Abstract
Relapse remains a major obstacle to the survival of patients with hematologic malignancies after allogeneic hematopoietic stem cell transplantation. A disintegrin-like and metalloprotease with a thrombospondin type 1 motif (ADMATS13), which cleaves von Willebrand factor multimers into less active fragments, is encoded by the ADAMTS13 gene and has a functional single-nucleotide polymorphism (SNP) rs2285489 (C > T). We retrospectively examined whether ADAMTS13 rs2285489 affected the transplant outcomes in a cohort of 281 patients who underwent unrelated human leukocyte antigen (HLA)-matched bone marrow transplantation for hematologic malignancies. The recipient ADAMTS13 C/C genotype, which putatively has low inducibility, was associated with an increased relapse rate (hazard ratio [HR], 3.12; 95% confidence interval [CI], 1.25–7.77; P = 0.015), resulting in a lower disease-free survival rate in the patients with a recipient C/C genotype (HR, 1.64; 95% CI, 1.01–2.67; P = 0.045). Therefore, ADAMTS13 rs2285489 genotyping in transplant recipients may be a useful tool for evaluating pretransplantation risks.
Keywords: ADAMTS13, unrelated donor, bone marrow transplantation, single nucleotide polymorphism
1. Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) has curative potential for patients with hematologic malignancies. However, life-threatening complications, such as severe infections, organ damage and graft-versus-host disease (GVHD), may develop eventually. In particular, disease relapse remains a major drawback due to the subsequent dismal outcomes as it accounts for 20–50% of the primary causes of death associated with allo-HSCT [1,2,3]. Although disease characteristics and HLA matching are the major determinants of a survival outcome after allo-HSCT, other factors, such as sex and its matching of recipients and donors, age of donors, pretransplantation cytomegalovirus (CMV) serostatus and genetic polymorphisms other than HLA, have also been suggested as predictors of the post-transplant survival [4,5,6,7,8]. A better understanding of these predictors for each patient may facilitate personalized adaptability and procedures for allo-HSCT.
A disintegrin-like and metalloprotease with thrombospondin type 1 motif (ADMATS13) produced by vascular endothelial cells, hepatic stellate cells, platelets and kidney podocytes is a protease that can cleave von Willebrand factor (vWF) multimers, thereby governing platelet aggregation and inflammation [9]. A low level of ADAMTS13 activity is known to be associated with not only thrombotic microangiopathy (TMA) but also cancer metastasis, the diagnosis of acute leukemia and disease relapse after allo-HSCT [10,11,12,13]. ADAMTS13 is encoded by the ADAMTS13 gene on chromosome 9q34 and has one important single-nucleotide polymorphism (SNP) rs2285489 (C > T) located in an intronic region that is functional, high (>0.3) in the minor allele frequency irrespective of the populations and not linked to any other SNPs [14]. The major allele (C) of the ADAMTS13 rs2285489 SNP has been shown to be associated with lower serum levels of ADAMTS13 than the minor allele (T).
We therefore hypothesized that the ADAMTS13 rs2285489 SNP might be associated with disease relapse after allo-HSCT. To test this hypothesis, we investigated the influence of the ADAMTS13 rs2285489 SNP on transplant outcomes in a cohort of patients undergoing unrelated HLA-matched bone marrow transplantation (BMT) for hematologic malignancies through the Japan Marrow Donor Program (JMDP).
2. Methods
2.1. Patients
ADAMTS13 genotyping was performed on 281 transplantation recipients, including 165 with acute myeloid leukemia (AML) (55.9%), 65 with acute lymphoblastic leukemia (ALL) (23.1%) and 51 with myelodysplastic syndrome (MDS) (18.1%), and their unrelated donors who underwent BMT through the JMDP with T cell-replete marrow from HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQB1 and HLA-DPB1 allele-matched donors between January 2006 and December 2009 (Table 1). The study cohort included only Asian patients and excluded those with any history of transplantation. The final clinical data analyses of these patients were completed by 1 September 2014.
Table 1.
Recipient and donor characteristics according to the recipient ADAMTS13 genotype.
| Recipient ADAMTS13 Genotype, n (%) | ||||
|---|---|---|---|---|
| Variable | Total | C/C, 206 (73.3%) | C/T, 70 (24.9%) T/T, 5 (1.8%) | P |
| Number of cases | 281 | 206 | 75 | |
| Recipient age, years, median (range) | 47 (1–66) | 47 (2–66) | 46 (1–65) | 0.874 |
| Donor age, years, median (range) | 34 (20–66) | 34 (20–66) | 34 (21–55) | 0.582 |
| Year of HSCT, median (range) | 2008 (2006–2009) | 2008 (2006–2009) | 2008 (2006–2009) | 0.787 |
| Donor ADAMTS13 genotype, n (%) | ||||
| C/C | 203 (72.2) | 152 (73.8) | 51 (68.0) | 0.447 |
| C/T | 71 (25.3) | 50 (24.3) | 21 (28.0) | |
| T/T | 7 (2.5) | 4 (1.9) | 3 (4.0) | |
| Patient sex, n (%) | ||||
| Male | 150 (53.4) | 111 (53.9) | 39 (52.0) | 0.789 |
| Female | 131 (46.6) | 95 (46.1) | 36 (48.0) | |
| Donor sex, n (%) | ||||
| Male | 193 (68.7) | 138 (67.0) | 55 (73.3) | 0.383 |
| Female | 88 (31.3) | 68 (33.0) | 20 (26.7) | |
| Recipient/Donor sex match, n (%) | ||||
| Sex-matched | 158 (56.2) | 119 (57.8) | 39 (52.0) | 0.416 |
| Not sex-matched | 123 (43.8) | 87 (42.2) | 36 (48.0) | |
| Disease, n (%) | ||||
| AML | 165 (55.9) | 124 (60.2) | 41 (54.7) | 0.509 |
| ALL | 65 (23.1) | 44 (21.4) | 21 (28.0) | |
| MDS | 51 (18.1) | 38 18.4) | 13 (17.3) | |
| Disease stage, n (%) | ||||
| Standard risk | 198 (70.5) | 148 (71.8) | 50 (66.7) | 0.460 |
| High risk | 83 (29.5) | 58 (28.2) | 25 (33.3) | |
| ABO matching, n (%) | ||||
| ABO-matched | 157 (55.9) | 112 (54.4) | 45 (60.0) | 0.641 |
| Major mismatch | 74 (26.3) | 53 (25.7) | 21 (28.0) | 0.756 |
| Minor mismatch | 69 (24.6) | 55 (26.7) | 14 (18.7) | 0.155 |
| Bidirectional | 19 (6.8) | 14 (6.8) | 5 (6.7) | 1.000 |
| Conditioning regimen, n (%) | 69 (25.1) | 57 (28.1) | 12 (16.7) | |
| Reduced intensity | 209 (74.4) | 147 (71.4) | 62 (82.7) | 0.064 |
| Myeloablative | 72 (25.6) | 59 (28.6) | 13 (17.3) | |
| GVHD prophylaxis, n (%) | ||||
| Cyclosporine | 66 (23.5) | 54 (26.2) | 12 (16.0) | 0.082 |
| Tacrolimus | 215 (76.5) | 152 (73.8) | 63 (84.0) | |
| PS at transplant, n (%) | ||||
| 2–4 | 17 (6.0) | 12 (5.8) | 5 (6.7) | 0.748 |
| Pretransplantation CMV serostatus, n (%) | ||||
| CMV-positive recipient | 222 (82.5) | 162 (78.6) | 60 (80.0) | 0.854 |
| Missing | 12 (4.3) | 10 (4.9) | 2 (2.7) | |
| TNC, ×108/kg, median (range) | 2.77 (0.54–8.83) | 2.66 (0.77–6.29) | 2.91 (0.54–8.83) | 0.502 |
HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; GVHD, graft-versus-host disease; PS, performance status; CMV, cytomegalovirus; TNC, total number of nucleated cells harvested.
The recipients were defined as having high-risk disease if they had AML or ALL during the first complete remission. All other patients were designated as having standard-risk disease. The conditioning regimen varied according to the underlying disease and the condition of the patient. The combination of cyclophosphamide (CY) combined with total body irradiation (TBI) was mainly used for the myeloablative conditioning (MAC) regimen, whereas the combination of fludarabine and melphalan or busulfan was mainly used for the reduced-intensity conditioning (RIC) regimen [15]. Cyclosporine or tacrolimus with short-term methotrexate was used for GVHD prophylaxis [16,17]. No patients received anti-T cell therapy, such as antithymocyte globulin or ex vivo T cell depletion, in this study.
All patients and donors gave their informed consent at the time of transplantation to take part in molecular studies of this nature, which was carried out in accordance with the Declaration of Helsinki. This project was approved by the Institutional Review Board of Aichi Medical University School of Medicine and the JMDP. All methods were performed in accordance with the approved guidelines and regulations.
2.2. Genotyping
Real-time polymerase chain reaction (PCR) genotyping of ADAMTS13 was performed using the TaqMan-Allelic discrimination method as previously described [7]. The genotyping assay was conducted in 96-well PCR plates using specific TaqMan probes for the ADAMTS13 gene SNP rs2285489 (C___3183341_20) in a StepOnePlus Real-time PCR system (Applied Biosystems, Foster City, CA, USA).
2.3. Data Management and Statistical Analyses
Data were collected by the JMDP using a standardized report form. Follow-up reports were submitted after 100 days, 1 year and then annually after transplantation. Only recipients were routinely measured for the pretransplantation CMV serostatus. The time to neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count of more than 0.5 × 109/L. Acute GVHD developing within the first 100 days post-transplantation was diagnosed and graded based on the established criteria [18]. The classification of chronic GVHD observed in patients who survived beyond day 100 was based on the Seattle criteria [19]. The overall survival (OS) was calculated from the date of transplantation to the date of death from any cause. Disease relapse was defined as the number of days from transplantation to disease relapse or progression. The non-relapse mortality (NRM) was defined as death due to any cause other than relapse or disease progression. The disease-free survival (DFS) was defined as the survival without disease relapse or progression. Any patients who were alive at the last follow-up date were censored. Data regarding the clinical and microbiological characteristics of infections, postmortem changes, prophylaxis against infections and therapy for GVHD given on an institutional basis were not considered in this study.
All of the statistical analyses were carried out using the EZR software package [20]. The probabilities of the OS and DFS were calculated using the Kaplan–Meier method and the comparisons between groups were performed via the log-rank test. The occurrence of NRM, disease relapse, acute GVHD and chronic GVHD were compared using the Gray test [21] and analyzed using the cumulative incidence analysis [22] with relapse, death without disease relapse, death without acute GVHD, death without chronic GVHD and death without engraftment taken into consideration as separate competing risks. A multivariate Cox model was constructed for the OS and DFS while a Fine-Gray competing risk regression model was constructed for NRM, relapse, acute GVHD and chronic GVHD to evaluate the hazard ratio (HR) associated with the ADAMTS13 genotypes. Recipient characteristics (age and performance status [PS] at the time of BMT, sex, pretransplantation CMV serostatus, disease and disease risk at transplantation), donor characteristics (age, sex, sex compatibility and ABO compatibility), transplant characteristics (MAC or RIC, total number of nucleated cells [TNC] harvested per recipient weight and the use of cyclosporine or tacrolimus as GVHD prophylaxis) and year of transplantation were all used as confounding factors in the multivariate analyses. The Chi-squared and Mann–Whitney tests were used to compare the results of two groups. For all analyses, P < 0.05 was considered to be statistically significant.
3. Results
3.1. Frequencies of ADAMTS13 Genotypes
The frequencies of C/C, C/T and T/T in the ADAMTS13 rs2285489 C > T were 73.3%, 24.9% and 1.8% among donors and 72.2%, 25.3% and 2.5% among recipients (P = 0.801), respectively (Table 1). Since the T/T genotype frequency was low, we decided to compare the C/C genotype and the C/T or T/T genotype in the subsequent analyses.
3.2. Transplant Outcomes According to ADAMTS13 Genotypes
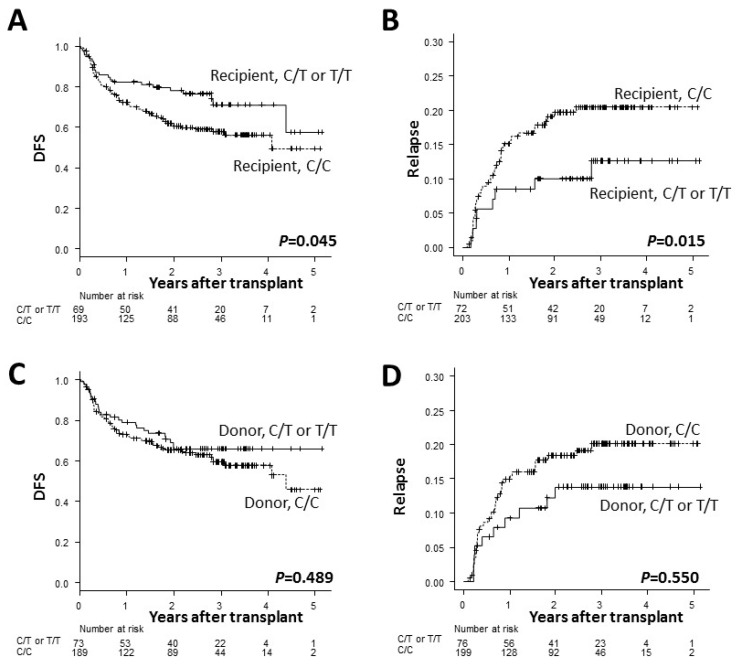
The transplant outcomes according to the ADAMTS13 genotype are summarized in Table 2. After adjusting for confounding factors in the multivariate model (Supplementary Table S1), the recipient C/C genotype was found to be associated with a significantly lower 3-year DFS (58% vs. 71%; HR, 1.64; 95% confidence interval [CI], 1.01–2.67; P = 0.045; Figure 1A) and a significantly higher incidence of relapse (21% vs. 13%; HR, 3.12; 95% CI, 1.25–7.77; P = 0.015; Figure 1B) than the recipient C/T or T/T genotype. These effects were not seen when the donor genotype was examined (Figure 1C,D). Three years was set as the study timepoint according to the median follow-up period among the survivors (median, 825 days; range, 8–1879 days). The proportion of all confounding factors according to the recipient ADAMTS13 genotype was shown in Table 1, in which no significant differences that would have impaired the reliability of the multivariate analysis were noted. The recipient and donor ADAMTS13 genotypes were found to have no significant effect on the OS, NRM or GVHD (Table 2). When distinguishing between the recipient C/T and T/T genotypes and comparing them with the recipient C/C genotype, the recipient T/T genotype tended to be associated with a favorable DFS (Supplementary Figure S1). However, it should be noted that only four patients possessed the T/T genotype.
Table 2.
The results of a multivariate analysis regarding the association between ADAMTS13 variations and clinical outcomes after transplantation.
| Variable | n | Adjusted 3-y DFS | HR (95% CI) | P | Adjusted 3-y OS | HR (95% CI) | P | Adjusted 3-y Relapse | HR (95% CI) | P |
| Overall | 281 | 61% | 63% | 18% | ||||||
| Recipient ADAMTS13, C/C | 206 | 58% | 1.64 (1.01–2.67) | 0.045 | 61% | 1.40 (0.86–2.29) | 0.180 | 21% | 3.12 (1.25–7.77) | 0.015 |
| Recipient ADAMTS13, C/T or T/T | 75 | 71% | 71% | 13% | ||||||
| Donor ADAMTS13, C/C | 203 | 60% | 1.18 (0.74–1.89) | 0.489 | 62% | 1.23 (0.76–2.01) | 0.403 | 20% | 1.26 (0.60–2.66) | 0.550 |
| Donor ADAMTS13, C/T or T/T | 78 | 66% | 67% | 14% | ||||||
| Variable | n | Adjusted 3-y NRM | HR (95% CI) | P | Adjusted grades 2–4 acute GVHD | HR (95% CI) | P | Adjusted chronic GVHD | HR (95% CI) | P |
| Overall | 281 | 23% | 34% | 28% | ||||||
| Recipient ADAMTS13, C/C | 206 | 23% | 1.03 (0.55–1.94) | 0.920 | 35% | 1.08 (0.64–1.81) | 0.770 | 28% | 1.00 (0.57–1.73) | 0.990 |
| Recipient ADAMTS13, C/T or T/T | 75 | 25% | 32% | 27% | ||||||
| Donor ADAMTS13, C/C | 203 | 24% | 1.03 (0.55–1.91) | 0.930 | 34% | 0.93 (0.58–1.49) | 0.750 | 27% | 0.74 (0.46–1.22) | 0.240 |
| Donor ADAMTS13, C/T or T/T | 78 | 22% | 34% | 33% |
DFS, disease-free survival; HR, hazard ratio; OS, overall survival; CI, confidence interval; NRM, non-relapse mortality. Bolded results regarding the genotype represent P < 0.05.
Figure 1.
The Kaplan–Meier curves of the adjusted disease-free survival (DFS) rates (A,C) and the cumulative incidence curves of the adjusted relapse rates (B,D) after transplantation according to the recipient (A,B) and donor (C,D) ADAMTS13 genotypes. The solid lines represent the C/T or T/T genotype while the dashed lines represent the C/C genotype.
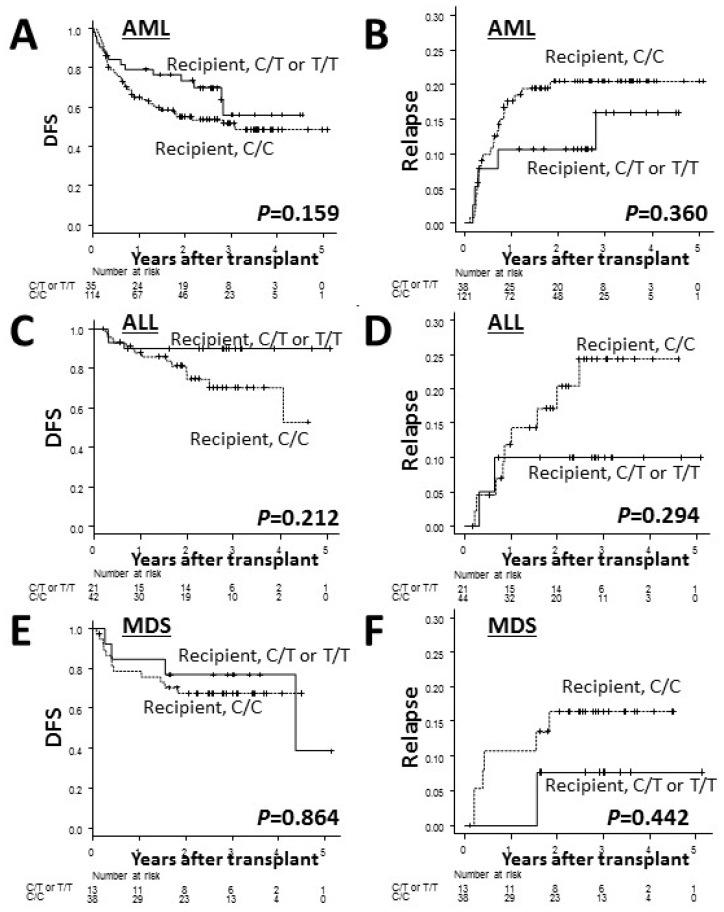
When conducting analyses separately for each disease, the recipient C/C genotype showed a lower adjusted DFS and higher relapse rates than the recipient C/T or T/T genotype although no significant difference was found (Figure 2).
Figure 2.
The Kaplan–Meier curves of the adjusted disease-free survival (DFS) rates (A,C,E) and the cumulative incidence curves of the adjusted relapse rates (B,D,F) after transplantation in patients with AML (A,B), ALL (C,D) and MDS (E,F) according to the recipient ADAMTS13 genotype. The solid lines represent the C/T or T/T genotype while the dashed lines represent the C/C genotype.
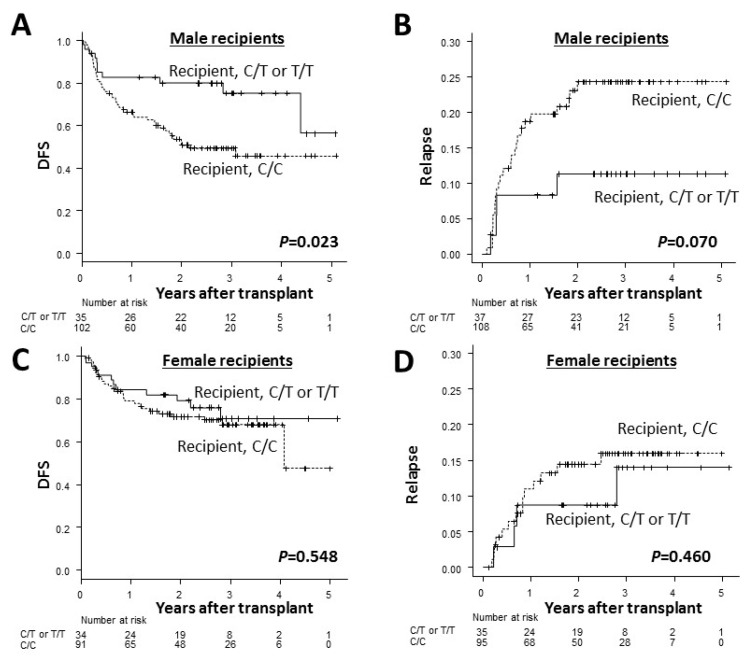
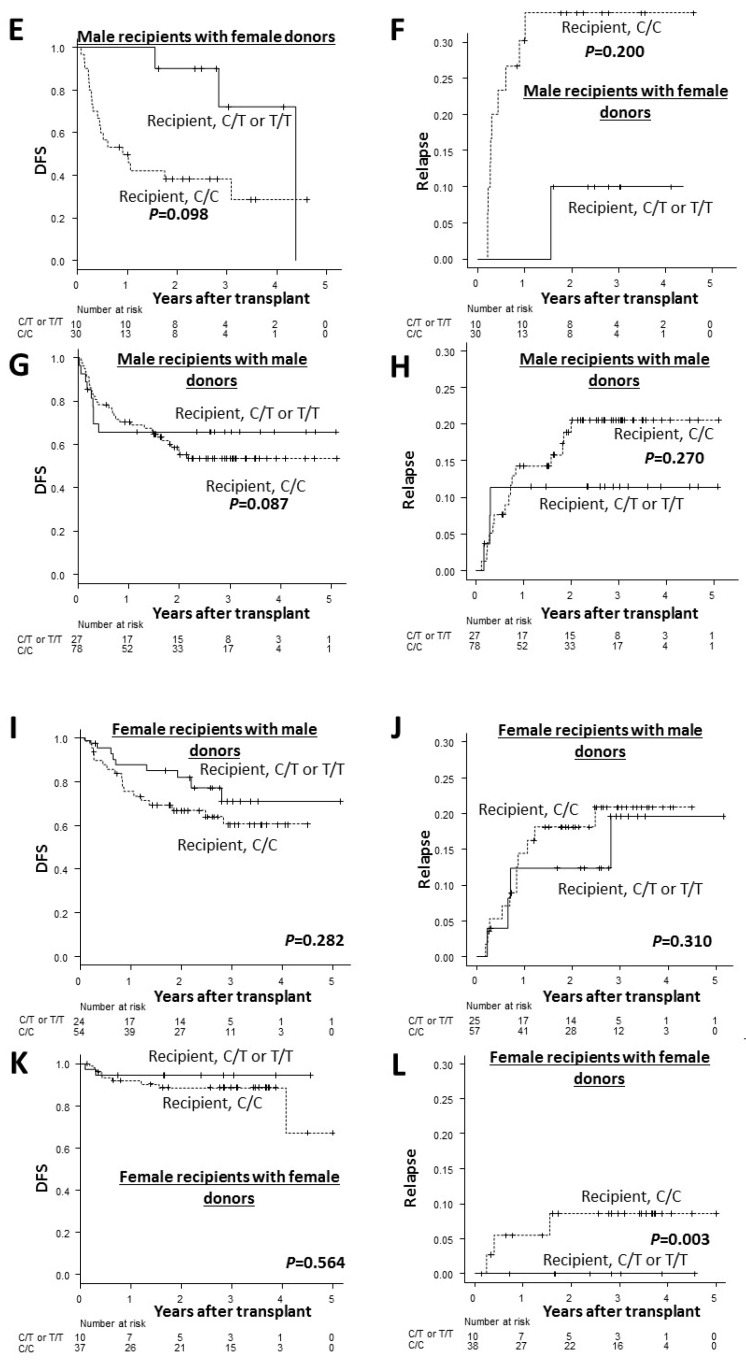
Since recipient sex and sex matching between recipients and donors had a significant influence on the DFS and relapse rates (Supplementary Table S1), a subgroup analysis was performed with a focus on each recipient’s sex and sex matching between recipients and donors. Figure 3 and Supplementary Table S2 show the adjusted DFS and relapse rates according to the recipient ADAMTS13 genotype in each subgroup. In male recipients, the recipient C/C genotype adversely affected the DFS and relapse rate (Figure 3A,B), with this same trend found in the DFS results, regardless of sex mismatches with donors (Figure 3E,G). In contrast, the adverse effects of the recipient C/C genotype on the DFS and relapse rate were not evident in female recipients as a whole (Figure 3C,D). However, when the data were analyzed further by the presence or absence of sex mismatches with donors, the recipient C/C genotype tended to result in a lower DFS and higher relapse rate relative to the recipient C/T and C/T genotypes in both groups (Figure 3J,L), particularly with regard to the relapse rate among female recipients with male donors.
Figure 3.
The Kaplan–Meier curves of the adjusted disease-free survival (DFS) rates (A,C,E,G,I,K) and the cumulative incidence curves of the adjusted relapse rates (B,D,F,H,J,L) after transplantation in male recipients (A,B), female recipients (C,D), male recipients with female donors (E,F), male recipients with male donors (G,H), female recipients with male donors (I,J) and female recipients with female donors (K,L) according to the recipient ADAMTS13 genotype. The solid lines represent the C/T or T/T genotype while the dashed lines represent the C/C genotype.
4. Discussion
The current study showed that the recipient ADAMTS13 rs2285489 C/C genotype, which putatively has lower inducibility of ADAMTS13 than the C/T or T/T genotype [14], was associated with an increased rate of relapse, leading to a lower DFS in patients with hematologic malignancies receiving unrelated BMT than the recipient C/T or T/T genotype.
The mechanism through which the recipient ADAMTS13 rs2285489 C/C genotype exerts its detrimental effects remains unclear. A recent report [14] demonstrated that the ADAMTS13 rs2285489 was functional and the ADAMTS13 major allele (C) was associated with a reduced ADAMTS13 activity, increasing the risk of pediatric stroke compared with its minor allele (T). The lower translational activity associated with the ADAMTS13 rs2285489 C/C genotype in hepatic stellate cells, vascular endothelial cells and kidney podocytes might contribute to an increased incidence of relapse. Evidence of a link between the lower ADAMTS13 activity and the higher relapse rate after allo-HSCT was provided in a recent study [13], which showed that lower ADAMTS13 levels before allo-HSCT predicted higher relapse rates after transplantation in AML patients. This hypothesis may also be supported by the decreased ADAMTS13 levels in AML and ALL patients compared to those in normal individuals [11,12]. Furthermore, it is suggested that endothelial dysfunction due to the increased vWF activity may be related to the progress of acute leukemia [23,24]. Therefore, the reduced ADAMTS13 activity associated with the recipient C/C genotype may result in increased vWF, possibly promoting the progression of hematologic malignancies due to a decreased function of vascular endothelial cells, although these considerations remain speculation because the blood vWF levels were not measured in the current study. Furthermore, it is still unclear whether the reduced risk of relapse associated with the ADAMTS13 minor allele (T) was caused by its anti-malignancy effect or by its induction of graft-versus-malignancy effects. Clarifying these mechanisms may lead to the development of specific anti-hematologic-malignancy therapies and prophylaxis.
The current multivariate analyses showed that the male recipient and sex mismatch between recipients and donors were risk factors for the DFS and relapse rate as well as the recipient ADAMTS13 C/C genotype (Supplementary Table S1). These findings are consistent with previous reports [4,5], in which male recipients with female donors were associated with a lower survival rate after allo-HSCT for hematologic malignancies. Furthermore, in a recent large-scale retrospective study [6], male recipients were associated with a reduced DFS and increase relapse rate compared to female recipients, independent of the donor sex. Our subgroup analyses according to the recipient sex and sex mismatch showed that adverse effects of the recipient C/C genotype on the DFS and relapse rate were consistently observed in male recipients. However, these effects were not apparent in female recipients, except for the relapse rate in female recipients with female donors (Figure 3L). The reason why the effects of the ADAMTS13 SNP on the survival outcomes were not found in the female recipients is unknown. A recent study [25] has reported that blood levels of ADAMTS13 antigens were higher in females than in males, which may suggest that female recipients have a greater amount of ADAMTS13 antigen in the blood and are less likely to be affected by the ADAMTS13 SNP than male recipients, although this is highly speculative. Further studies will be needed to clarify whether the ADAMTS13 antigen can effectively protect transplant recipients against relapse.
Three major limitations associated with the present study warrant mention. One limitation is that the functional roles of the ADAMTS13 SNP in relapse after allo-HSCT remain speculative due to the lack of data from the blood samples of allo-HSCT patients and their donors. Likewise, this study did not determine whether there was an association between the serum concentrations of ADAMTS13 and the ADAMTS13 SNP. The second limitation is that detailed information on sinusoidal obstruction syndrome (SOS)/veno-occlusive syndrome (VOD), such as its incidence and treatment outcomes, was not available in the present study. Furthermore, the effects of the recipient genotypes on the development and treatment outcome of SOS/VOD were unclear, although previous reports [26,27] have suggested an association of a low ADAMTS13 activity with the development of SOS/VOD. Finally, a validation cohort study was not performed to ascertain the current observations because it was beyond the scope of the study, making this an urgent issue to address in the future.
In conclusion, the findings of the present data suggested that the recipient ADAMTS13 genotype predicted the relapse rate and survival outcome after allo-HSCT from unrelated donors. Therefore, ADAMTS13 genotyping in transplant recipients may be a useful tool for evaluating the pretransplantation risks that can form a basis for appropriately tailoring transplantation strategies when combined with other currently known risk factors. If ADAMTS13 genotype information can be used to identify patients prone to relapse in advance, appropriate prophylactic measures and preemptive treatment can be performed prior to hematologic relapse, which may lead to better survival outcomes. Even treatment alternatives other than allo-HSCT should be considered. Aside from HLA and killer-cell immunoglobulin-like receptors [28,29,30], genetic variations that affect the survival outcomes by influencing relapse are rare. Given the plausible functional roles of this SNP, the current findings suggest the pivotal role of ADAMTS13 in disease progression, which may contribute to the development of novel prophylactic and therapeutic strategies for relapse, such as alterations in the in vivo regulation of the ADAMTS13 gene. Further studies are warranted to ascertain whether or not the findings of this study can be expanded to other stem cell sources or to HLA-mismatched transplantation and to validate the present data in other ethnic groups.
Acknowledgments
We thank the Japan Marrow Donor Program and the Japanese Data Center for Hematopoietic Cell Transplantation, and all of the Japan Marrow Donor Program transplant teams who provided valuable assistance in caring for the patients and donors investigated in this study.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/1/214/s1.
Author Contributions
Conceptualization, E.M. and A.T.; Data curation, H.N. and A.T.; Funding acquisition, E.M. and A.T.; Investigation, H.N.; Methodology, H.N. and J.L.E.; Project administration, E.M.; Resources, M.O., K.K., Y.M., T.F., Y.K., N.D., K.M., and T.M.; Supervision, S.N.; Visualization, A.T.; Writing—original draft, A.T. and H.N.; Writing—review & editing, A.T.
Funding
This study was supported by grants from the Ministry of Education, Culture, Sports and Technology of Japan, and the Ministry of Health, Labour and Welfare of Japan. The funders played no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
References
- 1.Schriber J., Agovi M.A., Ho V., Ballen K.K., Bacigalupo A., Lazarus H.M., Bredeson C.N., Gupta V., Maziarz R.T., Hale G.A., et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol. Blood Marrow Transplant. 2010;16:1099–1106. doi: 10.1016/j.bbmt.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takami A., Yano S., Yokoyama H., Kuwatsuka Y., Yamaguchi T., Kanda Y., Morishima Y., Fukuda T., Miyazaki Y., Nakamae H., et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2014;20:1785–1790. doi: 10.1016/j.bbmt.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Schmid C., Labopin M., Nagler A., Niederwieser D., Castagna L., Tabrizi R., Stadler M., Kuball J., Cornelissen J., Vorlicek J., et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 4.Ayuk F., Beelen D.W., Bornhauser M., Stelljes M., Zabelina T., Finke J., Kobbe G., Wolff D., Wagner E.M., Christopeit M., et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2018;24:2558–2567. doi: 10.1016/j.bbmt.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Nakasone H., Remberger M., Tian L., Brodin P., Sahaf B., Wu F., Mattsson J., Lowsky R., Negrin R., Miklos D.B., et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica. 2015;100:1477–1485. doi: 10.3324/haematol.2015.125294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.T., Zhang M.J., Woolfrey A.E., St Martin A., Chen J., Saber W., Perales M.A., Armand P., Eapen M. Donor and recipient sex in allogeneic stem cell transplantation: What really matters. Haematologica. 2016;101:1260–1266. doi: 10.3324/haematol.2016.147645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinoza J.L., Takami A., Onizuka M., Sao H., Akiyama H., Miyamura K., Okamoto S., Inoue M., Kanda Y., Ohtake S., et al. NKG2D gene polymorphism has a significant impact on transplant outcomes after HLA-fully-matched unrelated bone marrow transplantation for standard risk hematologic malignancies. Haematologica. 2009;94:1427–1434. doi: 10.3324/haematol.2009.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinoza J.L., Takami A., Onizuka M., Morishima Y., Fukuda T., Kodera Y., Akiyama H., Miyamura K., Mori T., Nakao S., et al. Recipient PTPN22-1123 C/C genotype predicts acute graft-versus-host disease after HLA fully matched unrelated bone marrow transplantation for hematologic malignancies. Biol. Blood Marrow Transplant. 2013;19:240–246. doi: 10.1016/j.bbmt.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Fujimura Y., Matsumoto M. Registry of 919 patients with thrombotic microangiopathies across Japan: Database of Nara Medical University during 1998–2008. Intern. Med. 2010;49:7–15. doi: 10.2169/internalmedicine.49.2706. [DOI] [PubMed] [Google Scholar]
- 10.Oleksowicz L., Bhagwati N., DeLeon-Fernandez M. Deficient Activity of von Willebrand’s Factor-cleaving Protease in Patients with Disseminated Malignancies. Cancer Res. 1999;59:2244–2250. [PubMed] [Google Scholar]
- 11.Liu C., Zhao L., Zhao J., Xu Q., Song Y., Wang H. Reduced ADAMTS-13 level negatively correlates with inflammation factors in plasma of acute myeloid leukemia patients. Leuk. Res. 2017;53:57–64. doi: 10.1016/j.leukres.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Liu C., Zhao L., Zhao J., Xu Q., Song Y., Wang H. Decreased ADAMTS-13 level is related to inflammation factors and risk stratification of acute lymphoblastic leukemia patients. Medicine. 2017;96:e6136. doi: 10.1097/MD.0000000000006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C., Han M., Zhao L., Zhu M., Xu Q., Song Y., Wang H. ADAMTS-13 activity reduction in plasma of acute myeloid leukemia predicts poor prognosis after bone marrow transplantation. Hematology (Amsterdam, Netherlands) 2019;24:129–133. doi: 10.1080/10245332.2018.1532648. [DOI] [PubMed] [Google Scholar]
- 14.Stoll M., Ruhle F., Witten A., Barysenka A., Arning A., Strauss C., Nowak-Gottl U. Rare Variants in the ADAMTS13 Von Willebrand Factor-Binding Domain Contribute to Pediatric Stroke. Circ. Cardiovasc. Genet. 2016;9:357–367. doi: 10.1161/CIRCGENETICS.115.001184. [DOI] [PubMed] [Google Scholar]
- 15.Giralt S., Ballen K., Rizzo D., Bacigalupo A., Horowitz M., Pasquini M., Sandmaier B. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storb R., Deeg H.J., Whitehead J., Appelbaum F., Beatty P., Bensinger W., Buckner C.D., Clift R., Doney K., Farewell V., et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 17.Nash R.A., Antin J.H., Karanes C., Fay J.W., Avalos B.R., Yeager A.M., Przepiorka D., Davies S., Petersen F.B., Bartels P., et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 18.Przepiorka D., Weisdorf D., Martin P., Klingemann H.G., Beatty P., Hows J., Thomas E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Shulman H.M., Sullivan K.M., Weiden P.L., McDonald G.B., Striker G.E., Sale G.E., Hackman R., Tsoi M.S., Storb R., Thomas E.D. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am. J. Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 20.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooley T.A., Leisenring W., Crowley J., Storer B.E. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat. Med. 1999;18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 22.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: An easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 23.Hatzipantelis E.S., Athanassiou-Metaxa M., Gombakis N., Tzimouli V., Taparkou A., Sidi-Fragandrea V., Garipidou V., Papageorgiou T., Kleta D., Koliouskas D.E., et al. Thrombomodulin and von Willebrand factor: Relation to endothelial dysfunction and disease outcome in children with acute lymphoblastic leukemia. Acta Haematol. 2011;125:130–135. doi: 10.1159/000322120. [DOI] [PubMed] [Google Scholar]
- 24.Athale U., Moghrabi A., Nayiager T., Delva Y.L., Thabane L., Chan A.K. von Willebrand factor and thrombin activation in children with newly diagnosed acute lymphoblastic leukemia: An impact of peripheral blasts. Pediatr. Blood Cancer. 2010;54:963–969. doi: 10.1002/pbc.22466. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z., Dou M., Du X., Ma L., Sun P., Cao H., Ye S., Jiang P., Liu F., Lin F., et al. Influences of ABO blood group, age and gender on plasma coagulation factor VIII, fibrinogen, von Willebrand factor and ADAMTS13 levels in a Chinese population. PeerJ. 2017;5:e3156. doi: 10.7717/peerj.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y.D., Yoshioka A., Kawa K., Ishizashi H., Yagi H., Yamamoto Y., Matsumoto M., Fujimura Y. Impaired activity of plasma von Willebrand factor-cleaving protease may predict the occurrence of hepatic veno-occlusive disease after stem cell transplantation. Bone Marrow Transplant. 2002;29:789–794. doi: 10.1038/sj.bmt.1703544. [DOI] [PubMed] [Google Scholar]
- 27.Kentouche K., Zintl F., Angerhaus D., Fuchs D., Hermann J., Schneppenheim R., Budde U. von Willebrand factor-cleaving protease (ADAMTS13) in the course of stem cell transplantation. Semin. Thromb. Hemost. 2006;32:98–104. doi: 10.1055/s-2006-939765. [DOI] [PubMed] [Google Scholar]
- 28.Bari R., Rujkijyanont P., Sullivan E., Kang G., Turner V., Gan K., Leung W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 2013;31:3782–3790. doi: 10.1200/JCO.2012.47.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer B.C., Hsu K.C. How important is NK alloreactivity and KIR in allogeneic transplantation? Best Pract. Res. Clin. Haematol. 2016;29:351–358. doi: 10.1016/j.beha.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleischhauer K., Beelen D.W. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin. Hematol. 2016;53:57–64. doi: 10.1053/j.seminhematol.2016.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.