Abstract
A simple, economical and metal-free approach to the synthesis of 2-substituted benzoxazoles and 2-substituted benzothiazoles from 2-aminophenols, 2-aminothiophenols and DMF derivatives, only using imidazolium chloride (50% mmol) as promoter without any other additive, was reported. Various 2-substituted benzoxazoles and 2-substituted benzothiazoles were thus prepared in moderate to excellent yields.
Keywords: imidazolium chloride, benzoxazoles and benzothiazoles derivatives, syntheses
1. Introduction
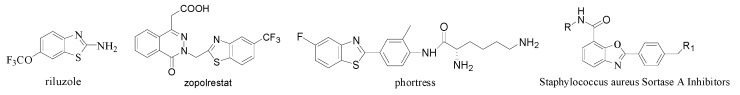
The 2-substituted benzoxazoles and 2-substituted benzothiazoles are well-known substructures in a variety of biologically active natural compounds [1,2], photoluminescent materials [3,4] or pharmaceutical agents such as riluzole, a treatment for amyotrophic lateral sclerosis [5], zopolrestat, an aldose reductase inhibitor used for the treatment of diabetes mellitus [6], the antitumor agent phortress, which acts via binding to aryl hydrocarbon receptors [7], 2-phenylbenzo[d]oxazole-7-carboxamide derivatives which are potential Staphylococcus aureus sortase A Inhibitors [8], and so on [9,10] (Figure 1).
Figure 1.
Biologically active benzoxazole and benzothiazole derivatives.
Because of this importance, much effort has been devoted to the development of synthetic methods for the synthesis of benzoxazole and benzothiazole derivatives. Several methods developed to synthesize 2-substituted and unsubstituted benzoxazoles or benzothiazoles via the condensation O-aminophenols/-thiophenols with aldehydes [11], acids [12], amides [13], acyl chlorides [14], nitriles [15] or esters [16], as well as cyclization of thiobenzanilides have been reported [17,18,19,20].
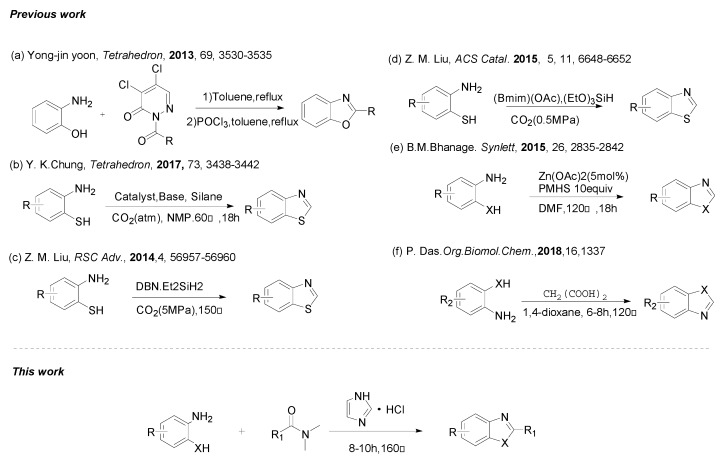
Since these reports, many improvements to these reactions have been made by the use of alternative catalysts. In 2013, Yoon et al. [21] reported an improved method to form benzimidazoles using 2-acyl-4,5-dichloropyridazin-3(2H)-one as acyl transfer agent under transition-metal-free conditions, (Scheme 1a), but, this reagent is expensive and additional POCl3 is needed. Liu and Chung have reported [22,23,24] the synthesis of benzothiazoles from 2-aminobenzenethiol using silane as a CO2 fixing agent. However, the practicality of these methods is offset by the need for a special reactor, the stoichiometric amount of catalyst and limited substrate scope which diminish their synthetic utility in practical application (Scheme 1b–d). Bhanage et al. [25] have reported an improved method to form benzimidazoles from O-phenylenediamines and DMF. In their reports, a metal catalyst (Zn(OAc)2) was employed (Scheme 1e). Recently, Das et al. [26] reported oxalic/malonic acids as carbon building blocks for benzoxazole, quinazoline and quinazolinone synthesis. In their reports, a large amount (4–8 equiv.) of corrosive oxalic/malonic acids were employed (Scheme 1f).
Scheme 1.
Syntheses of benzoxazole and benzothiazole derivatives.
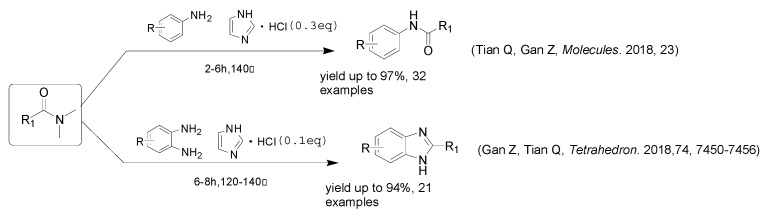
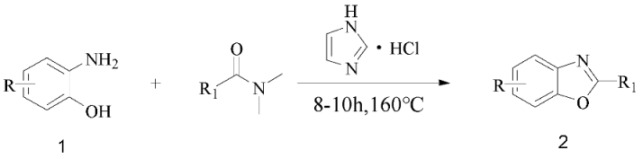
However, most of these methods suffer from one or more disvantages, including the use of some environmental unfriendly catalysts, the use of expensive reagents, long reaction times, toxic or non-reusable catalysts, metal catalysts and additional reagents. Therefore, efficient and environmentally friendly syntheses of 2-substituted benzoxazoles and 2-substituted benzothiazoles heterocycles are of great importance. We have recently reported [27,28] the use of imidazolium chloride as catalyst and DMF derivatives as eco-friendly carbon sources in the formation of amido bonds and benzimidazole derivatives (Scheme 2). We envisioned that this process might be extended to 2-aminophenols and 2-aminothiophenols, which would provide access to benzoxazole and benzothiazole derivatives using inexpensive, stable, and easy to synthesize DMF derivatives as carbon sources and imidazolium chloride as the promoter [29,30,31].
Scheme 2.
Previously explored and reported routes.
2. Results and Discussion
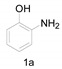
Considering the imidazolium chloride catalyst system has been successfully applied in the formation of amido bonds and benzimidazole derivatives, next, we attempted to synthesize 2-substituted benzoxazole derivatives by reacting O-aminophenol with DMF derivatives under the same conditions, however, the results showed that the main products were acetamide intermediates due to the less reactivity of the hydroxyl group compared to the amino group under these conditions (Table 1, entries 1–4).
Table 1.
Synthesis of 2-substituted-benzoxazoles a.
| Entry | Substance | Product 1 | Yield b (%) | |
|---|---|---|---|---|
| Substance 1 | Substance 2 | |||
| 1 |
|
DMAc |
|
18 |
| 2 |
|

|
|
8 |
| 3 |
|
DMA |
|
15 |
| 4 |
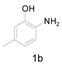
|
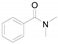
|
|
10 |
aConditions: O-aminophenol derivatives (5.5 mmol, 1 equiv.), DMA (5 mL), N,N-dimethylbenzamide (11 mmol, 2 equiv.), imidazolium chloride (30 mol %), 140 °C, 6 h. b Isolated yields are given. CN,N-dimethyl-acetamide.
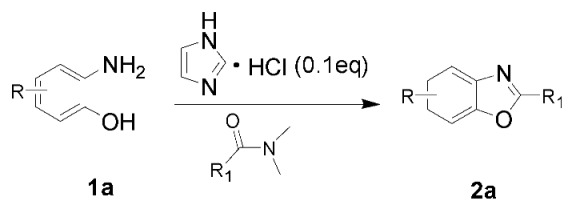
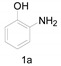
The results of our experiments to optimize the reaction conditions for the synthesis of 2-substituted benzoxazoles, using 2-aminophenol (1a) as a model substrate, are listed in Table 2.
Table 2.
Optimization of reaction conditions a.

| Entry | Cat (equiv.) | Solvent | Temp | Time | Yield b (%) |
|---|---|---|---|---|---|
| 1 | - | DMA | 140 | 8 | - |
| 2 | HCl (0.5) | DMA | 140 | 8 | trace |
| 3 | Imidazolium chloride (0.3) | DMA | 140 | 8 | 18 |
| 4 | Imidazolium chloride (0.3) | DMA | 150 | 8 | 43 |
| 5 | Imidazolium chloride (0.3) | DMA | 160 | 8 | 60 |
| 6 | Imidazolium chloride (0.3) | DMA | 170 | 8 | 62 |
| 7 | Imidazolium chloride (0.3) | DMA | 180 | 8 | 65 |
| 8 | Imidazolium chloride (0.5) | DMA | 160 | 8 | 87 |
| 9 | Imidazolium chloride (0.8) | DMA | 160 | 8 | 85 |
| 10 | Imidazolium chloride (1.0) | DMA | 160 | 8 | 88 |
| 11 c | Imidazolium chloride (0.5) | Xylenes | 140 | 10 | 15 |
| 12 c | Imidazolium chloride (0.5) | H2O | 100 | 10 | Trace |
| 13 c | Imidazolium chloride (0.5) | Benzene | 90 | 10 | Trace |
a All reactions were carried out on an approximately 0.6 g scale using O-aminophenol (1a) (5.5 mmol, 1 equiv.), solvent (5 mL), imidazolium chloride (30–100 mol %). b Isolated yields are given. c DMA (0.54 mL, 6.6 mmol, 1.2 equiv.) and solvent (5 mL).
Initially, the reaction did not occur in DMA without imdazolium chloride (Table 2, entry 1) and 0.5 equiv. of HCl was found to give only traces of product 2a (Table 2, entry 2). The reaction was performed in DMA at 140 °C and we obtained a 18% yield of the product (Table 2, entry 3). Furthermore, on increasing the temperature to 150 and 160 °C for 8 h the product was obtained in 43% and 60% yield, respectively (Table 2, entries 4, 5), indicating that the reaction was highly sensitive to temperature. Encouraged by this promising result, next, with the aim to decrease the imidazolium chloride loading, we then carried out the reaction at 160 °C. It was observed that under these conditions 0.5 equiv. of imidazolium chloride were sufficient to obtain a good yield, and no further improvements in the yield of the target product were observed with higher mole equivalents of imidazolium chloride (Table 2, entries 8, 9 and 10). In addition, we tried various solvents like xylenes, water and benzene, but unsatisfactory yields of the product was obtained (Table 2, entries 11, 12 and 13).
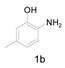
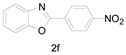
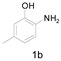
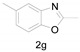
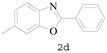
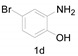
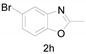
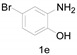
Under the optimized conditions, we next set out to examine the scope and limitations of this reaction and the results are shown in Table 3. All the substrates listed afforded the corresponding target product, under the standard conditions, in moderate to good yield (52–87%). Both electron-donating and electron-withdrawing groups were tolerated under the reaction conditions. Mono-substituents on the benzene moiety showed no obvious influence on the reaction outcome and produced desired products in good yields (Table 3, 2a, 2c, 2g and 2h). It is noteworthy to mention that a functional group like bromo (Table 3, 2h) is tolerable under this condition. With aromatic-substituted substrates, the reaction affords the corresponding benzoxazole derivatives 2b–f, 2d and 2g in 52–86% yields, respectively (Table 2, entries 2, 3, 4, 7 and 9), although a longer reaction time is required. However, when the phenyl ring bore electron-withdrawing substituents, such as a nitro group (Table 3, 2f), the yield was decreased. These results suggest that the electron-withdrawing groups disfavor the formation of the activated reaction intermediate.
Table 3.
Substrate scope with respect to substituted 2-aminophenols a.
| Entry | Substance | Time (h) | Product | Yield b (%) | |
|---|---|---|---|---|---|
| Substance 1 | Substance 2 | ||||
| 1 |
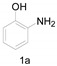
|
DMA | 8 |
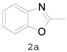
|
80 |
| 2 C |
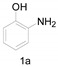
|
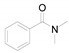
|
10 |

|
86 |
| 3 C |

|
|
10 |
|
88 |
| 4 C |
|
|
10 |
|
52 |
| 5 |
|
DMA | 8 |

|
86 |
| 6 |

|
DMA | 8 |
|
84 |
| 7 C |

|
|
10 |
|
83 |
| 8 |
|
DMA | 8 |
|
87 |
| 9 C |
|
|
10 |
|
80 |
a Experiments were performed with substance 1 (5 mmol) and DMA (5 mL) at 160 °C for 8 h. b Yields after column purification. C Experiments were performed with substance 1 (5 mmol) and DMA derivatives (10 mmol) at 160 °C for 10 h.
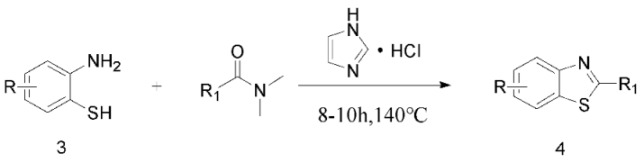
Next, to evaluate further the scope of this methodology, the reaction was carried out with a series of O-aminothiophenol and DMF derivatives, affording the corresponding target products in moderate to good yields (Table 4), under the same optimized conditions.
Table 4.
Substrate scope with respect to substituted 2-aminothiophenols a.
| Entry | Substance | Time (h) | Product | Yield b (%) | |
|---|---|---|---|---|---|
| Substance 1 | Substance 2 | ||||
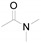
| 1 |
|
|
10 |
|
82 |
| 2 |
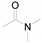
|
|
10 |
|
85 |
| 3 |
|
|
10 |
|
80 |
| 4 |
|
|
10 |
|
75 |
| 5 |
|
|
10 |
|
87 |
| 6 |
|

|
10 |
|
79 |
| 7 |
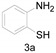
|
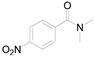
|
10 |
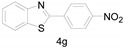
|
60 |
| 8 |
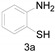
|
|
10 |

|
82 |
| 9 |

|
|
10 |
|
79 |
| 10 |

|
|
10 |
|
75 |
| 11 |
|
|
10 |

|
82 |
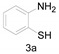
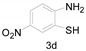
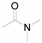
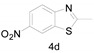
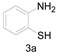
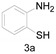
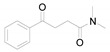
a Experiments were performed with 2-amino-benzenethiol derivatives (5 mmol), DMA derivatives (10 mmol) and DMA (5 mL) at 140 °C for 8–10 h. b Yields after column purification.
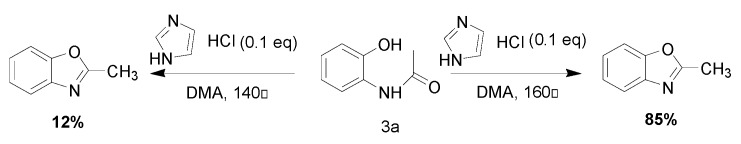
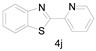
Different para substituents on the O-aminothiophenols 3a–d also gave good yields (75–85%, Table 4, 4a–4k). Similarly, the cyclization reactions of O-aminophenol with aromatic-substituted DMF derivatives also gave the target products 4e–4k in excellent yields. 2-amino-5-nitrobenzenethiol and N,N-Dimethyl-4-nitrobenzamide acid dimethylamide substrate were also found to be stabilized under the conditions and gave the desired products 4d and 4g in moderate yields (75%, 60% and 75%). In addition, a heteroaromatic substrate was well tolerated and gave the desired target product 4j in 75% yield. To explore the reaction mechanism, control experiments were conducted under the standard reaction conditions. By stirring N-(2-hydroxyphenyl)-acetamide with DMA at 140 °C for 8 h in the presence of 0.1 eq imidazolium chloride only 12% yield of target product was obtained (Scheme 3), while a satisfactory yield (85%) was observed at 160 °C, indicating that the reaction was highly sensitive to temperature.
Scheme 3.
Control experiments to elucidate the mechanism.
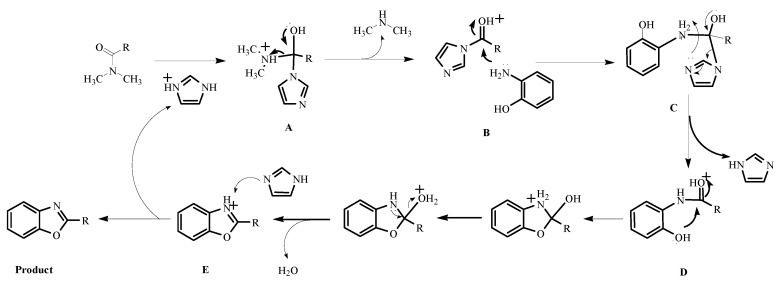
Therefore, based on these experiment results and literature [27,28,32], a plausible mechanism for the formation of benzoxazoles is proposed in Scheme 4. First, DMA is activated by imidazolium chloride to afford the tetrahedral intermediate A. Breakdown of tetrahedral intermediate A leads to the formation of intermediate B (N-acylimidazole) and dimethylamine gas. Next the intermediate B reacts with O-aminophenol to form compound C, followed by extrusion of one molecule of imidazole to generate compound D. Afterwards, the intermediate E was formed by intramolecular cyclization of compound D, and one molecule of H2O was eliminated. Finally, intermediate E furnishes the target product, recycling the imidazole chloride.
Scheme 4.
A plausible mechanism for the imidazolium chloride-promoted cyclization.
3. Conclusions
In conclusion, in the present work, we have reported a new, efficient, metal free and economical method to synthesize 2-substituted benzoxazoles and 2-substituted benzothiazoles from 2-amino-phenols or 2-aminothiophenols and DMF derivatives in the presence of imidazolium chloride without any other catalysts or additives. This method has wide substrate scope, providing moderate to excellent yields of the target products. Further applications of imidazolium chloride in the synthesis of other heterocycles or important medical intermediates are currently under investigation in our laboratory.
4. Experimental
4.1. General Information
All reagents were purchased from Ltd. (Shenzhen, China), Meyer Reagent Co., Ltd. (Shanghai, China), Macklin Reagent Co., Ltd. (Shanghai, China), Chongqing Chuandong Chemical Co., Ltd. (Chongqing, China). etc., and used without further purification. 1H- (600 MHz) and 13C-NMR (151 MHz) spectra were recorded on an Avance III NMR spectrometer (Bruker, Bruker, Fällande, Switzerland) in CDCl3 using tetramethylsilane (TMS) or the residual CHCl3 signals as internally reference. Chemical shifts are reported in ppm and coupling constants (J) in Hz. Open-bed chromatography was carried out on silica gel (200–300 mesh, Qingdao, China) using gravity flow. All substrates are known compounds according to the literature.
4.2. General Procedure for the Synthesis of Benzoxazole Derivatives 2a–2d
A mixture of 1a (0.6 g, 5.5 mmol, 1 equiv.), imidazolium chloride (0.17 g, 1.65 mmol, 0.3 equiv.) and DMA (5 mL) was stirred at 140 °C for 8 h. When the reaction was completed, water (15 mL) and ethyl acetate (20 mL) were added with stirring to the reaction mixture. The organic layer was extracted and dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by column chromatography on silica gel using PE/EA as eluent to give the target product 2a.
4.3. General Procedure for the Synthesis of Benzoxazole Derivatives 2a, 2c, 2g and 2h and Benzothiazole Derivatives 4a–4d
A tube-type Schlenk flask was charged with 1a (0.6 g, 5.5 mmol, 1 equiv.), imidazolium chloride (0.28 g, 2.75 mmol, 0.5 equiv.) and DMA (5 mL) and the mixture was stirred at 160 °C for 8 h. When the reaction was complete water (15 mL) and ethyl acetate (20 mL) were added with stirring to the reaction mixture. The organic layer was extracted and dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by column chromatography on silica gel using PE/EA as eluent to give the corresponding product 4a.
4.4. General Procedure for the Synthesis of Benzoxazole Derivatives 2b, 2d, 2e, 2f, 2i and Benzothiazole Derivatives 4e–4k
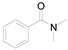
To a mixture of 1a (0.6 g, 5.5 mmol, 1 equiv.), imidazolium chloride (0.25 g, 2.3 mmol, 0.5 equiv.) and N,N-dimethylbenzamide (1.43 g, 11.0 mmol, 2 equiv.) was added. The mixture was stirred at 160 °C for 10 h. After completion of the reaction water (15 mL) was added and the resulting mixture was extracted thrice with EA (20 mL), and the combined organic layers were dried over anhydrous Na2SO4 and concentrated. The residue was purified by column chromatography on silica gel using PE/EA as eluent to obtain the pure desired product.
4.5. Product Characterization Data
2-Methylbenzo[d]oxazole (2a) [33]: The product was obtained as yellow liquid in 80% yield. 1H-NMR δ 7.64 (d, J = 7.2 Hz, 1H), 7.45 (d, J = 7.6 Hz, 1H), 7.29–7.25 (m, 2H), 2.61 (s, 3H). 13C-NMR δ 163.74, 150.94, 141.49, 124.39, 124.04, 119.38, 110.15, 14.46. (See Supplementary Materials).
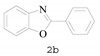
2-Phenylbenzo[d]oxazole (2b) [34]: The product was obtained as white solid in 86% yield. m.p.: 101–103 °C. 1H-NMR δ 8.27 (d, J = 6.3 Hz, 2H), 7.80–7.78 (m, 1H), 7.60–7.58 (m, 1H), 7.53 (d, J = 7.0 Hz, 3H), 7.36 (dd, J = 6.0, 3.1 Hz, 2H). 13C-NMR δ 162.02, 149.72, 140.96, 130.55, 127.90, 126.63, 126.07, 124.12, 123.59, 118.97, 109.58. (See Supplementary Materials).
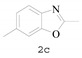
2,6-Dimethylbenzo[d]oxazole (2c) [35]: The product was obtained as yellow liquid in 86% yield. 1H-NMR δ 7.42 (d, J = 8.1 Hz, 1H), 7.17 (s, 1H), 7.01 (d, J = 8.0 Hz, 1H), 2.51 (s, 3H), 2.37 (s, 3H). 13C-NMR δ 163.23, 151.26, 139.25, 134.71, 125.22, 118.70, 110.39, 21.63, 14.46. (See Supplementary Materials).
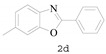
6-Methyl-2-phenylbenzo[d]oxazole (2d) [36]: The product was obtained as yellow solid in 83% yield. m.p.: 90–92 °C. 1H-NMR δ 8.17–8.15 (m, 1H), 7.57 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 1.6 Hz, 1H), 7.31 (s, 1H), 7.10 (d, J = 8.1 Hz, 0H), 2.43 (s, 2H). 13C-NMR δ 161.55, 150.03, 138.87, 134.56, 130.26, 127.85, 126.44, 126.32, 124.79, 118.31, 109.74, 20.78. (See Supplementary Materials).
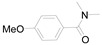
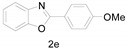
2-(4-Methoxyphenyl)benzo[d]oxazole (2e) [37]: The product was obtained as white solid in 88% yield. m.p.: 97–100 °C. 1H-NMR δ 8.20 (d, J = 8.5 Hz, 2H), 7.74 (d, J = 8.7 Hz, 1H), 7.56 (d, J = 6.8 Hz, 1H), 7.32 (h, J = 6.7, 6.1 Hz, 2H), 7.03 (d, J = 8.3 Hz, 2H), 3.89 (s, 3H).13C-NMR δ 163.17, 162.34, 150.66, 142.21, 129.42, 124.61, 124.43, 119.63, 114.37, 110.39, 55.47. (See Supplementary Materials).
2-(4-Nitrophenyl)benzo[d]oxazole (2f) [38]: The product was obtained as yellow solid in 52% yield. m.p.: 166–167 °C.1H-NMR δ 7.74 (d, J = 6.9 Hz, 2H), 7.52 (t, J = 7.5 Hz, 2H), 7.42 (t, J = 7.8 Hz, 4H), 7.30–7.26 (m, 1H). 13C-NMR δ 160.67, 151.04, 149.42, 141.90, 132.80, 128.42, 126.37, 125.25, 124.25, 120.70, 110.96. (See Supplementary Materials).
2,5-Dimethylbenzo[d]oxazole (2g) [39]: The product was obtained as yellow liquid in 84% yield. 1H-NMR δ 7.42 (s, 1H), 7.30 (d, J = 22.9 Hz, 1H), 7.07 (d, J = 7.6 Hz, 1H), 2.60 (s, 3H), 2.44 (s, 3H). 13C-NMR δ 163.85, 149.19, 141.68, 133.82, 125.43, 119.34, 109.51, 21.38, 14.49. (See Supplementary Materials).
5-Bromo-2-methylbenzo[d]oxazole (2h) [40]: The product was obtained as yellow liquid in 87% yield. 1H-NMR δ 7.78 (d, J = 1.9 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H), 2.64 (s, 3H). 13C-NMR δ 165.10, 149.94, 143.11, 127.45, 122.46, 116.83, 111.42, 14.56. (See Supplementary Materials).
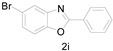
5-Bromo-2-phenylbenzo[d]oxazole (2i) [41]: The product was obtained as white solid in 80% yield. m.p.: 108–110 °C. 1H-NMR δ 8.24 (d, J = 7.5 Hz, 2H), 7.91 (s, 1H), 7.58–7.52 (m, 3H), 7.46 (s, 2H).13C-NMR δ 164.16, 149.74, 143.64, 131.97, 128.99, 128.10, 127.78, 126.60, 122.96, 117.33, 111.81. (See Supplementary Materials).
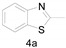
2-Methylbenzo[d]thiazole (4a) [42]: The product was obtained as yellow liquid in 82% yield. 1H-NMR δ 7.95 (d, J = 8.0 Hz, 1H), 7.80 (d, J = 7.3 Hz, 1H), 7.43 (t, J = 7.7 Hz, 1H), 7.34–7.31 (m, 1H), 2.81 (s, 3H). 13C-NMR δ 166.90, 153.36, 135.64, 125.90, 124.68, 122.37, 121.38, 20.09. (See Supplementary Materials).
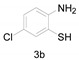
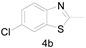
6-Chloro-2-methylbenzo[d]thiazole (4b) [43]: The product was obtained as yellow solid in 85% yield. m.p.: 79–82 °C 1H-NMR δ 7.80 (d, J = 8.6 Hz, 1H), 7.74 (s, 1H), 7.35 (d, J = 8.6 Hz, 1H), 2.78 (s, 3H). 13C-NMR δ 167.78, 151.49, 136.64, 130.79, 126.85, 123.05, 121.08, 20.13. (See Supplementary Materials).
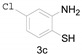
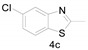
5-Chloro-2-methylbenzo[d]thiazole (4c) [43]: The product was obtained as white solid in 80% yield. m.p.: 60–62 °C. 1H-NMR δ 7.93 (d, J = 2.0 Hz, 1H), 7.73 (d, J = 8.5 Hz, 1H), 7.33 (d, J = 6.5 Hz, 1H), 2.84 (s, 3H). 13C-NMR δ 169.04, 154.13, 133.85, 132.00, 125.24, 122.29, 122.09, 20.23. (See Supplementary Materials).
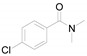
2-Methyl-6-nitrobenzo[d]thiazole (4d) [44]: The product was obtained as yellow solid in 75% yield. m.p.: 161–162 °C. 1H-NMR δ 8.78 (s, 1H), 8.34 (d, J = 6.7 Hz, 1H), 8.04 (d, J = 8.9 Hz,1H), 2.93 (s, 3H). 13C-NMR δ 173.30, 157.09, 144.81, 136.02, 122.65, 121.59, 118.01, 20.71. (See Supplementary Materials).
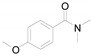
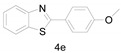
2-(4-Methoxyphenyl)benzo[d]thiazole (4e) [21]: The product was obtained as white solid in 87% yield. m.p.: 119–120 °C. 1H-NMR δ 8.04 (d, J = 8.6 Hz, 3H), 7.87 (d, J = 7.6 Hz, 1H), 7.47 (t, J = 7.4 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H), 3.87 (s, 3H).13C-NMR δ 167.91, 161.96, 154.11, 134.80, 129.15, 126.36, 126.25, 124.83, 122.80, 121.53, 114.57, 114.39, 77.26, 77.13, 55.49. (See Supplementary Materials).
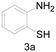
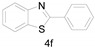
2-Phenylbenzo[d]thiazole (4f) [21]: The product was obtained as white solid in 79% yield. m.p.: 111–113 °C. 1H-NMR δ 8.12–8.08 (m, 3H), 7.91 (d, J = 8.0 Hz, 1H), 7.50 (p, J = 4.1 Hz, 4H), 7.39 (t, J = 7.6 Hz, 1H). 13C-NMR δ 168.15, 153.99, 135.00, 133.52, 131.06, 129.07, 127.62, 126.39, 125.26, 123.23, 121.66. (See Supplementary Materials).
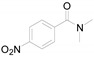
2-(4-Nitrophenyl)benzo[d]thiazole (4g) [45]: The product was obtained as yellow solid in 60% yield. m.p.: 224–226 °C. 1H-NMR δ 8.29 (d, J = 8.9 Hz, 2H), 8.21 (d, J = 8.8 Hz, 2H), 8.07 (d, J = 8.2 Hz, 1H), 7.89 (d, J = 7.8 Hz, 1H), 7.51–7.48 (m, 1H), 7.42–7.39 (m,1H). 13C-NMR δ 163.82, 153.08, 148.02, 138.16, 134.46, 127.23, 125.90, 125.21, 123.30, 122.92, 120.82. (See Supplementary Materials).
2-(4-Chlorophenyl)benzo[d]thiazole (4h) [46]: The product was obtained as white solid in 82% yield. m.p.: 114–115 °C. 1H-NMR) δ 8.07 (d, J = 8.2 Hz, 1H), 8.04–8.01 (m, 2H), 7.90 (dd, J = 8.0, 1.1 Hz, 1H), 7.50 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.47–7.45 (m, 2H), 7.41–7.38 (m, 1H). 13C-NMR δ 166.65, 154.02, 137.06, 135.04, 132.08, 129.29, 128.73, 126.52, 125.45, 123.30, 121.67. (See Supplementary Materials).
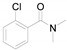
2-(2-Chlorophenyl)benzo[d]thiazole (4i) [46]: The product was obtained as white solid in 79% yield. m.p.: 81–83 °C. 1H-NMR δ 8.24–8.19 (m, 1H), 8.16–8.12 (m, 1H), 7.95 (dd, J = 8.1, 1.1 Hz, 1H), 7.57–7.51 (m, 2H), 7.47–7.39 (m, 3H). 13C-NMR δ 164.20, 152.38, 136.05, 132.71, 132.19, 131.76, 131.17, 130.81, 127.12, 126.32, 125.47, 123.43, 121.40. (See Supplementary Materials).
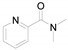
2-(Pyridin-2-yl)benzo[d]thiazole (4j) [47]: The product was obtained as white solid in 75% yield. m.p.: 133–135 °C. 1H-NMR) δ 8.70 (d, J = 4.0 Hz, 1H), 8.39 (d, J = 8.0 Hz, 1H), 8.10 (d, J = 8.2 Hz, 1H), 7.97 (d, J = 7.6 Hz, 1H), 7.86 (t, J = 7.7 Hz, 1H), 7.51 (t, J = 7.0 Hz, 1H), 7.44–7.38 (m, 2H). 13C-NMR δ 169.35, 154.22, 151.37, 149.65, 137.05, 136.11, 126.30, 125.67, 125.29, 123.57, 122.02, 120.81. (See Supplementary Materials).
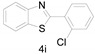
3-Benzothiazol-2-yl-1-phenyl-propan-1-one (4k) [48]: The product was obtained as white solid in 82% yield. m.p.: 93–95 °C. 1H-NMR δ 8.02 (d, J = 7.8 Hz, 2H), 7.95 (d, J = 8.1 Hz, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.57 (t, J = 7.4 Hz, 1H), 7.50–7.40 (m, 3H), 7.34 (t, J = 7.6 Hz, 1H), 3.66 (t, J = 7.1 Hz, 2H), 3.57 (t, J = 7.1 Hz, 2H). 13C-NMR δ 197.87, 170.59, 153.09, 136.50, 135.24, 133.36, 128.69, 128.14, 125.97, 124.85, 122.52, 121.54, 37.64, 28.29. (See Supplementary Materials).
Supplementary Materials
The following are available online, general procedure for 2-substituted benzoxazoles and 2-substituted benzothiazoles of cyclization reaction and 1H-NMR and 13C-NMR spectra of all products.
Author Contributions
Q.T. and J.Y. conceived and designed the experiments; Q.T. and W.L. performed the experiments; Z.G. data curation; D.L. and X.W. analyzed the data; H.W., Z.D. and D.L. contributed reagents/materials/analysis tools; Q.T. wrote the paper.
Funding
This study was financially supported by the Chongqing science and Technology Commission (cstc2018jscx-msybx0294).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Viirre R.D., Ghotas E., Batey R.A. Copper-catalyzed domino annulation approaches to the synthesis of benzoxazoles under microwave-accelerated and conventional thermal conditions. J. Org. Chem. 2008;39:3452–3459. doi: 10.1021/jo702145d. [DOI] [PubMed] [Google Scholar]
- 2.Kaplancikli Z.A., Gülhan T.Z., Revial G., Guven K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl] acetylamino]thiazoles. Arch. Pharm. Res. 2004;27:1081. doi: 10.1007/BF02975108. [DOI] [PubMed] [Google Scholar]
- 3.Carayon C., Fery-Forgues S. 2-Phenylbenzoxazole derivatives: A family of robust emitters of solid-state fluorescence. Photochem. Photobiol. Sci. 2017;16:1020–1035. doi: 10.1039/C7PP00112F. [DOI] [PubMed] [Google Scholar]
- 4.Benelhadj K., Muzuzu W., Massue J., Retailleau P., Charaf-Eddin A., Laurent A.D., Jacquemin D., Ulrich G., Ziessel R. White emitters by tuning the excited-state intramolecular proton-transfer fluorescence emission in 2-(2′-hydroxybenzofuran)benzoxazole dyes. Chemistry. 2014;20:12843–12857. doi: 10.1002/chem.201402717. [DOI] [PubMed] [Google Scholar]
- 5.Aiello S., Wells G., Stone E.L., Kadri H., Bazzi R., Bell D.R., Stevens M.F., Matthews C.S., Bradshaw T.D., Westwell A.D. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648) J. Med. Chem. 2008;51:5135–5139. doi: 10.1021/jm800418z. [DOI] [PubMed] [Google Scholar]
- 6.Mylari B.L., Beyer T.A., Scott P.J., Aldinger C.E., Dee M.F., Siegel T.W., Zembrowski W.J. Potent, orally active aldose reductase inhibitors related to zopolrestat: Surrogates for benzothiazole side chain. J. Med. Chem. 1992;35:457–465. doi: 10.1021/jm00081a006. [DOI] [PubMed] [Google Scholar]
- 7.Ian H., Sharon A.J.B., Rao V., Andrew D.W., Malcolm F.G.S. Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J. Med. Chem. 2002;45:744–747. doi: 10.1021/jm011025r. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Bao J., Deng X.X., He W., Fan J.J., Jiang F.Q., Fu L. Synthesis, biological evaluation and molecular docking of 2-phenyl-benzo[d]oxazole-7-carboxamide derivatives as potential Staphylococcus aureus Sortase A inhibitors. Bioorg. Med. Chem. Lett. 2016;26:4081–4085. doi: 10.1016/j.bmcl.2016.06.074. [DOI] [PubMed] [Google Scholar]
- 9.Kaur A., Pathak D.P., Sharma V., Wakode S. Synthesis, biological evaluation and docking study of a new series of di-substituted benzoxazole derivatives as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. 2018;26:891–902. doi: 10.1016/j.bmc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Satyendra R.V., Vishnumurthy K.A., Vagdevi H.M., Dhananjaya B.L., Shruthi A. Synthesis, in vitro anthelmintic, and molecular docking studies of novel 5-nitro benzoxazole derivatives. Med. Chem. Res. 2015;24:1342–1350. doi: 10.1007/s00044-014-1207-6. [DOI] [PubMed] [Google Scholar]
- 11.Suvendu S., Sudipto D., Papu B. Photocatalysis by 3,6-Disubstituted-s-Tetrazine: Visible-Light Driven Metal-Free Green Synthesis of 2-Substituted Benzimidazole and Benzothiazole. J. Org. Chem. 2014;45:11184–11193. doi: 10.1021/jo401445j. [DOI] [PubMed] [Google Scholar]
- 12.Wen X., Bakali J.E., Deprez-Poulain R., Deprez B. Efficient propylphosphonic anhydride (®T3P) mediated synthesis of benzothiazoles, benzoxazoles and benzimidazoles. Tetrahedron Lett. 2012;53:2440–2443. doi: 10.1016/j.tetlet.2012.03.007. [DOI] [Google Scholar]
- 13.Kozlov N.S., Kiselev B.I. Catalytic synthesis of 2-alkylbenzoxazoles. Chem. Heterocycl. Compd. 1967;2:248–249. doi: 10.1007/BF00742357. [DOI] [Google Scholar]
- 14.Pottorf R.S., Chadha N.K., Katkevics M., Ozola V., Suna E., Ghane H., Regberg T., Player M.R. Parallel synthesis of benzoxazoles via microwave-assisted dielectric heating. Tetrahedron Lett. 2003;44:175–178. doi: 10.1016/S0040-4039(02)02495-4. [DOI] [Google Scholar]
- 15.Yadong S., Huanfeng J., Wanqing W., Wei Z., Xia W. Copper-catalyzed synthesis of substituted benzothiazoles via condensation of 2-aminobenzenethiols with nitriles. Org. Lett. 2013;15:1598–1601. doi: 10.1021/ol400379z. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita H., Lee S.-H., Joung M., Clapham B., Janda K.D. Smart cleavage reactions: The synthesis of benzimidazoles and benzothiazoles from polymer-bound esters. Tetrahedron Lett. 2004;45:313–316. doi: 10.1016/j.tetlet.2003.10.168. [DOI] [Google Scholar]
- 17.Sahoo S.K., Khatun N., Gogoi A., Deb A., Patel B.K. Cu(ii) catalysed chemoselective oxidative transformation of thiourea to thioamidoguanidine/2-aminobenzothiazole. RSC Adv. 2013;3:438–446. doi: 10.1039/C2RA22240J. [DOI] [Google Scholar]
- 18.Liu K., Jia F., Xi H., Li Y., Zheng X., Guo Q., Shen B., Li Z. Direct benzothiophene formation via oxygen-triggered intermolecular cyclization of thiophenols and alkynes assisted by manganese/PhCOOH. Org. Lett. 2013;15:2026–2029. doi: 10.1021/ol400719d. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T., Mase T. A novel practical synthesis of benzothiazoles via Pd-catalyzed thiol cross-coupling. Org. Lett. 2007;9:3687. doi: 10.1021/ol7015737. [DOI] [PubMed] [Google Scholar]
- 20.Evindar G., Batey R.A. Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J. Org. Chem. 2010;37:1802–1808. doi: 10.1002/chin.200629107. [DOI] [PubMed] [Google Scholar]
- 21.Sung G.H., Lee I.-H., Kim B.R., Shin D.-S., Kim J.-J., Lee S.-G., Yoon Y.-J. Eco-friendly atom-economical synthesis of 2-substituted-benzo[d]thiazoles and 2-substituted-benzo[d]oxazoles using 2-acylpyridazin-3(2H)-ones. Tetrahedron. 2013;69:3530–3535. doi: 10.1016/j.tet.2013.02.093. [DOI] [Google Scholar]
- 22.Gao X., Yu B., Zhao Y., Hao L., Liu Z. Hydrosilane-promoted cyclization of 2-aminothiophenols by CO2to benzothiazoles. RSC Adv. 2014;4:56957–56960. doi: 10.1039/C4RA09372K. [DOI] [Google Scholar]
- 23.Gao X., Yu B., Yang Z., Zhao Y., Zhang H., Hao L., Han B., Liu Z. Ionic Liquid-Catalyzed C–S Bond Construction using CO2 as a C1 Building Block under Mild Conditions: A Metal-Free Route to Synthesis of Benzothiazoles. ACS Catal. 2015;5:6648–6652. doi: 10.1021/acscatal.5b01874. [DOI] [Google Scholar]
- 24.Chun S., Yang S., Chung Y.K. Synthesis of benzothiazoles from 2-aminobenzenethiols in the presence of a reusable polythiazolium precatalyst under atmospheric pressure of carbon dioxide. Tetrahedron. 2017;73:3438–3442. doi: 10.1016/j.tet.2017.05.003. [DOI] [Google Scholar]
- 25.Bhanage B., Nale D. N-Substituted Formamides as C1-Sources for the Synthesis of Benzimidazole and Benzothiazole Derivatives by Using Zinc Catalysts. Synlett. 2015;26:2835–2842. doi: 10.1055/s-0035-1560319. [DOI] [Google Scholar]
- 26.Sharma S., Bhattacherjee D., Das P. Oxalic/malonic acids as carbon building blocks for benzazole, quinazoline and quinazolinone synthesis. Org. Biomol. Chem. 2018;16:1337–1342. doi: 10.1039/C7OB03064A. [DOI] [PubMed] [Google Scholar]
- 27.Tian Q., Gan Z., Wang X., Li D., Luo W., Wang H., Dai Z., Yuan J. Imidazolium Chloride: An Efficient Catalyst for Transamidation of Primary Amines. Molecules. 2018;23:2234. doi: 10.3390/molecules23092234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan Z., Tian Q., Shang S., Luo W., Dai Z., Wang H., Li D., Wang X., Yuan J. Imidazolium chloride-catalyzed synthesis of benzimidazoles and 2-substituted benzimidazoles from o-phenylenediamines and DMF derivatives. Tetrahedron. 2018;74:7450–7456. doi: 10.1016/j.tet.2018.11.014. [DOI] [Google Scholar]
- 29.Srinivas M., Hudwekar A.D., Venkateswarlu V., Reddy G.L., Kumar K.A.A., Vishwakarma R.A., Sawant S.D. A metal-free approach for transamidation of amides with amines in aqueous media. Tetrahedron Lett. 2015;56:4775–4779. doi: 10.1016/j.tetlet.2015.06.052. [DOI] [Google Scholar]
- 30.Muzart J. N,N-Dimethylformamide: Much more than a solvent. Tetrahedron. 2009;65:8313–8323. doi: 10.1016/j.tet.2009.06.091. [DOI] [Google Scholar]
- 31.Le Bras J., Muzart J. Recent Uses of N,N-Dimethylformamide and N,N-Dimethylacetamide as Reagents. Molecules. 2018;23:1939. doi: 10.3390/molecules23081939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suchy M., Elmehriki A.A.H., Hudson R.H.E. A Remarkably Simple Protocol for the N-Formylation of Amino Acid Esters and Primary Amines. Org. Lett. 2011;13:3952–3955. doi: 10.1021/ol201475j. [DOI] [PubMed] [Google Scholar]
- 33.Mayo M.S., Yu X., Zhou X., Feng X., Yamamoto Y., Bao M. Convenient synthesis of benzothiazoles and benzimidazoles through Bronsted acid catalyzed cyclization of 2-amino thiophenols/anilines with beta-diketones. Org. Lett. 2014;16:764–767. doi: 10.1021/ol403475v. [DOI] [PubMed] [Google Scholar]
- 34.Bochatay V.N., Boissarie P.J., Murphy J.A., Suckling C.J., Lang S. Mechanistic exploration of the palladium-catalyzed process for the synthesis of benzoxazoles and benzothiazoles. J. Org. Chem. 2013;78:1471–1477. doi: 10.1021/jo302544d. [DOI] [PubMed] [Google Scholar]
- 35.Rancan E., Aricò F., Quartarone G., Ronchin L., Vavasori A. Acid Catalyzed Direct-Amidation–Dehydrocyclization of 2-Hydroxy-acetophenones to Benzoxazoles by a One-Pot Sustainable Synthesis. Catal. Lett. 2015;145:939–946. doi: 10.1007/s10562-014-1466-3. [DOI] [Google Scholar]
- 36.Wang Y., Wu C., Nie S., Xu D., Yu M., Yao X. Ligand-promoted, copper nanoparticles catalyzed one-pot synthesis of substituted benzoxazoles from 2-bromoanilines and acyl chlorides. Tetrahedron Lett. 2015;56:6827–6832. doi: 10.1016/j.tetlet.2015.10.078. [DOI] [Google Scholar]
- 37.Teo Y.C., Riduan S.N., Zhang Y. Iodine-mediated arylation of benzoxazoles with aldehydes. Green Chem. 2013;15:2365–2368. doi: 10.1039/c3gc41027g. [DOI] [Google Scholar]
- 38.Azizian J., Torabi P., Noei J. Synthesis of benzimidazoles and benzoxazoles using TiCl 3 OTf in ethanol at room temperature. Tetrahedron Lett. 2016;57:185–188. doi: 10.1016/j.tetlet.2015.11.092. [DOI] [Google Scholar]
- 39.Aksenov N.A., Aksenov A.V., Nadein O.N., Aksenov D.A., Smirnov A.N., Rubin M. One-pot synthesis of benzoxazoles via the metal-free ortho-C–H functionalization of phenols with nitroalkanes. RSC Adv. 2015;5:71620–71626. doi: 10.1039/C5RA15128G. [DOI] [Google Scholar]
- 40.Zhang X., Huang R., Marrot J., Coeffard V., Xiong Y. Hypervalent iodine-mediated synthesis of benzoxazoles and benzimidazoles via an oxidative rearrangement. Tetrahedron. 2015;71:700–708. doi: 10.1016/j.tet.2014.11.066. [DOI] [Google Scholar]
- 41.Wang L., Ma Z.-G., Wei X.-J., Meng Q.-Y., Yang D.-T., Du S.-F., Chen Z.-F., Wu L.-Z., Liu Q. Synthesis of 2-substituted pyrimidines and benzoxazoles via a visible-light-driven organocatalytic aerobic oxidation: Enhancement of the reaction rate and selectivity by a base. Green Chem. 2014;16:3752–3757. doi: 10.1039/C4GC00337C. [DOI] [Google Scholar]
- 42.Mortimer C.G., Wells G., Crochard J.P., Stone E.L., Bradshaw T.D., Stevens M.F., Westwell A.D. Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem. 2006;49:179–185. doi: 10.1021/jm050942k. [DOI] [PubMed] [Google Scholar]
- 43.Huang X., Tang J. Solid phase synthesis of benzothiazole and thiophene derivatives based on resin-bound cyclic malonic acid ester. Tetrahedron. 2003;59:4851–4856. doi: 10.1016/S0040-4020(03)00688-4. [DOI] [Google Scholar]
- 44.Shimada T., Yamamoto Y. Carbon—Carbon Bond Cleavage of Diynes Through the Hydroamination with Transition Metal Catalysts. J. Am. Chem. Soc. 2003;125:6646–6647. doi: 10.1021/ja034105o. [DOI] [PubMed] [Google Scholar]
- 45.Ranjit S., Liu X. Direct arylation of benzothiazoles and benzoxazoles with aryl boronic acids. Chemistry. 2011;17:1105–1108. doi: 10.1002/chem.201002787. [DOI] [PubMed] [Google Scholar]
- 46.Kumar P., Meenakshi, Kumar S., Kumar A., Hussain K., Kumar S. Solvent-Free One Pot Synthesis of 2-aryl/Heteroarylbenzothiazoles Using Hypervalent Iodine (III) Reagents. J. Heterocycl. Chem. 2012;49:1243–1249. doi: 10.1002/jhet.962. [DOI] [Google Scholar]
- 47.Huang Y., Yan D., Wang X., Zhou P., Wu W., Jiang H. Controllable assembly of the benzothiazole framework using a C[triple bond, length as m-dash]C triple bond as a one-carbon synthon. Chem. Commun. 2018;54:1742–1745. doi: 10.1039/C7CC09855C. [DOI] [PubMed] [Google Scholar]
- 48.Lu S.C., Li H.S., Xu S., Duan G.Y. Silver-catalyzed C2-selective direct alkylation of heteroarenes with tertiary cycloalkanols. Org. Biomol. Chem. 2017;15:324–327. doi: 10.1039/C6OB02330D. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.