavathi V., Reddy G.S., Reddy G.D., Payani T. Synthesis and Antimicrobial Activity of Pyrazolyl 1,3,4-Oxadiazoles. Synth. Commun. 2010;40:482–493. doi: 10.1080/00397910902985531. [DOI] [Google Scholar]
- 46.Zhang X.M., Qiu M., Sun J., Zhang Y.B., Yang Y.S., Wang X.L., Tang J.F., Zhu H.L. Synthesis, biological evaluation, and molecular docking studies of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan moiety as potential anticancer agents. Bioorg. Med. Chem. 2011;19:6518–6524. doi: 10.1016/j.bmc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Wang F., Qin Z., Huang Q. Synthesis and fungicidal activity of 1,3,4-oxadiazole substituted acylthioureas. Front. Chem. China. 2006;1:112–114. doi: 10.1007/s11458-005-0020-7. [DOI] [Google Scholar]
- 48.Almasirad A., Mousavi Z., Tajik M., Assarzadeh M., Shafiee A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1,3,4-oxadiazoles and 1,2,4-triazoles. DARU J. Pharm. Sci. 2014;22:22. doi: 10.1186/2008-2231-22-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhary S.K., Chaudhary M., Chaudhari A., Parmar S.S. Anticonvulsant and antiproteolytic properties of 3,5-disubstituted oxadiazole-2-thiones and their inhibition of respiration in rat brain homogenates. J. Pharm. Sci. 1978;67:1507–1509. doi: 10.1002/jps.2600671104. [DOI] [PubMed] [Google Scholar]
- 50.El-Emam A.A., Al-Deeb O.A., Al-Omar M., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 51.Karaca Gençer H., Acar Çevik U., Levent S., Sağlık B.N., Korkut B., Özkay Y., Ilgın S., Öztürk Y. New benzimidazole-1,2,4-triazole hybrid compounds: Synthesis, anticandidal activity and cytotoxicity evaluation. Molecules. 2017;22:507. doi: 10.3390/molecules22040507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplancıklı Z.A., Levent S., Osmaniye D., Sağlık B.N., Çevik U.A., Çavuşoğlu B.K., Özkay Y., Ilgın S. Synthesis and anticandidal activity evaluation of new benzimidazole-thiazole derivatives. Molecules. 2017;22:2051. doi: 10.3390/molecules22122051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Tudela J.L., Barchiesi F. Subcommittee on Antifungal Susceptibility Testing (AFST). EUCAST Definitive Document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeast. Clin. Microbiol. Infect. 2008;14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 54.Hargrove T.Y., Friggeri L., Wawrzak Z., Qi A., Hoekstra W.J., Schotzinger R.J., York J.D., Guengerich F.P., Lepesheva G.I. Structural analyses of Candida albicans sterol 14 α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017;292:6728–6743. doi: 10.1074/jbc.M117.778308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Can N.Ö., Çevik U.A., Sağlık B.N., Özkay Y., Atlı Ö., Baysal M., Özkay Ü.D., Can Ö.D. Pharmacological and Toxicological Screening of Novel Benzimidazole-Morpholine Derivatives as Dual-Acting Inhibitors. Molecules. 1374;22:1374. doi: 10.3390/molecules22081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Can Ö.D., Osmaniye D., Demir Ö.Ü., Sağlık B.N., Levent S., Ilgın S., Baysal M., Özkay Y., Kaplancıklı Z.A. MAO enzymes inhibitory activity of new benzimidazole derivatives including hydrazone and propargyl side chains. Eur. J. Med. Chem. 2017;131:92–106. doi: 10.1016/j.ejmech.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Breivik O.N., Owades J.L. Yeast analysis, spectrophotometric semimicrodetermination of ergosterol in yeast. Agric. Food Chem. 1957;5:360–363. doi: 10.1021/jf60075a005. [DOI] [Google Scholar]
- 58.Maestro, version 10.6. Schrödinger, LLC; New York, NY, USA: 2016. [Google Scholar]
- 59.Levent S., Kaya Çavuşoğlu B., Sağlık B.N., Osmaniye D., Acar Çevik U., Atlı Ö., Özkay Y., Kaplancıklı Z.A. Synthesis of oxadiazole-thiadiazole hybrids and their anticandidal activity. Molecules. 2017;22:2004. doi: 10.3390/molecules22112004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osmaniye D., Kaya Çavuşoğlu B., Sağlık B.N., Levent S., Acar Çevik U., Atlı Ö., Özkay Y., Kaplancıklı Z.A. Synthesis and anticandidal activity of new ımidazole-chalcones. Molecules. 2018;23:831. doi: 10.3390/molecules23040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.